Pesticide Type Distinctly Shapes Soil Resistomes: A Comparative Analysis of Antibiotic and Non-Antibiotic Agro-Chemicals
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Compilation
2.2. Taxonomic and Pathogen Annotation
2.3. ARG Annotation and Host Analyses
2.4. Statistical Analyses
3. Results
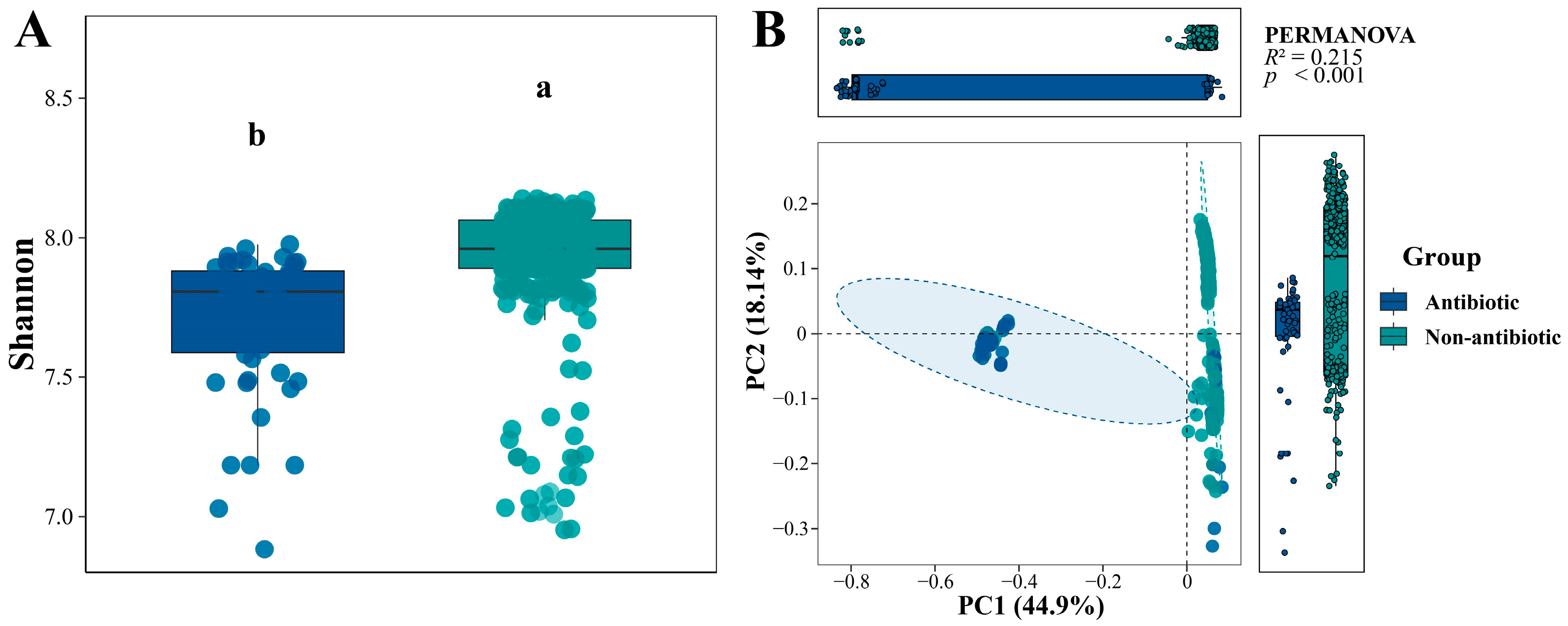
3.1. Pesticide Type Differentially Shapes Soil ARG Composition and Diversity
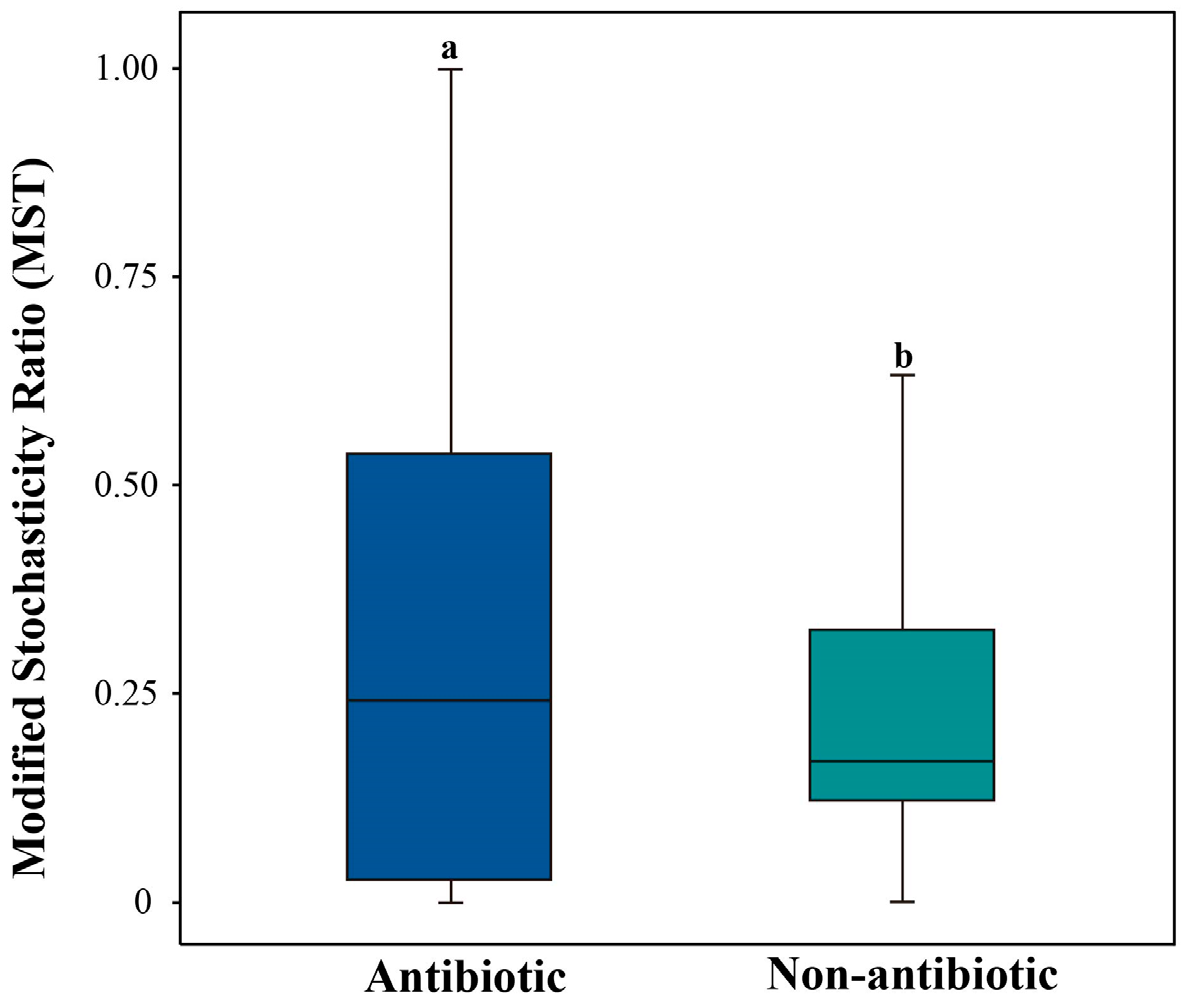
3.2. Deterministic Processes Dominate ARG Assembly Across Different Pesticide-Use Soils
3.3. ARG Co-Occurrence Patterns Vary with Pesticide Type
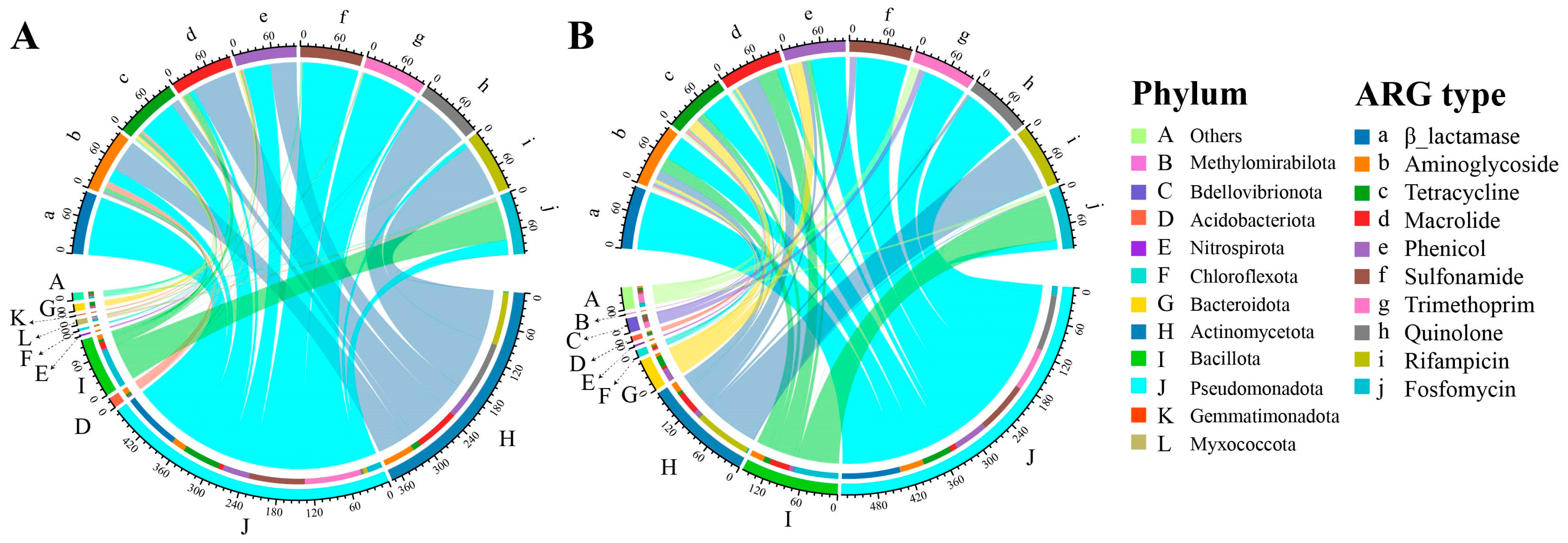
3.4. Distinct ARG Host Community Under Antibiotic and Non-Antibiotic Pesticides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Achmon, Y.; Cao, Y.; Liang, X.; Chen, L.; Wang, H.; Siame, B.A.; Leung, K.Y. Distribution of Antibiotic Resistance Genes in the Environment. Environ. Pollut. 2021, 285, 117402. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Hu, H.-W.; Maestre, F.T.; Guerra, C.A.; Eisenhauer, N.; Eldridge, D.J.; Zhu, Y.-G.; Chen, Q.-L.; Trivedi, P.; Du, S.; et al. The Global Distribution and Environmental Drivers of the Soil Antibiotic Resistome. Microbiome 2022, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Li, H.; Duan, C.-S.; Zhou, X.-Y.; An, X.-L.; Zhu, Y.-G.; Su, J.-Q. Metagenomic and Viromic Analysis Reveal the Anthropogenic Impacts on the Plasmid and Phage Borne Transferable Resistome in Soil. Environ. Int. 2022, 170, 107595. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Zhu, Q.; Zhong, G.; Liu, J. Biodegradation of Soil Agrochemical Contamination Mitigates the Direct Horizontal Transfer Risk of Antibiotic Resistance Genes to Crops. Sci. Total Environ. 2023, 901, 166454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Cheng, L.-C.; Zhao, F.-J.; Chen, M.-M.; Wang, P. Chiral Pesticides Selectively Influence the Dissemination of Antibiotic Resistance Genes: An Overlooked Environmental Risk. Environ. Sci. Technol. 2025, 59, 13374–13384. [Google Scholar] [CrossRef]
- Liao, H.; Li, X.; Yang, Q.; Bai, Y.; Cui, P.; Wen, C.; Liu, C.; Chen, Z.; Tang, J.; Che, J.; et al. Herbicide Selection Promotes Antibiotic Resistance in Soil Microbiomes. Mol. Biol. Evol. 2021, 38, 2337–2350. [Google Scholar] [CrossRef]
- Zeng, Y.; Feng, R.; Huang, C.; Liu, J.; Yang, F. Antibiotic Resistance Genes in Agricultural Soils: A Comprehensive Review of the Hidden Crisis and Exploring Control Strategies. Toxics 2025, 13, 239. [Google Scholar] [CrossRef]
- Huang, C.; Cui, M.; Li, T.; Zheng, C.; Qiu, M.; Shan, M.; Li, B.; Zhang, L.; Yu, Y.; Fang, H. Migration of Fungicides, Antibiotics and Resistome in the Soil-Lettuce System. J. Hazard. Mater. 2025, 484, 136725. [Google Scholar] [CrossRef]
- Qiu, D.; Ke, M.; Zhang, Q.; Zhang, F.; Lu, T.; Sun, L.; Qian, H. Response of Microbial Antibiotic Resistance to Pesticides: An Emerging Health Threat. Sci. Total Environ. 2022, 850, 158057. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S.; Zhang, Q.; Long, Z.; Yu, Y.; Fang, H. Fungicides Enhanced the Abundance of Antibiotic Resistance Genes in Greenhouse Soil. Environ. Pollut. 2020, 259, 113877. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, K.; Athiappan, M.; Devarajan, N.; Parray, J.A. Emergence of Multi Drug Resistance among Soil Bacteria Exposing to Insecticides. Microb. Pathog. 2017, 105, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Ferreira, J.P.; LeJeune, J.T. Antimicrobial Use and Resistance in Plant Agriculture: A One Health Perspective. Agriculture 2022, 12, 289. [Google Scholar] [CrossRef]
- USEPA What Are Antimicrobial Pesticides? Available online: https://www.epa.gov/pesticide-registration/what-are-antimicrobial-pesticides (accessed on 18 April 2025).
- WHO Critically Important Antimicrobials for Human Medicine: 6th Revision. Available online: https://www.who.int/publications/i/item/9789241515528 (accessed on 18 April 2025).
- Sundin, G.W.; Monks, D.E.; Bender, C.L. Distribution of the Streptomycin-Resistance Transposon Tn 5393 among Phylloplane and Soil Bacteria from Managed Agricultural Habitats. Can. J. Microbiol. 1995, 41, 792–799. [Google Scholar] [CrossRef]
- Verhaegen, M.; Bergot, T.; Liebana, E.; Stancanelli, G.; Streissl, F.; Mingeot-Leclercq, M.-P.; Mahillon, J.; Bragard, C. On the Use of Antibiotics to Control Plant Pathogenic Bacteria: A Genetic and Genomic Perspective. Front. Microbiol. 2023, 14, 1221478. [Google Scholar] [CrossRef]
- Shentu, J.; Zhang, K.; Shen, D.; Wang, M.; Feng, H. Effect from Low-Level Exposure of Oxytetracycline on Abundance of Tetracycline Resistance Genes in Arable Soils. Environ. Sci. Pollut. Res. 2015, 22, 13102–13110. [Google Scholar] [CrossRef] [PubMed]
- Herbert, A.; Hancock, C.N.; Cox, B.; Schnabel, G.; Moreno, D.; Carvalho, R.; Jones, J.; Paret, M.; Geng, X.; Wang, H. Oxytetracycline and Streptomycin Resistance Genes in Xanthomonas Arboricola Pv. Pruni, the Causal Agent of Bacterial Spot in Peach. Front. Microbiol. 2022, 13, 821808. [Google Scholar] [CrossRef]
- Cerqueira, F.; Matamoros, V.; Bayona, J.M.; Berendonk, T.U.; Elsinga, G.; Hornstra, L.M.; Piña, B. Antibiotic Resistance Gene Distribution in Agricultural Fields and Crops. A Soil-to-Food Analysis. Environ. Res. 2019, 177, 108608. [Google Scholar] [CrossRef]
- Guo, Y.; Qiu, T.; Gao, M.; Sun, Y.; Cheng, S.; Gao, H.; Wang, X. Diversity and Abundance of Antibiotic Resistance Genes in Rhizosphere Soil and Endophytes of Leafy Vegetables: Focusing on the Effect of the Vegetable Species. J. Hazard. Mater. 2021, 415, 125595. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, G.; Zhang, Y.; Wang, N.; Zhang, Y.; Wang, X.; Zhao, F.; Xu, Y.; Shen, Q.; Wei, Z. MBPD: A Multiple Bacterial Pathogen Detection Pipeline for One Health Practices. iMeta 2023, 2, e82. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Chen, M.; Yan, X.; Tang, Q.; Liu, M.; Yang, M.; Chai, Y.; Wei, Y.; Shen, P.; Zhang, J. Particle Size Transfer of Antibiotic Resistance Genes in Typical Processes of Municipal Wastewater Treatment Plant. Bioresour. Technol. 2025, 424, 132288. [Google Scholar] [CrossRef] [PubMed]
- Dželalija, M.; Kvesić-Ivanković, M.; Jozić, S.; Ordulj, M.; Kalinić, H.; Pavlinović, A.; Šamanić, I.; Maravić, A. Marine Resistome of a Temperate Zone: Distribution, Diversity, and Driving Factors across the Trophic Gradient. Water Res. 2023, 246, 120688. [Google Scholar] [CrossRef]
- Gao, Y.-X.; Li, X.; Zhao, J.-R.; Zhang, Z.-X.; Fan, X.-Y. Response of Microbial Communities Based on Full-Scale Classification and Antibiotic Resistance Genes to Azithromycin and Copper Combined Pollution in Activated Sludge Nitrification Laboratory Mesocosms at Low Temperature. Bioresour. Technol. 2021, 341, 125859. [Google Scholar] [CrossRef]
- Kemp, P.F.; Aller, J.Y. Bacterial Diversity in Aquatic and Other Environments: What 16S RDNA Libraries Can Tell Us. FEMS Microbiol. Ecol. 2004, 47, 161–177. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Stanek, M.; Rola, K.; Zubek, S. Herbaceous Plant Species Support Soil Microbial Performance in Deciduous Temperate Forests. Sci. Total Environ. 2022, 810, 151313. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Kent, A.D.; Brisson, V.L.; Gaudin, A.C.M.M. Agricultural Management and Plant Selection Interactively Affect Rhizosphere Microbial Community Structure and Nitrogen Cycling. Microbiome 2019, 7, 146. [Google Scholar] [CrossRef]
- Ning, D.; Deng, Y.; Tiedje, J.M.; Zhou, J. A General Framework for Quantitatively Assessing Ecological Stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [CrossRef]
- Wang, F.; Han, W.; Chen, S.; Dong, W.; Qiao, M.; Hu, C.; Liu, B. Fifteen-Year Application of Manure and Chemical Fertilizers Differently Impacts Soil ARGs and Microbial Community Structure. Front. Microbiol. 2020, 11, 62. [Google Scholar] [CrossRef]
- Jauregi, L.; Epelde, L.; Alkorta, I.; Garbisu, C. Antibiotic Resistance in Agricultural Soil and Crops Associated to the Application of Cow Manure-Derived Amendments From Conventional and Organic Livestock Farms. Front. Vet. Sci. 2021, 8, 633858. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; Zhang, B.; Wang, J.; Zhu, L.; Hu, B. Deterministic Effect of PH on Shaping Soil Resistome Revealed by Metagenomic Analysis. Environ. Sci. Technol. 2023, 57, 985–996. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Y.; Wen, Y.; Peng, Z.; Xu, D.; Xiao, J. Divergent Risk Gene Profiles in Smallholder and Large-Scale Paddy Farms. Environ. Res. 2025, 285, 122406. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ning, D. Stochastic Community Assembly: Does It Matter in Microbial Ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Guo, Y.; Isabwe, A.; Chen, H.; Wang, Y.; Zhang, Y.; Zhu, Z.; Yang, J. Urbanization Drives Riverine Bacterial Antibiotic Resistome More than Taxonomic Community at Watershed Scale. Environ. Int. 2020, 137, 105524. [Google Scholar] [CrossRef]
- Vanwonterghem, I.; Jensen, P.D.; Dennis, P.G.; Hugenholtz, P.; Rabaey, K.; Tyson, G.W. Deterministic Processes Guide Long-Term Synchronised Population Dynamics in Replicate Anaerobic Digesters. ISME J. 2014, 8, 2015–2028. [Google Scholar] [CrossRef]
- Zhao, H.; Brearley, F.Q.; Huang, L.; Tang, J.; Xu, Q.; Li, X.; Huang, Y.; Zou, S.; Chen, X.; Hou, W.; et al. Abundant and Rare Taxa of Planktonic Fungal Community Exhibit Distinct Assembly Patterns Along Coastal Eutrophication Gradient. Microb. Ecol. 2023, 85, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, Y.; Ma, L.; Ju, F.; Guo, F.; Tiedje, J.M.; Zhang, T. Metagenomic and Network Analysis Reveal Wide Distribution and Co-Occurrence of Environmental Antibiotic Resistance Genes. ISME J. 2015, 9, 2490–2502. [Google Scholar] [CrossRef]
- Zheng, D.; Yin, G.; Liu, M.; Hou, L.; Yang, Y.; Van Boeckel, T.P.; Zheng, Y.; Li, Y. Global Biogeography and Projection of Soil Antibiotic Resistance Genes. Sci. Adv. 2022, 8, eabq8015. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Gu, J.; Feng, Y.; He, K.; Jiang, H. Effects of Soil Solarization Combined with Manure-Amended on Soil ARGs and Microbial Communities during Summer Fallow. Environ. Pollut. 2023, 333, 121950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, D.; Zhang, Y.; Xie, J.; Xiong, H.; Wan, Y.; Zhang, Y.; Chen, X.; Shi, X. Soil Type Shapes the Antibiotic Resistome Profiles of Long-Term Manured Soil. Sci. Total Environ. 2021, 786, 147361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-M.; Liu, X.; Wang, S.-L.; Fang, L.-X.; Sun, J.; Liu, Y.-H.; Liao, X.-P. Distribution Patterns of Antibiotic Resistance Genes and Their Bacterial Hosts in Pig Farm Wastewater Treatment Systems and Soil Fertilized with Pig Manure. Sci. Total Environ. 2021, 758, 143654. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Nakashima, T. Actinomycetes, an Inexhaustible Source of Naturally Occurring Antibiotics. Antibiotics 2018, 7, 45. [Google Scholar] [CrossRef]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef]
- Fatahi-Bafghi, M. Antibiotic Resistance Genes in the Actinobacteria Phylum. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1599–1624. [Google Scholar] [CrossRef]
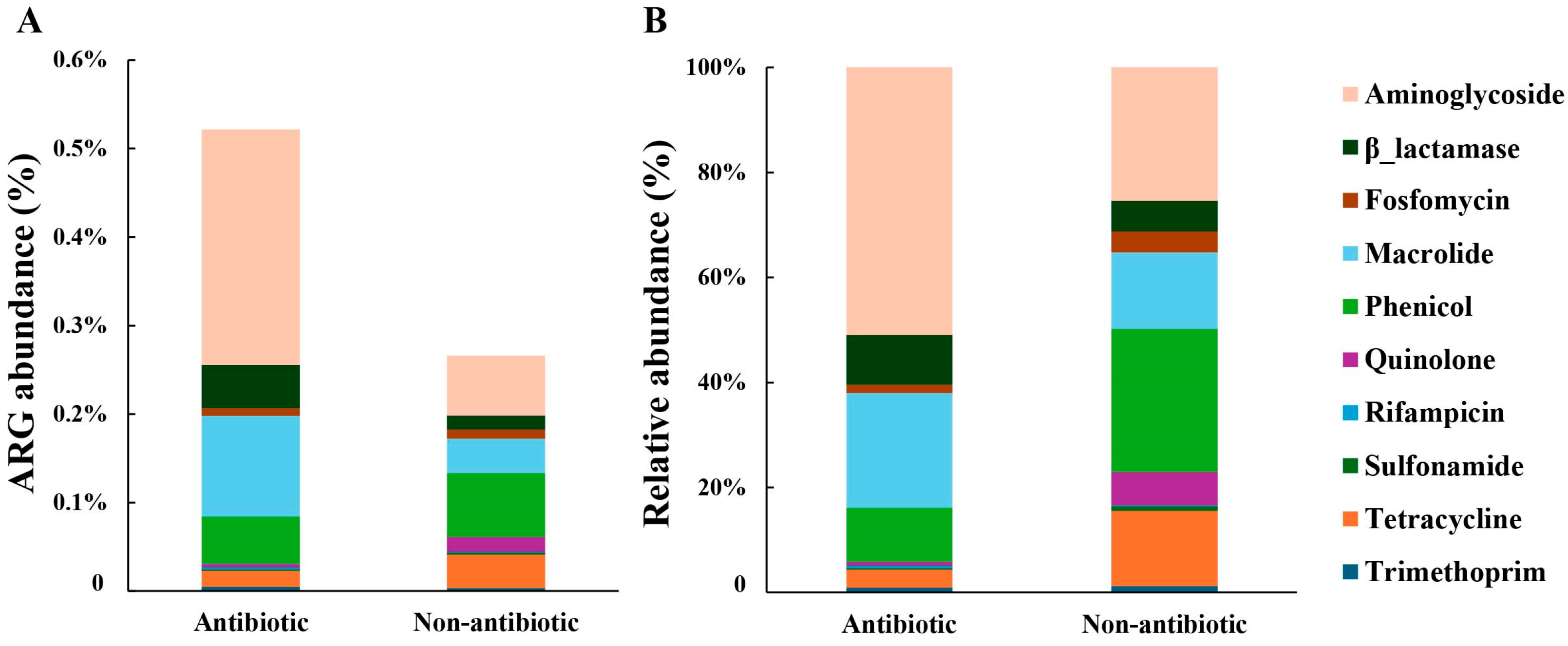

| Arg Type | Antibiotic | Non-Antibiotic | p-Value |
|---|---|---|---|
| Aminoglycoside | 0.30225 | 0.05103 | p < 0.001 |
| β-lactamase | 0.03797 | 0.00617 | p < 0.001 |
| Fosfomycin | 0.00283 | 0.00660 | p < 0.001 |
| Macrolide | 0.13594 | 0.03074 | p < 0.001 |
| Phenicol | 0.05936 | 0.06717 | p < 0.001 |
| Quinolone | 0.00086 | 0.01135 | p < 0.001 |
| Rifampicin | 0.00129 | 0.00030 | p < 0.001 |
| Sulfonamide | 0.00105 | 0.00113 | p < 0.001 |
| Tetracycline | 0.01599 | 0.03075 | p < 0.001 |
| Trimethoprim | 0.00079 | 0.00093 | p < 0.001 |
| Total ARGs | 0.65274 | 0.20158 | p < 0.001 |
| Pathogenic Bacteria Type | Antibiotic | Non-Antibiotic | p-Value |
|---|---|---|---|
| Animal | 0.200 | 1.760 | p < 0.001 |
| Plant | 0.015 | 0.030 | p > 0.05 |
| Zoonotic | 0.180 | 9.380 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, L.; Lu, Q.; Huang, C.; Zhang, X.; Yao, J.; Zhao, H.; Zou, C. Pesticide Type Distinctly Shapes Soil Resistomes: A Comparative Analysis of Antibiotic and Non-Antibiotic Agro-Chemicals. Agriculture 2025, 15, 2015. https://doi.org/10.3390/agriculture15192015
Lyu L, Lu Q, Huang C, Zhang X, Yao J, Zhao H, Zou C. Pesticide Type Distinctly Shapes Soil Resistomes: A Comparative Analysis of Antibiotic and Non-Antibiotic Agro-Chemicals. Agriculture. 2025; 15(19):2015. https://doi.org/10.3390/agriculture15192015
Chicago/Turabian StyleLyu, Lilan, Qinyu Lu, Chanchan Huang, Xiyu Zhang, Jinjie Yao, Huaxian Zhao, and Chengwu Zou. 2025. "Pesticide Type Distinctly Shapes Soil Resistomes: A Comparative Analysis of Antibiotic and Non-Antibiotic Agro-Chemicals" Agriculture 15, no. 19: 2015. https://doi.org/10.3390/agriculture15192015
APA StyleLyu, L., Lu, Q., Huang, C., Zhang, X., Yao, J., Zhao, H., & Zou, C. (2025). Pesticide Type Distinctly Shapes Soil Resistomes: A Comparative Analysis of Antibiotic and Non-Antibiotic Agro-Chemicals. Agriculture, 15(19), 2015. https://doi.org/10.3390/agriculture15192015






