Molecular and Phytopathological Characterization of Fusarium Wilt-Resistant Chickpea Genotypes for Breeding Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Research Field Experiments and Field Trials
2.3. Identification of the Pathogen
2.4. Evaluation of Fusarium Wilt Resistance
2.5. Evaluation of Normalized Difference Vegetation Index (NDVI)
2.6. Meteorological Conditions During the Study Period
2.7. Hydrothermal Coefficient (HTC) Determination
2.8. Molecular Analyses
2.9. Statistical Treatments
3. Results
3.1. Meteorological Conditions and HTC During the Study Period
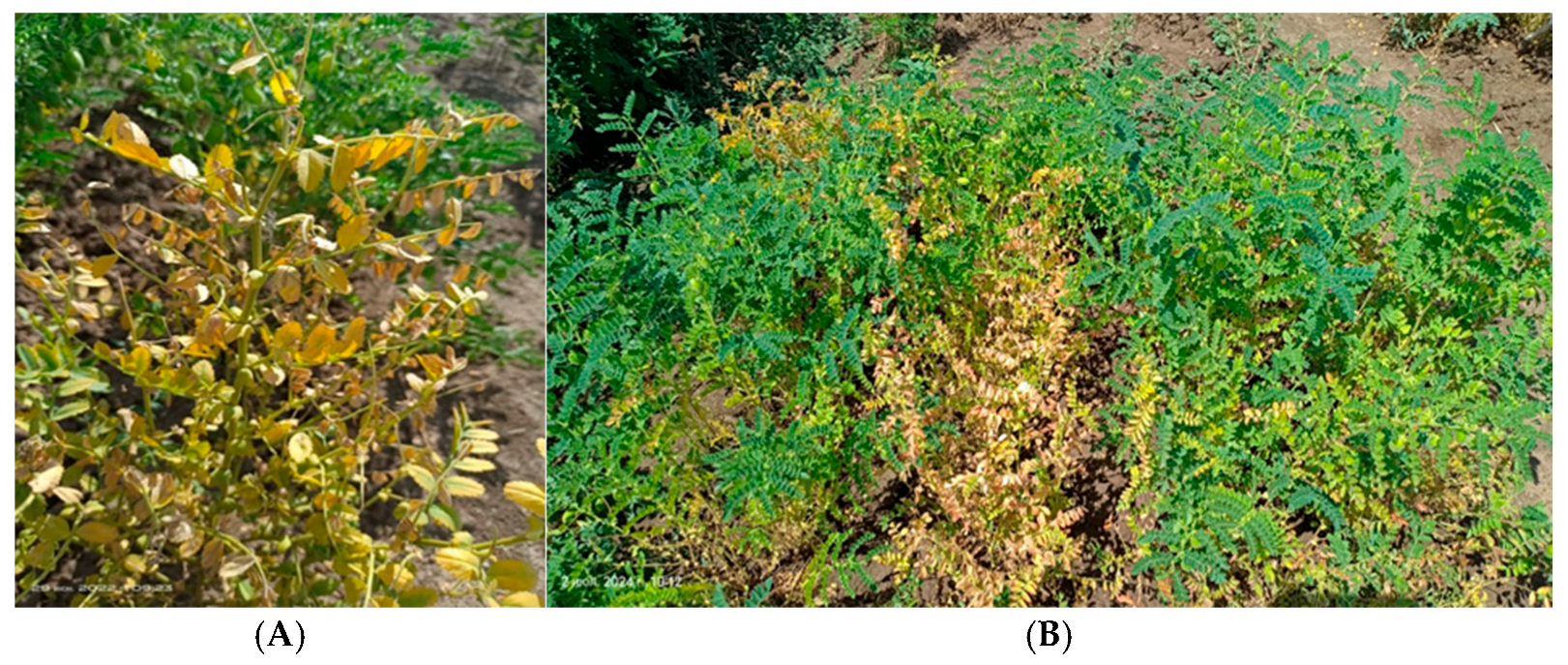
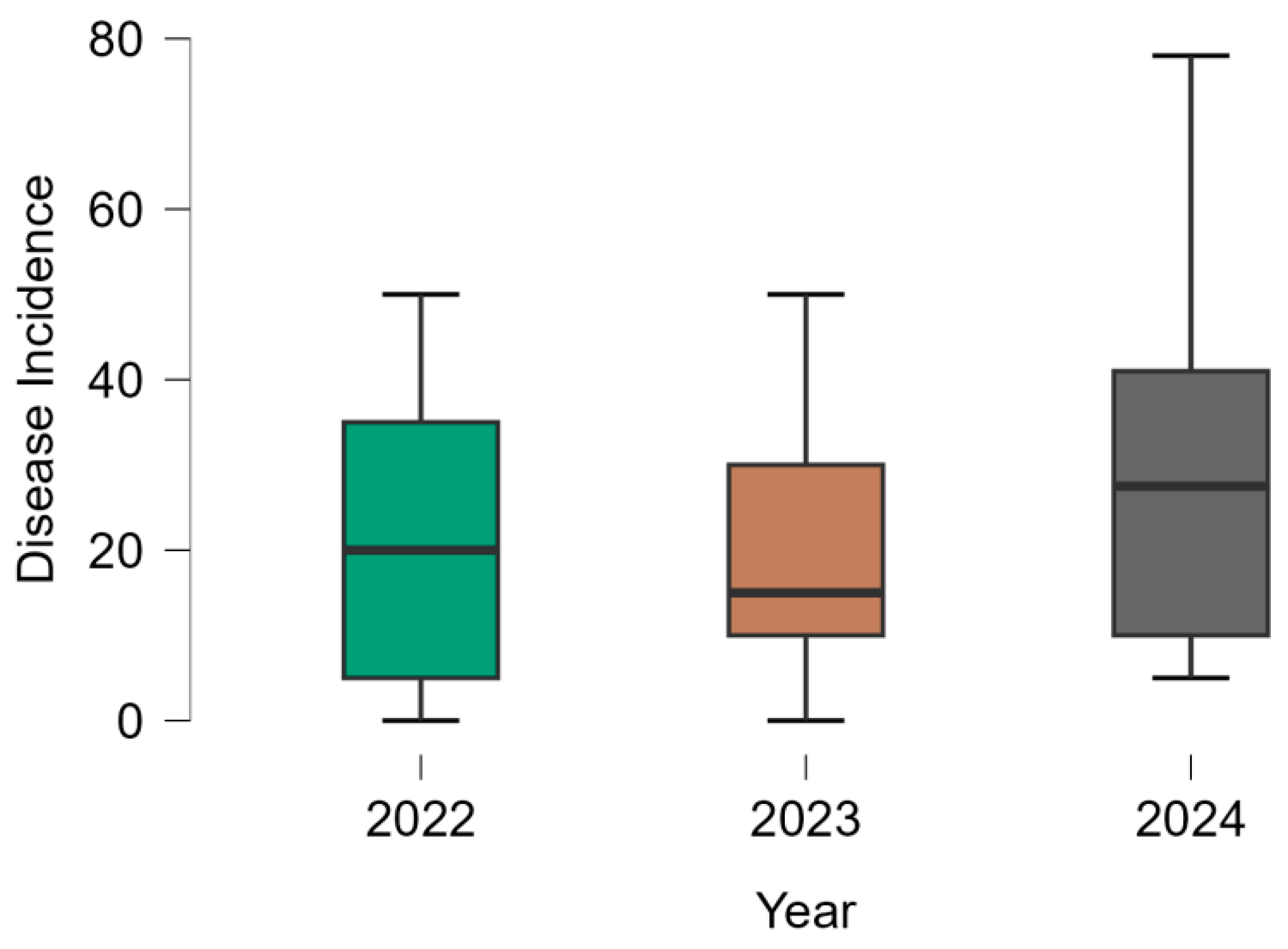
3.2. Fusarium Wilt Resistance Assessment
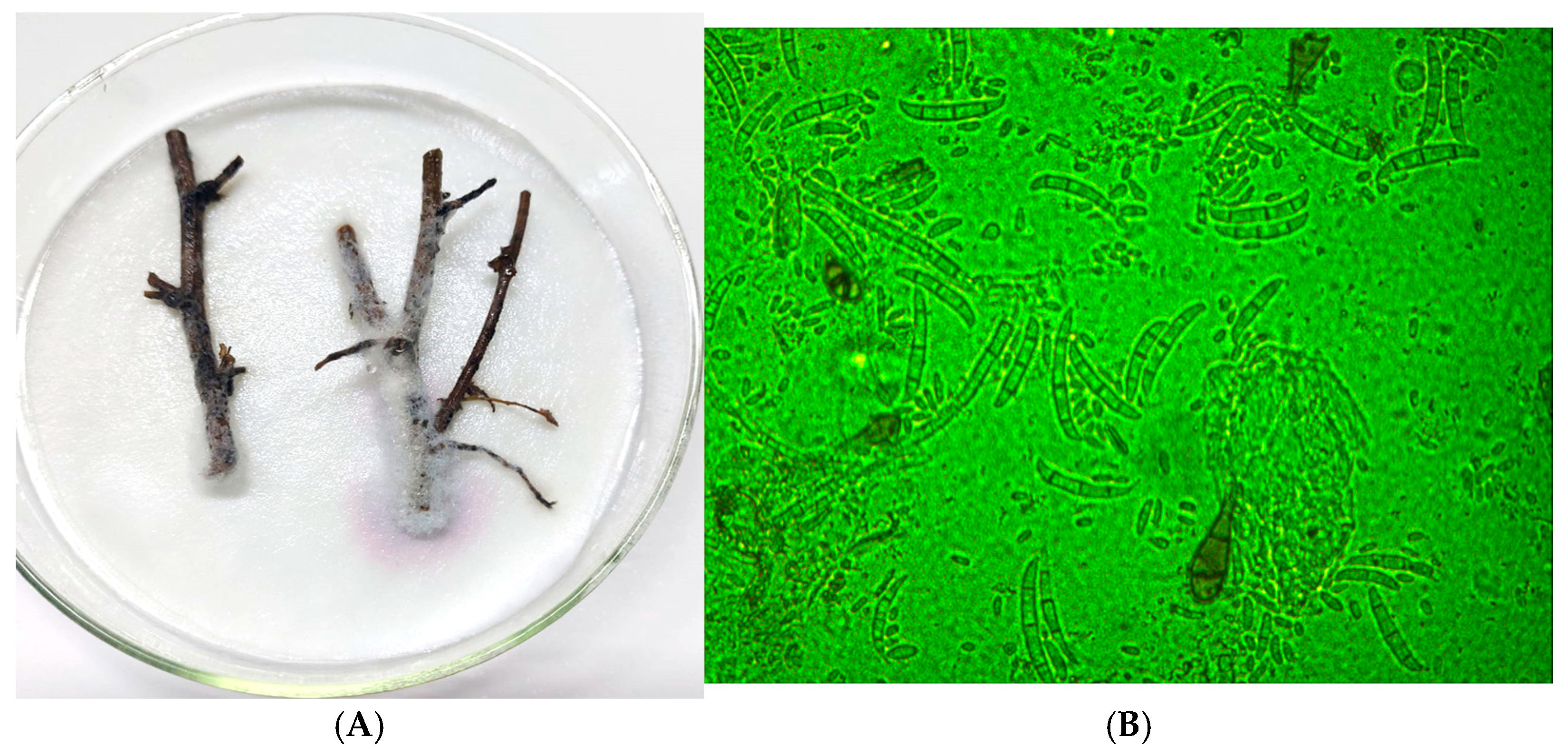
3.3. Pathogen Identification and Characterization
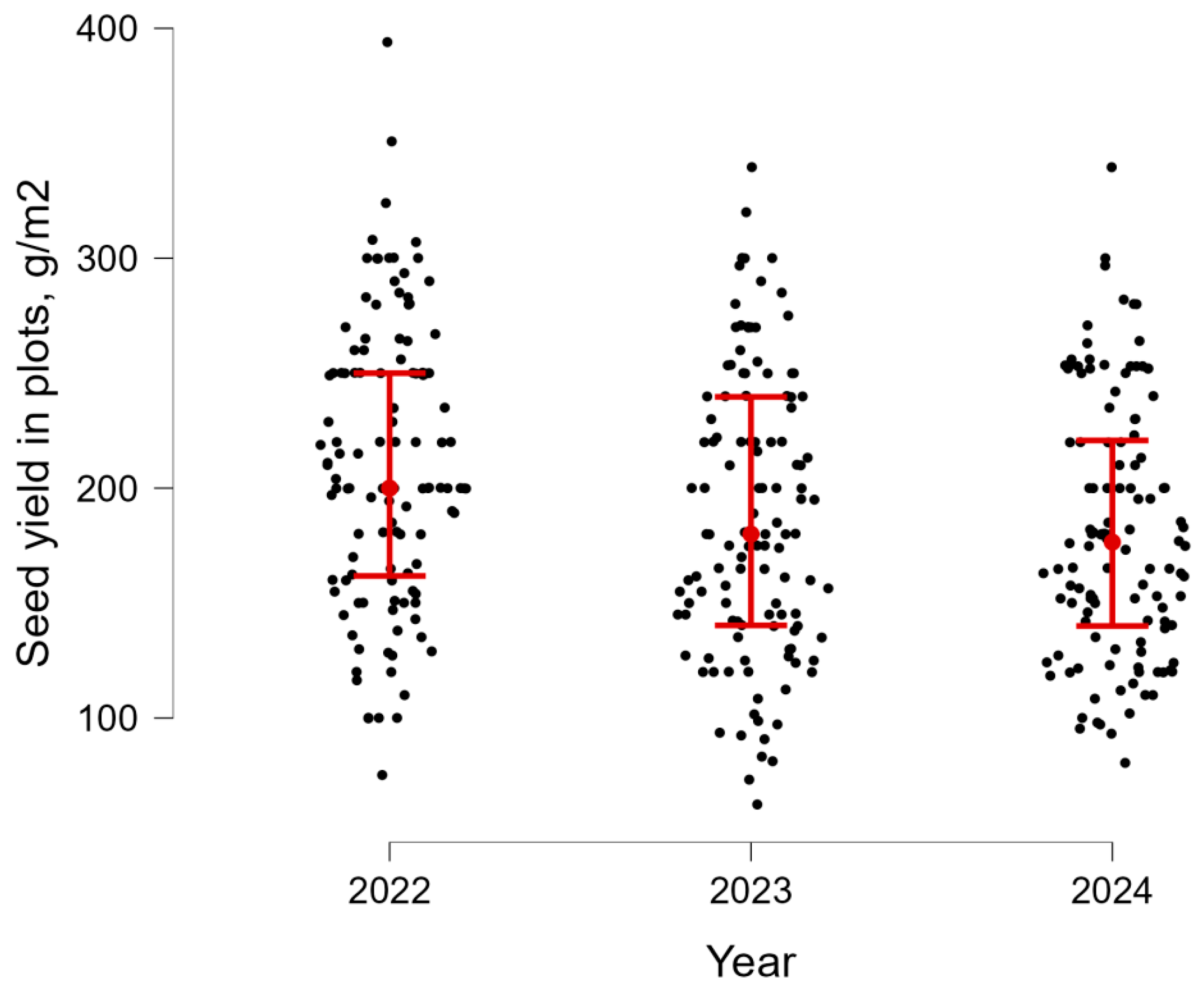
3.4. SY and TSW Evaluation Under Natural Field Conditions
3.5. Normalized Difference Vegetation Index (NDVI)
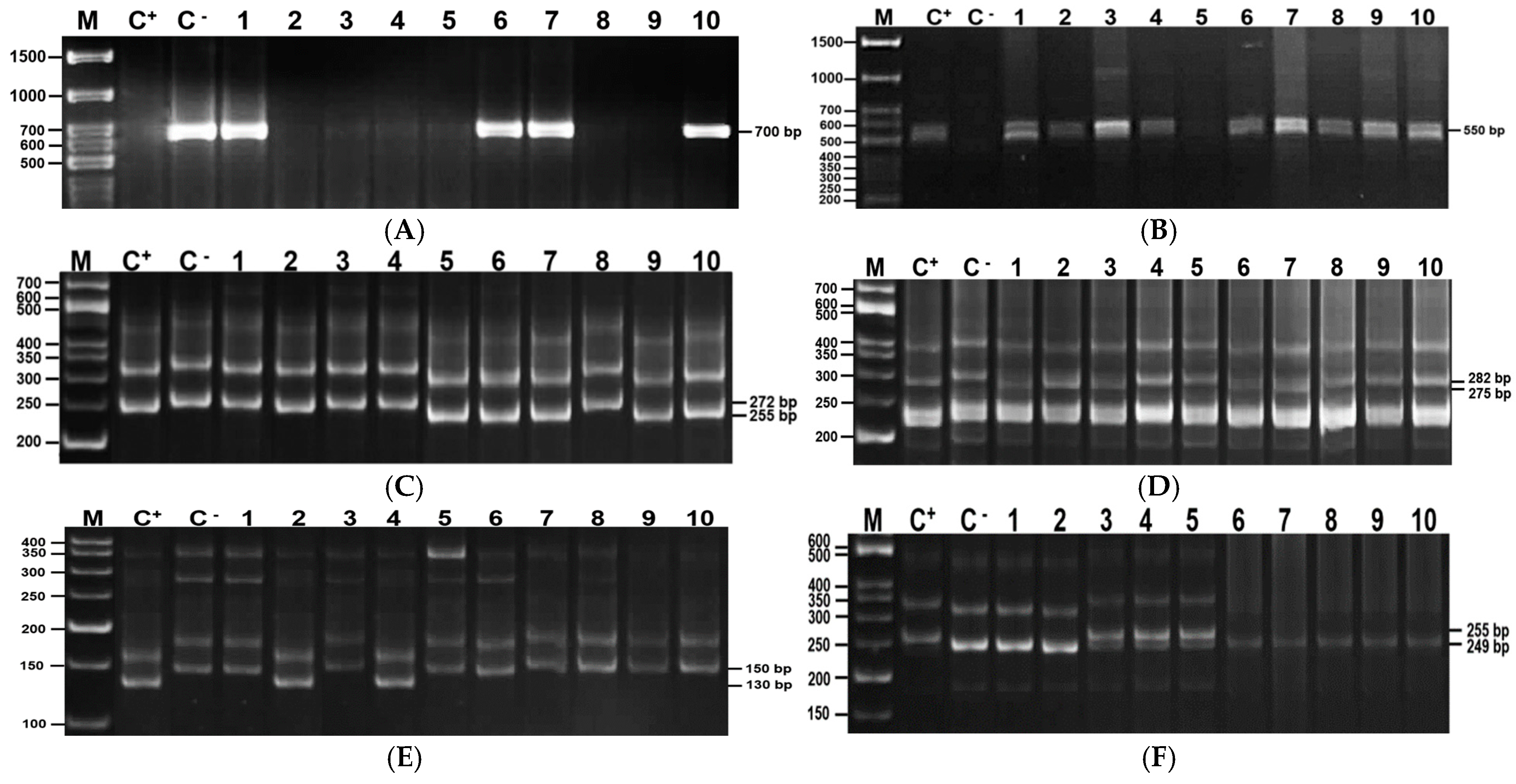
3.6. Genotyping of Foc Genes
3.7. Comparison of Genotypic and Phenotypic Data of Chickpea Accessions
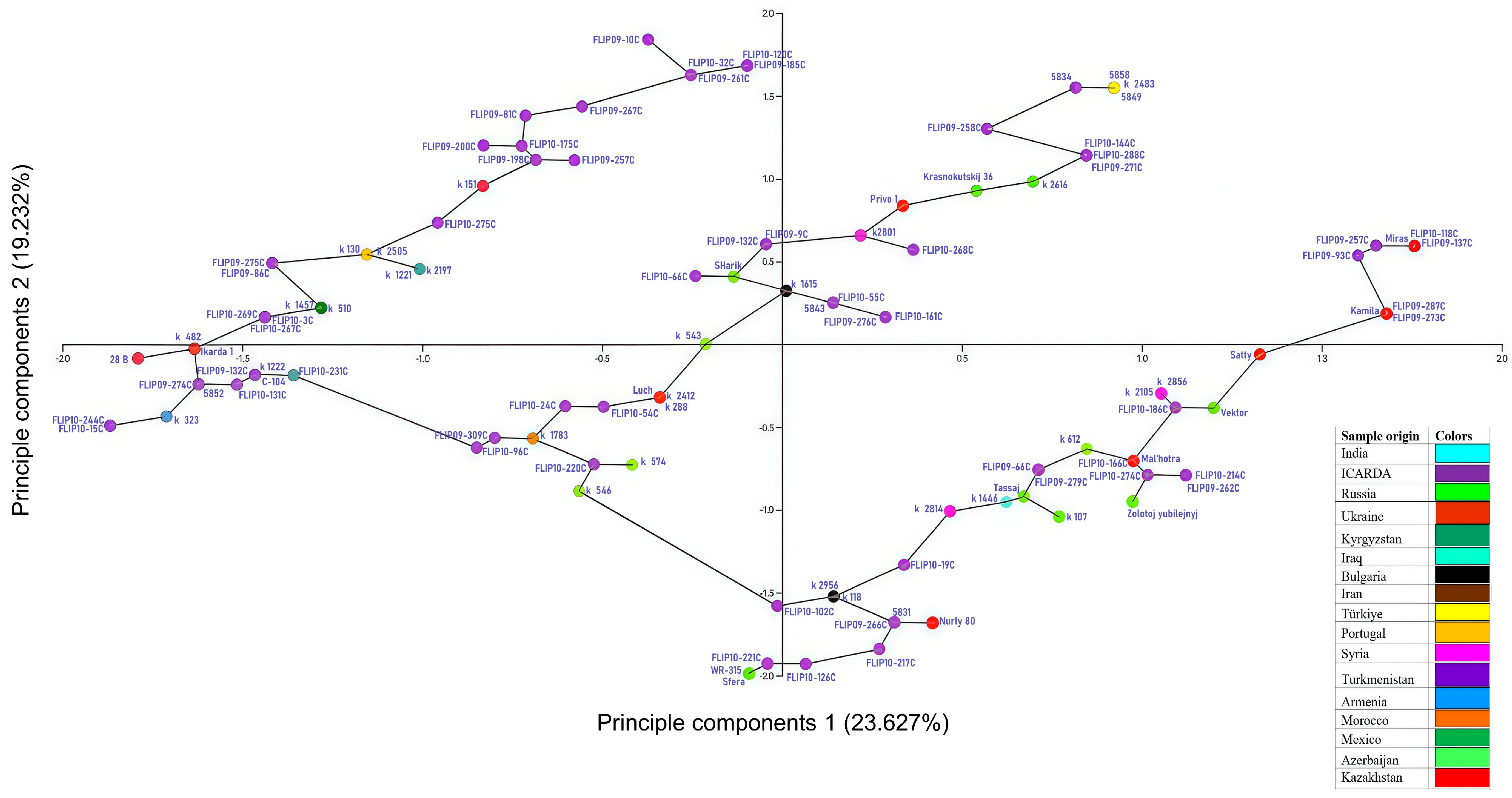
3.8. Analysis of Genetic Diversity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Wang, J.; Zhu, C.; Singh, R.P.; Chen, W. Chickpea: Its origin, distribution, nutrition, benefits, breeding, and symbiotic relationship with Mesorhizobium species. Plants 2024, 13, 429. [Google Scholar] [CrossRef]
- Jha, U.C.; Nayyar, H.; Thudi, M.; Beena, R.; Vara Prasad, P.V.; Siddique, K.H. Unlocking the nutritional potential of chickpea: Strategies for biofortification and enhanced multinutrient quality. Front. Plant Sci. 2024, 15, 1391496. [Google Scholar] [CrossRef]
- Sandhu, J.S.; Tripathi, S.; Chaturvedi, S.K. Chickpea Nutritional Status and Value Chain for Sustainable Development. In Sustainable Food Value Chain Development; Narula, S.A., Raj, S.P., Eds.; Springer: Singapore, 2023; pp. 175–183. [Google Scholar] [CrossRef]
- Iqbal, A.; Ateeq, N.; Khalil, I.A.; Perveen, S.; Saleemullah, S. Physicochemical characteristics and amino acid profile of chickpea cultivars grown in Pakistan. J. Foodserv. 2010, 17, 94–101. [Google Scholar] [CrossRef]
- Anwar, R.; Borbi, M.; Rakha, A. Significance and the use of legumes in developing weaning foods with a balanced nutrition—A review. Legume Sci. 2024, 6, e249. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Olías, R.; Marín-Manzano, M.C.; Seiquer, I.; Clemente, A. Chickpea seed flours improve the nutritional and the antioxidant profiles of traditional shortbread biscuits: Effects of in vitro gastrointestinal digestion. Antioxidants 2024, 13, 118. [Google Scholar] [CrossRef]
- David, L.S.; Nalle, C.L.; Abdollahi, M.R.; Ravindran, V. Feeding value of lupins, field peas, faba beans and chickpeas for poultry: An overview. Animals 2024, 14, 619. [Google Scholar] [CrossRef] [PubMed]
- Bampidis, V.A.; Christodoulou, V. Chickpeas (Cicer arietinum L.) in animal nutrition: A review. Anim. Feed Sci. Technol. 2011, 168, 1–20. [Google Scholar] [CrossRef]
- Muehlbauer, F.J.; Sarker, A. Economic Importance of Chickpea: Production, Value, and World Trade. In The Chickpea Genome; Varshney, R., Thudi, M., Muehlbauer, F., Eds.; Springer: Cham, Switzerland, 2017; pp. 5–12. [Google Scholar] [CrossRef]
- FAO. FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 28 June 2025).
- Mazkirat, S.; Baitarakova, K.; Kudaybergenov, M.; Babissekova, D.; Bastaubayeva, S.; Bulatova, K.; Shavrukov, Y. SSR genotyping and marker–trait association with yield components in a Kazakh germplasm collection of Chickpea (Cicer arietinum L.). Biomolecules 2023, 13, 1722. [Google Scholar] [CrossRef] [PubMed]
- Kudaibergenov, M.S.; Baitarakova, K.; Saikenova, A.; Kanatkyzy, M.; Abdrakhmanov, K.A.; Saken, G.S. Chickpea genotype selection based on economically valuable traits to develop high-yielding types. SABRAO J. Breed. Genet. 2024, 56, 1. [Google Scholar] [CrossRef]
- Bureau of National Statistics of the Agency for Strategic Planning and Reforms of the Republic of Kazakhstan. Available online: https://stat.gov.kz/ (accessed on 14 July 2025).
- Bolatova, Z.; Bulkhairova, Z.; Kulshigashova, M. Modeling scenarios of climate change impacts on leguminous crop production: A case study in Kazakhstan. Int. J. Agric. Biosci. 2024, 13, 367–377. [Google Scholar] [CrossRef]
- Verma, S.; Gupta, S.; Nitesh, B.; Kumar, T.; Bharadwaj, C.; Bhatia, S. High-density linkage map construction and mapping of seed trait QTLs in chickpea (Cicer arietinum L.) using Genotyping-by-Sequencing (GBS). Sci. Rep. 2015, 5, 17512. [Google Scholar] [CrossRef]
- Jendoubi, W.; Bouhadida, M.; Boukteb, A.; Béji, M.; Kharrat, M. Fusarium wilt affecting chickpea crop. Agriculture 2017, 7, 23. [Google Scholar] [CrossRef]
- Jiménez-Díaz, R.M.; Castillo, P.; Jiménez-Gasco, M.M.; Landa, B.B.; Navas-Cortés, J.A. Fusarium wilt of chickpeas: Biology, ecology and management. Crop Prot. 2015, 73, 16–27. [Google Scholar] [CrossRef]
- Jiménez-Gasco, M.M.; Milgroom, M.G.; Jiménez-Díaz, R.M. Gene genealogies support Fusarium oxysporum f. sp. ciceris as a monophyletic group. Plant Pathol. 2002, 51, 72–77. [Google Scholar] [CrossRef]
- Demers, J.E.; Garzón, C.D.; Jiménez-Gasco, M.M. Striking genetic similarity between races of Fusarium oxysporum f. sp. ciceris confirms a monophyletic origin and clonal evolution of the chickpea vascular wilt pathogen. Eur. J. Plant Pathol. 2014, 139, 309–324. [Google Scholar] [CrossRef]
- Jiménez-Gasco, M.M.; Navas-Cortés, J.A.; Jiménez-Díaz, R.M. The Fusarium oxysporum f. sp. ciceris/Cicer arietinum pathosystem: A case study of the evolution of plant-pathogenic fungi into races and pathotypes. Int. Microbiol. 2004, 7, 95–104. [Google Scholar] [PubMed]
- Jiménez-Fernández, D.; Landa, B.B.; Kang, S.; Jiménez-Díaz, R.M.; Navas-Cortés, J.A. Quantitative and microscopic assessment of compatible and incompatible interactions between chickpea cultivars and Fusarium oxysporum f. sp. ciceris races. PLoS ONE 2013, 8, e61360. [Google Scholar] [CrossRef] [PubMed]
- Haware, M.P.; Nene, Y.L. Races of Fusarium oxysporum f. sp. ciceri. Plant Dis. 1982, 66, 809–810. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Iqbal, S.M.; Ayub, N.; Yasmin, A.; Akram, A. Identification of resistant sources in chickpea against Fusarium wilt. Pak. J. Bot. 2010, 42, 417–426. [Google Scholar]
- Navas-Cortés, J.A.; Hau, B.; Jiménez-Díaz, R.M. Yield loss in chickpeas in relation to development of Fusarium wilt epidemics. Phytopathology 2000, 90, 1269–1278. [Google Scholar] [CrossRef]
- Sunkad, G.; Deepa, H.; Shruthi, T.H.; Singh, D. Chickpea wilt: Status, diagnostics and management. Indian Phytopathol. 2019, 72, 619–627. [Google Scholar] [CrossRef]
- Dhawale, S.N.; Dhale, D.A. Effects of Fusarium wilt on chickpea in India: A review. Int. J. Bot. Stud. 2021, 6, 884–890. [Google Scholar]
- Sampaio, A.M.; Araujo, S.D.S.; Rubiales, D.; Vaz Patto, M.C. Fusarium wilt management in legume crops. Agronomy 2020, 10, 1073. [Google Scholar] [CrossRef]
- Amine, E.; Douira, A.; Ilyass, M.; Ahmed, S. Integrating sowing date with chickpea genotypes in managing Fusarium wilt in Morocco. Agriculture 2022, 12, 773. [Google Scholar] [CrossRef]
- Maleki, H.H.; Pouralibaba, H.R.; Ghiasi, R.; Mahmodi, F.; Sabaghnia, N.; Samadi, S.; Pellegrini, M. Exploring resistant sources of chickpea against Fusarium oxysporum f. sp. ciceris in dryland areas. Agriculture 2024, 14, 824. [Google Scholar] [CrossRef]
- Maisuria, H.J.; Patel, R.M.; Suthar, K.P. Validation of molecular markers linked to Fusarium wilt resistance in chickpea genotypes. Int. J. Pure Appl. Biosci. 2017, 5, 254–260. [Google Scholar] [CrossRef]
- Padaliya, R.V.; Suthar, K.P.; Singh, D.; Mahatma, M.K.; Patil, V.R. Marker assisted characterization of chickpea genotypes for wilt resistance. Afr. J. Biotechnol. 2013, 12, 6907. [Google Scholar]
- Jha, U.C.; Bohra, A.; Pandey, S.; Parida, S.K. Breeding, genetics, and genomics approaches for improving Fusarium wilt resistance in major grain legumes. Front. Genet. 2020, 11, 1001. [Google Scholar] [CrossRef]
- Fikre, A.; Korbu, L.; Eshete, M.; Bekele, D.; Girma, N.; Mohamed, R.; Assefe, S.; Admasu, D.; Tilahun, G.; Tesfaye, T. A decade of research progress in chickpea and lentil breeding and genetics. Ethiop. J. Crop Sci. 2018, 6, 101–113. [Google Scholar]
- Sharma, M.; Babu, T.K.; Gaur, P.; Ghosh, R.; Rameshwar, T.; Chaudhary, R.; Upadhyay, J.; Gupta, O.; Saxena, D.; Kaur, L.; et al. Identification and multi-environment validation of resistance to Fusarium oxysporum f. sp. ciceris in chickpea. Field Crop. Res. 2012, 135, 82–88. [Google Scholar] [CrossRef]
- Yadav, R.K.; Tripathi, M.K.; Tiwari, S.; Tripathi, N.; Asati, R.; Patel, V.; Sikarwar, R.S.; Payasi, D.K. Breeding and Genomic Approaches towards Development of Fusarium Wilt Resistance in Chickpea. Life 2023, 13, 988. [Google Scholar] [CrossRef]
- International Union for the Protection of New Varieties of Plants (UPOV). Chickpea (Cicer arietinum L.), UPOV Code: CICER_ARI. Available online: https://www.upov.int (accessed on 14 September 2025).
- Sharma, M.; Ghosh, R.; Tarafdar, A.; Rathore, A.; Chobe, D.R.; Kumar, A.V.; Harer, P.N. Exploring the genetic cipher of chickpea (Cicer arietinum L.) through identification and multi-environment validation of resistant sources against Fusarium wilt (Fusarium oxysporum f. sp. ciceris). Front. Plant Sci. 2019, 10, 963. [Google Scholar] [CrossRef]
- Vishniyakova, M.A.; Seferova, I.V.; Buravtseva, T.V.; Burlyaeva, M.O.; Semenova, E.V.; Filipenko, G.I.; Aleksandrova, T.G.; Egorova, G.P.; Yankov, I.I.; Bulyntsev, S.V. VIR Global Collection of Grain Legume Crop Genetic Resources: Replenishment, Conservation and Studying; Ministry of Science and Higher Education: St. Petersburg, Russia, 2018; 143p. (In Russian) [Google Scholar]
- International Seed Testing Association (ISTA). International Rules for Seed Testing; ISTA: Bassersdorf, Switzerland, 2024; Available online: https://www.seedtest.org/api/rm/882F39FEQGF349B/ista-rules-2024-00-introduction-final.pdf (accessed on 14 September 2025).
- GOST 12044-93; Interstate Standard. Seeds of Agricultural Crops. Methods for Determining Disease Infection. Russian GOST Committee: Moscow, Russia, 1996. (In Russian)
- Moss Instruments Co., Ltd. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 14 September 2025).
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: University Park, PA, USA, 1983. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006. [Google Scholar]
- Shahjahan, M.; Inam-ul-Haq, M.; Mukhtar, T.; Khalid, A. Incidence of major fungal root diseases of chickpea in Layyah and Bhakkar districts of Punjab, Pakistan. Pak. J. Phytopathol. 2016, 28, 75–79. [Google Scholar]
- Haware, M.P.; Nene, Y.L. Symptomless carriers of the chickpea wilt Fusarium. Phytopathology 1982, 72, 193–195. [Google Scholar]
- Maheshwari, S.K.; Satyavir, S.; Chauhan, R. Morphological and molecular characterization of Fusarium oxysporum f. sp. ciceris causing wilt in chickpea (Cicer arietinum L.). Afr. J. Biotechnol. 2013, 12, 2630–2635. [Google Scholar]
- Trapero-Casas, A.; Jiménez-Díaz, R.M. Fungal wilt and root rot diseases of chickpea in southern Spain. Phytopathology 1985, 75, 1146–1151. [Google Scholar] [CrossRef]
- GreenSeeker Handheld Crop Sensor. Available online: https://ww2.agriculture.trimble.com/product/greenseeker-handheld-crop-sensor/ (accessed on 7 July 2025).
- Beck, H.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Lutsko, N.J.; Dufour, A.; Zeng, Z.; Jiang, X.; van Dijk, A.I.J.M.; Miralles, D.G. High-resolution (1 km) Köppen-Geiger maps for 1901–2099 based on constrained CMIP6 projections. Sci. Data 2023, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Climate in Kazakhstan. Climate Data. Available online: https://ru.climate-data.org (accessed on 17 July 2024).
- Evarte-Bundere, G.; Evarts-Bunders, P. Using of the hydrothermal coefficient (HTC) for interpretation of distribution of non-native tree species in Latvia on example of cultivated species of genus Tilia. Acta Biol. Univ. Daugavp. 2012, 12, 135–148. [Google Scholar]
- Selyaninov, G.L. About the agricultural evaluation of the climate. Trudy GGO 1928, 20, 177–185. (In Russian) [Google Scholar]
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- JASP Version 0.19.3. Available online: https://jasp-stats.org/ (accessed on 12 May 2025).
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- Altay, Y.A.; Kremlev, A.S.; Gandalipov, E.R.; Yerzhebayeva, R.S. On the use of the statistical methods for biomedical signals and data processing. In Proceedings of the 2019 IEEE Conference of Russian Young Researchers in Electrical and Electronic Engineering (EIConRus), Moscow, Russia; Saint Petersburg, Russia, 28–31 January 2019; pp. 1129–1134. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Education: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Cohen’s Kappa. Available online: https://datatab.net/tutorial/cohens-kappa (accessed on 15 September 2025).
- Past 5—The Past of the Future, Version 4.09. Available online: https://www.chinamedevice.com/ChinaSuppliers/5815/ (accessed on 5 September 2025).
- Jolliffe, I.T. Principal Component Analysis; Springer Series in Statistics; Springer: New York, NY, USA, 2002. [Google Scholar]
- Botstein, D.; White, R.L.; Skolnick, M.H.; Davies, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Serrote, C.M.L.; Reiniger, L.R.S.; Silva, K.B.; dos Santos Rabaiolli, S.M.; Stefanel, C.M. Determining the polymorphism information content of a molecular marker. Gene 2020, 726, 144175. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.S.; Tullu, A.; Simon, C.J.; Kumar, J.; Kaiser, W.J.; Kraft, J.M.; Muehlbauer, F.J. Development of a DNA marker for Fusarium wilt resistance in chickpea. Crop Sci. 1997, 37, 1625–1629. [Google Scholar] [CrossRef]
- Soregaon, C.D.; Ravikumar, R.L. Marker assisted characterization of wilt resistance in productive chickpea genotypes. Electron. J. Plant Breed. 2010, 1, 1159–1163. [Google Scholar]
- Winter, P.; Pfaff, T.; Udupa, S.M.; Huttel, B.; Sharma, P.C.; Sahi, S.; Arreguin-Espinoza, R.; Weigand, F.; Muehlbauer, F.J.; Kahl, G. Characterization and mapping of sequence-tagged microsatellite sites in the chickpea (Cicer arietinum L.) genome. Mol. Gen. Genet. 1999, 262, 90–101. [Google Scholar] [CrossRef]
- Pratap, A.; Chaturvedi, S.K.; Tomar, R.; Rajan, N.; Malviya, N.; Thudi, M.; Saabale, P.R.; Prajapati, U.; Varshney, R.K.; Singh, N.P. Marker assisted introgression of resistance to Fusarium wilt race 2 in Pusa 256, an elite cultivar of desi chickpea. Mol. Genet. Genom. 2017, 292, 1237–1245. [Google Scholar] [CrossRef]
- Sharma, K.D.; Winter, P.; Kahl, G.; Muehlbauer, F.J. Molecular mapping of Fusarium oxysporum f. sp. ciceris race 3 resistance gene in chickpea. Theor. Appl. Genet. 2004, 108, 1243–1248. [Google Scholar] [CrossRef]
- Ali, H.; Haq, M.A.; Shah, T.M.; Mehboob, R.; Chen, W. Validation of molecular markers for resistance among Pakistani chickpea germplasm to races of Fusarium oxysporum f. sp. ciceris. Eur. J. Plant Pathol. 2012, 132, 237–244. [Google Scholar] [CrossRef]
- Castro, P.; Pistón, F.; Madrid, E.; Millan, T.; Gil, J.; Rubio, J. Development of chickpea near-isogenic lines for Fusarium wilt. Theor. Appl. Genet. 2010, 121, 1519–1526. [Google Scholar] [CrossRef]
- Kukreja, S.; Singh, K.; Sharma, K.D.; Bhatia, S.; Kaur, J. Identification and validation of candidate genes associated with resistance to Fusarium wilt in chickpea using comparative transcriptome analysis. Front. Plant Sci. 2018, 9, 909. [Google Scholar] [CrossRef]
- Meriem Wafa, K.; Hannane, A.; Noureddine, R. Screening for chickpea germplasm resistant to Fusarium wilt disease under natural conditions of infection. J. Crop Prot. 2022, 11, 229–242. [Google Scholar]
- Kapoor, P.; Khichar, M.L.; Dhankar, S.; Al, A.M.G. Assessing the Suitability of Different Modeling Techniques for Meteorological Forecasting on Chickpea Wilt. Mausam 2025, 76, 351–364. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, C.; Long, J.; Zhang, Q.; Luo, Y.; Li, L. Soil moisture deficits triggered by increasing compound drought and heat events during the growing season in Arid Central Asia. J. Hydrol. 2025, 660, 133397. [Google Scholar] [CrossRef]
- Mina, U.; Dubey, S.C. Effect of environmental variables on development of Fusarium wilt in chickpea (Cicer arietinum) cultivars. Indian J. Agric. Sci. 2010, 80, 231–234. [Google Scholar]
- Landa, B.B.; Navas-Cortés, J.A.; Hervás, A.; Jiménez-Díaz, R.M. Influence of Temperature and Inoculum Density of Fusarium oxysporum f. sp. ciceris on Suppression of Fusarium Wilt of Chickpea by Rhizosphere Bacteria. Phytopathology 2001, 91, 807–816. [Google Scholar] [CrossRef]
- Dubey, S.C.; Singh, B.; Srinivasa, N. Evaluation of Chickpea Genotypes against Fusarium Wilt for Resistant Sources. Indian Phytopathol. 2017, 70, 254–255. [Google Scholar] [CrossRef]
- Samiksha, K.S.; Goyal, V.; Kumar, S. Screening of Chickpea Genotypes from Different Agro-Climatic Areas against Fusarium oxysporum f. sp. ciceris (Race 3) Using Morphological and Molecular Marker. Plant Sci. Today 2023, 10, 335–342. [Google Scholar] [CrossRef]
- Yadav, R.K.; Tripathi, M.K.; Asati, R.; Tripathi, N. Microsatellite Markers-Based Genotyping, Population Structure Analysis and Field Screening of Chickpea (Cicer arietinum L.) Genotypes against Fusarium Wilt. Horizon 2025, 12, 1–11. [Google Scholar] [CrossRef]
- Maisuria, H.J.; Patel, R.M.; Suthar, K.P. Assessment of Genetic Diversity among Chickpea (Cicer arietinum L.) Genotypes Using Seed Storage Protein Profiling and RAPD Markers. Appl. Biol. Res. 2017, 19, 248–256. [Google Scholar] [CrossRef]
- Ahmad, Z.; Mumtaz, A.S.; Ghafoor, A.; Ali, A.; Nisar, M. Marker Assisted Selection (MAS) for Chickpea Fusarium oxysporum Wilt Resistant Genotypes Using PCR Based Molecular Markers. Mol. Biol. Rep. 2014, 41, 6755–6762. [Google Scholar] [CrossRef]
- Rani, R.; Tripathi, S.; Srinivasa, N.; Kumari, N.; Singh, G. Genetic Resistance in Chickpea (Cicer arietinum L.) against Race 3 and 4 of Fusarium Wilt. Indian Phytopathol. 2022, 75, 713–721. [Google Scholar] [CrossRef]
- Iruela, M.; Castro, P.; Rubio, J.; Cubero, J.I.; Jacinto, C.; Millán, T.; Gil, J. Validation of a QTL for resistance to ascochyta blight linked to resistance to fusarium wilt race 5 in chickpea (Cicer arietinum L.). Eur. J. Plant Pathol. 2007, 119, 29–37. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, S. Screening and Evaluation of Cicer arietinum Genotypes against Fusarium Wilt under Sick Field and Artificial Condition. Asian J. Microbiol. Biotechnol. Environ. Sci. 2019, 21, 1068–1075. [Google Scholar]
| Month | 2022 | 2023 | 2024 | Average |
|---|---|---|---|---|
| May | 2.47 | 0.81 | 2.22 | 1.83 |
| June | 0.49 | 0.06 | 0.27 | 0.27 |
| July | 0.18 | 0.40 | 1.10 | 0.56 |
| Average | 1.04 | 0.42 | 1.20 |
| Trait | Year | Statistical Parameters of Chickpea Accessions | ||||
|---|---|---|---|---|---|---|
| Mean | Std.D | CV | Min | Max | ||
| DI | 2022 | 21.5 | 14.2 | 0.66 | 0 | 50 |
| 2023 | 18.0 | 12.5 | 0.69 | 0 | 50 | |
| 2024 | 26.8 | 18.0 | 0.67 | 5 | 78 | |
| DS | 2022 | 1.57 | 0.51 | 0.33 | 1 | 3 |
| 2023 | 1.35 | 0.57 | 0.43 | 0 | 3 | |
| 2024 | 1.58 | 0.60 | 0.38 | 1 | 3 | |
| SY | 2022 | 209.9 | 60.8 | 0.29 | 75.2 | 394.0 |
| 2023 | 187.1 | 61.1 | 0.31 | 62.4 | 339.6 | |
| 2024 | 181.9 | 55.1 | 0.31 | 80.5 | 340.7 | |
| TSW | 2022 | 284.1 | 48.1 | 0.16 | 162.0 | 384.0 |
| 2023 | 284.2 | 51.7 | 0.18 | 175.0 | 391.0 | |
| 2024 | 284.3 | 48.3 | 0.17 | 168.0 | 389.0 | |
| NDVI | 2022 | 0.58 | 0.09 | 0.16 | 0.28 | 0.78 |
| 2023 | 0.52 | 0.09 | 0.18 | 0.24 | 0.75 | |
| 2024 | 0.62 | 0.10 | 0.16 | 0.36 | 0.84 | |
| Parameter | Year | Genotype | ||||
|---|---|---|---|---|---|---|
| SS | MS | F | SS | MS | F | |
| SY | 53,427.2 | 26,713.6 | 7.6 *** | 1.007 × 106 | 9008.6 | 9.2 *** |
| TSW | 657.5 | 328.8 | 0.13 ns | 809,724.3 | 6804.4 | 25.2 *** |
| NDVI | 0.53 | 0.27 | 29.2 *** | 1.7 | 0.01 | 1.6 *** |
| DI | 4703.5 | 2351.7 | 10.3 *** | 53,200.8 | 447.1 | 3.2 *** |
| DS | 4.0 | 2 | 6.3 ** | 68.6 | 0.57 | 2.8 *** |
| df = 2 | df = 119 | |||||
| Resistant Gene | Chickpea Accessions |
|---|---|
| Foc-1 | WR-315, FLIP09-261C, FLIP09-93C, FLIP10-3C, FLIP10-15C, FLIP10-19C, FLIP10-54C, FLIP10-96C, FLIP10-102C, FLIP10-131C, FLIP10-267C, FLIP10-269C, FLIP10-275C, 28-B, Tassaj, Privo 1, Krasnokutskij 36, Sfera and k2814 |
| Foc-2 | WR-315, FLIP09-81C, FLIP09-276C, FLIP09-287C, FLIP09-309C, FLIP09-273C, FLIP09-271C, FLIP09-261C, FLIP09-185C, FLIP09-74C, FLIP09-267C, FLIP09-61C, FLIP10-32C, FLIP10-120C, FLIP10-144C, FLIP10-161C, FLIP10-214C, FLIP10-274C, FLIP10-288C, k107, k1221, k2197, k 2616, Kamila, Krasnokutskij 36, Privo 1, Satty |
| Foc-3 | WR-315, 28-B, FLIP09-309C, FLIP10-15C, FLIP10-102C, FLIP10-126C, FLIP10-221C, FLIP10-244C, Ikarda 1, k107, k118, k1221, k1615, k1783, k2197, k2956, k323, k482, k612, Sfera |
| Foc-5 | WR-315, FLIP0-255C, FLIP09-137C, FLIP09-66C, FLIP09-287C, FLIP09-309C, FLIP09-273C, FLIP09-94C, 5831, FLIP09-279C, FLIP09-265C, 5846, 5849, FLIP09-93C, 5852, 5855, 5859, FLIP09-262C, FLIP10-19C, FLIP10-66C, FLIP10-102C, FLIP10-118C, FLIP10-126C, FLIP10-186C, FLIP10-214C, FLIP10-217C, FLIP10-221C, FLIP10-274C, Vektor, Zolotoj yubilejnyj, k107, k118, k1446, k2105, k2814, k2856, k612, Mal’hotra, Miras, Nurly 80, Satty, Sfera, Tassaj |
| Foc-1, Foc-2 | 5832, Krasnokutskij 36, Privo 1 |
| Foc-1, Foc-3 | WR-315, 28-B, FLIP10-15C, FLIP10-102C, Sfera |
| Foc-1, Foc-5 | WR-315, FLIP09-93C, FLIP10-19C, FLIP10-102C, к2814, Sfera, Tassaj |
| Foc-2, Foc-3 | WR-315, k107, k1221, k2197 |
| Foc-2, Foc-5 | WR-315, Satty, k 107, FLIP10-214C, FLIP09-61C, FLIP09-273C, FLIP09-309C, FLIP09-287C |
| Foc-3, Foc-5 | WR-315, FLIP09-287C, Sfera, k612, k107, k118, FLIP10-221C, FLIP10-126C, FLIP10-1, FLIP10-102C |
| Foc-1, Foc-3, Foc-5 | Sfera, FLIP10-102C |
| PC | Eigenvalue | % Variance | Eig 2.5% | Eig 97.5% |
|---|---|---|---|---|
| 1 | 0.77 | 23.6 | 19.6 | 28.3 |
| 2 | 0.63 | 19.6 | 15.6 | 24.8 |
| 3 | 0.52 | 15.8 | 12.5 | 19.0 |
| 4 | 0.40 | 12.2 | 8.5 | 15.9 |
| 5 | 0.37 | 11.2 | 8.8 | 14.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yerzhebayeva, R.; Abekova, A.; Baitarakova, K.; Kudaibergenov, M.; Yesserkenov, A.; Maikotov, B.; Didorenko, S. Molecular and Phytopathological Characterization of Fusarium Wilt-Resistant Chickpea Genotypes for Breeding Applications. Agriculture 2025, 15, 1992. https://doi.org/10.3390/agriculture15191992
Yerzhebayeva R, Abekova A, Baitarakova K, Kudaibergenov M, Yesserkenov A, Maikotov B, Didorenko S. Molecular and Phytopathological Characterization of Fusarium Wilt-Resistant Chickpea Genotypes for Breeding Applications. Agriculture. 2025; 15(19):1992. https://doi.org/10.3390/agriculture15191992
Chicago/Turabian StyleYerzhebayeva, Raushan, Alfiya Abekova, Kuralay Baitarakova, Mukhtar Kudaibergenov, Aydarkhan Yesserkenov, Bekzhan Maikotov, and Svetlana Didorenko. 2025. "Molecular and Phytopathological Characterization of Fusarium Wilt-Resistant Chickpea Genotypes for Breeding Applications" Agriculture 15, no. 19: 1992. https://doi.org/10.3390/agriculture15191992
APA StyleYerzhebayeva, R., Abekova, A., Baitarakova, K., Kudaibergenov, M., Yesserkenov, A., Maikotov, B., & Didorenko, S. (2025). Molecular and Phytopathological Characterization of Fusarium Wilt-Resistant Chickpea Genotypes for Breeding Applications. Agriculture, 15(19), 1992. https://doi.org/10.3390/agriculture15191992






