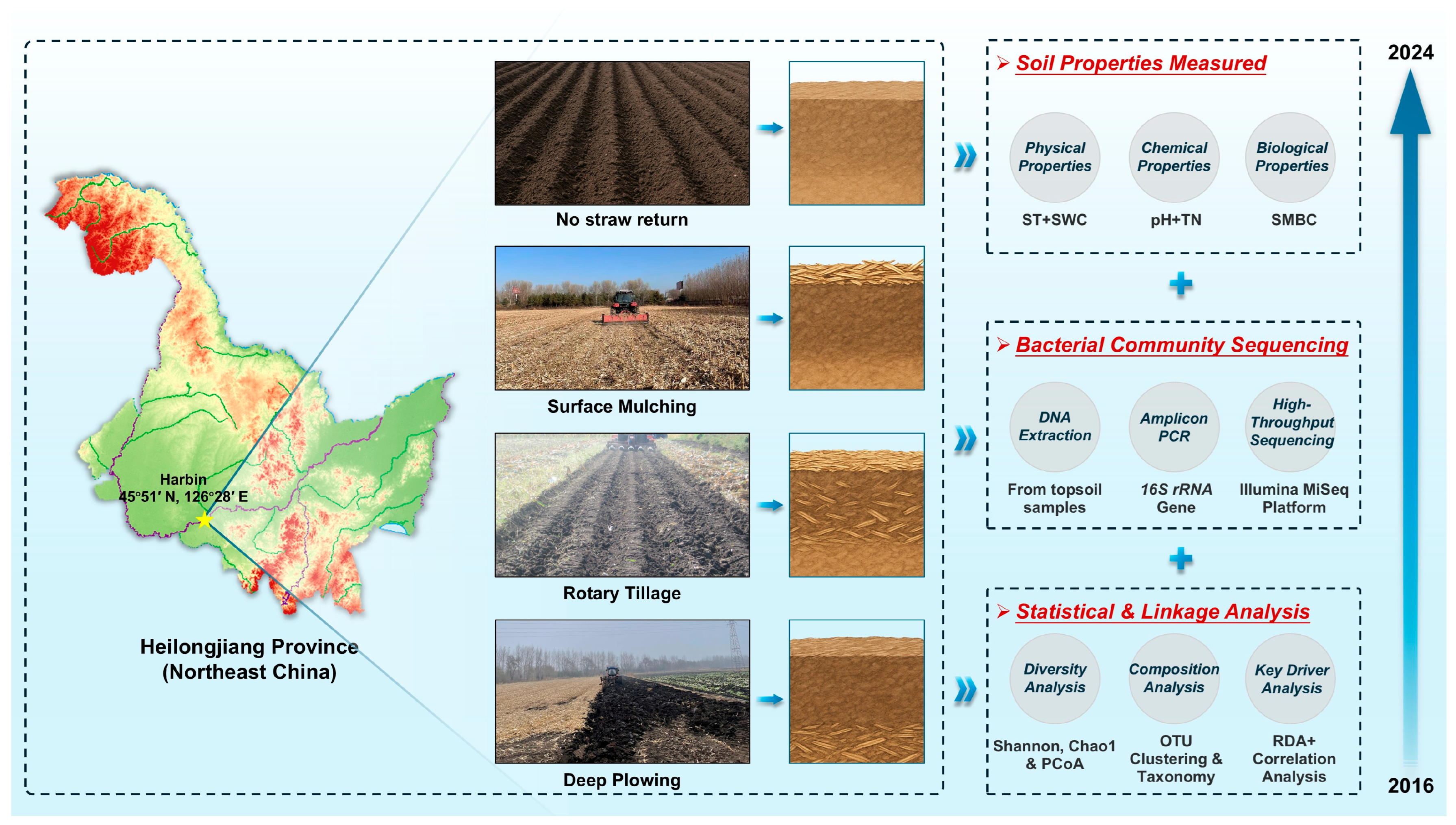
1. Introduction
Maintaining the health of agricultural soils is foundational to global food security and ecological stability. Mollisols, colloquially known as “black soils”, represent some of the planet’s most fertile and productive lands, functioning as critical breadbaskets that underpin the global food supply [
1,
2]. The Mollisol region of Northeast China is a paramount example; remarkably, these limited black soil areas have contributed approximately one-quarter of China’s total grain production since 2013 [
3]. In response to signs of soil degradation from decades of intensive cultivation, the return of crop straw—predominantly maize (
Zea mays L.) straw in this region—to the soil has been widely advocated as a cornerstone practice for sustainable agriculture. This strategy aims to replenish soil organic matter, enhance nutrient cycling, improve soil structure and water retention, and ultimately reduce the reliance on synthetic fertilizers [
4,
5]. While crop yield responses to straw return have been extensively documented, the underlying soil mechanisms governing these responses remain poorly understood, particularly the long-term soil-microbe interactions that drive sustainable productivity.
Despite its clear theoretical benefits, the practical implementation of straw return in temperate and cold-climate agroecosystems like Northeast China is constrained by a persistent agronomic paradox. The region’s climate, characterized by cold, dry winters and short, cool springs, dramatically slows the decomposition of straw residues left on the soil surface [
6]. This leads to the accumulation of a thick, insulating mat that, while highly effective at conserving soil moisture, simultaneously acts as a potent thermal barrier, significantly suppressing soil warming during the critical spring sowing period [
7,
8]. The resulting “cold and wet” soil conditions are well-documented to trigger a cascade of negative agronomic consequences, including delayed seed germination, impaired seedling vigor, and increased susceptibility to pathogens, which collectively compromise crop yields and farmer profitability [
9,
10]. This acute conflict between the long-term ecological goal of soil conservation and the short-term economic imperative of crop establishment presents a powerful disincentive for its widespread adoption, posing a significant barrier to the region’s sustainable development [
11].
To navigate this complex trade-off, a considerable body of research has explored various management strategies, focusing on two key variables: the incorporation method and the application rate. These studies have provided valuable foundational insights, confirming, for example, that surface mulching excels at water conservation at the cost of soil warmth, whereas incorporation via tillage can accelerate nutrient release. However, a comprehensive understanding is hindered by two critical limitations. First, the vast majority of studies are conducted over short-term periods (typically 1–3 years), a timescale often insufficient to move beyond transient effects and characterize the stable, long-term ecological realignment of the soil system [
12,
13]. Second, while foundational studies have documented individual responses of soil physical, chemical, or biological properties, a systems-level integration that mechanistically links these domains remains less developed. [
14,
15,
16]. The full causal chain—from management practice to physical environment, to biogeochemical transformation, and ultimately to microbial community assembly—has not been fully elucidated. Consequently, a fundamental knowledge gap persists: how do the long-term, interactive effects of straw placement and quantity collaboratively shape the soil’s integrated physicochemical environment to determine the final, stable structure of its resident microbial communities? Addressing this interactive, long-term dimension is the key to resolving the region’s central agronomic paradox.
Current research directly addresses this critical knowledge gap by leveraging a comprehensive, nine-year factorial field experiment established in a representative Mollisol. This long-term platform, which systematically evaluates three distinct incorporation methods (surface mulching, rotary tillage, and deep plowing), each applied at three rates (30%, 50%, and 100%), was designed explicitly to disentangle the complex, interactive effects after the system had reached a mature state. Objectives in this research were threefold: (1) to quantify how the long-term interplay of the straw return method and rate reshapes the soil’s physical environment, particularly the temperature-moisture dynamics that define the central agronomic paradox; (2) to determine how these integrated management regimes, acting as sustained ecological filters, drive transformations in soil biogeochemistry (total nitrogen, microbial biomass) and ultimately shape the resultant, stable structure and diversity of the soil bacterial community; and (3) to synthesize these findings to identify an optimal, evidence-based strategy that reconciles the competing demands of crop productivity and long-term soil health.
Based on the premise that the physical placement of residue fundamentally dictates its ecological function, it was hypothesized that after nine years of consistent application, the incorporation method would emerge as a far more dominant driver of change in soil properties and bacterial community assembly than the residue rate. Specifically, it was predicted that a strategy of incorporating a moderate amount of residue (50%) via rotary tillage would prove superior. It is assumed that this approach would strike an optimal balance by effectively enhancing nutrient cycling and microbial health through improved soil-straw contact, while simultaneously mitigating the severe soil cooling penalty associated with high-residue surface mulching. By testing this hypothesis, this work aims to shift the prevailing management paradigm. Activities in this research move beyond the simplistic, quantity-focused question of “how much” residue to return and instead address the more critical and functionally relevant question of “how” and “where it should be placed within the soil ecosystem. This transition from a quantity-centric to a method-driven framework provides a robust scientific pathway for achieving both agricultural productivity and long-term soil sustainability in temperate agroecosystems worldwide.
2. Materials and Methods
2.1. Site Description and Experimental Design
The long-term field experiment was initiated in autumn 2015 at the Crop Breeding Center of the Harbin Academy of Agricultural Sciences, located in Heilongjiang Province, China (45°51′ N, 126°28′ E). The experimental site is situated in the Songnen Plain, a region representative of the Mollisols of Northeast China. This region is characterized by a temperate continental monsoon climate, with a mean annual temperature of 3.6 °C and mean annual precipitation of 533 mm. The soil is classified as a Mollisol with a clay loam texture (35% clay, 40% silt, and 25% sand). The site follows a continuous maize (Zea mays L.) monoculture system, which is the dominant cropping pattern in the region. To isolate the effects of the straw return strategies, all experimental plots, including the control, received identical mineral fertilization management throughout the nine-year period, based on local agronomic recommendations for maize (200 kg N ha−1, 100 kg P2O5 ha−1, and 90 kg K2O ha−1). The site operates under a rainfed agricultural system; therefore, no supplementary irrigation was applied during the experiment. All straw used in the experiment was derived from the previous season’s maize harvest.
For this study, the no-straw return (NSR) treatment serves as the experimental control, providing the baseline against which all straw return strategies are compared. After nine years of consistent management, the core physicochemical and biological properties of the topsoil (0–20 cm) in these control plots, reflecting a state of degradation due to long-term residue removal, were as follows: pH 6.85, organic matter 23.45 g/kg, total nitrogen (TN) 1.40 g/kg, and soil microbial biomass carbon (SMBC) 180 mg/kg.
The experiment, maintained for nine consecutive years (2016–2024), consisted of ten treatments arranged in a randomized complete block design with three replications. To align with standard agronomic practices while optimizing land use, individual plots were established with a size of 30 m in length by 6.5 m in width, accommodating 10 rows of maize at a 65 cm row spacing. A 2 m buffer zone was maintained between adjacent plots to minimize cross-contamination. To ensure data representativeness and avoid edge effects, all measurements and sampling were conducted within a central core area of each plot, comprising the middle 6 rows over a 25 m length. The experimental treatments included the no-straw return control (NSR) and a 3 × 3 factorial design comprising three straw incorporation methods—Surface Mulching (MUL), Rotary Tillage (ROT), and Deep Plowing (PLW)—each applied at three distinct rates. Detailed descriptions of the ten treatments are provided in
Table 1.
2.2. Soil Sampling and Physicochemical Analysis
Soil sampling for physicochemical analysis (ST, SWC, pH, TN, SMBC) was conducted annually in mid-May from 2016 to 2024, consistently capturing the pre-sowing period. For the comprehensive bacterial community analysis reported herein, soil samples from the final year (mid-May 2024) were used. This final sampling point was selected as it represents the mature, stabilized state of the soil ecosystem after nine consecutive years of treatment application, allowing for a definitive assessment of the long-term impacts on microbial community structure. From each plot, five soil cores (0–20 cm depth) were randomly collected using a spiral auger and subsequently pooled to create a single composite sample. After manually removing visible roots and plant debris, each composite sample was passed through a 2 mm sieve. Each sieved sample was then partitioned into two subsamples. One was immediately stored at −80 °C for subsequent molecular analysis, while the other was air-dried for the determination of physicochemical properties.
Soil temperature (ST) was measured using an automatic temperature recorder (TR-71U, T&D Corporation, Matsumoto, Japan) equipped with a digital thermocouple probe. Soil water content (SWC) was determined gravimetrically by oven-drying of fresh soil samples at 105 °C to a constant weight. Soil pH was measured in a 1:2.5 (w/v) soil-to-deionized water suspension using a benchtop pH meter. Soil total nitrogen (TN) was determined using the semi-micro Kjeldahl method following digestion with concentrated H2SO4.
Soil microbial biomass carbon (SMBC) was quantified using the chloroform fumigation-extraction method. Briefly, fumigated and non-fumigated soil samples were extracted with 0.5 M K
2SO
4, and the carbon content in the extracts was measured with a total organic carbon (TOC) analyzer (Vario TOC cube, Elementar Analysensysteme, Langenselbold, Germany). SMBC was calculated according to the established formula:
where
EC represents the difference in extractable organic carbon between fumigated and non-fumigated samples, and
kEC is the extraction efficiency coefficient. A standard
kEC value of 0.45 was used in this study, consistent with the recommendations of Joergensen et al. [
17].
2.3. Soil DNA Extraction and 16S rRNA Gene Sequencing
Total genomic DNA was extracted from 0.5 g of each frozen soil subsample using the DNeasy PowerSoil Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. Three biological replicates were processed for each treatment. The concentration and purity of the extracted DNA were assessed via spectrophotometry using a NanoDrop 2000 instrument (Thermo Fisher Scientific, Waltham, MA, USA), with DNA concentrations ranging from 15 to 45 ng/μL and A260/A280 ratios between 1.8 and 2.0.
The V4–V5 hypervariable regions of the bacterial 16S rRNA gene were amplified using the universal primer pair 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 926R (5′-CCGTCAATTCMTTTGAGTTT-3′), a protocol widely adopted since its description by 157 Zhao et al. [
18] Amplicon libraries were prepared for sequencing through a two-step PCR 158 protocol. PCR amplification was performed in 25 μL reactions containing 12.5 μL of 2× Taq PCR Master Mix, 1 μL each of forward and reverse primers (10 μM), 2 μL of template DNA, and 8.5 μL of nuclease-free water. The thermal cycling program consisted of initial denaturation at 95 °C for 3 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s, with a final extension at 72 °C for 10 min. Amplicon libraries were sequenced on an Illumina MiSeq platform (Illumina, Inc., San Diego, CA, USA) using a 2 × 250 bp paired-end strategy, generating approximately 50,000–80,000 raw reads per sample.
2.4. Bioinformatic and Statistical Analysis
Raw paired-end reads were subjected to quality control using Cutadapt (v4.5) for primer and adapter removal. High-quality reads were merged using FLASH2 (v2.2.0) with minimum overlap of 50 bp and maximum mismatch ratio of 0.1. Merged sequences were quality-filtered using Trimmomatic (v0.39) with a sliding window of 4 bp and average quality threshold of 25; sequences shorter than 350 bp were discarded.
Quality-filtered sequences were clustered into Operational Taxonomic Units (OTUs) at 97% similarity using Usearch (v11.0), with chimeric sequences removed using the UCHIME algorithm. Singleton OTUs were excluded from downstream analysis. Taxonomic classification was assigned to representative sequences against the SILVA (v138.1) database. The OTU table was rarefied to 25,000 reads per sample to normalize sequencing depth. Alpha diversity indices (Chao1, Ace, Shannon, and Simpson) were calculated in Mothur (v1.48.0).
Prior to statistical analysis, data were tested for normality and variance homogeneity using Shapiro–Wilk and Levene’s tests, respectively. For the nine straw return treatments, a two-way ANOVA was conducted using the General Linear Model (GLM) procedure in IBM SPSS Statistics (v29.0) to test the main effects of incorporation method (MUL, ROT, PLW), return rate (30%, 50%, 100%), and their interaction. The no-straw return (NSR) control was compared with other treatments using planned contrasts or Dunnett’s post hoc test. Beta diversity was assessed via Principal Coordinate Analysis (PCoA) based on Bray–Curtis dissimilarity. A two-way permutational multivariate analysis of variance (PERMANOVA) was used to test the effects of method, rate, and their interaction on bacterial community structure. Redundancy analysis (RDA) in Canoco (v5.0) explored relationships between soil properties and bacterial community composition, with significance validated by Monte Carlo permutation tests (999 permutations). Visualizations were created using OriginPro (2024).
To provide a holistic overview, the entire experimental and analytical framework, encompassing field site characteristics, management practices, laboratory procedures, and statistical analyses, is visually summarized in
Figure 1.
4. Discussion
4.1. Spatial Placement of Residue, Not Quantity, Resolves the Hydrothermal and Biogeochemical Trade-Offs of Straw Return
The nine-year field experiment in present study unequivocally demonstrated that the spatial placement of crop residue, not its application rate, is the decisive factor shaping the soil physicochemical environment. This pivotal insight provides a direct resolution to the long-standing “agronomic paradox” that has historically hindered straw return adoption in cold-climate breadbaskets like the Mollisol region of Northeast China. By systematically deconstructing the interconnected trade-offs between soil hydrothermal regulation and biogeochemical enrichment, it was revealed that management strategies, through their control of residue placement at the soil-atmosphere interface, fundamentally govern the ecological function of returned straw and the long-term trajectory of soil health.
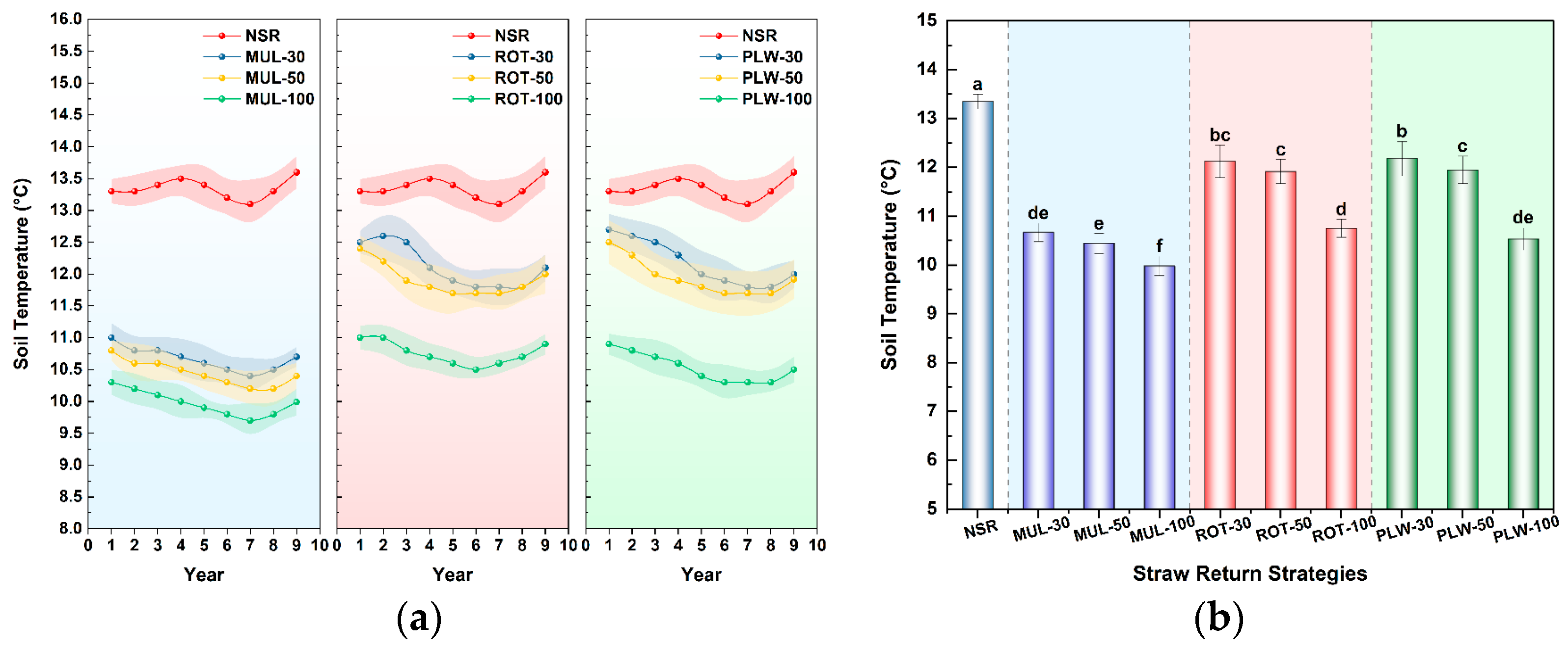
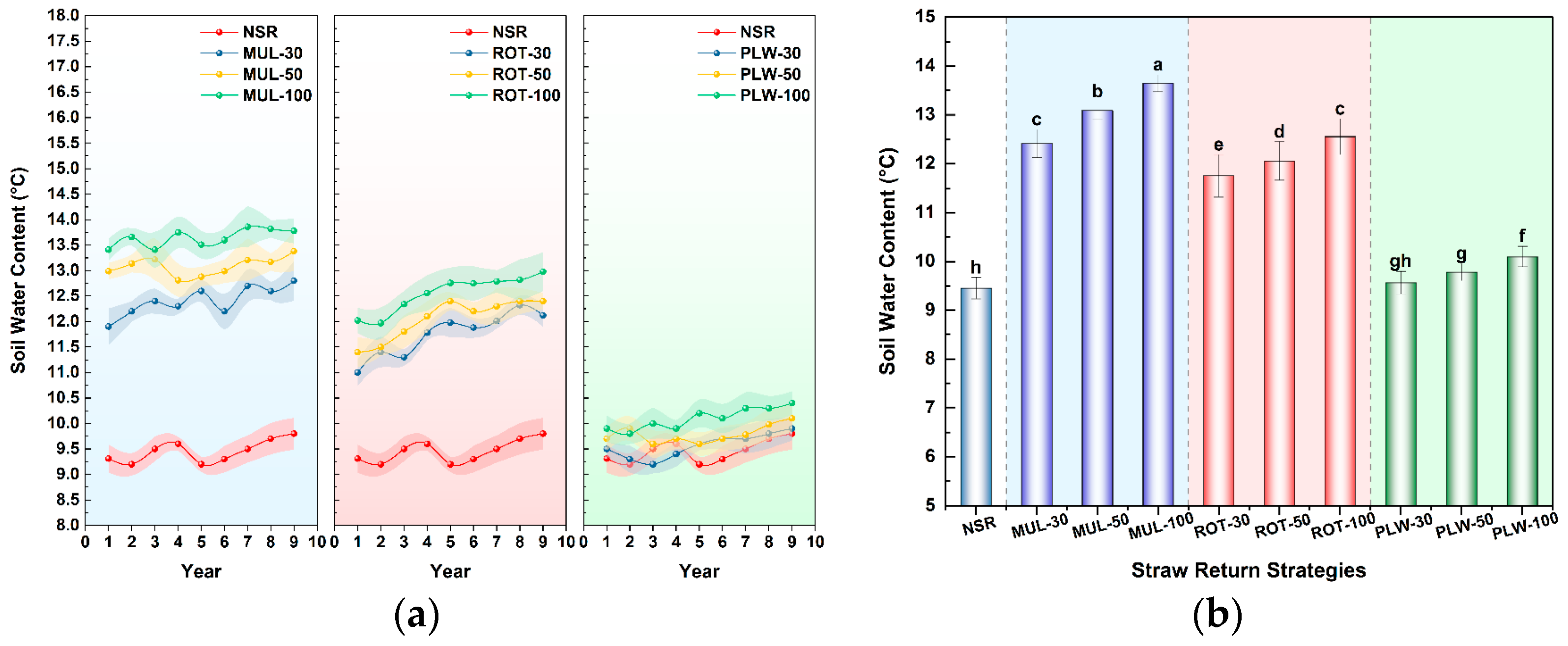
The most immediate and consequential trade-off manifested in the soil hydrothermal regime, creating a direct conflict between water conservation and the thermal conditions required for spring crop establishment. Obtained results confirmed that surface mulching (MUL) represents an extreme form of single-objective optimization. Its efficacy for water conservation was unparalleled; the MUL-50 treatment, for instance, increased soil water content by a remarkable 44% over the no-straw control (NSR). This is a direct consequence of the physical barrier formed by the straw mat, a well-documented ‘blanket effect’ that curtails evaporative losses. This phenomenon, as also reported by Gómez-Muñoz et al. [
19] and Neha et al. [
20] in other temperate agroecosystems, effectively preserves vital moisture. However, this hydrological gain was acquired at a severe and agronomically unacceptable thermal cost. The same insulating layer that trapped moisture also impeded solar energy transfer into the soil, suppressing spring soil temperatures by as much as 3.7 °C under the MUL-100 treatment compared to the control. Such “cold and wet” conditions precipitate delayed germination and impaired seedling vigor, as maize, being a C4 plant species, is particularly susceptible to chilling stress through membrane damage, photoinhibition, and disturbed enzyme activity as demonstrated by Farooq et al. [
21]. These effects, quantified in controlled studies showing 15–30% reductions in germination rate under similar temperature conditions, represent the primary barrier to farmer adoption in regions with short growing seasons, as detailed by Xia et al. [
22].
In stark contrast, physical incorporation of straw via rotary tillage (ROT) and deep plowing (PLW) offered a direct and effective solution to this water-heat conflict. By breaking the surface insulating layer and mixing residue into the soil, these methods allowed for more effective soil warming, with ROT treatments mitigating the chilling effect to a mere 1.1–1.7 °C reduction relative to the control. While this thermal gain involved a predictable compromise on water retention compared to surface mulching, it is critical to note that all incorporation treatments still maintained significantly more moisture than the control. This demonstrates that incorporation does not negate the hydrological benefits of straw return but rather strikes a more agronomically favorable balance, trading a fraction of the maximum potential water savings for a vital improvement in thermal conditions necessary for timely crop establishment.
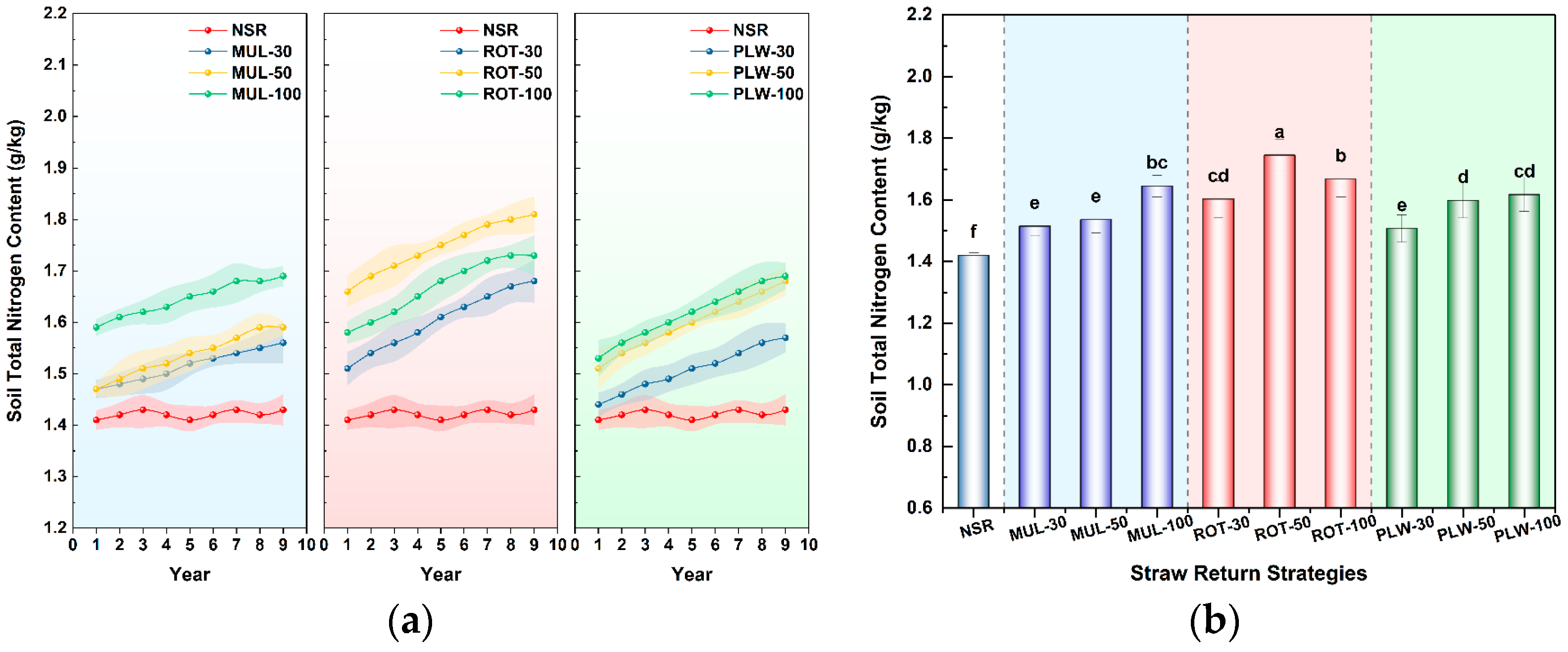
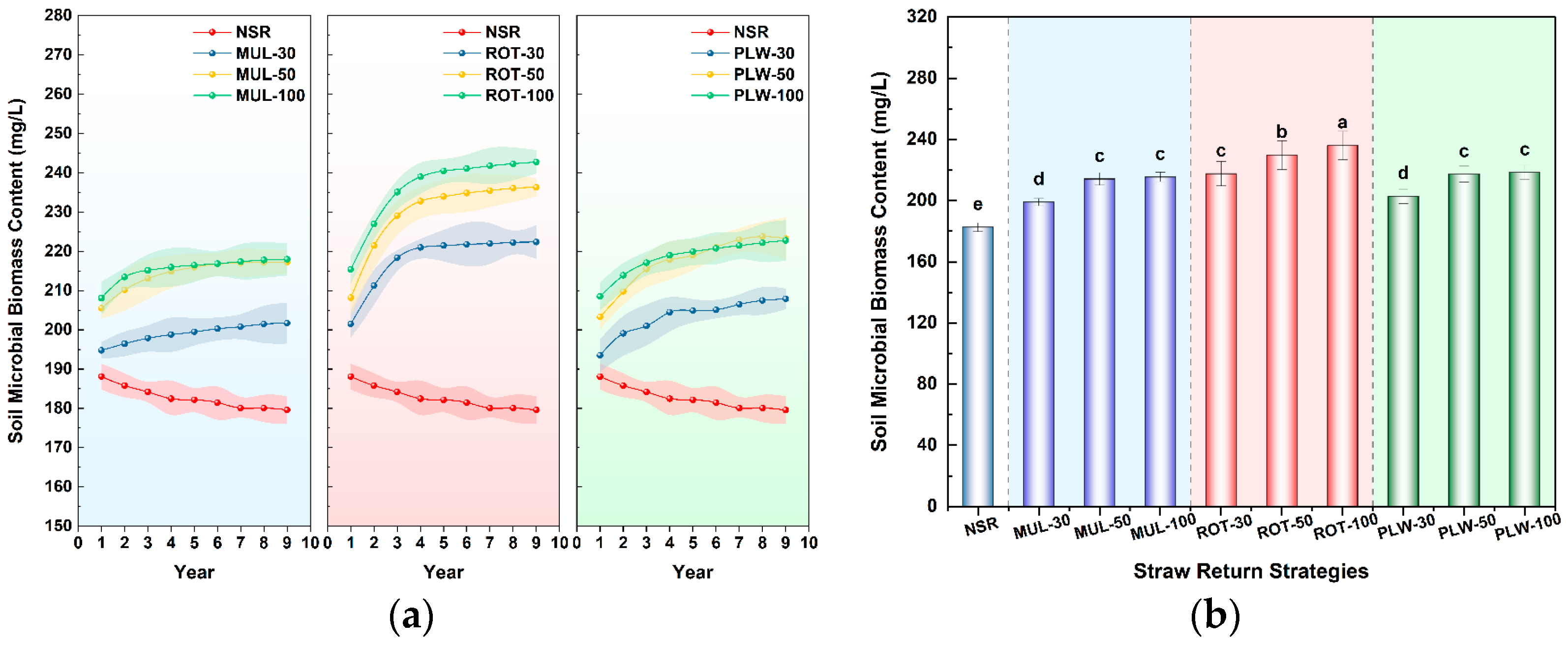
Beyond this immediate physical recalibration, the long-term divergence in soil biogeochemistry was even more profound, revealing the superior capacity of incorporation methods to build lasting soil fertility. After nine years, incorporation strategies (ROT and PLW) were systematically superior to surface mulching (MUL) in enhancing core fertility indicators like total nitrogen (TN) and soil microbial biomass carbon (SMBC). This superiority is rooted in a fundamental principle: substrate-microbe contact efficiency. When left on the surface, straw decomposition is slow and inefficient, limited by harsh environmental fluctuations and minimal contact with the bulk soil microbial community. As described by Liu et al. [
23] and Li et al. [
24], mechanically incorporating residue into the soil matrix radically transforms this dynamic. It creates innumerable “microbial hotspots” where straw fragments are brought into intimate contact with soil aggregates, water films, and a diverse consortium of decomposers. This close association dramatically increases the accessibility of carbon and nutrients, while the soil matrix provides a buffered, more stable environment and supplies the mineral nutrients required by microorganisms, thereby accelerating decomposition. This mechanism directly explains the significant enrichment of TN and SMBC pools observed under ROT and PLW treatments, where straw is effectively transformed from a surface barrier into a potent fuel for the soil’s biological engine.
Furthermore, the obtained results revealed a critical distinction between the two incorporation methods, highlighting the importance of distribution uniformity within the plow layer. The superior performance of rotary tillage (ROT), particularly the ROT-50 treatment in accumulating total nitrogen, suggests a distinct advantage over deep plowing. Deep plowing (PLW), which inverted the soil, tends to bury residue in a concentrated layer near the bottom of the plow zone. This practice, as supported by previous work from Liu et al. [
25] and Jin et al. [
26], can create localized anaerobic zones that inhibit optimal aerobic decomposition and places the fresh substrate in a deeper, often less biologically active and colder stratum. In contrast, rotary tillage (ROT) distributes the residue more homogeneously throughout the topsoil. This uniform distribution fosters a more consistently aerated and biologically active plow layer, optimizing conditions for nutrient cycling across the entire volume and preventing the temporary nitrogen immobilization that can occur in concentrated residue pockets. This superior physical framework for decomposition ultimately translates into more efficient nutrient sequestration and a healthier, more productive soil ecosystem.
In synthesis, this long-term study provided compelling, multi-faceted evidence that the spatial placement of straw is paramount. Surface mulching, while maximizing water retention, imposes an unacceptable thermal penalty and fails to efficiently translate residue into soil fertility, thus perpetuating the agronomic paradox. In contrast, soil incorporation resolves this primary conflict and, more importantly, unlocks the full biogeochemical potential of the returned residue by maximizing substrate-microbe interactions. This establishes a robust, process-based framework for optimizing straw management, decisively shifting the focus from the simplistic question of “how much” residue to return, to the more critical and functionally relevant question of “where it is placed within the soil ecosystem.
4.2. Straw Return Strategies as Deterministic Filters Shaping Bacterial Community Assembly
4.2.1. Altered Soil Conditions Modulate Bacterial Alpha Diversity
A consistent outcome across all straw return treatments was an enhancement of bacterial alpha diversity relative to the no-straw control, yet the magnitude and nature of this enhancement were intricately linked to the incorporation method. Present results strongly support the Resource Availability Hypothesis, which, as proposed by Duan et al. [
27] and Yang et al. [
28], posits that increased resource abundance and heterogeneity can support greater species diversity. The soil incorporation treatments (ROT and PLW) created environments rich in freshly supplied, labile carbon and subsequently elevated total nitrogen (TN) and soil microbial biomass carbon (SMBC). This enrichment of key limiting resources effectively expanded the number of available ecological niches, thereby supporting a more species-rich bacterial community. This is directly evidenced by the significant positive correlations observed between SMBC, TN, and the richness and diversity indices (
Table 4).
Interestingly, the obtained data revealed a nuanced distinction between community richness and evenness. While deep plowing (PLW) treatments fostered some of the highest species richness (Chao1 index), the rotary tillage (ROT) treatments consistently yielded the highest Shannon diversity, an index that accounts for both richness and evenness. This suggests different mechanisms of diversity enhancement. Deep plowing creates stable microniches in the lower topsoil that support specialist taxa, while rotary tillage produces uniformly resource-rich conditions favoring copiotrophic species coexistence as established by Gaitanis et al. [
29] and Pingthaisong et al. [
30]. The latter promotes more evenly structured communities with enhanced functional resilience.
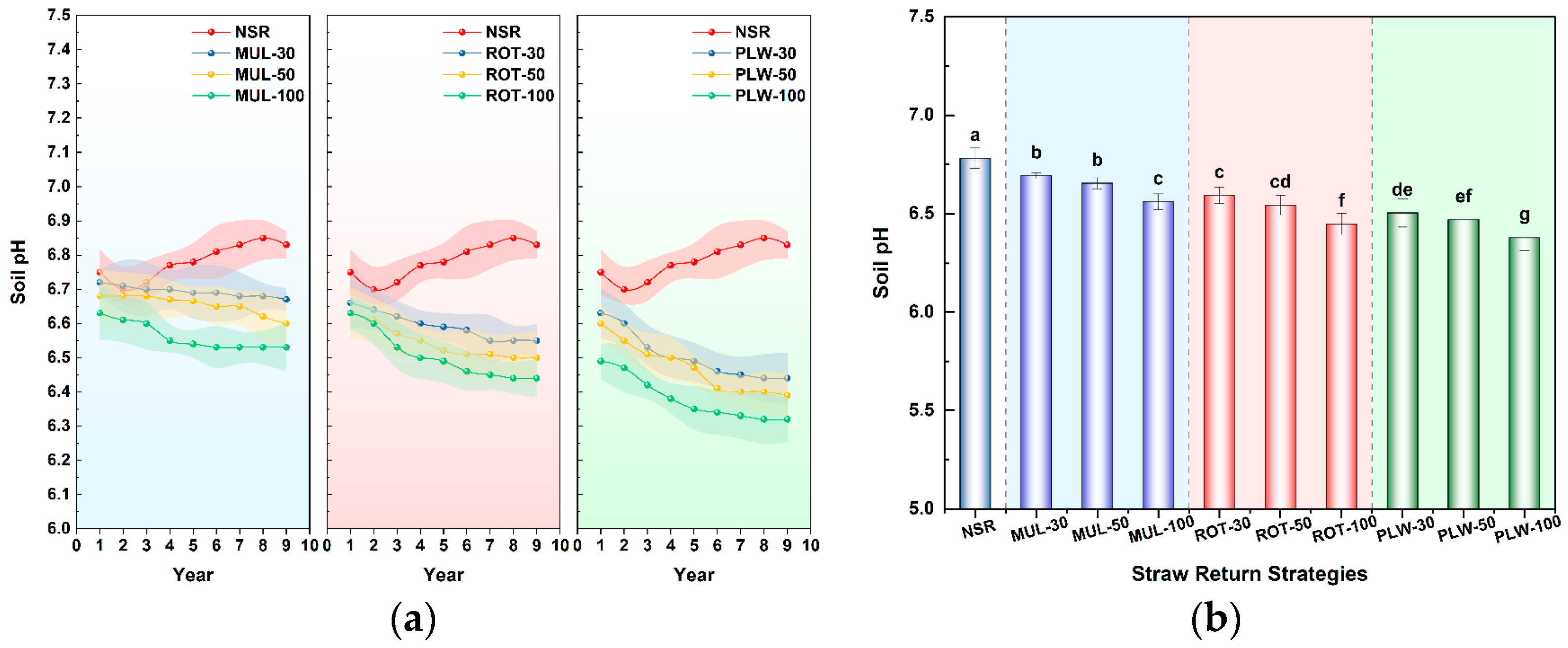
Crucially, this study also clarifies the role of soil pH in modulating bacterial diversity in this context. The significant negative correlation between pH and diversity indices might initially seem paradoxical. However, this correlation represents a concomitant effect rather than a direct causal driver of diversity. The acidification itself is a predictable consequence of intensified biological activity fueled by straw incorporation. Acidification occurs through three established mechanisms as documented by Bian et al. [
31], Macreadie et al. [
32] and Lewoyehu et al. [
33]: organic acid release during straw decomposition including acetic and oxalic acids, increased CO
2 production from enhanced microbial respiration forming carbonic acid, and proton release during intensified nitrification processes. Therefore, the observed pH decline is a signature of a highly active, resource-rich system. The overwhelmingly positive influence of this enhanced resource availability on niche partitioning far outweighed any potential negative selective pressure from the moderate acidification.
4.2.2. Deterministic Assembly Drives a Fundamental Shift in Bacterial Composition
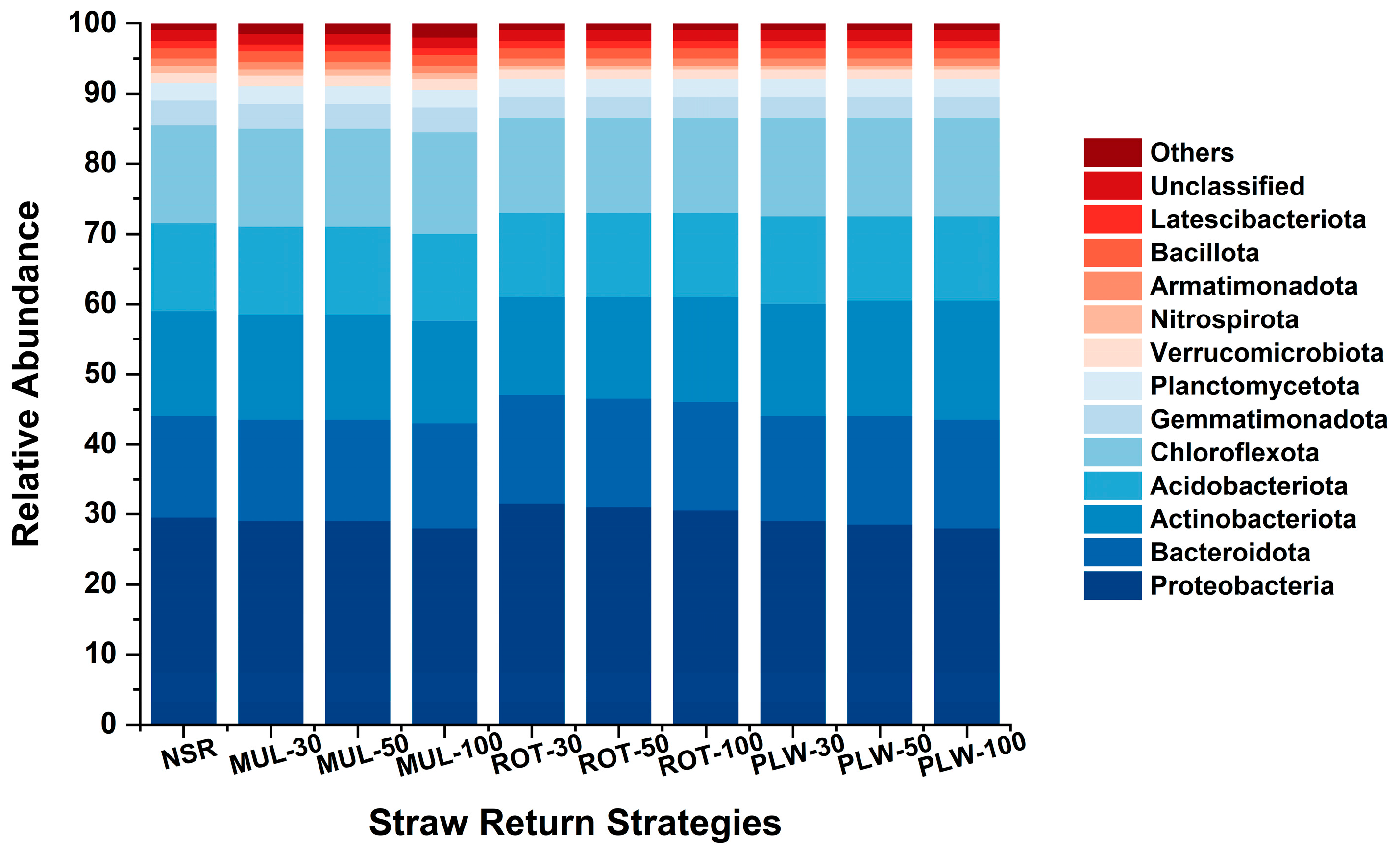
Beyond modulating overall diversity, the straw incorporation method acted as a powerful deterministic force driving a fundamental and predictable shift in the taxonomic composition of the bacterial community. The observed changes align perfectly with the established copiotroph-oligotroph framework, a cornerstone of microbial ecology. The low-disturbance, relatively resource-poor environments of the no-straw control (NSR) and surface mulching (MUL) treatments selected for oligotrophic taxa (often termed K-strategists). Phyla such as Acidobacteriota and Chloroflexota, which dominated these treatments, are well-known for their metabolic efficiency and ability to thrive under stable, low-nutrient conditions, as documented in numerous studies by Zhang et al. [
34] and Wei et al. [
35].
In stark contrast, the incorporation of straw via rotary tillage (ROT) and deep plowing (PLW) represented a regular, high-intensity resource pulse. This condition created a strong selective pressure favoring copiotrophic taxa (r-strategists), which are adapted for rapid growth and resource monopolization when labile carbon is abundant. The significant enrichment of phyla like Proteobacteria and Bacteroidota in these treatments was a classic signature of such a copiotrophic response. This profound taxonomic reorganization from an oligotroph-dominated to a copiotroph-dominated community represented the primary ecological consequence of long-term straw incorporation.
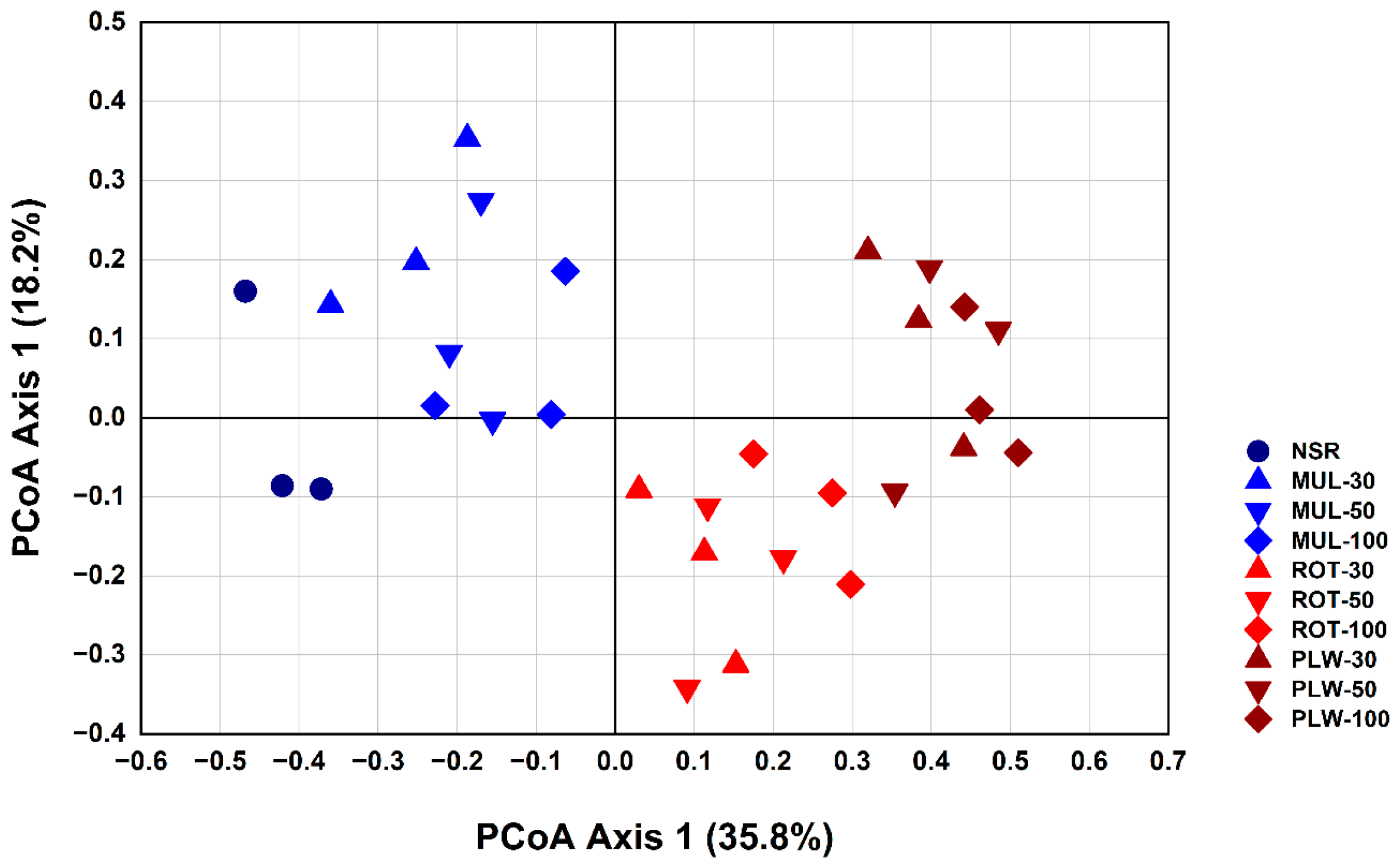
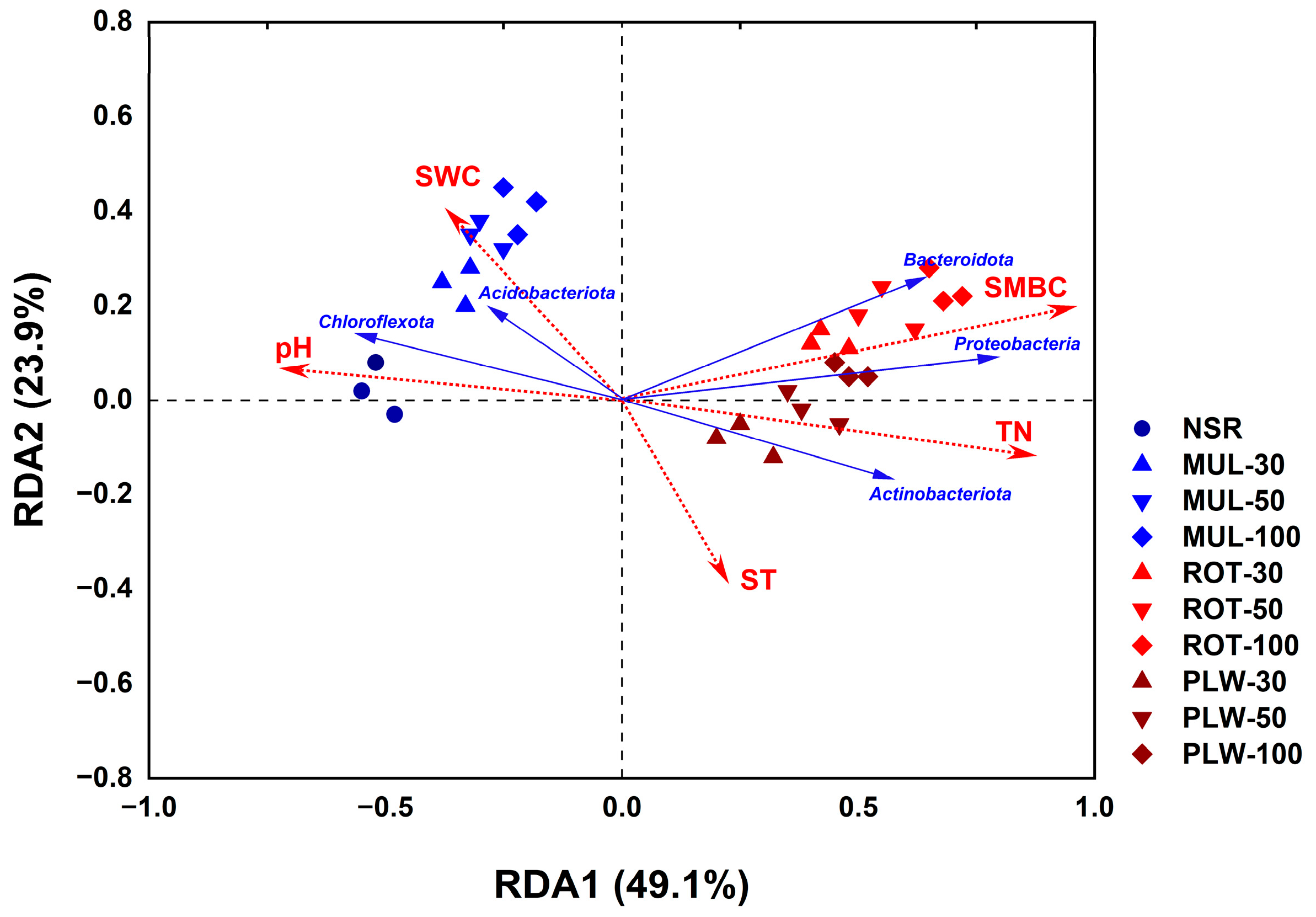
Principal Coordinate Analysis (PCoA) provided unequivocal evidence for this deterministic assembly process. The ordination plot starkly segregated the treatments into two distinct, non-overlapping clusters along the primary axis, which alone explained a substantial 35.8% of the community variation. This powerful separation confirms that the physical act of soil incorporation is the primary ecological filter shaping the bacterial community structure. The fact that samples from different application rates (30%, 50%, and 100%) were intermingled within their respective method-based clusters further reinforces central conclusion of the current paper: the method of incorporation is a far more influential driver of community assembly than the rate of application.
Finally, the Redundancy Analysis (RDA) mechanistically linked these structural shifts to the altered soil environment. The analysis confirmed that the community structure was most strongly governed by SMBC and TN, not the physical parameters of temperature or moisture. The RDA biplot clearly illustrates that the proliferation of copiotrophic phyla (Proteobacteria, Bacteroidota) was fueled by the elevated SMBC and TN in the ROT and PLW treatments. This establishes a clear causal chain: straw placement dictates substrate-microbe contact, which in turn governs biogeochemical cycling, and this altered biogeochemical landscape ultimately selects for a predictable and functionally distinct microbial community.
4.3. An Evidence-Based Framework for Optimal Straw Management in Temperate Agroecosystems
The ultimate goal of agricultural science is to translate complex ecological understanding into actionable strategies that enhance productivity while safeguarding natural resources. This comprehensive, long-term investigation moves beyond the evaluation of isolated variables to construct a holistic, systems-level understanding of how management choices propagate through the soil ecosystem. By synthesizing the interconnected responses of soil physics, biogeochemistry, and microbial ecology, it could now be definitively identified as a management practice that resolves the critical “agronomic paradox” and provides a scientifically validated pathway towards sustainable intensification in the Mollisol region. This synthesis unequivocally points to rotary tillage with a 50% straw return rate (ROT-50) as the optimal strategy.
The superiority of the ROT-50 practice is rooted in its ability to achieve a synergistic balance across multiple, often competing, soil health objectives. Its most immediate and critical advantage is the effective resolution of the water-heat conflict. Unlike surface mulching (MUL), which imposes a severe thermal penalty that renders it agronomically unviable in cold spring climates, the ROT-50 strategy facilitates crucial soil warming. Simultaneously, it represents a judicious compromise on moisture management, maintaining significantly higher soil water content than the no-straw control, thereby conserving precious water without sacrificing essential thermal gains. This practice directly addresses the primary barrier to straw return adoption by ensuring favorable conditions for the current crop’s germination and establishment.
However, the most profound benefits of the ROT-50 strategy lie in its capacity to function as a powerful biogeochemical engine, transforming straw residue into lasting soil fertility. The obtained results demonstrate that this practice achieved the highest accumulation of soil total nitrogen (TN) among all ten treatments, while concurrently fostering a robust and highly active pool of soil microbial biomass carbon (SMBC). This indicates that the ROT-50 treatment created an ideal “bioreactor” environment. The rotary tillage action ensures an optimal substrate-microbe contact efficiency, thoroughly mixing the high-carbon straw (the energy source) with the mineral soil, which contains the nitrogen and other nutrients required by the microbial decomposers. This efficient integration fuels the copiotroph-dominated microbial community that excels at rapidly cycling nutrients and incorporating them into stable microbial biomass and, eventually, long-term soil organic matter. This stands in stark contrast to the inefficient decomposition observed under surface mulching and the potentially suboptimal conditions created by deep plowing’s concentrated residue layer.
Furthermore, the ROT-50 strategy was instrumental in cultivating a highly resilient soil microbial community. It consistently yielded one of the highest numerical values for the Shannon diversity index. While the differences in mean diversity among treatments were not statistically significant in a direct ANOVA comparison, the correlation analysis revealed a crucial underlying principle: Shannon diversity was strongly and significantly correlated with the key fertility indicators of SMBC (r = 0.628, p < 0.05) and TN (r = 0.589, p < 0.05). Therefore, by creating the most biologically active and nitrogen-rich environment, the ROT-50 strategy promoted a community structure trending towards the highest diversity and its associated functional resilience.
Finally, this research provides a clear, evidence-based refutation of the simplistic “more is better” approach to straw return. The data obtained demonstrate that applying 100% of the residue (ROT-100) leads to diminishing returns in cold-climate agroecosystems, likely because low temperature constrains straw degradation, as Huang et al. [
36] established. Incorporating 100% of the residue can overwhelm soil decomposer capacity in cold conditions, where high C:N, lignin, cellulose, and hemicellulose contents slow decomposition and mineralization; consequently, undecomposed straw may persist and impair seedbed structure, as Hassan et al. [
37] reported for rice straw residues under controlled temperature–moisture regimes. These constraints create excessive porosity and hinder intimate seed-to-soil contact required for water uptake and successful germination and are likely compounded by transient N immobilization associated with high C:N. In contrast, the 50% rate (ROT-50) provides substantial organic matter input without triggering these adverse physical and biogeochemical feedback, thereby reconciling the short-term need for a warm, well-structured seedbed with the long-term goal of soil conservation through organic matter accrual.
5. Conclusions
This nine-year field experiment provides a scientifically grounded resolution to the foundational agronomic conflict that has long constrained sustainable agriculture in temperate Mollisols. The present findings fundamentally reframe the management paradigm for crop residue, demonstrating that the spatial placement of straw, not its application rate, is the paramount factor governing the long-term trajectory of soil health and microbial community assembly. By establishing the mechanistic pathway from management to microbial succession, this work provides an evidence-based framework for transforming returned residue from a passive surface barrier into a potent engine for soil regeneration. The principal conclusions are as follows:
(1) The method of straw incorporation, not the application rate, is the overwhelmingly dominant driver of ecosystem change. This principle is statistically validated by the obtained two-way ANOVA and PERMANOVA results. For nearly all the measured soil properties and for the overall bacterial community structure, the incorporation method showed a highly significant main effect, while the main effect of application rate and the method × rate interaction were often non-significant. This provides powerful, long-term evidence that the “how” and “where of residue placement far outweighs the “how much” in determining the final, stable state of the soil ecosystem.
(2) Straw incorporation acts as a deterministic ecological filter, driving a functional shift in the bacterial community. The physical placement of straw dictates the dominant microbial life-history strategy. Low-disturbance surface mulching selected for an oligotrophic community (e.g., Acidobacteriota), adapted to stable, low-nutrient conditions. In stark contrast, physically incorporating straw created a resource-rich environment that fueled a fundamental shift to a copiotrophic community (e.g., Proteobacteria, Bacteroidota). This transition from a slow-growing to a dynamic, fast-cycling microbial system is the core mechanistic pathway underpinning the enhanced nutrient cycling and fertility observed in incorporation treatments.
(3) Rotary tillage with 50% residue return (ROT-50) emerged as the optimal strategy among the tested alternatives through multi-objective synergy. This specific strategy proved superior by concurrently resolving multiple agronomic challenges. It successfully mitigated spring soil cooling without forfeiting crucial moisture gains, thus solving the primary water-heat conflict. Critically, it proved most effective for building long-term fertility, achieving the highest total nitrogen accumulation, and fostering a highly active microbial biomass. Furthermore, by fostering the most active microbial biomass and achieving the highest nitrogen accumulation, it cultivated a bacterial community that trended towards the highest Shannon diversity, suggesting enhanced functional redundancy and greater resilience to environmental stress. The ROT-50 strategy therefore represents the most effective, evidence-based pathway to reconcile immediate agronomic needs with long-term soil regeneration.
The present work establishes a new benchmark for residue management, framing these practices as powerful tools for applied microbial ecology that actively select for desired functional traits within the soil microbiome. Through this comprehensive nine-year investigation, we have demonstrated that the ROT-50 strategy provides a scientifically validated solution to the longstanding agronomic paradox in cold climate agriculture, shifting the paradigm from residue quantity to strategic placement for sustainable intensification. While our findings are robust within the scope of this single-site, Mollisol-based study focusing on bacterial communities, several areas warrant investigation to fully realize the global potential of this approach. It was acknowledged that a key frontier for future research is to transition from documenting these compositional shifts to directly validating their functional consequences. Priority research directions include: (1) multi-site validation across different soil types and climatic zones to establish broader applicability; (2) comprehensive assessment of the entire soil microbiome including fungal communities and their interactions with bacterial communities; (3) direct evaluation of crop yield responses and economic viability under the ROT-50 strategy; (4) long-term soil carbon sequestration studies to understand how the ROT-50 strategy influences the formation of stable soil organic matter, particularly its contribution to the mineral-associated organic matter (MAOM) pool, to verify its climate mitigation potential; (5) sustainable intensification research assessing the capacity of this optimized strategy to reduce the input of artificial nitrogen and phosphorus fertilizers while maintaining the same or higher yield of cultivated field crops, thereby validating its potential for truly sustainable intensification in global temperate agriculture. Despite these research priorities, this study provides compelling evidence that strategic straw placement can transform residue management from a simple waste disposal practice into a sophisticated tool for soil health enhancement. The ROT-50 strategy emerges not merely as a best practice but as a paradigm shift that reconciles immediate agronomic needs with long-term soil sustainability, offering a pathway forward for climate-smart agriculture in the world’s critical grain-producing regions.