Microbial Load, Physical–Chemical Characteristics, Ammonia, and GHG Emissions from Fresh Dairy Manure and Digestates According to Different Environmental Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Locations of Manure Sampling
2.2. Sampling Procedure
- 2 sub-samples of 1 L: physical–chemical analysis;
- 2 sub-samples of 500 mL: microbiological analysis;
- 8 sub-samples of 500 mL: gas emission trials designed to compare manure vs. digestate emissions and to assess the effect of temperature (18 °C and 28 °C) on gas release, with 4 samples per temperature. This allows evaluation of the impact of anaerobic digestion on greenhouse gas emissions and the influence of summer-like storage temperatures.
2.3. Physical–Chemical Analyses
2.4. Microbiological Analysis
- Coliforms (Gram-negative facultative anaerobes)
- Enterococci (Gram-positive facultative anaerobes)
- Lactobacilli (Gram-positive facultative anaerobes)
- Clostridia (Gram-positive, spore-forming anaerobes)
2.5. Gas Emissions Measurement
2.6. Statistical Analysis
3. Results
3.1. Physical–Chemical Composition of Manure and Digestate Samples
| Fresh Manure | Digested Manure | |
|---|---|---|
| TS, g kg−1 | 9.2 ± 0.3 | 8.25 ± 0.37 |
| VS, % | 81 ± 1.8 a | 72 ± 2.2 b |
| TKN, g kg−1 | 3.18 ± 0.09 A | 3.71 ± 0.11 B |
| TAN, g kg−1 | 1.5 ± 0.1 a | 1.9 ± 0.1 b |
| P, g kg−1 | 0.57 ± 0.18 | 1.15 ± 0.22 |
| K, g kg−1 | 2.51 ± 0.12 | 2.55 ± 0.14 |
| C/N | 19.8 ± 0.95 | 17 ± 0.67 |
| pH | 7.08 ± 0.11 a | 7.53 ± 0.13 b |
| Ashes, g kg−1 | 1.78 ± 0.19 | 2.44 ± 0.23 |
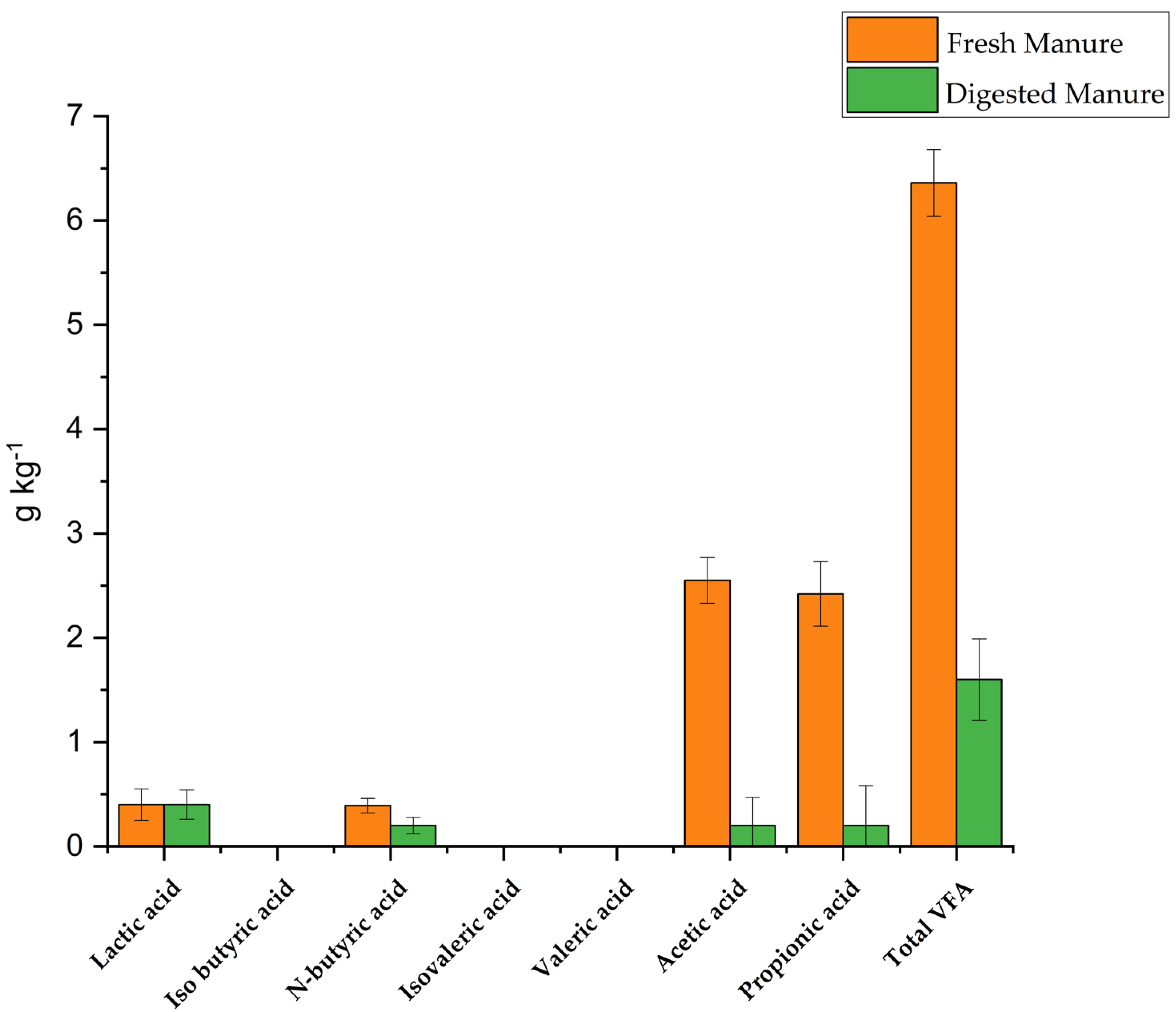
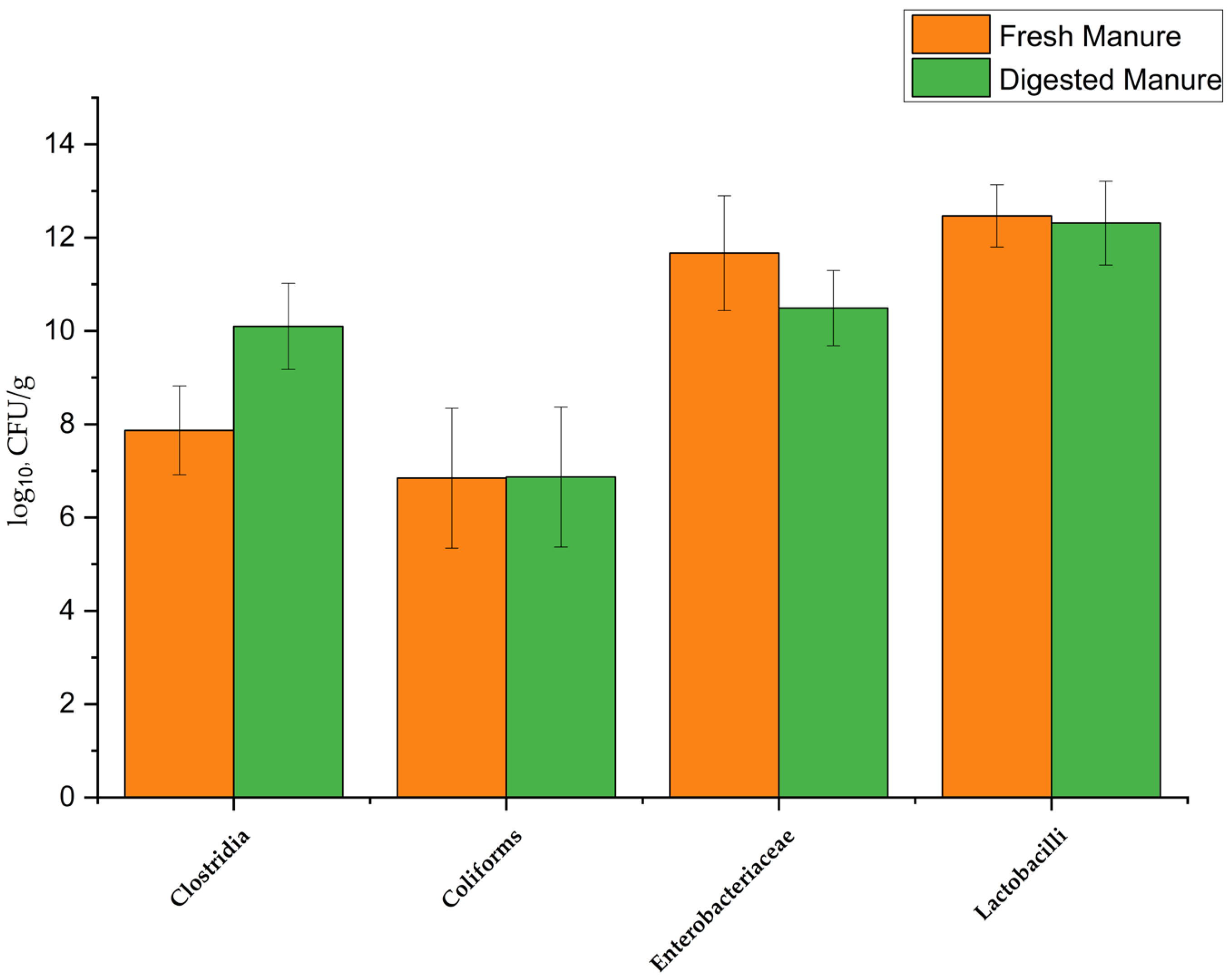
3.2. Pathogen Indicators
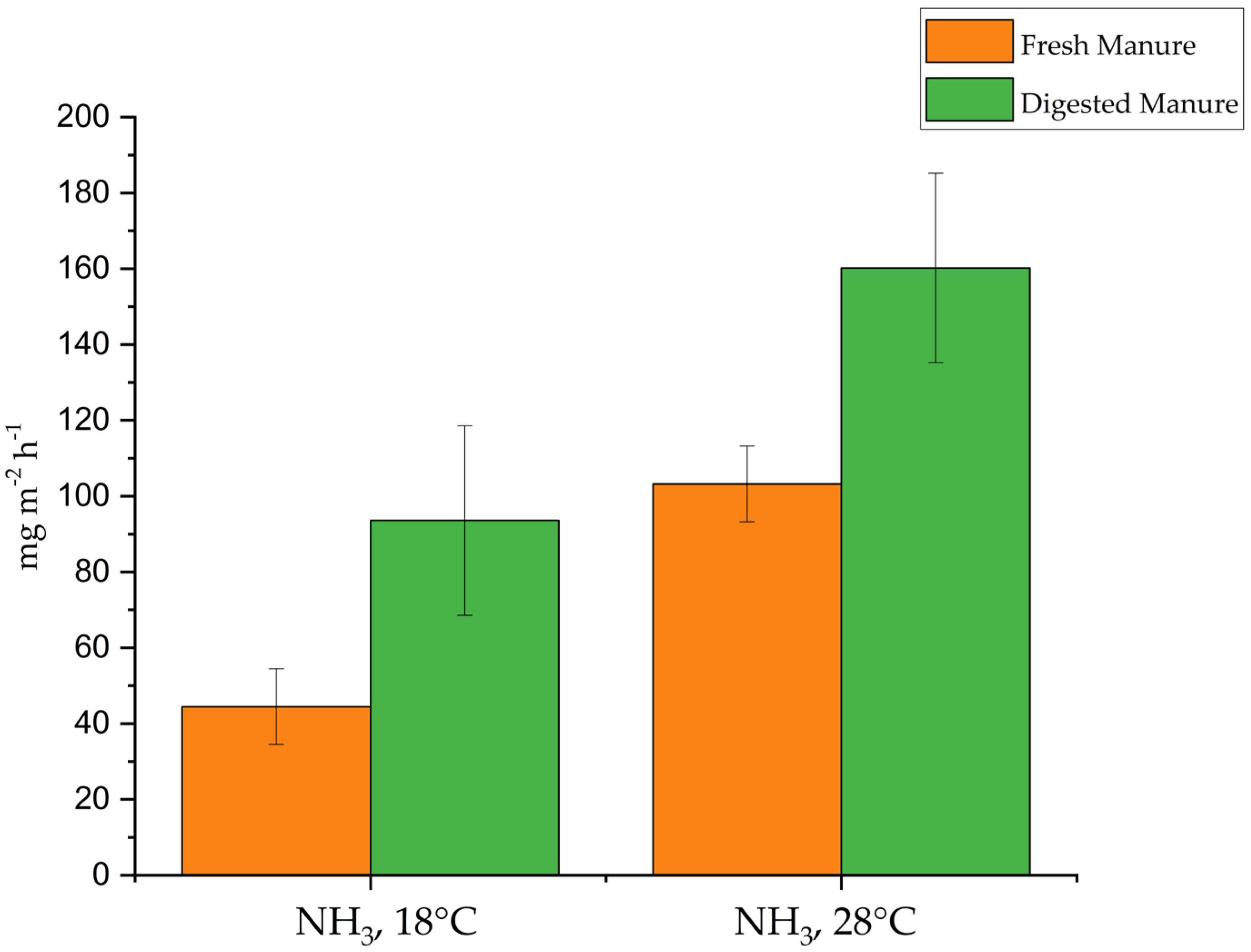
3.3. Ammonia and GHG Emissions

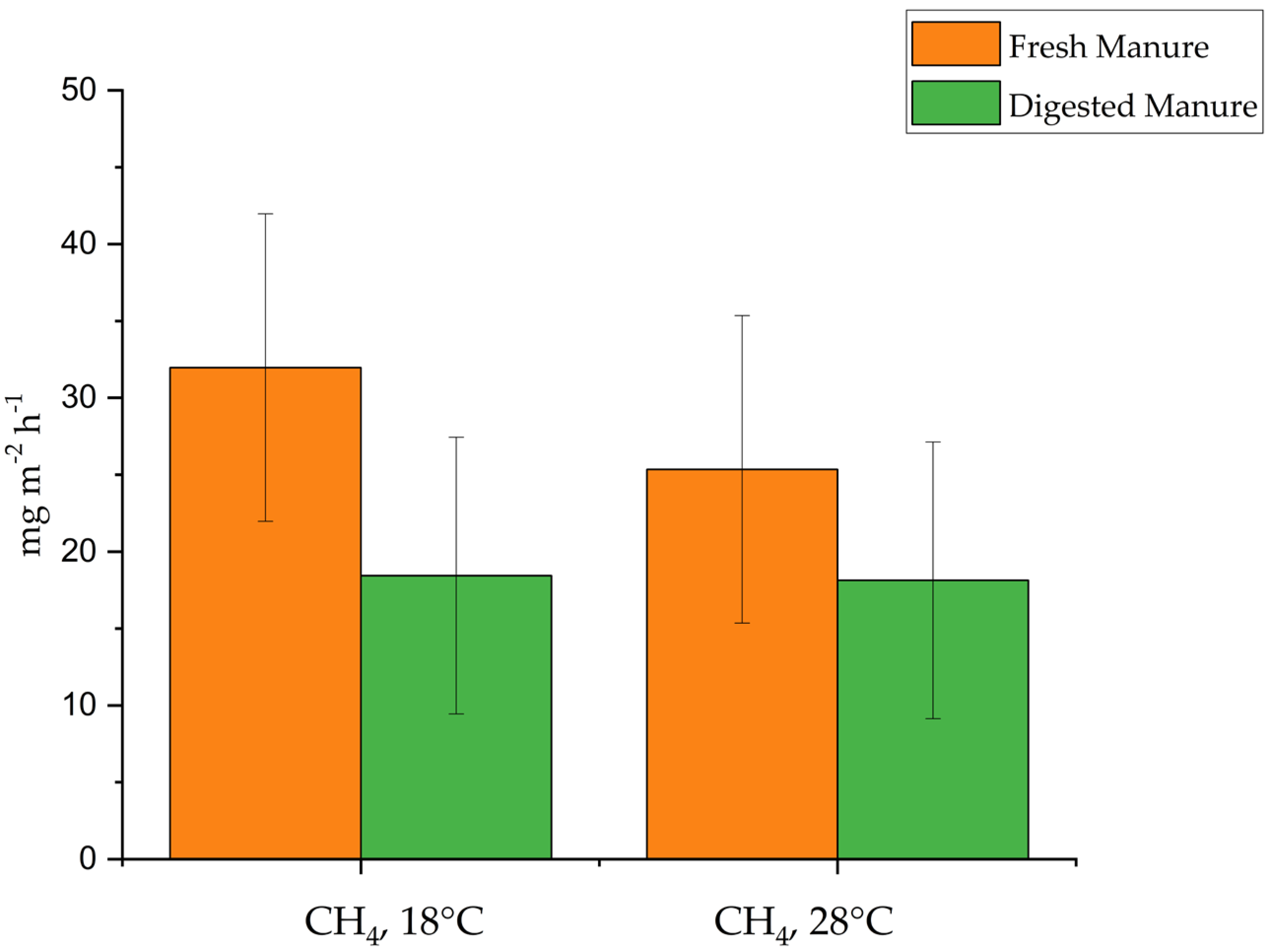
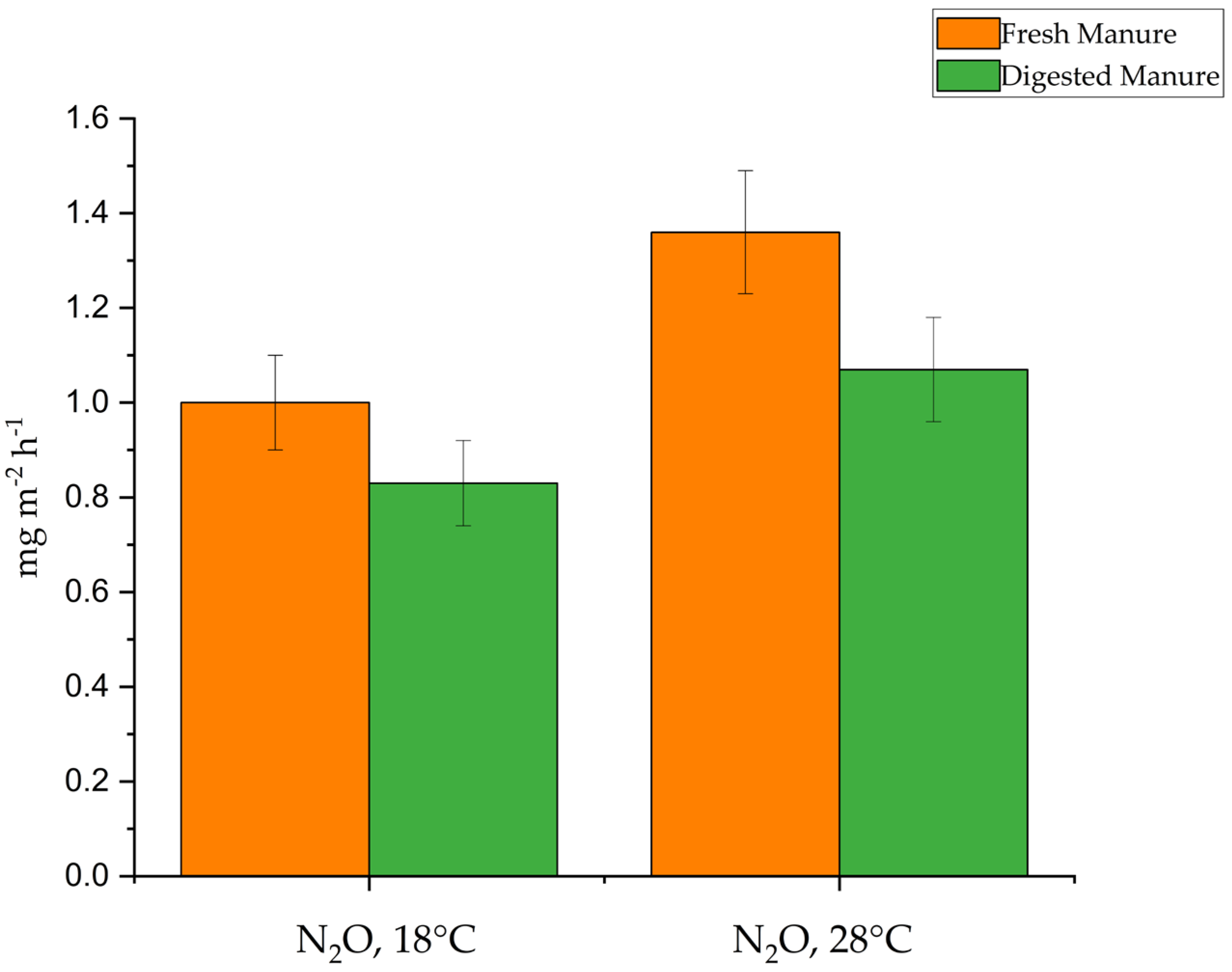
3.4. GHG Emissions as CO2 Equivalents
3.5. Pearson Correlations Among Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VFA | Volatile fatty acid |
| GHG | Green House Gases |
| HRT | Hydraulic retention time |
| TS | Total solids |
| VS | Volatile solids |
| TKN | Total Kjeldahl nitrogen |
| TAN | Total ammoniacal nitrogen |
| K | Potassium |
| CFU | Colony Forming Units |
| CO2 eq | CO2 equivalent |
References
- Regione Lombardia. Lombardy. In the North of Italy, in the Heart of Europe. Available online: https://www.en.regione.lombardia.it/wps/portal/site/en-regione-lombardia/discover-lombardy/territory-and-population (accessed on 10 June 2025).
- Clarisse, L.; Clerbaux, C.; Dentener, F.; Hurtmans, D.; Coheur, P.F. Global ammonia distribution derived from infrared satellite observations. Nat. Geosci. 2009, 2, 479–483. [Google Scholar] [CrossRef]
- Pain, B. Gaseous pollutants from organic waste use in agriculture. In Proceedings of the Eighth International Conference of the European Cooperative Research Network on Recycling of Agricultural Municipal and Industrial Residuals in Agriculture, Rennes, France, 26–29 May 1998; pp. 233–246. [Google Scholar]
- Costa, A.; Domeneghini, C. Pollutants in livestock buildings: Ammonia and dust interplay with the respiratory tract. In Air Quality and Livestock Farming; Banhazi, T., Aland, A., Hartung, J., Eds.; CRC Press: London, UK, 2018; pp. 49–58. ISBN 9781138027039. [Google Scholar]
- Renard, J.J.; Calidonna, S.E.; Henley, M.V. Fate of ammonia in the atmosphere—A review for applicability to hazardous releases. J. Hazard. Mater. 2004, 130, 29–60. [Google Scholar] [CrossRef]
- Costa, A. Ammonia Concentrations and Emissions from Finishing Pigs Reared in Different Growing Rooms. J. Environ. Qual. 2017, 46, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Danev, V.; Atanasova, T.; Dineva, K. Multi-Sensor Platform in Precision Livestock Farming for Air Quality Measurement Based on Open-Source Tools. Appl. Sci. 2024, 14, 8113. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; p. 2391. [Google Scholar] [CrossRef]
- Heng, K.; Tan, K.; Chan, A.; Lee, C.C.C. Food Waste Valorization: Leveraging Singapore’s Zero Waste Master Plan and 30-by-30 Goal. Sustainability 2024, 16, 7321. [Google Scholar] [CrossRef]
- Milich, L. The role of methane in global warming: Where might mitigation strategies be focused? Glob. Environ. Change 1999, 9, 179–201. [Google Scholar] [CrossRef]
- Jalota, S.K.; Vashisht, B.B.; Sharma, S.; Kaur, S. (Eds.) Emission of greenhouse gases and their warming effect. In Understanding Climate Change Impacts on Crop Productivity and Water Balance; Academic Press: Cambridge, MA, USA, 2018; pp. 1–53. [Google Scholar] [CrossRef]
- Møller, H.B.; Sørensen, P.; Olesen, J.E.; Petersen, S.O.; Nyord, T.; Sommer, S.G. Agricultural Biogas Production—Climate and Environmental Impacts. Sustainability 2022, 14, 1849. [Google Scholar] [CrossRef]
- ISPRA. Italian Emission Inventory 1990–2014. Informative Inventory Report 2016. Available online: https://www.isprambiente.gov.it/ (accessed on 1 July 2025).
- Costa, A.; Guarino, M. Particulate Matter Concentration and Emission Factor in Three Different Laying Hen Housing Systems. J. Agric. Eng. 2009, 3, 15–24. [Google Scholar] [CrossRef]
- Costa, A.; Ferrari, S.; Guarino, M. Yearly emissions factors of ammonia and particulate matter from three laying hens housing systems. Anim. Prod. Sci. 2012, 52, 1089–1098. [Google Scholar] [CrossRef]
- Buoio, E.; Cialini, C.; Cafiso, A.; Aidos, L.; Mazzola, S.M.; Rossi, R.; Livolsi, S.; Di Giancamillo, A.; Moretti, V.M.; Selli, E.; et al. From Photocatalysis to Photo-Electrocatalysis: An Innovative Water Remediation System for Sustainable Fish Farming. Sustainability 2022, 14, 9067. [Google Scholar] [CrossRef]
- Buoio, E.; Cialini, C.; Costa, A. Air Quality Assessment in Pig Farming: The Italian Classyfarm. Animals 2023, 13, 2297. [Google Scholar] [CrossRef]
- Costa, A.; Gusmara, C.; Gardoni, D.; Zaninelli, M.; Tambone, F.; Sala, V.; Guarino, M. The effect of anaerobic digestion and storage on indicator microorganisms in swine and dairy manure. Environ. Sci. Pollut. Res. 2017, 24, 24135–24146. [Google Scholar] [CrossRef]
- Sutton, M.A.; Reis, S.; Riddick, S.N.; Dragosits, U.; Nemitz, E.; Theobald, M.R.; Tang, Y.S.; Braban, C.F.; Vieno, M.; Dore, A.J.; et al. Towards a climate-dependent paradigm of ammonia emission and deposition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20130166. [Google Scholar] [CrossRef]
- Colombi, C.; D’Angelo, L.; Biffi, B.; Cuccia, E.; Dal Santo, U.; Lanzani, G. Monitoring ammonia concentrations in more than 10 stations in the Po Valley for the period 2007–2022 in relation to the evolution of different sources. Front. Environ. Health 2024, 3, 1249457. [Google Scholar] [CrossRef]
- Van Damme, M.; Clarisse, L.; Coheur, P.F. Industrial and agricultural ammonia point sources exposed. Nature 2018, 564, 99–103. [Google Scholar] [CrossRef]
- Kreidenweis, U.; Breier, J.; Herrmann, C.; Libra, J.; Prochnow, A. Greenhouse gas emissions from broiler manure treatment options are lowest in well-managed biogas production. J. Clean. Prod. 2021, 280, 124969. [Google Scholar] [CrossRef]
- Walsh, J.J.; Jones, D.L.; Edwards-Jone, G.; PrysorWilliams, A. Replacing inorganic fertilizer with anaerobic digestate may maintain agricultural productivity at less environmental cost. J. Plant. Nutr. Soil Sci. 2012, 175, 840–845. [Google Scholar] [CrossRef]
- Kuhn, E. Kofermentation; Arbeitspapier 249; Kuratorium für Technik und Bauwesen in der Landwirtschaft (KTBL): Darmstadt, Germany, 1998; ISBN 3-7843-1971-8. [Google Scholar]
- Rodhe, L.K.K.; Ascue, J.; Willen, A.; Vegerfors, B. Greenhouse gas emissions from storage and field application of anaerobically digested and non-digested cattle slurry. Agric. Ecosyst. Environ. 2015, 199, 358–368. [Google Scholar] [CrossRef]
- Maldaner, L.; Wagner-Riddle, C.; VanderZaag, A.C.; Gordon, R.; Duke, C. Methane emissions from storage of digestate at a dairy manure biogas facility. AFM 2018, 258, 96–107. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- EPA. Method EPA 3051: Microwave Assisted Acid Digestion of Sediments, Sludges, Soils and Oils; EPA: Washington, DC, USA, 1998. [Google Scholar]
- Canale, A.; Valente, M.E.; Ciotti, A. Determination of volatile carboxylic acids (C1–C5i) and lactic acid in aqueous acid extracts of silage by high performance liquid chromatography. J. Sci. Food Agric. 1984, 35, 1178–1182. [Google Scholar] [CrossRef]
- SAS Statistical Package, Version 9.4; SAS Institute: Cary, NC, USA, 2022.
- Balde, H.; VanderZaag, A.C.; Burtt, S.D.; Wagner-Riddle, C.; Crolla, A.; Desjardins, R.L.; MacDonald, D.J. Methane emissions from digestate at an agricultural biogas plant. Bioresour. Technol. 2016, 216, 914–922. [Google Scholar] [CrossRef]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Methane productivity of manure, straw and solid fractions of manure. Biomass Bioenergy 2003, 26, 485–495. [Google Scholar] [CrossRef]
- Fang, H.H.; Yu, H.Q. Effect of HRT on mesophilic acidogenesis of dairy wastewater. J. Environ. Eng. 2000, 126, 1145–1148. [Google Scholar] [CrossRef]
- Messner, H. Düngewirkung Anaerob Fermentierter und Unbehandelter Gülle. Master’s Thesis, Universität München-Weihenstephan, Freising, Germany, 1988. [Google Scholar]
- Sung, S.W.; Liu, T. Ammonia inhibition on thermophilic anaerobic digestion. Chemosphere 2003, 5, 43–52. [Google Scholar] [CrossRef]
- Zhuang, M.; Shan, N.; Wang, Y.; Caro, D.; Fleming, R.M.; Wang, L. Different characteristics of greenhouse gases and ammonia emissions from conventional stored dairy cattle and swine manure in China. Sci. Total Environ. 2020, 722, 137693. [Google Scholar] [CrossRef] [PubMed]
- Getabalew, M.; Alemneh, T.; Bzuneh, E. Review on Methanogenesis and its Role. World J. Agric. Soil Sci. 2020, 6, 1–7. [Google Scholar] [CrossRef]
- Bucci, L.; Ghiotto, G.; Zampieri, G.; Raga, R.; Favaro, L.; Treu, L.; Campanaro, S. Adaptation of anaerobic digestion microbial communities to high ammonium levels: Insights from strain-resolved metagenomics. Environ. Sci. Technol. 2023, 57, 580–590. [Google Scholar] [CrossRef]
- Vergote, T.L.I.; Bodè, S.; Dobbelaere, d.A.E.J.; Buysse, J.; Meers, E.; Volcke, E.I.P. Monitoring methane and nitrous oxide emissions from digestate storage following manure mono-digestion. Biosyst. Eng. 2020, 196, 159–171. [Google Scholar] [CrossRef]
- Cecchi, F.; Battistoni, P.; Pavan, P.; Bolzonella, D.; Innocenti, L. Digestione Anaerobica della Frazione Organica dei Rifiuti Solidi. Aspetti Fondamentali, Progettuali, Gestionali, di Impatto Ambientale ed Integrazione con la Depurazione delle Acque Reflue; Manuali e linee guida 13/2005; APAT, Agenzia per la Protezione dell’Ambiente e per i Servizi Tecnici o le persone: Roma, Italy, 2005. Available online: https://www.isprambiente.gov.it/contentfiles/00003400/3482-manuali-linee-guida-2005.pdf (accessed on 1 July 2025).
- Rusanowska, P.; Zielinski, M.; Kisielewska, M.; Dudek, M.; Paukszto, Ł.; Debowski, M. Methane Production, Microbial Community, and Volatile Fatty Acids Profiling During Anaerobic Digestion Under Different Organic Loading. Energies 2025, 18, 575. [Google Scholar] [CrossRef]
- Rajlakshmi, J.; Jadhav, D.A.; Dutta, S.; Sherpa, K.C.; Jayaswal, K.; Saravanabhupathy, S.; Mohanty, K.T.; Banerjee, R.; Kumar, J.; Rajak, R.C. Co-digestion processes of waste: Status and perspective. In Advanced Zero Waste Tools, Bio-Based Materials and Waste for Energy Generation and Resource Management; Hussain, C.M., Kushwaha, A., Bharagava, R.N., Goswami, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 5, pp. 207–241. [Google Scholar]
- Speece, R.E. Anaerobic Biotechnology for Industrial Wastewaters; ArchaePress: Nashville, TN, USA, 1996. [Google Scholar]
- Colonna, N.; Alfano, V.; Gaeta, M. La Stima del Potenziale di Biogas da Biomasse di Scarto del Settore Zootecnico in Italia; Report Ricerca Sistema Elettrico, RSE/2009/201; 2009. Available online: https://www.researchgate.net/publication/265279702_La_stima_del_potenziale_di_biogas_da_biomasse_di_scarto_del_settore_zootecnico_in_Italia (accessed on 1 July 2025).
- Wu, J.; Zhang, H.; Zhao, Y.; Yuan, X.; Cui, Z. Characteristics of Biogas Production Activity and Microbial Community during Sub-Moderate Temperature Anaerobic Digestion of Wastewater. Fermentation 2023, 9, 903. [Google Scholar] [CrossRef]
- Hirsh, D.C.; Zee, Y.C. Veterinary Microbiology; Blackwell Science, Inc.: Oxford, UK, 1999. [Google Scholar]
- McFeters, G.A.; Stuart, D.G. Survival of coliform bacteria in natural waters: Field and laboratory studies with membrane-filter chambers. Appl. Microbiol. 1972, 24, 805–811. [Google Scholar] [CrossRef]
- Parhad, N.M.; Rao, U.N. Effect of pH on survival of Escherichia coli. J. Water Pollut. Control Fed. 1974, 46, 980–986. [Google Scholar]
- Sahlström, L. A review of survival of pathogenic bacteria in organic waste used in biogas plants. Bioresour. Technol. 2003, 87, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Wang, A.; Ren, L.; Qiao, W.; Wandera, S.M.; Dong, R. Challenges of pathogen inactivation in animal manure through anaerobic digestion: A short review. Bioengineered 2022, 13, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.G. Ammonia volatilization from farm tanks containing anaerobically digested animal slurry. Atmos. Environ. 1997, 31, 863–868. [Google Scholar] [CrossRef]
- Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N-emissions, and soil biological activity. A review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Nartey, O.D.; Liu, D.; Uwamungu, J.Y.; Luo, J.; Lindser, S.; Di, H.J.; Chen, Z.; Yuan, J.; Ding, W. Corn cobs efficiently reduced ammonia volatilization and improved nutrient value of stored dairy effluents. Sci. Total Environ. 2021, 769, 144712. [Google Scholar] [CrossRef]
- Efosa, N.; Häni, C.; Kupper, T.; Krause, H.-M.; Six, J.; Bünemann, E.K. Ammonia emissions after trailing hose application of digestates and cattle slurry. Nutr. Cycl. Agroecosyst. 2025, 130, 419–426. [Google Scholar] [CrossRef]
- Denmead, O.; Freney, L.; Simpson, J. Dynamics of ammonia volatilization during furrow irrigation of maize. Soil Sci. Soc. Am. J. 1982, 46, 149–155. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Z.; Li, X.; Yang, J.; Liang, L.; Sui, Q.; Wang, B.; Dong, H. NH3, N2O, and NO emissions from digested pig slurry stored under different temperatures: Characteristics and microbial mechanisms. J. Clean. Prod. 2021, 319, 128560. [Google Scholar] [CrossRef]
- Hüther, L. Entwicklung Analytischer Methoden und Untersuchung von Einflußfaktoren auf Ammoniak-, Methan- und Distickstoffmonoxidemissionen aus Flussig- und Festmist; Landbauforschung Volkenrode, Sonderheft: Braunschweig, Germany, 1999. [Google Scholar]
- Sommer, S.G.; Petersen, S.O.; Sogaard, H.T. Greenhouse gas emission from stored livestock slurry. J. Environ. Qual. 2000, 29, 744–751. [Google Scholar] [CrossRef]
- Kebreab, E.; Clark, K.; Wagner-Riddle, C.; France, J. Methane and nitrous oxide emissions from Canadian animal agriculture: A review. Can. J. Anim. Sci. 2006, 86, 135–158. [Google Scholar] [CrossRef]
- Wulf, S.; Maeting, M.; Clemens, J. Effect of application technique on the emission of trace gases (NH3, N2O, CH4) after spreading cofermented slurry on arable and grassland. Part II. Greenhouse gas emissions. J. Environ. Qual. 2002, 31, 1795–1801. [Google Scholar]
- Cárdenas, A.; Ammon, C.; Schumacher, B.; Stinner, W.; Herrmann, C.; Schneider, M.; Weinrich, S.; Fischer, P.; Amon, T.; Amon, B. Methane emissions from the storage of liquid dairy manure: Influences of season, temperature and storage duration. Waste Manag. 2021, 121, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Im, S.; Petersen, S.O.; Lee, D.; Kim, D.H. Effects of storage temperature on CH4 emissions from cattle manure and subsequent biogas production potential. Waste Manag. 2020, 101, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, Y.S.; Min, H.G.; Kim, J.G. Applicability of the dynamic chamber-capture system (DCS) for estimating the flux of ammonia emission during liquid fertilizer spreading. Atmos. Pollut. Res. 2020, 11, 723729. [Google Scholar] [CrossRef]
| Temperature | NH3 | CH4 | CO2 | N2O | |
|---|---|---|---|---|---|
| 18 °C | Fresh manure | 0.71 ± 0.11 | 0.87 ± 0.15 | 0.99 ± 0.01 | 0.98 ± 0.01 |
| Digested manure | 0.77 ± 0.10 | 0.76 ± 0.04 | 0.99 ± 0.01 | 0.98 ± 0.01 | |
| 28 °C | Fresh manure | 0.65 ± 0.06 | 0.74 ± 0.12 | 0.96 ± 0.00 | 0.94 ± 0.03 |
| Digested manure | 0.65 ± 0.12 | 0.84 ± 0.03 | 0.88 ± 0.12 | 0.96 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buoio, E.; Ighina, E.; Costa, A. Microbial Load, Physical–Chemical Characteristics, Ammonia, and GHG Emissions from Fresh Dairy Manure and Digestates According to Different Environmental Temperatures. Agriculture 2025, 15, 1931. https://doi.org/10.3390/agriculture15181931
Buoio E, Ighina E, Costa A. Microbial Load, Physical–Chemical Characteristics, Ammonia, and GHG Emissions from Fresh Dairy Manure and Digestates According to Different Environmental Temperatures. Agriculture. 2025; 15(18):1931. https://doi.org/10.3390/agriculture15181931
Chicago/Turabian StyleBuoio, Eleonora, Elena Ighina, and Annamaria Costa. 2025. "Microbial Load, Physical–Chemical Characteristics, Ammonia, and GHG Emissions from Fresh Dairy Manure and Digestates According to Different Environmental Temperatures" Agriculture 15, no. 18: 1931. https://doi.org/10.3390/agriculture15181931
APA StyleBuoio, E., Ighina, E., & Costa, A. (2025). Microbial Load, Physical–Chemical Characteristics, Ammonia, and GHG Emissions from Fresh Dairy Manure and Digestates According to Different Environmental Temperatures. Agriculture, 15(18), 1931. https://doi.org/10.3390/agriculture15181931








