Land Use Changes Influence Tropical Soil Diversity: An Assessment Using Soil Taxonomy and the World Reference Base for Soil Classifications
Abstract
1. Introduction
2. Materials and Methods
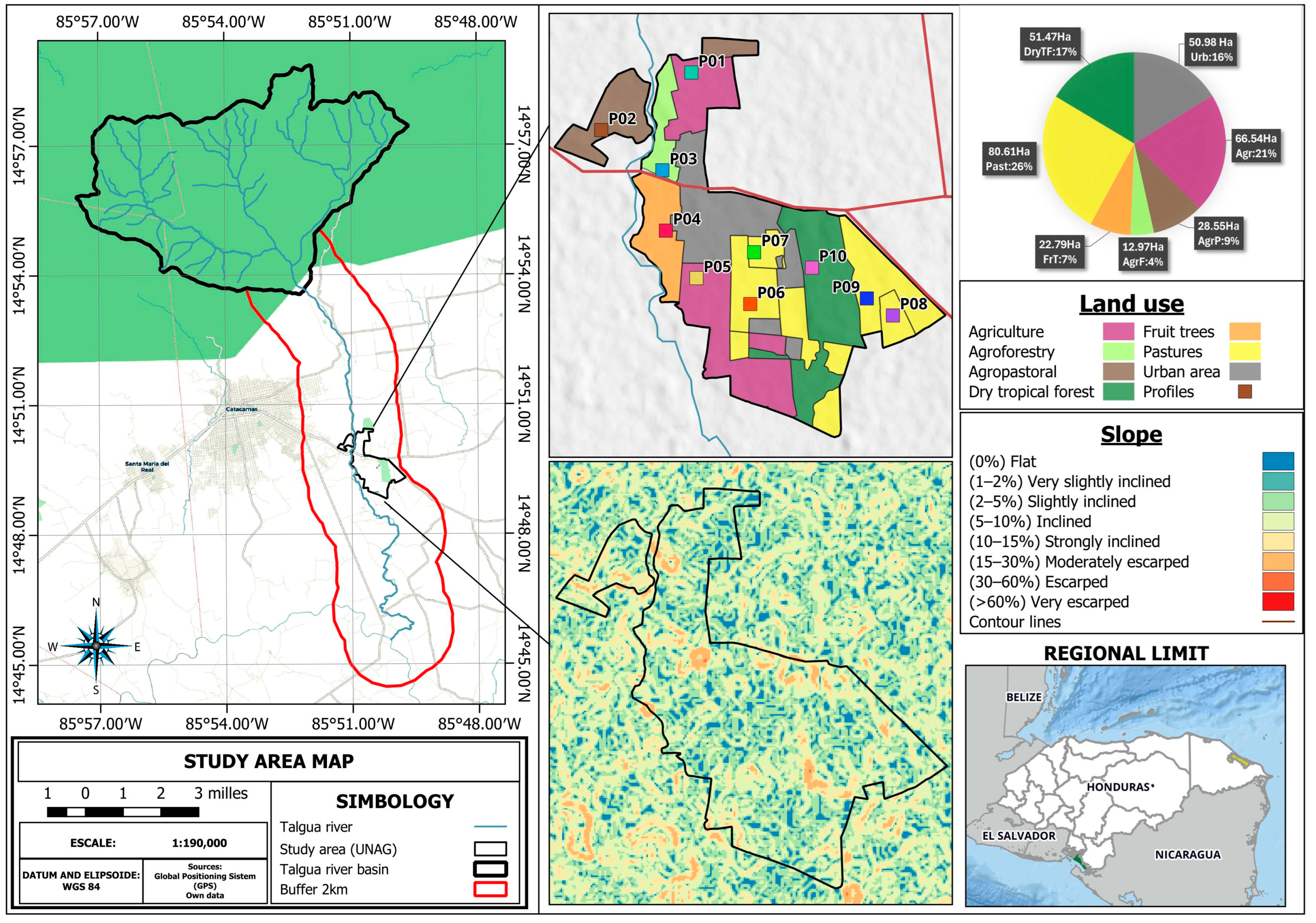
2.1. Characteristics of the Study Area
2.2. Influence of the Talgua River Basin on the Study Area
2.3. Description and Soil Profile Sampling
2.4. Soil Sample Preparation and Laboratory Analysis
3. Results and Discussion
3.1. Site Characterization and Description of Soil Profiles
3.2. Physical Properties of the Horizons
3.3. Physicochemical Properties of the Profiles
3.4. Soil Classification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LULC | Land Use/Land Cover |
| ST | Soil Taxonomy |
| WRB | World Reference Base for Soil Resources |
| RSGs | Reference Soil Groups |
| SDGs | Sustainable Development Goals |
| cv. | Cultivate |
| AU | Animal Unit |
References
- Govoni, C.; D’Odorico, P.; Pinotti, L.; Rulli, M.C. Preserving global land and water resources through the replacement of livestock feed crops with agricultural by-products. Nat. Food 2023, 4, 1047–1057. [Google Scholar] [CrossRef]
- Sugihara, S.; Shibata, M.; Mvondo Ze, A.D.; Araki, S.; Funakawa, S. Effect of vegetation on soil C, N, P and other minerals in Oxisols at the forest-savanna transition zone of central Africa. Soil Sci. Plant Nutr. 2014, 60, 45–59. [Google Scholar] [CrossRef]
- Hoogsteen, M.J.J.; Lantinga, E.A.; Bakker, E.J.; Groot, J.C.J.; Tittonell, P.A. Estimating soil organic carbon through loss on ignition: Effects of ignition conditions and structural water loss. Eur. J. Soil Sci. 2015, 66, 320–328. [Google Scholar] [CrossRef]
- Tellen, V.S.; Yerima, B.P.K. Effects of land use change on soil physicochemical properties in selected areas in the North West region of Cameroon. Environ. Syst. Res. 2018, 7, 1–29. [Google Scholar] [CrossRef]
- Fujii, K.; Shibata, M.; Kitajima, K.; Ichie, T.; Kitayama, K.; Turner, B.L. Plant-soil interactions maintain biodiversity and functions of tropical forest ecosystems. Ecol. Res. 2018, 33, 149–160. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.; Qiu, Y.; Chen, L. Soil nutrients in relation to land use and landscape position in the semi-arid small catchment on the loess plateau in China. J. Arid. Environ. 2001, 48, 537–550. [Google Scholar] [CrossRef]
- Li, Y.; Duan, X.; Li, Y.; Li, Y.; Zhang, L. Interactive effects of land use and soil erosion on soil organic carbon in the dry-hot valley region of southern China. Catena 2021, 201, 105187. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Zamanian, K. Reviews and syntheses: Agropedogenesis—Humankind as the sixth soil-forming factor and attractors of agricultural soil degradation. Biogeosciences 2019, 16, 4783–4803. [Google Scholar] [CrossRef]
- Jia, S.; Yuan, D.; Li, W.; He, W.; Raza, S.; Kuzyakov, Y.; Zamanian, K.; Zhao, X. Soil Chemical Properties Depending on Fertilization and Management in China: A Meta-Analysis. Agronomy 2022, 12, 2501. [Google Scholar] [CrossRef]
- Yang, Z.; Hu, Y.; Zhang, S.; Raza, S.; Wei, X.; Zhao, X. The Thresholds and Management of Irrigation and Fertilization Earning Yields and Water Use Efficiency in Maize, Wheat, and Rice in China: A Meta-Analysis (1990–2020). Agronomy 2022, 12, 709. [Google Scholar] [CrossRef]
- Zhang, H.; Ouyang, Z.; Jiang, P.; Li, M.; Zhao, X. Spatial distribution patterns and influencing factors of soil carbon, phosphorus, and C:P ratio on farmlands in southeastern China. Catena 2022, 216, 106409. [Google Scholar] [CrossRef]
- Isola, J.O.; Asunlegan, O.A.; Abiodun, F.O.; Smart, M.O. Effect of soil depth and topography on physical and chemical properties of soil along Federal College of Forestry, Ibadan North West, Oyo State. J. Res. For. Wildl. Environ. 2020, 12, 382–390. Available online: https://www.ajol.info/index.php/jrfwe/article/view/198640 (accessed on 10 February 2025).
- Shpedt, A.A.; Trubnikov, Y.N.; Zharinova, N.Y. Agrogenic degradation of soils in Krasnoyarsk forest-steppe. Eurasian. Soil Sci. 2017, 50, 1209–1216. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Guidelines for Soil Description, 4th ed.; FAO: Rome, Italy, 2006; Available online: https://www.fao.org/4/a0541e/a0541e.pdf (accessed on 5 September 2024).
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; U.S. Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2022. Available online: https://nrcspad.sc.egov.usda.gov/DistributionCenter/pdf.aspx?productID=1709 (accessed on 5 February 2025).
- WRB (IUSS Working Group). World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; Available online: https://wrb.isric.org/files/WRB_fourth_edition_2022-12-18.pdf (accessed on 5 February 2025).
- Dazzi, C.; Monteleone, S. Anthropogenic processes in the evolution of a soil chronosequence on marly-limestone substrata in an Italian Mediterranean environment. Geoderma 2007, 141, 201–209. [Google Scholar] [CrossRef]
- Rossiter, D.G. Classification of Urban and Industrial Soils in the World Reference Base for Soil Resources (5pp). J. Soils Sediments 2007, 7, 96–100. [Google Scholar] [CrossRef]
- Sarmast, M.; Farpoor, M.H.; Borujeni, I.E. Comparing Soil Taxonomy (2014) and updated WRB (2015) for describing calcareous and gypsiferous soils, Central Iran. Catena 2016, 145, 83–91. [Google Scholar] [CrossRef]
- Esfandiarpour–Borujeni, I.; Mosleh, Z.; Farpoor, M.H. Comparing the ability of Soil Taxonomy (2014) and WRB (2015) to distinguish lithologic discontinuity and an abrupt textural change in major soils of Iran. Catena 2018, 165, 63–71. [Google Scholar] [CrossRef]
- Salehi, M.H. Challenges of Soil Taxonomy and WRB in classifying soils: Some examples from Iranian soils. Phys. Geogr. Ser. 2018, 14, 63–70. [Google Scholar] [CrossRef]
- Lee, D.-B.; Kim, Y.-N.; Sonn, Y.-K.; Kim, K.-H. Comparison of Soil Taxonomy (2022) and WRB (2022) Systems for Classifying Paddy Soils with Different Drainage Grades in South Korea. Land 2023, 12, 1204. [Google Scholar] [CrossRef]
- Meng, Q.; Li, S.; Liu, B.; Hu, J.; Liu, J.; Chen, Y.; Ci, E. Appraisal of Soil Taxonomy and the World Reference Base for Soil Resources Applied to Classify Purple Soils from the Eastern Sichuan Basin, China. Agronomy 2023, 13, 1837. [Google Scholar] [CrossRef]
- ICF (Instituto de Conservación Forestal). Mapa Cobertura Forestal. 2018. Available online: https://geoportal.icf.gob.hn/geoportal/main (accessed on 17 February 2025).
- NASA (National Aeronautics and Space Administration). 2025. Available online: https://power.larc.nasa.gov/data-access-viewer/ (accessed on 1 February 2025).
- Mapa Geológico de Honduras. 2025. Available online: https://www.arcgis.com/home/webmap/viewer.html?webmap=47a212626db54b68bdb356d8e3a3477c (accessed on 2 February 2025).
- FAO (Food and Agriculture Organization of the United Nations). Guía para la Descripción de Suelos, 4th ed.; FAO: Roma, Italy, 2009; ISBN 978-92-5-305521-0. Available online: https://openknowledge.fao.org/handle/20.500.14283/a0541s (accessed on 5 September 2024).
- Vera-Macías, L.R.; Hernández-Jiménez, A.; Mesías-Gallo, F.W.; Cedeño-Sacón, Á.F.; Guzmán-Cedeño, Á.M.; Ormaza-Cedeño, K.P.; López-Alava, G.A. Principales suelos y particularidades de su formación del sistema Carrizal Chone, Manabí, Ecuador. Cultiv. Trop. 2019, 40, 1–35. Available online: http://scielo.sld.cu/pdf/ctr/v40n2/1819-4087-ctr-40-02-e06.pdf (accessed on 5 January 2025).
- Soil Survey Staff. Field Book for Describing and Sampling Soils; Version 4.0; USDA, Natural Resources Conservation Service: Washington, DC, USA; U.S. Government Printing Office: Washington, DC, USA, 2024. Available online: https://www.nrcs.usda.gov/sites/default/files/2022-09/field-book.pdf (accessed on 5 September 2024).
- ISO 11272–2017; Soil Quality—Determination of Dry Bulk Density, 2nd ed. International Organization for Standardization: Geneva, Switzerland, 2017.
- Bouyoucos, G.J. Hydrometer method improvement for making particle size analysis of soils. Agron. J. 1962, 54, 179–181. [Google Scholar] [CrossRef]
- Soil Survey Staff. Kellogg Soil Survey Laboratory Methods Manual; Soil Survey Investigations Report No. 42, Version 6.0; U.S. Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2022. Available online: https://www.nrcs.usda.gov/sites/default/files/2023-01/SSIR42.pdf (accessed on 2 February 2025).
- T 194-97; Standard Method of Test for Determination of Organic Matter in Soils by Wet Combustion. AASHTO (American Association of State Highway and Transportation Officials): Washington, DC, USA, 2018.
- Pansu, M.; Gautheyrou, J. Exchangeable Acidity. In Handbook of Soil Analysis; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Sharma, R.C.; Singh, R.; Singh, Y.; Singh, G. Sodic Soils of Shivari Experimental Farm Site Characteristics, Reclaim Ability and Use Potential for Different Land Uses; Central Soil Salinity Research Institute: Karnal, India, 2006; Available online: http://krishikosh.egranth.ac.in/handle/1/2046476 (accessed on 10 February 2025).
- Wakwoya, M.B.; Woldeyohannis, W.H.; Yimamu, F.K. Characterization and classification of soils of upper Hoha sub-watershed in Assosa District, Western Ethiopia. Heliyon 2023, 9, e14866. [Google Scholar] [CrossRef]
- Yitbarek, T.; Beyene, S.; Kibret, K. Characterization and Classification of Soils of Abobo Area, Western Ethiopia. Appl. Env. Soil Sci. 2016, 2016, 1–16. [Google Scholar] [CrossRef]
- Dinssa, B.; Elias, E. Characterization and classification of soils of Bako Tibe District, West Shewa, Ethiopia. Heliyon 2021, 7, e08279. [Google Scholar] [CrossRef]
- Mulugeta, D.; Sheleme, B. Characterization and classification of soils along the toposequence of Kindo Koye watershed in southern Ethiopia. E. Afr. J. Sci. 2010, 4, 65–77. [Google Scholar] [CrossRef]
- Yitbarek, T.; Gebrekidan, H.; Kibret, K.; Beyene, S. Impacts of Land Use on Selected Physicochemical Properties of Soils of Abobo Area, Western Ethiopia. Agric. For. Fish. 2013, 2, 177–183. [Google Scholar] [CrossRef]
- Yacob, A.; Nigussie, A. Characterization and Classification of Soils Along the Toposequence of Medo Sub-watershed at Wondo Genet District, Ethiopia. Int. J. Nat. Res. Eco. Man. 2022, 7, 73–85. [Google Scholar]
- Hargreaves, P.R.; Baker, K.L.; Graceson, A.; Bonnett, S.; Ball, B.C.; Cloy, J.M. Soil compaction effects on grassland silage yields and soil structure under different levels of compaction over three years. Eur. J. Agr. 2019, 109, 125916. [Google Scholar] [CrossRef]
- Batey, T. Soil compaction and soil management—A review. Soil Use Manag. 2009, 25, 335–345. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. A simple soil organic level metric beyond the organic carbon-to-clay ratio. Soil Use Manag. 2023, 39, 1057–1067. [Google Scholar] [CrossRef]
- Saravia–Maldonado, S.A.; Fernández–Pozo, L.F.; Ramírez–Rosario, B.; Rodríguez–González, M.Á. Analysis of Deforestation and Water Quality in the Talgua River Watershed (Honduras): Ecosystem Approach Based on the DPSIR Model. Sustainability 2024, 16, 5034. [Google Scholar] [CrossRef]
- Fragoso–Servón, P.; Bautista, F.; Pereira, A.; Frausto, O. Distribución de Suelos en ambientes tectokársticos en la porción este de la Península de Yucatán, México. GEOS 2016, 36, 265–273. Available online: https://geos.cicese.mx/index.php/geos/article/view/10 (accessed on 8 February 2025).
- Saravia–Maldonado, S.A.; Rodríguez–González, M.Á.; Ramírez–Rosario, B.; Fernández–Pozo, L.F. Change in Land Use Affects Soil Organic Carbon Dynamics and Distribution in Tropical Systems. Soil Syst. 2024, 8, 101. [Google Scholar] [CrossRef]
- Fink, J.R.; Inda, A.V.; Bayer, C.; Torrent, J.; Barrón, V. Mineralogy and phosphorus adsorption in soils of south and central-west Brazil under conventional and no-tillage systems. Acta Scientiarum. Agron. 2014, 36, 379–387. [Google Scholar] [CrossRef]
- Hao, S.; Wu, K.; Li, L.; Li, X.; Wei, H.; Wu, X.; Liu, B. Revised Proposed lassifications for Typical Anthropogenic Soils in China. Land 2023, 12, 1974. [Google Scholar] [CrossRef]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil science. Soil and human security in the 21st century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef] [PubMed]
- Schwilch, G.; Bernet, L.; Fleskens, L.; Giannakis, E.; Leventon, J.; Maranon, T.; Mills, J.; Short, C.; Stolte, J.; Delden, H.; et al. Operationalizing ecosystem services for the mitigation of soil threats: A proposed framework. Ecol. Indic. 2016, 67, 586–597. [Google Scholar] [CrossRef]
- McBratney, A.B.; Morgan, C.L.S.; Jarrett, L.E. The Value of Soil’s Contributions to Ecosystem Services. In Global Soil Security; Springer: Cham, Switzerland, 2017; pp. 227–235. [Google Scholar] [CrossRef]
| Profile | Coordinates | Elevation | Slope | Geoforms 1st; 2nd Level | Slope Position | Erosion/ Deposition | Drainage Class | Land Use/Land Cover |
|---|---|---|---|---|---|---|---|---|
| P01 | 14°28′35.89″ N 85°50′53.10″ W | 367 m | 5% | Lands at level; plain | Upper part | No erosion | Well-drained | Agriculture |
| After the primary forest was cleared, Zea mays and Oryza sativa were established between 1982 and 1984, and gravity irrigation and flooding were implemented starting in 1985. From 2007 to the present, the system has been modernized with mechanization and fertigation for horticultural production in rotation with Z. mays and Phaseolus vulgaris. | ||||||||
| P02 | 14°50′3.76″ N 85°51′8.82″ W | 375 m | 2% | Lands at level; plain | Middle part | No erosion | Well-drained | Agropastoral |
| After the primary forest was cleared, Z. mays was cultivated between 1982 and 1984. From 1993 to the present, the area has been managed as an integrated crop and livestock system (cattle, sheep, and goats), with practices that include soil preparation, fertilization, and weed control. | ||||||||
| P03 | 14°49′53.95″ N 85°50′53.65″ W | 369 m | 3% | Lands at level; plain | Middle part | Water deposition | Well-drained | Agroforestry |
| After the primary forest was cleared, it was used for horticultural production between 1984 and 1988. Subsequently, it was implemented as an agroforestry system, which includes Cordia alliodora, Tabebuia ochracea, Swietenia macrophylla, Cedrela odorata, Acacia mangium, and Salix alba, with native grass cover. | ||||||||
| P04 | 14°49′39.30″ N 85°50′52.85″ W | 363 m | 7% | Lands at level; plain | Middle part | Water deposition | Poorly drained | Tree crops (fruit trees) |
| After the deforestation of the primary forest, from 1983 to the present day, it has been managed as a fruit production system: Mangifera indica, Psidium guajava, Citrus x sinensis, Persea americana, Anacardium occidentale, Averrhoa carambola, Passiflora edulis, Carica papaya, Cocos nucifera, and Z. mays, with native grass cover. | ||||||||
| P05 | 14°49′28.21″ N 85°50′47.23″ W | 355 m | 6% | Lands at level; plain | Upper part | Laminar erosion, light | Poorly drained | Agriculture under flooding |
| After the deforestation of the primary forest, Z. mays and O. sativa were established between 1982 and 1985. From 1986 to 2002, gravity irrigation and flooding were used. From 2003 to the present, grains and cereals are produced: O. sativa under flooding and Sorghum bicolor, Z. mays, and P. vulgaris on ridges under flooding. | ||||||||
| P06 | 14°49′22.91″ N 85°50′36.52″ W | 360 m | 6% | Lands at level; plain | Middle part | No erosion | Well-drained | Pasture for forage |
| After the primary forest was cleared, it was used as natural pasture between 1984 and 1996.. In 1996, it was converted into a managed grazing system established with hybrid cultivars of Brachiaria (Mulato II and CIAT BR02/1794) and Panicum maximum cv. Mombasa. In addition, between 6 and 7 harvests per year are carried out using agricultural machinery, rest periods of 45 to 50 days are implemented, and occasional application of NPK fertilizers. | ||||||||
| P07 | 14°49′33.95″ N 85°50′30.90″ W | 356 m | 5% | Lands at level; plain | Middle part | Laminar erosion, light | Well-drained | Grazing pasture |
| After the primary forest was cleared, it was used as natural pasture between 1984 and 1988. In 1992, improved pastures were installed and have been maintained to date: Brachiaria brizantha cv. (Marandú, MG-4, Xaraes MG-5, and decumbens). In addition, the area has been managed as a rotational grazing system with a stocking rate of approximately 1.5–2 AU ha−1 year−1, with only weed control practices implemented. | ||||||||
| P08 | 14°49′18.45″ N 85°49′56.43″ W | 360 m | 2% | Lands at level; plain | Middle part | No erosion | Well-drained | Pasture for forage |
| After the primary forest was cleared, it was used as natural pasture between 1987 and 1988. In 1989, pastures for cutting were established and have been maintained to this day: Brachiaria brizantha cv. (MG-4). In addition, between 6 and 7 harvests per year are carried out using agricultural machinery, rest periods of 45 to 50 days are implemented, and no fertilization practices are applied. | ||||||||
| P09 | 14°49′22.61″ N 85°50′2.90″ W | 373 m | 8% | Lands at level; plain | Middle part | Laminar erosion, light | Well-drained | Grazing pasture |
| After the primary forest was cleared, it was used as natural pasture between 1984 and 1986. In 1988, it was established as improved pasture and has been maintained to this day: Brachiaria brizantha cv. (decumbens). In addition, the area has been managed as a rotational grazing system with a stocking rate of approximately 2–2.5 AU ha−1 year−1, with only weed control practices implemented. | ||||||||
| P10 | 14°49′30.14″ N 85°50′16.51″ W | 372 m | 5% | Lands at level; plain | Middle part | No erosion | Well-drained | Dry Tropical Forest |
| Primary forest without human disturbance, preserving its natural state and evolution, also used as a reference area in this study. | ||||||||
| Profile | Horizon (ST–WRB) | Horizon Depth (cm) | Horizon Boundary a | Soil Color | Structure b Grade; Type; Size | Consistency c | |||
|---|---|---|---|---|---|---|---|---|---|
| Dry | Moist | Dry | Moist | Wet | |||||
| P01 | Ap | 0–35 | D, S | 10YR 5/2 | 10YR 3/2 | MO; AS, GR; ME, FI | SHA | FR | SSS, SPP |
| AE | 35–50 | D, S | 10YR 4/2 | 10YR 2/2 | MO; AS; CO, ME | HA | FI | SSS, SPP | |
| Bt1 | 50–75 | D, S | 10YR 5/3 | 10YR 4/3 | MO; AB, GR; CO, ME | SO | FR | SST, SPL | |
| Bt2 | 75–100 | D, S | 10YR 5/3 | 10YR 4/2 | MO; AB, GR; CO, ME, | SHA | FR | SST, SPL | |
| Bt3 | 100–155 | D, S | 10YR 6/3 | 10YR 5/3 | MO; AB; CO, ME | SHA | FR | SST, SPL | |
| P02 | Ap | 0–50, 0–30 | D, W | 10YR 3/1 | 10YR 2/1 | MO; AS; CO, ME, FI | VHA | FI | SST, SPL |
| Eg | 50–70, 30–75 | D, W | 10YR 4/1 | 10YR 3/1 | MO; AB; CO, ME | EHA | EFI | SSS, SPP | |
| Btg–Btl | 70–135, 75–135 | D, W | 10YR 5/1 | 10YR 4/1 | MO; AB; CO, ME | EHA | EFI | SSS, SPP | |
| P03 | Ap | 0–18 | D, S | 10YR 6/2 | 10YR 5/2 | WE; AS, GR; ME, FI, VF | SO | VFR | NST, NPL |
| 2E1 | 25–70 | D, S | Whitish sands and silts | MA | LO | LO | NST, NPL | ||
| 2E2 | 70–100 | D, S | Whitish sands and silts | MA | LO | LO | NST, NPL | ||
| 3Ab1 | 100–127 | D, S | Reddish sands | MA | LO | LO | NST, NPL | ||
| 3Ab2 | 127–145 | D, S | 10YR 7/3 | 10YR 6/3 | WE; AS; ME, FI, VF | SO | VFR | NST, NPL | |
| P04 | Ap | 0–17 | D, S | 10YR 6/2 | 10YR 5/2 | MO; GR; ME, FI | SO | VFR | NST, NPL |
| 2Cg1–2Cg | 17–30 | D, S | 10YR 6/2 | 10YR 5/1 | WE; SG; ME, FI | SHA | FR | NST, NPL | |
| 2Cg2–2Cl1 | 30–47 | D, S | 5Y 6/2 | 5Y 5/2 | MA | SO | VFR | NST, NPL | |
| 2Cg3–2Cl2 | 47–90+ | D, S | 5Y 7/1 | 5Y 7/2 | MA | SO | VFR | NST, NPL | |
| P05 | Ap | 0–28 | D, S | 10YR 3/2 | 10YR 2/2 | ST; AS; CO, ME, FI | EHA | EFI | SSS, SPP |
| Btg | 28–60 | D, S | 5Y 7/1 | 5Y 5/1 | ST; AS; VC, CO | EHA | EFI | VST, VPL | |
| Bssg–Bil | 60–95 | D, S | 5Y 5/2 | 5Y 4/3 | ST; AS, WEG; EC, VC | EHA | EFI | VST, VPL | |
| Bkssg–Bkil | 95–155 | D, S | 5Y 5/1 | 5Y 4/1 | ST; AS, WEG; EC, VC | EHA | EFI | VST, VPL | |
| P06 | Ap | 0–28 | D, S | 10YR 3/2 | 10YR 2/2 | ST; GR, AS; ME, FI | HA | FI | SST, SPL |
| Bt1 | 28–55, 28–65 | D, W | 10YR 5/2 | 10YR 4/2 | MO; AS; CO, ME | HA | FI | SST, SPL | |
| Bt2 | 55–85, 65–85 | D, W | 10YR 6/3 | 10YR 5/3 | MO; AS; CO, ME | HA | FI | SST, SPL | |
| Bt3 | 85–107 | D, S | 10YR 6/4 | 10YR 5/4 | MO; AS; CO, ME | HA | FI | SST, SPL | |
| BCk | 107–150 | D, S | 10YR 6/4 | 10YR 5/4 | MO; AS; CO, ME | SHA | FR | SST, SPL | |
| P07 | Ap | 0–23 | D, S | 10YR 5/2 | 10YR 4/2 | MO; GR, AS; ME, FI | HA | FI | SST, SPL |
| Bt1 | 23–50 | D, S | 10YR 5/3 | 10YR 4/3 | MO; AS; CO, ME | HA | VFI | SST, SPL | |
| Bt2 | 50–75 | D, S | 10YR 6/3 | 10YR 5/3 | MO; AS; CO, ME | HA | VFI | SST, SPL | |
| Bt3 | 75–116 | D, S | 10YR 6/4 | 10YR 5/4 | MO; AS; CO, ME | HA | VFI | SST, SPL | |
| BCk | 116–140 | D, S | 10YR 7/4 | 10YR 6/4 | MO; AS; CO, ME | HA | VFI | SST, SPL | |
| P08 | Ap | 0–13, 0–18 | G, S | 10YR 5/2 | 10YR 4/2 | ST; GR; CO, ME | SO | VFR | SST, SPL |
| 2Cr–2C | 13–30, 18–57 | D, I | 10YR 3/2 | 10YR 2/2 | SG. WE; GR; ME, FI | LO | L | NST, NPL | |
| 3BEg | 30–70, 57–70 | D, I | 5Y 4/1 | 5Y 3/1 | MO; AB; CO, ME | SHA | FR | SSS, SPP | |
| 3Byn1 | 70–105 | D, S | 2.5Y 6/4 | 2.5Y 5/4 | ST; AS, WE; EC, VC | EHA | EFI | VST, VPL | |
| 3Byn2 | 105–150+ | D, S | 2.5Y 6/4 | 2.5Y 5/4 | ST; AS, WE; EC, VC | EHA | EFI | VST, VPL | |
| P09 | Ap | 0–10 | G, S | 10YR 5/2 | 10YR 4/2 | ST; GR, AS; ME, FI | SHA | FR | SST, SPL |
| BEg | 14–70 | D, S | 2.5Y 6/4 | 2.5Y 5/4 | MO; AS; CO, ME | EHA | EFI | VST, VPL | |
| Cr/Bsv–C/Bsv | 70–130 | D, S | 2.5Y 7/4 | 2.5Y 6/4 | MO; AS; CO, ME | HA | FI | SST, SPL | |
| P10 | A | 0–22 | D, S | 7.5YR 4/2 | 7.5YR 3/2 | ST; GR, AS; ME, FI | SHA | VFR | SST, SPL |
| E | 22–54 | D, S | 7.5YR 5/4 | 7.5YR 4/3 | MO; AS; CO, ME | HA | FR | SST, SPL | |
| Bt1 | 54–97 | D, S | 10YR 5/3 | 10YR 4/3 | MO; AS; CO, ME, | HA | FR | SST, SPL | |
| Bt2 | 97–117 | D, S | 10YR 5/3 | 10YR 4/3 | MO; AS; CO, ME | HA | FR | SST, SPL | |
| Btk | 117–147+ | D, S | 10YR 6/3 | 10YR 5/3 | MO; AS; CO, ME, | HA | FI | SSS, SPP | |
| Profile | Horizon (ST–WRB) | Depth (cm) | Sand (%) | Silt (%) | Clay (%) | Silt/ Clay | Rf (%) | Texture a | Bd (Mg m−3) |
|---|---|---|---|---|---|---|---|---|---|
| Ap | 0–35 | 38.4 | 44.0 | 17.6 | 2.5 | - | L | 1.24 | |
| AE | 35–50 | 79.4 | 16.0 | 4.6 | 3.5 | - | SL | 1.38 | |
| P01 | Bt1 | 50–75 | 26.0 | 47.8 | 26.2 | 1.8 | - | L | 1.41 |
| Bt2 | 75–100 | 32.4 | 43.0 | 24.6 | 1.8 | - | L | 1.68 | |
| Bt3 | 100–155 | 25.8 | 48.0 | 26.2 | 1.8 | - | L | 1.71 | |
| Ap | 0–50, 0–30 | 46.0 | 28.2 | 25.8 | 1.1 | - | L | 1.37 | |
| P02 | Eg | 50–70, 30–75 | 69.6 | 19.6 | 10.8 | 1.8 | 1.5 | SL | 1.57 |
| Btg–Btl | 70–135, 75–135 | 34.4 | 33.0 | 32.6 | 1.0 | 3.7 | CL | 1.73 | |
| Ap | 0–18 | 20.0 | 61.6 | 18.4 | 3.4 | - | SIL | 1.07 | |
| 2E1 | 25–70 | 67.4 | 28.0 | 4.6 | 6.1 | - | SL | 1.10 | |
| P03 | 2E2 | 70–100 | 51.4 | 40.6 | 8.0 | 5.1 | - | SL | 1.00 |
| 3Ab1 | 100–127 | 52.8 | 35.0 | 12.2 | 2.9 | - | SL | 1.09 | |
| 3Ab2 | 127–145 | 37.8 | 47.2 | 15.0 | 3.2 | - | L | 1.15 | |
| Ap | 0–17 | 52.0 | 33.8 | 14.2 | 2.4 | - | SL | 1.24 | |
| 2Cg1–2Cg | 17–30 | 35.2 | 50.4 | 14.4 | 3.5 | - | SIL | 1.32 | |
| P04 | 2Cg2–2Cl1 | 30–47 | 32.4 | 50.0 | 17.6 | 2.8 | - | SIL | 1.32 |
| 2Cg3–2Cl2 | 47–90+ | 33.6 | 50.2 | 16.2 | 3.1 | - | SIL | 0.97 | |
| Ap | 0–28 | 48.4 | 34.2 | 17.4 | 2.0 | 2.0 | L | 1.44 | |
| P05 | Btg | 28–60 | 18.0 | 43.4 | 38.6 | 1.1 | 2.0 | SICL | 1.62 |
| Bssg–Bil | 60–95 | 24.4 | 37.6 | 38.0 | 1.0 | 3.0 | CL | 1.65 | |
| Bkssg–Bkil | 95–155 | 30.4 | 34.4 | 35.2 | 1.0 | 2.5 | CL | 1.71 | |
| Ap | 0–28 | 62.4 | 30.8 | 6.8 | 4.5 | - | SL | 1.19 | |
| Bt1 | 28–55, 28–65 | 34.0 | 43.2 | 22.8 | 1.9 | - | L | 1.50 | |
| P06 | Bt2 | 55–85, 65–85 | 18.8 | 53.6 | 27.6 | 1.9 | - | SICL | 1.41 |
| Bt3 | 85–107 | 37.2 | 43.6 | 29.8 | 1.5 | - | L | 1.69 | |
| BCk | 107–150 | 34.4 | 50.6 | 15.0 | 3.4 | 18.4 | SIL | 1.57 | |
| Ap | 0–23 | 55.0 | 34.2 | 10.8 | 3.2 | - | SL | 1.48 | |
| Bt1 | 23–50 | 45.6 | 34.0 | 20.4 | 1.7 | - | L | 1.59 | |
| P07 | Bt2 | 50–75 | 36.4 | 38.0 | 25.6 | 1.5 | - | L | 1.60 |
| Bt3 | 75–116 | 24.0 | 46.2 | 29.8 | 1.6 | - | CL | 1.52 | |
| BCk | 116–140 | 30.2 | 43.6 | 26.2 | 1.7 | 15.4 | L | 1.60 | |
| Ap | 0–13, 0–18 | 40.0 | 37.8 | 22.2 | 1.7 | 19.2 | L | 1.41 | |
| 2Cr–2C | 13–30, 18–57 | 71.6 | 20.0 | 8.4 | 2.4 | 81.0 | SL | 1.31 | |
| P08 | 3BEg | 30–70, 57–70 | 30.4 | 64.0 | 5.6 | 11.4 | 61.5 | SIL | 1.79 |
| 3Byn1 | 70–105 | 25.2 | 31.6 | 43.2 | 0.7 | 3.0 | C | 1.58 | |
| 3Byn2 | 105–150+ | 26.8 | 35.0 | 38.2 | 0.9 | 2.5 | CL | 1.70 | |
| Ap | 0–10 | 66.4 | 27.4 | 6.2 | 4.4 | 10.8 | SL | 1.43 | |
| P09 | BEg | 14–70 | 39.6 | 55.4 | 5.0 | 11.1 | 54.3 | SIL | 1.73 |
| Cr/Bsv–C/Bsv | 70–130 | 37.0 | 58.7 | 4.3 | 13.7 | 58.4 | SIL | 1.53 | |
| A | 0–22 | 52.8 | 30.6 | 16.6 | 1.8 | - | L | 1.27 | |
| E | 22–54 | 70.0 | 20.2 | 9.8 | 2.1 | - | SL | 1.43 | |
| P10 | Bt1 | 54–97 | 38.4 | 35.6 | 26.0 | 1.4 | - | L | 1.50 |
| Bt2 | 97–117 | 40.8 | 36.0 | 23.2 | 1.6 | - | L | 1.60 | |
| Btk | 117–147+ | 40.8 | 35.0 | 24.2 | 1.5 | 24.9 | L | 1.74 |
| Profile | Horizon (ST–WRB) | Depth (cm) | pH (H2O) | SOC (%) | Av–P (mg kg−1) | Interchangeable Bases (cmolc kg−1) | Al3+ CEC (cmolc kg−1) | BS (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | Mg2+ | K+ | Na+ | |||||||||
| P01 | Ap | 0–35 | 7.20 | 2.12 | 33 | 15.09 | 1.24 | 0.37 | nd | nd | 13.4 | Sat |
| AE | 35–50 | 7.49 | 1.77 | 11 | 15.06 | 1.00 | 0.18 | nd | nd | 12.8 | Sat | |
| Bt1 | 50–75 | 7.75 | 0.62 | 5 | 9.17 | 0.77 | 0.13 | nd | nd | 6.2 | Sat | |
| Bt2 | 75–100 | 7.56 | 0.12 | 8 | 7.97 | 0.86 | 0.12 | nd | nd | 6.6 | Sat | |
| Bt3 | 100–155 | 7.63 | 0.25 | 9 | 7.82 | 0.74 | 0.12 | 0.15 | nd | 5.6 | Sat | |
| P02 | Ap | 0–50, 0–30 | 6.28 | 2.53 | 7 | 19.26 | 2.26 | 0.44 | nd | nd | 15.0 | Sat |
| Eg | 50–70, 30–75 | 6.91 | 0.62 | 1 | 12.94 | 2.11 | 0.26 | nd | nd | 16.2 | 95 | |
| Btg–Btl | 70–135, 75–135 | 7.15 | 0.31 | 2 | 11.58 | 2.17 | 0.18 | nd | nd | 13.4 | Sat | |
| P03 | Ap | 0–18 | 8.12 | 2.09 | 14 | 27.56 | 0.69 | 0.17 | 0.08 | nd | 9.6 | Sat |
| 2E1 | 25–70 | 8.26 | 0.29 | 9 | 12.63 | 0.66 | 0.07 | nd | nd | 4.2 | Sat | |
| 2E2 | 70–100 | 8.23 | 0.45 | 11 | 15.68 | 0.27 | 0.13 | 0.16 | nd | 5.4 | Sat | |
| 3Ab1 | 100–127 | 8.30 | 0.52 | 11 | 17.06 | 0.28 | 0.09 | nd | nd | 6.2 | Sat | |
| 3Ab2 | 127–145 | 8.26 | 0.91 | 13 | 23.31 | 0.30 | 0.11 | nd | nd | 10.0 | Sat | |
| P04 | Ap | 0–17 | 8.07 | 1.87 | 12 | 29.32 | 2.32 | 0.17 | nd | nd | 9.8 | Sat |
| 2Cg1–2Cg | 17–30 | 8.19 | 1.62 | 9 | 27.29 | 1.69 | 0.13 | nd | nd | 8.0 | Sat | |
| 2Cg2–2Cl1 | 30–47 | 8.22 | 0.88 | 5 | 21.49 | 0.42 | 0.07 | nd | nd | 6.2 | Sat | |
| 2Cg3–2Cl2 | 47–90+ | 8.26 | 0.85 | 6 | 26.64 | 0.12 | 0.08 | nd | nd | 4.0 | Sat | |
| P05 | Ap | 0–28 | 7.14 | 1.50 | 18 | 21.76 | 1.58 | 0.37 | 0.07 | nd | 12.6 | Sat |
| Btg | 28–60 | 7.59 | 0.57 | 2 | 15.53 | 1.67 | 0.26 | 0.32 | nd | 15.2 | Sat | |
| Bssg–Bil | 60–95 | 7.68 | 0.30 | 1 | 17.13 | 3.36 | 0.35 | 0.14 | nd | 17.6 | Sat | |
| Bkssg–Bkil | 95–155 | 8.23 | 0.19 | 1 | 31.21 | 3.72 | 0.35 | nd | nd | 16.6 | Sat | |
| P06 | Ap | 0–28 | 6.66 | 2.99 | 13 | 18.21 | 1.54 | 0.16 | nd | nd | 11.8 | Sat |
| Bt1 | 28–55, 28–65 | 7.11 | 1.52 | 2 | 10.01 | 1.78 | 0.16 | nd | nd | 10.0 | Sat | |
| Bt2 | 55–85, 65–85 | 7.23 | 0.42 | 2 | 10.11 | 1.63 | 0.04 | nd | nd | 7.6 | Sat | |
| Bt3 | 85–107 | 7.43 | 0.23 | 2 | 9.06 | 1.55 | 0.06 | nd | nd | 6.6 | Sat | |
| BCk | 107–150 | 8.28 | 0.16 | 2 | 23.37 | 0.44 | 0.03 | nd | nd | 4.0 | Sat | |
| P07 | Ap | 0–23 | 6.63 | 1.62 | 18 | 16.09 | 0.65 | 0.16 | nd | nd | 14.0 | Sat |
| Bt1 | 23–50 | 7.20 | 0.74 | 13 | 13.12 | 1.53 | 0.22 | nd | nd | 11.4 | Sat | |
| Bt2 | 50–75 | 8.12 | 0.46 | 26 | 12.79 | 2.18 | 0.34 | nd | nd | 11.0 | Sat | |
| Bt3 | 75–116 | 8.14 | 0.31 | 19 | 17.12 | 0.71 | 0.21 | nd | nd | 12.2 | Sat | |
| BCk | 116–140 | 8.38 | 0.21 | 10 | 26.63 | 1.08 | 0.19 | nd | nd | 7.4 | Sat | |
| P08 | Ap | 0–13, 0–18 | 5.05 | 3.05 | 6 | 6.09 | 0.82 | 0.13 | nd | 1.50 | 10.6 | 66 |
| 2Cr–2C | 13–30, 18–57 | 5.12 | 1.98 | 4 | 5.20 | 1.06 | 0.07 | nd | 1.10 | 12.0 | 54 | |
| 3BEg | 30–70, 57–70 | 5.24 | 0.62 | 1 | 23.52 | 2.40 | 0.11 | 2.31 | 0.50 | 20.4 | Sat | |
| 3Byn1 | 70–105 | 6.36 | 0.04 | 1 | 31.69 | 3.65 | 0.14 | 6.80 | nd | 21.6 | Sat | |
| 3Byn2 | 105–150+ | 6.83 | 0.10 | 1 | 28.88 | 2.80 | 0.13 | 7.09 | nd | 19.6 | Sat | |
| P09 | Ap | 0–10 | 5.20 | 3.01 | 6 | 4.45 | 1.64 | 0.12 | nd | 0.10 | 11.2 | 56 |
| BEg | 14–70 | 5.04 | 0.45 | 1 | 37.26 | 3.18 | 0.08 | 2.30 | 0.30 | 12.6 | Sat | |
| Cr/Bsv–C/Bsv | 70–130 | 5.18 | 0.21 | 2 | 25.33 | 6.95 | 0.13 | 2.34 | 0.50 | 18.0 | Sat | |
| P10 | A | 0–22 | 6.81 | 3.98 | 8 | 17.24 | 3.82 | 0.19 | nd | nd | 8.0 | Sat |
| E | 22–54 | 6.90 | 0.75 | 3 | 10.78 | 2.34 | 0.15 | nd | nd | 13.2 | Sat | |
| Bt1 | 54–97 | 7.08 | 0.35 | 2 | 10.98 | 4.11 | 0.13 | nd | nd | 14.2 | Sat | |
| Bt2 | 97–117 | 7.19 | 0.35 | 4 | 14.63 | 2.78 | 0.17 | nd | nd | 15.2 | Sat | |
| Btk | 117–147+ | 8.27 | 0.23 | 2 | 30.55 | 1.66 | 0.13 | nd | nd | 15.4 | Sat | |
| Profile | Classification System | |
|---|---|---|
| Soil Taxonomy (2022) | World Reference Base for Soil Resources (2022) | |
| P01 | Typic Argiustolls | Pachic Irragric Anthrosols (Pantoloamic, Abruptic, Differentic) |
| P02 | Vertic Haplustolls | Protovertic Phaeozems (Anoloamic, Endogleyic, Albic, Differentic) |
| P03 | Typic Ustipsamments | Albic Pantofluvic Fluvisols (Loaminovic, Calcaric, Litholinic, Panpaic, Someromollic) |
| P04 | Typic Epiaquents | Calcaric Pantofluvic Gleysols (Pantoloamic, Drainic, Reductigleyic, Someromollic) |
| P05 | Typic Calciusterts | Calcic Hydragric Anthrosols (Endoclayic, Loamic, Abruptic, Anthromollic, Gleyic, Vertic) |
| P06 | Typic Haplustolls | Haplic Phaeozems (Pantoloamic, Cambic, Mollic) |
| P07 | Typic Haplustolls | Haplic Phaeozems (Pantoloamic, Cambic, Mollic) |
| P08 | Vertic Haplustalfs | Protovertic Skeletic Gypsisols (Endoclayin, Loamic, Abruptic, Cutanic, Someromollic) |
| P09 | Typic Plinthustalfs | Skeletic Plinthosols (Pantoloamic, Litholinic, Plinthic, Someromollic) |
| P10 | Typic Calciustolls | Calcaric Phaeozems (Pantoloamic, Albic, Cambic, Mollic) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saravia-Maldonado, S.A.; Ramírez-Rosario, B.; Rodríguez-González, M.Á.; Fernández-Pozo, L.F. Land Use Changes Influence Tropical Soil Diversity: An Assessment Using Soil Taxonomy and the World Reference Base for Soil Classifications. Agriculture 2025, 15, 1893. https://doi.org/10.3390/agriculture15171893
Saravia-Maldonado SA, Ramírez-Rosario B, Rodríguez-González MÁ, Fernández-Pozo LF. Land Use Changes Influence Tropical Soil Diversity: An Assessment Using Soil Taxonomy and the World Reference Base for Soil Classifications. Agriculture. 2025; 15(17):1893. https://doi.org/10.3390/agriculture15171893
Chicago/Turabian StyleSaravia-Maldonado, Selvin Antonio, Beatriz Ramírez-Rosario, María Ángeles Rodríguez-González, and Luis Francisco Fernández-Pozo. 2025. "Land Use Changes Influence Tropical Soil Diversity: An Assessment Using Soil Taxonomy and the World Reference Base for Soil Classifications" Agriculture 15, no. 17: 1893. https://doi.org/10.3390/agriculture15171893
APA StyleSaravia-Maldonado, S. A., Ramírez-Rosario, B., Rodríguez-González, M. Á., & Fernández-Pozo, L. F. (2025). Land Use Changes Influence Tropical Soil Diversity: An Assessment Using Soil Taxonomy and the World Reference Base for Soil Classifications. Agriculture, 15(17), 1893. https://doi.org/10.3390/agriculture15171893






