Effects of Seed Priming with Talaromyces ruber Extracts on Tomato (Solanum lycopersicum) Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal and Plant Material
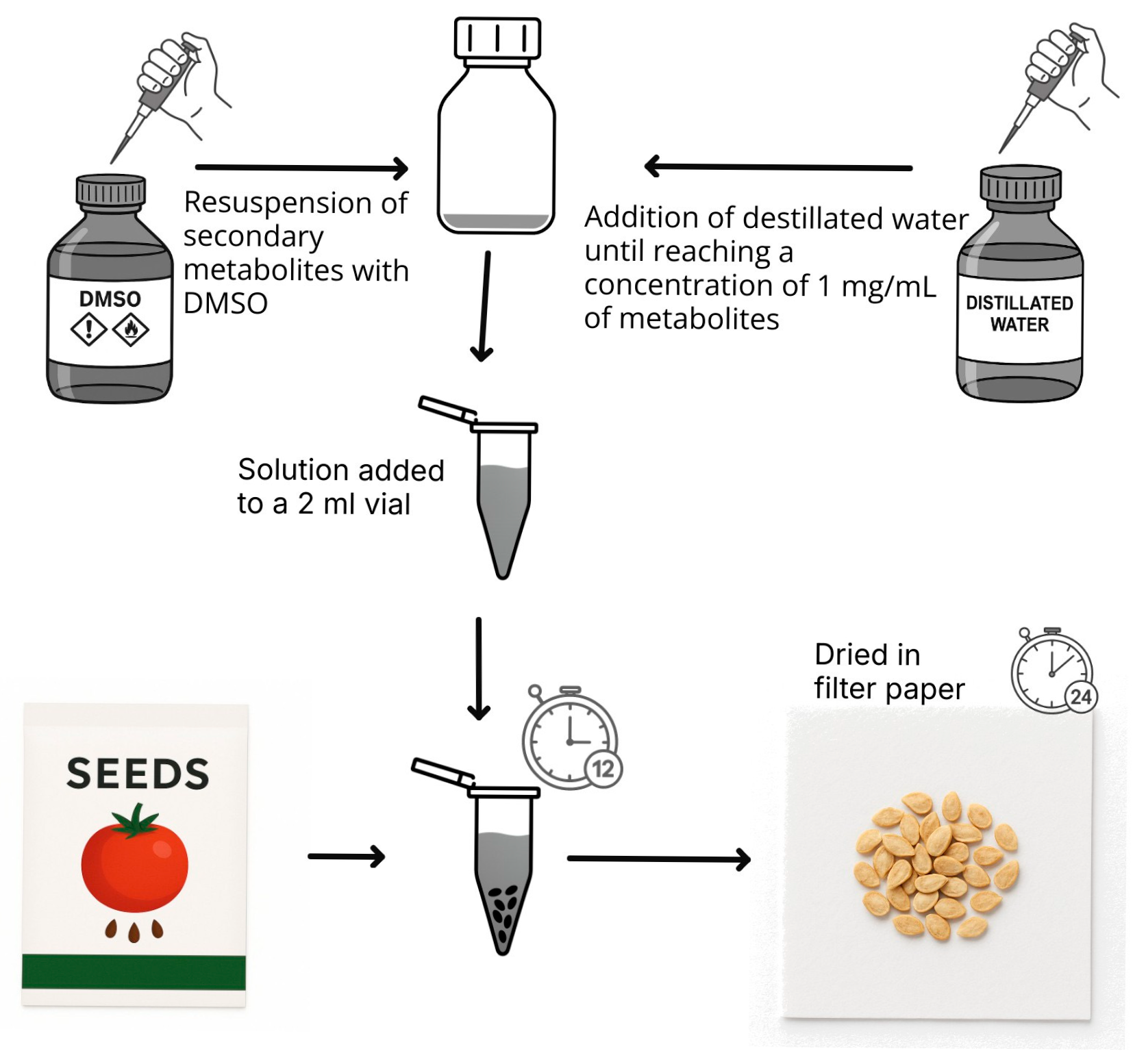
2.2. Obtaining Fungal Extracts from T. ruber
2.3. Seed Treatment
2.4. In Plant Experiments
2.5. Morphometric and Physiological Characteristics Analyzed
2.6. Statistical Analysis
3. Results
3.1. Effect of the Seed Treatments on Germination Rates
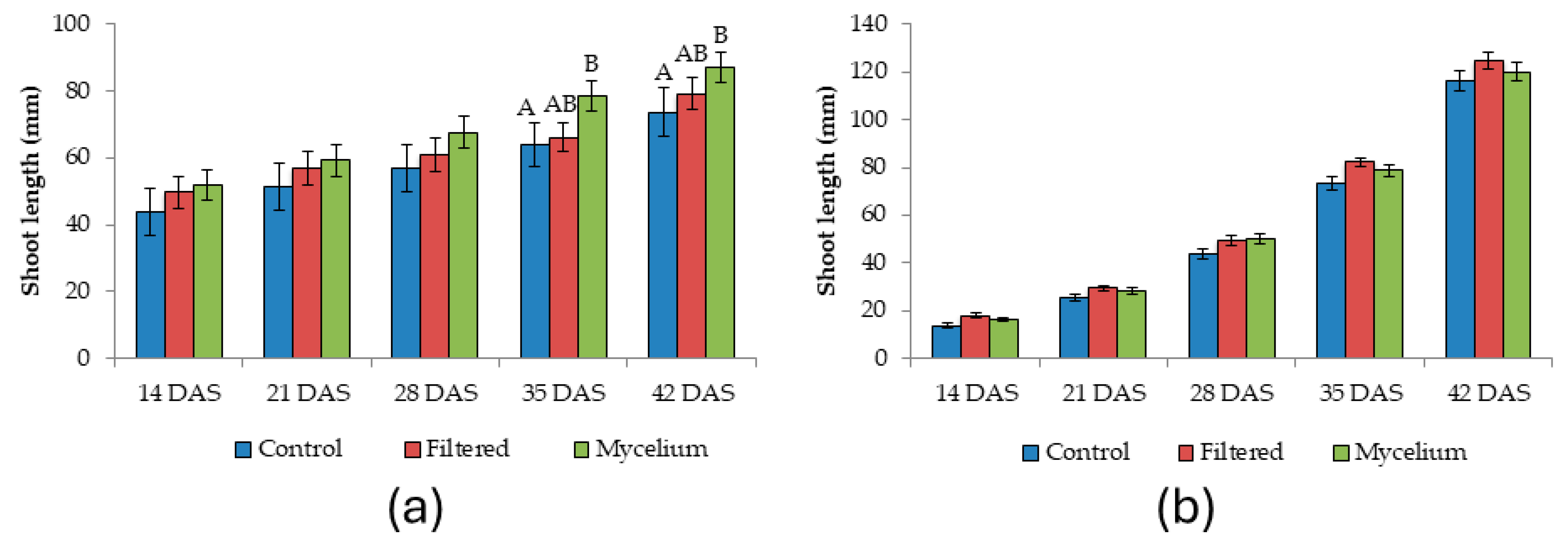
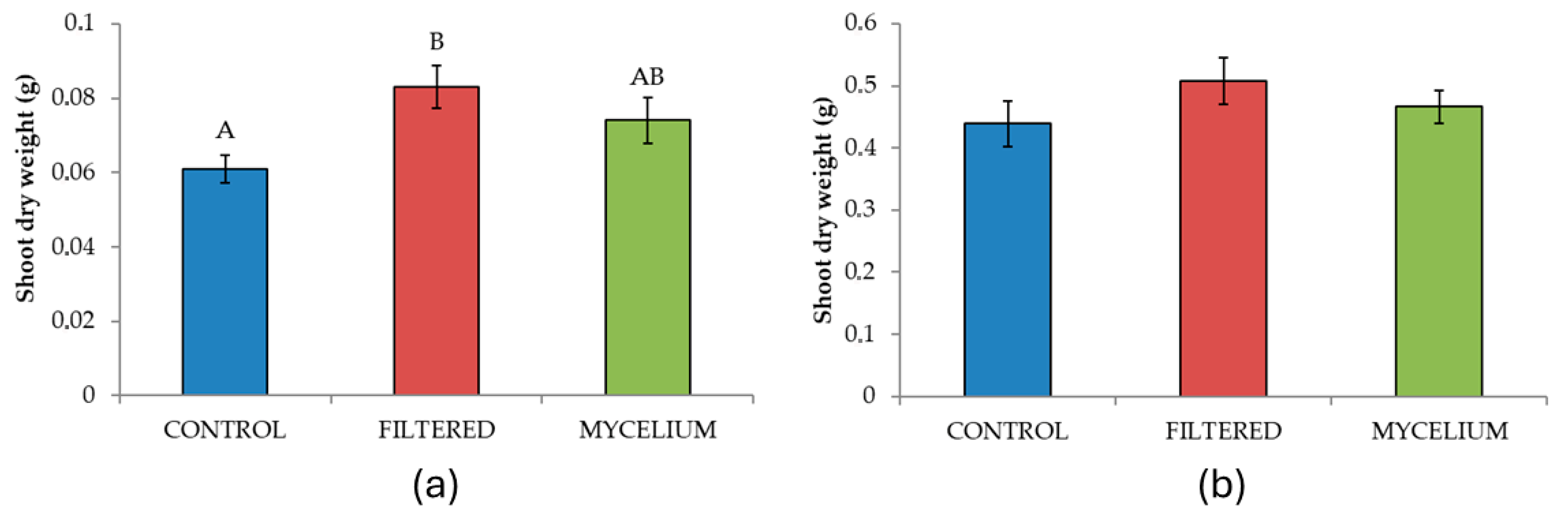
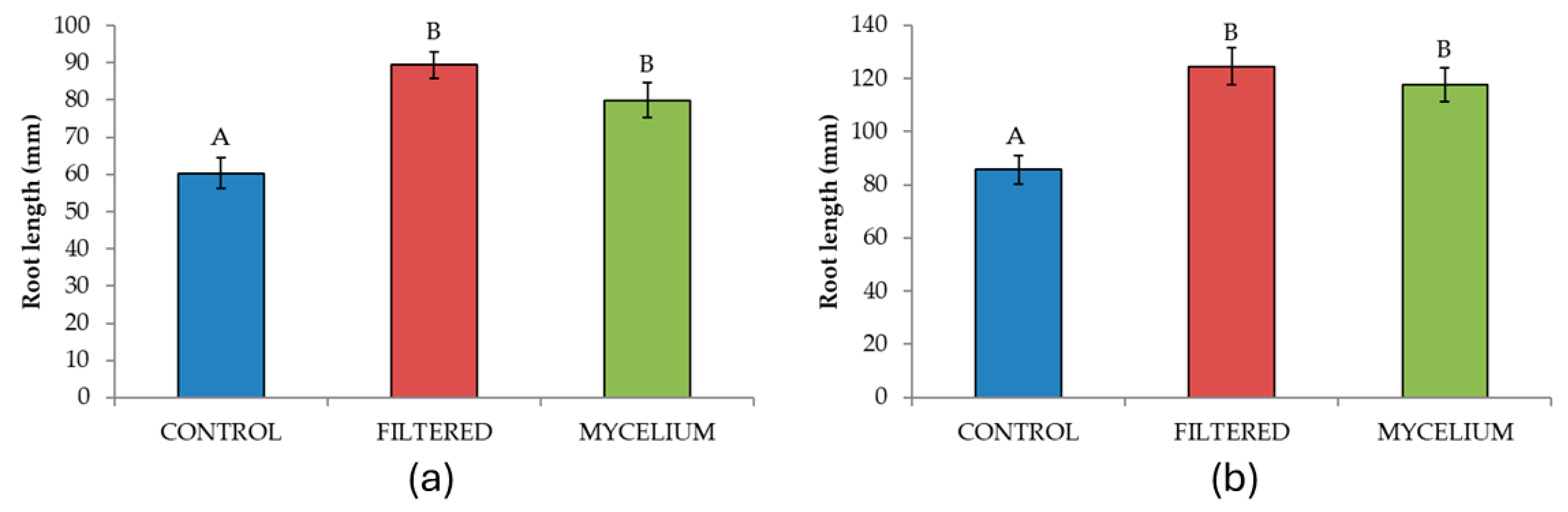
3.2. Effect of the Seed Treatments on the Morphometric and Physiological Characteristics Analyzed
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- European Commission. Farm to Fork Strategy; European Commission: Luxembourg, 2019. [Google Scholar]
- Smirnov, O.; Lahav, G.; Orbell, J.; Zhang, M.; Xiao, T. Climate Change, Drought, and Potential Environmental Migration Flows Under Different Policy Scenarios. Int. Migr. Rev. 2023, 57, 36–67. [Google Scholar] [CrossRef]
- Boros, A.; Szólik, E.; Desalegn, G.; Tőzsér, D. A Systematic Review of Opportunities and Limitations of Innovative Practices in Sustainable Agriculture. Agronomy 2025, 15, 76. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT Database. Available online: https://www.fao.org/statistics/en/ (accessed on 8 June 2025).
- Karačić, V.; Miljaković, D.; Marinković, J.; Ignjatov, M.; Milošević, D.; Tamindžić, G.; Ivanović, M. Bacillus Species: Excellent Biocontrol Agents against Tomato Diseases. Microorganisms 2024, 12, 457. [Google Scholar] [CrossRef]
- Janssen, D.G.C.; Ruiz, L.; de Cara-García, M.; Simón, A.; Martínez, A. Disease resistance in tomato crops produced in Spain. In Proceedings of the V International Symposium on Tomato Diseases: Perspectives and Future Directions in Tomato Protection, Malaga, Spain, 13–16 June 2016; pp. 63–68. [Google Scholar]
- Litskas, V.; Migeon, A.; Navajas, M.; Marie-Stephane, T.; Stavrinides, M. Impacts of climate change on tomato, a notorious pest and its natural enemy: Small scale agriculture at higher risk. Environ. Res. Lett. 2019, 14, 084041. [Google Scholar] [CrossRef]
- El-Banna, M.F.; Mosa, A. Exogenous application of proline mitigates deteriorative effects of salinity stress in NFT closed-loop system: An ultrastructural and physio-biochemical investigation on hydroponically grown tomato (Solanum lycopersicum L.). Sci. Hortic. 2024, 330, 113061. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate Change and Salinity Effects on Crops and Chemical Communication Between Plants and Plant Growth-Promoting Microorganisms Under Stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Cañizares, E.; Giovannini, L.; Gumus, B.O.; Fotopoulos, V.; Balestrini, R.; González-Guzmán, M.; Arbona, V. Seeds of Change: Exploring the transformative effects of seed priming in sustainable agriculture. Physiol. Plant. 2025, 177, e70226. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.; Sarwar, T.; Nawaz, A.; Ijaz, M.; Sattar, A.; Ahmad, S. Methods of Seed Priming. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Hasanuzzaman, M., Fotopoulos, V., Eds.; Springer Singapore: Singapore, 2019; pp. 1–10. [Google Scholar]
- Singh, P.; Vaishnav, A.; Liu, H.; Xiong, C.; Singh, H.B.; Singh, B.K. Seed biopriming for sustainable agriculture and ecosystem restoration. Microb. Biotechnol. 2023, 16, 2212–2222. [Google Scholar] [CrossRef]
- García-Latorre, C.; Rodrigo, S.; Santamaría, O. Evaluation of the Extract of Pseudopithomyces chartarum to be used as Biocontrol Agent Against Phytophthora cinnamomi in Lupinus luteus. J. Soil Sci. Plant Nutr. 2024, 24, 6325–6337. [Google Scholar] [CrossRef]
- Dwisandi, R.F.; Miranti, M.; Widiastuti, A.; Prismantoro, D.; Awal, M.A.; Mispan, M.S.; Joshi, R.C.; Doni, F. Microbial secondary metabolites for modulating plant biotic stress resistance: Bridging the lab-field gap. Plant Stress 2025, 15, 100720. [Google Scholar] [CrossRef]
- Jangir, M.; Sharma, S.; Sharma, S. Non-target Effects of Trichoderma on Plants and Soil Microbial Communities; Springer: Cham, Switzerland, 2019; pp. 239–251. [Google Scholar]
- Köhl, J. Use of beneficial microorganisms in crop production: Do current regulatory frameworks in the EU fit for purpose? BioControl 2025, 70, 433–450. [Google Scholar] [CrossRef]
- Pozo, M.I.; Herrero, B.; Martín-García, J.; Santamaría, Ó.; Poveda, J. Evaluating potential side effects of Trichoderma as biocontrol agent: A two-edges sword? Curr. Opin. Environ. Sci. Health 2024, 41, 100566. [Google Scholar] [CrossRef]
- Naeimi, S.; Hatvani, L.; Marik, T.; Balázs, D.; Dóczi, I.; Cai, F.; Vágvölgyi, C.; Druzhinina, I.S.; Kredics, L. Trichodermosis: Human Infections Caused by Trichoderma Species. In Advances in Trichoderma Biology for Agricultural Applications; Amaresan, N., Sankaranarayanan, A., Dwivedi, M.K., Druzhinina, I.S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 607–634. [Google Scholar]
- European Commission. Commission Delegated Regulation (EU) 2022/1439 of 31 August 2022 Amending Regulation (EU) No 283/2013 as Regards the Information to Be Submitted for Active Substances and the Specific Data Requirements for Microorganism; European Commission: Luxembourg, 2022; pp. 8–37. [Google Scholar]
- European Parliament and of the Council. Regulation (EC) No 1107/2009 of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, L309, 1–50. [Google Scholar]
- European Parliament and of the Council. Regulation (EU) 2019/1009 of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, L170, 1–114. [Google Scholar]
- James, D.; Gleena Mary, C.F.K.; Mathew, S. Evaluation of Culture Filtrates of Endophytic Microorganisms from Tomato against Ralstonia solanacearum. Int. J. Environ. Clim. Change 2022, 12, 1476–1481. [Google Scholar] [CrossRef]
- Sánchez-Gómez, T.; Santamaría, O.; Martín-García, J.; Poveda, J. Fungal Metabolites as Plant Growth Promoters in Crops. In Fungal Metabolites for Agricultural Applications; Poveda, J., Santamaría, Ó., Martín-García, J., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2025; pp. 59–84. [Google Scholar]
- Rodrigo, S.; García-Latorre, C.; Santamaria, O. Metabolites Produced by Fungi against Fungal Phytopathogens: Review, Implementation and Perspectives. Plants 2022, 11, 81. [Google Scholar] [CrossRef]
- da Silva, F.M.R.; Paggi, G.M.; Brust, F.R.; Macedo, A.J.; Silva, D.B. Metabolomic Strategies to Improve Chemical Information from OSMAC Studies of Endophytic Fungi. Metabolites 2023, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- El-Nagar, D.; Salem, S.H.; El-Zamik, F.I.; El-Basit, H.M.I.A.; Galal, Y.G.M.; Soliman, S.M.; Aziz, H.A.A.; Rizk, M.A.; El-Sayed, E.S.R. Bioprospecting endophytic fungi for bioactive metabolites with seed germination promoting potentials. BMC Microbiol. 2024, 24, 200. [Google Scholar] [CrossRef]
- García-Latorre, C.; Rodrigo, S.; Santamaria, O. Biological Control of Pseudomonas syringae in Tomato Using Filtrates and Extracts Produced by Alternaria leptinellae. Horticulturae 2024, 10, 334. [Google Scholar] [CrossRef]
- Mathur, P.; Chaturvedi, P.; Sharma, C.; Bhatnagar, P. Improved seed germination and plant growth mediated by compounds synthesized by endophytic Aspergillus niger (isolate 29) isolated from Albizia lebbeck (L.) Benth. 3 Biotech 2022, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Khan, A.; Hamayun, M.; Kang, S.-M.; Kim, Y.-H.; Lee, I.-J. Assessment of endophytic fungi cultural filtrate on soybean seed germination. Afr. J. Biotechnol. 2012, 11, 15135–15143. [Google Scholar] [CrossRef]
- Pablo, C.H. Plant growth-promoting characteristics of root fungal endophytes isolated from a traditional Cordillera rice landrace. Stud. Fungi 2020, 5, 536–549. [Google Scholar] [CrossRef]
- García-Latorre, C.; Rodrigo, S.; Marin-Felix, Y.; Stadler, M.; Santamaria, O. Plant-growth promoting activity of three fungal endophytes isolated from plants living in dehesas and their effect on Lolium multiflorum. Sci. Rep. 2023, 13, 7354. [Google Scholar] [CrossRef]
- Hashem, A.H.; Attia, M.S.; Kandil, E.K.; Fawzi, M.M.; Abdelrahman, A.S.; Khader, M.S.; Khodaira, M.A.; Emam, A.E.; Goma, M.A.; Abdelaziz, A.M. Bioactive compounds and biomedical applications of endophytic fungi: A recent review. Microb. Cell Factories 2023, 22, 107. [Google Scholar] [CrossRef]
- Zhai, M.-M.; Li, J.; Jiang, C.-X.; Shi, Y.-P.; Di, D.-L.; Crews, P.; Wu, Q.-X. The Bioactive Secondary Metabolites from Talaromyces species. Nat. Prod. Bioprospecting 2016, 6, 1–24. [Google Scholar] [CrossRef]
- Lei, L.-R.; Gong, L.-Q.; Jin, M.-Y.; Wang, R.; Liu, R.; Gao, J.; Liu, M.-D.; Huang, L.; Wang, G.-Z.; Wang, D.; et al. Research advances in the structures and biological activities of secondary metabolites from Talaromyces. Front. Microbiol. 2022, 13, 984801. [Google Scholar] [CrossRef]
- Houbraken, J.; Visagie, C.M.; Meijer, M.; Frisvad, J.C.; Busby, P.E.; Pitt, J.I.; Seifert, K.A.; Louis-Seize, G.; Demirel, R.; Yilmaz, N.; et al. A taxonomic and phylogenetic revision of Penicillium section Aspergilloides. Stud. Mycol. 2014, 78, 373–451. [Google Scholar] [CrossRef]
- Gupta, V.; Sharma, A.; Jamwal, G.; Gupta, S.; Razdan, V. Penicillium Genus as a Source of Metabolites for Agricultural Applications. In Fungal Metabolites for Agricultural Applications; Poveda, J., Santamaría, O., Martín-García, J., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 181–198. [Google Scholar]
- Hao, L.; Zheng, X.; Wang, Y.; Li, S.; Shang, C.; Xu, Y. Inhibition of Tomato Early Blight Disease by Culture Extracts of a Streptomyces puniceus Isolate from Mangrove Soil. Phytopathology 2019, 109, 1149–1156. [Google Scholar] [CrossRef]
- Feller, C.; Bleiholder, H.; Buhr, L.; Hack, H.; Heß, M.; Klose, R.; Meier, U.; Stauß, R.; Boom, T.v.d.; Weber, E. Phänologische Entwicklungsstadien von GemüsepflanzenII. Fruchtgemüse und Hülsenfrüchte: Codierung und Beschreibung nach der erweiterten BBCH-Skala—Mit Abbildungen. Heft 9 1995, 47, 217–232. [Google Scholar]
- Therneau, T. A Package for Survival Analysis in R. Available online: https://cran.r-project.org/package=survival (accessed on 14 April 2025).
- Kagithoju, S.; Sk, A.H.; Godishala, V.; Nanna, R.S. Role of Fungi and Fungal Extract on Strychnos potatorum Seed Germination. Res. J. Biotechnol. 2013, 8, 32–36. [Google Scholar]
- Parveen, S.; Wani, A.H.; Yaqub, M. Effect of culture filtrates of pathogenic and antagonistic fungi on seed germination of some economically important vegetables. Braz. J. Bot. 2019, 6, 133–139. [Google Scholar] [CrossRef]
- Ogórek, R.; Przywara, K.; Piecuch, A.; Cal, M.; Lejman, A.; Matkowski, K. Plant–Fungal Interactions: A Case Study of Epicoccoum nigrum Link. Plants 2020, 9, 1691. [Google Scholar] [CrossRef]
- Zheng, Y.; Zou, J.; Lin, S.; Jin, C.; Shi, M.; Yang, B.; Yang, Y.; Jin, D.; Li, R.; Li, Y.; et al. Effects of different light intensity on the growth of tomato seedlings in a plant factory. PLoS ONE 2023, 18, e0294876. [Google Scholar] [CrossRef]
- Abou Chehade, L.; Al Chami, Z.; De Pascali, S.A.; Cavoski, I.; Fanizzi, F.P. Biostimulants from food processing by-products: Agronomic, quality and metabolic impacts on organic tomato (Solanum lycopersicum L.). J. Sci. Food Agric. 2018, 98, 1426–1436. [Google Scholar] [CrossRef]
- Miceli, A.; Vetrano, F.; Moncada, A. Effects of Foliar Application of Gibberellic Acid on the Salt Tolerance of Tomato and Sweet Pepper Transplants. Horticulturae 2020, 6, 93. [Google Scholar] [CrossRef]
- Katsumi, M.; Ishida, K. The Gibberellin Control of Cell Elongation. In Gibberellins; Springer: New York, NY, USA, 1991; pp. 211–219. [Google Scholar]
- Ericsson, T. Growth and shoot: Root ratio of seedlings in relation to nutrient availability. Plant Soil 1995, 168, 205–214. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Yan, X.; Wang, C.; Guan, P.; Tang, Z. A response of biomass and nutrient allocation to the combined effects of soil nutrient, arbuscular mycorrhizal, and root-knot nematode in cherry tomato. Front. Ecol. Evol. 2023, 11, 1106122. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Sakthivel, A.; Ramesh, K.; Manisseeri, C.; Ganeshan, S.; Subramani, M.; Gnanajothi, K. Bioprospecting of exopolysaccharides from the endophytic fungi Epicoccum sorghinum AMFS4, for its structure, composition, bioactivities and application in seed priming. Nat. Prod. Res. 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Agrawal, P.; Ghosh, U.; Sharma, C.; Bhatnagar, P.; Chaturvedi, P. Growth promotion ability of endophytic Aspergillus niger on different species of Vigna. Vegetos 2024, 37, 192–201. [Google Scholar] [CrossRef]
- Chen, S.-M.; Zhang, C.-M.; Peng, H.; Qin, Y.-Y.; Li, L.; Li, C.-G.; Xing, K.; Liu, L.-L.; Qin, S. Exopolysaccharides from endophytic Glutamicibacter halophytocota KLBMP 5180 functions as bio-stimulants to improve tomato plants growth and salt stress tolerance. Int. J. Biol. Macromol. 2023, 253, 126717. [Google Scholar] [CrossRef] [PubMed]
- Nandini, B.; Hariprasad, P.; Shankara, H.N.; Prakash, H.S.; Geetha, N. Total crude protein extract of Trichoderma spp. induces systemic resistance in pearl millet against the downy mildew pathogen. 3 Biotech 2017, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Patel, A.; Patel, M.; Goswami, D. Talaromyces pinophilus strain M13: A portrayal of novel groundbreaking fungal strain for phytointensification. Environ. Sci. Pollut. Res. 2021, 28, 8758–8769. [Google Scholar] [CrossRef] [PubMed]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef]
- Flores-Velázquez, J.; Rojano, F.; Aguilar-Rodríguez, C.E.; Villagran, E.; Villarreal-Guerrero, F. Greenhouse Thermal Effectiveness to Produce Tomatoes Assessed by a Temperature-Based Index. Agronomy 2022, 12, 1158. [Google Scholar] [CrossRef]
- Stubbs, C.; Cook, D.; Niklas, K. A general review of the biomechanics of root anchorage. J. Exp. Bot. 2019, 70, 3439–3451. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Mannino, G.; Beekwilder, J.; Contartese, V.; Karlova, R.; Bertea, C.M. The application of a biostimulant based on tannins affects root architecture and improves tolerance to salinity in tomato plants. Sci. Rep. 2021, 11, 354. [Google Scholar] [CrossRef]
- Ismail, M.A.; Amin, M.A.; Eid, A.M.; Hassan, S.E.; Mahgoub, H.A.M.; Lashin, I.; Abdelwahab, A.T.; Azab, E.; Gobouri, A.A.; Elkelish, A.; et al. Comparative Study between Exogenously Applied Plant Growth Hormones versus Metabolites of Microbial Endophytes as Plant Growth-Promoting for Phaseolus vulgaris L. Cells 2021, 10, 1059. [Google Scholar] [CrossRef]

| First Trial | Second Trial | ||||
|---|---|---|---|---|---|
| Analyzed Parameters | Degrees of Freedom | F-Value | p-Value | F-Value | p-Value |
| Shoot length 14 DAS | 2 | 0.78 | 0.466 | 5.03 | 0.099 |
| Shoot length 21 DAS | 2 | 0.67 | 0.515 | 3.08 | 0.224 |
| Shoot length 28 DAS | 2 | 1.16 | 0.323 | 2.38 | 0.21 |
| Shoot length 35 DAS | 2 | 3.79 | 0.03 | 3.34 | 0.165 |
| Shoot length 42 DAS | 2 | 3.64 | 0.034 | 1.23 | 0.753 |
| Shoot dry weight | 2 | 3.50 | 0.04 | 1.05 | 0.355 |
| Root length | 2 | 12.73 | <0.001 | 11.22 | <0.001 |
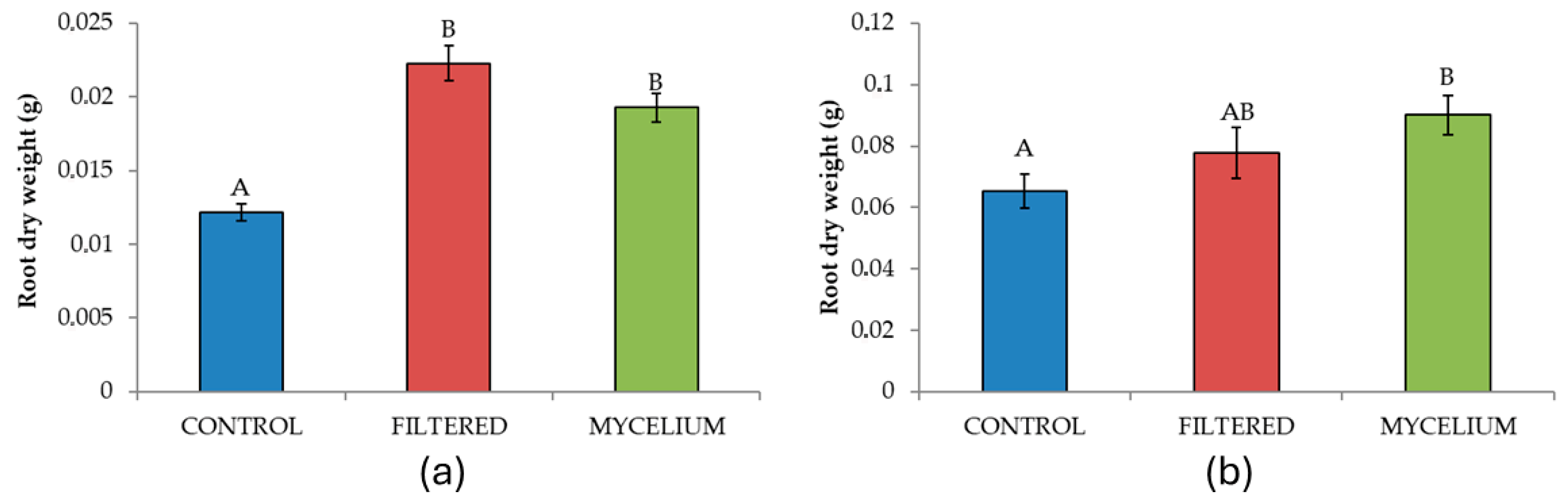
| Root dry weight | 2 | 34.78 | <0.001 | 3.48 | 0.037 |
| Chlorophyll content (µg/cm2) | 2 | 0.02 | 0.979 | 0.33 | 0.722 |
| Flavonol Index | 2 | 0.76 | 0.472 | 0.97 | 0.386 |
| Anthocyanins Index | 2 | 0.58 | 0.564 | 0.08 | 0.923 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias-Ganado, Á.; Poveda, J.; Santamaría, O.; Rodrigo, S.; Pozo, M.I.; Martín-García, J. Effects of Seed Priming with Talaromyces ruber Extracts on Tomato (Solanum lycopersicum) Growth. Agriculture 2025, 15, 1868. https://doi.org/10.3390/agriculture15171868
Iglesias-Ganado Á, Poveda J, Santamaría O, Rodrigo S, Pozo MI, Martín-García J. Effects of Seed Priming with Talaromyces ruber Extracts on Tomato (Solanum lycopersicum) Growth. Agriculture. 2025; 15(17):1868. https://doi.org/10.3390/agriculture15171868
Chicago/Turabian StyleIglesias-Ganado, Álvaro, Jorge Poveda, Oscar Santamaría, Sara Rodrigo, María I. Pozo, and Jorge Martín-García. 2025. "Effects of Seed Priming with Talaromyces ruber Extracts on Tomato (Solanum lycopersicum) Growth" Agriculture 15, no. 17: 1868. https://doi.org/10.3390/agriculture15171868
APA StyleIglesias-Ganado, Á., Poveda, J., Santamaría, O., Rodrigo, S., Pozo, M. I., & Martín-García, J. (2025). Effects of Seed Priming with Talaromyces ruber Extracts on Tomato (Solanum lycopersicum) Growth. Agriculture, 15(17), 1868. https://doi.org/10.3390/agriculture15171868









