Morphometric Variation and Production Constraints of Criollo Sheep in the High Andes of Southern Peru
Abstract
1. Introduction
2. Materials and Methods
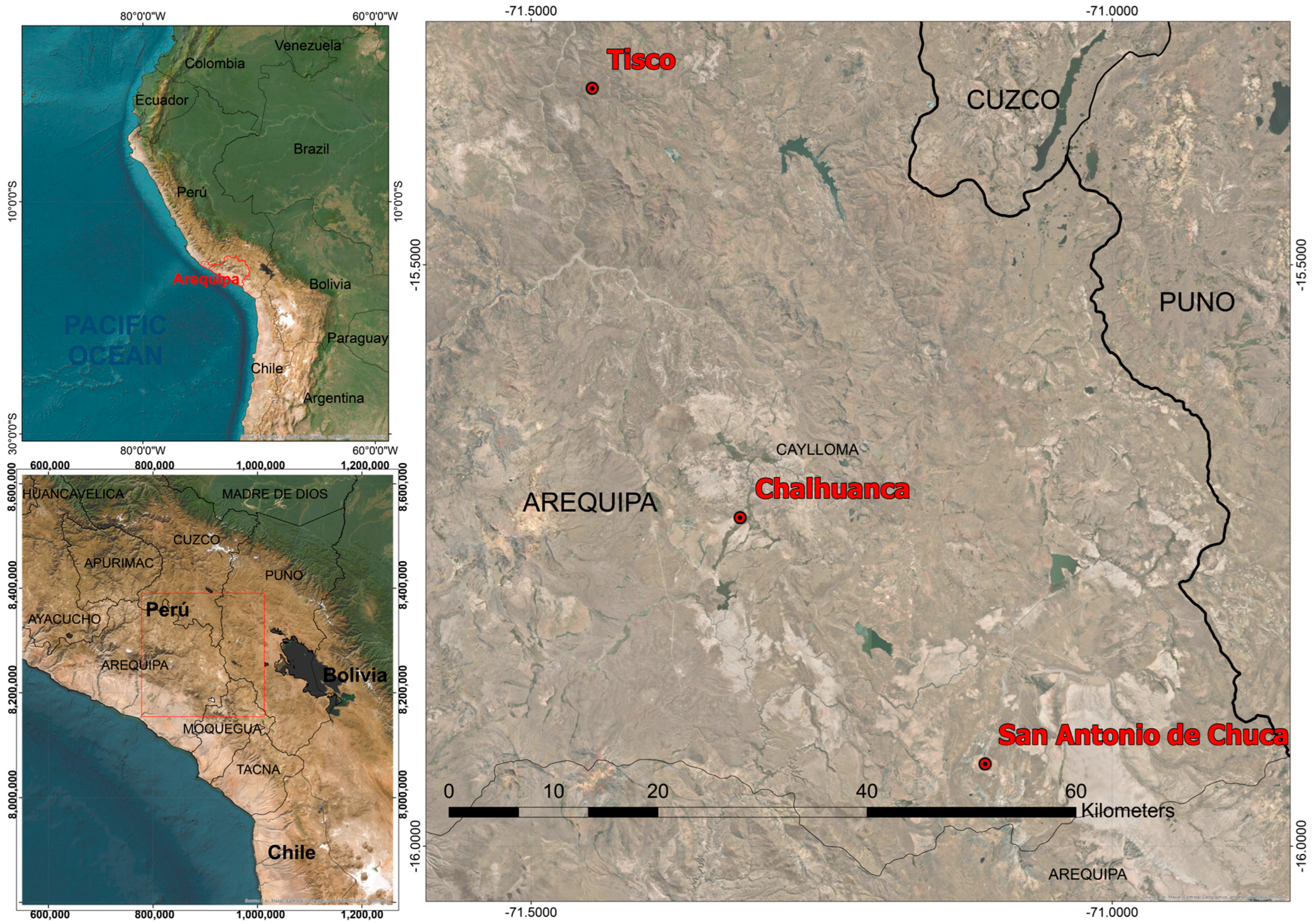
2.1. Study Area and Environmental
2.2. Study Design and Sampling Strategy
2.3. Morphometric Evaluation and Zoometric Indices
2.4. Questionnaire Design and Characterization of the Production System
2.5. Statistical Analysis
3. Results
3.1. Sociodemographic, Economic, and Productive Characterization of Sheep Producers
3.2. Health Management and Phenotypic Characterization of Criollo Sheep
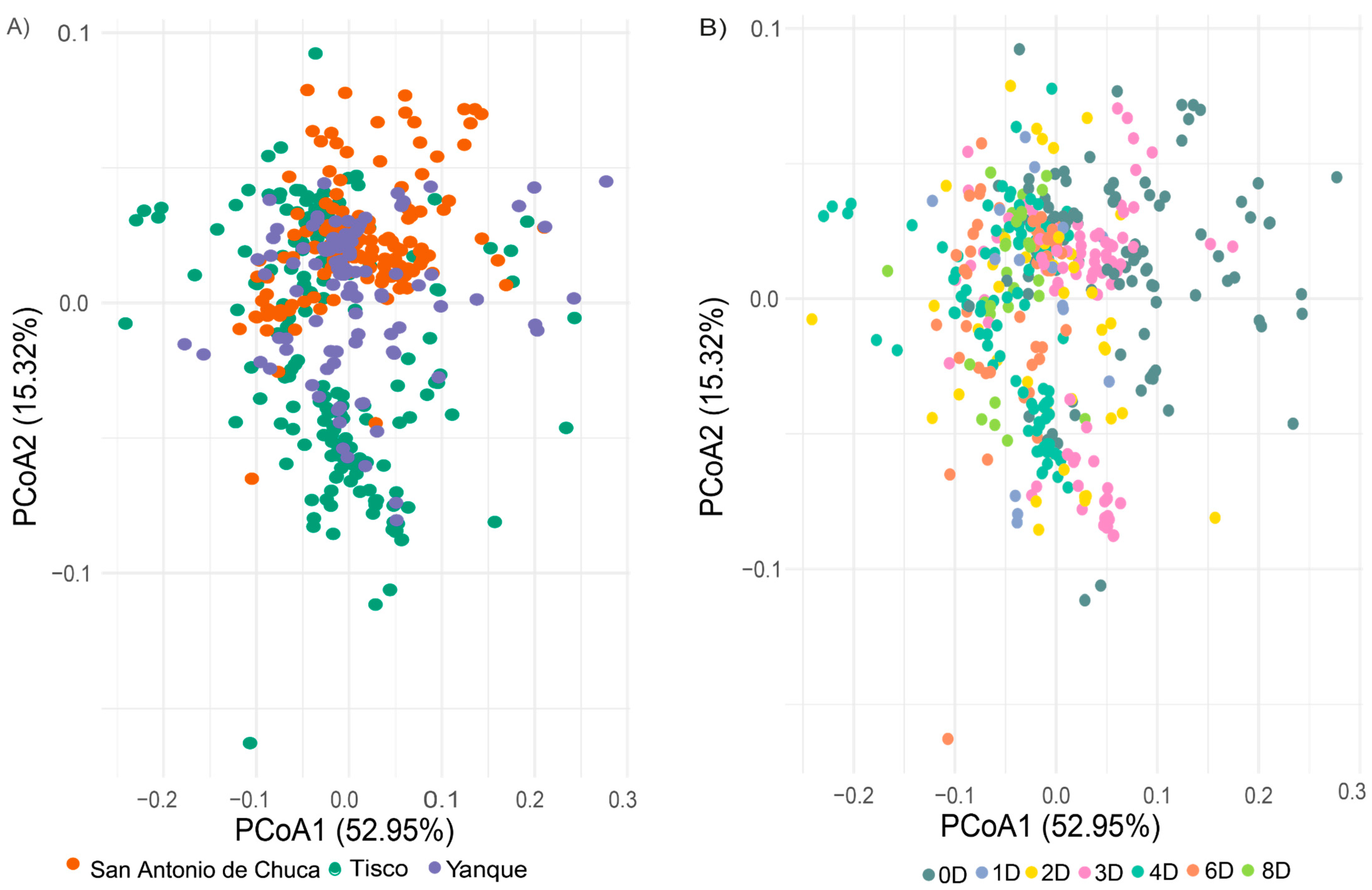
3.3. Multivariate Analysis of Morphometric Variation
3.4. Spearman Correlations Among Morphometric and Zoometric Traits
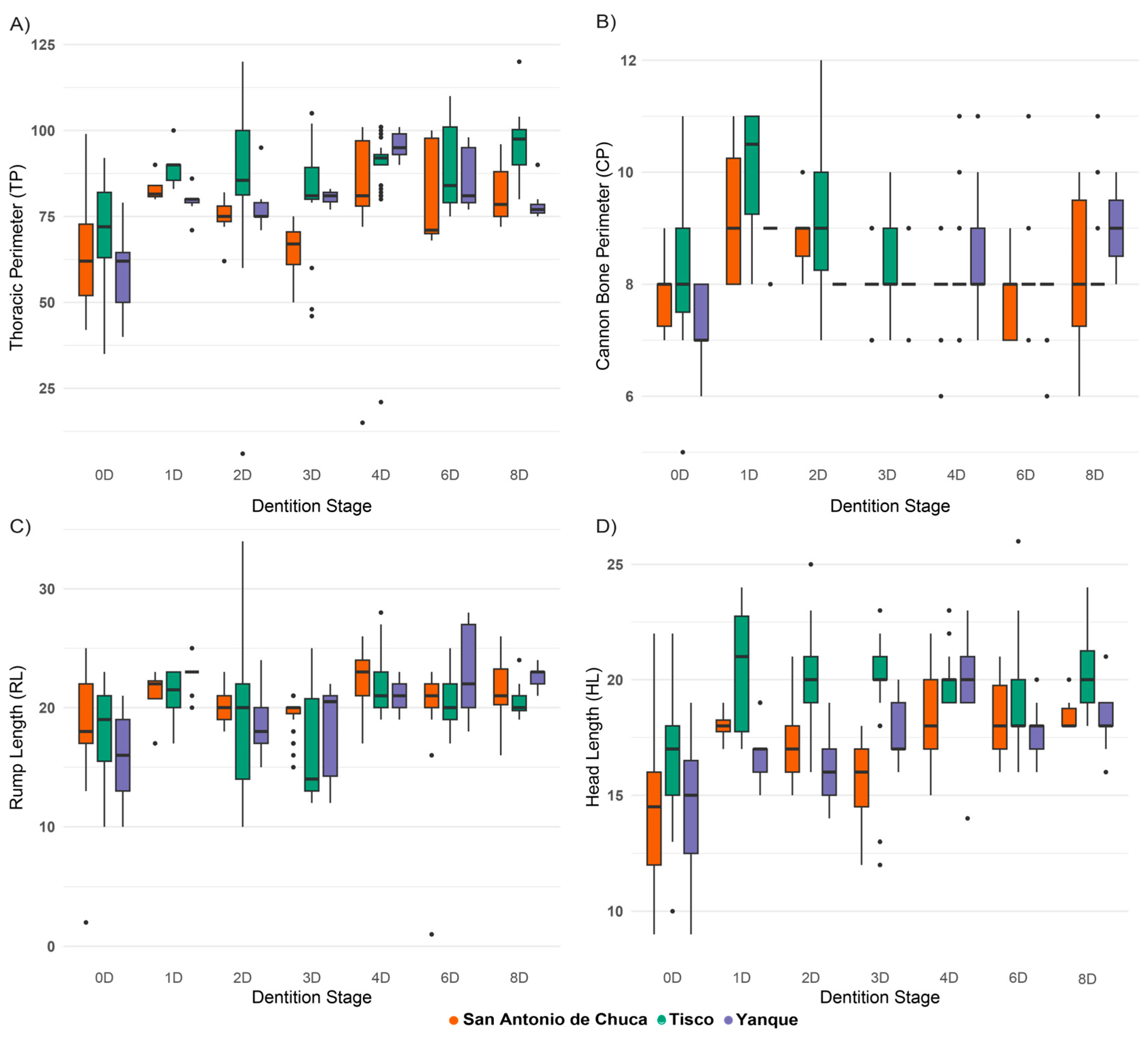
3.5. Effects of Sex and Dental Development on Morphometric Traits of Criollo Sheep
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojo, V.; Momo, F.R.; Arzamendia, Y.; Baldo, J.L.; Vilá, B.L. Sustainability of Animal Stocks in Traditional Pastoral Systems of the High-Altitude Andean Altiplano. Rangel. Ecol. Manag. 2023, 90, 195–207. [Google Scholar] [CrossRef]
- Revelo, H.A.; Landi, V.; López-Alvarez, D.; Palacios, Y.A.; Paiva, S.R.; McManus, C.; Ciani, E.; Alvarez, L.Á. New Insight into the Genome-Wide Diversity and Admixture of Six Colombian Sheep Populations. Genes 2022, 13, 1415. [Google Scholar] [CrossRef]
- Contreras, J.P.; Cordero, A.G.; Rojas, Y.C.; Carhuas, J.N.; Curasma, J.; Mayhua, P.; Salazar, K. Prediction Models for Live Body Weight and Body Compactness of Criollo Sheep in Huancavelica Region, Peru. Indian J. Anim. Sci. 2024, 94, 637–641. [Google Scholar] [CrossRef]
- Salinas-Rios, T.; Hernández-Bautista, J.; Mariscal-Méndez, A.; Aquino-Cleto, M.; Martínez-Martínez, A.; Rodríguez-Magadán, H.M. Genetic Characterization of a Sheep Population in Oaxaca, Mexico: The Chocholteca Creole. Animals 2021, 11, 1172. [Google Scholar] [CrossRef]
- Zhu, L.; Tang, L.; Zhang, K.; Nie, H.; Gou, X.; Kong, X.; Deng, W. Genetic and Epigenetic Adaptation Mechanisms of Sheep Under Multi-Environmental Stress Environment. Int. J. Mol. Sci. 2025, 26, 3261. [Google Scholar] [CrossRef]
- Ormachea, V.E.; Alencastre, D.R.G.; Olivera, M.L.V. Índices zoométricos del ovino criollo en el Centro Experimental Chuquibambilla, Puno, Perú. Rev. Investig. Vet. Perú 2020, 31, e17139. [Google Scholar] [CrossRef]
- Monosi, A.B.; Ketshephaone, T.; Patrick, M.K.; Phetogo, I.M.; Cosmos, M. On-Farm Phenotypic Characterization of Indigenous Tswana Sheep Population in Selected Districts of Southern Botswana. Afr. J. Agric. Res. 2021, 17, 1268–1280. [Google Scholar] [CrossRef]
- De La Barra, R.; Martínez, M.E.; Carvajal, A. Morphostructural Relationships and Productive Functionality of Sheep Breeds Used for Terminal Crossbreeding in Chile. Int. J. Morphol. 2016, 34, 958–962. [Google Scholar] [CrossRef]
- Bernardi, R.F.; Vieira, M.M.; Sanchez, N.; Vargas, M.I.; Mendes, N.; Cavalcante, S.O. Correlation of Zoometric Indices and Morphometric Measurements in Dorper Lambs. Boletim de Indústria Animal 2022, 79, e1857. Instituto de Zootecnia, APTA, Secretaria de Agricultura e Abastecimento do Estado de São Paulo: Nova Odessa, Brazil. Available online: https://bia.iz.sp.gov.br/index.php/bia/article/view/1857 (accessed on 2 June 2025).
- Corredor, F.-A.; Figueroa, D.; Estrada, R.; Burgos-Paz, W.; Salazar, W.; Cruz, W.; Lobato, R.; Injante, P.; Godoy, D.; Barrantes, C.; et al. Genome-Wide Single Nucleotide Polymorphisms Reveal the Genetic Diversity and Population Structure of Creole Goats from Northern Peru. Livest. Sci. 2024, 283, 105473. [Google Scholar] [CrossRef]
- Demir, E.; Ceccobelli, S.; Bilginer, U.; Pasquini, M.; Attard, G.; Karsli, T. Conservation and Selection of Genes Related to Environmental Adaptation in Native Small Ruminant Breeds: A Review. Ruminants 2022, 2, 255–270. [Google Scholar] [CrossRef]
- Salamanca Montesinos, I.; Gómez Urviola, N.; Soares Fioravanti, M.C.; Bezerra Sereno, J.R. Caracterización de los ovinocultores y sus sistemas productivos en el litoral sur del Perú. An. Científicos 2018, 79, 182. [Google Scholar] [CrossRef]
- Sánchez Dávila, M.E. La organización social de la agricultura andina: Una mirada desde la antropología. Anthroposentido 2019, 3, 39–53. Universidad Peruana de Ciencias Aplicadas (UPC): Lima, Perú. Available online: https://www.researchgate.net/publication/343682496 (accessed on 2 June 2025).
- Loayza-Aguilar, J.; Blanco-Capia, L.E.; Bernabé-Uño, A.; Ayala-Flores, G. Saberes locales sobre tecnologías y estrategias de producción agropecuaria para la resiliencia climática Local knowledge of agricultural production technologies and strategies for climate resilience. J. Selva Andin. Biosph. 2020, 8, 32–41. [Google Scholar] [CrossRef]
- Rubio, V.G.G. Potencialidades de la Ovinocultura y los Hongos Comestibles (Pleurotus spp.) en la Seguridad Alimentaria y el Desarrollo Rural; Laberinto Ediciones: Edmonton, AB, Canada, 2022. [Google Scholar]
- Poma, C.P.; Jiménez, E.; Ordoñez, N.; Maguiña, R.M.; Hidalgo, N. Componentes estructurales del sistema de producción ovina en la Comunidad Campesina de San Pedro de Pirca, Huaral, Perú. Peruvian Agric. Res. 2021, 3, 1–12. [Google Scholar] [CrossRef]
- Pérez-Abadia, D.F.; Medina-Arroyo, H.H.; Navarro-Hevia, J. Tipificación y caracterización de sistemas productivos agroforestales en comunidades del departamento del Chocó, Colombia. Cienc. Tecnol. Agropecu. 2024, 25, e3176. [Google Scholar] [CrossRef]
- Ellis, J.A.; Chavera, A.E.V.; DeMartini, J.C. Disease Conditions in Slaughtered Sheep from Small Holder Flocks in Peru. Small Rumin. Res. 1993, 10, 243–250. [Google Scholar] [CrossRef]
- Olivares, F.; Marchant, C.; Ibarra, J.T. “The Climate Itself Must Have Hidden Some Medicines”: Traditional Veterinary Medicine of Indigenous and Non-Indigenous Campesinos of the Southern Andes. J. Ethnobiol. Ethnomed. 2022, 18, 36. [Google Scholar] [CrossRef]
- Abera, B.; Kebede, K.; Gizaw, S. Indigenous Breeding Practices and Selection Criteria of Sheep Breed in Selale Area, Central Ethiopia. Int. J. Livest. Res. 2014, 4, 49. [Google Scholar] [CrossRef]
- Kosgey, I.S.; Okeyo, A.M. Genetic Improvement of Small Ruminants in Low-Input, Smallholder Production Systems: Technical and Infrastructural Issues. Small Rumin. Res. 2007, 70, 76–88. [Google Scholar] [CrossRef]
- Hernández, J.A.; Lepe, M.; Macedo, R.; Arredondo, V.; Cortez, C.E.; García, L.J.; Prado, O. Morphological Study of Socorro Island Merino Sheep and Its Crosses with Hair Breeds. Trop. Anim. Health Prod. 2017, 49, 173–178. [Google Scholar] [CrossRef]
- Burfening, P.J.; Chavez, C.J. The Criollo Sheep in Peru. Anim. Genet. Resour. Inf. 1996, 17, 115–125. [Google Scholar] [CrossRef]
- Edwin, O.V.; Bilo, C.C.; Eyner, A.S.; Buenaventura, O.V.; Henry, G.C.; Yecenia, M.M.G. Principal Component Analysis of Morphological Characteristics in Creole Sheep (Ovis Aries). Adv. Anim. Vet. Sci. 2023, 11, 903–909. [Google Scholar] [CrossRef]
- Megdiche, S. The Tunisian Barbary Sheep: A Look at the Morphostructural Characteristics of Purebred Ewes Reared under Arid Conditions. J. Saudi Soc. Agric. Sci. 2022, 21, 160–170. [Google Scholar] [CrossRef]
- Getaneh, M.; Taye, M.; Kebede, D.; Andualem, D. Structural Indices of Indigenous Goats Reared under Traditional Management Systems in East Gojjam Zone, Amhara Region, Ethiopia. Heliyon 2022, 8, e09180. [Google Scholar] [CrossRef] [PubMed]
- Storz, J.F.; Cheviron, Z.A. Physiological Genomics of Adaptation to High-Altitude Hypoxia. Annu. Rev. Anim. Biosci. 2021, 9, 149–171. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, S.; He, Z.; Ma, X. Physiological and Genetic Basis of High-Altitude Indigenous Animals’ Adaptation to Hypoxic Environments. Animals 2024, 14, 3031. [Google Scholar] [CrossRef] [PubMed]
- Pitt, D.; Bruford, M.W.; Barbato, M.; Orozco-terWengel, P.; Martínez, R.; Sevane, N. Demography and Rapid Local Adaptation Shape Creole Cattle Genome Diversity in the Tropics. Evol. Appl. 2019, 12, 105–122. [Google Scholar] [CrossRef]
- Wuletaw, Z.; Wurzinger, M.; Holt, T.; Dessie, T.; Sölkner, J. Assessment of Physiological Adaptation of Indigenous and Crossbred Cattle to Hypoxic Environment in Ethiopia. Livest. Sci. 2011, 138, 96–104. [Google Scholar] [CrossRef]
- Kumari, P.; Bharti, V.K.; Kumar, K.; Sharma, I. Hypobaric Hypoxia Affects the Reproductive Physiology of Dairy Cattle. Anim. Reprod. Update 2023, 3, 6–17. [Google Scholar] [CrossRef]
- Tella, A. Regression Analysis of Phenotypic Traits of West African Dwarf Goats (Capra hircus) in Derived Savanna Zone of Nigeria. Anoa J. Anim. Husb. 2025, 4, 51–61. [Google Scholar]
- Hagos, T.; Banerjee, A.K.; Mummed, Y. Live Body Weight and Linear Body Measurements of Indigenous Sheep Population in Their Production System for Developing Suitable Selection Criteria in Central Zone of Tigray, Northern Ethiopia. Afr. J. Agric. Res. 2017, 12, 1087–1095. [Google Scholar] [CrossRef]
- Assan, N.; Musasira, M.; Mpofu, M.; Mwayera, N.; Mokoena, K.; Tyasi, T.L. Relationship Between Body Weight and Linear Body Measurements at Various Stages of Permanent Tooth Eruption in Indigenous Matebele Female Goats of Zimbabwe. Adv. Anim. Vet. Sci. 2024, 12, 1818–1828. [Google Scholar] [CrossRef]

| Variable | Tisco | San Antonio de Chuca | Yanque |
|---|---|---|---|
| Mean age (±SE) | 46.89 ± 3.87 | 50.00 ± 2.57 | 42.43 ± 2.92 |
| Age (min–max) | 18–77 | 29–71 | 22–65 |
| % Male/Female | 66.7/33.3 | 66.7/33.3 | 56.2/43.8 |
| Predominant education level | Incomplete primary (33.3%) | Incomplete primary (38.9%) | Incomplete primary (50.0%) |
| Years of livestock farming (mean ± SE) | 9.00 ± 0.46 | 9.16 ± 0.45 | 10.00 ± 0.00 |
| Monthly income (S/.) (mean ± SD) | 1107.69 ± 507.7 | 878.75 ± 304.08 | 820.00 ± 240.05 |
| Income range (S/.) | 150–2000 | 180–1200 | 300–1000 |
| Variable | Tisco | San Antonio de Chuca | Yanque |
|---|---|---|---|
| Campaign-based production (%) | 16.6 (Yes)/83.4 (No) | 50.0 (Yes)/50.0 (No) | 37.5 (Yes)/62.5 (No) |
| Livestock as main income source (%) | Not specified | Not specified | Not specified |
| Technical assistance (%) | 72.2 (Yes)/27.8 (No) | 72.2 (Yes)/27.8 (No) | 87.5 (Yes)/12.5 (No) |
| Main reason for raising sheep | Family tradition | Family tradition | Family tradition |
| Change in sheep population over the last 5 years | 75% Decrease/15% Stable/10% Increase | 75% Decrease/15% Stable/10% Increase | 75% Decrease/15% Stable/10% Increase |
| Main causes of change in the sheep population | Lack of pasture (18), Market demand (4), Others (4) | Lack of pasture (18), Market demand (4), Others (4) | Lack of pasture (18), Market demand (4), Others (4) |
| Variables | Df | SumOfSqs | R2 | F | p-Value |
|---|---|---|---|---|---|
| Sex | 1 | 0.02 | 0.005 | 3.29 | 0.0225 * |
| Teeth | 6 | 1.11 | 0.255 | 30.49 | 0.0001 *** |
| District | 2 | 0.29 | 0.066 | 23.82 | 0.0001 *** |
| Sex×Teeth | 6 | 0.06 | 0.013 | 1.56 | 0.0714 |
| Sex×District | 2 | 0.02 | 0.005 | 1.96 | 0.0639 |
| Teeth×District | 12 | 0.24 | 0.056 | 3.35 | 0.0001 *** |
| Sex × Teeth × District | 9 | 0.09 | 0.021 | 1.64 | 0.0322 * |
| Residual | 416 | 2.53 | 0.579 | ||
| Total | 454 | 4.37 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrada, R.; Guelac-Mori, E.; Pedemonte-Cruz, C.; Chiqui-Condori, K.M.; Pacherres, K.M.; Cerdan-Ramos, D.; Zúñiga-Aranibar, D.M. Morphometric Variation and Production Constraints of Criollo Sheep in the High Andes of Southern Peru. Agriculture 2025, 15, 1860. https://doi.org/10.3390/agriculture15171860
Estrada R, Guelac-Mori E, Pedemonte-Cruz C, Chiqui-Condori KM, Pacherres KM, Cerdan-Ramos D, Zúñiga-Aranibar DM. Morphometric Variation and Production Constraints of Criollo Sheep in the High Andes of Southern Peru. Agriculture. 2025; 15(17):1860. https://doi.org/10.3390/agriculture15171860
Chicago/Turabian StyleEstrada, Richard, Elias Guelac-Mori, Cristian Pedemonte-Cruz, Katherine M. Chiqui-Condori, Klinsmann Montero Pacherres, Dilser Cerdan-Ramos, and Dayana M. Zúñiga-Aranibar. 2025. "Morphometric Variation and Production Constraints of Criollo Sheep in the High Andes of Southern Peru" Agriculture 15, no. 17: 1860. https://doi.org/10.3390/agriculture15171860
APA StyleEstrada, R., Guelac-Mori, E., Pedemonte-Cruz, C., Chiqui-Condori, K. M., Pacherres, K. M., Cerdan-Ramos, D., & Zúñiga-Aranibar, D. M. (2025). Morphometric Variation and Production Constraints of Criollo Sheep in the High Andes of Southern Peru. Agriculture, 15(17), 1860. https://doi.org/10.3390/agriculture15171860






