Balancing Tradition and Innovation: A 5-Year Review of Modern Approaches to Livestock Breed Conservation
Abstract
1. Introduction
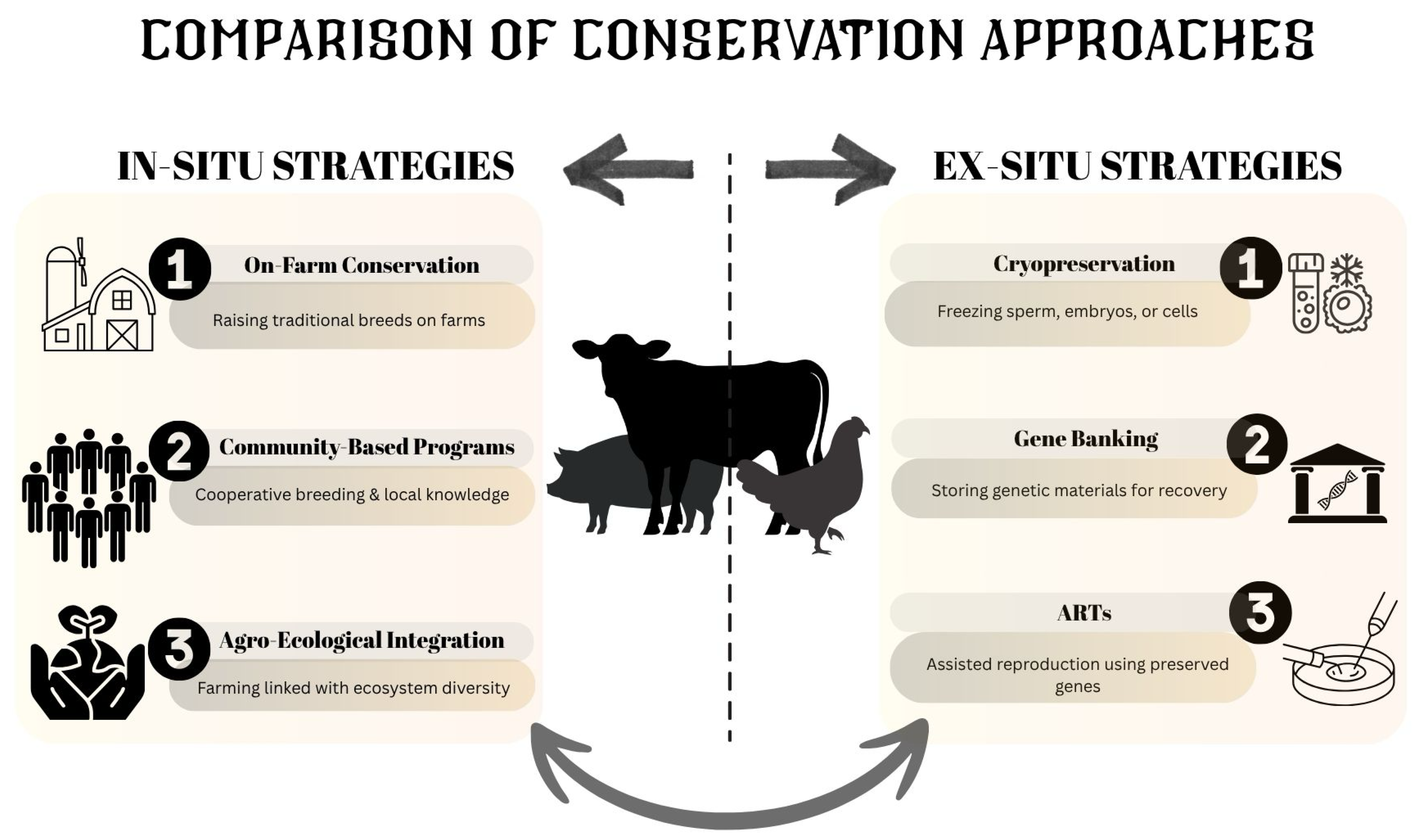
2. Traditional Conservation Methods
| Methodology | Breeds Investigated | Outcome Measured | Main Findings | Ref. |
|---|---|---|---|---|
| - Maraichine Association’s new system for population control - Mating plans with sociological input - Farmer surveys (2004, 2018, 2019) | Maraichine cattle, Charolais cattle | - Ease of calving (percentage of births requiring no assistance) - Calving rate (percentage) - Milk production (kg/day) - Carcass characteristics (EUROP classification) - Disease resistance (veterinary expenses per livestock unit per year, EUR) | - Population grew from 8 to 1666 in 30 years; breeders from 3 to 127 - Inbreeding rate kept at 3.9% (2015) - Veal meat sales yielded 45% higher margin than weanlings | [24] |
| Three-step strategy (Discover, Secure, Sustain): - Identify candidate populations via historical, phenotypic, and genetic analysis - Apply rescue and breeding protocols to recover genetic variation - Stimulate market demand to ensure long-term sustainability | - American Spanish goats - Texas Longhorn cattle - Navajo Churro sheep - Pineywoods cattle - Randall Lineback cattle - Heritage turkeys - Santa Cruz Island sheep | - Recognition and validation of breeds as repeatable genomic packages - Securing genetic variation through rescue protocols - Maintaining production potential and genetic structure - Sustaining market demand for breeds and their products | - Livestock breeds should be viewed as repeatable genomic packages with predictable performance - The Discover–Secure–Sustain model effectively protects livestock populations - Successful conservation requires attention to biological, genetic, and cultural factors | [25] |
| Understanding livestock breeds genetic adaptation to environmental conditions in order to update in situ conservation strategies by landscape genetics. | - Rathi, Siri cattle breeds - Indian sheep breeds - Goat, chicken, swine, yak: Various breeds | Gene flow, impact of global change on genetic patterns, adaptive pattern of species at different geo-climatic conditions, functional connectivity, genetic connectivity | - 94% increase in hatching rate by introducing new genes - More males and fewer stillbirths in Scandinavian adder population - Improved survival in migrant and inbreeding treatment groups vs. control (no exact values) | [26] |
| - Semen cryopreservation and spermatological diagnostics - Intraspecies cloning via somatic cell nuclear transfer (SCNT) - Cryobanking of germplasm (semen and embryos) - Development of genetically stable primary cell cultures - Generation of SCNT embryos using somatic cells | - White-backed, Polish Red, Polish Black and White, Polish Red and White cattle breeds - Puławska, Złotnicka Spotted pig breeds | Efficiency of cryopreservation methods for semen and embryos; Success rates of generating embryos through SCNT to the blastocyst stage (e.g., 30.1% for Polish Red cattle, 34.1% for Puławska pigs, 27.9% for Złotnicka Spotted pigs) | - 80.9% sperm motility, 56.7% viability, 60.1% acrosomal integrity with BHT extender - 87% farrowing rate, 11 piglets per litter - NT embryo efficiency: 30.1% (bovine), 34.1% (Puławska pigs), 27.9% (Złotnicka Spotted pigs) | [27] |
| In situ conservation of livestock breeds in Slovakia is primarily based on animal breeding in natural conditions without limitations to breed improvement. | - Slovak Pinzgau cattle - Valachian sheep - Improved Valachian and Tsigai sheep - Askanian Merino sheep - Lipitsa, Shagya Arab, Hutsul, Furioso, Slovak Sport Pony horses - Noric of Murany horses - Nonius horses | Population size and number of registered purebred females | - Slovak Pinzgau cows declined from 2500 (2003) to 1600 (2006), then rose to 2000 (2010) - Valachian sheep increased from 50 (2003) to 907 (2020) - Improved Valachian and Tsigai sheep declined until 2016, then stabilized - Askanian Merino sheep maintained a stable population with one registered breeder - Lipitsa, Shagya Arab, Hutsul, Furioso, and Slovak Sport Pony horses saw an increase in purebred females since 2003 - Norik of Murany horses declined; Nonius horses remain at low levels - The open conservation system allows adding new breeds and subsidies support stability, but the lack of national legislation remains a key issue | [28] |
| Methodology | Breeds Investigated | Outcome Measured | Main Findings | Ref. |
|---|---|---|---|---|
| - Development of bioreservoirs via cryogenic and lyophilization methods for ex situ conservation - Use of ARTs such as SCNT and IVF - Cryopreservation and lyophilization of somatic/stem cells and germplasm (sperm, oocytes, embryos) - Establishment of ex vivo-expanded permanent cell lines from tissue biopsies - Research on factors influencing proliferation and genetic stability of donor cell lines - Application of preserved semen in ARTs, especially for in vitro embryo production | - Merino, Blackhead, Polish Heath, Olkuska sheep - Polish Red cattle - Carpathian goats - Złotnicka Spotted, Puławska, Złotnicka White pigs | - Blastocyst formation rate. - Efficiency of SCNT-based cloning. - Number of somatic and stem cell lines. - Number of germplasm-based biological materials | - Bioreservoirs for ex situ conservation developed using cryopreservation and lyophilization - SCNT success depends on somatic/stem cell line quality - Preserved semen supports ARTs and genetic resource conservation | [42] |
| - Semen cryopreservation - Spermatological diagnostics - Intraspecies cloning by SCNT - Establishment and cryopreservation of genetically stable somatic cell lines - Generation of nuclear-transferred embryos | - White-backed, Polish Red, Polish Black and White, Polish Red and White cattle - Puławska, Złotnicka Spotted pigs | - Ne - Efficiency of generating nuclear-transferred embryos to reach the blastocyst stage (%) - Cryosurvival rate (%) | - Reproductive biotechnologies like semen cryopreservation and SCNT are key to conserving endangered Polish breeds - Efficient cryopreservation protocols developed, especially for cattle - Genetically stable somatic cell lines established for Polish Red cattle, Puławska, and Złotnicka Spotted pigs | [42] |
| - Development of conservation management surveys Breed characterization and conservation through consortia - Efficient resource use via international cooperation (e.g., CONBIAND network) Systematic coordination by national committees - Use of both conventional and modern tools (e.g., multi-criteria analysis) - Proactive emergency response via national alert systems and inventory coordination - Interdisciplinary approach integrating genetics, economics, and ecology | - Pigs (European Gene Banking Project, PIGBIODIV 2) - Cattle (European Cattle Genetic Diversity Consortium, BIOBOVIS) - Small ruminants (ECONOGENE, BIOGOAT) - Horses (Equine Genetic Diversity Consortium, BIOHORSE) - Chickens (AVIANDIV) - Donkeys (BIODONKEY) - Turkeys (BIOTURKEY) - Guinea pigs (BIOCUY) | - Maintenance of domestic animal diversity - Conservation of breeds - Maximization of intrapopulation genetic variability - Monitoring and reporting of breed status - Establishment and management of germplasm banks - Establishment and management of ex situ conservation centers | - National committees for domestic animal genetic resources are recommended to organize conservation efforts. - The CONBIAND network is a model for efficient conservation in low-resource settings through international cooperation. - National committees coordinate conservation activities, including alert systems and ex situ conservation centers. | [43] |
| - Multi-period chance-constrained model for optimizing endangered breed collections - Uses stochastic linear programming to minimize costs under cryotank limits and in situ uncertainties - Applied to semen collection, implemented in AIMMS 2018—Modeling Guide—Integer Programming Tricks and solved with IBM ILOG CPLEX Optimization Studio (v12.8; IBM, Armonk, NY, USA). | - Cattle: 8 non-endangered, 31 endangered - Sheep: 36 non-endangered, 8 endangered - Goats: 20 non-endangered, 3 endangered - Pigs: 3 non-endangered, 12 endangered - Chicken: 1 non-endangered, 16 endangered - Equine (horse and donkey): 1 non-endangered, 14 endangered | - Total cost of all gene banks over the planned period. - Sensitivity analysis of ‘acceptable level of risk’ versus total collection costs. | - A model was developed to optimize collection sites, timing, and quantities for endangered livestock breeds at minimal cost - Based on data from 18 gene banks, it links ex situ collection costs to in situ extinction risk - Strategic use of local gene banks and a national backup can reduce extinction risk cost-effectively | [30] |
3. Modern Approaches in Livestock Breed Conservation Methods
3.1. Genomic Selection and Genomic Diversity Monitoring
3.2. Advanced Reproductive Technologies
3.3. Digital Herdbooks and Blockchain for Traceability
3.4. Machine Learning for Breed Identification and Risk Assessment
4. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NE | Effective Population Size |
| SNPs | Single Nucleotide Polymorphisms |
| IVF | In Vitro Fertilization |
| SCNT | Somatic Cell Nuclear Transfer |
| ARTs | Assisted Reproductive Technologies |
| AI | Artificial Intelligence |
| ICSI | Intra-Cytoplasmic Sperm Injection |
References
- Eusebi, P.G.; Martinez, A.; Cortes, O. Genomic Tools for Effective Conservation of Livestock Breed Diversity. Diversity 2019, 12, 8. [Google Scholar] [CrossRef]
- Menger, A.K.; Hamm, U. Consumers’ Knowledge and Perceptions of Endangered Livestock Breeds: How Wording Influences Conservation Efforts. Ecol. Econ. 2021, 188, 107117. [Google Scholar] [CrossRef]
- Hemachand, N.S.; Bharath, V.S.; Phanindra, J.; Athota, H. Exploring Adaptive Genetic Traits in Domestic Farm Animals: A Comprehensive Review. Act. Sci. Vet. Sci. 2023, 5, 64–67. [Google Scholar] [CrossRef]
- Khandoker, M.Y.; Ali, M.Y.; Akter, T.; Akhtar, M.M.; Kona, M.K.; Meki, N.J.; Sompa, M.R.; Meem, I.J. Population Distribution and Breeding Practices of Livestock in Different Districts of Bangladesh. Asian Australas. J. Biosci. Biotechnol. 2023, 8, 38–48. [Google Scholar] [CrossRef]
- Malcom, A. Integrating Indigenous Knowledge into Livestock Policy Formulation. IJLP 2024, 3, 30–42. [Google Scholar] [CrossRef]
- Tenzin, J.; Chankitisakul, V.; Boonkum, W. Current Status and Conservation Management of Farm Animal Genetic Resources in Bhutan. Vet. Sci. 2023, 10, 281. [Google Scholar] [CrossRef]
- Aguilera-Alcalá, N.; Arrondo, E.; Pascual-Rico, R.; Morales-Reyes, Z.; Gil-Sánchez, J.M.; Donázar, J.A.; Moleón, M.; Sánchez-Zapata, J.A. The Value of Transhumance for Biodiversity Conservation: Vulture Foraging in Relation to Livestock Movements. Ambio 2022, 51, 1330–1342. [Google Scholar] [CrossRef]
- Root-Bernstein, M.; Vargas, B.H.; Bondoux, A.; Guerrero-Gatica, M.; Zorondo-Rodríguez, F.; Huerta, M.; Valenzuela, R.; Bello, Á.V. Silvopastoralism, Local Ecological Knowledge and Woodland Trajectories in a Category V-Type Management Area. Biodivers. Conserv. 2022, 31, 543–564. [Google Scholar] [CrossRef]
- Jablonski, K.E.; Merishi, J.; Dolrenry, S.; Hazzah, L. Ecological Doctors in Maasailand: Identifying Herding Best Practices to Improve Livestock Management and Reduce Carnivore Conflict. Front. Sustain. Food Syst. 2020, 4, 118. [Google Scholar] [CrossRef]
- Osman, A.; Sbhatu, D.B.; Giday, M. Medicinal Plants Used to Manage Human and Livestock Ailments in Raya Kobo District of Amhara Regional State, Ethiopia. Evid. Based Complement. Altern. Med. 2020, 2020, 1329170. [Google Scholar] [CrossRef]
- Swain, S.; Bhattacharya, S.; Chakrabarty, S. Ethnoveterinary Practice Followed by Santhal Tribes to Treat Foot and Mouth Diseases of Livestock in Ghatshila Block, Jharkhand, India. JMHE 2020, 6, 48–51. [Google Scholar] [CrossRef]
- Rahman, I.U.; Ijaz, F.; Bussmann, R.W. Editorial: Ethnoveterinary Practices in Livestock: Animal Production, Healthcare, and Livelihood Development. Front. Vet. Sci. 2023, 9, 1086311. [Google Scholar] [CrossRef] [PubMed]
- Azhar, B.; Tohiran, K.A.; Nobilly, F.; Zulkifli, R.; Syakir, M.I.; Ishak, Z.; Razi, N.; Oon, A.; Shahdan, A.; Maxwell, T.M.R. Time to Revisit Oil Palm-Livestock Integration in the Wake of United Nations Sustainable Development Goals (SDGs). Front. Sustain. Food Syst. 2021, 5, 640285. [Google Scholar] [CrossRef]
- Musara, J.P.; Tibugari, H.; Moyo, B.; Mutizira, C. Crop-Livestock Integration Practices, Knowledge, and Attitudes among Smallholder Farmers: Hedging against Climate Change-Induced Shocks in Semi-Arid Zimbabwe. Open Life Sci. 2021, 16, 1330–1340. [Google Scholar] [CrossRef]
- Fedorca, A.; Mergeay, J.; Akinyele, A.O.; Albayrak, T.; Biebach, I.; Brambilla, A.; Burger, P.A.; Buzan, E.; Curik, I.; Gargiulo, R.; et al. Dealing with the Complexity of Effective Population Size in Conservation Practice. Evol. Appl. 2024, 17, e70031. [Google Scholar] [CrossRef]
- Ladyka, V.; Skliarenko, Y.; Kovtun, P.S.; Tatiana, D.; Viktoriy, V.; Malikova, A. Evaluation of Stud Bulls by Beta-Casein Genotype in the Context of Conservation of Local Cattle Breeds in Ukraine. Sci. Pap. Ser. D. Anim. Sci. 2023, LXVI, 42–47. [Google Scholar]
- Surlea, C.; Mateescu, C.; Dinita, G.; Cringanu, I.; Grosu, H.; Nicolae, C.G. Research on Characters Conservation in Mountain Ecotype Queen Bees. Sci. Pap. Ser. D. Anim. Sci. 2024, LXVII, 307. [Google Scholar]
- Davidescu, M.; Madescu, B.; Ciorpac, M.; Dascalu, L.; Bugeac, T.; Andrei, M.; Porosnicu, I.; Steofil, C. Genetic Diversity of Pinzgau Cattle Breed: A Systematic Review. Sci. Pap. Ser. D. Anim. Sci. 2024, LXIV, 2. [Google Scholar]
- Davidescu, M.-A.; Simeanu, D.; Simeanu, C.; Panzaru, C.; Usturoi, A.; Creanga, S. Analysis of Blood Biochemical Profile of Endangered Romanian Grey Cows According to Age. Sci. Pap. Ser. D. Anim. Sci. 2024, LXVII, 2. [Google Scholar]
- Mureithi, S.; Verdoodt, A.; Njoka, J.T.; Olesarioyo, J.S.; Van Ranst, E. Community-Based Conservation: An Emerging Land Use at the Livestock-Wildlife Interface in Northern Kenya. In Wildlife Management—Failures, Successes and Prospects; Kideghesho, J.R., Rija, A.A., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-291-2. [Google Scholar]
- Velado-Alonso, E.; Morales-Castilla, I.; Rebollo, S.; Gómez-Sal, A. Relationships between the Distribution of Wildlife and Livestock Diversity. Divers. Distrib. 2020, 26, 1264–1275. [Google Scholar] [CrossRef]
- Gladkikh, T.M.; Collazo, J.A.; Torres-Abreu, A.; Reyes, A.M.; Molina, M. Factors That Influence Participation of Puerto Rican Coffee Farmers in Conservation Programs. Conserv. Sci. Pr. 2020, 2, e172. [Google Scholar] [CrossRef]
- Medina, G.; Isley, C.; Arbuckle, J. Iowa Farm Environmental Leaders’ Perspectives on the U.S. Farm Bill Conservation Programs. Front. Sustain. Food Syst. 2020, 4, 497943. [Google Scholar] [CrossRef]
- Roche, B.; Farruggia, A.; Pousin, M.; Riga, P.; Chataigner, C.; Boutifard, V.; Prieur, M.; Roux, P.; Cooke, A.S.; Rivero, M.J. The Maraichine Cattle Breed Supports Breeders and Researchers in the Atlantic Coastal Marshlands. Ruminants 2022, 2, 173–187. [Google Scholar] [CrossRef]
- Sponenberg, D.P.; Martin, A.; Couch, C.; Beranger, J. Conservation Strategies for Local Breed Biodiversity. Diversity 2019, 11, 177. [Google Scholar] [CrossRef]
- Yadav, N.; Mukherjee, A.; Pannu, U.; Gujar, G. Approach and Prospects of Landscape Genetics in Livestock: A Review. ELSR 2023, 9, 33–39. [Google Scholar] [CrossRef]
- Trzcińska, M.; Samiec, M. Ex. Situ Conservation and Genetic Rescue of Endangered Polish Cattle and Pig Breeds with the Aid of Modern Reproductive Biotechnology—A Review. Ann. Anim. Sci. 2021, 21, 1193–1207. [Google Scholar] [CrossRef]
- Tomka, J.; Huba, J.; Pavlík, I. State of Conservation of Animal Genetic Resources in Slovakia. GenResJ 2022, 3, 49–63. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Zong, Y.; Mehaisen, G.M.K.; Chen, J. Poultry Genetic Heritage Cryopreservation and Reconstruction: Advancement and Future Challenges. J. Anim. Sci. Biotechnol. 2022, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Silva, R.; Cortes Gardyn, O.; Hiemstra, S.-J.; Oliveira Marques, J.G.; Tixier-Boichard, M.; Moran, D. Rationalizing Ex. Situ Collection of Reproductive Materials for Endangered Livestock Breed Conservation. Ecol. Econ. 2021, 181, 106916. [Google Scholar] [CrossRef]
- Farhadinia, M.S.; Johnson, P.J.; Zimmermann, A.; McGowan, P.J.K.; Meijaard, E.; Stanley-Price, M.; Macdonald, D.W. Ex Situ Management as Insurance against Extinction of Mammalian Megafauna in an Uncertain World. Conserv. Biol. 2020, 34, 988–996. [Google Scholar] [CrossRef]
- Yang, H.; Huo, Y.; Yee, J.C.; Rikard, S.; Walton, W.C.; Saillant, E. Sperm Repository for a Breeding Program of the Eastern Oyster Crassostrea Virginica: Sample Collection, Processing, Cryopreservation, and Data Management Plan. Animals 2021, 11, 2836. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gao, L.; Hou, Y.; Zhu, S.; Fu, X. Cryopreservation of Farm Animal Gametes and Embryos: Recent Updates and Progress. Front. Agr. Sci. Eng. 2019, 6, 42. [Google Scholar] [CrossRef]
- Elango, K.; Kumaresan, A.; Ashokan, M.; Karuthadurai, T.; Nag, P.; Bhaskar, M.; Prasad, B.A.; Jeyakumar, S.; Manimaran, A.; Bhat, V.; et al. Dynamics of Mitochondrial Membrane Potential and DNA Damage during Cryopreservation of Cattle and Buffalo Bull Spermatozoa. Indian. J. Anim. Sci. 2021, 91, 9–14. [Google Scholar] [CrossRef]
- Dhali, A.; Kolte, A.P.; Mishra, A.; Roy, S.C.; Bhatta, R. Cryopreservation of Oocytes and Embryos: Current Status and Opportunities. In Infertility, Assisted Reproductive Technologies and Hormone Assays; Sultan Sheriff, D., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Nikitkina, E.V.; Dementieva, N.V.; Shcherbakov, Y.S.; Atroshchenko, M.M.; Kudinov, A.A.; Samoylov, O.I.; Pozovnikova, M.V.; Dysin, A.P.; Krutikova, A.A.; Musidray, A.A.; et al. Genome-Wide Association Study for Frozen-Thawed Sperm Motility in Stallions across Various Horse Breeds. Anim. Biosci. 2022, 35, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, R.; Fan, X.; Lv, Y.; Zheng, Y.; Hoque, S.A.M.; Wu, D.; Zeng, W. Resveratrol Improves Boar Sperm Quality via 5′ AMP-Activated Protein Kinase Activation during Cryopreservation. Oxidative Med. Cell. Longev. 2019, 2019, 5921503. [Google Scholar] [CrossRef]
- Ebenezer Samuel King, J.P.; Sinha, M.K.; Kumaresan, A.; Nag, P.; Das Gupta, M.; Arul Prakash, M.; Talluri, T.R.; Datta, T.K. Cryopreservation Process Alters the Expression of Genes Involved in Pathways Associated with the Fertility of Bull Spermatozoa. Front. Genet. 2022, 13, 1025004. [Google Scholar] [CrossRef]
- Pimprasert, M.; Kheawkanha, T.; Boonkum, W.; Chankitisakul, V. Influence of Semen Collection Frequency and Seasonal Variations on Fresh and Frozen Semen Quality in Thai Native Roosters. Animals 2023, 13, 573. [Google Scholar] [CrossRef]
- Tăpăloagă, P.-R.; Gheorghe-Irimia, R.A.; Tăpăloagă, T. Genomic Precision in Animal Reproduction: A Pathway to Enhanced Breeding Programs. Rev. Rom. Med. Vet. 2023, 33, 79–84. [Google Scholar]
- Rather, H.A.; Kumaresan, A.; Nag, P.; Kumar, V.; Nayak, S.; Batra, V.; Ganaie, B.A.; Baithalu, R.K.; Mohanty, T.K.; Datta, T.K. Spermatozoa Produced during Winter Are Superior in Terms of Phenotypic Characteristics and Oviduct Explants Binding Ability in the Water Buffalo (Bubalus bubalis). Reprod. Domest. Anim. 2020, 55, 1629–1637. [Google Scholar] [CrossRef]
- Trzcińska, M.; Samiec, M.; Duda, M. Creating Ex Situ Protected Bioreservoirs as a Powerful Strategy for the Reproductive Biotechnology-Mediated Rescue of Threatened Polish Livestock Breeds. Agriculture 2023, 13, 1426. [Google Scholar] [CrossRef]
- Delgado Bermejo, J.V.; Martínez Martínez, M.A.; Rodríguez Galván, G.; Stemmer, A.; Navas González, F.J.; Camacho Vallejo, M.E. Organization and Management of Conservation Programs and Research in Domestic Animal Genetic Resources. Diversity 2019, 11, 235. [Google Scholar] [CrossRef]
- Li, W.; Gao, C.; Cai, Z.; Yan, S.; Lei, Y.; Wei, M.; Sun, G.; Tian, Y.; Wang, K.; Kang, X. Assessing the Conservation Impact of Chinese Indigenous Chicken Populations between Ex-Situ and In-Situ Using Genome-Wide SNPs. J. Integr. Agric. 2024, 23, 975–987. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wang, S.; Zhang, H.; Sun, M.; Wang, J.; Wu, K. Conservation of Genomic Diversity and Breed-Specific Characteristics of Domestic Chickens in China. Genet. Sel. Evol. 2020, 22, 92. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, P.; Trivedi, M.M.; Modi, R.J.; Patel, Y.G. A Look at Genomic Selection Techniques for Climate Change Adaptation and Production in Livestock. JSRR 2024, 30, 427–436. [Google Scholar] [CrossRef]
- Biscarini, F.; Mastrangelo, S.; Catillo, G.; Senczuk, G.; Ciampolini, R. Insights into Genetic Diversity, Runs of Homozygosity and Heterozygosity-Rich Regions in Maremmana Semi-Feral Cattle Using Pedigree and Genomic Data. Animals 2020, 10, 2285. [Google Scholar] [CrossRef] [PubMed]
- Szmatoła, T.; Gurgul, A.; Jasielczuk, I.; Ząbek, T.; Ropka-Molik, K.; Litwińczuk, Z.; Bugno-Poniewierska, M. A Comprehensive Analysis of Runs of Homozygosity of Eleven Cattle Breeds Representing Different Production Types. Animals 2019, 9, 1024. [Google Scholar] [CrossRef] [PubMed]
- Selokar, N.L.; Sharma, P.; Saini, M.; Sheoran, S.; Rajendran, R.; Kumar, D.; Sharma, R.K.; Motiani, R.K.; Kumar, P.; Jerome, A.; et al. Successful Cloning of a Superior Buffalo Bull. Sci. Rep. 2019, 9, 11366. [Google Scholar] [CrossRef] [PubMed]
- Fatira, E.; Havelka, M.; Labbé, C.; Depincé, A.; Pšenička, M.; Saito, T. A Newly Developed Cloning Technique in Sturgeons; an Important Step towards Recovering Endangered Species. Sci. Rep. 2019, 9, 10453. [Google Scholar] [CrossRef]
- Carolino, N.; Vitorino, A.; Carolino, I.; Pais, J.; Henriques, N.; Silveira, M.; Vicente, A. Genetic Diversity in the Portuguese Mertolenga Cattle Breed Assessed by Pedigree Analysis. Animals 2020, 10, 1990. [Google Scholar] [CrossRef]
- Markov, N.; Stoycheva, S.; Hristov, M.; Mondeshka, L. Digital Management of Technological Processes in Cattle Farms: A Review. J. Cent. Eur. Agric. 2022, 23, 486–495. [Google Scholar] [CrossRef]
- Yadav, A.; Shivani, S.; Manda, V.K.; Sangwan, V.; Demkiv, A. Blockchain Technology for Ecological and Environmental Applications. EQ 2024, 35, 91–102. [Google Scholar] [CrossRef]
- Kamble, S.S.; Gunasekaran, A.; Sharma, R. Modeling the Blockchain Enabled Traceability in Agriculture Supply Chain. Int. J. Inf. Manag. 2020, 52, 101967. [Google Scholar] [CrossRef]
- Gao, J.; Sun, L.; Zhang, S.; Xu, J.; He, M.; Zhang, D.; Wu, C.; Dai, J. Screening Discriminating SNPs for Chinese Indigenous Pig Breeds Identification Using a Random Forests Algorithm. Genes 2022, 13, 2207. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, W.; Falzon, G.; Kwan, P.; Guo, L.; Sun, Z.; Li, C. Livestock Classification and Counting in Quadcopter Aerial Images Using Mask R-CNN. Int. J. Remote Sens. 2020, 41, 8121–8142. [Google Scholar] [CrossRef]
- Lee, W.; Ham, Y.; Ban, T.-W.; Jo, O. Analysis of Growth Performance in Swine Based on Machine Learning. IEEE Access 2019, 7, 161716–161724. [Google Scholar] [CrossRef]
- Seo, D.; Cho, S.; Manjula, P.; Choi, N.; Kim, Y.-K.; Koh, Y.J.; Lee, S.H.; Kim, H.-Y.; Lee, J.H. Identification of Target Chicken Populations by Machine Learning Models Using the Minimum Number of SNPs. Animals 2021, 11, 241. [Google Scholar] [CrossRef]
- Darmawan, H.; Chang, H.-L.; Wu, H.-H. A Community-Based Breeding Program as a Genetic Resource Management Strategy of Indonesian Ongole Cattle. Sustainability 2023, 15, 6013. [Google Scholar] [CrossRef]
- Wurzinger, M.; Gutiérrez, G.A.; Sölkner, J.; Probst, L. Community-Based Livestock Breeding: Coordinated Action or Relational Process? Front. Vet. Sci. 2021, 8, 613505. [Google Scholar] [CrossRef]
- Juvančič, L.; Slabe-Erker, R.; Ogorevc, M.; Drucker, A.G.; Erjavec, E.; Bojkovski, D. Payments for Conservation of Animal Genetic Resources in Agriculture: One Size Fits All? Animals 2021, 11, 846. [Google Scholar] [CrossRef]
- Gicquel, E.; Boettcher, P.; Besbes, B.; Furre, S.; Fernández, J.; Danchin-Burge, C.; Berger, B.; Baumung, R.; Feijóo, J.R.J.; Leroy, G. Impact of Conservation Measures on Demography and Genetic Variability of Livestock Breeds. Animal 2020, 14, 670–680. [Google Scholar] [CrossRef]
- Mongke, T.; Budsuren, U.; Tiemuqier, A.; Bozlak, E.; Wallner, B.; Dulamsuren, S.; Daidiikhuu, D.; Amgalan, S.; An, T.; Mongkejargal, B.; et al. Genomic Conservation of Mongolian Horses Promoted by Preservation of the Intangible Cultural Heritage of Naadam in Mongolia. Conserv. Lett. 2024, 17, e13019. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, H.; Qadri, Q.R.; Wang, Q.; Pan, Y.; Su, G. Long-Term Impact of Conventional and Optimal Contribution Conservation Methods on Genetic Diversity and Genetic Gain in Chinese Indigenous Pig Breeds. Heredity 2021, 127, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Baumung, R.; Boettcher, P.; Scherf, B.; Besbes, B.; Leroy, G. Monitoring and Progress in the Implementation of the Global Plan of Action on Animal Genetic Resources. Sustainability 2021, 13, 775. [Google Scholar] [CrossRef]
- Abu, E. Review on Population Status and Conservation Activity of Indigenous Cattle Breeds of Ethiopia. JBAH 2021, 4, 32–39. [Google Scholar] [CrossRef]
- Roessler, R. Selection Decisions and Trait Preferences for Local and Imported Cattle and Sheep Breeds in Peri-/Urban Livestock Production Systems in Ouagadougou, Burkina Faso. Animals 2019, 9, 207. [Google Scholar] [CrossRef]
- Houessou, S.O.; Dossa, L.H.; Diogo, R.V.C.; Ahozonlin, M.C.; Dahouda, M.; Schlecht, E. Confronting Pastoralists’ Knowledge of Cattle Breeds Raised in the Extensive Production Systems of Benin with Multivariate Analyses of Morphological Traits. PLoS ONE 2019, 14, e0222756. [Google Scholar] [CrossRef]
- Kaumbata, W.; Nakimbugwe, H.; Nandolo, W.; Banda, L.J.; Mészáros, G.; Gondwe, T.; Woodward-Greene, M.J.; Rosen, B.D.; Van Tassell, C.P.; Sölkner, J.; et al. Experiences from the Implementation of Community-Based Goat Breeding Programs in Malawi and Uganda: A Potential Approach for Conservation and Improvement of Indigenous Small Ruminants in Smallholder Farms. Sustainability 2021, 13, 1494. [Google Scholar] [CrossRef]
- Okpeku, M.; Ogah, D.M.; Adeleke, M.A. A Review of Challenges to Genetic Improvement of Indigenous Livestock for Improved Food Production in Nigeria. AJFAND 2019, 19, 13959–13978. [Google Scholar] [CrossRef]
- Giovannini, S.; Chessari, G.; Riggio, S.; Marletta, D.; Sardina, M.T.; Mastrangelo, S.; Sarti, F.M. Insight into the Current Genomic Diversity, Conservation Status and Population Structure of Tunisian Barbarine Sheep Breed. Front. Genet. 2024, 15, 1379086. [Google Scholar] [CrossRef]
- Velado-Alonso, E.; Gómez-Sal, A.; Bernués, A.; Martín-Collado, D. Disentangling the Multidimensional Relationship between Livestock Breeds and Ecosystem Services. Animals 2021, 11, 2548. [Google Scholar] [CrossRef]
- Santillo, A.; Della Malva, A.; Albenzio, M. Preserving Biodiversity of Sheep and Goat Farming in the Apulia Region. Animals 2025, 15, 1610. [Google Scholar] [CrossRef]
- Lauvie, A.; Alexandre, G.; Angeon, V.; Couix, N.; Fontaine, O.; Gaillard, C.; Meuret, M.; Mougenot, C.; Moulin, C.-H.; Naves, M.; et al. Is the Ecosystem Services Concept Relevant to Capture the Multiple Benefits from Farming Systems Using Livestock Biodiversity? A Framework Proposal. GenResJ 2023, 4, 15–28. [Google Scholar] [CrossRef]
- Leroy, G.; Boettcher, P.; Besbes, B.; Peña, C.R.; Jaffrezic, F.; Baumung, R. Food Securers or Invasive Aliens? Trends and Consequences of Non-Native Livestock Introgression in Developing Countries. Glob. Food Secur. 2020, 26, 100420. [Google Scholar] [CrossRef]
- Arndt, S.S.; Goerlich, V.C.; Van Der Staay, F.J. A Dynamic Concept of Animal Welfare: The Role of Appetitive and Adverse Internal and External Factors and the Animal’s Ability to Adapt to Them. Front. Anim. Sci. 2022, 3, 908513. [Google Scholar] [CrossRef]
- Chazhaev, M. Economic Potential of Biotechnologies: Challenges and Windows of Opportunity. BIO Web Conf. 2023, 76, 10002. [Google Scholar] [CrossRef]
- Al Hakim, R.R.; Saputro, T.A. Development of Somatic Cell Nuclear Transfer Biotechnology for Cloning of Animals: A Mini-Review. J. Biol. Instr. 2021, 1, 20–27. [Google Scholar] [CrossRef]
- Gouveia, C.; Huyser, C.; Egli, D.; Pepper, M.S. Lessons Learned from Somatic Cell Nuclear Transfer. Int. J. Mol. Sci. 2020, 21, 2314. [Google Scholar] [CrossRef] [PubMed]
- Alberio, R.; Wolf, E. Nuclear Transfer and the Development of Genetically Modified/Gene Edited Livestock. Reproduction 2021, 162, F59–F68. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Liu, J. Formality or Reality: Student Teachers’ Experiences of Ethical Dilemmas and Emotions During the Practicum. Front. Psychol. 2022, 13, 870069. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, Y.; Liao, Z.; Xu, Y.; Wang, Y.; Wang, Z.; Jiang, X.; Li, Y.; Lu, Y.; Nie, Y.; et al. Cloning of a Gene-Edited Macaque Monkey by Somatic Cell Nuclear Transfer. Natl. Sci. Rev. 2019, 6, 101–108. [Google Scholar] [CrossRef]
- Klinger, B.; Schnieke, A. Twenty-Five Years after Dolly—How Far Have We Come? Reproduction 2021, 162, F1–F10. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Li, Z.-K.; Wang, L.-B.; Liu, C.; Sun, X.-H.; Feng, G.-H.; Wang, J.-Q.; Li, Y.-F.; Qiao, L.-Y.; Nie, H.; et al. Overcoming Intrinsic H3K27me3 Imprinting Barriers Improves Post-Implantation Development after Somatic Cell Nuclear Transfer. Cell Stem Cell 2020, 27, 315–325.e5. [Google Scholar] [CrossRef]
- Yeung, T.; Bhatia, S.K. Embryo Editing Technology and Its Effect on Conceptions of Motherhood. In Proceedings of the 8th World Congress on New Technologies, ICBB 005, Prague, Czech Republic, 3–5 August 2022; pp. 1–8. [Google Scholar] [CrossRef]
- Peng, Y.; Lv, J.; Ding, L.; Gong, X.; Zhou, Q. Responsible Governance of Human Germline Genome Editing in China. Biol. Reprod. 2022, 107, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Qiu, R. Ethical and Regulatory Issues in Human Gene Editing: Chinese Perspective. Biotech. Appl. Biochem. 2020, 67, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Astarăstoae, V.; Ioan, B.G.; Rogozea, L.M.; Hanganu, B. Advances in Genetic Editing of the Human Embryo. Am. J. Ther. 2023, 30, e126–e133. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Ranisch, R. Beyond Safety: Mapping the Ethical Debate on Heritable Genome Editing Interventions. Humanit. Soc. Sci. Commun. 2022, 9, 139. [Google Scholar] [CrossRef]
- Abuhammad, S.; Khabour, O.F.; Alzoubi, K.H. Researchers Views about Perceived Harms and Benefits of Gene Editing: A Study from the MENA Region. Heliyon 2021, 7, e06860. [Google Scholar] [CrossRef]
- Zhang, D.; Hussain, A.; Manghwar, H.; Xie, K.; Xie, S.; Zhao, S.; Larkin, R.M.; Qing, P.; Jin, S.; Ding, F. Genome Editing with the CRISPR-Cas System: An Art, Ethics and Global Regulatory Perspective. Plant Biotechnol. J. 2020, 18, 1651–1669. [Google Scholar] [CrossRef]
- Ammari, A.A.; ALGhadi, M.G.; Amran, R.A.; Al Malahi, N.M.; Alhimaidi, A.R. Interspecific Nuclear Transfer Blastocysts Reconstructed from Arabian Oryx Somatic Cells and Domestic Cow Ooplasm. Vet. Sci. 2022, 10, 17. [Google Scholar] [CrossRef]
- Cwik, B. Intergenerational Monitoring in Clinical Trials of Germline Gene Editing. J. Med. Ethics 2020, 46, 183–187. [Google Scholar] [CrossRef]
- Konno, S.; Wakayama, S.; Ito, D.; Kazama, K.; Hirose, N.; Ooga, M.; Wakayama, T. Removal of Remodeling/Reprogramming Factors from Oocytes and the Impact on the Full-Term Development of Cloned Embryos. Development 2020, 147, dev190777. [Google Scholar] [CrossRef] [PubMed]
- Diana, I.M. Ethics in Gene Editing. Nat. Sci. Eng. Technol. J. 2021, 1, 12–16. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, W.; Liu, W.; Kou, X.; Zhao, Y.; Liang, Z.; Wang, L.; Zhang, Z.; Xiao, J.; Gao, R.; et al. Differential Transcriptomes and Methylomes of Trophoblast Stem Cells From Naturally-Fertilized and Somatic Cell Nuclear-Transferred Embryos. Front. Cell Dev. Biol. 2021, 9, 664178. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, D.; Soudré, A.; Yougbaré, B.; Ouédraogo-Koné, S.; Zoma-Traoré, B.; Khayatzadeh, N.; Traoré, A.; Sanou, M.; Mészáros, G.; Burger, P.A.; et al. Genetic Improvement of Local Cattle Breeds in West Africa: A Review of Breeding Programs. Sustainability 2021, 13, 2125. [Google Scholar] [CrossRef]
- Mapiye, C.; Chikwanha, O.C.; Chimonyo, M.; Dzama, K. Strategies for Sustainable Use of Indigenous Cattle Genetic Resources in Southern Africa. Diversity 2019, 11, 214. [Google Scholar] [CrossRef]
- Smith, M.; Dawson, J.C. Participatory Plant Breeding Programs to Optimize Use of Crop Genetic Resources; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; pp. 251–270. [Google Scholar]
- Puneeth, G.M.; Deepak, D.A.; Sneha, G.R.; Gowthami, R.; Chander, S.; Bhatt, K.C.; Vasudeva, R.; Archak, S. A Review of Landraces’ On-Farm Conservation, Practices and Management: An Indian Perspective. J. Sci. Res. Rep. 2024, 30, 910–923. [Google Scholar] [CrossRef]
- Korir, L.; Manning, L.; Moore, H.L.; Lindahl, J.F.; Gemechu, G.; Mihret, A.; Berg, S.; Wood, J.L.N.; Nyokabi, N.S. Adoption of Dairy Technologies in Smallholder Dairy Farms in Ethiopia. Front. Sustain. Food Syst. 2023, 7, 1070349. [Google Scholar] [CrossRef]
- Mutenje, M.; Chipfupa, U.; Mupangwa, W.; Nyagumbo, I.; Manyawu, G.; Chakoma, I.; Gwiriri, L. Understanding Breeding Preferences among Small-Scale Cattle Producers: Implications for Livestock Improvement Programmes. Animal 2020, 14, 1757–1767. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tăpăloagă, D.; Gheorghe-Irimia, R.-A.; Șonea, C.; Ilie, L.; Ciocîrlie, N.; Tăpăloagă, P.-R. Balancing Tradition and Innovation: A 5-Year Review of Modern Approaches to Livestock Breed Conservation. Agriculture 2025, 15, 1855. https://doi.org/10.3390/agriculture15171855
Tăpăloagă D, Gheorghe-Irimia R-A, Șonea C, Ilie L, Ciocîrlie N, Tăpăloagă P-R. Balancing Tradition and Innovation: A 5-Year Review of Modern Approaches to Livestock Breed Conservation. Agriculture. 2025; 15(17):1855. https://doi.org/10.3390/agriculture15171855
Chicago/Turabian StyleTăpăloagă, Dana, Raluca-Aniela Gheorghe-Irimia, Cosmin Șonea, Lucian Ilie, Nicoleta Ciocîrlie, and Paul-Rodian Tăpăloagă. 2025. "Balancing Tradition and Innovation: A 5-Year Review of Modern Approaches to Livestock Breed Conservation" Agriculture 15, no. 17: 1855. https://doi.org/10.3390/agriculture15171855
APA StyleTăpăloagă, D., Gheorghe-Irimia, R.-A., Șonea, C., Ilie, L., Ciocîrlie, N., & Tăpăloagă, P.-R. (2025). Balancing Tradition and Innovation: A 5-Year Review of Modern Approaches to Livestock Breed Conservation. Agriculture, 15(17), 1855. https://doi.org/10.3390/agriculture15171855







