Abstract
Common bean (Phaseolus vulgaris L.), the world’s most widely grown legume crop, is not only of great commercial importance but is also a vital smallholder crop in low-to-medium-income countries. In sub-Saharan Africa common bean provides consumers with a major proportion of their dietary protein and micronutrients. However, productivity is constrained by viruses, particularly those vectored by aphids and whiteflies, and problems are further compounded by seed-borne transmission. We describe common bean’s major viral threats including the aphid-transmitted RNA viruses bean common mosaic virus and bean common mosaic necrosis virus, and the whitefly-transmitted begomoviruses bean golden mosaic virus and bean golden yellow mosaic virus and discuss how high-throughput sequencing is revealing emerging threats. We discuss how recent work on indirect and direct viral ‘manipulation’ of vector behaviour is influencing modelling of viral epidemics. Viral extended phenotypes also modify legume interactions with beneficial organisms including root-associated microbes, pollinators and the natural enemies of vectors. While problems with common bean tissue culture have constrained transgenic and gene editing approaches to crop protection, topical application of double-stranded RNA molecules could provide a practical protection system compatible with the wide diversity of common bean lines grown in sub-Saharan Africa.
1. Introduction: Viruses Are Major Threats to Common Bean, but Are Also Useful Tools for Improved Understanding of This Vital Crop
Common bean (Phaseolus vulgaris L.) is the most widely planted legume in the world. A review published in 2006 reported common bean was cultivated across 27 million hectares across the Americas (its centre of domestication), Europe, Africa and Asia [1]. According to recent figures from the Food and Agriculture Organisation (FAO), the area used for common bean cultivation has expanded and now likely exceeds 34.5 million hectares [2,3]. Common bean is of special importance for smallholder farmers across sub-Saharan Africa, where growing and marketing beans provides income that benefits healthcare and education for rural and peri-urban families [4]. Furthermore, for 200 million African citizens common bean provides a far cheaper source of protein than meat and a vital supply of micronutrients (iron and zinc) in the diet [5,6]. These micronutrients are of critical dietary importance in a region where anaemia is prevalent [7]. Unfortunately, much of the iron and zinc present in bean is chelated by phytic acid and indigestible by humans [5,6]. Thus, an important aim of current breeding programmes is to decrease phytate levels [6].
Compared to other crops, common bean and other legumes are environmentally ‘friendly’ for at least two reasons. Firstly, legume cultivation is associated with the lowest rates of greenhouse gas (GHG) emission per unit of nutritional protein and energy produced for any agricultural product [8,9]. Secondly, legumes require fewer inputs since their roots can be colonised by dinitrogen-fixing bacteria (‘rhizobia’) and simultaneously by phosphate-mining arbuscular mycorrhizal fungi; mutualistic microorganisms that synergistically improve legume growth [10]. Additionally, because common bean and other legumes enrich fixed nitrogen in the rhizosphere, their use as intercrops enhances growth of neighbouring plants, making them sustainable mainstays for smallholder agriculture in east and central Africa [5,11], as well as important rotation crops for agriculture at larger scales [12,13].
Common bean production is constrained by a range of abiotic and biotic stresses. Increasing incidences of drought due to a warming climate may exacerbate pre-existing problems and lead to further decreases in common bean yield and nutritional quality, with some of the worst effects predicted to occur in Africa [14]. Indeed, climate warming on its own may render some regions no longer able to support common bean cultivation [14]. Viruses are among the most damaging pathogens of common bean. The effects of viruses on their hosts, seed-borne transmission rates and the geographical ranges, behaviour, physiology and fecundity of viruses’ invertebrate vectors (predominantly aphids and whiteflies) may also be affected in various ways by changes in temperature, humidity and atmospheric CO2 concentration [15,16,17,18,19,20]. Thus, the breeding of common bean lines for enhanced climate-resilience will need to account for changed or novel impacts of pests and pathogens.
Viruses have long been recognised as important common bean pathogens [1]. Among the best studied positive-sense single stranded RNA viruses of common bean are the potyviruses bean common mosaic virus (BCMV, Potyvirus phaseovulgaris), bean common mosaic necrosis virus (BCMNV, Potyvirus phaseoli) [21], bean yellow mosaic virus (BYMV, Potyvirus phaseoluteum) [1], and the cucumovirus cucumber mosaic virus (CMV, Cucumovirus CMV) [1,22]. These viruses are vectored by aphids and can also be transmitted vertically though seed. DNA viruses, most notably begomoviruses (viruses in the Family Geminiviridae with single-stranded circular DNA genomes, of one or two genomic components) such as bean golden mosaic virus (BGMV, Bean golden mosaic virus), which are transmitted by members of the whitefly Bemisia tabaci species complex, are also problems for common bean cultivation, but predominantly in the Americas [23].
Currently, the aphid- and seed-transmitted RNA viruses BCMV and BCMNV still present the main challenges to common bean production in Africa, but novel CMV strains are appearing [24,25,26,27,28]. Meanwhile, reports from Tanzania and Zambia indicate that the beetle-transmitted southern bean mosaic virus (SBMV, Sobemovirus SBMV) may be an increasing threat [27,29,30]. Virus surveys of crops in Africa have increasingly used high-throughput sequencing (HTS) methods such as Illumina and Nanopore. HTS is valuable not only for its ability to monitor the incidence and distribution of established viruses, but also because it enables discovery of emerging, sometimes previously unknown viruses, as well as other novel pathogens [30]. HTS has vastly expanded the number and variety of viruses known to infect common bean under field conditions. At least 83 viruses naturally infect this crop, including viral species belonging to 24 genera within 12 viral families [31]. Interestingly, HTS use has led to increasing reports of detection of the endornaviruses Phaseolus vulgaris endornavirus 1 (PvEV1), PvEV2 and PvEV3 in common bean lines deployed in Africa [26,32,33,34,35,36]. These vertically transmitted RNA viruses cause no obvious disease symptoms, and it has been suggested they may even be beneficial (Section 5).
In this article we discuss recent findings on the interactions of common bean with viruses. We will explore the current major and emerging viral threats to common bean and possible solutions, with particular emphasis on African agriculture (Section 2). However, we also consider how the study of virus-common bean interactions has revealed some unexpected and fascinating insights into the fundamental ecology and evolution of virus–plant–insect interactions (Section 3 and Section 4), some of which may provide useful avenues of further research for the improvement of resilience and yield in this important crop (Section 5).
2. The Main Viral Threats to Common Bean
Common bean can be experimentally infected by around 270 viruses with at least 83 occurring in natural infections [31,37,38]. The viral pathogens that are currently the major problems for common bean cultivation are the RNA viruses BCMV, BCMNV, CMV, and cowpea mild mottle virus (CPMMV, a species within the genus Carlavirus), and the DNA viruses BGMV and bean golden yellow mosaic virus (BGYMV, Bean golden yellow mosaic virus). However, there are also novel viral and suspected viral threats emerging. In this section, we briefly review these viruses, roughly in order of their importance as crop pathogens.
2.1. Bean-Infecting Potyviruses
The potyviruses BCMV, BCMNV are vectored by aphids in a non-persistent manner (Section 3), but they are also transmitted through infected seed and by wounding. This combination of horizontal and vertical transmission routes complicates containment and management efforts [21]. Another potyvirus, BYMV, was first discovered in common bean and causes problems in many pulse crops [39]. BYMV causes serious losses in common bean production in the Americas [1], but from the available literature it does not appear to be as important a problem for African common bean cultivation.
Severe BCMV and BCMNV infection symptoms in common bean include mosaic patterns of light and dark green on leaves, accompanied often by leaf curling, blistering, and distortion [21]. Infected plants typically exhibit stunted growth, delayed flowering, but symptom severity varies depending on varietal properties, environmental conditions, and the developmental stage at which plants become infected (Figure 1) [1,21]. BCMNV infection is characterised by induction of systemic necrosis in common bean varieties carrying the dominant I gene for resistance against BCMV (black root disease) [21]. Certain BCMV strains and BCMV/BCMNV recombinants can also induce necrosis in plants carrying the I gene, and expression of necrosis is sometimes associated with exposure of infected plants to temperatures greater than 30 °C [40,41]. Modern bean varieties can be bred to contain not only the I gene but also recessive resistance alleles such as bc-3 to provide combined resistance against BCMV and BCMNV (for example, see [42]), with decreased likelihood of black root disease. Given the prevalence of BCMNV in Africa, combining determinants of genetic resistance in this way is extremely valuable. However, the sheer diversity of common bean varieties in terms of, among other things, regional consumer preferences and suitability for agroecological conditions in specific localities makes deployment of lines carrying multiple resistance genes more difficult (for example, see [43] and references therein). This may also present difficulties when devising protection approaches involving genetic modification or gene editing, which are discussed in Section 5.

Figure 1.
Systemic disease symptoms of bean common mosaic virus (BCMV), bean common mosaic necrosis virus (BCMNV) and cucumber mosaic virus (CMV). The appearance of common bean plants of the variety Wairemu Dwarf 10 days following mechanical inoculation on a lower leaf with either a suspension of the indicated virus, or a control solution (Mock). BCMNV can induce vein necrosis on this variety [21], but this was not yet visible. Scale bars are c. 5 cm (Photo credits: Francis O. Wamonje).
2.2. Bean Golden Mosaic Virus and Bean Golden Yellow Mosaic Virus
BGMV and BGYMV are begomoviruses with bipartite single-stranded DNA genomes, and under natural conditions are both vectored by B. tabaci [1,31]. Infected plants exhibit characteristic chlorotic mosaic symptoms, leaf crumpling, and deformation, often accompanied by stunted growth and poor pod development. These symptoms reduce photosynthetic efficiency and reproductive output, resulting in major yield losses that can reach 100% [44]. Both geminiviruses emerged in the Americas where they cause significant crop losses [1,23]. To aid in breeding for resistance, several loci were identified, including the recessive bgm-1 gene and quantitative trait loci such as BGY4.1, BGY7.1, and BGY8.1, which act additively to confer enhanced resistance, and can be combined with resistance to BCMV [45,46]. Marker-assisted selection and genomic tools have been employed to accelerate development of durable regionally adapted cultivars. Nevertheless, the dynamic nature of virus–vector–host interactions, compounded by climate variability, necessitates continued research and the development of adaptive management strategies. Tomato yellow leaf curl virus (TYLCV), a begomovirus that causes serious epidemics in tomato (Solanum lycopersicum) crops, has been identified in common bean in some locations including Greece, Spain and Australia [47,48,49]. To our knowledge, BGMV, BGYMV and TYLCV are not associated with major losses of common bean in Africa. However, recent observations suggest that bean-infecting geminiviruses are emerging in east Africa (Section 2.5).
2.3. Cowpea Mild Mottle Virus and Bean Yellow Disorder Virus
Cowpea mild mottle virus (CPMMV) is a species in the Carlavirus genus of monopartite positive-sense RNA viruses. CPMMV is non-persistently transmitted by B. tabaci (Section 3) to several crops, including common bean [50,51]. First reported in cowpea (Vigna unguiculata), CPMMV is endemic in and probably indigenous to Africa but has spread worldwide [26,27,29,52,53,54]. CPMMV infects many legume crops including groundnut (Arachis hypogaea), but it also infects tomato. This is significant because east and central African smallholders frequently intercrop groundnut and tomato with common bean, and these hosts may provide additional reservoirs for CPMMV. In common bean CPMMV causes a yellow ‘angular’ mosaic, leaf deformation and curling symptoms [52].
Transmitted by B. tabaci in a non-circulative, semi-persistent manner (Section 3) [55], bean yellow disorder virus (BnYDV) is a species (Crinivirus flavibetae) in the Crinivirus genus of bipartite single-stranded positive-sense RNA viruses [56]. BnYDV may be an emerging threat to common bean production in east Africa. Although BnYDV was first detected in Spain [37,38] it was subsequently detected in a survey of common bean plantings in Tanzania [27]. An alarming feature of BnYDV is that it can recombine with other criniviruses, expanding its otherwise restricted host range [57]. BnYDV recombined with lettuce chlorosis virus (LCV), to produce a novel pathogen, LCV-SP, which infects bean but can no longer infect lettuce [37]. Emergence of bean-infecting recombinant criniviruses may increase the difficulty of breeding resistant lines.
2.4. Cucumoviruses
Cucumoviruses have tripartite single-stranded positive-sense RNA genomes [58]. Two are known to infect common bean: CMV and peanut stunt virus (Cucumovirus PSV) [1]. PSV infection of common bean occurs in the Americas [1] and in Europe [59]; however, we are not aware of reports of PSV in common bean in Africa. This contrasts with CMV, which has been detected in surveys of common bean and other grain legumes across the continent [26,27,29,53,54]. Common bean is one of >1000 plant species that can host CMV [60]. However, not all CMV strains and isolates are compatible with common bean or other legumes. The ability to infect legumes (or in some cases to elicit defence reactions) depends on specific amino acid residues within the 2a RNA-directed RNA polymerase protein encoded by CMV RNA2 [22,61]. Residues in the 1a replicase component encoded CMV RNA1 determine seed transmissibility of CMV in common bean [62].
Seed transmission is an important factor in CMV epidemiology; the phenomenon enables CMV to survive when its hosts are not actively growing, when its vectors are absent (during winter for example), and it allows longer distance transport of the virus and introduction of novel strains into new areas when seed is traded [63]. Even when seed infection rates are low, a few infected seedlings can act as inoculum sources for vectors, which then initiate an epidemic [63]. The insect vectors of CMV (and PSV) are aphids, which transmit the virus in the non-persistent manner (Section 3), and at least 80 aphid species vector CMV [64]. CMV epidemics in common bean have been studied in greatest detail in the US where virus in-field incidence can reach 100%, with vector dynamics being the dominant factor driving epidemic development [65]. Typically, CMV infection of common bean results in inhibited growth and leaf distortion (Figure 1). However, disease severity and the extent of losses due to CMV are influenced also by the environment, the characteristics of the common bean varieties deployed, the properties of the CMV strain, and whether it is parasitised by a satellite RNA.
Certain isolates of CMV and PSV act as ‘helpers’ for satellite RNAs [66]. These non-coding RNAs depend upon a virus (the helper) for replication and encapsidation and that can modify disease symptoms, sometimes ameliorating disease, or, in some cases, exacerbating disease severity to the point of inducing systemic necrosis and complete crop loss [66]. Isolates of CMV and PSV that support satellite RNAs occur in common bean [67,68], and in surveys of viruses infecting legume crops in Greece it was found that common bean acts as a reservoir for a CMV-satellite RNA combination that induces necrosis in neighbouring tomato plants [69]. At this point, we are not aware that any CMV-satellite RNA combinations have been detected in Africa. However, it seems unlikely that such combinations are not present. It is plausible that their emergence would not only increase the disease threat to common bean, but also to other CMV hosts such as tomato, with which common bean is frequently intercropped.
Mutuku and colleagues [26] found a CMV isolate in common bean in Kenya (CMV-Kirinyaga) that was a reassortant strain comprising an RNA 3 arising from a different CMV subgroup (Subgroup IB) than its RNAs 1 and 2 (Subgroup IA), and with all three RNA components having sequence similarities to corresponding RNAs of Asian CMV strains. Such inter-subgroup reassortant viruses have been associated with significant CMV outbreaks in Spanish tomato growing areas [70]. The discovery of CMV-Kirinyaga in Kenya raises two concerning possibilities. Firstly, that non-indigenous common bean-infecting CMV strains are entering Africa, presumably through increasing intercontinental trade in seed. Secondly, that entry into Africa of previously geographically separated strains of CMV is creating the conditions for mixed infections and the genesis of reassortant viruses with completely novel and potentially highly destructive properties.
2.5. Emerging and Unassigned Viral-like Diseases in Common Bean
Recent surveys in east Africa using HTS [71] and serological methods [53] detected viruses never seen before in common bean, and which may be emerging threats to the crop. These include pararetroviruses, nicked double-stranded circular DNA viruses that replicate using reverse transcriptase [71], and the RNA virus maize chlorotic mosaic virus (MCMV: Genus Machlomovirus) [53]. The apparent discovery of MCMV infecting common bean may be of significance from the perspective of food security since this virus, which was somehow introduced from Asia, synergises the effects of sugarcane mosaic virus and other potyviruses infecting maize to induce maize lethal necrosis, a disease that has spread to epidemic scales across east and central Africa [72,73,74]. It is important to confirm the results of Osogo and colleagues [53] because maize and common bean are often intercropped. If common bean provides an MCMV reservoir, this may facilitate maize lethal necrosis disease in neighbouring maize plants.
Since 2023, there has been an emergence of golden yellow mosaic-like symptoms observed on plants in common bean breeding nurseries across Kenya, Ethiopia, Rwanda, and Uganda (pers. comm. W.A., breeders affiliated to the Pan-African Bean Research Alliance) (Figure 2). Incidences of these symptoms appear to be on the increase, raising concerns of a novel pathogen threatening regional bean production. Notably, in early 2025, similar symptoms were observed in a farmer’s field in Kenya. Affected plants were frequently colonised by B. tabaci (Figure 2), suggesting the possible involvement of a whitefly-transmitted geminivirus, such as BGYMV. This is particularly significant as BGYMV, a major pathogen in the Americas [23], has not yet been reported in African bean plantings. This requires urgent investigation to identify the causal agent and assess its epidemiological implications. The example highlights the need for integrated virological surveillance and mechanistic studies to detect new threats and inform development of sustainable disease management strategies [30].
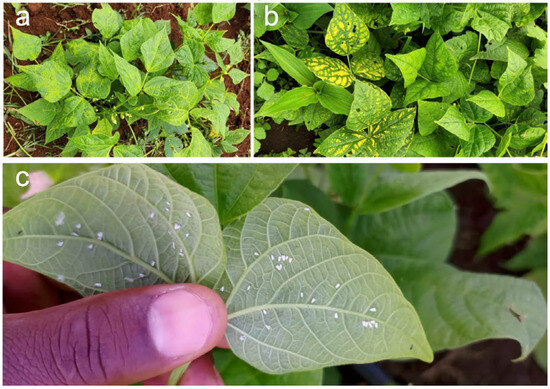
Figure 2.
Golden yellow-like mosaic symptoms observed on plants in a common bean nursery in Kenya at early (a) and late (b) infection stages. The golden yellow mosaic virus-like disease is associated with whitefly colonisation (c), shown here on the abaxial surfaces of plants in an on-farm demonstration plot in Kenya (Photo credits: Warren Arinaitwe).
3. Viral Modification of Vector–Host Interactions: Implications for Epidemiology and Control
Viruses that cause major problems for the cultivation of common bean are predominantly, but not exclusively, vectored by whiteflies and aphids, which are hemipterans, i.e., insects with probing mouthparts called stylets [15,21,23]. Although a common approach to controlling these diseases is through the use of insecticides to limit the availability of vectors, this topic is beyond the remit of this article and in this section we discuss the mechanisms of virus transmission by arthropods, and how in some cases viruses appear to ‘manipulate’ plant–vector interactions to enhance their own transmission.
3.1. Modes of Plant Virus Transmission by Arthropod Vectors
Plant viruses are primarily vectored by herbivorous arthropods through two distinct pathways: non-circulative and circulative (Table 1). Non-circulative transmission is classified into non-persistent and semi-persistent modes (Table 1). In the non-persistent mode, exemplified by aphid-mediated transmission of potyviruses (such as BCMV or BCMNV) or CMV, virus acquisition occurs during brief probes by the aphid stylet into epidermal cells of an infected host plant [75,76,77,78]. Electrical penetration graph (EPG) studies of aphid feeding behaviour (Figure 3), and studies of inoculation using CMV modified to express a fluorescent protein, indicated that acquisition of non-persistently transmitted viruses can occur from brief stylet penetrations of just 3–15 s on epidermal cells of infected plants [75,76,77,78]. Following virus acquisition, aphids may retain inoculum for a period lasting only minutes to up to 2–4 h. Viral inoculum is present on the insect stylets only, and there is no circulation within the vector’s body. Vectors are thought to lose all inoculum when they salivate into a new host.

Table 1.
Plant virus transmission by arthropod vectors 1.
Southern bean mosaic virus (SBMV, Genus Sobemovirus), a positive-sense single-stranded RNA virus exhibits semi-persistent transmission by bean leaf beetles (Cerotoma trifurcata) and Mexican bean beetles (Epilachna varivestis) (Family Chrysomelidae). Virions adhere to the beetle foregut with no circulation within the insect body and vectors remain competent to transmit the virus for several hours to days [82,83].
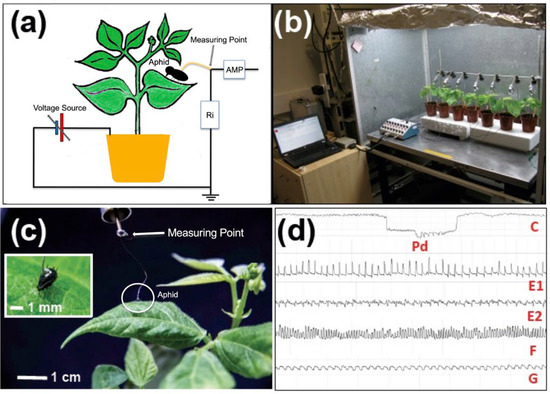
Figure 3.
Overview of the electrical penetration graph (EPG) method for monitoring aphid feeding behaviour. (a) A diagrammatic representation of the electrical penetration graph method showing an aphid attached to a gold wire as part of a DC circuit (Ri, resistor). An amplifier (AMP) provides the output for analysis; nowadays a computer loaded with specialist software. (b) EPG is carried out in a Faraday cage to minimise radiofrequency interference. (c) A black bean aphid (Aphis fabae) feeding on a leaf of a virus-infected common bean plant was attached to the measuring point using a fine gold wire and electrically conductive adhesive. (d) Examples of waveforms produced by EPG corresponding to: C, activity during stylet pathway including a potential drop (Pd) when the stylet punctured a cell membrane; E1 and E2, respectively, saliva secretion into and sap ingestion from the phloem; F, feeding difficulties, and accessing hydration from the xylem (G). Based on [84] (Photo credits: Francis O. Wamonje).
Circulative transmission is classified into propagative and non-propagative modes (Table 1). ‘Propagative’ indicates when a plant virus also replicates within its arthropod vector’s cells. Few such viruses appear to infect common bean, but a rhabdovirus (monopartite negative-sense, single-stranded RNA) and a tospovirus (tripartite negative/ambisense, single-stranded RNA) that replicate, respectively, in their whitefly and thrips vectors have been found associated with bean [23,85,86]. Conversely, persistent ‘non-propagative’ describes transmission of that are unable to infect arthropods, despite which virions enter the vector’s haemocoel, circulate in the haemolymph, before accumulating in the accessory salivary glands, from whence they can be inoculated into plant cells when the vector feeds [87,88]. Persistent non-propagative transmission is exemplified by begomoviruses, including the major bean pathogens BGMV and BGYMV, which are whitefly-transmitted [23,89,90].
3.2. ‘Manipulation Strategies’ in Virus–Vector–Host Interactions Involving Non-Persistently Transmitted Viruses
Plant viruses can modify host–vector interactions in ways that may facilitate onward virus transmission, providing examples of extended phenotypes in which a parasite induces changes in hosts or vectors to benefit the parasite [91]. Plant viruses can influence vector behaviour directly or indirectly. Direct effects on vector behaviour and their interactions with infected versus non-infected plants are best studied for viruses transmitted in circulative/persistent manners, not only when the viruses replicate in the insect vectors, but also for viruses transmitted in the non-propagative mode (reviewed in [92]). It remains unclear how viruses transmitted in the circulative/persistent mode, but which do not replicate in the vector, modify vector preferences, although recent work found there are changes in vector gene expression [93], discussed further in Section 3.3.
A recently discovered mechanism of direct manipulation of a vector, in this case reprogramming of vector development to facilitate longer-range transmission, was revealed by a study of CMV, its Y-satellite RNA and the aphid, M. persicae [94]. During infection of tobacco (Nicotiana tabacum), small RNAs derived from sequences within the Y-satellite RNA accumulated in infected plant tissue and were ingested by aphids. Remarkably, when taken up by the vector, these small RNAs interfered with development of aphid embryos, causing increases in the birth of winged nymphs [94]. Since winged aphids spread viral (and satellite RNA) inoculum over greater distances [95], it appears that the satellite RNA is exerting an extended phenotype on the aphid that potentially ‘pays back’ the helper virus by increasing the likelihood of long-range transmission.
As far as we are aware, for bean-infecting viruses specifically, studies to date have focused on indirect effects on viruses on vector–host interactions. Indirect effects on vectors are mediated by virus-induced changes in arthropod-perceivable cues presented by host plants. These changes in the plant phenotype increase the likelihood of virus acquisition and subsequent dispersal by vectors [96]. A common mechanism involves altering the blends of volatile organic compounds (VOCs) emitted by infected plants, as odour cues can affect aphid host-selection behaviour [97,98]. Studies of bean-infecting viruses and their insect vectors illustrate this clearly. Two aphid species, Aphis fabae (a bean specialist) and M. persicae (a generalist) can efficiently spread BCMV, BCMNV and CMV, although A. fabae predominates in fields of common bean [99]. Analysis by gas chromatography-coupled mass spectrometry showed that common bean plants infected with BCMV, BCMNV or CMV showed significant qualitative changes in VOC profiles, including for compounds that are electrophysiologically active on aphid antennal preparations [100]. Among other effects, virus infection suppressed emission of an aphid-attractive sesquiterpene, α-copaene, and nonanal and octanal were elevated in blends produced by plants infected by BCMV and CMV, while (E)-4,8-dimethyl-1,3,7-nonatriene emission was elevated in the blend emitted by BCMNV-infected plants. Levels of some electrophysiologically active compounds such as (Z)-3-hexenyl acetate were unaffected by virus infection [100].
Decreases in levels of the aphid-attracting compound α-copaene, for example, were consistent with results of settling assays, that showed that aphids were more likely to migrate from infected plants and settle on uninfected plants (thus, more likely to transmit inoculum). But olfactometry assays indicated that changes in VOCs did not solely explain the tendency of aphids to be deterred from settling on infected plants. Using EPG, it was found that on common bean plants infected with CMV, BCMV or BCMNV aphids (both M. persicae and A. fabae) had difficulties with phloem feeding and increased exploratory stylet activity [101]. Despite this, both A. fabae and M. persicae commenced probing more rapidly when placed on infected plants than on uninfected plants. It was concluded that the various effects of these three aphid-transmitted viruses on common bean combined to increase the likelihood that aphids, particularly A. fabae, would acquire inoculum from infected plants but then disperse towards uninfected plants [100,101].
Similar patterns have been seen for other plants infected with viruses that are non-persistently transmitted by aphids. CMV-infected squash and cucumber plants emit VOC blends that attract aphids (M. persicae and A. gossypii). However, parallel biochemical changes in plant tissue render plants distasteful, prompting aphids to disperse after superficial probing, thus facilitating efficient virus spread [102,103]. Work using the model plants Arabidopsis thaliana and N. benthamiana with various CMV strains and the potyviruses turnip mosaic virus (TuMV; Potyvirus rapae) and potato virus Y (PVY; Potyvirus yituberosi) has enabled the detailed dissection of the molecular interactions and biochemical changes governing expression of viral extended phenotypes affecting aphid–host interactions [104,105,106,107,108,109,110,111,112,113,114]. These model systems have provided important mechanistic insights into how aphid-transmitted viruses evoke “attract-and-repel” extended phenotypes (explained further in Section 3.4); however, this level of detail is lacking for key crops including common bean, and this represents an important knowledge gap.
In some cases, viruses that are non-persistently transmitted by aphids induce emission of non-attractive VOC blends and do not cause accumulation of repellent substances. For TuMV in A. thaliana, Casteel and colleagues [106] found that infected plants became more hospitable to M. persicae. Meanwhile, and in contrast to effects seen with cucurbits and A. thaliana, two CMV strains (particularly LS-CMV) decreased the resistance of tobacco plants to M. persicae, increasing survival and fecundity of aphids confined on infected plants. Although CMV infection altered the VOC blend emitted by tobacco, this neither increased nor decreased the plants’ attractiveness to aphids [115,116,117].
It should also be noted that not all aphids respond to virus-induced cues in the same way, and that cues can change over the course of infection. Using CMV-infected A. thaliana, it was found that two crucifer-specialist aphids (Brevicoryne brassicae and Lipaphis erysimi) where less affected by two CMV-induced anti-aphid resistance mechanisms than M. persicae (a generalist aphid) [118]. In tomato (Solanum lycopersicum), CMV induced resistance to aphids, causing them to move from infected to uninfected plants (as in A. thaliana and common bean). However, it was observed that during the pre-symptomatic period, infected plants attracted M. persicae and were susceptible to settlement, but when symptoms appeared the aphids were repelled and preferred to settle on uninfected plants [119]. Thus, CMV-induced attraction and deterrence occurred at distinct points in infection. This contrasts with observations with cucurbits that indicated CMV induced simultaneous production of attractive olfactory cues and deterrent substances in leaves [102,103]. Contrastingly, aphids of the species Macrosiphum euphorbiae (a solanaceous plant specialist) were not attracted to pre-symptomatic tomato plants, although, like M. persicae, they were repelled by symptomatic plants [119]. These studies comparing the responses to virus-infected plants of specialist and non-specialist aphids suggest that a generalist may be more tractable to manipulation by the viral extended phenotype, and this may make it a more efficient vector.
3.3. Manipulation Strategies’ in Other Virus–Vector–Host Interactions
Importantly, indirect manipulation of vector behaviour by viruses through their effects on hosts is neither exclusive to non-persistently transmitted viruses nor to arthropod vectors. Indeed, changes in below-ground VOC emission by N. benthamiana roots attracted trichodorid nematode vectors when plants were infected by the nematode-transmitted virus tobacco rattle virus (Tobravirus tabaci) [120].
Semi-persistently transmitted viruses can also manipulate vectors. SBMV infection results in visually conspicuous yellow chlorotic and mosaic symptoms on infected bean plants, which increase the visual appeal of infected plants to beetle vectors [82]. CPMMV, which is semi-persistently transmitted virus by whiteflies, presents a different form of manipulation, one that also benefits the vector. Transmission efficiency from individual whiteflies can be quite low [121]. Nevertheless, CPMMV-infected plants are not only more attractive to vectors but are also better hosts for the vector. On CPMMV-infected hosts whitefly emergence rates were increased, revealing a virus-induced extended phenotype that increases the whitefly population that in turn increases the probability of virus transmission [121].
Several examples of host–vector manipulation have been investigated for persistently transmitted positive-sense RNA viruses. For example, in the case of M. persicae, which vectors turnip yellows virus (TuYV: Polerovirus TUYV) and potato leafroll virus (Polerovirus PLRV) in the non-propagative circulative persistent mode, acquisition of inoculum modifies some of its behavioural and physiological responses, possibly through effects on the vector’s transcriptome and its complement of small RNAs [93] despite not being truly infected, i.e., in the sense of supporting virus replication [122,123]. These effects include modification of settling preferences on infected versus non-infected plants, feeding patterns and reproduction, and are thought to increase the likelihood of both acquiring and transmitting TuYV [123]. Although the molecular basis of these effects remains to be elucidated, it appears that for TuYV the coat protein is a key factor in engendering changes in both the plant and vector and in increasing access of the vector to phloem tissue to enhance virus acquisition [124].
The effects of some begomoviruses and their satellite DNAs on interactions of their host plants with B. tabaci vectors have been investigated in significant detail. These persistently transmitted viruses increase the fitness of whiteflies settled on infected plants by inhibiting anti-insect defences, which is likely to improve transmission (reviewed in detail by [125]). However, most work has focused on tomato yellow leaf curl virus, tomato yellow leaf curl China virus and the viral DNA satellite βC1 protein [125]. In contrast, little is known of the effects of the major begomoviruses of common bean, BGMV and BGYMV, on vector-host interactions.
3.4. Implications of Virus-Induced Extended Phenotypes for Epidemiology and Disease Control
Understanding viruses’ influence on vector behaviours and how these in turn affect virus transmission dynamics is vital for improvement of viral epidemiology. In particular, it is important for development of models that consider the extended phenotypes that viruses exert on their vectors. Improved modelling will improve our ability to devise means of decreasing outbreak intensity, while minimising the occurrence of unintended consequences, particularly if we want to move beyond the control of arthropod vectors using pesticides. For example, mathematical modelling of intercropping and trap cropping that incorporated vector behaviour indicated that placing attractant companion plants can significantly reduce disease incidence by decoying viruliferous vectors [126]. This suggests there is scope to develop equivalents of the ‘push–pull’ system used to disrupt corn borer infestation of maize plants by surrounding them with plants emitting attractant VOCs. For aphids or whiteflies carrying viruses, this could be done by finding and utilising plants that produce repellent VOCs acting as ‘push’ to deter viruliferous insects from crops, while an attractant plant ‘pulls’ vectors to trap them directly or lure them to decoys. While push–pull has not yet been exploited to protect common bean or to control viral vectors, common bean and soybean have been used for push–pull management of maize against fall armyworm and stemborers, providing a starting point [127,128].
Some newer epidemiological models account for vector behaviour and phenology and increasingly incorporate recent work on virus-induced extended phenotypes, which has made older modelling assumptions, e.g., that acquisition or transmission occur randomly, no longer tenable [129,130]. In recent modelling of non-persistent transmission by aphids, virus-induced expression of attractive olfactory or visual cues together with accumulation of repellent or antifeedant compounds is sometimes referred to as a virally induced ‘attract-and-deter’ extended phenotype [95]. Induction of this extended phenotype is predicted to accelerate virus spread to immediately surrounding plants by -winged (alate) and (the usually much more abundant) non-winged (apterous) morphs [95]. Modelling has also suggested that if aphids can remain infective for more than one stylet probe this may amplify the effects of the ‘attract-and-deter’ phenotype and dramatically increase transmission rates [130].
Persistently transmitted viruses pose another challenge to modelling. These viruses are present long-term in vectors and are sometimes passed down intergenerationally, which might enable populations of viruliferous vectors to persist between cropping seasons. In contrast to non-persistently transmitted viruses, efficient acquisition and transmission of persistently transmitted viruses by vectors require prolonged feeding bouts (Table 1). Whether vectored by aphids or whiteflies, persistently transmitted viruses often suppress plant defences and/or induce host plants to exhibit attractive cues [125,131]. There are two potential consequences of the evocation of such ‘retain’ or ‘attract-and-retain’ extended phenotypes [95]. Firstly, they will increase the likelihood of virus acquisition and inoculation and secondly, they may foster increased vector reproduction. The latter effect has been proposed as the possible genesis of superabundant whitefly populations associated with epidemics of cassava mosaic disease in sub-Saharan Africa [132].
It was thought that induction of ‘retain’ or ‘attract-and-retain’ extended phenotypes by non-persistently transmitted viruses should be deleterious to their transmission. However, as noted in Section 3.2, both CMV and TuMV induce ‘retain’ phenotypes in tobacco and A. thaliana, respectively [106,115]. A possibility considered was that these viruses, or the virus strains used in the experiments, are poorly adapted to the host plants. However, modelling that accounted for aphids’ tendency to produce more alate morphs in response to increased population density [133] suggested that although the ‘retain’ phenotype would discourage rapid localised virus transmission, it would eventually encourage longer distance dissemination of inoculum by flying aphids [95].
Models are becoming increasingly sophisticated with further development and refinement [134]. Although recent models that consider vector phenology and the effects of viral extended phenotypes are compelling, it is necessary to validate these models: first, under controlled conditions, and subsequently in the field. It will also be useful to consider specialist versus non-specialist vectors, which are not always affected in the same ways by virally induced extended phenotypes (Section 3.2). The direct effects of a virus or viral satellite on aphid biology, as seen in the example of satellite RNA-induced wing formation [94] (Section 3.2), are also likely to influence epidemics. Models for whitefly transmitted viruses will need to account for the distinct biology of these vectors (sexually reproducing, egg-producing, winged adults) compared to aphids (predominantly parthenogenetic reproduction with live birth, distinct alate and apterous morphs).
4. Viral Modification of Common Bean Interactions with Beneficial Organisms
A growing body of work indicates that the virally induced extended phenotypes introduced in Section 3.2 influence not only vectors but also affect plant interactions with beneficial organisms. In common bean viral infections affect key biotic partners including nitrogen-fixing microbes, pollinators, and the natural enemies of insect vectors. Understanding these interactions may be critical for improving sustainable management practices for common bean and other important crops.
4.1. Effects on the Rhizobium–Legume Symbiosis
Nitrogen fixation through symbiosis with rhizobia is central to the productivity of P. vulgaris (Section 1). Viral infections of legumes can disrupt nodulation and dinitrogen fixation. Pea necrotic yellow dwarf virus (PNYDV) is a multicomponent single-stranded circular DNA virus classified in the Nanovirus genus. PNYDV infection of broad bean, Vicia faba, severely impairs nodulation and N2 fixation, especially in early-stage infections [135]. SBMV, when introduced very early in plant development (or if present in seed) disrupts common bean root anatomy and decreases the sites available for R. leguminosarum colonisation [136]. Virus-infected legumes develop fewer and smaller root nodules through various mechanisms including interference with plant gene expression and crosstalk between hormone signalling pathways. Modelling of then-available datasets indicated that infection with BCMV and other viruses would decrease the fixed nitrogen benefit in soils gained from growing alfalfa, common bean and red clover [137]. Marchetto and Power [137] attributed these predicted negative effects of virus infection to disruption of plant–rhizobia interactions by the defensive hormone salicylic acid. But this seems an unlikely general explanation as salicylic acid only inhibits nodulation of the indeterminate type [138]. Other hormones including abscisic acid and ethylene may be more important since they are stronger inhibitors of plant–rhizobia interactions [139].
Not all virus–legume interactions inhibit nodulation. Root colonisation of Medicago truncatula plants by Sinorhizobium meliloti is unaffected by alfalfa mosaic virus (AMV: Alfamovirus AMV) infection [140]. Furthermore, rhizobia partially mitigate viral effects, restoring plant growth, immunity, and yield. For example, pea enation mosaic virus (Enamovirus PEMV) infection decreased nodule formation, root and shoot mass in peas (Pisum sativum). However, higher R. leguminosarum abundance mitigated these impacts, especially early in plant growth, indicating that while viral infection inhibits bacteria–plant mutualism, rhizobia can buffer the virus’ effects [139]. It should be noted that this study examined a mixed virus infection since enamovirus infection requires a helper umbravirus [141]. Further evidence that colonisation of legume roots with rhizobia, as well as another beneficial but non-dinitrogen fixing bacterium (Delftia acidovorans), mitigates the effects of viral infection was provided by a study of soybean’s (Glycine max) interaction with bean pod mottle virus (BPMV: Comovirus siliquae) [142]. BPMV infection suppressed wound-induced VOC emission, and this protected beetle vectors (E. varivestis: Section 3.1) from detection by parasitoid wasps. But if the plant roots were colonised by Bradyrhizobium japonicum and D. acidovorans, this negated the effect of BPMV, and parasitoids were able to locate beetles by tracing the plant VOCs produced during feeding [142]. In the AMV–M. truncatula–S. meliloti system it was found that root colonisation with rhizobia did not decrease viral titre but did increase viral fitness by enhancing the AMV-induced ‘attract-and-deter’ extended phenotype (Section 3.4) which promoted virus transmission by the pea aphid Acyrthosiphon pisum [140]. Research on virus–legume–rhizobia interactions focusing on common bean is still relatively scarce and such fascinating three-way interactions merit further investigation.
4.2. Modification of Pollinator-Plant Interactions
While common bean is mainly autogamous, with far less outcrossing compared to V. faba [143], its ability to produce maximal seed yield benefits from visitation by bees [144,145]. Legumes are most effectively pollinated by larger bee types that are powerful enough to mechanically open the flowers’ hull and wing petals to access a nectar reward [146]. In Europe, the main bean pollinators are bumblebees such as Bombus terrestris and B. pascuorum [147], while in sub-Saharan Africa carpenter bees fulfil this role [148] (Figure 4).

Figure 4.
Common bean is predominantly pollinated by larger bees, such as (a) bumblebees (shown, Bombus terrestris on common bean) and (b) carpenter bees (shown, Xylocopa sp.) ranging in size between 14–17 and 13–30 mm [149,150] [Photo credits: Alex M. Murphy (a) and John P. Carr (b)].
Viral infection alters floral traits, and this influences pollinators. This may have influenced plant evolution under natural conditions and in domesticated plants it may affect yield [151,152]. In tomato, CMV exerts an extended phenotype that influences B. terrestris behaviour through changes in VOC emission and possibly other cues. Reacting to these virus-induced cues, bumblebees rescue seed production of CMV-infected tomato plants to levels statistically indistinguishable from those of non-infected plants [151]. The bees also show a 10-fold bias in transferring pollen from flowers of CMV-infected plants to those of non-infected plants [152]. Mathematical modelling indicated that if these phenomena occurred in wild plants under natural conditions this would favour inheritance of alleles for susceptibility and decrease the benefits of resistance alleles in host populations [151,152].
P. vulgaris plants infected with BCMV, BCMNV or CMV show changes in interactions with bumblebees that may improve seed production. Virus-infected plants emit VOC blends that are changed quantitatively and qualitatively; their flowers produce larger volumes of nectar reward with higher sucrose concentrations, and changes in flowering time and modified floral optical properties are likely to attract bumblebees [153,154]. Consistent with these changes, when pollinators were present, seed yield from BCMV-infected common bean plants was increased [154]. Curiously, bumblebee-visited flowers on infected plants gave rise to fewer virus-carrying seeds: whereas ~30% of seed was infected from undisturbed flowers, only ~12% of seeds from flowers visited by bees were infected [147]. Since BCMV transmission can occur via pollen [155,156,157] a possible explanation is that bumblebees importing pollen from uninfected plants diluted infected pollen resulting in decreased seed infection [147]. However, for pollen-transmitted viruses, increased pollinator attraction to infected plants might increase overall vertical virus transmission.
4.3. Influence of Virus Infection of Legumes on the Natural Enemies of Vectors
As described in Section 4.1 for BPMV-infected soybean, the virus damps down host plant responses to herbivory which protects its beetle vectors against oviposition by parasitoid wasps [142]. Overall, however, there appear to be few studies on the effects of plant viruses on the relationships of vectors with their natural enemies in the context of legumes. In non-legume systems, several plant viruses have been shown to alter emission of VOCs and thereby influence natural enemies and their interactions with whiteflies and aphids.
Natural enemies of the whitefly B. tabaci, which vectors a wide range of begomoviruses (Section 2.2), include parasitoid wasps such as Encarsia formosa. The B. tabaci species complex contains many morphologically indistinguishable subspecies or ‘biotypes’. On tomato plants infected with TYLCV, Q biotype whiteflies were significantly more attractive to E. formosa than those of the B biotype, and oviposition took place more frequently on Q biotype individuals. On uninfected plants, wasps showed no discrimination between biotypes and parasitised both equally [158]. The difference in host preference on TYLCV-infected versus uninfected tomato plants is explainable by virus-induced changes in plant VOC emission, which interferes with wasps’ perception of olfactory cues associated with whiteflies and the effects of their feeding activity [158]. The refocusing of parasitoid activity against Q biotype whiteflies, however, appears paradoxical if TYLCV-induced changes in VOC emission represent a virally induced extended phenotype that evolved to promote transmission. This is because Q biotype whiteflies, females especially, are more competent TYLCV vectors than biotype B [159,160]. Does this represent an unsuccessful virally induced phenotype? A different interpretation is that increased attack by wasps motivates the more efficient Q biotype vectors to leave infected plants and disperse to uninfected plants, thereby increasing TYLCV transmission.
Interactions of aphids with parasitoid wasps, including the popular study models Aphidius colemani and A. ervi, are altered on virus-infected plants. Wasps were attracted to VOCs that pepper plants (Capsicum annuum) emit when infected with CMV or PVY, despite no aphid prey being present [161]. This may make sense for the parasitoids since aphids feeding on CMV-infected squash plants are better hosts for A. colemani than aphids on non-infected plants [162]. Scattering of aphids in their attempts to evade parasitoids and predators can result in increased virus transmission, with examples described for both non-circulative transmitted viruses including for BYMV transmission by A. pisum [163] and for persistently transmitted viruses [164,165]. Interestingly, the presence of parasitoids increased birthrates for winged aphids (A. pisum) on V. faba [166], which may drive longer range virus transmission (Section 3.2).
In the case of the aphid predator ladybird beetle Hippodamia convergens, it was found that the chemical cues left in its footprints on Brassica napus leaves caused scattering of aphids (M. persicae), but this did not increase TuMV transmission [167]. In contrast, transmission of broad bean wilt virus 1 (Fabavirus alphaviciae) by M. persicae between pepper plants was promoted by encounter with two-spotted ladybird beetle (Adalia bipunctata) adults, although not significantly by ladybird larvae or larvae of the hoverfly Sphaerophoria rueppellii [168]. It is not clear if virus-induced effects on plants influence aphid hunters like ladybird beetles or hoverflies as much as they do for parasitoids.
Generalisable rules for understanding interactions of plant viruses, their hosts, vectors and their vectors’ enemies remain difficult to formulate. However, to parameterise increasingly realistic and useful models such understanding will be needed. Additional studies will be important to determine whether virus-induced tritrophic interactions operate more widely in legume and other agroecosystems and if they are usable in crop protection.
5. Future Prospects
Controlling and monitoring existing common bean diseases as well as identifying novel threats is important everywhere but is especially critical in sub-Saharan Africa (Section 1). Wamonje [30] has made the case for ensuring that in anticipation of novel and emerging crop pathogens, sub-Saharan Africa needs improved infrastructure for state-of-the-art diagnostics, HTS-based virus discovery and vector biology research to underpin specialist training and inform crop breeding programmes. Other needs include training for epidemiological modellers to tackle vector-borne crop pathogens in the African context. Establishment of clean seed supply chains is also essential in addressing the challenge of seed-transmitted viruses, since contaminated bean seed provides foci for vector-mediated transmission, and this is already an important area of research [13]. The lack of reliable tissue culture and regeneration protocols for common bean stands in the way of both tissue culture-based production of clean planting materials and application of certain biotechnological approaches. This major roadblock to progress will be discussed further.
In sub-Saharan Africa, myriad common bean lines are used due to the agronomic requirements of diverse agroecological conditions and in response to local consumer preferences [43]. This makes traditional common bean breeding challenging and is a factor to be considered in applying biotechnological solutions to protection of this crop. Biotechnological approaches including genetic modification and gene editing may have potential in protecting common bean against viruses and other pathogens and pests in the long term, but their use faces significant technical hurdles.
Genetic modification of common bean plants to produce virus-resistant plants is possible. This was demonstrated by Bonfim and colleagues [169] who induced RNA silencing-mediated resistance to BGMV in a transgenic line of ‘Olathe Pinto’ by constitutive expression of an RNA hairpin construct targeting a sequence in the transcript of the BGMV Rep protein gene, AC1. A newer approach for engineering plants is gene editing, which introduces targeted mutations into the DNA sequences of existing genes. The most common gene editing approach adapts the bacterial CRISPR (clustered regularly interspaced palindromic repeats) antiviral system in which the DNA endonuclease Cas9 (CRISPR-associated 9) or a similar enzyme is directed by a short guide RNA to target a specific DNA sequence. Gene editing allows generation of non-transgenic plants rendered resistant to specific viruses by mutation of plant genes conditioning virus susceptibility. Successful engineering for loss-of-susceptibility was pioneered in cucumber and A. thaliana, and in subsequent studies has been expanded to other cucurbits, tobacco, maize, sugar beet, and cassava [170,171,172,173,174,175,176,177]. The common feature of these loss-of-susceptibility examples is that the editing targets are genes encoding eIF4E-type translation initiation factors that interact with VPg proteins covalently linked to potyvirid genomic RNAs. VPg– eIF4E interactions support potyvirid gene expression, replication and movement. In common bean, the recessive resistance genes bc-u, bc-1, bc-2, and bc-3 (which variously protect against BCMNV or BCMV) encode eIF4E variants [178] and, in principle, gene editing could be used to convert susceptible common bean varieties to resistant by modifying the corresponding virus-supporting alleles. Gene editing can also modify and improve dominant resistance genes [179]. An additional advantage for gene editing is that if plants can be regenerated from single protoplasts there is no need to generate transgenic plants expressing Cas-9 and guide RNA transgenes at the initial stages of the work, since transient expression can be used [179]. Furthermore, the CRISPR-Cas9 system can directly protect plants against DNA viruses. Transgenic tobacco plants expressing Cas-9 and guide RNAs directed against non-coding intergenic regions of geminiviral genomes were protected [180], and in principle this could be used against BGMV and BGYMV in common bean.
Unfortunately, common bean is recalcitrant to tissue culture and even in the most amenable varieties plant regeneration from callus occurs inefficiently [181,182]. Although it is possible to produce BGMV-resistant transgenic plants this appears to have been successful for only one cultivar, ‘Olathe Pinto’ [169]. In that work, transformation efficiency was only 0.66%, and although 18 transgenic lines were produced, only one (line 5.1) gave high levels of resistance against whitefly-mediated transmission of BGMV [169]. This work was a remarkable achievement, especially since common bean has low susceptibility to Agrobacterium tumefaciens [182]. However, until major breakthroughs occur in tissue culture and plant regeneration for common bean, the wider use of genetic modification, gene editing, or meristem tip culture to generate virus-free lineages, remains a distant prospect. Even when biotechnological methods can be incorporated more routinely into the production of new common bean lines, it will be important to be able to pyramid resistance since lines resistant to one pest or pathogen are still vulnerable to others as seen for BGMV-resistant transgenic lines, which remain susceptible to other whitefly transmitted viruses such as CPMMV [183].
A promising approach to inducing resistance against viruses and their vectors utilises RNA silencing but without the need to make transgenic plants. This uses synthetic double-stranded RNA molecules with homology to sequences of the target pest or pathogen that are sprayed on plants [184]. The stability and effectiveness of these molecules can be enhanced using various forms of encapsulation. Topical application has been shown to be effective in protecting plants (including legumes) against several viruses and it can inhibit virus transmission by aphids and whiteflies [185,186,187]. Optimistically, topical application of synthetic double-stranded RNA would be compatible with the vast array of farmer- and consumer-preferred varieties present in sub-Saharan Africa.
Developing improved legume crops while balancing yield and defence may be challenging [188], but certain viruses may be potential allies in achieving this. Plant ‘persistent’ viruses, so-called because they are inherited at a rate of 100% through seed and pollen [189], may have beneficial effects in plants including common bean [190]. The persistent viruses PvEV1, PvEV2 and PvEV3 occur frequently in common bean lines, including in varieties widely grown in sub-Saharan Africa (Section 1). Potentially, such inherited mutualistic viruses could be usefully employed in breeding programmes to increase the resilience and yield in future common bean lines.
Author Contributions
All authors participated in conceptualization, original draft preparation, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
JPC and AMM are supported by grants from the Leverhulme Trust (RPG-2022–134) and the UK Biotechnological and Biological Sciences Research Council (BBSRC) (APP65186). Earlier phases of research on virus–plant–vector interactions have been funded by the Royal Society (ICA\R1\201221) and BBSRC (SCPRID grant number BB/J011762/1, GCRF grant number BB/P023223/1, and 21ROMITIGATIONFUND CAMBRIDGE BB/W510609/1). FOW’s work was funded by a Royal Society Future Leaders-African Independent Research (FLAIR) Fellowship (FLR/R1/190462) and a Royal Society FLAIR Collaboration Grant 2020 (FCG/R1/201005). NM was supported by studentships from the Schlumberger Foundation, Cambridge Trust, and Magdalene College Cambridge. WA was funded by a Cambridge Africa studentship and grants from the Cambridge University Department of Plant Sciences Frank Smart Studentship, the Cambridge Philosophical Society, and Global Affairs Canada (BRAINS project: P011585).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank Josiah Musembi Mutuku, Ruairí Donnelly, Nik Cunniffe, Appolinaire Djikeng, Jagger Harvey, Chris Gilligan, Peter Palukaitis, John Pickett, Toby Bruce, Lizzie Worrall, Heiko Ziebell, Luke Braidwood and Neena Mitter for very many useful discussions on common bean, its viruses, their vectors and its various other pathogens over so many years. We also thank Adrienne Pate for keeping the lab going flawlessly.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Morales, F.J. Common beans. In Natural Resistance Mechanisms of Plants to Viruses; Loebenstein, G., Carr, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 367–382. ISBN 978-1-4020-3780-1. [Google Scholar]
- Dutta, A.; Trivedi, A.; Nath, C.P.; Gupta, D.S.; Hazra, K.K. A comprehensive review on grain legumes as climate-smartcrops: Challenges and prospects. Environ. Chall. 2022, 7, 100479. [Google Scholar] [CrossRef]
- Nigam, S.N.; Chaudhari, S.; Deevi, K.C.; Saxena, K.B.; Janila, P. Trends in Legume Production and Future Outlook. In Genetic Enhancement in Major Food Legumes; Saxena, K.B., Saxena, R.K., Varshney, R.K., Eds.; Springer International Publishing: Cham, Germany, 2021; pp. 7–48. ISBN 978-3-030-64499-4. [Google Scholar]
- Farrow, A.; Muthoni-Andriatsitohaina, R. Atlas of Common Bean Production in Africa, 2nd ed.; Pan-Africa Bean Research Alliance (PABRA): Nairobi, Kenya; International Center for Tropical Agriculture (CIAT): Palmira, Colombia, 2020; Available online: https://hdl.handle.net/10568/110556 (accessed on 20 April 2025).
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)—Model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Huertas, R.; Karpinska, B.; Ngala, S.; Mkandawire, B.; Maling’A, J.; Wajenkeche, E.; Kimani, P.M.; Boesch, C.; Stewart, D.; Hancock, R.D.; et al. Biofortification of common bean (Phaseolus vulgaris L.) with iron and zinc: Achievements and challenges. Food Energy Secur. 2022, 12, e406. [Google Scholar] [CrossRef] [PubMed]
- De Benoist, B.; McLean, E.; Egli, I.; Cogswell, M. Worldwide Prevalence of Anaemia 1993–2005; WHO Publication: Geneva, Switzerland, 2008; Available online: https://apps.who.int/iris/handle/10665/43894 (accessed on 21 April 2025).
- United Nations Climate Action. Available online: https://www.un.org/en/climatechange/science/climate-issues/food (accessed on 18 April 2025).
- Uebersax, M.A.; Cichy, K.A.; Gomez, F.E.; Porch, T.G.; Heitholt, J.; Osorno, J.M.; Kamfwa, K.; Snapp, S.S.; Bales, S. Dry beans (Phaseolus vulgaris L.) as a vital component of sustainable agriculture and food security—A review. Legume Sci. 2022, 5, e155. [Google Scholar] [CrossRef]
- Razakatiana, A.T.E.; Trap, J.; Baohanta, R.H.; Raherimandimby, M.; Le Roux, C.; Duponnois, R.; Ramanankierana, H.; Becquer, T. Benefits of dual inoculation with arbuscular mycorrhizal fungi and rhizobia on Phaseolus vulgaris planted in a low-fertility tropical soil. Pedobiologia 2020, 83, 150685. [Google Scholar] [CrossRef]
- Breen, C.; Ndlovu, N.; McKeown, P.C.; Spillane, C. Legume seed system performance in sub-Saharan Africa: Barriers, opportunities, and scaling options. A review. Agron. Sustain. Dev. 2024, 44, 20. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Beillouin, D.; Lambers, H.; Yang, Y.D.; Smith, P.; Zeng, Z.H.; Olesen, J.E.; Zang, H.D. Global systematic review with meta-analysis reveals yield advantage of legume-based rotations and its drivers. Nat. Commun. 2022, 13, 4926. [Google Scholar] [CrossRef] [PubMed]
- The Alliance of Biodiversity International and CIAT. Importance of Bean Crop Research. Available online: https://alliancebioversityciat.org/crops/beans/importance (accessed on 20 April 2025).
- Hummel, M.; Hallahan, B.F.; Brychkova, G.; Ramirez-Villegas, J.; Guwela, V.; Chataika, B.; Curley, E.; McKeown, P.C.; Morrison, L.; Talsma, E.F.; et al. Reduction in nutritional quality and growing area suitability of common bean under climate change induced drought stress in Africa. Sci. Rep. 2018, 8, 16187. [Google Scholar] [CrossRef]
- Jones, R.A.C. Future scenarios for plant virus pathogens as climate change progresses. Adv. Virus. Res. 2016, 95, 87–147. [Google Scholar]
- Aguilar, E.; Cutrona, C.; Del Toro, F.J.; Vallarino, J.G.; Osorio, S.; Perez-Bueno, M.L.; Baron, M.; Ching, B.N.; Tenllado, F. Virulence determines beneficial trade-offs in the response of virus-infected plants to drought via induction of salicylic acid. Plant Cell Environ. 2017, 40, 2909–2930. [Google Scholar] [CrossRef]
- del Toro, F.J.; Rakhshandehroo, F.; Larruy, B.; Aguilar, E.; Tenllado, F.; Canto, T. Effects of simultaneously elevated temperature and CO2 levels on Nicotiana benthamiana and its infection by different positive-sense RNA viruses are cumulative and virus type-specific. Virology 2017, 511, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Delafuente, A.; Vinuela, E.; Fereres, A.; Medina, P.; Trebicki, P. Simultaneous increase in CO2 and temperature alters wheat growth and aphid performance differently depending on virus infection. Insects 2020, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Sánchez, Á.; Cobos, A.; López-Herranz, M.; Canto, T.; Pagán, I. Environmental conditions modulate plant virus vertical transmission and survival of infected seeds. Phytopathology 2023, 113, 1773–1787. [Google Scholar] [CrossRef]
- Worrall, E.A.; Wamonje, F.O.; Mukeshimana, G.; Harvey, J.J.W.; Carr, J.P.; Mitter, N. Bean common mosaic virus and bean common mosaic necrosis virus: Relationships, biology, and prospects for control. Adv. Virus Res. 2015, 93, 1–46. [Google Scholar]
- Thompson, J.R.; Langenhan, J.L.; Fuchs, M.; Perry, K.L. Genotyping of Cucumber mosaic virus isolates in western New York State during epidemic years: Characterization of an emergent plant virus population. Virus Res. 2015, 210, 169–177. [Google Scholar] [CrossRef]
- Ferreira, A.L.; Ghanim, M.; Xu, Y.; Pinheiro, P.V. Interactions between common bean viruses and their whitefly vector. Viruses 2024, 16, 1567. [Google Scholar] [CrossRef]
- Spence, N.J.; Walkey, D.G.A. Variation for pathogenicity among isolates of bean common mosaic virus in Africa and a reinterpretation of the genetic relationship between cultivars of Phaseolus vulgaris and pathotypes of BCMV. Plant Pathol. 1995, 44, 527–546. [Google Scholar] [CrossRef]
- Sengooba, T.N.; Spence, N.J.; Walkey, D.G.A.; Allen, D.J.; Femi Lana, A. The occurrence of bean common mosaic necrosis virus in wild and forage legumes in Uganda. Plant Pathol. 1997, 46, 95–103. [Google Scholar] [CrossRef]
- Mutuku, J.M.; Wamonje, F.O.; Mukeshimana, G.; Njuguna, J.; Wamalwa, M.; Choi, S.K.; Tungadim, T.; Djikeng, A.; Kelly, K.; Domelevo Entfellner, J.B.; et al. Metagenomic analysis of plant virus occurrence in common bean (Phaseolus vulgaris) in Central Kenya. Front. Microbiol. 2018, 9, 2939. [Google Scholar] [CrossRef]
- Mwaipopo, B.; Nchimbi-Msolla, S.; Njau, P.J.R.; Mark, D.; Mbanzibwa, D.R. Comprehensive surveys of Bean common mosaic virus and Bean common mosaic necrosis virus and molecular evidence for occurrence of other Phaseolus vulgaris viruses in Tanzania. Plant Dis. 2018, 102, 2361–2370. [Google Scholar] [CrossRef]
- Wainaina, J.M.; Kubatko, L.; Harvey, J.; Ateka, E.; Makori, T.; Karanja, D.; Boykin, L.M.; Kehoe, M.A. Evolutionary insights of bean common mosaic necrosis virus and cowpea aphid-borne mosaic virus. PeerJ 2019, 7, e6792. [Google Scholar] [CrossRef]
- Mulenga, R.M.; Miano, D.W.; Kaimoyo, E.; Akello, J.; Felister, M.; Al Rwahnih, M.; Chikoti, P.C.; Chiona, M.; Simulundu, E.; Alabi, O.J. First report of southern bean mosaic virus infecting common bean in Zambia. Dis. Notes 2020, 104, 1880. [Google Scholar] [CrossRef]
- Wamonje, F.O. Post-COVID-19 action: Guarding Africa’s crops against viral epidemics requires research capacity building that unifies a trio of transdisciplinary interventions. Viruses 2020, 12, 1276. [Google Scholar] [CrossRef] [PubMed]
- Chatzivassiliou, E.K. An annotated list of legume-infecting viruses in the light of metagenomics. Plants 2021, 10, 1413. [Google Scholar] [CrossRef]
- Valverde, R.A.; Khalifa, M.E.; Okada, R.; Fukuhara, T.; Sabanadzovic, S. ICTV Virus Taxonomy Profile: Endornaviridae. J. Gen. Virol. 2019, 100, 1204–1205. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Yong, C.K.; Valverde, R.A.; Sabanadzovic, S.; Aoki, N.; Hotate, S.; Kiyota, E.; Moriyama, H.; Fukuhara, T. Molecular characterization of two evolutionarily distinct endornaviruses co-infecting common bean (Phaseolus vulgaris). J. Gen. Virol. 2013, 94, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Nordenstedt, N.; Marcenaro, D.; Chilagane, D.; Mwaipopo, B.; Rajamäki, M.-L.; Nchimbi-Msolla, S.; Njau, P.J.R.; Mbanzibwa, D.; Valkonen, J.P.T. Pathogenic seedborne viruses are rare but Phaseolus vulgaris endornaviruses are common in bean varieties grown in Nicaragua and Tanzania. PLoS ONE 2017, 12, e0178242. [Google Scholar]
- Brine, T.J.; Crawshaw, S.; Murphy, A.M.; Pate, A.E.; Carr, J.P.; Wamonje, F.O. Identification and Characterization of Phaseolus vulgaris Endornavirus 1, 2 and 3 in Common Bean Cultivars of East Africa. Virus Genes 2023, 59, 741–751. [Google Scholar] [CrossRef]
- Brine, T.J.; Viswanathan, S.B.; Murphy, A.M.; Pate, A.E.; Wamonje, F.O.; Carr, J.P. Investigating the interactions of endornaviruses with each other and with other viruses in common bean, Phaseolus vulgaris. Virol. J. 2023, 20, 216. [Google Scholar] [CrossRef]
- Garcia, L.R.; Janssen, D. Epidemiology and control of emerging criniviruses in bean. Virus Res. 2020, 280, 197902. [Google Scholar] [CrossRef] [PubMed]
- Segundo, E.; Martín, G.; Cuadrado, I.M.; Janssen, D. A new yellowing disease in Phaseolus vulgaris associated with a whitefly-transmitted virus. Plant Pathol. 2004, 53, 517. [Google Scholar] [CrossRef]
- Jones, R.A.C. Australian Cool-season pulse seed-borne virus research: 2. Bean yellow mosaic virus. Viruses 2025, 17, 668. [Google Scholar] [CrossRef]
- Silbernagel, M.J.; Mink, G.I.; Zhao, R.L.; Zheng, G.Y. Phenotypic recombination between bean common mosaic and bean common mosaic necrosis potyviruses in vivo. Arch. Virol. 2001, 146, 1007–1020. [Google Scholar] [CrossRef]
- Feng, X.; Poplawsky, A.R.; Nikolaeva, O.V.; Myers, J.R.; Karasev, A.V. Recombinants of bean common mosaic virus (BCMV) and genetic determinants of BCMV involved in overcoming resistance in common bean. Phytopathology 2014, 104, 786–793. [Google Scholar] [CrossRef]
- Beaver, J.S.; González, A.; Mateo, B.; Lutz, G.G.; Miranda, A.; Rosas, J.C.; Porch, T.G. Release of multiple virus and bruchid resistant Mesoamerican bean germplasm lines PR1303-129 and PR1743-44. J. Plant Regist. 2024, 18, 149–156. [Google Scholar] [CrossRef]
- Asiimwe, R.; Katungi, E.; Marimo, P.; Mukankusi, C.; Rubyogo, J.C.; Anthony, V. Evaluating consumer preferences for reduced cooking time, taste and colour of beans in rural and urban communities in Uganda. Agric. Food Sec. 2024, 13, 19. [Google Scholar] [CrossRef]
- Bianchini, A. Resistance to bean golden mosaic virus in bean genotypes. Plant Dis. 1999, 83, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.W.; Rodriguez, L.M.; Pedraza, F.; Morales, F.; Beebe, S. Genetic mapping of the Bean golden yellow mosaic geminivirus resistance gene bgm-1 and linkage with potyvirus resistance in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2007, 114, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Beaver, J.S.; Porch, T.G.; Zapata, M. Registration of ‘Verano’ white bean. J. Plant Regist. 2008, 2, 187–189. [Google Scholar] [CrossRef]
- Anon. Tomato Yellow Leaf Curl Virus. Agriculture Victoria. 2024. Available online: https://agriculture.vic.gov.au/biosecurity/plant-diseases/vegetable-diseases/tomato-yellow-leaf-curl-virus (accessed on 6 July 2025).
- Papayiannis, L.C.; Paraskevopoulos, A.; Katis, N.I. First report of tomato yellow leaf curl virus infecting common bean (Phaseolus vulgaris) in Greece. Plant Dis. 2007, 91, 465. [Google Scholar] [CrossRef] [PubMed]
- Navas-Castillo, J.; Sánchez-Campos, S.; Díaz, J.A.; Sáez-Alonso, E.; Moriones, E. Tomato yellow leaf curl virus-Is causes a novel disease of common bean and severe epidemics in tomato in Spain. Plant Dis. 1999, 83, 29–32. [Google Scholar] [CrossRef]
- Naidu, R.A.; Gowda, S.; Satyanarayana, T.; Boyko, V.; Reddy, A.S.; Dawson, W.O.; Reddy, D.V. Evidence that whitefly-transmitted cowpea mild mottle virus belongs to the genus Carlavirus Arch. Virol. 1998, 143, 769–780. [Google Scholar]
- Lamas, N.S.; Matos, V.O.R.L.; Alves-Freitas, D.M.T.; Melo, F.L.; Costa, A.F.; Faria, J.C.; Ribeiro, S.G. Occurrence of Cowpea mild mottle virus in common bean and associated weeds in Northeastern Brazil. Plant Dis. 2017, 101, 1828. [Google Scholar] [CrossRef]
- Brown, J.K. Cowpea Mild Mottle Virus (Angular Mosaic of Beans). CABI Compendium Datasheet 15735. 2020. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.15735 (accessed on 27 July 2025).
- Osogo, A.K.; Muyekho, F.; Okoth, P.; Were, H.; Ayaaga, G. Occurrence, distribution, incidence, and severity of common bean viral diseases in resource-limited smallholder farms of western Kenya. Crop Prot. 2025, 194, 107231. [Google Scholar] [CrossRef]
- Ogunsola, K.E.; Fatokun, C.A.; Boukar, O.; Kumar, P.L. Inheritance of resistance to three endemic viral diseases of cowpea in Nigeria. J. Crop Improv. 2023, 37, 291–308. [Google Scholar] [CrossRef]
- Duffus, J.E.; Liu, H.-Y.; Wisler, G.C.; Li, R. Lettuce chlorosis virus—A new whitefly-transmitted Closterovirus. Eur. J. Plant Pathol. 1996, 102, 591–596. [Google Scholar] [CrossRef]
- Fuchs, M.; Bar-Joseph, M.; Candresse, T.; Maree, H.J.; Martelli, G.P.; Melzer, M.J.; Menzel, W.; Minafra, A.; Sabanadzovic, S.; ICTV Report Consortium. ICTV Virus Taxonomy Profile: Closteroviridae. J. Gen. Virol. 2020, 101, 364–365. [Google Scholar] [CrossRef]
- Martín, G.; Cuadrado, I.M.; Janssen, D. Bean yellow disorder virus: Parameters of transmission by Bemisia tabaci and host plant range. Insect Sci. 2011, 1, 50–56. [Google Scholar] [CrossRef]
- Thompson, J.R.; Canto, T.; Carr, J.P.; Pallás, V.; Šafářová, D. ICTV Virus Taxonomy Profile: Bromoviridae 2025. J. Gen. Virol. 2025, 106, 002069. [Google Scholar] [CrossRef]
- Pasev, G.; Radeva-Ivanova, V.; Manoussopoulos, Y.; Turina, M.; Kostova, D. First report of Peanut stunt virus on beans in Bulgaria. New Dis. Rep. 2018, 38, 9. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Palukaitis, P.; Choi, S.K. Chapter 1: Host Range. In Cucumber Mosaic Virus; Palukaitis, P., García-Arenal, F., Eds.; American Phytopathological Society Press: St. Paul, MN, USA, 2019; pp. 15–18. [Google Scholar]
- Kim, C.H.; Palukaitis, P. The plant defense response to cucumber mosaic virus in cowpea is elicited by the viral polymerase gene and affects virus accumulation in single cells. EMBO J. 1997, 16, 4060–4068. [Google Scholar] [CrossRef]
- Hampton, R.O.; Francki, R.I.B. RNA-1 dependent seed transmissibility of cucumber mosaic virus in Phaseolus vulgaris. Phytopathology 1992, 82, 127–130. [Google Scholar] [CrossRef]
- Pagán, I. Chapter 16: Movement Between Plants: Vertical Transmission. In Cucumber Mosaic Virus; Palukaitis, P., García-Arenal, F., Eds.; American Phytopathological Society Press: St. Paul, MN, USA, 2019; pp. 185–198. [Google Scholar]
- Fereres, A.; Perry, K.L. Chapter 15: Movement between plants: Horizontal transmission. In Cucumber Mosaic Virus; Palukaitis, P., García-Arenal, F., Eds.; American Phytopathological Society Press: St. Paul, MN, USA, 2019; pp. 173–184. [Google Scholar]
- Nault, B.A.; Shah, D.A.; Straight, K.E.; Bachmann, A.C.; Sackett, W.M.; Dillard, H.R.; Fleischer, S.J.; Gildow, F.E. Modeling temporal trends in aphid vector dispersal and cucumber mosaic virus epidemics in snap bean. Environ. Entomol. 2009, 38, 1347–1359. [Google Scholar] [CrossRef]
- Palukaitis, P. Satellite RNAs and satellite viruses. Mol. Plant-Microbe Interact. 2016, 29, 181–186. [Google Scholar] [CrossRef]
- Obrępalska-Stęplowska, A.; Renaut, J.; Planchon, S.; Przybylska, A.; Wieczorek, P.; Barylski, J.; Palukaitis, P. Effect of temperature on the pathogenesis, accumulation of viral and satellite RNAs and on plant proteome in peanut stunt virus and satellite RNA-infected plants. Front. Plant Sci. 2015, 6, 903. [Google Scholar] [CrossRef]
- Tarquini, G.; Martini, M.; Maestri, S.; Firrao, G.; Ermacora, P. The virome of ‘Lamon Bean’: Application of MinION sequencing to investigate the virus population associated with symptomatic beans in the Lamon Area, Italy. Plants 2022, 11, 779. [Google Scholar] [CrossRef]
- Giakountis, A.; Tsarmpopoulos, I.; Chatzivassiliou, E.K. Cucumber mosaic virus isolates from Greek legumes are associated with satellite RNAs that are necrogenic for tomato. Plant Dis. 2018, 102, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Tepfer, M.; García-Arenal, F. Chapter 3: Epidemiology and Ecology. In Cucumber Mosaic Virus; Palukaitis, P., García-Arenal, F., Eds.; American Phytopathological Society Press: St. Paul, MN, USA, 2019; pp. 37–45. [Google Scholar]
- Wainaina, J.M.; Ateka, E.; Makori, T.; Kehoe, M.A.; Boykin, L.M. A metagenomic study of DNA viruses from samples of local varieties of common bean in Kenya. PeerJ 2019, 7, e6465. [Google Scholar] [CrossRef] [PubMed]
- Wangai, A.W.; Redinbaugh, M.G.; Kinyua, Z.M.; Miano, D.W.; Leley, P.K.; Kasina, M.; Mahuku, G.; Scheets, K.; Jeffers, D. First Report of Maize chlorotic mottle virus and Maize Lethal Necrosis in Kenya. Plant Dis. 2012, 96, 1582. [Google Scholar] [CrossRef]
- Wamaitha, M.J.; Nigam, D.; Maina, S.; Stomeo, F.; Wangai, A.; Njuguna, J.N.; Holton, T.A.; Wanjala, B.W.; Wamalwa, M.; Lucas, T.; et al. Metagenomic analysis of viruses associated with maize lethal necrosis in Kenya. Virol. J. 2018, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Braidwood, L.; Quito-Avila, D.F.; Cabanas, D.; Bressan, A.; Wangai, A.; Baulcombe, D.C. Maize chlorotic mottle virus exhibits low divergence between differentiated regional sub-populations. Sci. Rep. 2018, 8, 1173. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Collar, J.L.; Tjallingii, W.F.; Fereres, A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J. Gen. Virol. 1997, 78, 2701–2705. [Google Scholar] [CrossRef]
- Powell, G. Intracellular salivation is the aphid activity associated with inoculation of non-persistently transmitted viruses. J. Gen. Virol. 2005, 86, 469–472. [Google Scholar] [CrossRef]
- Tjallingii, W.F.; Garzo, E.; Fereres, A. New structure in cell puncture activities by aphid stylets: A dual-mode EPG study. Entomol. Exp. Appl. 2010, 135, 193–207. [Google Scholar] [CrossRef]
- Krenz, B.; Bronikowski, A.; Lu, X.; Ziebell, H.; Thompson, J.R.; Perry, K.L. Visual monitoring of Cucumber mosaic virus infection in Nicotiana benthamiana following transmission by the aphid vector Myzus persicae. J. Gen. Virol. 2015, 96, 2904–2912. [Google Scholar] [CrossRef]
- Hull, R. Chapter 12. Plant to plant movement. In Plant Virology, 5th ed.; Academic Press: New York, NY, USA, 2014; pp. 669–751. [Google Scholar]
- Liang, Y.; Gao, X.W. The cuticle protein gene MPCP4 of Myzus persicae (Homoptera: Aphididae) plays a critical role in cucumber mosaic virus acquisition. J. Econ. Entomol. 2017, 110, 848–853. [Google Scholar] [CrossRef]
- Webster, C.G.; Pichon, E.; van Munster, M.; Monsion, B.; Deshoux, M.; Gargani, D.; Calevro, F.; Jimenez, J.; Moreno, A.; Krenz, B.; et al. Identification of plant virus receptor candidates in the stylets of their aphid vectors. J. Virol. 2018, 92, e00432-18. [Google Scholar] [CrossRef]
- Musser, R.O.; Hum-Musser, S.M.; Felton, G.W.; Gergerich, R.C. Increased larval growth and preference for virus-infected leaves by the Mexican bean beetle, Epilachna varivestis Mulsant, a plant virus vector. J. Insect Behav. 2003, 16, 247–256. [Google Scholar] [CrossRef]
- Wielkopolan, B.; Jakubowska, M.; Obrępalska-Stęplowska, A. Beetles as plant pathogen vectors. Front. Plant Sci. 2021, 12, 748093. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Sieve element acceptance by aphids. Eur. J. Entomol. 1994, 91, 47–52. [Google Scholar]
- Pinheiro-Lima, B.; Pereira-Carvalho, R.C.; Alves-Freitas, D.M.T.; Kitajima, E.W.; Vidal, A.H.; Lacorte, C.; Godinho, M.T.; Fontenele, R.S.; Faria, J.C.; Abreu, E.F.M.; et al. Transmission of the bean-associated cytorhabdovirus by the whitefly Bemisia tabaci MEAM1. Viruses 2020, 12, 1028. [Google Scholar] [CrossRef]
- de Oliveira, A.S.; Bertran, A.G.; Inoue-Nagata, A.K.; Nagata, T.; Kitajima, E.W.; Oliveira Resende, R. An RNA-dependent RNA polymerase gene of a distinct Brazilian tospovirus. Virus Genes 2011, 43, 385–389. [Google Scholar] [CrossRef]
- Brault, V.; Uzest, M.; Monsion, B.; Jacquot, E.; Blanc, S. Aphids as transport devices for plant viruses. Comptes Rendus Biol. 2010, 333, 524–538. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect vector-mediated transmission of plant viruses. Virology 2015, 479–480, 278–289. [Google Scholar] [CrossRef]
- Hunter, W.B.; Hiebert, E.; Webb, S.E.; Tsai, J.H.; Polston, J.E. Location of geminiviruses in the whitefly Bemisia tabaci (Homoptera: Aleyrodidae). Plant. Dis. 1998, 82, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.; Cilia, M.; Ghanim, M. Circulative, “nonpropagative” virus transmission: An orchestra of virus-, insect-, and plant-derived instruments. Adv. Virus Res. 2014, 89, 141–199. [Google Scholar] [PubMed]
- Dawkins, R. The Extended Phenotype: The Long Reach of the Gene; Oxford University Press: Oxford, UK, 1982; 496p. [Google Scholar]
- Carr, J.P.; Murphy, A.M.; Tungadi, T.; Yoon, J.Y. Plant defense signals: Players and pawns in plant-virus-vector interactions. Plant Sci. 2019, 279, 87–95. [Google Scholar] [CrossRef] [PubMed]
- He, M.-J.; Wang, Y.; Zhao, M.; Zuo, D.-P.; Wang, Y.; Zhang, Z.-Y.; Wang, Y.; Han, C.-G. Molecular characterization of miRNAs in Myzus persicae carrying brassica yellows virus. Biology 2024, 13, 941. [Google Scholar] [CrossRef]
- Jayasinghe, W.H.; Kim, H.; Nakada, Y.; Masuta, C. A plant virus satellite RNA directly accelerates wing formation in its insect vector for spread. Nat. Commun. 2021, 12, 7087. [Google Scholar] [CrossRef]
- Donnelly, R.; Cunniffe, N.J.; Carr, J.P.; Gilligan, C.A. Pathogenic modification of plants enhances long-distance dispersal of nonpersistently transmitted viruses to new hosts. Ecology 2019, 100, e02725. [Google Scholar] [CrossRef]
- Groen, S.C.; Wamonje, F.O.; Murphy, A.M.; Carr, J.P. Engineering resistance to virus transmission. Curr. Opin. Virol. 2017, 26, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.; Bruce, T.; Pickett, J.; Hardie, J. Volatiles functioning as host cues in a blend become nonhost cues when presented alone to the black bean aphid. Anim. Behav. 2010, 79, 451–457. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects—Finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Wamonje, F.O.; Michuki, G.N.; Braidwood, L.A.; Njuguna, J.N.; Musembi Mutuku, J.; Djikeng, A.; Harvey, J.J.W.; Carr, J.P. Viral metagenomics of aphids present in bean and maize plots on mixed-use farms in Kenya reveals the presence of three dicistroviruses including a novel Big Sioux River virus-like dicistrovirus. Virol. J. 2017, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Wamonje, F.O.; Tungadi, T.D.; Murphy, A.M.; Pate, A.E.; Woodcock, C.; Caulfield, J.C.; Mutuku, J.M.; Cunniffe, N.J.; Bruce, T.J.A.; Gilligan, C.A.; et al. Three aphid-transmitted viruses encourage vector migration from infected common bean (Phaseolus vulgaris) plants through a combination of volatile and surface cues. Front. Plant Sci. 2020, 11, 613772. [Google Scholar] [CrossRef]
- Wamonje, F.O.; Donnelly, R.; Tungadi, T.D.; Murphy, A.M.; Pate, A.E.; Woodcock, C.; Caulfield, J.; Mutuku, J.M.; Bruce, T.J.A.; Gilligan, C.A.; et al. Different plant viruses induce changes in feeding behavior of specialist and generalist aphids on common bean that are likely to enhance virus transmission. Front. Plant Sci. 2020, 10, 1811. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef]
- Carmo-Sousa, M.; Moreno, A.; Garzo, E.; Fereres, A. A non-persistently transmitted-virus induces a pull-push strategy in its aphid vector to optimise transmission and spread. Virus Res. 2014, 186, 38–46. [Google Scholar] [CrossRef]
- Westwood, J.H.; Groen, S.C.; Du, Z.; Murphy, A.M.; Anggoro, D.T.; Tungadi, T.; Luang-In, V.; Lewsey, M.G.; Rossiter, J.T.; Powell, G.; et al. A trio of viral proteins tunes aphid-plant interactions in Arabidopsis thaliana. PLoS ONE 2013, 8, e83066. [Google Scholar] [CrossRef]
- Westwood, J.H.; Lewsey, M.G.; Murphy, A.M.; Tungadi, T.; Bates, A.; Gilligan, C.A.; Carr, J.P. Interference with jasmonic acid-regulated gene expression is a general property of viral suppressors of RNA silencing but only partly explains virus-induced changes in plant–aphid interactions. J. Gen. Virol. 2014, 95, 733. [Google Scholar] [CrossRef] [PubMed]
- Casteel, C.L.; Yang, C.; Nanduri, A.C.; De Jong, H.N.; Whitham, S.A.; Jander, G. The NIa-Pro protein of turnip mosaic virus improves growth and reproduction of the aphid vector, Myzus persicae (green peach aphid). Plant. J. 2014, 77, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Casteel, C.L.; De Alwis, M.; Bak, A.; Dong, H.; Whitham, S.A.; Jander, G. Disruption of ethylene responses by turnip mosaic virus mediates suppression of plant defense against the green peach aphid vector. Plant. Physiol. 2015, 169, 209–218. [Google Scholar] [CrossRef]
- Rhee, S.J.; Watt, L.G.; Bravo-Cazar, A.; Murphy, A.M.; Carr, J.P. Effects of the cucumber mosaic virus 2a protein on aphid–plant interactions in Arabidopsis thaliana. Mol. Plant Pathol. 2020, 21, 1248–1254. [Google Scholar] [CrossRef]
- Ray, S.; Murad, T.; Arena, G.D.; Arshad, K.; Arendsee, Z.; Herath, V.; Whitham, S.A.; Casteel, C.L. Turnip mosaic virus infection cleaves MEDIATOR SUBUNIT16 in plants increasing plant susceptibility to the virus and its aphid vector Myzus persicae. BMC Plant Biol. 2025, 25, 411. [Google Scholar] [CrossRef]
- Herath, V.; Casteel, C.L.; Verchot, J. Comprehensive transcriptomic analysis reveals turnip mosaic virus infection and its aphid vector Myzus persicae cause large changes in gene regulatory networks and co-transcription of alternative spliced mRNAs in Arabidopsis thaliana. BMC Plant Biol. 2025, 25, 128. [Google Scholar] [CrossRef]
- Nihranz, C.T.; Garg, P.; Shin, J.; Dumas, M.; McCalla, S.G.; Roy, S.; Casteel, C.L. Transcriptomic analysis reveals vector attraction to potato virus Y is mediated through temporal regulation of TERPENE SYNTHASE 1 (TPS1). Plant Stress 2025, 16, 100862. [Google Scholar] [CrossRef]
- Watt, L.G.; Crawshaw, S.; Rhee, S.-J.; Murphy, A.M.; Canto, T.; Carr, J.P. The cucumber mosaic virus 1a protein regulates interactions between the 2b protein and ARGONAUTE 1 while maintaining the silencing suppressor activity of the 2b protein. PLoS Pathog. 2020, 16, e1009125. [Google Scholar] [CrossRef]
- Crawshaw, S.; Murphy, A.M.; Rowling, P.J.E.; Nietlispach, D.; Itzhaki, L.S.; Carr, J.P. Investigating the interactions of the cucumber mosaic virus 2b protein with the viral 1a replicase component and the cellular RNA silencing factor Argonaute 1. Viruses 2024, 16, 676. [Google Scholar] [CrossRef]
- Crawshaw, S.; Watt, L.G.; Murphy, A.M.; Carr, J.P. Strain-specific differences in the interactions of the cucumber mosaic virus 2b protein with the viral 1a and host Argonaute 1 proteins. J. Virol. 2024, 98, e00993-24. [Google Scholar] [CrossRef]
- Ziebell, H.; Murphy, A.M.; Groen, S.C.; Tungadi, T.; Westwood, J.H.; Lewsey, M.G.; Moulin, M.; Kleczkowski, A.; Smith, A.G.; Stevens, M.; et al. Cucumber mosaic virus and its 2b RNA silencing suppressor modify plant-aphid interactions in tobacco. Sci. Rep. 2011, 1, 187. [Google Scholar] [CrossRef]
- Tungadi, T.; Groen, S.C.; Murphy, A.M.; Pate, A.E.; Iqbal, J.; Bruce, T.J.; Cunniffe, N.J.; Carr, J.P. Cucumber mosaic virus and its 2b protein alter emission of host volatile organic compounds but not aphid vector settling in tobacco. Virol. J. 2017, 14, 91. [Google Scholar] [CrossRef]
- Tungadi, T.; Donnelly, R.; Qing, L.; Iqbal, J.; Murphy, A.M.; Pate, A.E.; Cunniffe, N.J.; Carr, J.P. Cucumber mosaic virus 2b proteins inhibit virus-induced aphid resistance in tobacco. Mol. Plant Pathol. 2020, 21, 250–257. [Google Scholar] [CrossRef]
- Tungadi, T.; Watt, L.G.; Groen, S.C.; Murphy, A.M.; Du, Z.; Pate, A.E.; Westwood, J.H.; Fennell, T.G.; Powell, G.; Carr, J.P. Infection of Arabidopsis by cucumber mosaic virus triggers jasmonate-dependent resistance to aphids that relies partly on the pattern-triggered immunity factor BAK1. Mol. Plant Pathol. 2021, 22, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Arinaitwe, W.; Guyon, A.; Tungadi, T.D.; Cunniffe, N.J.; Rhee, S.-J.; Khalaf, A.; Mhlanga, N.M.; Pate, A.E.; Murphy, A.M.; Carr, J.P. The effects of cucumber mosaic virus and its 2a and 2b proteins on interactions of tomato plants with the aphid vectors Myzus persicae and Macrosiphum euphorbiae. Viruses 2022, 14, 1703. [Google Scholar] [CrossRef] [PubMed]
- Van Griethuysen, P.A.; Redeker, K.R.; MacFarlane, S.A.; Neilson, R.; Hartley, S.E. Virus-induced changes in root volatiles attract soil nematode vectors to infected plants. New Phytol. 2024, 241, 2275–2286. [Google Scholar] [CrossRef]
- Bello, V.H.; Barreto da Silva, F.; Watanabe, L.F.M.; Vicentin, E.; Muller, C.; de Freitas Bueno, R.C.O.; Santos, J.C.; De Marchi, B.R.; Nogueira, A.M.; Yuki, V.A.; et al. Detection of Bemisia tabaci Mediterranean cryptic species on soybean in São Paulo and Paraná States (Brazil) and interaction of cowpea mild mottle virus with whiteflies. Plant Pathol. 2021, 70, 1508–1520. [Google Scholar] [CrossRef]
- Rajabaskar, D.; Bosque-Pérez, N.A.; Eigenbrode, S.D. Preference by a virus vector for infected plants is reversed after virus acquisition. Virus Res. 2014, 186, 32–37. [Google Scholar] [CrossRef]
- Chesnais, Q.; Caballero Vidal, G.; Coquelle, R.; Yvon, M.; Mauck, K.; Brault, V.; Ameline, A. Post-acquisition effects of viruses on vector behavior are important components of manipulation strategies. Oecologia 2020, 194, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Verdier, M.; Boissinot, S.; Baltenweck, R.; Negrel, L.; Brault, V.; Ziegler-Graff, V.; Hugueney, P.; Scheidecker, D.; Krieger, C.; Chesnais, Q.; et al. The turnip yellows virus capsid protein promotes access of its main aphid vector Myzus persicae to phloem tissues. Plant Cell Environ. 2025, 48, 2434–2444. [Google Scholar] [CrossRef]
- Pan, L.-L.; Miao, H.; Wang, Q.; Walling, L.L.; Liu, S.-S. Virus-induced phytohormone dynamics and their effects on plant-insect interactions. New Phytol. 2021, 230, 1305–1320. [Google Scholar] [CrossRef]
- Allen-Perkins, A.; Estrada, E. Mathematical modelling for sustainable aphid control in agriculture via intercropping. Proc. R. Soc. A-Math. Phys. Eng. Sci. 2019, 475, 20190136. [Google Scholar] [CrossRef]
- Khan, Z.R.; Midega, C.A.; Wanyama, J.M.; Amudavi, D.M.; Hassanali, A.; Pittchar, J.; Pickett, J.A. Integration of edible beans (Phaseolus vulgaris L.) into the push–pull technology developed for stemborer and Striga control in maize-based cropping systems. Crop Prot. 2009, 28, 997–1006. [Google Scholar] [CrossRef]
- Hailu, G.; Niassy, S.; Zeyaur, K.R.; Ochatum, N.; Subramanian, S. Maize–legume intercropping and push–pull for management of Fall Armyworm, stemborers, and Striga in Uganda. Agron. J. 2018, 110, 2513–2522. [Google Scholar] [CrossRef]
- Cunniffe, N.J.; Taylor, N.P.; Hamelin, F.M.; Jeger, M.J. Epidemiological and ecological consequences of virus manipulation of host and vector in plant virus transmission. PLoS Comput. Biol. 2021, 17, e1009759. [Google Scholar] [CrossRef]
- Falla, E.K.; Cunniffe, N.J. Why aphid virus retention needs more attention: Modelling aphid behaviour and virus manipulation in non-persistent plant virus transmission. PLoS Comput. Biol. 2024, 20, e1012479. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Ding, H.; Shiel, P.; Berger, P.H. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. Roy. Soc. B 2002, 269, 455–460. [Google Scholar] [CrossRef]
- Donnelly, R.; Gilligan, C.A. What is pathogen-mediated insect superabundance? J. Roy. Soc. Interface 2020, 17, 20200229. [Google Scholar] [CrossRef] [PubMed]
- Braendle, C.; Davis, G.K.; Brisson, J.A.; Stern, D.L. Wing dimorphism in aphids. Heredity 2006, 97, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Zaffaroni, M.; Rimbaud, L.; Mailleret, L.; Cunniffe, N.J.; Bevacqua, D. Modelling interference between vectors of non-persistently transmitted plant viruses to identify effective control strategies. PLoS Comput. Biol. 2021, 17, e1009727. [Google Scholar] [CrossRef] [PubMed]
- Seeger, J.N.; Ziebell, H.; Saucke, H. Impact of Pea necrotic yellow dwarf virus (PNYDV) on nodulation, N2 fixation and yield in faba bean (Vicia faba, L.). J. Plant Dis. Prot. 2022, 129, 1437–1450. [Google Scholar] [CrossRef]
- López, M.; Muñoz, N.; Lascano, H.R.; Izaguirre-Mayoral, M.L. The seed-borne Southern bean mosaic virus hinders the early events of nodulation and growth in Rhizobium-inoculated Phaseolus vulgaris L. Funct. Plant Biol. 2017, 44, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Marchetto, K.M.; Power, A.G. Viral infection can reduce the net nitrogen inputs of legume break crops and cover crops. Ecol. Appl. 2021, 31, e02241. [Google Scholar] [CrossRef]
- van Spronsen, P.C.; Tak, T.; Rood, A.M.; van Brussel, A.A.; Kijne, J.W.; Boot, K.J. Salicylic acid inhibits indeterminate-type nodulation but not determinate-type nodulation. Molec. Plant-Microbe Interact. 2003, 16, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, P.; Basu, S.; Lee, B.W.; Oeller, L.; Crowder, D.W. Effects of soil rhizobia abundance on interactions between a vector, pathogen, and legume plant host. Genes 2024, 15, 273. [Google Scholar] [CrossRef]
- Nenadić, M.; Grandi, L.; Mescher, M.C.; De Moraes, C.M.; Mauck, K.E. Transmission-enhancing effects of a plant virus depend on host association with beneficial bacteria. Arthropod-Plant Interact. 2022, 16, 15–31. [Google Scholar] [CrossRef]
- Demler, S.A.; Rucker, D.G.; Nooruddin, L.; de Zoeten, G.A. Replication of the satellite RNA of pea enation mosaic virus is controlled by RNA 2-encoded functions. J. Gen. Virol. 1994, 75, 1399–1406. [Google Scholar] [CrossRef]
- Pulido, H.; Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Combined effects of mutualistic rhizobacteria counteract virus-induced suppression of indirect plant defences in soya bean. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190211. [Google Scholar] [CrossRef]
- Adhikari, K.N.; Burrows, L.; Sadeque, A.; Chung, C.; Cullis, B.; Trethowan, R. Frequency of outcrossing and isolation distance in faba beans (Vicia faba L.). Agronomy 2023, 13, 1893. [Google Scholar] [CrossRef]
- Elisante, F.; Ndakidemi, P.; Arnold, S.E.J.; Belmain, S.R.; Gurr, G.M.; Darbyshire, I.; Xie, G.; Stevenson, P.C. Insect pollination is important in a smallholder bean farming system. PeerJ 2020, 8, e10102. [Google Scholar] [CrossRef]
- Franceschinelli, E.V.; Ribeiro, P.L.M.; Mesquita-Neto, J.N.; Bergamini, L.L.; Madureira de Assis, I.; Elias, M.A.S.; Fernandes, P.M.; Carvalheiro, L.G. Importance of biotic pollination varies across common bean cultivars. J. Appl. Entomol. 2022, 146, 32–43. [Google Scholar] [CrossRef]
- Bailes, E.J.; Pattrick, J.G.; Glover, B.J. An analysis of the energetic reward offered by field bean (Vicia faba) flowers: Nectar, pollen, and operative force. Ecol. Evol. 2018, 8, 3161–3171. [Google Scholar] [CrossRef]
- Mhlanga, N.M.; Pate, A.E.; Arinaitwe, W.; Carr, J.P.; Murphy, A.M. Reduction in vertical transmission rate of bean common mosaic virus in bee-pollinated common bean plants. Virol. J. 2024, 21, 147. [Google Scholar] [CrossRef]
- Kingha, B.M.T.; Fohouo, F.-N.T.; Ngakou, A.; Brückner, D. Foraging and pollination activities of Xylocopa olivacea (Hymenoptera, Apidae) on Phaseolus vulgaris (Fabaceae) flowers at Dang (Ngaoundere-Cameroon). J. Agric. Ext. Rural Dev. 2012, 4, 330–339. [Google Scholar]
- Anon. The Buff-Tailed Bumblebee. Nature Spot: Wildlife and Wild Places of Leicestershire and Rutland. Available online: https://www.naturespot.org/species/buff-tailed-bumblebee (accessed on 15 August 2025).
- Ruiz, C.; Suárez, D.; Naranjo, M.; De la Rúa, P. First record of the carpenter bee Xylocopa pubescens (Hymenoptera, Apidae) in the Canary Islands confirmed by DNA barcoding. J. Hymenoptera Res. 2020, 80, 169–175. [Google Scholar] [CrossRef]
- Groen, S.C.; Jiang, S.; Murphy, A.M.; Cunniffe, N.J.; Westwood, J.H.; Davey, M.P.; Bruce, T.J.A.; Caulfield, J.C.; Furzer, O.J.; Reed, A.; et al. Virus infection of plants alters pollinator preference: A payback for susceptible hosts? PLoS Pathog. 2016, 12, e1005790. [Google Scholar] [CrossRef]
- Murphy, A.M.; Jiang, S.; Elderfield, J.A.D.; Pate, A.E.; Halliwell, C.; Glover, B.J.; Cunniffe, N.J.; Carr, J.P. Biased pollen transfer between virus-infected and non-infected plants by bumblebees favors the paternity of infected plants in cross-pollination. iScience 2023, 26, 106116. [Google Scholar] [CrossRef]
- Mhlanga, N.M. Effects of Plant Viral Pathogens on Plant-Pollinator Relationships. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2020. [Google Scholar]
- Mhlanga, N.M.; Murphy, A.M.; Wamonje, F.O.; Cunniffe, N.J.; Caulfield, J.C.; Glover, B.J.; Carr, J.P. An innate preference of bumblebees for volatile organic compounds emitted by Phaseolus vulgaris plants infected with three different viruses. Front. Ecol. Evol. 2021, 9, 626851. [Google Scholar] [CrossRef]
- Medina, A.C.; Grogan, R.G. Seed transmission of bean common mosaic viruses. Phytopathology 1961, 51, 452–456. [Google Scholar]
- Schippers, B. Transmission of bean common mosaic virus by seed of Phaseolus vulgaris L. cultivar Beka. Acta Bot. Neerlandica. 1963, 12, 433–497. [Google Scholar] [CrossRef]
- Fetters, A.M.; Ashman, T.L. The pollen virome: A review of pollen-associated viruses and consequences for plants and their interactions with pollinators. Am. J. Bot. 2023, 110, e16144. [Google Scholar] [CrossRef]
- Liu, X.; Chen, G.; Zhang, Y.J.; Xie, W.; Wu, Q.J.; Wang, S.L. Virus-infected plants altered the host selection of Encarsia formosa, a parasite of whiteflies. Front. Physiol. 2017, 8, 937. [Google Scholar] [CrossRef]
- Liu, B.; Preisser, E.L.; Chu, D.; Pan, H.; Xie, W.; Wang, S.; Wu, Q.; Zhou, X. Multiple forms of vector manipulation by a plant-infecting virus: Bemisia tabaci and tomato yellow leaf curl virus. J. Virol. 2013, 87, 4929–4937. [Google Scholar] [CrossRef]
- Ning, W.; Shi, X.; Liu, B.; Pan, H.; Wei, W.; Zeng, Y.; Sun, X.; Xie, W.; Wang, S.; Wu, Q.; et al. Transmission of tomato yellow leaf curl virus by Bemisia tabaci as affected by whitefly sex and biotype open. Sci. Rep. 2015, 5, 10744. [Google Scholar] [CrossRef]
- Milonas, P.G.; Anastasaki, E.; Psoma, A.; Partsinevelos, G.; Fragkopoulos, G.N.; Kektsidou, O.; Vassilakos, N.; Kapranas, A. Plant viruses induce plant volatiles that are detected by aphid parasitoids. Sci. Rep. 2023, 13, 8721. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Infection of host plants by Cucumber mosaic virus increases the susceptibility of Myzus persicae aphids to the parasitoid Aphidius colemani. Sci. Rep. 2015, 5, 10963. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Hardie, J.; Powell, G. Parasitoids aid dispersal of a nonpersistently transmitted plant virus by disturbing the aphid vector. Agric. For. Entomol. 2011, 13, 83–88. [Google Scholar] [CrossRef]
- Christiansen-Weniger, P.; Powell, G.; Hardie, J. Plant virus and parasitoid interactions in a shared insect vector ⁄ host. Entomol. Exp. Appl. 1998, 86, 205–213. [Google Scholar] [CrossRef]
- Hodge, S.; Powell, G. Complex interactions between a plant pathogen and insect parasitoid via the shared vector-host: Consequences for host plant infection. Oecologia 2008, 157, 387–397. [Google Scholar] [CrossRef]
- Sloggett, J.J.; Weisser, W.W. Parasitoids induce production of the dispersal morph of the pea aphid, Acyrthosiphon pisum. Oikos 2002, 98, 323–333. [Google Scholar] [CrossRef]
- Norris, R.H.; Silva-Torres, C.S.; Lujan, M.; Wilson-Rankin, E.E.; Mauck, K.E. Footprints of predatory lady beetles stimulate increased dispersal of aphid prey, but do not alter feeding behavior or spread of a non-persistently transmitted plant virus. Food Webs 2023, 37, e00325. [Google Scholar] [CrossRef]
- Belliure, B.; Amorós-Jiménez, R.; Fereres, A.; Marcos-García, M.Á. Antipredator behaviour of Myzus persicae affects transmission efficiency of broad bean wilt virus 1. Virus Res. 2011, 159, 206–214. [Google Scholar] [CrossRef]
- Bonfim, K.; Faria, J.C.; Nogueira, E.O.P.L.; Mendes, É.A.; Aragão, F.J.L. RNAi-mediated resistance to bean golden mosaic virus in genetically engineered common bean (Phaseolus vulgaris). Mol. Plant-Microbe Interact. 2007, 20, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef]
- Pyott, D.E.; Sheehan, E.; Molnar, A. Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol. Plant Pathol. 2016, 17, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Zhao, D.; Zhao, C.; Yu, H.; Zeng, J.; Tong, Z.; Yuan, C.; Li, Z.; Huang, C. Simultaneous knockout of multiple eukaryotic translation initiation factor 4E genes confers durable and broad-spectrum resistance to potyviruses in tobacco. aBIOTECH 2025, 6, 232–248. [Google Scholar] [CrossRef]
- Li, M.; Qiu, Y.; Zhu, D.; Xu, X.; Tian, S.; Wang, J.; Yu, Y.; Ren, Y.; Gong, G.; Zhang, H.; et al. Editing eIF4E in the watermelon genome using CRISPR/Cas9 technology confers resistance to ZYMV. Int. J. Mol. Sci. 2024, 25, 11468. [Google Scholar] [CrossRef]
- Wen, Z.; Lu, F.; Jung, M.; Humbert, S.; Marshall, L.; Hastings, C.; Wu, E.; Jones, T.; Pacheco, M.; Martinez, I.; et al. Edited eukaryotic translation initiation factors confer resistance against maize lethal necrosis. Plant Biotechnol. J. 2024, 22, 3523–3535. [Google Scholar] [CrossRef]
- Rollwage, L.; Van Houtte, H.; Hossain, R.; Wynant, N.; Willems, G.; Varrelmann, M. Recessive resistance against beet chlorosis virus is conferred by the eukaryotic translation initiation factor (iso)4E in Beta vulgaris. Plant Biotechnol. J. 2024, 22, 2129–2141. [Google Scholar] [CrossRef]
- Gomez, M.A.; Lin, Z.D.; Moll, T.; Chauhan, R.D.; Hayden, L.; Renninger, K.; Beyene, G.; Taylor, N.J.; Carrington, J.C.; Staskawicz, B.J.; et al. Simultaneous CRISPR/Cas9-mediated editing of cassava eIF4E isoforms nCBP-1 and nCBP-2 reduces cassava brown streak disease symptom severity and incidence. Plant Biotechnol. J. 2019, 17, 421–434. [Google Scholar] [CrossRef]
- Le, N.T.; Tran, H.T.; Bui, T.P.; Nguyen, G.T.; Van Nguyen, D.; Ta, D.T.; Trinh, D.D.; Molnar, A.; Pham, N.B.; Chu, H.H.; et al. Simultaneously induced mutations in eIF4E genes by CRISPR/Cas9 enhance PVY resistance in tobacco. Sci. Rep. 2022, 12, 14627. [Google Scholar] [CrossRef]
- Feng, X.; Orellana, G.E.; Myers, J.R.; Karasev, A.V. Recessive resistance to Bean common mosaic virus conferred by the bc-1 and bc-2 genes in common bean (Phaseolus vulgaris) affects long-distance movement of the virus. Phytopathology 2018, 108, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, C.; Liu, Y.; Zeng, J.; Yu, H.; Tong, Z.; Yuan, X.; Sui, X.; Fang, D.; Xiao, B.; et al. CRISPR/Cas9-mediated seamless gene replacement in protoplasts expands the resistance spectrum to TMV-U1 strain in regenerated Nicotiana tabacum. Plant Biotechnol. J. 2023, 21, 2641–2653. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Ali, S.; Tashkandi, M.; Zaidi, S.S.; Mahfouz, M.M. CRISPR/Cas9-mediated immunity to geminiviruses: Differential interference and evasion. Sci. Rep. 2016, 6, 26912. [Google Scholar] [CrossRef]
- Mukeshimana, G.; Ma, Y.; Walworth, A.E.; Song, G.; Kelly, J.D. Factors influencing regeneration and Agrobacterium tumefaciens-mediated transformation of common bean (Phaseolus vulgaris L.). Plant Biotechnol. Rep. 2013, 7, 59–70. [Google Scholar] [CrossRef]
- Moura, M.d.C.; Pinheiro, P.V.; Vianello, R.P.; Sousa, N.L.d.; Faria, J.C.d.; Aragão, F.J.L. Genetic transformation of common beans (Phaseolus vulgaris): Achievements and challenges. Agriculture 2024, 14, 2060. [Google Scholar] [CrossRef]
- Quintela, E.D.; de Souza, T.L.; Faria, J.C.; Aragão, F.J.; e Silva, J.F.; Del Peloso, M.J.; Arthurs, S.P. Comparison of Bemisia tabaci infestation, virus infection, and yield in conventional and transgenic Bean golden mosaic virus-resistant common bean elite lines. Fla. Entomol. 2023, 106, 29–37. [Google Scholar] [CrossRef]
- Hoang, B.T.L.; Fletcher, S.J.; Brosnan, C.A.; Ghodke, A.B.; Manzie, N.; Mitter, N. RNAi as a foliar spray: Efficiency and challenges to field applications. Int. J. Mol. Sci. 2022, 23, 6639. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.; Robinson, K.; Li, P.; Jain, R.; Taochy, C. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef]
- Worrall, E.; Bravo-Cazar, A.; Nilon, A.; Fletcher, S.; Robinson, K.; Carr, J.P.; Mitter, N. Exogenous application of RNAi-inducing double-stranded RNA inhibits aphid-mediated transmission of a plant virus. Front. Plant Sci. 2019, 10, 265. [Google Scholar] [CrossRef]
- Jain, R.G.; Fletcher, S.J.; Manzie, N.; Robinson, K.E.; Li, P.; Lu, E.; Brosnan, C.A.; Xu, Z.P.; Mitter, N. Foliar application of clay-delivered RNA interference for whitefly control. Nat. Plants 2022, 8, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, K.; Peng, J.; Liu, B.; Kong, F.; Sang, Q.; Du, H. Strategies for balancing growth and defence against biotic stress in legumes. Plant Cell Environ. 2025. [Google Scholar] [CrossRef] [PubMed]
- Roossinck, M.J. Lifestyles of plant viruses. Philos. Trans. R. Soc. B 2010, 365, 1899–1905. [Google Scholar] [CrossRef]
- Khankhum, S.; Valverde, R.A. Physiological traits of endornavirus-infected and endornavirus-free common bean (Phaseolus vulgaris) cv Black Turtle Soup. Arch. Virol. 2018, 163, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).