Quarter-Level Milk Yield Recovery Following Clinical Mastitis: Associations with Milk Loss, Somatic Cell Count, Clinical Severity, and Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection, Selection, and Preprocessing
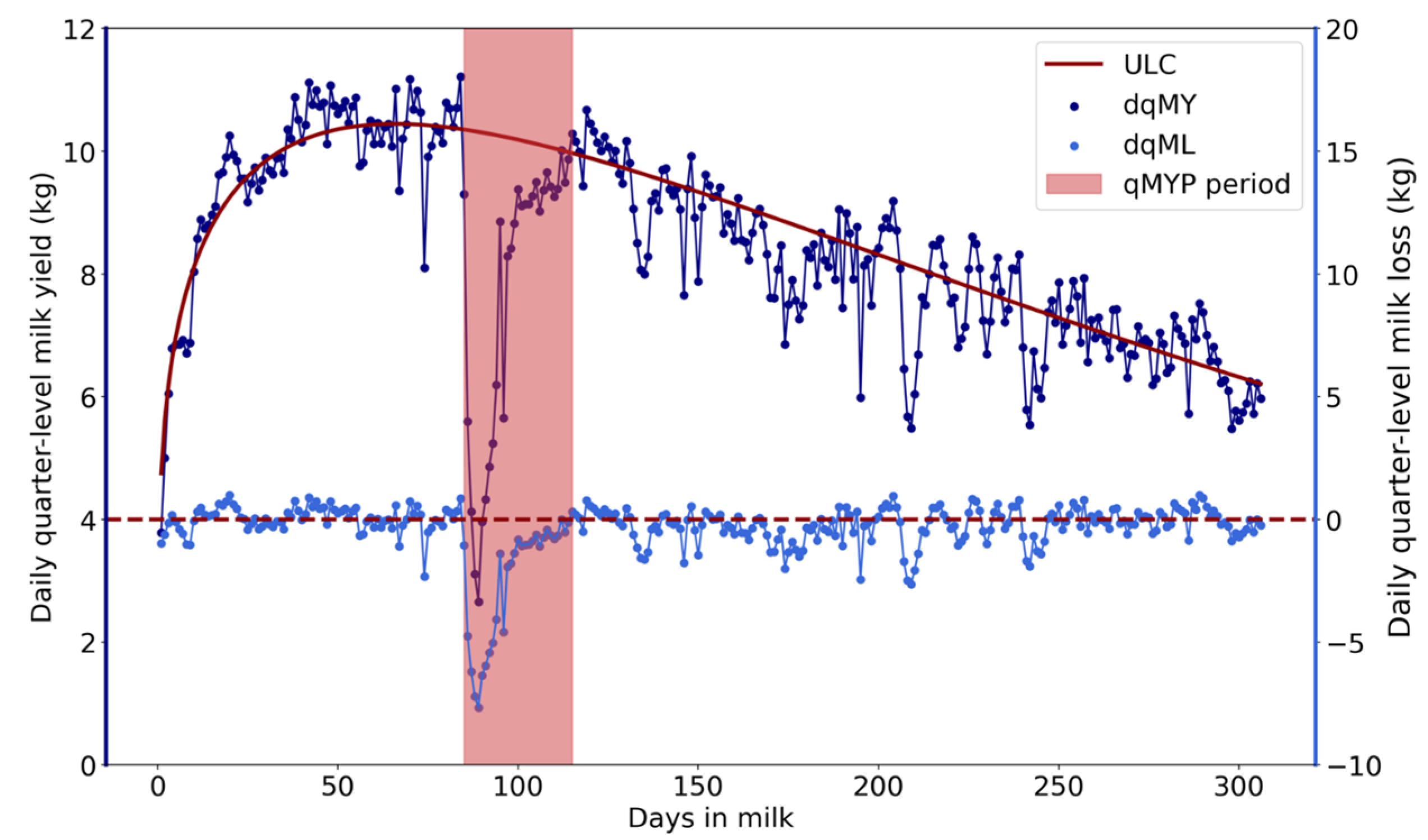
2.2. Detection of Quarter-Level Milk Yield Perturbations Caused by CM
2.3. Percentage Recovery
2.4. Statistical Analysis
2.4.1. Recovery Patterns Between Inflamed and Uninflamed Quarters
2.4.2. Associations Between Quarter-Level Milk Yield Recovery and Milk Loss, Somatic Cell Count, Clinical Severity, and Pathogens
3. Results
3.1. Description of the Clinical Mastitis Cases
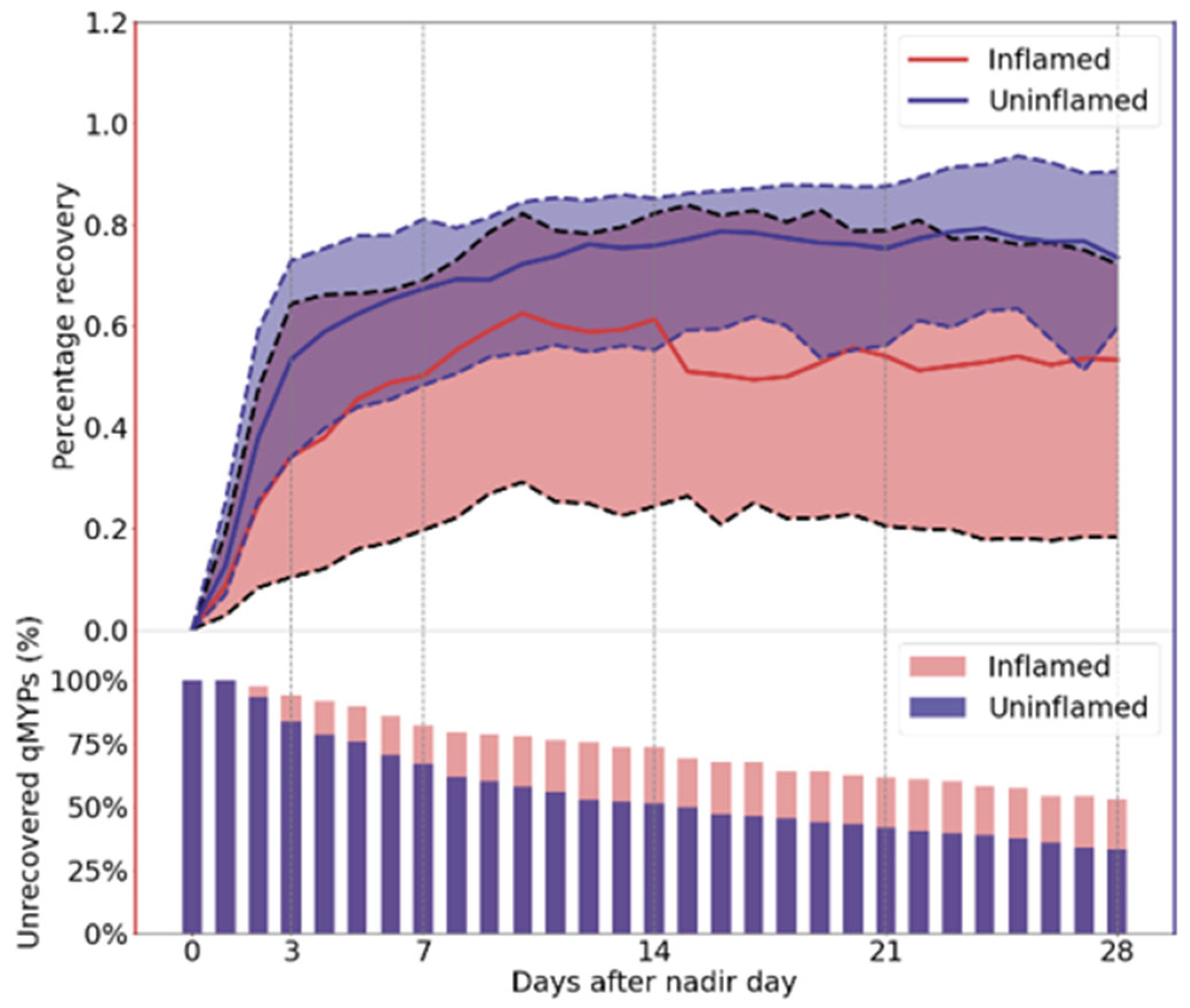
3.2. Percentage Recovery
3.3. Recovery Between Inflamed and Uninflamed Quarters
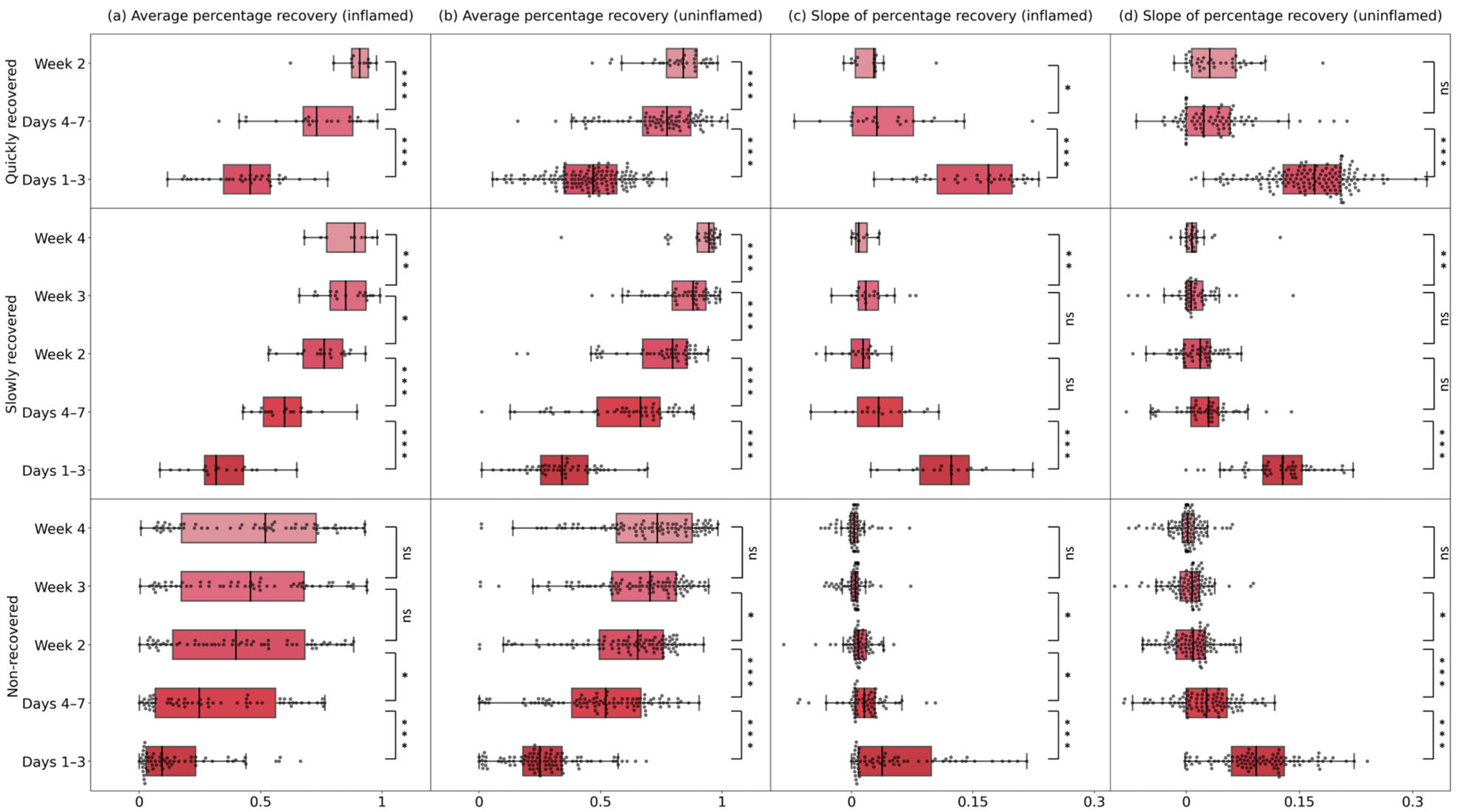
3.4. Recovery Between Adjacent Time Intervals in Inflamed and Uninflamed Quarters
3.5. Associations Between Quarter-Level Milk Yield Recovery and Milk Loss, Somatic Cell Count, Clinical Severity, and Pathogens
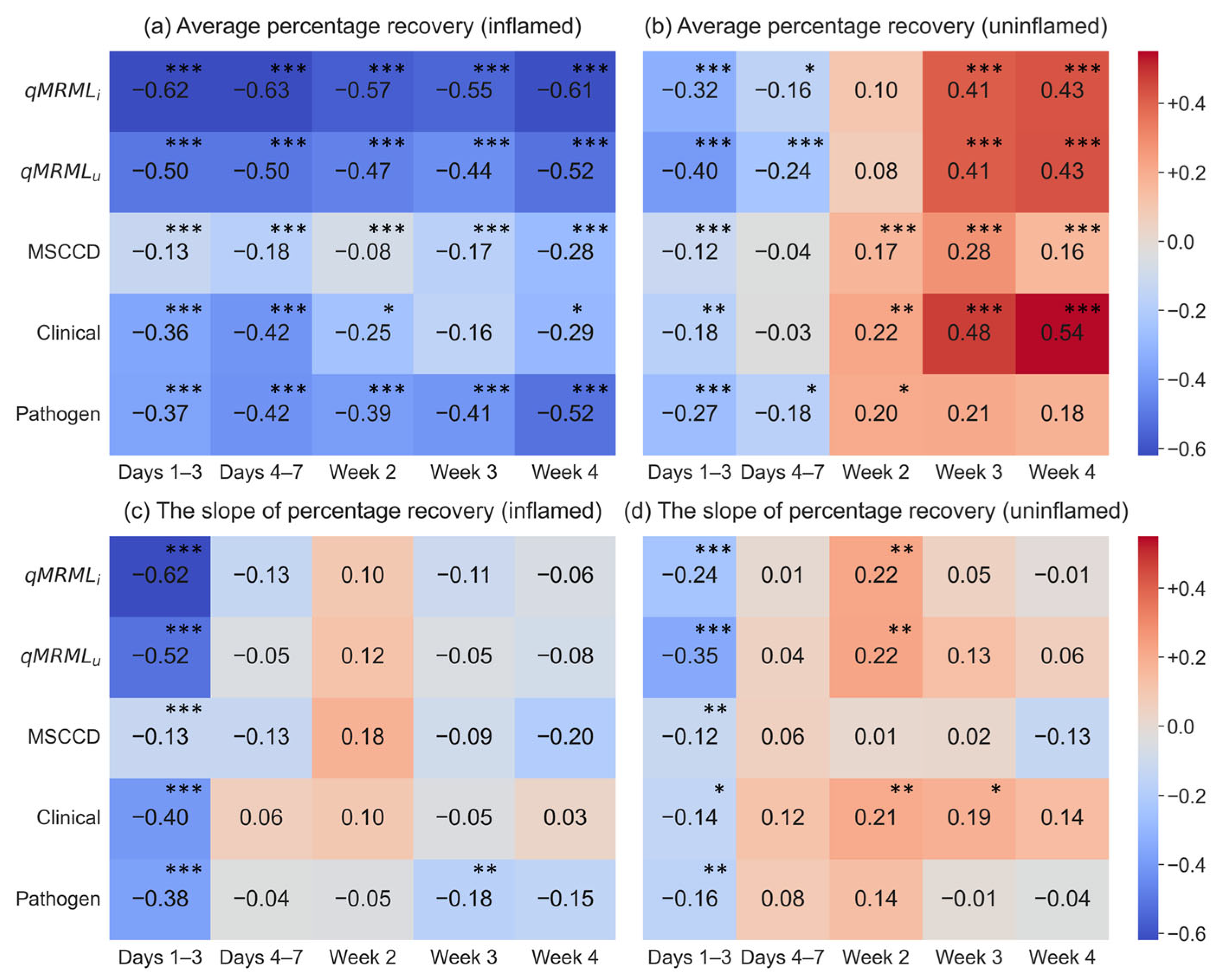
3.5.1. Correlation Analysis
3.5.2. Regression Analysis
4. Discussion
4.1. Description of Clinical Mastitis Cases
4.2. Quantification of the Recovery via Quarter-Level Milk Yield Perturbations
4.3. Percentage Recovery
4.4. Associations Between Quarter-Level Milk Yield Recovery and Milk Loss, Somatic Cell Count, Clinical Severity, and Pathogens
4.5. Application Potential and Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMS | Automatic milking system |
| APR | Average percentage recovery |
| CM | Clinical mastitis |
| CI | Confidence interval |
| dqMY | Daily quarter-level milk yield |
| dqML | Daily quarter-level milk loss |
| IQR | Interquartile range |
| MML | Maximum milk loss |
| MSCCD | Maximum somatic cell count deviation |
| qMRML | Quarter-level maximum relative milk loss |
| qMY | Quarter-level milk yield |
| qMYP | Quarter-level milk yield perturbation |
| SCC | Somatic cell count |
| SPR | Slope of percentage recovery |
| ULC | Unperturbed lactation curve |
| VIF | Variance inflation factor |
References
- He, W.; Ma, S.; Lei, L.; He, J.; Li, X.; Tao, J.; Wang, X.; Song, S.; Wang, Y.; Wang, Y.; et al. Prevalence, Etiology, and Economic Impact of Clinical Mastitis on Large Dairy Farms in China. Vet. Microbiol. 2020, 242, 108570. [Google Scholar] [CrossRef]
- Richardet, M.; Solari, H.G.; Cabrera, V.E.; Vissio, C.; Agüero, D.; Bartolomé, J.A.; Bó, G.A.; Bogni, C.I.; Larriestra, A.J. The Economic Evaluation of Mastitis Control Strategies in Holstein-Friesian Dairy Herds. Animals 2023, 13, 1701. [Google Scholar] [CrossRef] [PubMed]
- Lavon, Y.; Gilad, D.; Leitner, G. Recovery Rates of Treated vs. Non-Treated Dairy Cows with Subclinical Mastitis. Dairy 2021, 2, 576–584. [Google Scholar] [CrossRef]
- Leitner, G.; Blum, S.E.; Krifuks, O.; Edery, N.; Merin, U. Correlation between Milk Bacteriology, Cytology and Mammary Tissue Histology in Cows: Cure from the Pathogen or Recovery from the Inflammation. Pathogens 2020, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.L. Standards for Somatic Cells in Milk: Physiological and Regulatory. Mastitis Newsletter, Int. Dairy Fed. 1996; No. 21. 7–9. [Google Scholar]
- De Vliegher, S.; Fox, L.K.; Piepers, S.; McDougall, S.; Barkema, H.W. Invited Review: Mastitis in Dairy Heifers: Nature of the Disease, Potential Impact, Prevention, and Control. J. Dairy Sci. 2012, 95, 1025–1040. [Google Scholar] [CrossRef]
- Detilleux, J. Tolerance to Bovine Clinical Mastitis: Total, Direct, and Indirect Milk Losses. J. Dairy Sci. 2018, 101, 3334–3343. [Google Scholar] [CrossRef]
- de Jong, E.; McCubbin, K.D.; Speksnijder, D.; Dufour, S.; Middleton, J.R.; Ruegg, P.L.; Lam, T.J.G.M.; Kelton, D.F.; McDougall, S.; Godden, S.M.; et al. Invited Review: Selective Treatment of Clinical Mastitis in Dairy Cattle. J. Dairy Sci. 2023, 106, 3761–3778. [Google Scholar] [CrossRef]
- Stevens, M.; Piepers, S.; De Vliegher, S. Mastitis Prevention and Control Practices and Mastitis Treatment Strategies Associated with the Consumption of (Critically Important) Antimicrobials on Dairy Herds in Flanders, Belgium. J. Dairy Sci. 2016, 99, 2896–2903. [Google Scholar] [CrossRef]
- Rees, A.; Fischer-Tenhagen, C.; Heuwieser, W. Udder Firmness as a Possible Indicator for Clinical Mastitis. J. Dairy Sci. 2017, 100, 2170–2183. [Google Scholar] [CrossRef]
- Fredebeul-Krein, F.; Schmenger, A.; Wente, N.; Zhang, Y.; Krömker, V. Factors Associated with the Severity of Clinical Mastitis. Pathogens 2022, 11, 1089. [Google Scholar] [CrossRef]
- Steeneveld, W.; van Werven, T.; Barkema, H.W.; Hogeveen, H. Cow-Specific Treatment of Clinical Mastitis: An Economic Approach. J. Dairy Sci. 2011, 94, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, I.; van den Brulle, I.; D’Anvers, L.; Statham, J.M.E.; Geerinckx, K.; De Vliegher, S.; Piepers, S.; Aernouts, B. Milk Losses and Dynamics during Perturbations in Dairy Cows Differ with Parity and Lactation Stage. J. Dairy Sci. 2021, 104, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, I.; Huybrechts, T.; Aernouts, B.; Geerinckx, K.; Piepers, S.; De Ketelaere, B.; Saeys, W. Method for Short-Term Prediction of Milk Yield at the Quarter Level to Improve Udder Health Monitoring. J. Dairy Sci. 2018, 101, 10327–10336. [Google Scholar] [CrossRef]
- Ranzato, G.; Aernouts, B.; Lora, I.; Adriaens, I.; Ben Abdelkrim, A.; Gote, M.J.; Cozzi, G. Comparison of 3 Mathematical Models to Estimate Lactation Performance in Dairy Cows. J. Dairy Sci. 2024, 107, 6888–6901. [Google Scholar] [CrossRef]
- Adriaens, I.; Van Den Brulle, I.; Geerinckx, K.; D’Anvers, L.; De Vliegher, S.; Aernouts, B. Milk Losses Linked to Mastitis Treatments at Dairy Farms with Automatic Milking Systems. Prev. Vet. Med. 2021, 194, 105420. [Google Scholar] [CrossRef]
- Sguizzato, A.L.L.; da Silva, T.E.; Chagas, J.C.C.; Argüelo, A.M.; Gonçalves, N.M.; Marcondes, M.I. Understanding the Dynamics of Mastitis in Milk Yield: Decoding Onset and Recovery Patterns in Response to Mastitis Occurrence. JDS Commun. 2024, 5, 669–673. [Google Scholar] [CrossRef]
- D’Anvers, L.; Adriaens, I.; Piepers, S.; Gote, M.J.; De Ketelaere, B.; Aernouts, B. Association between Management Practices and Estimated Mastitis Incidence and Milk Losses on Robotic Dairy Farms. Prev. Vet. Med. 2023, 220, 106033. [Google Scholar] [CrossRef]
- Shinozuka, Y.; Kaneko, S.; Kurose, T.; Watanabe, A.; Kuruhara, K.; Kawai, K. Factors Associated with Marketable Milk Production Recovery after Treatment of Naturally Occurring Acute Coliform Mastitis. J. Vet. Med. Sci. 2016, 78, 917–920. [Google Scholar] [CrossRef]
- Kirkeby, C.; Schwarz, D.; Denwood, M.; Farre, M.; Nielsen, S.S.; Gussmann, M.; Toft, N.; Halasa, T. Dynamics of Somatic Cell Count (SCC) and Differential SCC during and Following Intramammary Infections. J. Dairy Sci. 2021, 104, 3427–3438. [Google Scholar] [CrossRef]
- Ferrero, F.J.; Valledor, M.; Campo, J.C. Screening Method for Early Detection of Mastitis in Cows. Measurement 2014, 47, 855–860. [Google Scholar] [CrossRef]
- Fogsgaard, K.K.; Løvendahl, P.; Bennedsgaard, T.W.; Østergaard, S. Changes in Milk Yield, Lactate Dehydrogenase, Milking Frequency, and Interquarter Yield Ratio Persist for up to 8 Weeks after Antibiotic Treatment of Mastitis. J. Dairy Sci. 2015, 98, 7686–7698. [Google Scholar] [CrossRef]
- Hammer, J.; Morton, J.; Kerrisk, K. Quarter-milking-, Quarter-, Udder- and Lactation-level Risk Factors and Indicators for Clinical Mastitis during Lactation in Pasture-fed Dairy Cows Managed in an Automatic Milking System. Aust. Vet. J. 2012, 90, 167–174. [Google Scholar] [CrossRef]
- Lusis, I.; Antane, V.; Laurs, A. Effectiveness of Mastitis Detection Index for Cow Monitoring and Abnormal Milk Detection in Milking Robots. Eng. Rural. Dev. 2017, 16, 1383–1387. [Google Scholar] [CrossRef]
- ICAR. ICAR Guidelines for testing & certification of measuring, recording and sampling devices or sensor systems. Available online: https://www.icar.org/Guidelines/11-Milk-recording-devices-Overview.pdf (accessed on 8 August 2024).
- ICAR. ICAR Guidelines for milk analysis. Available online: https://www.icar.org/Guidelines/12-Milk-Analysis.pdf (accessed on 7 May 2024).
- ISO. Milk: Enumeration of Somatic Cells. Available online: https://www.iso.org/standard/40260.html#:~:text=ISO%2013366%2D2%7CIDF%20148,applied%20in%20the%20counting%20section (accessed on 7 May 2024).
- Fernandes, L.; Guimaraes, I.; Noyes, N.R.; Caixeta, L.S.; Machado, V.S. Effect of Subclinical Mastitis Detected in the First Month of Lactation on Somatic Cell Count Linear Scores, Milk Yield, Fertility, and Culling of Dairy Cows in Certified Organic Herds. J. Dairy Sci. 2021, 104, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Rainard, P.; Foucras, G.; Boichard, D.; Rupp, R. Invited Review: Low Milk Somatic Cell Count and Susceptibility to Mastitis. J. Dairy Sci. 2018, 101, 6703–6714. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; D’Anvers, L.; Gote, M.; Geerinckx, K.; Piepers, S.; de Vliegher, S.; Aernouts, B.; Adriaens, I. Evaluating Clinical Mastitis in Four Dimensions: Definition and Correlation Analysis of the Production, Somatic Cell Count, Clinical and Pathogen Severity. Prev. Vet. Med. 2025; under review. [Google Scholar] [CrossRef]
- Salsberg, E.; Meek, A.H.; Martin, S.W. Somatic Cell Counts: Associated Factors and Relationship to Production. Can. J. Comp. Med. 1984, 48, 251. [Google Scholar] [PubMed]
- NMC. NMC Guidelines. Available online: https://www.nmconline.org/nmc-protocols-guidelines-and-procedures/ (accessed on 13 June 2023).
- MCC Standard Culture. Available online: https://www.mcc-vlaanderen.be/nl/content/interpretatie-0 (accessed on 18 August 2025).
- Hogan, J.; Gonzalez, R.; Harmon, R.; Nickerson, S.C.; Oliver, S.; Pankey, J.; Smith, K.L. Laboratory Handbook on Bovine Mastitis; National Mastitis Council: Madison, WI, USA, 1999. [Google Scholar]
- Sol, J. Effect of Preculture Freezing and Incubation on Bacteriological Isolation from Subclinical Mastitis Samples. Vet. Microbiol. 2002, 85, 241–249. [Google Scholar] [CrossRef]
- Poppe, M.; Veerkamp, R.F.; van Pelt, M.L.; Mulder, H.A. Exploration of Variance, Autocorrelation, and Skewness of Deviations from Lactation Curves as Resilience Indicators for Breeding. J. Dairy Sci. 2020, 103, 1667–1684. [Google Scholar] [CrossRef]
- Nielsen, C. Economic Impact of Mastitis in Dairy Cows. Doctoral Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2009. [Google Scholar]
- Winter, P.; Hofrichter, J.; Obritzhauser, W.; Zottl, K.; Egger-Danner, C. Health Monitoring in Austria–Statistical Models Based on Somatic Cell Count at Cow Level for Early Detection of Udder Health Problems Developed. In Proceedings of the ICAR 37th Annual Meeting, Riga, Latvia, 31 May–4 June 2010; pp. 153–157. [Google Scholar]
- Ruegg, P.L. New Perspectives in Udder Health Management. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 149–163. [Google Scholar] [CrossRef]
- Verbeke, J.; Piepers, S.; Supré, K.; De Vliegher, S. Pathogen-Specific Incidence Rate of Clinical Mastitis in Flemish Dairy Herds, Severity, and Association with Herd Hygiene. J. Dairy Sci. 2014, 97, 6926–6934. [Google Scholar] [CrossRef]
- Rowe, S.M.; Godden, S.M.; Royster, E.; Timmerman, J.; Boyle, M. Postcalving Udder Health and Productivity in Cows Approaching Dry-off with Intramammary Infections Caused by Non-Aureus Staphylococcus, Aerococcus, Enterococcus, Lactococcus, and Streptococcus Species. J. Dairy Sci. 2021, 104, 6061–6079. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Ding, T.; Liu, Y.; Zhou, X.; Du, J. The Yeast and Hypha Phases of Candida Krusei Induce the Apoptosis of Bovine Mammary Epithelial Cells via Distinct Signaling Pathways. Animals 2023, 13, 3222. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, D.; Mizomoto, T.; Ueda, C.; Takagi, N.; Shimizu, N.; Matsuura, Y.; Makuuchi, Y.; Watanabe, A.; Shinozuka, Y.; Kawai, K. Factors Affecting the Incidence and Outcome of Trueperella pyogenes Mastitis in Cows. J. Vet. Med. Sci. 2017, 79, 626–631. [Google Scholar] [CrossRef] [PubMed]
- D’Anvers, L.; Adriaens, I.; Van Den Brulle, I.; Valckenier, D.; Salamone, M.; Piepers, S.; De Vliegher, S.; Aernouts, B. Key Udder Health Parameters on Dairy Farms with an Automated Milking System. Livest. Sci. 2024, 287, 105522. [Google Scholar] [CrossRef]
- Levison, L.J.; Miller-Cushon, E.K.; Tucker, A.L.; Bergeron, R.; Leslie, K.E.; Barkema, H.W.; DeVries, T.J. Incidence Rate of Pathogen-Specific Clinical Mastitis on Conventional and Organic Canadian Dairy Farms. J. Dairy Sci. 2016, 99, 1341–1350. [Google Scholar] [CrossRef]
- Ballou, M.A. GROWTH AND DEVELOPMENT SYMPOSIUM: Inflammation: Role in the Etiology and Pathophysiology of Clinical Mastitis in Dairy Cows1. J. Anim. Sci. 2012, 90, 1466–1478. [Google Scholar] [CrossRef]
- Zhao, X.; Lacasse, P. Mammary Tissue Damage during Bovine Mastitis: Causes and Control1. J. Anim. Sci. 2008, 86 (Suppl. 13), 57–65. [Google Scholar] [CrossRef]
- Wellnitz, O.; Bruckmaier, R.M. The Innate Immune Response of the Bovine Mammary Gland to Bacterial Infection. Vet. J. 2012, 192, 148–152. [Google Scholar] [CrossRef]
- Oliveira, L.; Hulland, C.; Ruegg, P.L. Characterization of Clinical Mastitis Occurring in Cows on 50 Large Dairy Herds in Wisconsin. J. Dairy Sci. 2013, 96, 7538–7549. [Google Scholar] [CrossRef]
- Tomazi, T.; Ferreira, G.C.; Orsi, A.M.; Gonçalves, J.L.; Ospina, P.A.; Nydam, D.V.; Moroni, P.; dos Santos, M.V. Association of Herd-Level Risk Factors and Incidence Rate of Clinical Mastitis in 20 Brazilian Dairy Herds. Prev. Vet. Med. 2018, 161, 9–18. [Google Scholar] [CrossRef]
- Hertl, J.A.; Schukken, Y.H.; Welcome, F.L.; Tauer, L.W.; Gröhn, Y.T. Pathogen-Specific Effects on Milk Yield in Repeated Clinical Mastitis Episodes in Holstein Dairy Cows. J. Dairy Sci. 2014, 97, 1465–1480. [Google Scholar] [CrossRef]
- Ben Abdelkrim, A.; Tribout, T.; Martin, O.; Boichard, D.; Ducrocq, V.; Friggens, N.C. Exploring Simultaneous Perturbation Profiles in Milk Yield and Body Weight Reveals a Diversity of Animal Responses and New Opportunities to Identify Resilience Proxies. J. Dairy Sci. 2021, 104, 459–470. [Google Scholar] [CrossRef]
- Vilar, M.J.; Rajala-Schultz, P.J. Dry-off and Dairy Cow Udder Health and Welfare: Effects of Different Milk Cessation Methods. Vet. J. 2020, 262, 105503. [Google Scholar] [CrossRef]
- Ranzato, G.; Lora, I.; Aernouts, B.; Adriaens, I.; Gottardo, F.; Cozzi, G. Sensor-Based Behavioral Patterns Can Identify Heat-Sensitive Lactating Dairy Cows. Int. J. Biometeorol. 2023, 67, 2047–2054. [Google Scholar] [CrossRef]
- Rajala-Schultz, P.J.; Gröhn, Y.T.; McCulloch, C.E.; Guard, C.L. Effects of Clinical Mastitis on Milk Yield in Dairy Cows. J. Dairy Sci. 1999, 82, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Diegelmann, R.F. Wound Healing: An Overview of Acute, Fibrotic and Delayed Healing. Front. Biosci. 2004, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, A.-M.; Liski, E.; Pyörälä, S.; Taponen, S. Pathogen-Specific Production Losses in Bovine Mastitis. J. Dairy Sci. 2018, 101, 9493–9504. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.S. THE DEPRESSION OF QUARTER MILK YIELD CAUSED BY BOVINE MASTITIS, AND THE RESPONSE OF YIELD TO SUCCESSFUL THERAPY. Aust. Vet. J. 1973, 49, 153–156. [Google Scholar] [CrossRef]
- Hamann, J.; Reichmuth, J. Compensatory Milk Production within the Bovine Udder: Effects of Short-Term Non-Milking of Single Quarters. J. Dairy Res. 1990, 57, 17–22. [Google Scholar] [CrossRef]
- Hagnestam-Nielsen, C.; Emanuelson, U.; Berglund, B.; Strandberg, E. Relationship between Somatic Cell Count and Milk Yield in Different Stages of Lactation. J. Dairy Sci. 2009, 92, 3124–3133. [Google Scholar] [CrossRef]
- Morin, D.E.; Constable, P.D. Characteristics of Dairy Cows during Episodes of Bacteriologically Negative Clinical Mastitis or Mastitis Caused by Corynebacterium Spp. J. Am. Vet. Med. Assoc. 1998, 213, 855–861. [Google Scholar] [CrossRef]
- Gonzalez, R.N.; Jasper, D.E.; Kronlund, N.C.; Farver, T.B.; Cullor, J.S.; Bushnell, R.B.; Dellinger, J.D. Clinical Mastitis in Two California Dairy Herds Participating In Contagious Mastitis Control Programs. J. Dairy Sci. 1990, 73, 648–660. [Google Scholar] [CrossRef]
- Gröhn, Y.T.; Wilson, D.J.; González, R.N.; Hertl, J.A.; Schulte, H.; Bennett, G.; Schukken, Y.H. Effect of Pathogen-Specific Clinical Mastitis on Milk Yield in Dairy Cows. J. Dairy Sci. 2004, 87, 3358–3374. [Google Scholar] [CrossRef]
- Tsugami, Y.; Chiba, T.; Obayashi, T.; Higuchi, H.; Watanabe, A.; Isobe, N.; Kawai, K. Differences in Antimicrobial Components between Bacterial Culture-positive and Culture-negative Bovine Clinical Mastitis Milk. Anim. Sci. J. 2022, 93, e13771. [Google Scholar] [CrossRef]
- Kuehn, J.S.; Gorden, P.J.; Munro, D.; Rong, R.; Dong, Q.; Plummer, P.J.; Wang, C.; Phillips, G.J. Bacterial Community Profiling of Milk Samples as a Means to Understand Culture-Negative Bovine Clinical Mastitis. PLoS ONE 2013, 8, e61959. [Google Scholar] [CrossRef]

| Classification | Pathogens |
|---|---|
| Major pathogens | Staphylococcus aureus (S. aureus), Streptococcus, Strep-like organisms, Coliforms, Yeasts, Serratia spp., Klebsiella spp., and Trueperella pyogenes |
| Minor pathogens | Citrobacter spp., Corynebacterium spp., Enterococcus spp., Lactococcus spp., Non-aureus Staphylococcen, and Staphylococcus spp. (except S. aureus) |
| Culture-negative | Culture-negative, Aerococcus spp., Bacillus cereus, or Bacillus spp. |
| Quarter-Level Maximum Relative Milk Loss | Maximum Somatic Cell Count Deviation (Mean ± Std) | Number of Cases (Percentage *) | ||
|---|---|---|---|---|
| Inflamed (Mean ± Std) | Uninflamed (Mean ± Std) | |||
| Clinical severity | ||||
| Mild | 0.50 ± 0.25 | 0.24 ± 0.20 | 3.30 ± 0.97 | 48 (41%) |
| Moderate | 0.67 ± 0.27 | 0.36 ± 0.22 | 3.92 ± 0.64 | 36 (31%) |
| Severe | 0.81 ± 0.21 | 0.59 ± 0.28 | 3.88 ± 1.13 | 33 (28%) |
| Causative pathogens | ||||
| Culture-negative | 0.54 ± 0.24 | 0.29 ± 0.22 | 3.9 ± 1.20 | 29 (25%) |
| Minor pathogens | 0.46 ± 0.22 | 0.20 ± 0.12 | 3.25 ± 0.86 | 17 (15%) |
| Major pathogens | 0.73 ± 0.27 | 0.46 ± 0.28 | 3.86 ± 0.84 | 71 (61%) |
| Total | 0.64 ± 0.28 | 0.38 ± 0.27 | 3.65 ± 0.97 | 117 (100%) |
| Quarter-Level Milk Yield Perturbations NUMBERS (Percentage) | ||
|---|---|---|
| Inflamed | Uninflamed | |
| Recovered within day 1–3 | 10 (9%) | 64 (21%) |
| Recovered within day 4–7 | 13 (11%) | 50 (17%) |
| Recovered within week 2 | 13 (11%) | 36 (12%) |
| Recovered within week 3 | 10 (9%) | 27 (9%) |
| Recovered within week 4 | 9 (8%) | 26 (9%) |
| Not recovered | 62 (53%) | 96 (32%) |
| Total | 117 (100%) | 299 (100%) |
| Number of Cases | Time Interval | Average Percentage Recovery | Slope of Percentage Recovery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Inflamed | Uninflamed | Median ± IQR* | p-Value | Median ± IQR* | p-Value | ||||
| Inflamed | Uninflamed | Inflamed | Uninflamed | ||||||
| Quickly recovered | 36 | 150 | Days 1–3 | 0.46 ± 0.19 | 0.47 ± 0.22 | 0.65 | 0.1691 ± 0.0921 | 0.1700 ± 0.0766 | 0.32 |
| Days 4–7 | 0.73 ± 0.20 | 0.77 ± 0.20 | 0.84 | 0.0314 ± 0.0752 | 0.0232 ± 0.0581 | 0.32 | |||
| Week 2 | 0.91 ± 0.07 | 0.84 ± 0.13 | 0.06 | 0.0277 ± 0.0250 | 0.0313 ± 0.0584 | 0.16 | |||
| Slowly recovered | 19 | 53 | Days 1–3 | 0.32 ± 0.16 | 0.34 ± 0.19 | 0.84 | 0.1233 ± 0.0607 | 0.1279 ± 0.0516 | 0.574 |
| Days 4–7 | 0.60 ± 0.16 | 0.66 ± 0.26 | 0.36 | 0.0366 ± 0.0554 | 0.0298 ± 0.0369 | 0.51 | |||
| Week 2 | 0.76 ± 0.16 | 0.80 ± 0.18 | 0.51 | 0.0142 ± 0.0227 | 0.0187 ± 0.0351 | 0.41 | |||
| Week 3 | 0.85 ± 0.15 | 0.88 ± 0.14 | 1 | 0.0177 ± 0.0249 | 0.0068 ± 0.0220 | 0.02 * | |||
| Week 4 | 0.89 ± 0.16 | 0.95 ± 0.07 | 0.14 | 0.0092 ± 0.0140 | 0.0079 ± 0.0133 | 0.43 | |||
| Non-recovered | 62 | 96 | Days 1–3 | 0.09 ± 0.20 | 0.25 ± 0.16 | <0.001 * | 0.0377 ± 0.0899 | 0.0928 ± 0.0697 | <0.001 * |
| Days 4–7 | 0.25 ± 0.50 | 0.52 ± 0.29 | <0.001 * | 0.0160 ± 0.0248 | 0.0271 ± 0.0540 | 0.11 | |||
| Week 2 | 0.40 ± 0.54 | 0.65 ± 0.26 | <0.001 * | 0.0087 ± 0.0150 | 0.0094 ± 0.0384 | 0.96 | |||
| Week 3 | 0.46 ± 0.51 | 0.70 ± 0.27 | <0.001 * | 0.0051 ± 0.0079 | 0.0080 ± 0.0254 | 0.14 | |||
| Week 4 | 0.52 ± 0.55 | 0.73 ± 0.31 | <0.001 * | 0.0033 ± 0.0089 | 0.0020 ± 0.0159 | 0.54 | |||
| Total | 117 | 299 | Days 1–3 | 0.24 ± 0.35 | 0.36 ± 0.25 | <0.001 * | 0.0902 ± 0.1276 | 0.1381 ± 0.0959 | <0.001 * |
| Days 4–7 | 0.50 ± 0.50 | 0.66 ± 0.31 | <0.001 * | 0.0210 ± 0.0352 | 0.0279 ± 0.0512 | 0.46 | |||
| Week 2 | 0.62 ± 0.51 | 0.74 ± 0.25 | <0.001 * | 0.0094 ± 0.0188 | 0.0174 ± 0.0351 | 0.22 | |||
| Week 3 | 0.63 ± 0.56 | 0.78 ± 0.26 | <0.001 * | 0.0069 ± 0.0118 | 0.0079 ± 0.0217 | 0.67 | |||
| Week 4 | 0.55 ± 0.57 | 0.78 ± 0.33 | <0.001 * | 0.0038 ± 0.0086 | 0.0031 ± 0.0148 | 0.64 | |||
| Model Parameters | VIF * | Days 1–3 | Days 4–7 | Week 2 | Week 3 | Week 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflamed (95% CI *) | Uninflamed (95% CI *) | Inflamed (95% CI *) | Uninflamed (95% CI *) | Inflamed (95% CI *) | Uninflamed (95% CI *) | Inflamed (95% CI *) | Uninflamed (95% CI *) | Inflamed (95% CI *) | Uninflamed (95% CI *) | ||
| qMRMLi * | 1.97 | −0.07 *** [−0.11, −0.03] | 0.01 [−0.01, 0.04] | −0.10 *** [−0.16, −0.04] | 0.02 [−0.01, 0.07] | −0.13 ** [−0.20, −0.05] | 0.02 [−0.02, 0.04] | −0.11 * [−0.21, −0.03] | 0.03 [0.00, 0.06] | −0.09 [−0.20, 0.01] | 0.04 [−0.01, 0.09] |
| qMRMLu * | 2.1 | −0.06 ** [−0.09, −0.01] | −0.09 *** [−0.12, −0.07] | −0.08 ** [−0.14, −0.02] | −0.09 *** [−0.12, −0.04] | −0.11 ** [−0.17, −0.05] | −0.04 ** [−0.06, 0.00] | −0.09 * [−0.18, −0.04] | 0.00 [−0.04, 0.03] | −0.07 [−0.14, 0.02] | 0.02 [−0.03, 0.07] |
| MSCCD * | 1.15 | 0.03 * [0.02, 0.08] | 0.00 [−0.01, 0.02] | 0.05 * [0.01, 0.10] | 0.00 [−0.04, 0.02] | 0.08 ** [0.03, 0.13] | 0.02 [−0.02, 0.04] | 0.07 * [0.01, 0.13] | 0.02 [−0.00, 0.05] | 0.04 [−0.02, 0.11] | −0.03 [−0.05, 0.01] |
| Clinical severity | 1.45 | ||||||||||
| Mild | |||||||||||
| Moderate | −0.09 * [−0.14, −0.01] | 0.02 [−0.02, 0.07] | −0.13 * [−0.24, −0.03] | 0.07 [−0.02, 0.12] | −0.01 [−0.17, 0.07] | 0.06 [−0.02, 0.10] | −0.08 [−0.20, 0.10] | 0.06 * [0.00, 0.13] | −0.08 [−0.26, 0.06] | 0.15 *** [0.06, 0.23] | |
| Severe | −0.03 [−0.09, 0.06] | 0.02 [−0.03, 0.07] | −0.01 [−0.14, 0.11] | 0.07 [−0.01, 0.14] | 0.12 [0.00, 0.27] | 0.12 *** [0.04, 0.17] | 0.15 [−0.05, 0.36] | 0.15 *** [0.07, 0.23] | 0.08 [−0.10, 0.25] | 0.22 *** [0.11, 0.33] | |
| Pathogens | 1.17 | ||||||||||
| Culture-negative | |||||||||||
| Minor pathogens | 0.07 [−0.01, 0.17] | −0.05 [−0.11, 0.01] | −0.03 [−0.17, 0.12] | −0.12 * [−0.22, −0.03] | −0.07 [−0.26, 0.09] | −0.13 ** [−0.21, −0.03] | −0.13 [−0.35, 0.08] | −0.17 *** [−0.25, −0.08] | −0.09 [−0.30, 0.17] | −0.07 [−0.16, 0.08] | |
| Major pathogens | −0.04 [−0.13, 0.01] | −0.07 ** [−0.12, −0.02] | −0.13 * [−0.23, −0.02] | −0.07 * [−0.14, 0.00] | −0.13 * [−0.26, −0.04] | 0.06 [0.00, 0.13] | −0.21 ** [−0.33, −0.04] | −0.04 [−0.09, 0.03] | −0.24 ** [−0.38, −0.03] | −0.07 [−0.16, 0.01] | |
| Intercept | 21.51 | 0.32 *** [0.25, 0.38] | 0.41 *** [0.37, 0.45] | 0.60 *** [0.50, 0.70] | 0.65 *** [0.59, 0.73] | 0.64 *** [0.54, 0.76] | 0.62 *** [0.57, 0.69] | 0.72 *** [0.55, 0.84] | 0.71 *** [0.64, 0.77] | 0.75 *** [0.55, 0.89] | 0.69 *** [0.61, 0.78] |
| R2 | 50.52% | 30.14% | 52.93% | 13.44% | 48.01% | 23.69% | 43.07% | 46.62% | 44.28% | 45.38% | |
| Model Parameters | VIF * | Days 1–3 | Days 4–7 | Week 2 | Week 3 | Week 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflamed (95% CI *) | Uninflamed (95% CI *) | Inflamed (95% CI *) | Uninflamed (95% CI *) | Inflamed (95% CI *) | Uninflamed (95% CI *) | Inflamed (95% CI *) | Uninflamed (95% CI *) | Inflamed (95% CI *) | Uninflamed (95% CI *) | ||
| qMRMLi * | 1.97 | −0.0182 * [−0.0332, −0.0057] | 0.0067 [−0.0028, 0.0160] | −0.0143 ** [−0.0246, −0.0039] | −0.0070 [−0.0143, 0.0004] | 0.0042 [−0.0008, 0.0094] | −0.0028 [−0.0087, 0.0040] | −0.0017 [−0.0052, 0.0050] | −0.0098 *** [−0.0116, −0.0017] | −0.0007 [−0.0049, 0.0035] | −0.0001 [−0.0061, 0.0039] |
| qMRMLu * | 2.1 | −0.0223 ** [−0.0360, −0.0077] | −0.0332 *** [−0.0418, −0.0232] | 0.0022 [−0.0076, 0.0119] | 0.0012 [−0.0048, 0.0092] | −0.0020 [−0.0059, 0.0026] | 0.0060 * [0.0007, 0.0120] | 0.0008 [−0.0028, 0.0053] | 0.0069 * [0.0007, 0.0104] | −0.0001 [−0.0042, 0.0029] | 0.0008 [−0.0042, 0.0056] |
| MSCCD * | 1.15 | 0.0122 * [0.0016, 0.0227] | −0.0016 [−0.0085, 0.0054] | −0.0072 * [−0.0144, 0.0000] | 0.0044 [−0.0016, 0.0086] | 0.0020 [−0.0009, 0.0057] | −0.0016 [−0.0069, 0.0017] | −0.0027 [−0.0063, 0.0007] | −0.002 [−0.0066, 0.0007] | −0.0037 * [−0.0052, 0.0000] | −0.0012 [−0.0044, 0.0010] |
| Clinical severity | 1.45 | ||||||||||
| Mild | |||||||||||
| Moderate | −0.0434 *** [−0.0686, −0.0195] | 0.0101 [−0.0073, 0.0265] | 0.0212 * [0.0043, 0.0380] | 0.0081 [−0.0066, 0.0209] | 0.0020 [−0.0075, 0.0081] | 0.0022 [−0.0071, 0.0168] | 0.0073 [−0.0036, 0.0131] | 0.0182 *** [0.0081, 0.0261] | 0.0012 [−0.0077, 0.0059] | −0.0011 [−0.0069, 0.0105] | |
| Severe | −0.0143 [−0.0436, 0.0144] | 0.0107 [−0.0100, 0.0275] | 0.0290 ** [0.0084, 0.0497] | 0.0122 [−0.0038, 0.0245] | 0.0039 [−0.0074, 0.0112] | 0.0125 * [0.0026, 0.0263] | 0.0036 [−0.0071, 0.0105] | 0.0147 * [0.0034, 0.0244] | 0.0038 [−0.0054, 0.0099] | 0.0028 [−0.0062, 0.0157] | |
| Pathogens | 1.17 | ||||||||||
| Culture-negative | |||||||||||
| Minor pathogens | 0.0096 [−0.0172, 0.0489] | −0.0193 [−0.0437, 0.0008] | −0.0075 [−0.0292, 0.0143] | 0.0226 * [0.0049, 0.0416] | 0.0022 [−0.0112, 0.0111] | 0.0141 [−0.0042, 0.0282] | −0.0083 [−0.0203, 0.0037] | −0.0116 [−0.0109, 0.0137] | 0.0134 [−0.0023, 0.0248] | 0.0136 * [0.0046, 0.0194] | |
| Major pathogens | −0.0273 * [−0.0513, −0.0014] | −0.0118 [−0.0292, 0.0059] | 0.0066 [−0.0105, 0.0237] | 0.0132 [0.0005, 0.0275] | −0.0076 [−0.0177, 0.0020] | 0.0094 [−0.0038, 0.0197] | −0.0089 * [−0.0191, −0.0022] | −0.0063 [−0.0132, 0.0033] | 0.0017 [−0.0021, 0.0117] | 0.003 [−0.0071, 0.0108] | |
| Intercept | 21.51 | 0.1295 *** [0.1058, 0.1525] | 0.1396 *** [0.1239, 0.1567] | 0.0080 [−0.0080, 0.0239] | 0.0093 [−0.0031, 0.0227] | 0.0142 *** [0.0098, 0.0252] | 0.0001 [−0.0130, 0.0109] | 0.0103 * [0.0032, 0.0204] | 0.0006 [−0.0087, 0.0082] | 0.0014 [−0.0063, 0.0077] | −0.0003 [−0.0088, 0.0085] |
| R2 | 51.46% | 22.47% | 16.48% | 7.71% | 9.19% | 10.69% | 15.25% | 19.27% | 15.82% | 6.75% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; D’Anvers, L.; Gote, M.J.; Adriaens, I.; Aernouts, B. Quarter-Level Milk Yield Recovery Following Clinical Mastitis: Associations with Milk Loss, Somatic Cell Count, Clinical Severity, and Pathogens. Agriculture 2025, 15, 1805. https://doi.org/10.3390/agriculture15171805
Song Y, D’Anvers L, Gote MJ, Adriaens I, Aernouts B. Quarter-Level Milk Yield Recovery Following Clinical Mastitis: Associations with Milk Loss, Somatic Cell Count, Clinical Severity, and Pathogens. Agriculture. 2025; 15(17):1805. https://doi.org/10.3390/agriculture15171805
Chicago/Turabian StyleSong, Yifan, Lore D’Anvers, Martin Julius Gote, Ines Adriaens, and Ben Aernouts. 2025. "Quarter-Level Milk Yield Recovery Following Clinical Mastitis: Associations with Milk Loss, Somatic Cell Count, Clinical Severity, and Pathogens" Agriculture 15, no. 17: 1805. https://doi.org/10.3390/agriculture15171805
APA StyleSong, Y., D’Anvers, L., Gote, M. J., Adriaens, I., & Aernouts, B. (2025). Quarter-Level Milk Yield Recovery Following Clinical Mastitis: Associations with Milk Loss, Somatic Cell Count, Clinical Severity, and Pathogens. Agriculture, 15(17), 1805. https://doi.org/10.3390/agriculture15171805






