Effect of Cutting Age on Seed Production of Flemingia Macrophylla for the Optimisation of Cropping Systems, Cotopaxi-Ecuador
Abstract
1. Introduction
2. Materials and Methods
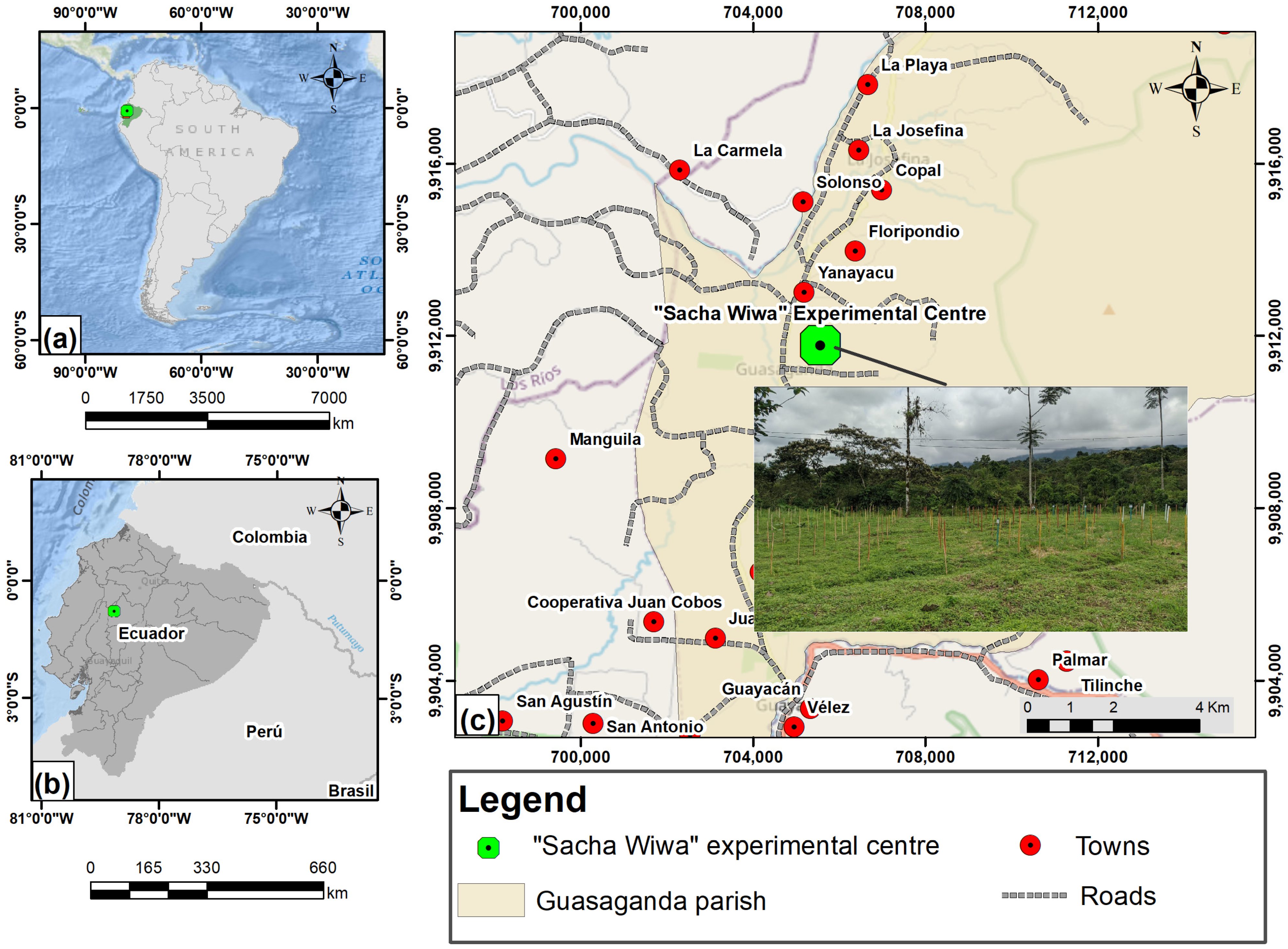
2.1. Case Study
2.2. Methods
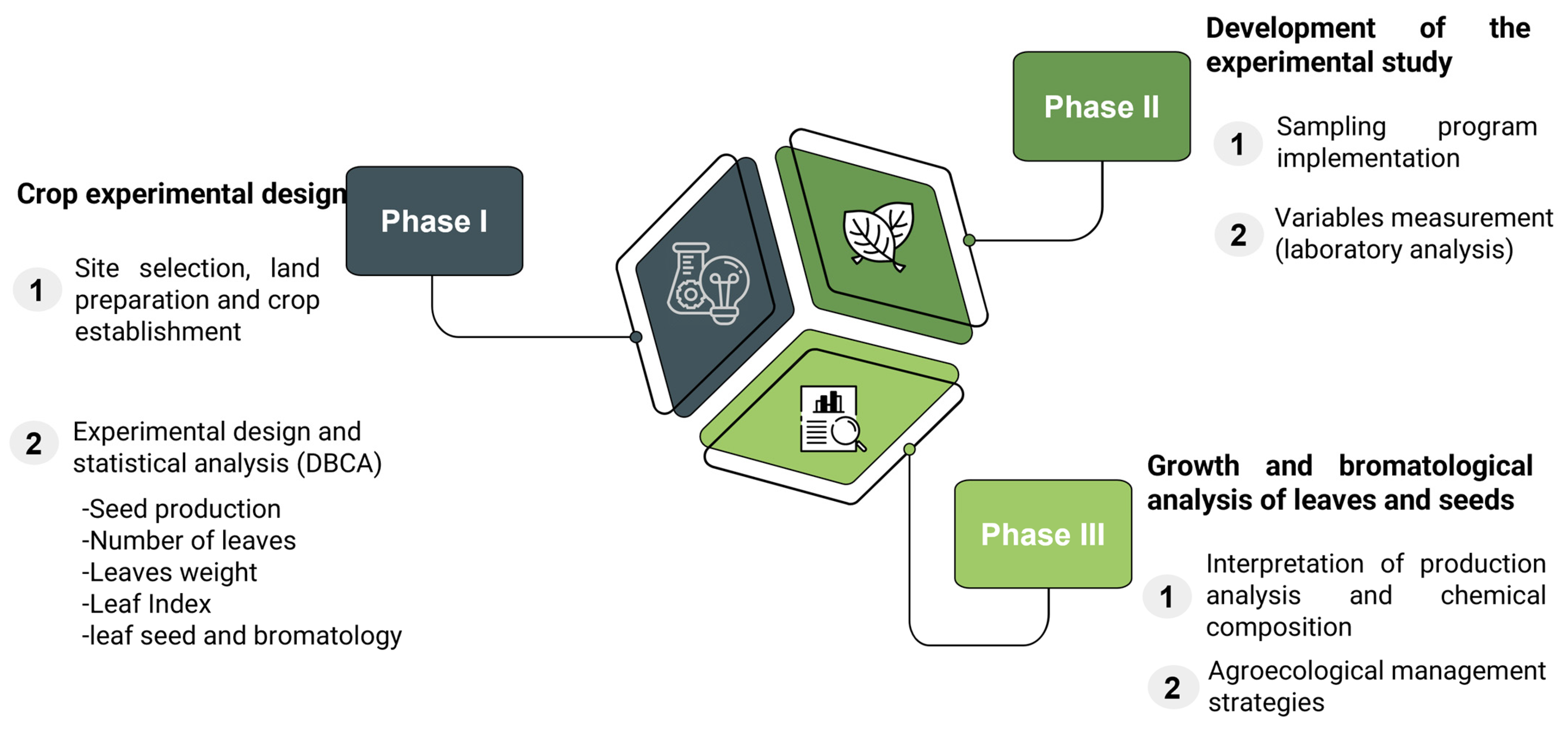
2.2.1. Crop Experimental Design
- Site selection, land preparation, and crop establishment
- Experimental design and statistical analysis
2.2.2. Crop Sampling
2.2.3. Agroecological Management Strategies
3. Results and Discussion
3.1. Soil Analysis
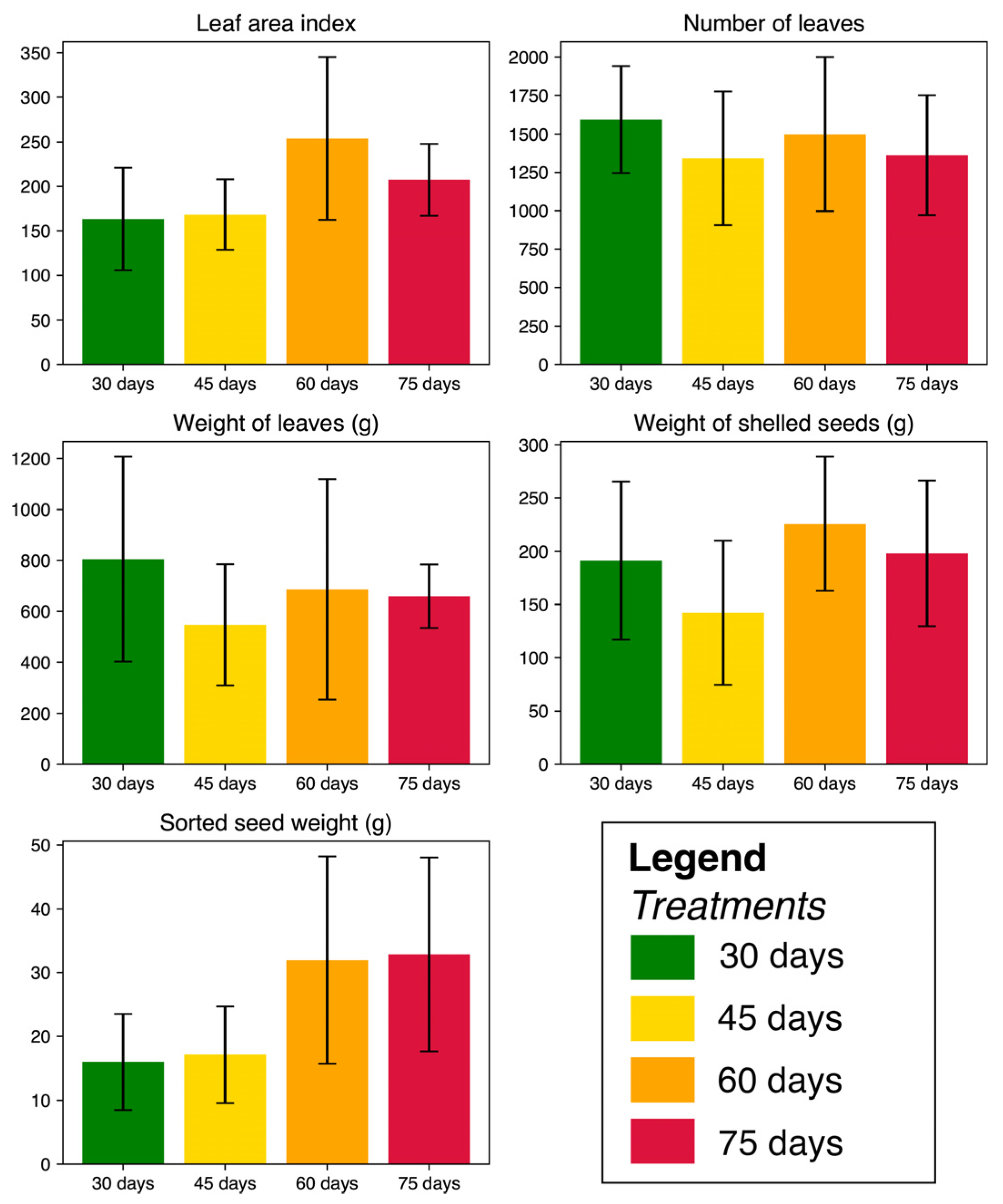
3.2. Analysis of Biological Parameters of the Plant
3.3. Variation in the Chemical Composition of Leaves and Seeds
3.4. SWOT Analysis of Production of F. macrophylla in the Local Context
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiebe, K.; Robinson, S.; Cattaneo, A. Climate Change, Agriculture and Food Security. In Sustainable Food and Agriculture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 55–74. [Google Scholar]
- Herrera-Franco, G.; Sánchez-Arizo, V.; Escandón-Panchana, P.; Caicedo-Potosí, J.; Jaya-Montalvo, M.; Zambrano-Mendoza, J. Analysis of Scientific Contributions to Agricultural Development and Food Security in Ecuador. Int. J. Des. Nat. Ecodynamics 2023, 18, 1129–1139. [Google Scholar] [CrossRef]
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Dittmer, K.M.; Rose, S.; Snapp, S.S.; Kebede, Y.; Brickman, S.; Shelton, S.; Egler, C.; Stier, M.; Wollenberg, E. Agroecology Can Promote Climate Change Adaptation Outcomes Without Compromising Yield In Smallholder Systems. Environ. Manag. 2023, 72, 333–342. [Google Scholar] [CrossRef]
- Sijpestijn, G.F.; Wezel, A.; Chriki, S. Can agroecology help in meeting our 2050 protein requirements? Livest. Sci. 2022, 256, 104822. [Google Scholar] [CrossRef]
- UNESCO. WWAP Informe Mundial de las Naciones Unidas Sobre el Desarrollo de los Recursos Hídricos 2019. No Dejar a Nadie Atrás; UNESCO: Paris, France, 2019; ISBN 978-92-3-300108-4. [Google Scholar]
- Pretty, J.; Morison, J.I.; Hine, R. Reducing food poverty by increasing agricultural sustainability in developing countries. Agric. Ecosyst. Environ. 2003, 95, 217–234. [Google Scholar] [CrossRef]
- Wezel, A.; Herren, B.G.; Kerr, R.B.; Barrios, E.; Gonçalves, A.L.R.; Sinclair, F. Agroecological principles and elements and their implications for transitioning to sustainable food systems. A review. Agron. Sustain. Dev. 2020, 40, 40. [Google Scholar] [CrossRef]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.-F.; Ferrer, A.; Peigné, J. Agroecological practices for sustainable agriculture. A review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef]
- Isbell, F. Agroecology: Agroecosystem diversification. Nat. Plants 2015, 1, 15041. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.K.; Boyer, C.N.; Griffith, A.P.; Waller, J.C.; Bates, G.E.; Keyser, P.D.; Larson, J.A.; Holcomb, E. The cost of feeding bred dairy heifers on native warm-season grasses and harvested feedstuffs. J. Dairy Sci. 2016, 99, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, S.P.; MacAdam, J.W.; Villalba, J.J. The Use of Temperate Tannin Containing Forage Legumes to Improve Sustainability in Forage–Livestock Production. Agronomy 2021, 11, 2264. [Google Scholar] [CrossRef]
- Alvarez-García, W.Y.; Diaz Herrera, A.; Becerra, Y.; Vallejos-Fernández, L.A.; Florián, R.; Carrasco-Chilón, W.; Cervantes-Peralta, M.; Quilcate, C.; Muñoz-Vilchez, Y. Sustainability Potential of Kikuyu Grass (Pennisetum clandestinum) in Livestock Farming of Peru’s Highland Regions. Sustainability 2024, 16, 11021. [Google Scholar] [CrossRef]
- Alexandre, G.; Rodriguez, L.; Arece, J.; Delgadillo, J.; Garcia, G.W.; Habermeier, K.; Almeida, A.M.; Fanchone, A.; Gourdine, J.-L.; Archimède, H. Agroecological practices to support tropical livestock farming systems: A Caribbean and Latin American perspective. Trop. Anim. Health Prod. 2021, 53, 111. [Google Scholar] [CrossRef] [PubMed]
- Merrill, E.D. Flemingia macrophylla (Willd.) Merr. Philipp. J. Sci. 1910, 5, 130. [Google Scholar]
- Sharma, A.; Sharma, C.; Shah, O.P.; Chigurupati, S.; Ashokan, B.; Meerasa, S.S.; Rashid, S.; Behl, T.; Bungau, S.G. Understanding the mechanistic potential of plant based phytochemicals in management of postmenopausal osteoporosis. Biomed. Pharmacother. 2023, 163, 114850. [Google Scholar] [CrossRef]
- Heider, B.; Andersson, M.S.; Schultze-Kraft, R. RAPD variation among North Vietnamese Flemingia macrophylla (Willd.) Kuntze ex Merr. accessions. In Plant Conservation and Biodiversity; Hawksworth, D.L., Bull, A.T., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 43–57. [Google Scholar]
- Phesatcha, B.; Viennasay, B.; Wanapat, M. Potential use of Flemingia (Flemingia macrophylla) as a protein source fodder to improve nutrients digestibility, ruminal fermentation efficiency in beef cattle. Anim. Biosci. 2021, 34, 613–620. [Google Scholar] [CrossRef]
- Yun, J.; Wang, C.; Zhang, F.; Chen, L.; Sun, Z.; Cai, Y.; Luo, Y.; Liao, J.; Wang, Y.; Cha, Y.; et al. A nitrogen fixing symbiosis-specific pathway required for legume flowering. Sci. Adv. 2023, 9, eade1150. [Google Scholar] [CrossRef]
- Vallejos, A.; Cardona, R. AGRIS—International System for Agricultural Science and Technology. Available online: https://agris.fao.org/search/en/providers/123819/records/64735b192c1d629bc97bdf8f (accessed on 20 January 2025).
- Tjahjandarie, T.S.; Tanjung, M.; Saputri, R.D.; Aldin, M.F.; Susanti, R.A.; Pertiwi, N.P.; Wibawa, R.S.; Halizah, I.N. Cytotoxicity evaluation of two new chalcones from the leaves of Flemingia macrophylla (Willd.) Merr. Phytochem. Lett. 2021, 44, 78–81. [Google Scholar] [CrossRef]
- Weeraratna, S. Control of Land Degradation. In Understanding Land Degradation; Springer: Cham, Switzerland, 2022; pp. 39–51. [Google Scholar]
- Oppong, F.; Osei-Bonsu, K.; Amoah, F.; Acheampong, K. Potential use of Flemingia macrophylla as mulch for managing weeds in young cocoa in Ghana. Ghana J. Agric. Sci. 1998, 31, 67–72. [Google Scholar] [CrossRef]
- Andersson, M.S.; Schultze-Kraft, R.; Peters, M.; Hincapié, B.; Lascano, C.E. Morphological, agronomic and forage quality diversity of the Flemingia macrophylla world collection. Field Crops Res. 2006, 96, 387–406. [Google Scholar] [CrossRef]
- Andersson, M.S.; Lascano, C.E.; Schultze-Kraft, R.; Peters, M. Forage quality and tannin concentration and composition of a collection of the tropical shrub legume Flemingia macrophylla. J. Sci. Food Agric. 2006, 86, 1023–1031. [Google Scholar] [CrossRef]
- Kang, S.; Wanapat, M.; Phesatcha, K.; Norrapoke, T.; Foiklang, S.; Ampapon, T.; Phesatcha, B. Using krabok (Irvingia malayana) seed oil and Flemingia macrophylla leaf meal as a rumen enhancer in an in vitro gas production system. Anim. Prod. Sci. 2017, 57, 327. [Google Scholar] [CrossRef]
- Binh, D.; Tien, N.; Mui, N. Study on biomass yield and quality of Flemingia macrophylla and on soil fertility. Proc. Work. Anim. Nutr. Sci. 1998, 137. [Google Scholar]
- Becker, M.; Johnson, D.E. Legumes as dry season fallow in upland rice-based systems of West Africa. Biol. Fertil. Soils 1998, 27, 358–367. [Google Scholar] [CrossRef]
- Wanapat, M. Potential uses of local feed resources for ruminants. Trop. Anim. Health Prod. 2009, 41, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Tierras, Y.; Díaz, A.; Caicedo, C.; Macas, J.; Suárez-Tapia, A.; Viera, W. Benefits of Legume Species in an Agroforestry Production System of Yellow Pitahaya in the Ecuadorian Amazon. Sustainability 2021, 13, 9261. [Google Scholar] [CrossRef]
- Hinojosa, L.; Leguizamo, A.; Carpio, C.; Muñoz, D.; Mestanza, C.; Ochoa, J.; Castillo, C.; Murillo, A.; Villacréz, E.; Monar, C.; et al. Quinoa in Ecuador: Recent Advances under Global Expansion. Plants 2021, 10, 298. [Google Scholar] [CrossRef]
- Caulfield, M.; Bouniol, J.; Fonte, S.J.; Kessler, A. How rural out-migrations drive changes to farm and land management: A case study from the rural Andes. Land Use Policy 2019, 81, 594–603. [Google Scholar] [CrossRef]
- Hess, C.G. “Moving up-Moving down”: Agro-Pastoral Land-Use Patterns in the Ecuadorian Paramos. Mt. Res. Dev. 1990, 10, 333. [Google Scholar] [CrossRef]
- Mihai, R.A.; Melo Heras, E.J.; Terán Maza, V.A.; Espinoza Caiza, I.A.; Pinto Valdiviezo, E.A.; Catana, R.D. The Panoramic View of Ecuadorian Soil Nutrients (Deficit/Toxicity) from Different Climatic Regions and Their Possible Influence on the Metabolism of Important Crops. Toxics 2023, 11, 123. [Google Scholar] [CrossRef]
- Del Vecchio, R.J. Design of Experiments. In Handbook of Vinyl Formulating, 2nd ed.; Wiley: Hoboken, NJ, USA, 2007; pp. 515–527. [Google Scholar] [CrossRef]
- Tukey, J.W.T. The problem of multiple comparisons. In The Collected Works of John W.Tukey: Volume VIII. Multiple Comparisons: 1948–1983; Braun, H.I., Ed.; Chapman and Hall: New York, NY, USA, 1953; pp. 1–300. ISBN 0-412-98281-1. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. Infostat. Available online: https://www.infostat.com.ar/ (accessed on 10 December 2024).
- Carrión-mero, P.; Borja-bernal, C.; Herrera-franco, G.; Morante-carballo, F.; Jaya-montalvo, M.; Maldonado-zamora, A.; Paz-salas, N.; Berrezueta, E. Geosites and geotourism in the local development of communities of the andes mountains. A case study. Sustainability 2021, 13, 4624. [Google Scholar] [CrossRef]
- Salonius, P.O.; Fisher, R.A.; Mahendrappa, M.K. An alternative method of measuring fertilizer effects in forest stands. Can. J. For. Res. 1982, 12, 146–150. [Google Scholar] [CrossRef]
- Siahaan, A.S.A.; Harahap, E.M.; Hanum, C.; Karim, A. The growth and yield of coffee arabica in shade conditions on different treatment of pruning and fertilizing. Russ. J. Agric. Socio-Econ. Sci. 2020, 3, 40–46. [Google Scholar] [CrossRef]
- Schnabel, F.; de Melo Virginio Filho, E.; Xu, S.; Fisk, I.D.; Roupsard, O.; Haggar, J. Shade trees: A determinant to the relative success of organic versus conventional coffee production. Agrofor. Syst. 2018, 92, 1535–1549. [Google Scholar] [CrossRef]
- Luna Murillo, R.A.; Cadme Arévalo, M.L.; Reyes Bermeo, M.R.; Zambrano Burgos, D.A.; Vargas Burgos, J.C.; Chacón Marcheco, E.; Ramírez de la Ribera, J.L. Calidad y microorganismos asociados de cuatros especies forrajeras en dos regiones del Ecuador. REDVET Rev. Electrónica Vet. 2016, 17, 1–9. [Google Scholar]
- Siegenthaler, A.; Buttler, A.; Grosvernier, P.; Gobat, J.-M.; Mitchell, E. Discrepancies in Growth Measurement Methods of Mosses: An Example from Two Keystone Species Grown under Increased CO2 and N Supply in a Restored Peatland. Am. J. Plant Sci. 2014, 05, 2354–2371. [Google Scholar] [CrossRef]
- Palm, C.A.; Gachengo, C.N.; Delve, R.J.; Cadisch, G.; Giller, K.E. Organic inputs for soil fertility management in tropical agroecosystems: Application of an organic resource database. Agric. Ecosyst. Environ. 2001, 83, 27–42. [Google Scholar] [CrossRef]
- Barat-Carnino, M.; Santachiara, G.A.; Borras, L.; Rotundo, J.L. Sowing date affects soybean biological nitrogen fixation. Crop Sci. 2022, 62, 2428–2438. [Google Scholar] [CrossRef]
- Padbhushan, R.; Kumar, A.; Kumar, U.; Kumar, A.; Kumari, R.; Kohli, A. Novel Potassium Management Strategies for Improvement of Soil Health. In Soil Management For Sustainable Agriculture; Apple Academic Press: Boca Raton, FL, USA, 2022; pp. 355–377. [Google Scholar]
- Das, I.; Pradhan, M. Potassium-Solubilizing Microorganisms and Their Role in Enhancing Soil Fertility and Health. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 281–291. [Google Scholar]
- Bērtiņš, M.; Klūga, A.; Dubova, L.; Petrēvics, P.; Alsiņa, I.; Vīksna, A. Study of Rhizobia Impact on Nutritional Element Concentration in Legumes. Proc. Latv. Acad. Sci. Nat. Exact Appl. Sci. 2021, 75, 457–462. [Google Scholar] [CrossRef]
- Sale, P.W.G.; Campbell, L.C. Patterns of mineral nutrient accumulation in soybean seed. Field Crops Res. 1980, 3, 157–163. [Google Scholar] [CrossRef]
- Andrew, D.M.; Adewale, A.L.; Aminu, H.; Aliyu, S.H. Evaluation of micronutrient status of unburnt and burnt coffee plantation in Ibadan. Glob. J. Res. Sci. Technol. 2022, 1, 022–025. [Google Scholar] [CrossRef]
- Pottier, M.; Masclaux-Daubresse, C.; Yoshimoto, K.; Thomine, S. Autophagy as a possible mechanism for micronutrient remobilization from leaves to seeds. Front. Plant Sci. 2014, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, X.; Qi, G.; Cui, D.; Huang, C.; Sui, X.; Li, G.; Fan, Q. Mechanisms of autophagy function and regulation in plant growth, development, and response to abiotic stress. Crop J. 2023, 11, 1611–1625. [Google Scholar] [CrossRef]
- Ferreira, A.C.M.; de Souza, H.A.; Sagrilo, E.; Barbosa da Silva Júnior, G.; Natale, W.; Sobral, A.H.S.; de Souza Vera, G.; da Conceicao Borges dos Santos, S.F. Absorption, partitioning, and export of nutrients by phenological stage in maize cultivated in Eastern Maranhão, Brazil. J. Plant Nutr. 2024, 47, 240–256. [Google Scholar] [CrossRef]
- Zharare, G.E.; Asher, C.J.; Blamey, F.P.C. Magnesium antagonizes pod-zone calclum and zinc uptake by developing peanut pods. J. Plant Nutr. 2010, 34, 1–11. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R.; El-Serehy, H.A.; George, T.S.; Neugebauer, K. Linear relationships between shoot magnesium and calcium concentrations among angiosperm species are associated with cell wall chemistry. Ann. Bot. 2018, 122, 221–226. [Google Scholar] [CrossRef]
- Gao, Y.-M.; Ma, B.L. Nitrogen, Phosphorus, and Zinc Supply on Seed and Metal Accumulation in Canola Grain. J. Plant Nutr. 2015, 38, 473–483. [Google Scholar] [CrossRef]
- Atar, B.; Uygur, V.; Sukusu, E. Effects of Priming with Copper, Zinc and Phosphorus on Seed and Seedling Composition in Wheat and Barley. Türk Tarım Doğa Bilim. Derg. 2020, 7, 104–111. [Google Scholar] [CrossRef]
- Rose, T.; Kretzschmar, T.; Liu, L.; Lancaster, G.; Wissuwa, M. Phosphorus Deficiency Alters Nutrient Accumulation Patterns and Grain Nutritional Quality in Rice. Agronomy 2016, 6, 52. [Google Scholar] [CrossRef]
- Garcia, C.B.; Grusak, M.A. Mineral accumulation in vegetative and reproductive tissues during seed development in Medicago truncatula. Front. Plant Sci. 2015, 6, 622. [Google Scholar] [CrossRef]
- Wysokinski, A.; Lozak, I.; Kuziemska, B. The Dynamics of Molybdenum, Boron, and Iron Uptake, Translocation and Accumulation by Pea (Pisum sativum L.). Agronomy 2022, 12, 935. [Google Scholar] [CrossRef]
- Sinha, A.; Haider, T.; Narula, K.; Ghosh, S.; Chakraborty, N.; Chakraborty, S. Integrated Seed Proteome and Phosphoproteome Analyses Reveal Interplay of Nutrient Dynamics, Carbon–Nitrogen Partitioning, and Oxidative Signaling in Chickpea. Proteomics 2020, 20, 1900267. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, D.; Yu, K.; Guo, H.; Zhang, R.; Wang, J.; Zhou, Q.; Niu, S. Seed nutrient is more stable than leaf in response to changing multiple resources in an alpine meadow. Ecol. Process. 2023, 12, 43. [Google Scholar] [CrossRef]
- Sauvadet, M.; Trap, J.; Damour, G.; Plassard, C.; Van den Meersche, K.; Achard, R.; Allinne, C.; Autfray, P.; Bertrand, I.; Blanchart, E.; et al. Agroecosystem diversification with legumes or non-legumes improves differently soil fertility according to soil type. Sci. Total Environ. 2021, 795, 148934. [Google Scholar] [CrossRef] [PubMed]
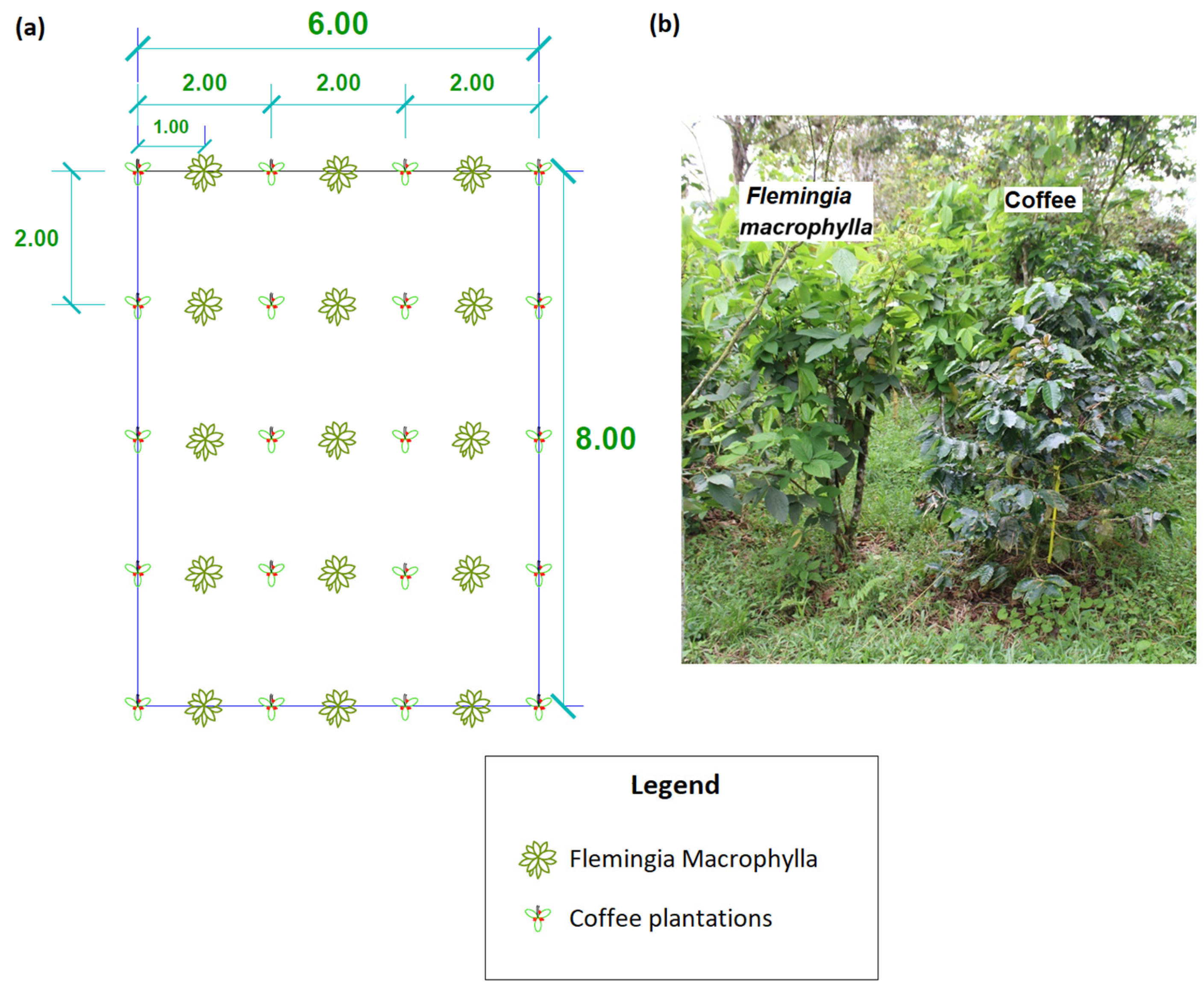
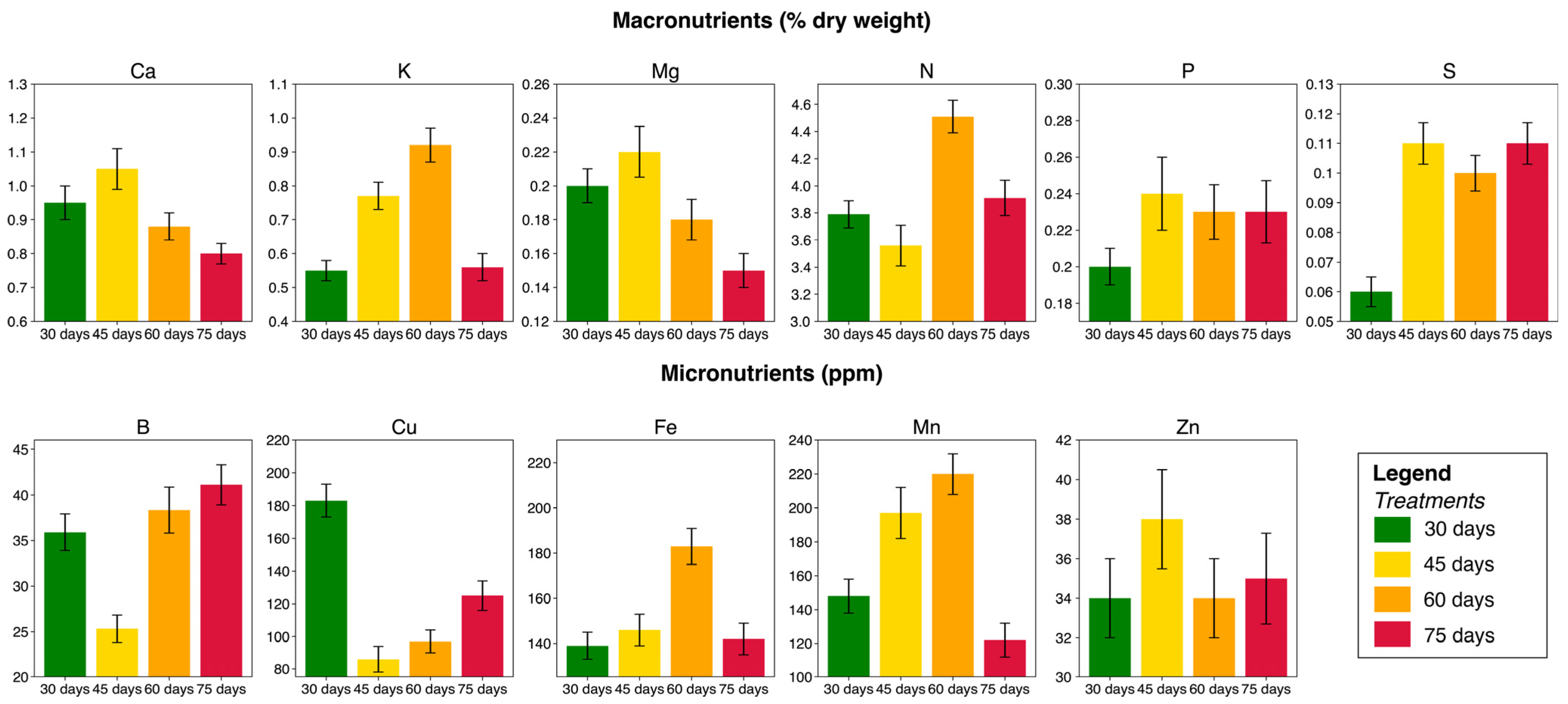
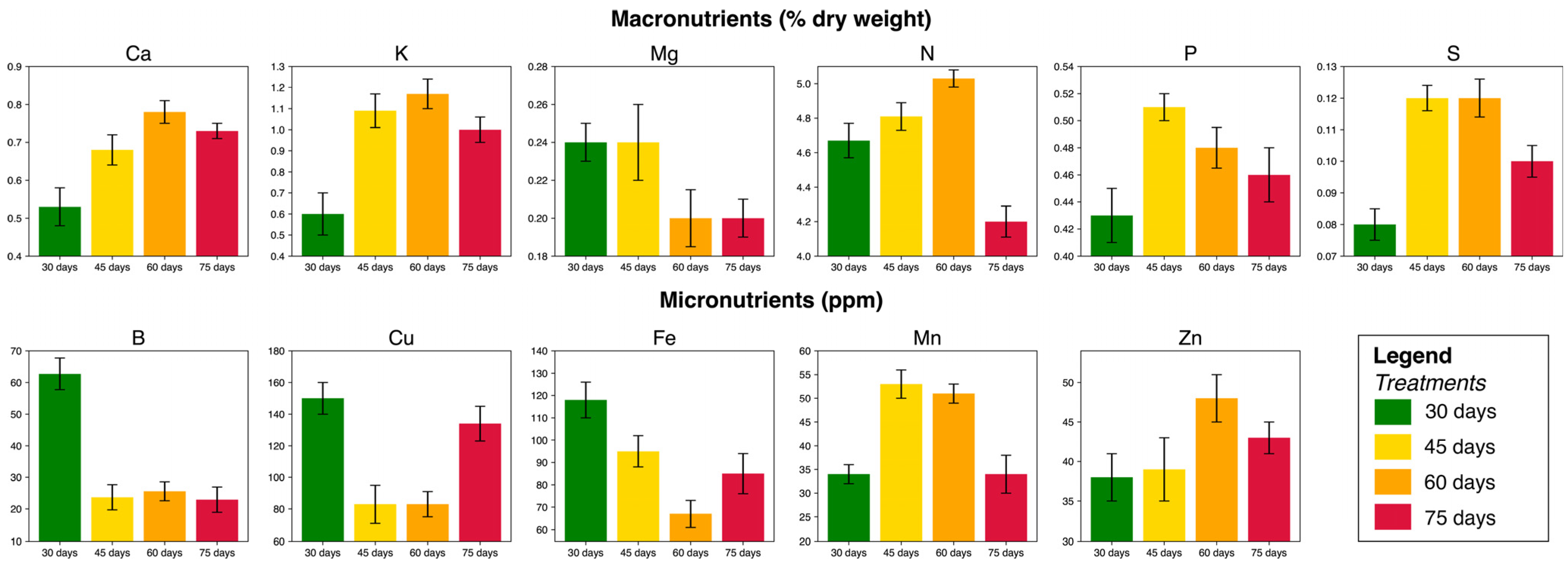
| Treatments | Replications * (Number of Blocks) | Experimental Units (Plants) | Total |
|---|---|---|---|
| T1: Flemingia at 30 days | 5 | 6 | 30 |
| T2: Flemingia at 45 days | 5 | 6 | 30 |
| T3: Flemingia at 60 days | 5 | 6 | 30 |
| T4: Flemingia at 75 days | 5 | 6 | 30 |
| Total | 120 |
| Variable | Experimental Characteristics | Measurement Units |
|---|---|---|
| Seed production | Seed collection and weight for each experimental unit and treatment at different cutting ages. | Grams (g) |
| Number of leaves | The number of leaves for each treatment, repeti-tion, and experimental unit at the different cutting ages was recorded. | Unit |
| Leaf weight | Leaf weight was recorded using a scale for each treatment. | Grams (g) |
| Leaf area index | The study used an LI-3100C Leaf Area (LA) Meter, which was measured by introducing each of the samples of green leaf blades taken for each treatment (100 leaves in total). The Leaf Area Index (LAI) was calculated by dividing the measured LA by the plantation area. The equation was used: LAI = | m2 m−2 |
| Chemical composition of F. macrophylla seeds and leaves/Nutrition characteristics of the seeds | Samples of 800 g of leaves and 100 g of F. macrophylla seeds were taken for each experimental unit, evaluated at 30, 45, 60, and 75 days, and sent to the AGROLAB laboratory. |
|
| Determination | Methodology | Extractant |
|---|---|---|
| P, NH4 | Colourimetry | Modified Olsen pH 8.5 |
| K, Ca, Mg, Zn, Cu, Fe, Mn | Atomic absorption | |
| S | Turbidimetry | Ca phosphate |
| B | Colourimetry | Monobasic |
| Cl | Volumetric analysis | Saturated Paste |
| Organic Matter | Walkley and Black | Not Applicable |
| Plot | Lot 1 (“Geisha Coffee”) | Lot 2 (“Sarchimor Coffee“) | ||||||
|---|---|---|---|---|---|---|---|---|
| Crop | Beginning Crop: Lot 1 Date: 5 May 2022 Depth: 20 cm | End Crop: F. macrophylla (Lot 2) Date: 23 July 2022 | Beginning Crop: Lot 2 Date: 5 May 2022 Depth: 20 cm | End Crop: F. macrophylla (Lot 2) Date: 23 July 2022 | ||||
| Parameters | Reading | Reading | Reading | Reading | ||||
| pH | 5.71 | Moderately acidic | 5.78 | Moderately acidic | 5.62 | Moderately acidic | 5.70 | Moderately acidic |
| Electrical Conductivity (ds/m) | 0.05 | Non-saline | 0.05 | Non-saline | 0.04 | Non-saline | 0.03 | Non-saline |
| Organic Matter (%) | 4.56 | Medium | 4.12 | Medium | 4.56 | Medium | 2.78 | Low |
| NH4 (ppm) | 25.15 | Low | 19.34 | Low | 24.50 | Low | 17.41 | Low |
| Phosphorus (ppm) | 7.89 | Low | 3.63 | Low | 9.08 | Low | 2.35 | Low |
| Sulphur (ppm) | 5.26 | Low | 24.99 | High | 5.50 | Low | 24.20 | High |
| Potassium (meq/100 g) | 0.20 | Medium | 0.30 | Medium | 0.22 | Medium | 0.20 | Medium |
| Calcium (meq/100 g) | 3.00 | Low | 8.90 | Medium | 3.00 | Low | 6.00 | Medium |
| Magnesium (meq/100 g) | 0.48 | Low | 1.01 | Low | 0.45 | Low | 0.57 | Low |
| Σ bases (meq/100 g) | 3.68 | Medium-Low | 10.21 | Low | 3.67 | Medium-Low | 6.77 | Low |
| Copper (ppm) | 4.00 | Medium | 3.50 | Medium | 4.20 | High | 3.20 | Medium |
| Boron (ppm) | 0.28 | Low | 0.37 | Medium | 0.27 | Low | 0.21 | Medium |
| Iron (ppm) | 223.6 | High | 102.0 | High | 217.8 | High | 74.00 | High |
| Zinc (ppm) | 1.30 | Low | 1.30 | Low | 1.40 | Low | 1.10 | Low |
| Manganese (ppm) | 17.80 | Medium | 3.60 | Low | 7.20 | Low | 1.40 | Low |
| Ca/Mg | 6.25 | High | 8.81 | High | 6.67 | High | 10.53 | High |
| Mg/K | 2.40 | Low | 3.37 | Optimal | 2.05 | Low | 2.85 | Optimal |
| (Ca + Mg)/K | 17.40 | Optimal | 33.03 | Optimal | 15.68 | Optimal | 32.85 | Optimal |
| Parameters | Bromatological Composition of Seeds | |||
|---|---|---|---|---|
| 30 Days | 45 Days | 60 Days | 75 Days | |
| Humidity (%) | 23.30 | 29.02 | 23.17 | 20.15 |
| Dry matter (%) | 76.70 | 70.98 | 76.83 | 79.85 |
| Protein (%) | 29.20 | 30.10 | 31.44 | 26.22 |
| Fatty ether extract (%) | 7.22 | 7.74 | 6.98 | 6.66 |
| Ash (%) | 7.80 | 4.00 | 7.30 | 6.80 |
| Fiber (%) | 8.17 | 9.60 | 10.40 | 11.20 |
| Non-Nitrogenous free extract-others | 47.61 | 48.56 | 43.88 | 49.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Murillo, R.; Solórzano, J.; Pacheco-Tigselema, I.; Dueñas-Tovar, J.; Bravo-Montero, L.; Jaya-Montalvo, M. Effect of Cutting Age on Seed Production of Flemingia Macrophylla for the Optimisation of Cropping Systems, Cotopaxi-Ecuador. Agriculture 2025, 15, 1781. https://doi.org/10.3390/agriculture15161781
Luna-Murillo R, Solórzano J, Pacheco-Tigselema I, Dueñas-Tovar J, Bravo-Montero L, Jaya-Montalvo M. Effect of Cutting Age on Seed Production of Flemingia Macrophylla for the Optimisation of Cropping Systems, Cotopaxi-Ecuador. Agriculture. 2025; 15(16):1781. https://doi.org/10.3390/agriculture15161781
Chicago/Turabian StyleLuna-Murillo, Ricardo, Joselyne Solórzano, Idalia Pacheco-Tigselema, Jairo Dueñas-Tovar, Lady Bravo-Montero, and María Jaya-Montalvo. 2025. "Effect of Cutting Age on Seed Production of Flemingia Macrophylla for the Optimisation of Cropping Systems, Cotopaxi-Ecuador" Agriculture 15, no. 16: 1781. https://doi.org/10.3390/agriculture15161781
APA StyleLuna-Murillo, R., Solórzano, J., Pacheco-Tigselema, I., Dueñas-Tovar, J., Bravo-Montero, L., & Jaya-Montalvo, M. (2025). Effect of Cutting Age on Seed Production of Flemingia Macrophylla for the Optimisation of Cropping Systems, Cotopaxi-Ecuador. Agriculture, 15(16), 1781. https://doi.org/10.3390/agriculture15161781








