Differential Responses of Two Sorghum Genotypes to Drought Stress at Seedling Stage Revealed by Integrated Physiological and Transcriptional Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Observations and Measurements
2.3.1. Determination of Physiological and Biochemical Indicators in Plant Growth
2.3.2. Determination of Antioxidant Enzymes
2.3.3. cDNA Library Preparation and RNA-Seq
2.4. Data Analysis
3. Results
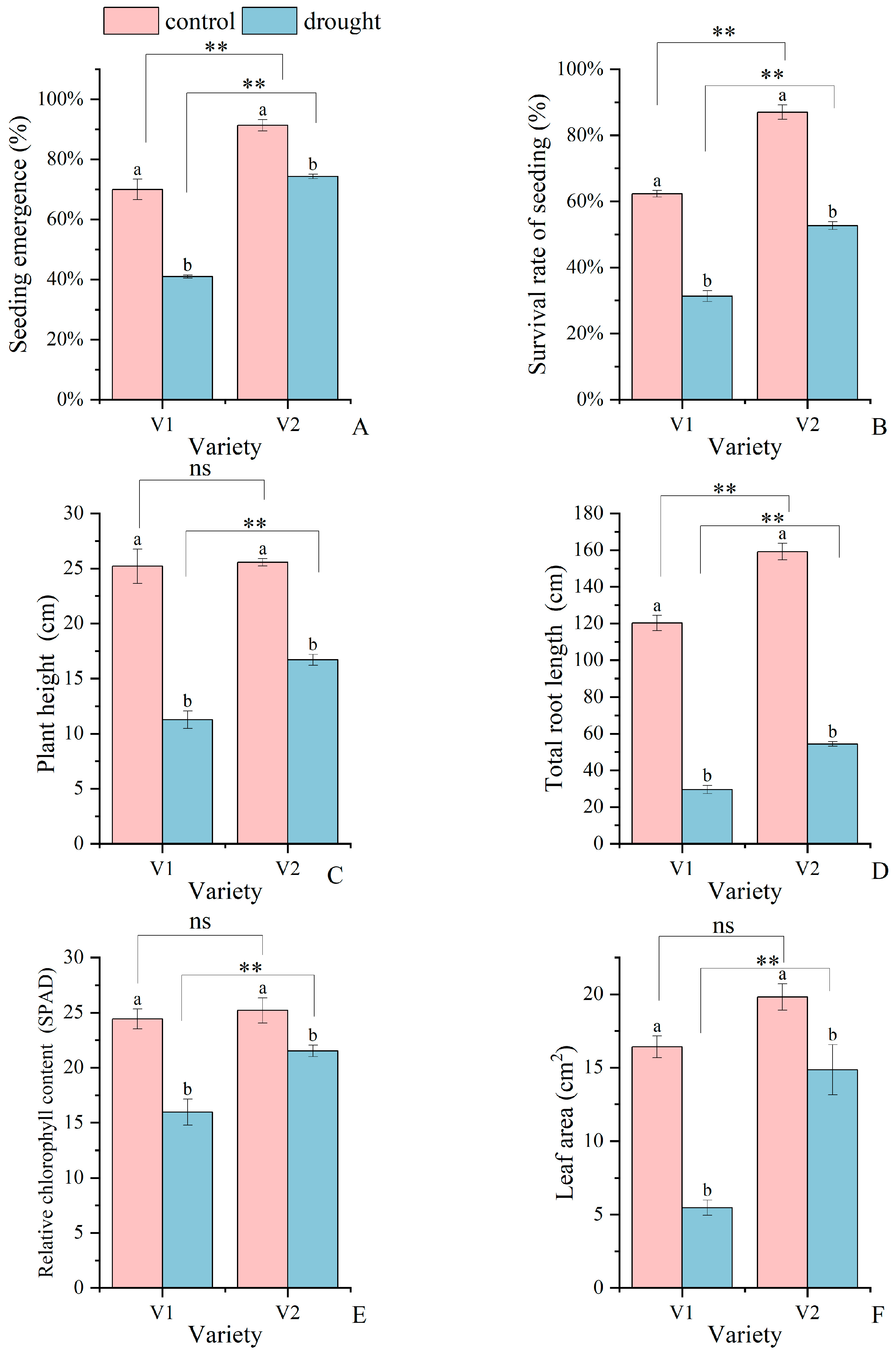
3.1. Phenotypic and Physiological Responses of Two Sorghum Varieties to Drought Stress
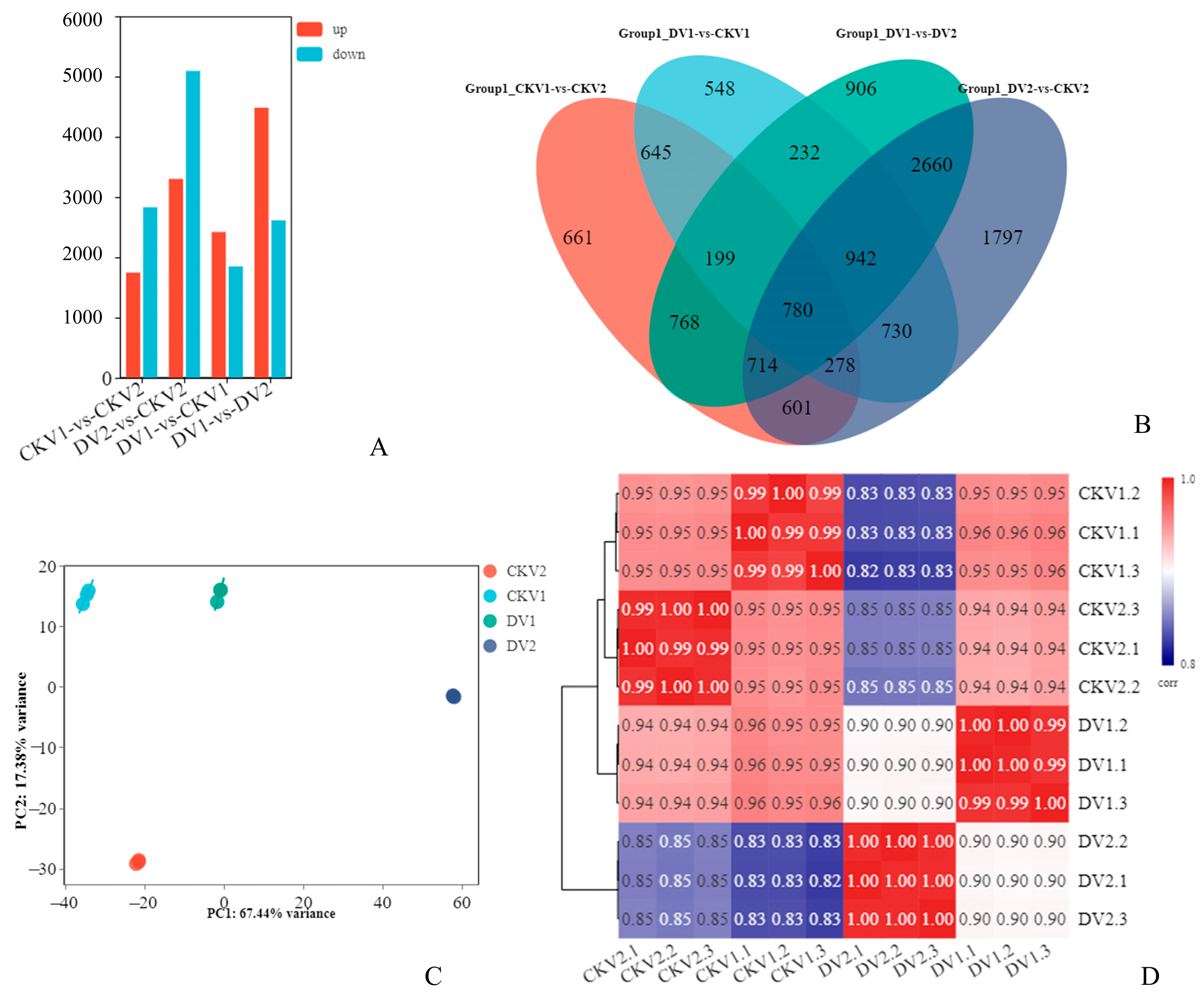
3.2. Transcriptome Sequencing and Differential Gene Expression Analysis
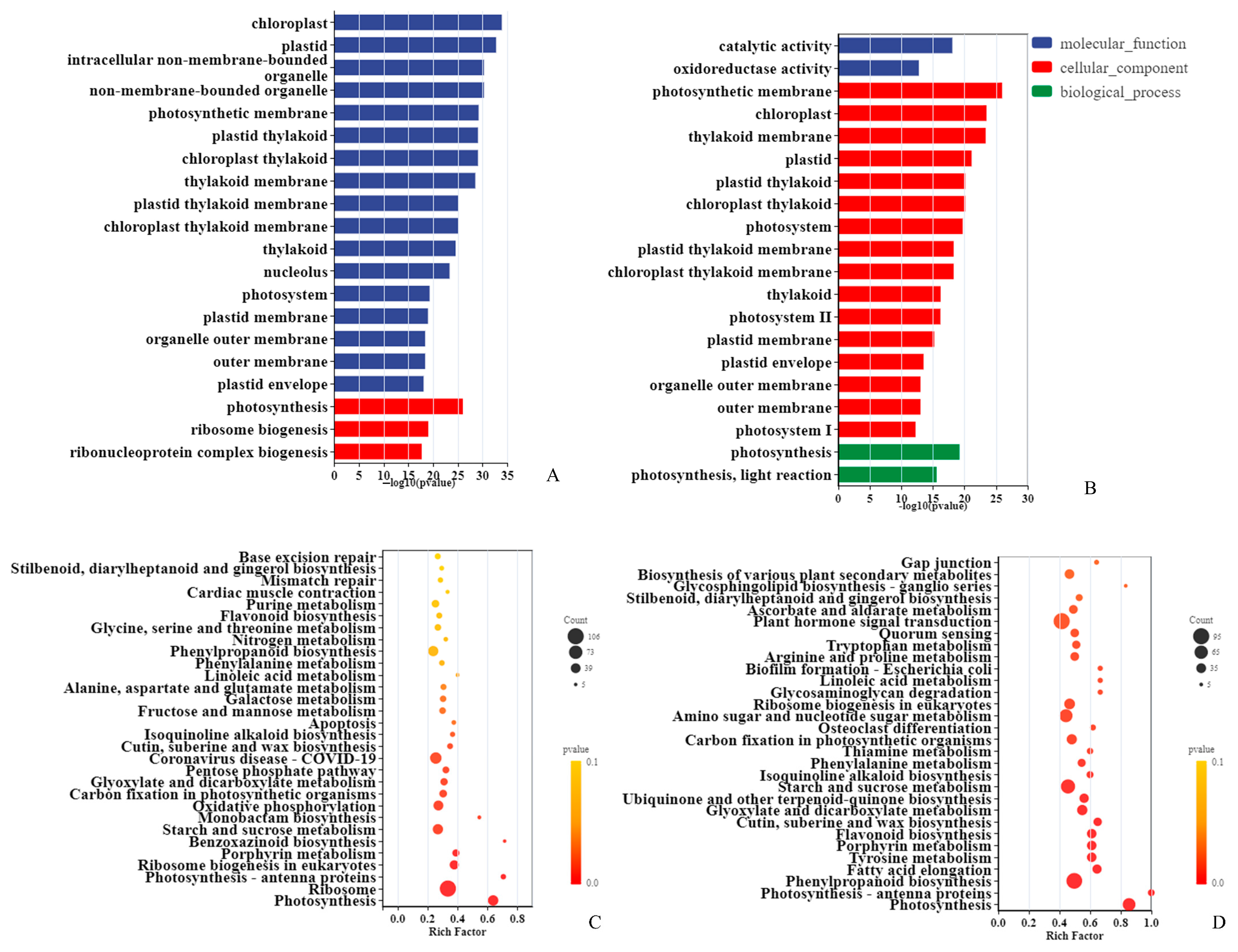
3.3. Response Pathways to Drought in Two Sorghum Varieties
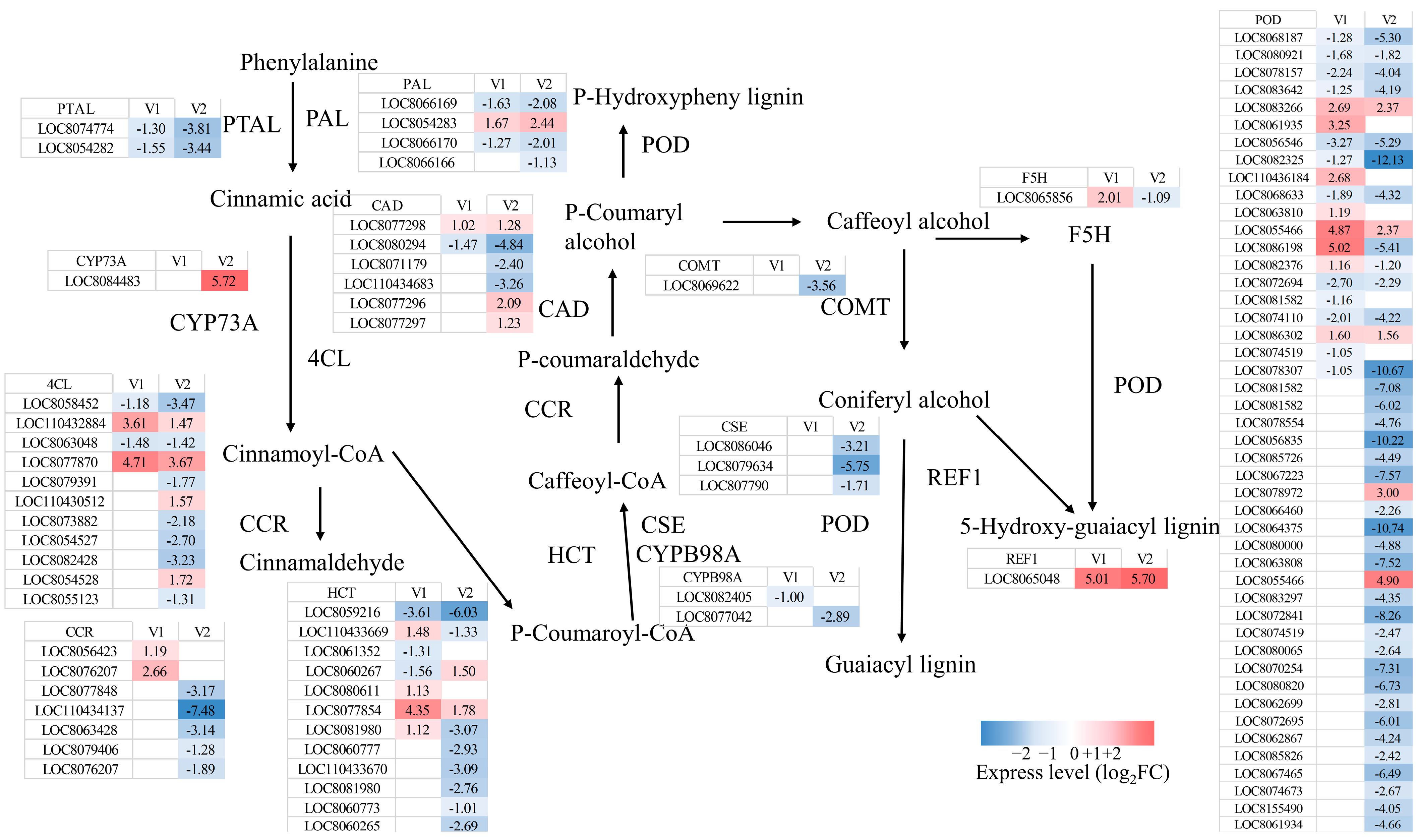
3.3.1. Response of Phenylpropane Metabolic Pathway to Drought Stress
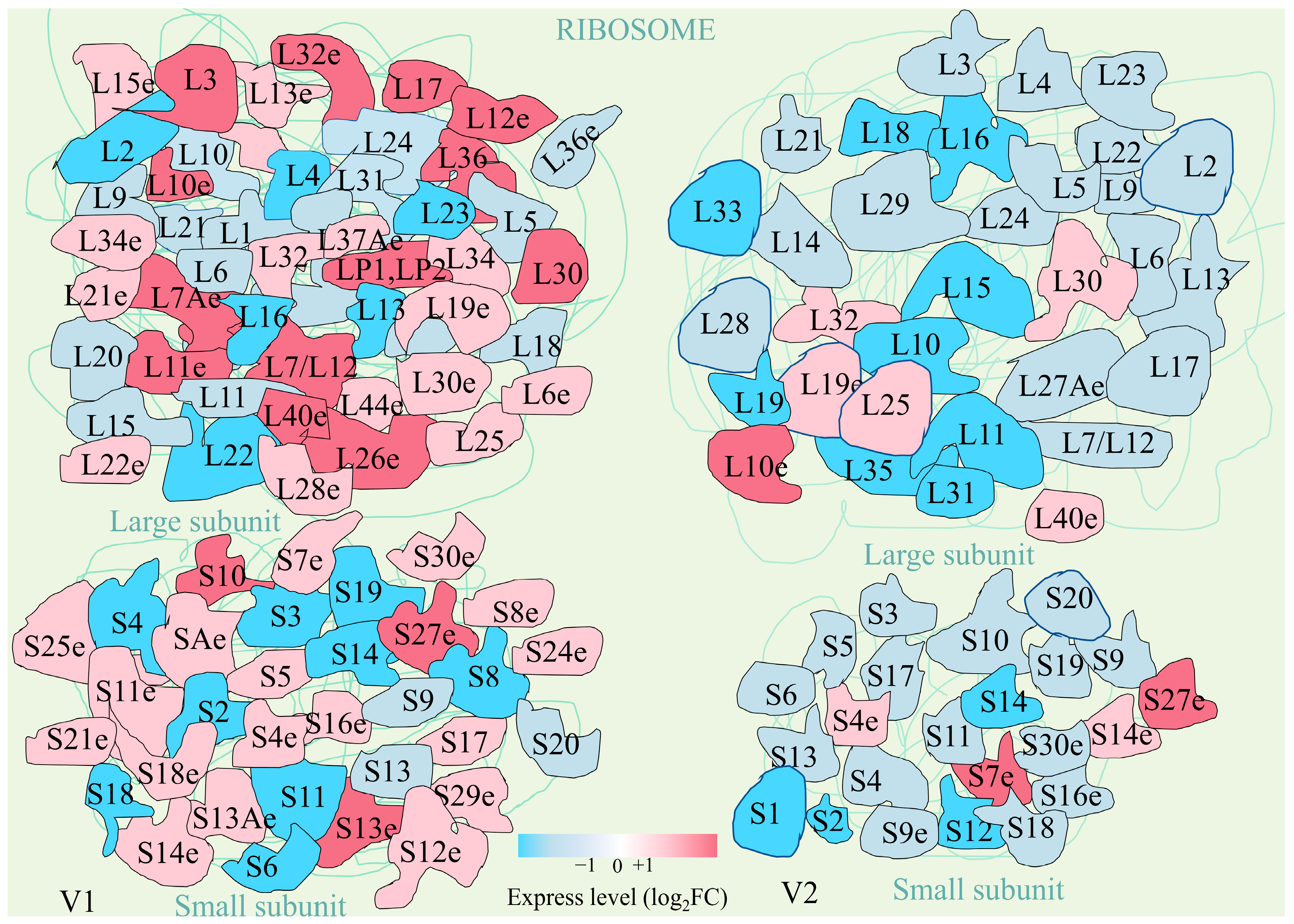
3.3.2. Ribosome Metabolic Pathways in Response to Drought Stress
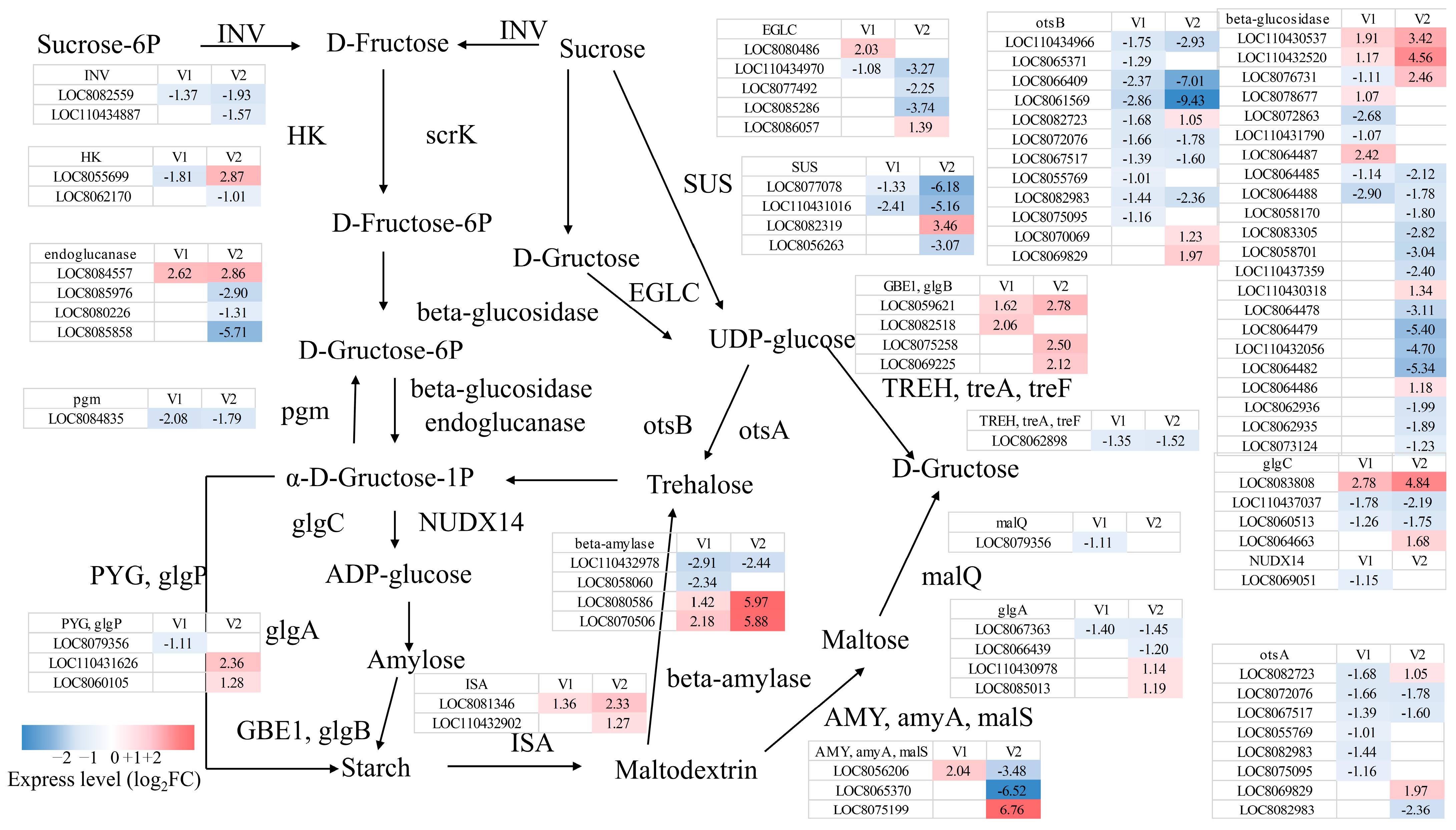
3.3.3. Sucrose Metabolic Pathways in Response to Drought Stress
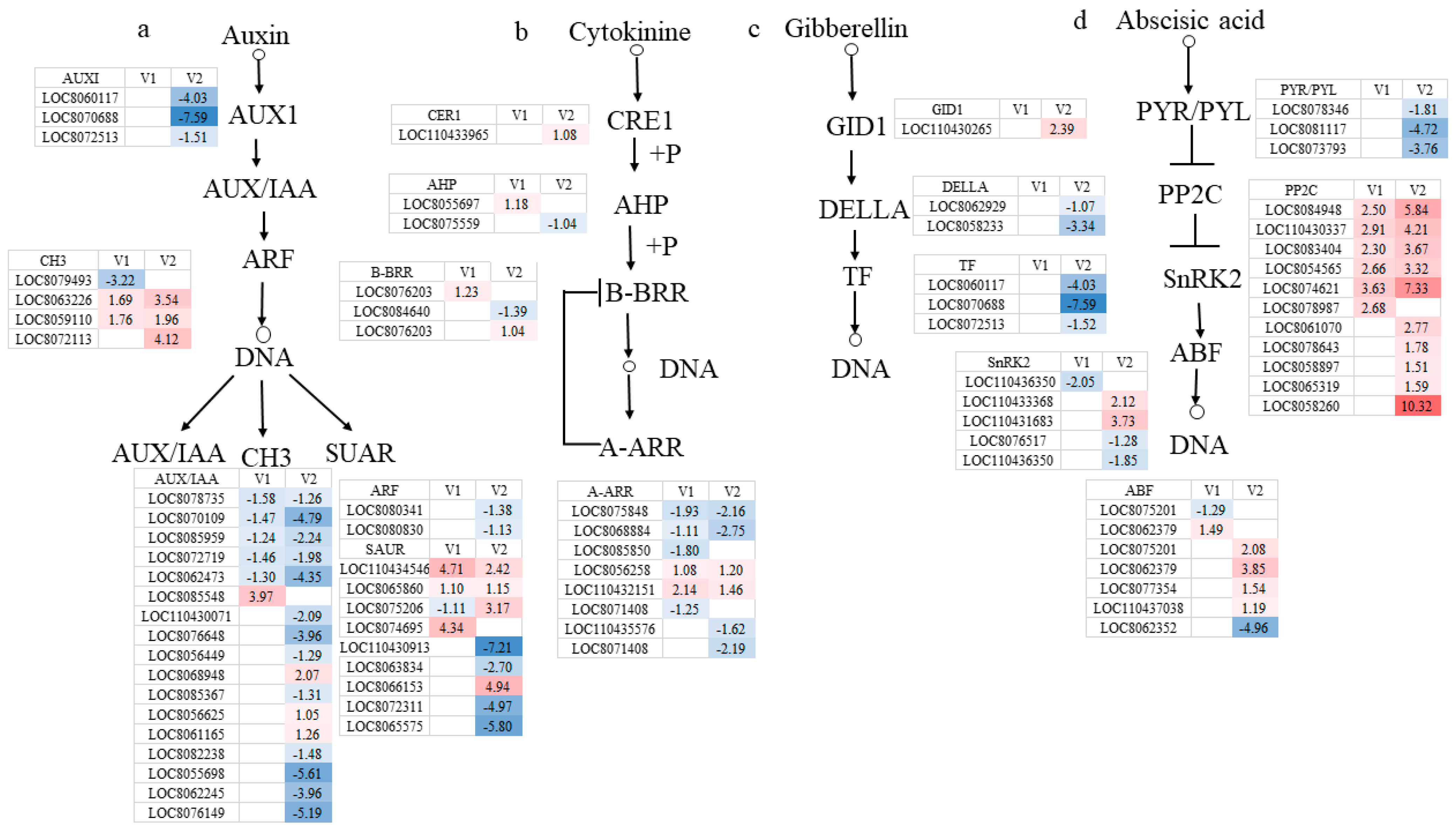
3.3.4. Hormone Metabolic Pathways in Response to Drought Stress
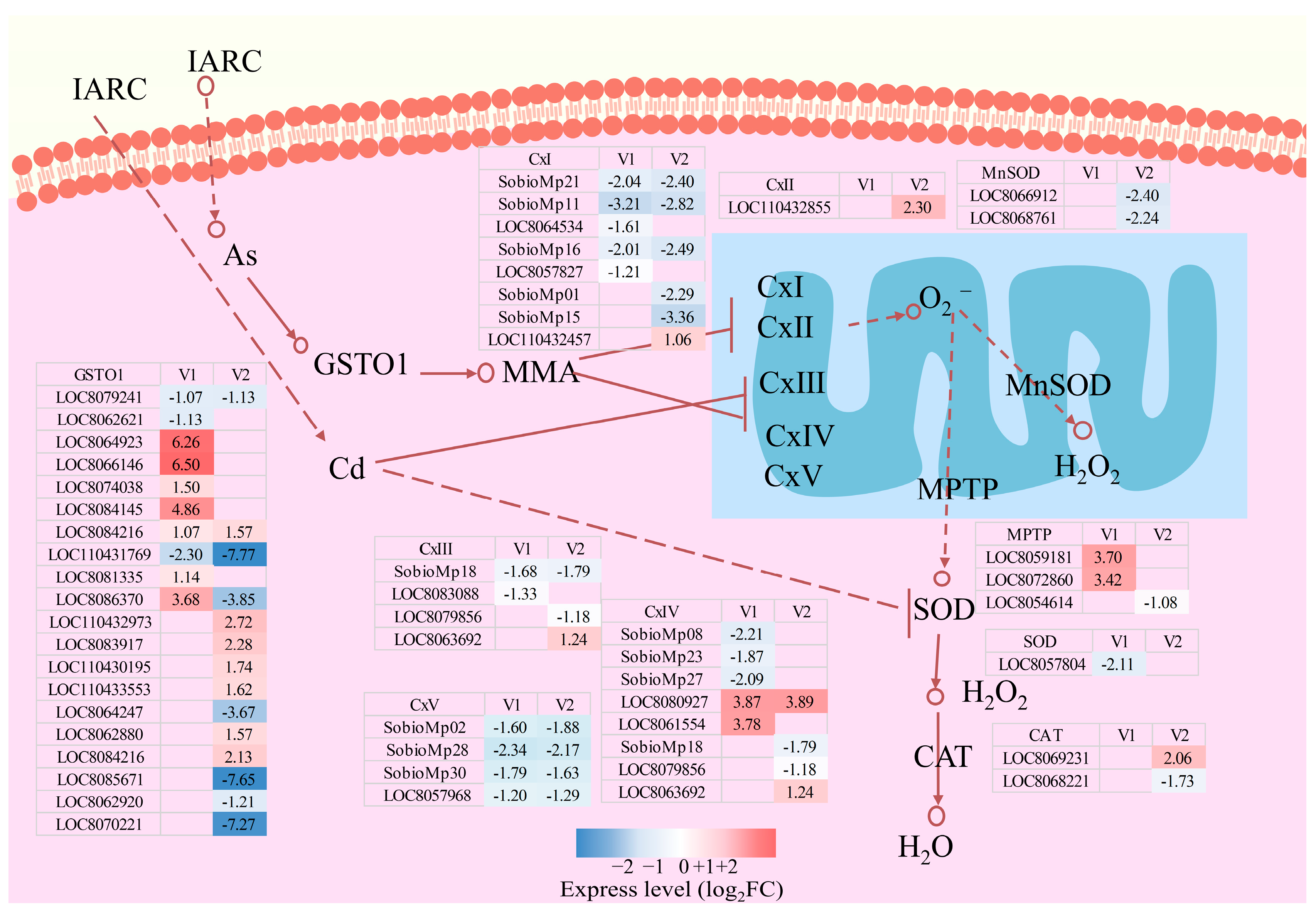
3.3.5. Response of Reactive Oxygen Species Metabolic Pathways to Drought Stress
4. Discussion
4.1. Response Mechanisms of Phenotypic and Physiological Changes in Sorghum in Response to Drought Stress
4.2. Response of the Phenylpropanoid Biosynthesis Pathway to Drought Stress
4.3. Response of Other Metabolic Pathways to Drought Stress
4.3.1. Starch and Sucrose Metabolism
4.3.2. Plant Hormone Signal Transduction
4.3.3. Ribosome Metabolism
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Wang, X.; Wu, H.; Zhu, Y.; Ahmad, I.; Dong, G.; Zhou, G.; Wu, Y. Association between Reactive Oxygen Species, Transcription Factors, and Candidate Genes in Drought-Resistant Sorghum. Int. J. Mol. Sci. 2024, 25, 6464. [Google Scholar] [CrossRef]
- Feller, U.; Vaseva, I. Extreme climatic events: Impacts of drought and high temperature on physiological processes in agronomically important plants. Front. Environ. Sci. 2014, 2, 39. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2023; FAO: Rome, Italy, 2023. [Google Scholar]
- Abreha, K.; Enyew, M.; Carlsson, A.; Vetukuri, R.; Feyissa, T.; Motlhaodi, T.; Ng’uni, D.; Geleta, M. Sorghum in dryland: Morphological, physiological, and molecular responses of sorghum under drought stress. Planta 2022, 255, 20. [Google Scholar] [CrossRef]
- Abdel-Ghany, S.; Ullah, F.; Ben-Hur, A.; Reddy, A. Transcriptome analysis of drought-resistant and drought-sensitive sorghum (sorghum bicolor) genotypes in response to PEG-induced drought stress. Int. J. Mol. Sci. 2020, 21, 772. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Wang, D.; Mu, Y.; Li, G.; Zhang, Z.; Pan, Y.; Zhu, L. Proteomic Analysis Revealed Different Molecular Mechanisms of Response to PEG Stress in Drought-Sensitive and Drought-Resistant Sorghums. Int. J. Mol. Sci. 2022, 23, 13297. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Ghaffar, A.; Kausar, A.; Zeidi, M.A.; Siddique, K.H.M.; Faroop, M. Plant photosynthesis under heat stress: Effects and management. Environ. Exp. Bot. 2023, 206, 105178. [Google Scholar] [CrossRef]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Pradyumna, K.; Dipali, S.; Poonam, T.; Madhu, T.; Giti, V.; Debasis, C. Drought tolerance in plants: Molecular mechanism and regulation of signaling molecules. Plant Signal. Mol. 2019, 105–123. [Google Scholar]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Dang, Y.; Diao, X.; Sui, N. Molecular mechanisms of stress resistance in sorghum: Implications for crop improvement strategies. J. Integr. Agric. 2024, 23, 741–768. [Google Scholar] [CrossRef]
- Wang, G.; Long, Y.; Jin, X.; Yang, Z.; Dai, L.; Yang, Y.; Lu, G.; Sun, B. SbMYC2 mediates jasmonic acid signaling to improve drought tolerance via directly activating SbGR1 in sorghum. Theor. Appl. Genet. 2024, 137, 72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Kang, W.; Sim, Y.; Koo, N.; Nam, J.; Lee, J.; Kim, N.; Jang, H.; Kim, Y.; Yeom, S. Transcriptome profiling of abiotic responses to heat, cold, salt, and osmotic stress of Capsicum annuum L. Sci. Data 2020, 7, 17. [Google Scholar]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.; Reiter, R.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef]
- Hu, W.; Ding, Z.; Tie, W.; Yan, Y.; Liu, Y.; Wu, C.; Liu, J.; Wang, J.; Peng, M.; Xu, B.; et al. Comparative physiological and transcriptomic analyses provide integrated insight into osmotic, cold, and salt stress tolerance mechanisms in banana. Sci. Rep. 2017, 22, 43007. [Google Scholar] [CrossRef]
- Yin, Q.; Feng, Z.; Ren, Z.; Wang, H.; Wu, D.; Jaisi, A.; Yang, M. Integrative physiological, metabolomic and transcriptomic insights into phenylpropanoids pathway responses in Nicotiana tabacum under drought stress. Plant Stress 2025, 16, 100815. [Google Scholar] [CrossRef]
- Ibrahim, M.E.; Adam, Y.; Zhou, G.; Elsiddig, A.; Zhu, G.; Nimir, E.; Ahmad, I. Biochar application affects forage sorghum under salinity stress Chilean. J. Agric. Res. 2020, 80, 317–325. [Google Scholar]
- Wu, H.; Ahmad, I.; Liu, J.; Zhang, Q.; Fei, H.; Bu, W.; Zhu, G.; Zhou, G. The Association of Sorghum Growth and Physiology with Soil Carbon Sink Source Captivity in Saline Soil. Plants 2025, 14, 670. [Google Scholar] [CrossRef]
- Qian, P.; Sun, R.; Ali, B.; Gill, R.; Xu, L.; Zhou, W. Effects of hydrogen sulfide on growth, antioxidative capacity, and ultrastructural changes in oilseed rape seedlings under aluminum toxicity. J. Plant Growth Regul. 2013, 33, 526–538. [Google Scholar] [CrossRef]
- Assaha, D.; Mekawy, A.; Liu, L.; Noori, M.; Saneoka, H. Na+ retention in the root is a key adaptive mechanism to low and high salinity in the glycophyte, talinum paniculatum (jacq.) gaertn. (portulacaceae). J. Agron. Crop Sci. 2017, 203, 56–67. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, Y.; Xu, Z.; Ahmad, I.; Nimir, N.E.A.; Zhou, G. Improving productivity of Sesbania pea in saline soils by enhancing antioxidant capacity with optimum application of nitrogen and phosphate combination. Front. Plant Sci. 2022, 13, 1027227. [Google Scholar] [CrossRef]
- Liu, F.; Pang, S.J. Stress tolerance and antioxidant enzymatic activities in the metabolisms of the reactive oxygen species in two intertidal red algae Grateloupia turuturu and Palmaria palmata. J. Exp. Mar. Biol. Ecol. 2010, 3822, 82–87. [Google Scholar] [CrossRef]
- Chen, F.; Ha, X.; Ma, T.; Ma, H. Comparative analysis of the physiological and transcriptomic profiles reveals alfalfa drought resistance mechanisms. BMC Plant Biol. 2024, 24, 954. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, G.; Xia, X.; Wang, M.; Yin, X.; Zhang, B.; Zhang, X.; Cui, Y. Deciphering the physiological and molecular mechanisms for copper tolerance in autotetraploid Arabidopsis. Plant Cell Rep. 2017, 36, 1585–1597. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, F.; Tao, Y.; Zhou, Y.; Bai, A.; Yu, Z.; Xiao, D.; Zhang, C.; Liu, T.; Hou, X.; et al. BcGR11, a cytoplasmic localized glutathione reductase, enhanced tolerance to copper stress in Arabidopsis thaliana. Antioxidants 2022, 11, 389. [Google Scholar] [CrossRef]
- Ksouri, N.; Jiménez, S.; Wells, C.; Contreras-Moreira, B.; Gogorcena, Y. Transcriptional Responses in Root and Leaf of Prunus persica under Drought Stress Using RNA Sequencing. Front. Plant Sci. 2016, 23, 1715. [Google Scholar] [CrossRef]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Qin, H.; Ding, H.; Li, Y.; Guo, T. Silicon application alleviates drought stress in wheat through transcriptional regulation of multiple antioxidant defense pathways. J. Plant Growth Regul. 2016, 35, 1–10. [Google Scholar] [CrossRef]
- Choi, S.; Lee, Z.; Kim, S.; Jeong, E.; Shim, J. Modulation of lignin biosynthesis for drought tolerance in plants. Front. Plant Sci. 2023, 14, 1116426. [Google Scholar] [CrossRef]
- Bonawitz, N.; Chapple, C. The genetics of lignin biosynthesis: Connecting genotype to phenotype. Annu. Rev. Genet. 2010, 44, 337–363. [Google Scholar] [CrossRef]
- Jun, S.; Sattler, S.; Cortez, G.; Vermerris, W.; Sattler, S.; Kang, C. Biochemical and Structural Analysis of Substrate Specificity of a Phenylalanine Ammonia-Lyase. Plant Physiol. 2018, 176, 1452–1468. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, F.; Tian, L.; Huang, M.; Deng, R.; Li, X.; Chen, W.; Wu, P.; Li, M.; Jiang, H.; et al. The Phenylalanine Ammonia Lyase Gene LjPAL1 Is Involved in Plant Defense Responses to Pathogens and Plays Diverse Roles in Lotus japonicus-Rhizobium Symbioses. Mol. Plant Microbe Interact. 2017, 30, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Escamilla-Treviño, L.; Sathitsuksanoh, N.; Shen, Z.; Shen, H.; Zhang, Y.; Dixon, R.; Zhao, B. Silencing of 4-coumarate: Coenzyme A ligase in switchgrass leads to reduced lignin content and improved fermentable sugar yields for biofuel production. New Phytol. 2011, 192, 611–625. [Google Scholar] [CrossRef]
- Afifi, O.; Tobimatsu, Y.; Lam, P.; Martin, A.; Miyamoto, T.; Osakabe, Y.; Osakabe, K.; Umezawa, T. Genome-edited rice deficient in two 4-COUMARATE: COENZYME A LIGASE genes displays diverse lignin alterations. Plant Physiol. 2022, 190, 2155–2172. [Google Scholar] [CrossRef]
- Ponniah, S.; Shang, Z.; Akbudak, M.; Srivastava, V.; Manoharan, M. Down-regulation of hydroxycinnamoyl CoA: Shikimate hydroxycinnamoyl transferase, cinnamoyl CoA reductase, and cinnamyl alcohol dehydrogenase leads to lignin reduction in rice (Oryza sativa L. ssp japonica cv. Nipponbare). Plant Biotechnol. Rep. 2017, 11, 17–27. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, L.; Chen, C.; Liu, X.; Yan, Y.; Wang, Z. Downregulation of cinnamoyl CoA reductase affects lignin and phenolic acids biosynthesis in Salvia miltiorrhiza Bunge. Plant Mol. Biol. Rep. 2012, 30, 1229–1236. [Google Scholar] [CrossRef]
- Fu, C.; Xiao, X.; Xi, Y.; Ge, Y.; Chen, F.; Bouton, J.; Dixon, R.; Wang, Z. Downregulation of cinnamyl alcohol dehydrogenase (CAD) leads to improved saccharification efficiency in switchgrass. BioEnergy Res. 2011, 4, 153–164. [Google Scholar] [CrossRef]
- Thevenin, J.; Pollet, B.; Letarnec, B.; Saulnier, L.; Gissot, L.; Maia-Grondard, A.; Lapierre, C.; Jouanin, L. The simultaneous repression of CCR and CAD, two enzymes of the lignin biosynthetic pathway, results in sterility and dwarfism in Arabidopsis thaliana. Mol. Plant 2011, 4, 70–82. [Google Scholar] [CrossRef]
- Joseph, N.; Amoah, M.; Albert, O. Unraveling the dynamics of starch metabolism and expression profiles of starch synthesis genes in millet under drought stress. Plant Gene 2024, 38, 100449. [Google Scholar] [CrossRef]
- Kaur, H.; Manna, M.; Thakur, T.; Gautam, V.; Salvi, P. Imperative role of sugar signaling and transport during drought stress responses in plants. Physiol. Plant. 2021, 171, 833–848. [Google Scholar] [CrossRef]
- Lyu, J.; Park, J.; Kim, J.; Bae, C.; Jeong, W.; Min, S.; Liu, J. Enhanced tolerance to heat stress in transgenic tomato seeds and seedlings overexpressing a trehalose-6-phosphate synthase/phosphatase fusion gene. Plant Biotechnol. Rep. 2018, 12, 399–408. [Google Scholar] [CrossRef]
- Liu, M.; Chien, C.; Lin, T. Constitutive components and induced gene expression are involved in the desiccation tolerance of Selaginella tamariscina. Plant Cell Physiol. 2008, 49, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, S.; Argyrokastritis, A.; Loukas, M.; Eliopoulos, E.; Tsakas, S.; Kaltsikes, P. Isolation and characterization of drought-related trehalose 6-phosphate-synthase gene from cultivated cotton (Gossypium hirsutum L.). Planta 2006, 223, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Ren, G.; Yue, G.; Li, Z.; Qu, X.; Hou, G.; Zhu, Y.; Zhang, J. Effects of water-deficit stress on the transcriptomes of developing immature ear and tassel in maize. Plant Cell Rep. 2007, 26, 2137–2147. [Google Scholar] [CrossRef]
- Liu, S.; Jin, J.; Ma, J.; Yao, M.; Ma, C.; Li, C.; Ding, Z.; Chen, L. Transcriptomic Analysis of Tea Plant Responding to Drought Stress and Recovery. PLoS ONE 2016, 20, e0147306. [Google Scholar] [CrossRef]
- Meraj, T.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef]
- Haider, M.; Zhang, C.; Kurjogi, M.; Pervaiz, T.; Zheng, T.; Zhang, C.; Lide, C.; Shangguan, L.; Fang, J. Insights into grapevine defense response against drought as revealed by biochemical, physiological and RNA-Seq analysis. Sci. Rep. 2017, 7, 13134. [Google Scholar] [CrossRef]
- Sah, S.; Reddy, K.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Zhao, S.; Guo, Y. The PYR-PP2C-CKL2 module regulates ABA-mediated actin reorganization during stomatal closure. New Phytol. 2022, 233, 2168–2184. [Google Scholar] [CrossRef]
- Liu, L.; Tang, C.; Zhang, Y.; Sha, X.; Tian, S.; Luo, Z.; Wei, G.; Zhu, L.; Li, Y.; Fu, J.; et al. The SnRK2.2-ZmHsf28-JAZ14/17 module regulates drought tolerance in maize. New Phytol. 2025, 245, 1985–2003. [Google Scholar] [CrossRef] [PubMed]
- Achard, P.; Renou, J.-P.; Berthomé, R.; Harberd, N.P.; Genschik, P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Krugman, T.; Chagué, V.; Peleg, Z.; Balzergue, S.; Just, J.; Korol, A.; Nevo, E.; Saranga, Y.; Chalhoub, B.; Fahima, T. Multilevel regulation and signalling processes associated with adaptation to terminal drought in wild emmer wheat. Funct. Integr. Genom. 2010, 10, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Omotajo, D.; Tate, T.; Cho, H.; Choudhary, M. Distribution and diversity of ribosome binding sites in prokaryotic genomes. BMC Genom. 2015, 16, 604. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, Z.; Lu, R.; Wu, B.; Tang, Q.; Xu, Y.; Cheng, C.; Su, J. Transcriptome Analysis of Two Species of Jute in Response to Polyethylene Glycol (PEG)- induced Drought Stress. Sci. Rep. 2017, 7, 16565. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chai, Q.; Liu, C.; Niu, X.; Li, W.; Shang, X.; Gu, A.; Zhang, D.; Guo, W. Cotton GhNAC4 promotes drought tolerance by regulating secondary cell wall biosynthesis and ribosomal protein homeostasis. Plant J. 2024, 117, 1052–1068. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Ahmad, I.; Hussien Ibrahim, M.E.; Qin, B.; Zhu, H.; Zhu, G.; Zhou, G. Differential Responses of Two Sorghum Genotypes to Drought Stress at Seedling Stage Revealed by Integrated Physiological and Transcriptional Analysis. Agriculture 2025, 15, 1780. https://doi.org/10.3390/agriculture15161780
Wang M, Ahmad I, Hussien Ibrahim ME, Qin B, Zhu H, Zhu G, Zhou G. Differential Responses of Two Sorghum Genotypes to Drought Stress at Seedling Stage Revealed by Integrated Physiological and Transcriptional Analysis. Agriculture. 2025; 15(16):1780. https://doi.org/10.3390/agriculture15161780
Chicago/Turabian StyleWang, Manhong, Irshad Ahmad, Muhi Eldeen Hussien Ibrahim, Bin Qin, Hailu Zhu, Guanglong Zhu, and Guisheng Zhou. 2025. "Differential Responses of Two Sorghum Genotypes to Drought Stress at Seedling Stage Revealed by Integrated Physiological and Transcriptional Analysis" Agriculture 15, no. 16: 1780. https://doi.org/10.3390/agriculture15161780
APA StyleWang, M., Ahmad, I., Hussien Ibrahim, M. E., Qin, B., Zhu, H., Zhu, G., & Zhou, G. (2025). Differential Responses of Two Sorghum Genotypes to Drought Stress at Seedling Stage Revealed by Integrated Physiological and Transcriptional Analysis. Agriculture, 15(16), 1780. https://doi.org/10.3390/agriculture15161780






