Foliar Ascorbic Acid Enhances Postharvest Quality of Cherry Tomatoes in Saline Hydroponic Substrate System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site Location
2.2. Treatments and Experimental Design
2.3. Studied Cultivar
2.4. Experimental Setup and Management
2.4.1. Preparation of Nutrient Solutions
2.4.2. Preparation and Management of Saline Nutrient Solutions
- C = concentration of salts to be applied (mmolc L−1);
- SNS = electrical conductivity of the nutrient solution, subtracting the control SNS (2.1 dS m−1).
2.4.3. Preparation and Application of Ascorbic Acid
2.4.4. Phytosanitary Control
2.5. Evaluated Traits
2.5.1. Yield
2.5.2. Physical Characterization of Fruits
2.5.3. Chemical Characterization of Fruits
- V = volume of 0.1 M NaOH solution used in the titration (mL);
- F = correction factor of the titrant solution;
- N = normality of the NaOH solution (0.1);
- 64.04 = conversion factor for citric acid;
- Pa = mass of the sample used (g).
- V = volume of DCPIP solution used in the titration (mL);
- F = correction factor of the DCPIP solution;
- Pa = sample mass (g).
2.6. Statistical Analysis
3. Results
4. Discussion
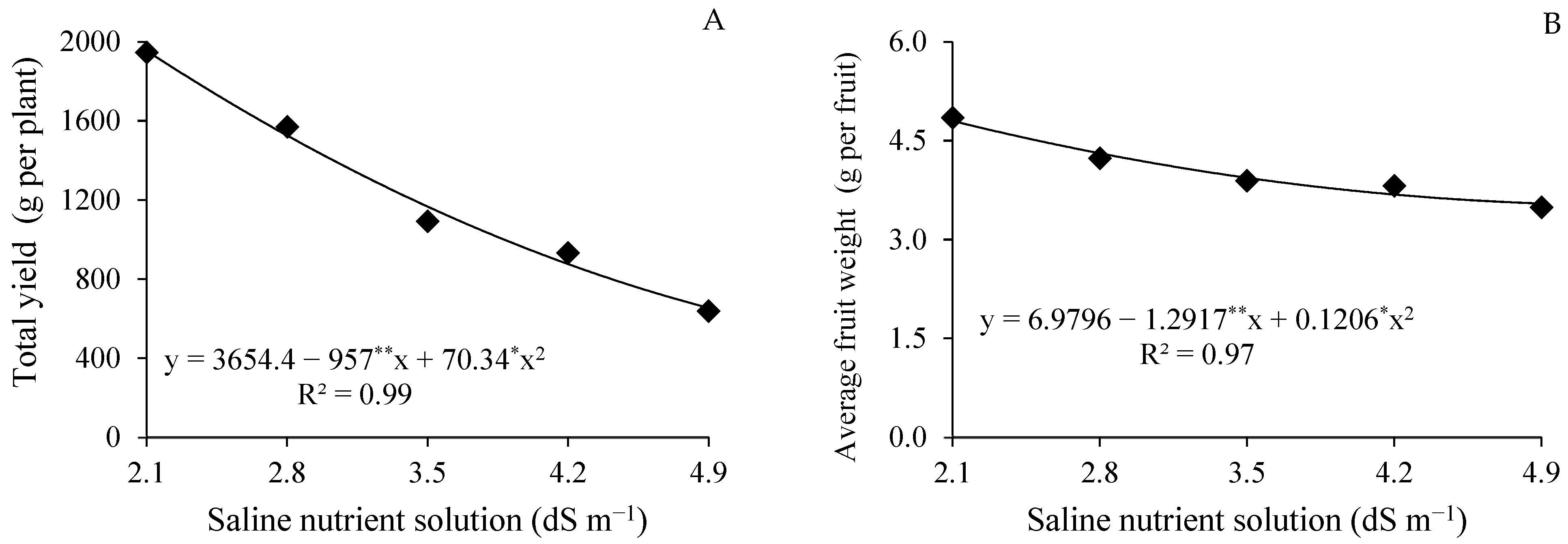
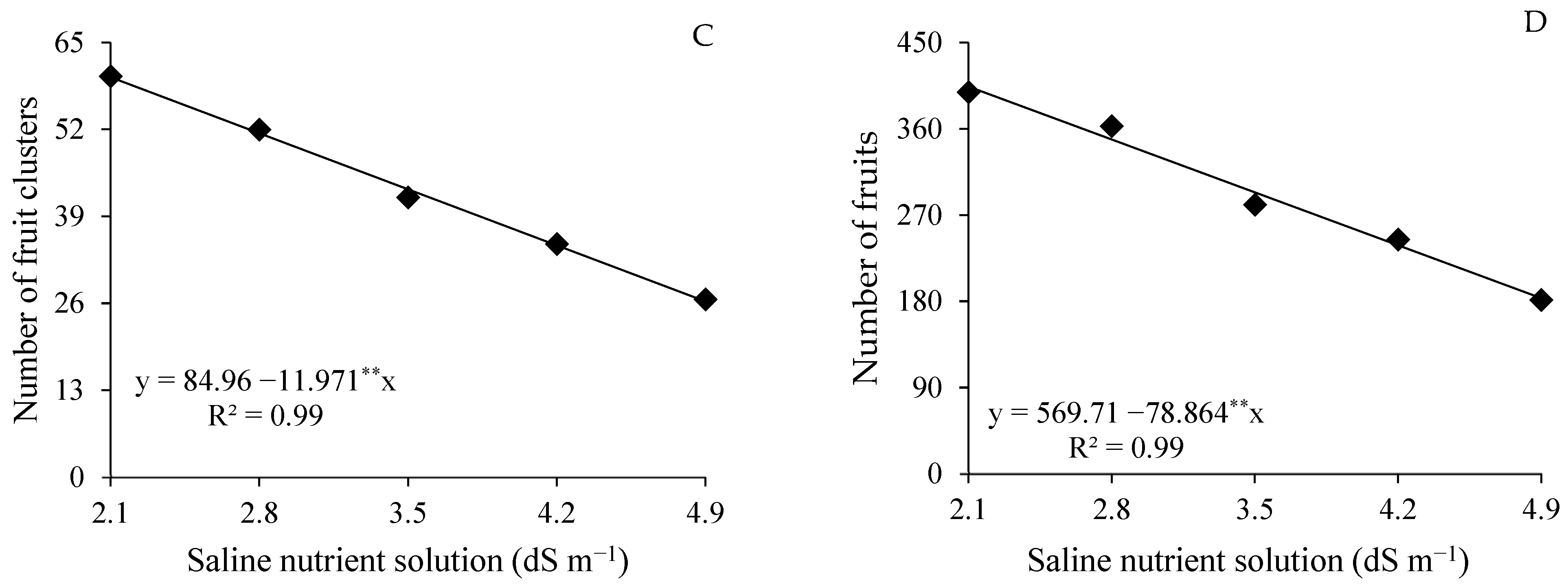
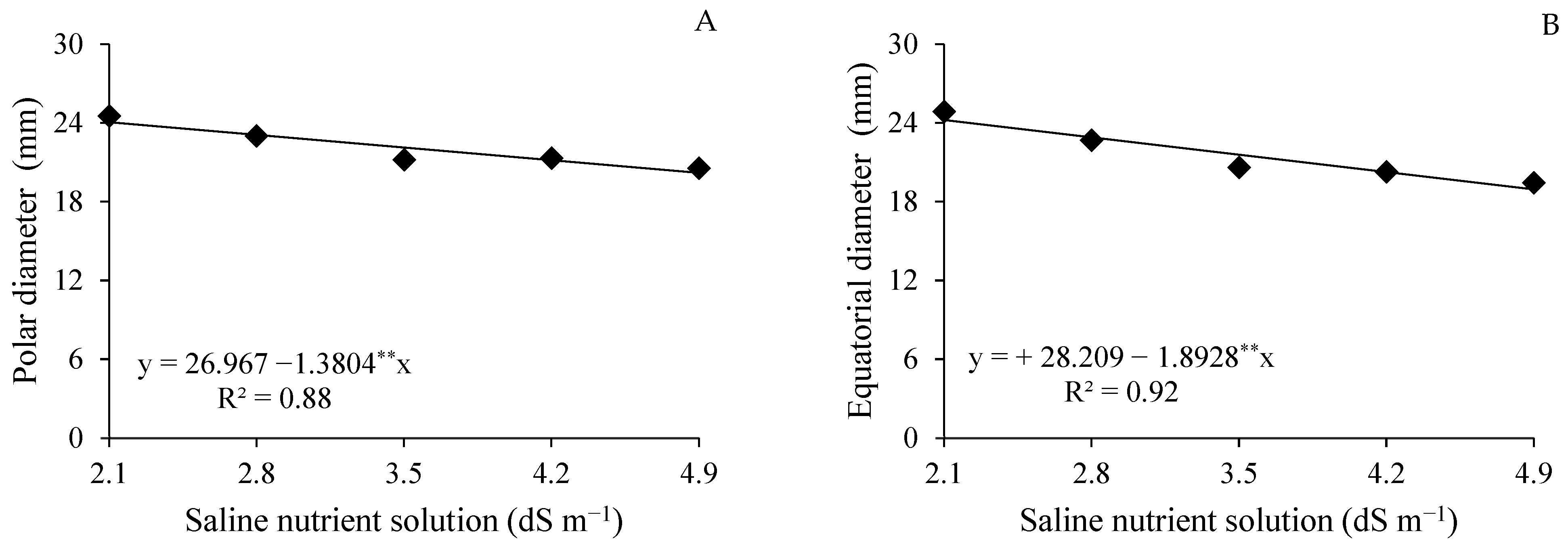
4.1. Effects of Saline Stress on Yield Components
4.2. Modulation of Postharvest Quality by Salinity and Ascorbic Acid Application
4.3. Correlations Between Yield Parameters and Postharvest Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonçalves, D.C.; dos S. Fernandes, C.H.; Tejo, D.P.; Vidal, T.C.M. Cultivo do tomate cereja sob sistema hidropônico: Influência do turno de rega. Uniciências 2018, 22, 20–23. [Google Scholar] [CrossRef]
- Roque, I.A.; dos A. Soares, L.A.; de Lima, G.S.; Lopes, I.A.P.; de A. Silva, L.; Fernandes, P.D. Biomass, gas exchange and production of cherry tomato cultivated under saline water and nitrogen fertilization. Rev. Caatinga 2022, 35, 686–696. [Google Scholar] [CrossRef]
- IBGE—Instituto Brasileiro de Geografia e Estatística. Produção Agrícola Municipal 2023. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/tomate/br (accessed on 8 September 2024).
- Nóbrega, J.S.; Guedes, M.A.; de Lima, G.S.; Gheyi, H.R.; dos A. Soares, L.A.; de A. Silva, L.; da Silva, S.S.; de A. Brito, L. Photosynthetic pigments, growth, and production of cherry tomato under salt stress and hydrogen peroxide. Rev. Bras. Eng. Agric. Ambient. 2024, 28, e275968. [Google Scholar] [CrossRef]
- Andrade, E.M.G.; de Lima, G.S.; de Lima, V.L.A.; da Silva, S.S.; Dias, A.S.; Gheyi, H.R. Hydrogen peroxide as attenuator of salt stress effects on the physiology and biomass of yellow passion fruit. Rev. Bras. Eng. Agrícola Ambient. 2022, 26, 571–578. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Liang, X.; Zhuang, J.; Wang, X.; Qin, F.; Jiang, C. A dirigent family protein confers variation of Casparian strip thickness and salt tolerance in maize. Nat. Commun. 2022, 13, 2222–2239. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.K.N.; da Silva, A.A.R.; de Lima, G.S.; dos A. Soares, L.A.; Gheyi, H.R.; de Lacerda, C.F.; de Azevedo, C.A.V.; Nobre, R.G.; Chaves, L.H.G.; Fernandes, P.D.; et al. Foliar application of salicylic acid mitigates saline stress on physiology, production, and post-harvest quality of hydroponic Japanese cucumber. Agriculture 2023, 13, 395. [Google Scholar] [CrossRef]
- Chen, L.; Meng, Y.; Yang, W.; Qian, L.V.; Zhou, L.; Liu, S.; Li, X. Genome-wide analysis and identification of TaRING-H2 gene family and TaSDIR1 positively regulates salt stress tolerance in wheat. Int. J. Biol. Macromol. 2023, 242, 125–162. [Google Scholar] [CrossRef]
- Sausen, D.F.; Lopes, C.; Marques, S.; Souza, L.; Alves, A.; Patrocínio, E.; Cordeiro, E.K. Cultivo fora do solo: Uma alternativa para áreas marginais. Braz. J. Dev. 2020, 6, 14888–14903. [Google Scholar] [CrossRef]
- Wendling, I.; Ferrari, M.P.; Dutra, L.F. Produção de Mudas de Corticeira-do-Mato por Miniestaquia a Partir de Propágulos Juvenis; Embrapa Florestas: Colombo, Sri Lanka, 2005; 5p. [Google Scholar]
- Hutchinson, G.K.; Nguyen, L.X.; Ames, Z.R.; Nemali, K.; Ferrarezi, R.S. Substrate system outperforms water-culture systems for hydroponic strawberry production. Front. Plant Sci. 2025, 16, 1469430. [Google Scholar]
- Martínez, J.P.; Fuentes, R.; Farías, K.; Lizana, C.; Alfaro, J.F.; Fuentes, L.; Lutts, S. Effects of salt stress on fruit antioxidant capacity of wild (Solanum chilense) and domesticated (Solanum lycopersicum var. cerasiforme) tomatoes. Agronomia 2020, 10, 1481. [Google Scholar] [CrossRef]
- Caetano, E.J.M.; da Silva, A.A.R.; de Lima, G.S.; de Azevedo, C.A.V.; de Veloso, L.L.S.A.; de Arruda, T.F.L.; de Souza, A.R.; dos Soares, L.A.A.; Gheyi, H.R.; dos Dias, M.S.; et al. Application techniques and concentrations of ascorbic acid to reduce saline stress in passion fruit. Plants 2024, 13, 2718. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Ahmad, I.; Basit, A.; Shehata, W.F.; Hassan, U.; Shah, S.T.; Ullah, A.; Mohamed, H.I. Ascorbic acid enhances growth and yield of sweet peppers (Capsicum annum) by mitigating salinity stress. Gesunde Pflanz. 2022, 74, 423–433. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Cong, Y.; Liu, X.; Yang, Q.; Xing, J.; Liu, H. Ascorbic acid mitigates salt stress in tomato seedlings by enhancing chlorophyll synthesis pathways. Agronomy 2024, 14, 1810. [Google Scholar] [CrossRef]
- Kamran, A.; Mushtaq, M.; Arif, M.; Rashid, S. Papel dos bioestimulantes (ácido ascórbico e ácido fúlvico) para sinergizar a atividade do Rhizobium na ervilha (Pisum sativum L. var. Meteor). Plant Physiol. Biochem. 2023, 196, 668–682. [Google Scholar] [CrossRef] [PubMed]
- Celi, G.E.A. Uso de Ácido Ascórbico em Plantas de Soja Submetidas à Salinidade. Dissertação Thesis, Universidade Estadual Paulista, Unesp, Jaboticabal, 25 March 2022. [Google Scholar]
- Naz, S.; Mushtaq, A.; Ali, S.; Muhammad, H.M.D.; Saddiq, B.; Ahmad, R.; Shahzad, M.; Ayaz, A.; Aamir, M.; Al-Saeed, F.A.; et al. Foliar application of ascorbic acid enhances growth and yield of lettuce (Lactuca sativa) under saline conditions by improving antioxidant defence mechanism. Funct. Plant Biol. 2024, 51, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Gonçalvez, J.D.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Guedes, M.A.; Gheyi, H.R.; de Lima, G.S.; dos A. Soares, L.A.; Sa, V.K.N.O.; Silva, L.A.; Silva, F.J.L.; Torres, R.A.F. Aplicação foliar de H2O2 no cultivo hidropônico de tomate cereja sob soluções nutritivas salinas. WRIM 2024, 13, 150–164. [Google Scholar]
- Gaafar, A.A.; Ali, S.I.; El-Shawadfy, M.A.; Salama, Z.A.; Sękara, A.; Ulrichs, C.; Abdelhamid, M.T. Ascorbic acid induces the increase of secondary metabolites, antioxidant activity, growth, and productivity of the common bean under water stress conditions. Plants 2020, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Agristar—Instituto de Ação Social. Available online: https://agristar.com.br/topseed-garden/blue-linehortalicas/tomate-cereja-laranja/1888147 (accessed on 9 October 2024).
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. Bull. 1950, 347, 1–32. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; U.S. Department of Agriculture USDA Handbook: Washington, DC, USA, 1954; Volume 60, 160p.
- IAL—Instituto Adolfo Lutz. Normas analíticas do Instituto Adolfo Lutz, 4th ed.; IAL: São Paulo, Brasil, 2008; pp. 102–104. [Google Scholar]
- Strohecker, R.R.; Henning, H.M. Analises de Vitaminas: Métodos Comprobados; Editora Paz Montalvo: Madrid, Spain, 1967; 428p. [Google Scholar]
- Benassi, M.T.; Antunes, A.J. A comparison of meta-phosphoric and oxalic acids as extractant solutions for the determination of vitamin C in selected vegetables. Braz. Arch. Biol. Technol. 1988, 31, 507–513. [Google Scholar]
- Vasconcelos, N.M.; Pinto, G.A.S.; Aragão, F.A.S. Determinação de Açúcares Redutores pelo Ácido 3,5-Dinitrosalicílico: Histórico do Desenvolvimento do Método e Estabelecimento de um Protocolo Para o Laboratório de Bioprocessos; Embrapa Agroindústria Tropical: Fortaleza, Brazil, 2013; 29p. [Google Scholar]
- Francis, F.J. Analysis of anthocyanins. In Anthocyanins as Food Colors; Markakis, P., Ed.; Academic Press: New York, NY, USA, 1982; pp. 182–205. [Google Scholar]
- Waterhouse, A. Folin-ciocalteau micro method for total phenol in wine. AJEV 2006, 11, 3–5. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer analysis system to fixed effects split-plot type designs. Rev. Bras. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
- Guedes, M.A.; Silva, A.A.R.; Lima, G.S.; Gheyi, H.R.; Soares, L.A.A.; Silva, L.A.; Oliveira, V.K.N.; Fatima, R.T.; Nobre, R.G.; Nobrega, J.S.; et al. Hydroponic cultivation of laranja cherry tomatoes under salt stress and foliar application of hydrogen peroxide. Agriculture 2023, 13, 1688. [Google Scholar] [CrossRef]
- Liang, H.; Shi, Q.; Li, X.; Gao, P.; Feng, D.; Zhang, X.; Ma, W. Synergistic effects of carbon cycle metabolism and photosynthesis in Chinese cabbage under salt stress. Hortic. Plant J. 2022, 9, 1–12. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, K.; Tian, Y.; Han, K.; El-Kassaby, Y.A.; Yang, H.; Li, Y. Timecourse transcriptomics analysis reveals key responses of populus to salt stress. Ind. Crops Prod. 2023, 194, 116–178. [Google Scholar] [CrossRef]
- Santos, C.A.; Carmo, M.G.F.; Abboud, A.C.S. Novo nicho: Tomate cereja orgânico. Campo Negócios HF 2016, 137, 16–20. [Google Scholar]
- Fernandes, C.; Corá, J.E.; Braz, L.T. Classificação de tomate-cereja em função do tamanho e peso dos frutos. Hortic. Bras. 2007, 25, 275–278. [Google Scholar] [CrossRef]
- Skider, R.K.; Wang, X.; Zhang, H.; Gui, H.; Dong, Q.; Jin, D.; Song, M. Nitrogen enhances salt tolerance by modulating the antioxidant defense system and osmoregulation substance content in Gossypium hirsutum. Plants 2020, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- de S. Viana, E.; Reis, R.C.; da Ronielli Cardoso, S.C.S.; das Neves, T.T.; de Jesus, J.L. Avaliação físico-química e sensorial de frutos de genótipos melhorados de mamoeiro. Pesqui. Agropecu. Trop. 2015, 45, 297–303. [Google Scholar] [CrossRef]
- Wu, P.; Li, B.; Liu, Y.; Bian, Z.; Xiong, J.; Wang, Y.; Zhu, B. Multiple physiological and biochemical functions of ascorbic acid in plant growth, development, and abiotic stress response. Int. J. Mol. Sci. 2024, 25, 1832. [Google Scholar] [CrossRef]
- Figueroa-Macías, J.P.; Ceballos-García, Y.; Núñez, M.; Díaz, K.; Olea, A.F.; Espinoza, L. Plant growth-defense trade-offs: Molecular processes leading to physiological changes. Int. J. Mol. Sci. 2021, 22, 693. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Pirjan, K.; Qasim, M.; Mahmood, A.; Javed, T.; Muhammad, H.; Yang, S.; Dong, R.; Ali, B.; Rahimi, M. Salinity stress improves antioxidant potential by modulating physio-biochemical responses in Moringa oleifera Lam. Sci. Rep. 2023, 13, 2895. [Google Scholar] [CrossRef]
- Brasil Ministério da Agricultura e do Abastecimento. Aprova o Regulamento Técnico Geral Para Fixação dos Padrões de Identidade e Qualidade Para Polpa de Fruta; Diário Oficial da União: Brasília, Brazil, 2018; 23p. [Google Scholar]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2020, 20, 207–220. [Google Scholar] [CrossRef]
- Zamuz, S.; Munekata, P.E.S.; Gullón, B.; Rocchetti, G.; Montesano, D.; Lorenzo, J.M. Citrullus lanatus as source of bioactive components: An up-to-date review. Trends Food Sci. Technol. 2021, 111, 208–222. [Google Scholar] [CrossRef]
- Da Silva, F.J.L.; de Lima, G.S.; da Silva, S.S.; dos A. Soares, L.A.; Torres, R.A.F.; Gheyi, H.R.; da Silva, J.S.; Mendonça, A.J.T.; Andrade, J.T.F.; da Silva, A.A.R.; et al. Effect of hydrogen peroxide application on physiology, production, and post-harvest quality of mini watermelon under salt stress. Arid Land Res. Manag. 2024, 38, 262–288. [Google Scholar]
- Ribeiro, E.P.; Seravalli, E.A.G. Química dos Alimentos, 2nd ed.; Editora Blucher: São Paulo, Brazil, 2007; 196p. [Google Scholar]
- Modesto Junior, E.N.; da S. Soares, S.; Gomes, P.W.P.; Ribeiro, C.D.F.A.; da Silva, R.M.V. Estudo do armazenamento da polpa do fruto ginja Eugenia uniflora L. e sua influência nos teores de ácido ascórbico e antocianinas. Sci. Plena 2016, 12, 1–8. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Baskar, V.; Venkatesh, R.; Ramalingam, S. Flavonoids (antioxidant systems) in higher plants and their response to stresses. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 253–268. [Google Scholar]
- UFRA-Universidade Federal Rural de Amazonas. Cartilha de Alimentos Funcionais: A Ciência no Seu Prato: Compostos Fenólicos e Flavonoides; Pará: Bélem, Brazil, 2024; 16p. [Google Scholar]
- González-García, Y.; Cárdenas-Álvarez, C.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Cabrera-De-La-Fuente, M.; Sandoval-Rangel, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Effect of three nanoparticles (Se, Si and Cu) on the bioactive compounds of bell pepper fruits under saline stress. Plants 2021, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, Y.K.; Singh, M.; Manorama, K.; Lakra, N.; Zaid, A.; Zulfiqar, F. Plant phenolics: Neglected secondary metabolites in plant stress tolerance. Braz. J. Bot 2024, 47, 703–721. [Google Scholar] [CrossRef]
- Queiroga, R.C.F.; Puiatti, M.; Fontes, P.C.R.; Cecon, P.R. Produtividade e qualidade do melão cantaloupe, cultivado em ambiente protegido, variando o número e a posição dos frutos na planta. Bragantia 2008, 67, 911–920. [Google Scholar] [CrossRef]
- Souza, A.V.D.; Vieira, M.R.D.S.; Putti, F.F. Correlações entre compostos fenólicos e atividade antioxidante em casca e polpa de variedades de uva de mesa. BJFT 2018, 21, e2017103. [Google Scholar] [CrossRef]
- Gouveia, A.M.F.Q. Qualidade Pós-Colheita dos Frutos de Passiflora cincinnata Armazenados sob Refrigeração. Dissertação Thesis, Universidade Federal de Campina Grande, Pombal, Portugal, 14 March 2023. [Google Scholar]

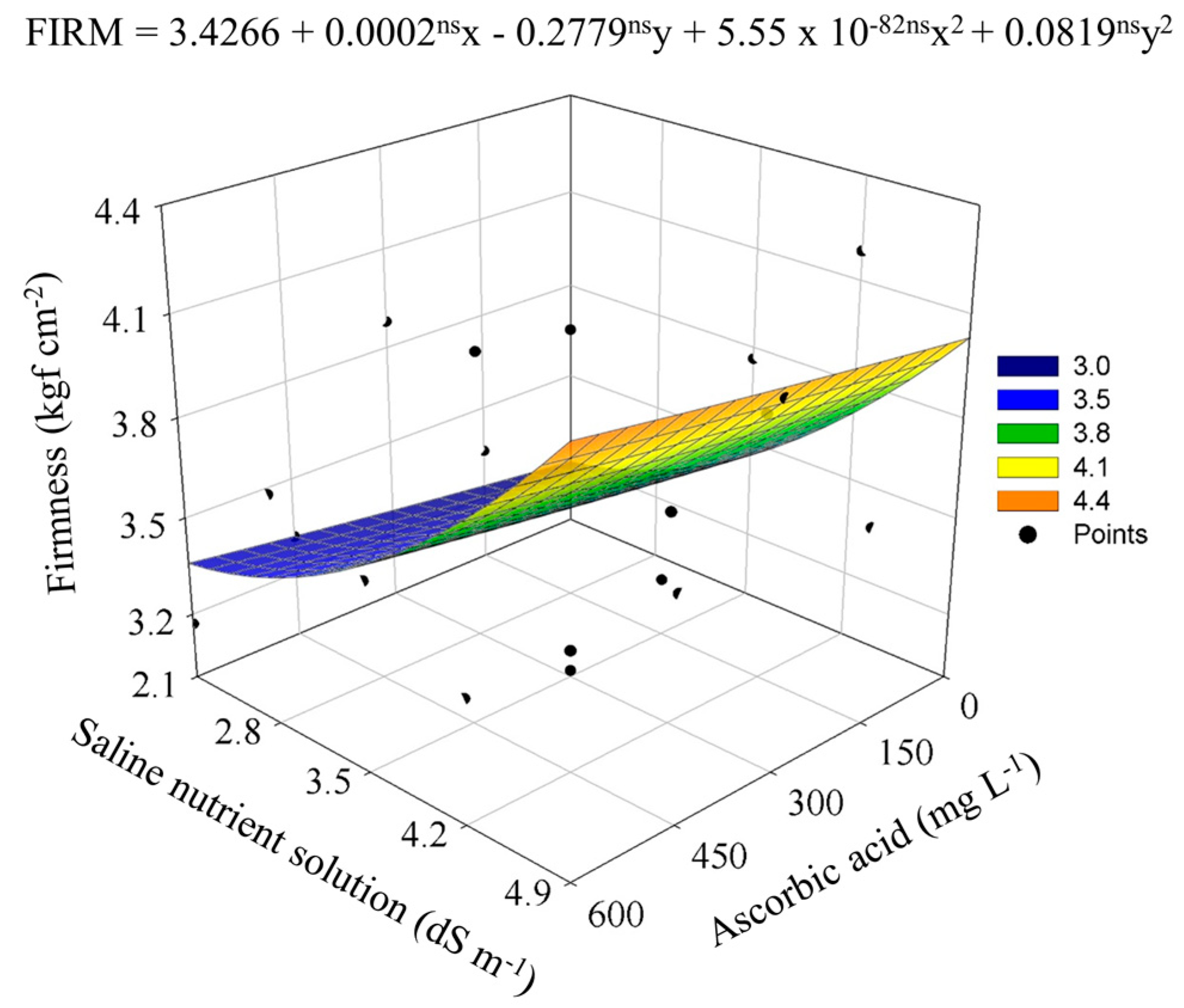
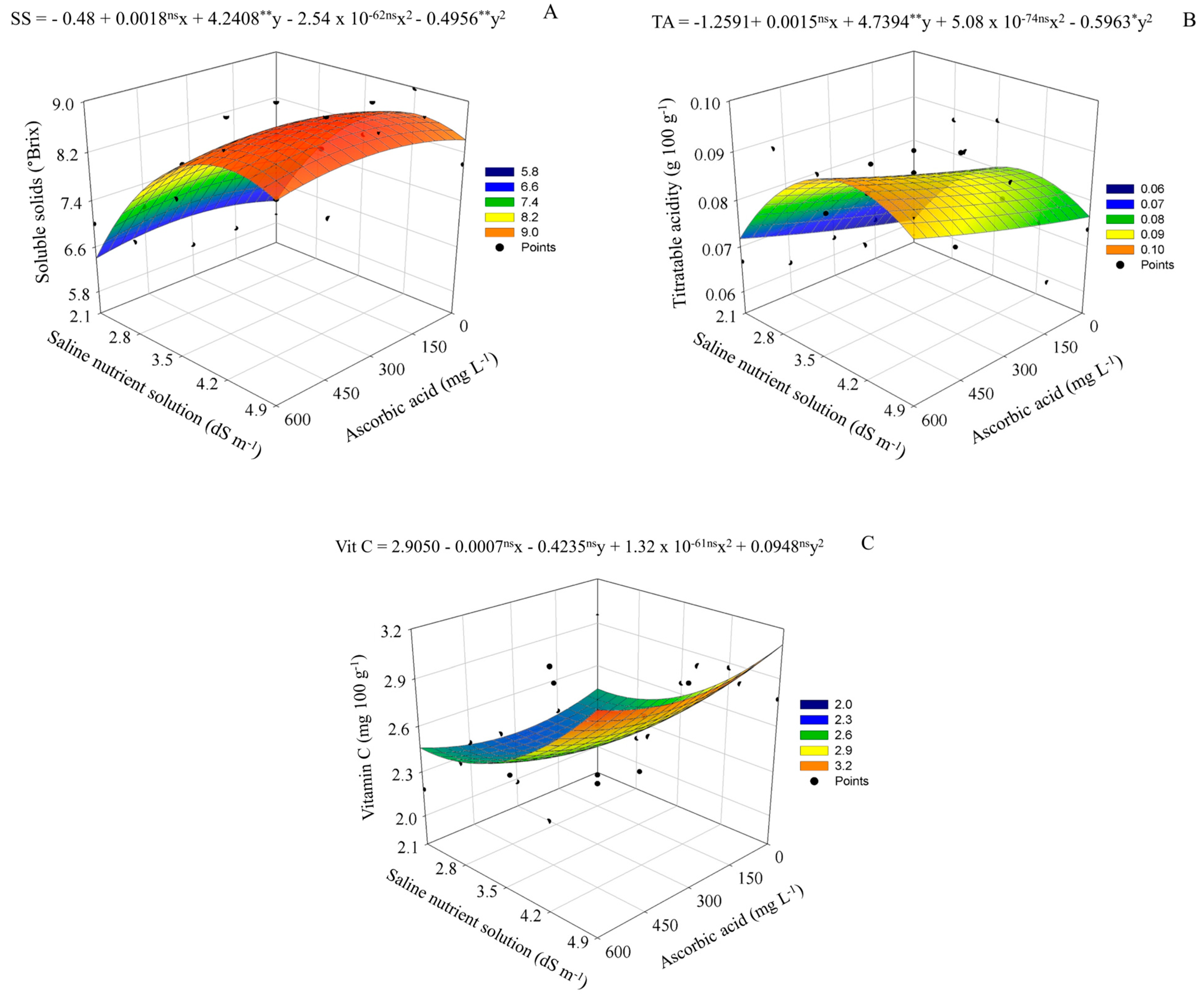
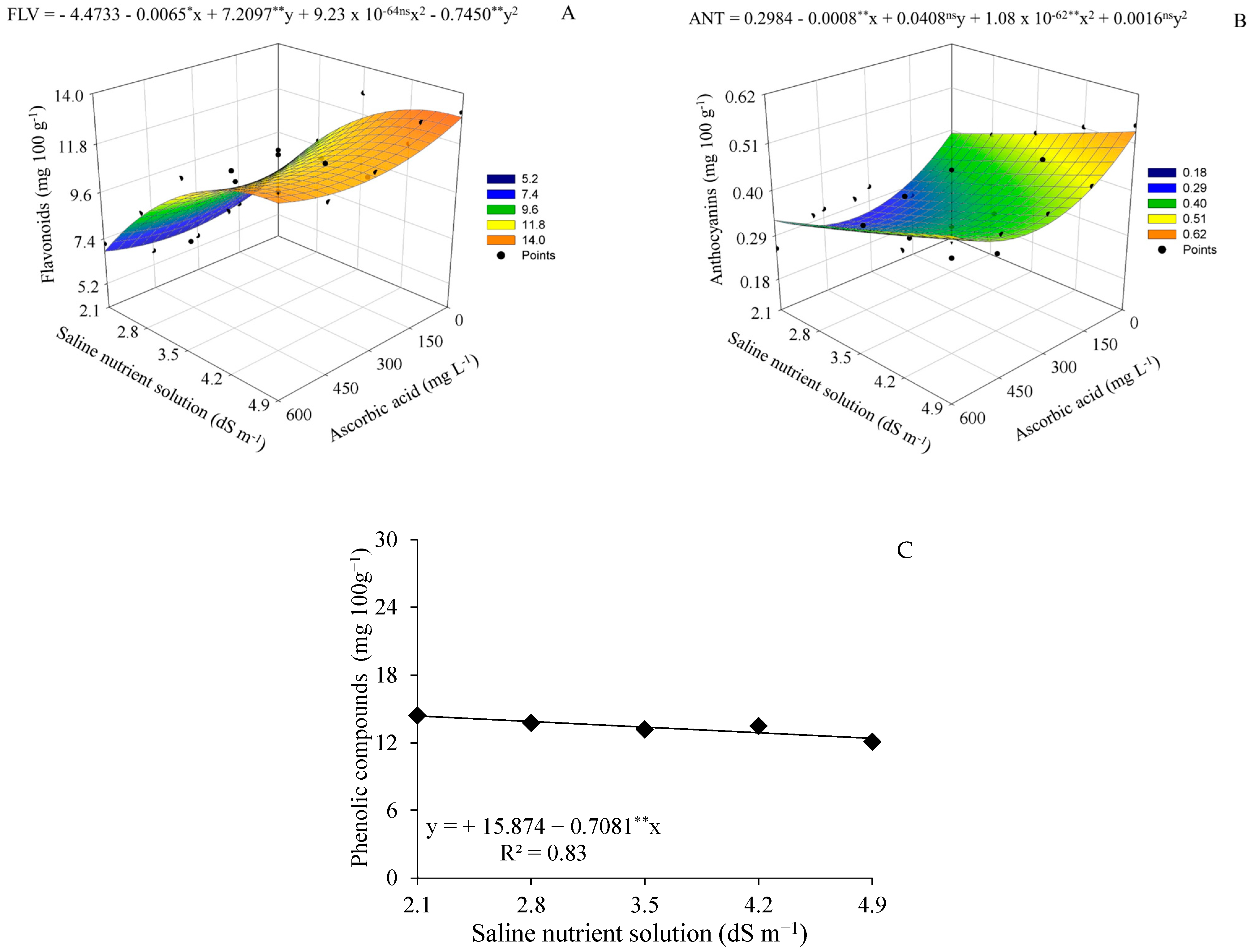

| Saline nutrient solution (dS m−1) | 2.1 | 2.8 | 3.5 | 4.2 | 4.9 |
| Water consumption (L per plant) | 134.60 | 128.70 | 71.60 | 64.81 | 61.86 |
| Source of Variation | DF | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| TY | AFW | NFC | NF | PD | ED | ||
| Saline nutrient solution (SNS) | 4 | 5,426,576.42 ** | 5.3012 ** | 3520.88 ** | 154,577.94 ** | 52.9045 ** | 95.862 ** |
| Linear regression | 1 | 21,155,649.94 ** | 19.6119 ** | 14,044.88 ** | 609,518.40 ** | 186.747 ** | 351.098 ** |
| Quadratic regression | 1 | 332,621.48 * | 0.9782 * | 8.93 ns | 150.09 ns | 16.835 ** | 27.462 ** |
| Ascorbic acid (AA) | 4 | 102,896.82 ns | 0.0420 ns | 150.21 ns | 4328.31 ns | 0.3280 ns | 1.083 ns |
| Linear regression | 1 | 4804.09 ns | 0.1072 ns | 136.12 ns | 6555.12 ns | 0.8115 ns | 1.654 ns |
| Quadratic regression | 1 | 167,600.58 ns | 0.0212 ns | 225.003 ns | 130,074.02 ns | 0.2286 ns | 0.0009 ns |
| Interaction (SNS × AA) | 16 | 59,568.68 ns | 0.2792 ns | 204.86 ns | 2668.73 ns | 0.4924 ns | 1.672 ns |
| Blocks | 3 | 112,677.83 ns | 0.9119 ns | 1989.77 ns | 1563.55 ns | 1.9993 ns | 2.473 ns |
| Residue | 72 | 83,852.47 | 0.2110 | 206.42 | 3192.17 | 0.5658 | 1.3072 |
| CV (%) | 23.44 | 11.33 | 33.37 | 19.24 | 3.40 | 5.30 | |
| Source of Variation | DF | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| FIRM | SS | TA | MI | pH | Vit C | ||
| Saline nutrient solution (SNS) | 4 | 2.3801 ** | 18.8 ** | 0.001784 ** | 0.0881 ns | 0.046 ns | 1.6172 ** |
| Linear regression | 1 | 8.5615 ** | 58.32 ns | 0.003135 ** | 0.1479 ns | 0.002 ns | 5.6558 ** |
| Quadratic regression | 1 | 0.4512 ns | 16.51 ns | 0.002390 ** | 0.0133 ns | 0.0232 ns | 0.6045 ** |
| Ascorbic acid (AA) | 4 | 0.1835 ns | 0.4 ** | 0.000592 ** | 0.0657 ns | 0.01 ns | 0.0762 ns |
| Linear regression | 1 | 0.3049 ns | 0.32 ns | 0.001546 ** | 0.1052 ns | 0.001 ns | 0.0081 ns |
| Quadratic regression | 1 | 0.0004 ns | 0.9143 ns | 0.000004 ns | 0.0082 ns | 0.0053 ns | 0.2470 * |
| Interaction (SNS × AA) | 16 | 1.4250 ** | 1.2 ** | 0.000259 ** | 0.0757 ns | 0.004 ns | 0.4843 ** |
| Blocks | 3 | 0.2362 ns | 1 ns | 0.00007 ns | 0.0036 ns | 0.0005 ns | 0.1644 ns |
| Residue | 72 | 0.1592 | 1 | 0.0687 | 0.0021 | 0.0001 | 0.0521 |
| CV (%) | 11.04 | 0 | 3.29 | 4.44 | 0.31 | 8.68 | |
| Source of Variation | DF | Mean Squares | |||
|---|---|---|---|---|---|
| RS | FLV | ANT | PC | ||
| Saline nutrient solution (SNS) | 4 | 6.5588 ns | 112.97 ** | 0.0688 ** | 14.7976 ** |
| Linear regression | 1 | 7.5355 ns | 390.014 ** | 0.265 ** | 49.1338 ** |
| Quadratic regression | 1 | 2.6076 ns | 37.3103 ** | 0.0001 ns | 0.5101 ns |
| Ascorbic acid (AA) | 4 | 3.1674 ns | 5.2190 ** | 0.0573 ** | 3.2250 ns |
| Linear regression | 1 | 5.4840 ns | 4.3630 ** | 0.0505 ** | 0.8141 ns |
| Quadratic regression | 1 | 0.0124 ns | 12.0723 ** | 0.1642 ** | 4.3152 ns |
| Interaction (SNS × AA) | 16 | 4.9383 ns | 1.9892 ** | 0.0110 ** | 18.1669 ns |
| Blocks | 3 | 0.1060 ns | 0.0682 ns | 0.0014 ns | 0.2835 ns |
| Residue | 72 | 0.0645 | 0.5130 | 0.0039 | 0.3509 |
| CV (%) | 2.82 | 7.03 | 16.48 | 7.84 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.J.L.d.; Gheyi, H.R.; Lima, G.S.d.; Soares, L.A.d.A.; Lima, V.L.A.d.; Paiva, F.J.d.S.; Silva, A.A.R.d.; Costa, D.S.; Torres, R.A.F.; Souza, A.R.d.; et al. Foliar Ascorbic Acid Enhances Postharvest Quality of Cherry Tomatoes in Saline Hydroponic Substrate System. Agriculture 2025, 15, 1767. https://doi.org/10.3390/agriculture15161767
Silva FJLd, Gheyi HR, Lima GSd, Soares LAdA, Lima VLAd, Paiva FJdS, Silva AARd, Costa DS, Torres RAF, Souza ARd, et al. Foliar Ascorbic Acid Enhances Postharvest Quality of Cherry Tomatoes in Saline Hydroponic Substrate System. Agriculture. 2025; 15(16):1767. https://doi.org/10.3390/agriculture15161767
Chicago/Turabian StyleSilva, Fellype Jonathar Lemos da, Hans Raj Gheyi, Geovani Soares de Lima, Lauriane Almeida dos Anjos Soares, Vera Lúcia Antunes de Lima, Francisco Jean da Silva Paiva, André Alisson Rodrigues da Silva, Denis Soares Costa, Rafaela Aparecida Frazão Torres, Allesson Ramos de Souza, and et al. 2025. "Foliar Ascorbic Acid Enhances Postharvest Quality of Cherry Tomatoes in Saline Hydroponic Substrate System" Agriculture 15, no. 16: 1767. https://doi.org/10.3390/agriculture15161767
APA StyleSilva, F. J. L. d., Gheyi, H. R., Lima, G. S. d., Soares, L. A. d. A., Lima, V. L. A. d., Paiva, F. J. d. S., Silva, A. A. R. d., Costa, D. S., Torres, R. A. F., Souza, A. R. d., Silva, V. M. B. d., Guedes, M. A., Oliveira, V. K. N., Lima, B. d. M., & Fátima, R. T. d. (2025). Foliar Ascorbic Acid Enhances Postharvest Quality of Cherry Tomatoes in Saline Hydroponic Substrate System. Agriculture, 15(16), 1767. https://doi.org/10.3390/agriculture15161767









