Shade Nets Increase Plant Growth but Not Fruit Yield in Organic Jalapeño Pepper (Capsicum annuum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site, Design, and Treatments
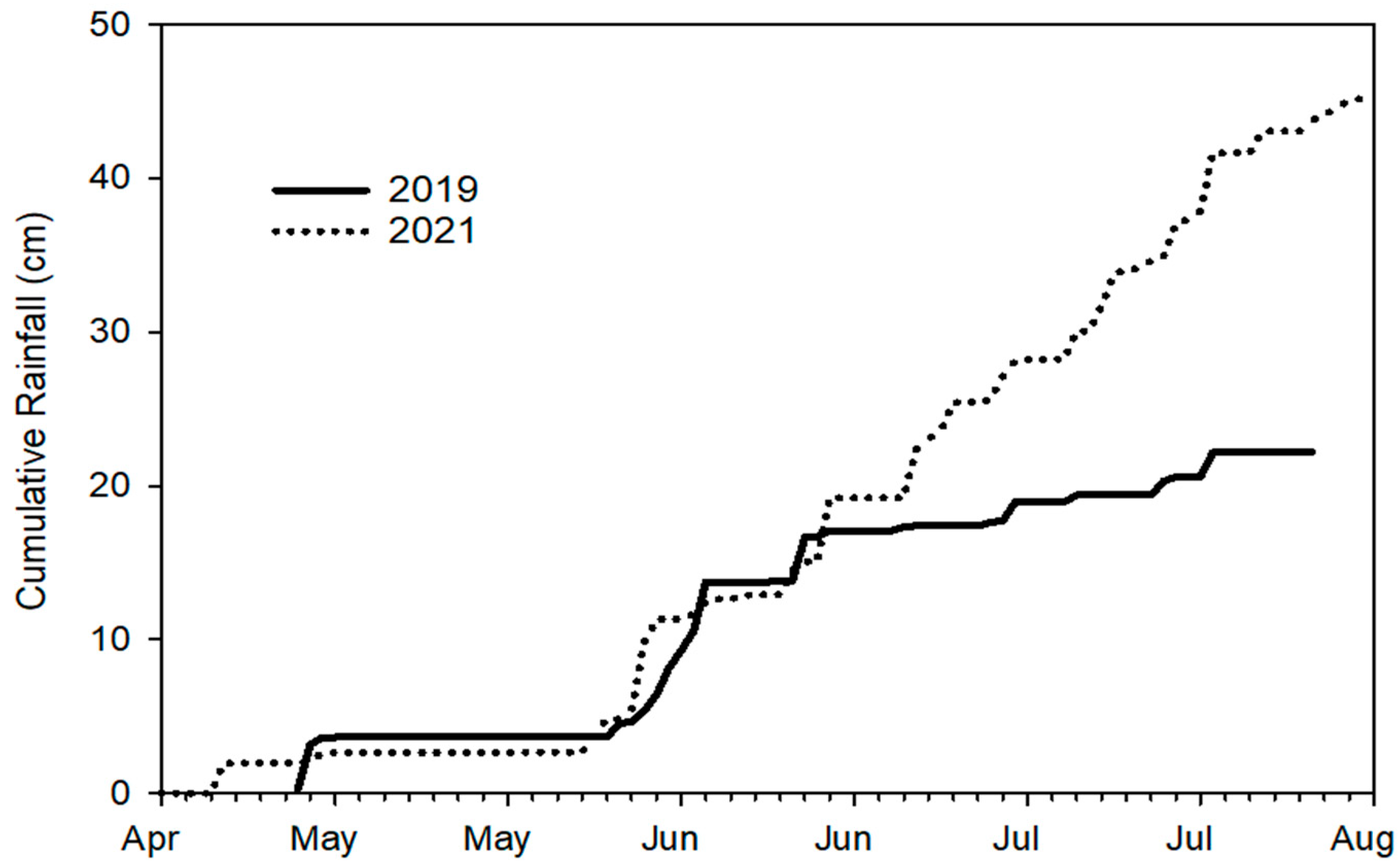
2.2. Microenvironment
2.3. Plant Growth and Leaf Chlorophyll Content
| Chlorophyll a | Chl a (µg/mL) = 13.36A664 − 5.19A649 |
| Chlorophyll b | Chl b (µg/mL) = 27.43A649 − 8.12A664 |
| Chlorophyll a + b | Chl (a + b) (µg/mL) = 5.24A664 + 22.24A649 |
| Carotenoids | Car (µg/mL) = (1000A470 − 2.13Chl a − 97.63Chl b)/209 |
2.4. Leaf Mineral Nutrients
2.5. Leaf Gas Exchange
2.6. Fruit Yield
2.7. Data Analysis
3. Results
3.1. Microenvironment
3.2. Plant Growth and Chlorophyll Content
3.3. Leaf Mineral Nutrients
3.4. Leaf Gas Exchange
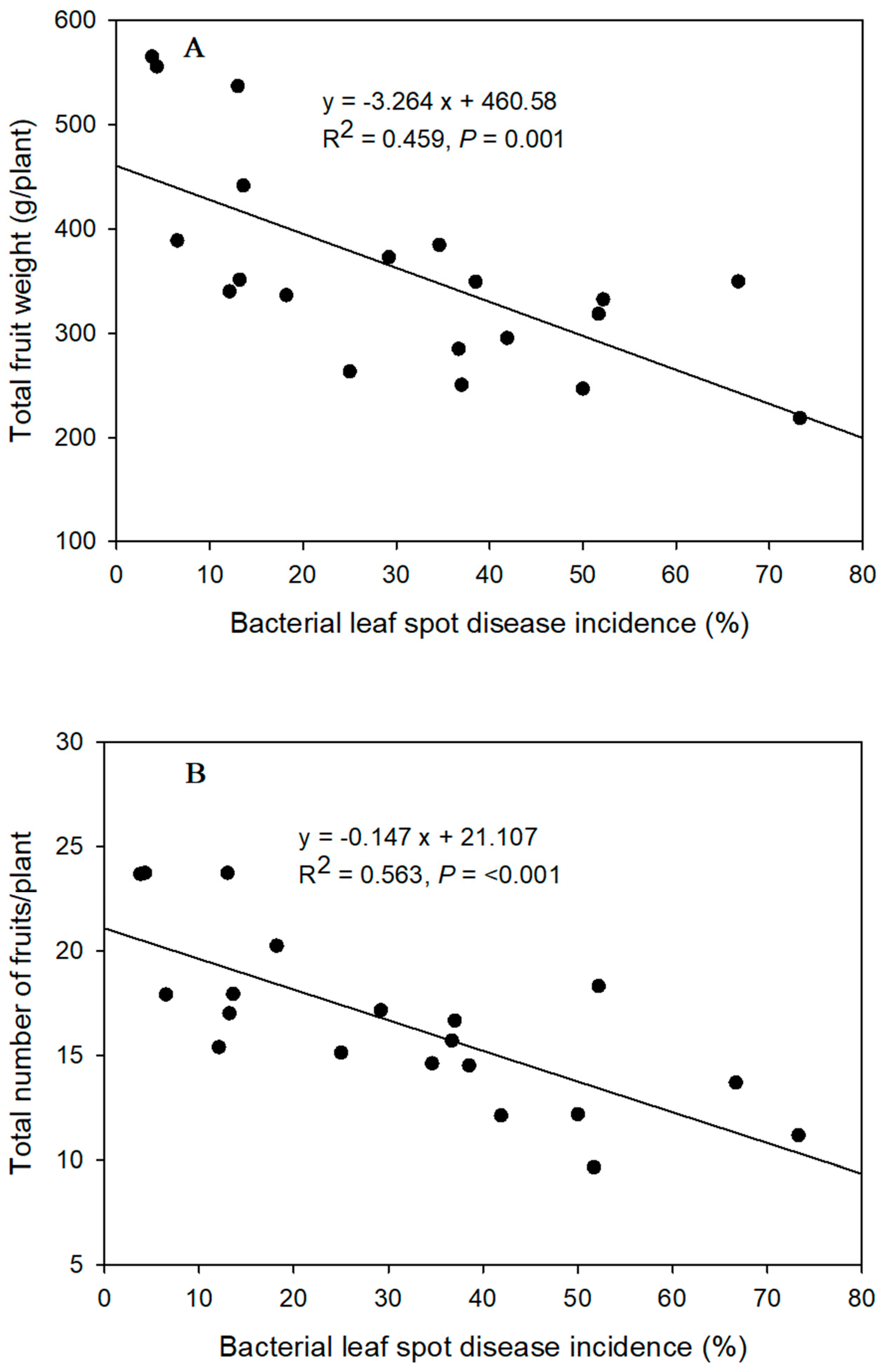
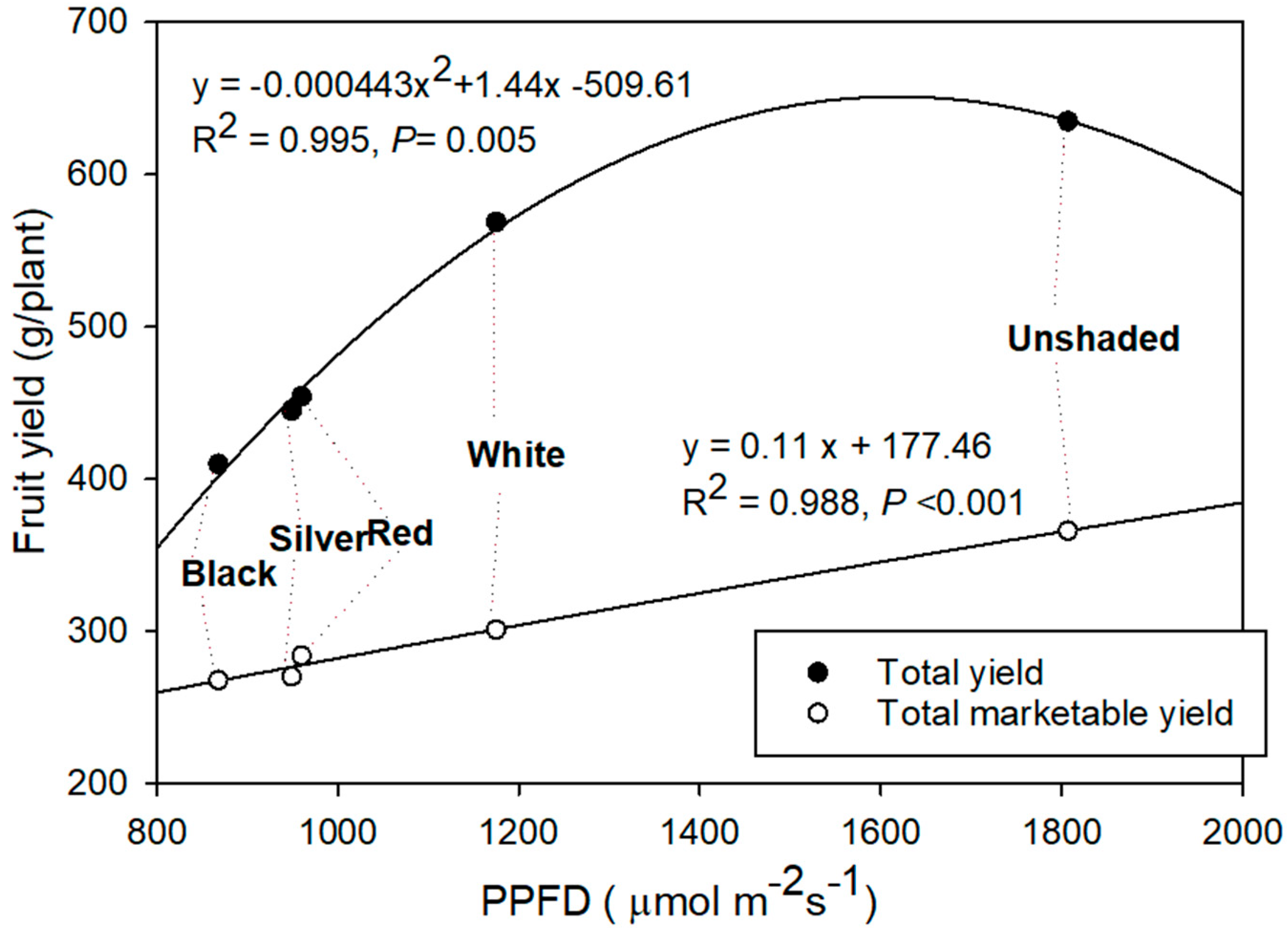
3.5. Fruit Yield
4. Discussion
4.1. Microenvironment
4.2. Plant Growth
4.3. Leaf Mineral Content
4.4. Leaf Gas Exchange
4.5. Fruit Yield
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snyder, R.L. Climate Change Impacts on Water Use in Horticulture. Horticulturae 2017, 3, 27. [Google Scholar] [CrossRef]
- Solankey, S.S.; Kumari, M.; Akhtar, S.; Singh, H.K.; Ray, P.K. Challenges and Opportunities in Vegetable Production in Changing Climate: Mitigation and Adaptation Strategies. In Advances in Research on Vegetable Production Under A Changing Climate Vol. 1; Springer: Berlin/Heidelberg, Germany, 2021; Volume 1, pp. 13–59. [Google Scholar] [CrossRef]
- Tanny, J. Microclimate and Evapotranspiration of Crops Covered by Agricultural Screens: A Review. Biosyst. Eng. 2013, 114, 26–43. [Google Scholar] [CrossRef]
- Ben-Yakir, D.; Antignus, Y.; Offir, Y.; Shahak, Y. Colored Shading Nets Impede Insect Invasion and Decrease the Incidences of Insect-transmitted Viral Diseases in Vegetable Crops. Entomol. Exp. Appl. 2012, 144, 249–257. [Google Scholar] [CrossRef]
- Stamps, R.H. Use of Colored Shade Netting in Horticulture. HortScience 2009, 44, 239–241. [Google Scholar] [CrossRef]
- Al-Helal, I.; Abdel-Ghany, A. Responses of Plastic Shading Nets to Global and Diffuse PAR Transfer: Optical Properties and Evaluation. NJAS-Wagening. J. Life Sci. 2010, 57, 125–132. [Google Scholar] [CrossRef]
- Castellano, S.; Russo, G.; Mugnozza, G.S. The Influence of Construction Parameters on Radiometric Performances of Agricultural Nets. Acta Hortic. 2006, 718, 283–290. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C.; John, K.S. Bell Pepper (Capsicum annuum L.) under Colored Shade Nets: Plant Growth and Physiological Responses. HortScience 2019, 54, 1795–1801. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Fallik, E. Effect of Colored Shade-nets on Plant Leaf Parameters and Tomato Fruit Quality. J. Sci. Food Agric. 2015, 95, 2660–2667. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Barać, S.; Mastilović, J.; Kevrešan, Ž.; Fallik, E. Effect of Shading by Colored Nets on Yield and Fruit Quality of Sweet Pepper. Zemdirb.-Agric. 2017, 104, 53–62. [Google Scholar] [CrossRef]
- Araujo, W.F.; Migliaccio, K.W.; Seal, D.R.; Schaffer, B.; Chagas, E.A. Evaluation of Jalapeño Peppers under Different Shade Cloths. Proc. Fla. State Hort. Soc. 2015, 128, 129–132. [Google Scholar]
- USDA-NASS. Vegetables-2020 Summary; National Agricultural Statistics Service: Washington, DC, USA, 2021.
- Dodd, M.; McGowan, A.; Power, I.; Thorrold, B. Effects of Variation in Shade Level, Shade Duration and Light Quality on Perennial Pastures. N. Z. J. Agric. Res. 2005, 48, 531–543. [Google Scholar] [CrossRef]
- López-Marín, J.; Galvez, A.; del Amor Saavedra, F.; Manera Bassa, F.J.; Carrero-Blanco, J.; Brotons Martínez, J. Photoselective Shade Netting in a Sweet Pepper Crop Accelerates Ripening Period and Enhances the Overall Fruits Quality and Yield. J. Agric. Sci. Technol. 2022, 24, 1171–1186. [Google Scholar]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R. Plant Responses to Red and Far-Red Lights, Applications in Horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Boini, A.; Muzzi, E.; Tixier, A.; Zwieniecki, M.; Manfrini, L.; Grappadelli, L.C. Photoselective Nets Alter Apple Canopy Air Temperature and Carbon Translocation during Dormancy and Budbreak. HortScience 2021, 56, 1166–1174. [Google Scholar] [CrossRef]
- Martinez-Luscher, J.; Chen, C.C.L.; Brillante, L.; Kurtural, S.K. Partial Solar Radiation Exclusion with Color Shade Nets Reduces the Degradation of Organic Acids and Flavonoids of Grape Berry (Vitis vinifera L.). J. Agric. Food Chem. 2017, 65, 10693–10702. [Google Scholar] [CrossRef] [PubMed]
- Maughan, T.; Drost, D.; Black, B.; Day, S. Using Shade for Fruit and Vegetable Production. 2017. Available online: https://digitalcommons.usu.edu/extension_curall/1654/ (accessed on 12 June 2023).
- Rylski, I.; Spigelman, M. Effect of Shading on Plant Development, Yield and Fruit Quality of Sweet Pepper Grown under Conditions of High Temperature and Radiation. Sci. Hortic. 1986, 29, 31–35. [Google Scholar] [CrossRef]
- Shahak, Y.; Ratner, K.; Zur, N.; Offir, Y.; Matan, E.; Yehezkel, H.; Messika, Y.; Posalski, I.; Ben-Yakir, D. Photoselective Netting: An Emerging Approach in Protected Agriculture. Acta Hortic. 2008, 807, 79–84. [Google Scholar] [CrossRef]
- Santana, J.; Balbino, M.; Tavares, T.; Bezerra, R.; Farias, J.; Ferreira, R. Effect of Photoselective Screens in the Development and Productivity of Red and Yellow Sweet Pepper. Acta Hortic. 2012, 956, 493–500. [Google Scholar] [CrossRef]
- Burden, D.; Huntrods, D. Bell and Chili Peppers; Iowa State University: Ames, IA, USA, 2012. [Google Scholar]
- Lillywhite, J.M.; Simonsen, J.E.; Uchanski, M.E. Spicy Pepper Consumption and Preferences in the United States. HortTechnology 2013, 23, 868–876. [Google Scholar] [CrossRef]
- Johnson, C.D.; Decoteau, D.R. Nitrogen and Potassium Fertility Affects Jalapeño Pepper Plant Growth, Pod Yield, and Pungency. HortScience 1996, 31, 1119–1123. [Google Scholar] [CrossRef]
- Rho, H.; Colaizzi, P.; Gray, J.; Paetzold, L.; Xue, Q.; Patil, B.; Rush, C. Yields, Fruit Quality, and Water Use in a Jalapeño Pepper and Tomatoes under Open Field and High-Tunnel Production Systems in the Texas High Plains. HortScience 2020, 55, 1632–1641. [Google Scholar] [CrossRef]
- Tanny, J.; Teitel, M.; Barak, M.; Esquira, Y.; Amir, R. The Effect of Height on Screenhouse Microclimate. Acta Hortic. 2007, 801, 107–114. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Jovicich, E.; Cantliffe, D.J.; Stoffella, P.J.; Haman, D.Z. Bell Pepper Fruit Yield and Quality as Influenced by Solar Radiation-Based Irrigation and Container Media in a Passively Ventilated Greenhouse. HortScience 2007, 42, 642–652. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing 2023. Available online: https://www.R-project.org/ (accessed on 10 June 2023).
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Reading, MA, USA, 1977. [Google Scholar]
- Kalcsits, L.; Musacchi, S.; Layne, D.R.; Schmidt, T.; Mupambi, G.; Serra, S.; Mendoza, M.; Asteggiano, L.; Jarolmasjed, S.; Sankaran, S. Above and Below-Ground Environmental Changes Associated with the Use of Photoselective Protective Netting to Reduce Sunburn in Apple. Agric. For. Meteorol. 2017, 237, 9–17. [Google Scholar] [CrossRef]
- Arthurs, S.P.; Stamps, R.H.; Giglia, F.F. Environmental Modification inside Photoselective Shadehouses. HortScience 2013, 48, 975–979. [Google Scholar] [CrossRef]
- Middleton, S.; McWaters, A. Hail Netting of Apple Orchards—Australian Experience. Compact Fruit Tree 2002, 35, 51–55. [Google Scholar]
- Mira-García, A.B.; Conejero, W.; Vera, J.; Ruiz-Sánchez, M.C. Leaf Water Relations in Lime Trees Grown under Shade Netting and Open-Air. Plants 2020, 9, 510. [Google Scholar] [CrossRef]
- Teitel, M.; Liang, H.; Tanny, J.; Garcia-Teruel, M.; Levi, A.; Ibanez, P.F.; Alon, H. Effect of Roof Height on Microclimate and Plant Characteristics in an Insect-Proof Screenhouse with Impermeable Sidewalls. Biosyst. Eng. 2017, 162, 11–19. [Google Scholar] [CrossRef]
- Mohawesh, O.; Albalasmeh, A.; Deb, S.; Singh, S.; Simpson, C.; AlKafaween, N.; Mahadeen, A. Effect of Colored Shading Nets on the Growth and Water Use Efficiency of Sweet Pepper Grown under Semi-Arid Conditions. HortTechnology 2022, 32, 21–27. [Google Scholar] [CrossRef]
- Goyal, A.; Karayekov, E.; Galvão, V.C.; Ren, H.; Casal, J.J.; Fankhauser, C. Shade Promotes Phototropism through Phytochrome B-Controlled Auxin Production. Curr. Biol. 2016, 26, 3280–3287. [Google Scholar] [CrossRef]
- Smith, H. Phytochromes and Light Signal Perception by Plants—An Emerging Synthesis. Nature 2000, 407, 585–591. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, S.; Pathania, V. Effect of Shading and Plant Density on Growth, Yield and Oil Composition of Clary Sage (Salvia sclarea L.) in North Western Himalaya. J. Essent. Oil Res. 2013, 25, 23–32. [Google Scholar] [CrossRef]
- Kitta, E.; Katsoulas, N.; Kandila, A.; González-Real, M.M.; Baille, A. Photosynthetic Acclimation of Sweet Pepper Plants to Screenhouse Conditions. HortScience 2014, 49, 166–172. [Google Scholar] [CrossRef]
- Field, R.J.; Jackson, D. Light Effects on Apical Dominance. Ann. Bot. 1975, 39, 369–374. [Google Scholar] [CrossRef]
- Luxmoore, R. A Source–Sink Framework for Coupling Water, Carbon, and Nutrient Dynamics of Vegetation. Tree Physiol. 1991, 9, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Awad-Allah, E.F.; Shams, A.H.; Helaly, A.A. Suppression of Bacterial Leaf Spot by Green Synthesized Silica Nanoparticles and Antagonistic Yeast Improves Growth, Productivity, and Quality of Sweet Pepper. Plants 2021, 10, 1689. [Google Scholar] [CrossRef] [PubMed]
- Bourque, D.P.; Naylor, A.W. Large Effects of Small Water Deficits on Chlorophyll Accumulation and Ribonucleic Acid Synthesis in Etiolated Leaves of Jack Bean (Canavalia ensiformis [L.] DC.). Plant Physiol. 1971, 47, 591. [Google Scholar] [CrossRef]
- Huang, W.; Ma, H.; Huang, Y.; Li, Y.; Wang, G.; Jiang, Q.; Wang, F.; Xiong, A. Comparative Proteomic Analysis Provides Novel Insights into Chlorophyll Biosynthesis in Celery under Temperature Stress. Physiol. Plant. 2017, 161, 468–485. [Google Scholar] [CrossRef]
- Horton, P. Optimization of Light Harvesting and Photoprotection: Molecular Mechanisms and Physiological Consequences. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3455–3465. [Google Scholar] [CrossRef]
- Ruban, A.V. Evolution under the Sun: Optimizing Light Harvesting in Photosynthesis. J. Exp. Bot. 2015, 66, 7–23. [Google Scholar] [CrossRef]
- Kosma, C.; Triantafyllidis, V.; Papasavvas, A.; Salahas, G.; Patakas, A. Yield and Nutritional Quality of Greenhouse Lettuce as Affected by Shading and Cultivation Season. Emir. J. Food Agric. 2013, 25, 974. [Google Scholar] [CrossRef]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional Quality of Ten Leafy Vegetables Harvested at Two Light Intensities. Food Chem. 2016, 199, 702–710. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M. Influence of Shade on Mineral Nutrient Status of Field-grown Cotton. J. Plant Nutr. 1998, 21, 1681–1695. [Google Scholar] [CrossRef]
- Singh, H.; Dunn, B.L.; Fontanier, C.; Singh, H.; Kaur, A.; Zhang, L. Shade Nets Reduced Growth, Nutrition, and Sugars of Hydroponic Lettuce and Basil. HortScience 2023, 58, 1383–1392. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C. Bell Pepper (Capsicum annuum L.) Crop as Affected by Shade Level: Microenvironment, Plant Growth, Leaf Gas Exchange, and Leaf Mineral Nutrient Concentration. HortScience 2013, 48, 175–182. [Google Scholar] [CrossRef]
- Gálvez, A.; Albacete, A.; del Amor, F.M.; López-Marín, J. The Use of Red Shade Nets Improves Growth in Salinized Pepper (Capsicum annuum L.) Plants by Regulating Their Ion Homeostasis and Hormone Balance. Agronomy 2020, 10, 1766. [Google Scholar] [CrossRef]
- Ashraf, M. Relationships between Growth and Gas Exchange Characteristics in Some Salt-Tolerant Amphidiploid Brassica Species in Relation to Their Diploid Parents. Environ. Exp. Bot. 2001, 45, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Matsubara, S.; Harbinson, J.; Heuvelink, E.; Marcelis, L.F. Acclimation of Photosynthesis to Lightflecks in Tomato Leaves: Interaction with Progressive Shading in a Growing Canopy. Physiol. Plant. 2018, 162, 506–517. [Google Scholar] [CrossRef]
- Kabir, M.Y.; Nambeesan, S.U.; Díaz-Pérez, J.C. Carbon Dioxide and Light Curves and Leaf Gas Exchange Responses to Shade Levels in Bell Pepper (Capsicum annuum L.). Plant Sci. 2023, 326, 111532. [Google Scholar] [CrossRef]
- Kitta, E.; Baille, A.D.; Katsoulas, N.; Rigakis, N.; González-Real, M.M. Effects of Cover Optical Properties on Screenhouse Radiative Environment and Sweet Pepper Productivity. Biosyst. Eng. 2014, 122, 115–126. [Google Scholar] [CrossRef]
- Kabir, M.Y.; Nambeesan, S.U.; Bautista, J.; Díaz-Pérez, J.C. Plant Water Status, Plant Growth, and Fruit Yield in Bell Pepper (Capsicum annuum L.) under Shade Nets. Sci. Hortic. 2022, 303, 111241. [Google Scholar] [CrossRef]
- Campany, C.E.; Tjoelker, M.G.; von Caemmerer, S.; Duursma, R.A. Coupled Response of Stomatal and Mesophyll Conductance to Light Enhances Photosynthesis of Shade Leaves under Sunflecks. Plant Cell Environ. 2016, 39, 2762–2773. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, Faster Stomata: Scaling of Stomatal Size, Rate of Response, and Stomatal Conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef]
- Fanourakis, D.; Giday, H.; Milla, R.; Pieruschka, R.; Kjaer, K.H.; Bolger, M.; Vasilevski, A.; Nunes-Nesi, A.; Fiorani, F.; Ottosen, C.-O. Pore Size Regulates Operating Stomatal Conductance, While Stomatal Densities Drive the Partitioning of Conductance between Leaf Sides. Ann. Bot. 2015, 115, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Medina, V.; Gilbert, M.E. Physiological Trade-Offs of Stomatal Closure under High Evaporative Gradients in Field-Grown Soybean. Funct. Plant Biol. 2015, 43, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, W.P.; Silva, J.R.; Ferreira, L.S.; Machado Filho, J.A.; Figueiredo, F.A.; Ferraz, T.M.; Bernado, W.P.; Bezerra, L.B.; De Abreu, D.P.; Cespom, L. Stomatal and Photochemical Limitations of Photosynthesis in Coffee (Coffea spp.) Plants Subjected to Elevated Temperatures. Crop Pasture Sci. 2018, 69, 317–325. [Google Scholar] [CrossRef]
- Jarial, K.; Jarial, R.S.; Gupta, S.K. Bacterial Spot (Xanthomonas cucurbitae) of Cucurbits: A Review. NBU J. Plant Sci. 2015, 9, 33–39. [Google Scholar] [CrossRef]
- Pruvost, O.; Boher, B.; Brocherieux, C.; Nicole, M.; Chiroleu, F. Survival of Xanthomonas axonopodis pv. citri in Leaf Lesions under Tropical Environmental Conditions and Simulated Splash Dispersal of Inoculum. Phytopathology 2002, 92, 336–346. [Google Scholar]
- Robinson, P.; Jones, J.; Pernezny, K. Bacterial Leaf Spot of Lettuce: Relationship of Temperature to Infection and Potential Host Range of Xanthomonas campestris pv. vitians. Plant Dis. 2006, 90, 465–470. [Google Scholar] [CrossRef]
- Maji, M.D.; Banerjee, R.; Kumar Das, N.; Ghosh, A. Forecasting Model of Bacterial Leaf Spot Disease of Mulberry Caused by Xanthomonas campestris pv. mori. Arch. Phytopathol. Plant Prot. 2012, 45, 1570–1574. [Google Scholar] [CrossRef]
- Pohronezny, K.; Volin, R. The Effect of Bacterial Spot on Yield and Quality of Fresh Market Tomatoes. HortScience 1983, 18, 69–70. [Google Scholar] [CrossRef]
- Jenkins, J.H. Studies on Bacterial Leaf Spot of Bell Pepper and the Casual Organism Xanthomonas vesicatoria (Doidge) Dowson. Ph.D. Thesis, Louisiana State University and Agricultural & Mechanical College, Baton Rouge, LA, USA, 1963. [Google Scholar]
- Díaz-Pérez, J.C.; John, K.S.; Kabir, M.Y.; Alvarado-Chávez, J.A.; Cutino-Jiménez, A.M.; Bautista, J.; Gunawan, G.; Nambeesan, S.U. Bell Pepper (Capsicum annuum L.) under Colored Shade Nets: Fruit Yield, Postharvest Transpiration, Color, and Chemical Composition. HortScience 2020, 55, 181–187. [Google Scholar] [CrossRef]
- Gent, M.P. Effect of Degree and Duration of Shade on Quality of Greenhouse Tomato. HortScience 2007, 42, 514–520. [Google Scholar] [CrossRef]
- Day, S.D. Biological and Mechanical Approaches to Sunscald Management in Bell Pepper Production. Master’s Thesis, Utah State University, Logan, UT, USA, 2014. [Google Scholar]
- Díaz-Pérez, J.C. Bell Pepper (Capsicum annuum L.) Crop as Affected by Shade Level: Fruit Yield, Quality, and Postharvest Attributes, and Incidence of Phytophthora Blight (Caused by Phytophthora capsici Leon.). HortScience 2014, 49, 891–900. [Google Scholar] [CrossRef]
- Laub, M.; Pataczek, L.; Feuerbacher, A.; Zikeli, S.; Högy, P. Contrasting Yield Responses at Varying Levels of Shade Suggest Different Suitability of Crops for Dual Land-Use Systems: A Meta-Analysis. Agron. Sustain. Dev. 2022, 42, 51. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C.; Batal, K.D. Colored Plastic Film Mulches Affect Tomato Growth and Yield via Changes in Root-Zone Temperature. J. Am. Soc. Hortic. Sci. 2002, 127, 127–135. [Google Scholar] [CrossRef]

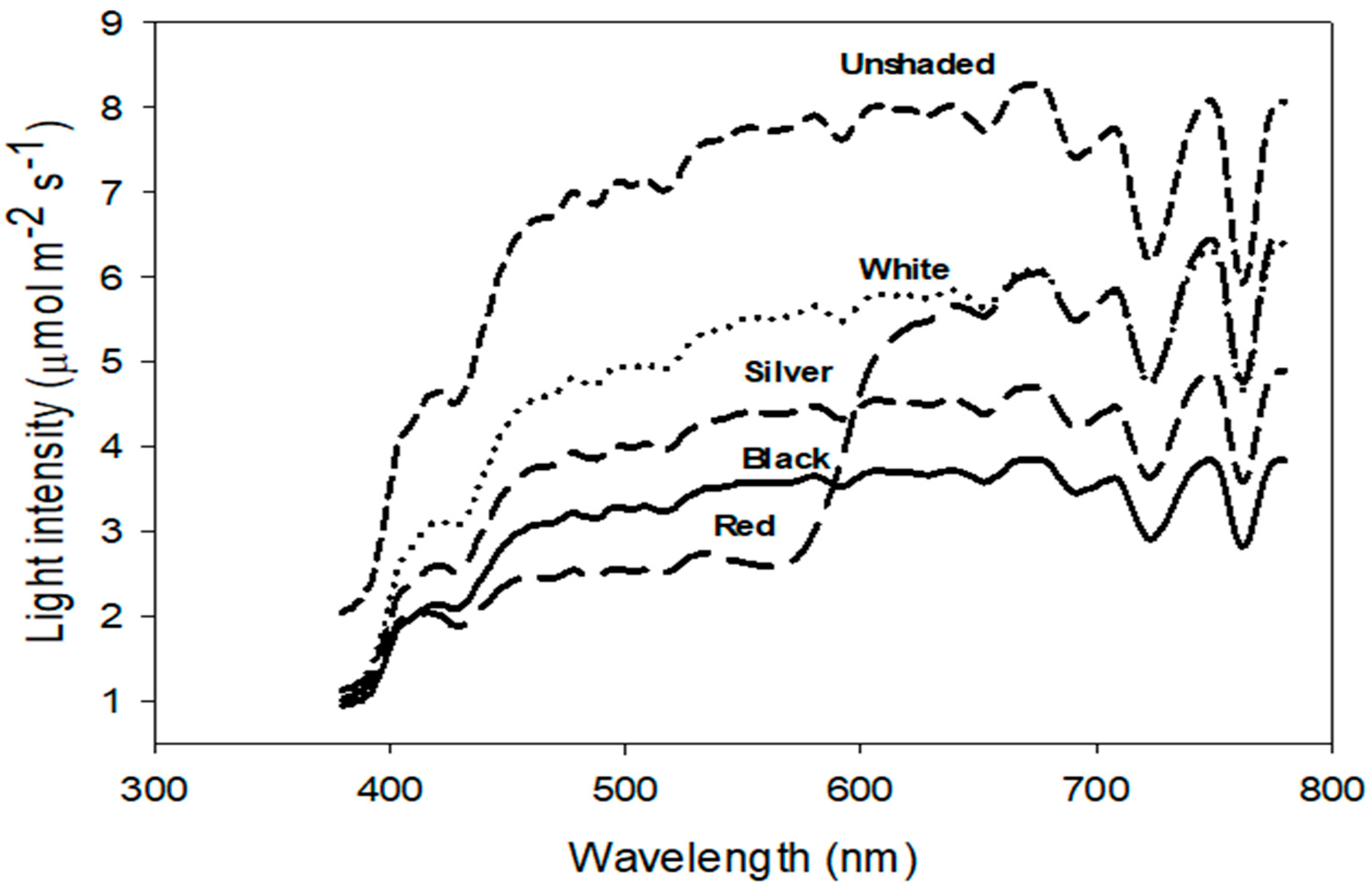
| Light Intensity (µmol·m−2·s−1) | Light Quality (Ratios) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shade Nets | PPFD | UV | Blue | Green | Red | Far-Red | Reduced | B/G | B/R | G/R | R/FR | PPFD/UV |
| (B) | (G) | (R) | (FR) | PPFD (%) | ||||||||
| Black | 962 d z | 24 c | 263 d | 341 d | 358 d | 263 d | 55 | 0.77 b | 0.73 a | 0.95 a | 1.36 ab | 40.5 b |
| Red | 1053 d | 26 c | 221 e | 281 e | 551 b | 444 b | 51 | 0.79 a | 0.4 c | 0.50 b | 1.23 d | 40.6 b |
| Silver | 1217 c | 29 b | 329 c | 433 c | 455 c | 343 c | 43 | 0.77 b | 0.72 a | 0.95 a | 1.32 bc | 41.3 b |
| White | 1534 b | 31 b | 402 b | 544 b | 588 b | 448 b | 29 | 0.74 c | 0.68 b | 0.93 a | 1.31 c | 48.9 a |
| Unshaded | 2151 a | 53 a | 587 a | 764 a | 800 a | 577 a | -- | 0.76 b | 0.73 a | 0.95 a | 1.38 a | 40.4 b |
| p-Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Tair (°C) | RZT (°C) | |||||
|---|---|---|---|---|---|---|
| Shade Nets | Minimum | Mean | Maximum | Minimum | Mean | Maximum |
| Black | 23.2 | 29.0 c z | 36.6 c | 26.7 d | 28.5 d | 30.6 b |
| Red | 22.9 | 29.9 b | 39.4 b | 26.6 d | 29.5 c | 33.1 a |
| Silver | 23 | 29.8 b | 40.2 b | 26.9 c | 28.6 d | 30.6 b |
| White | 22.9 | 30.4 a | 41.5 a | 27.3 b | 29.9 b | 33.2 a |
| Unshaded | 22.9 | 28.4 d | 33.9 d | 28.0 a | 30.5 a | 33.4 a |
| p-Value | 0.529 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Tair (°C) | RZT (°C) | |||||
|---|---|---|---|---|---|---|
| Shade Nets | Minimum | Mean | Maximum | Minimum | Mean | Maximum |
| Black | 22.8 | 27.3 ab z | 34.3 cd | 25.1 e | 26.7 d | 29.2 c |
| Red | 22.9 | 27.9 a | 39.3 b | 25.7 c | 27.3 c | 29.2 c |
| Silver | 22.9 | 26.6 b | 33.6 d | 25.5 d | 26.8 d | 28.7 d |
| White | 23 | 27.2 ab | 43.9 a | 25.8 b | 27.6 b | 29.9 b |
| Unshaded | 22.9 | 27.5 ab | 35.7 c | 26.7 a | 28.9 a | 31.5 a |
| p-Value | 0.843 | 0.021 | <0.001 | <0.001 | <0.001 | <0.001 |
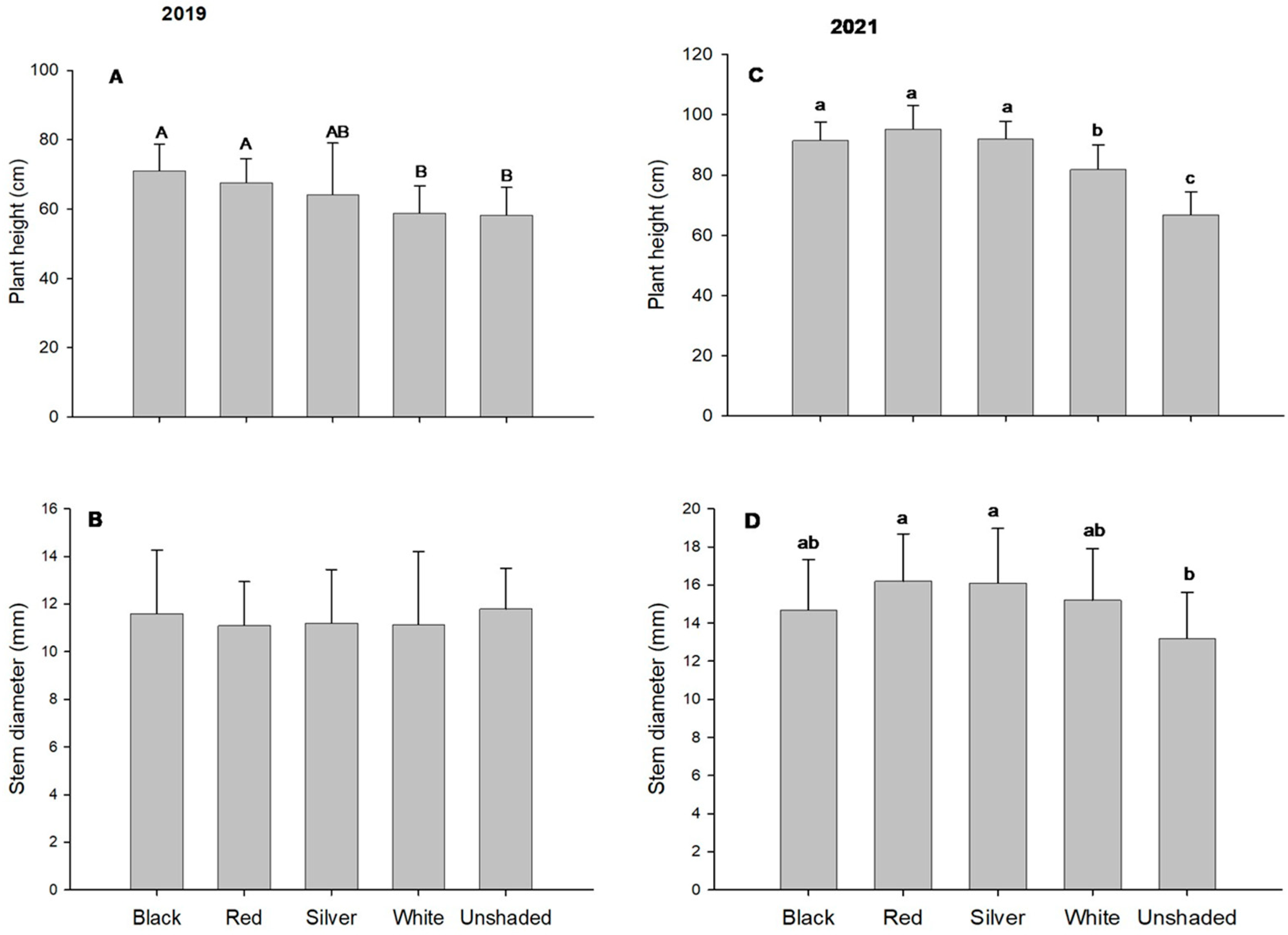
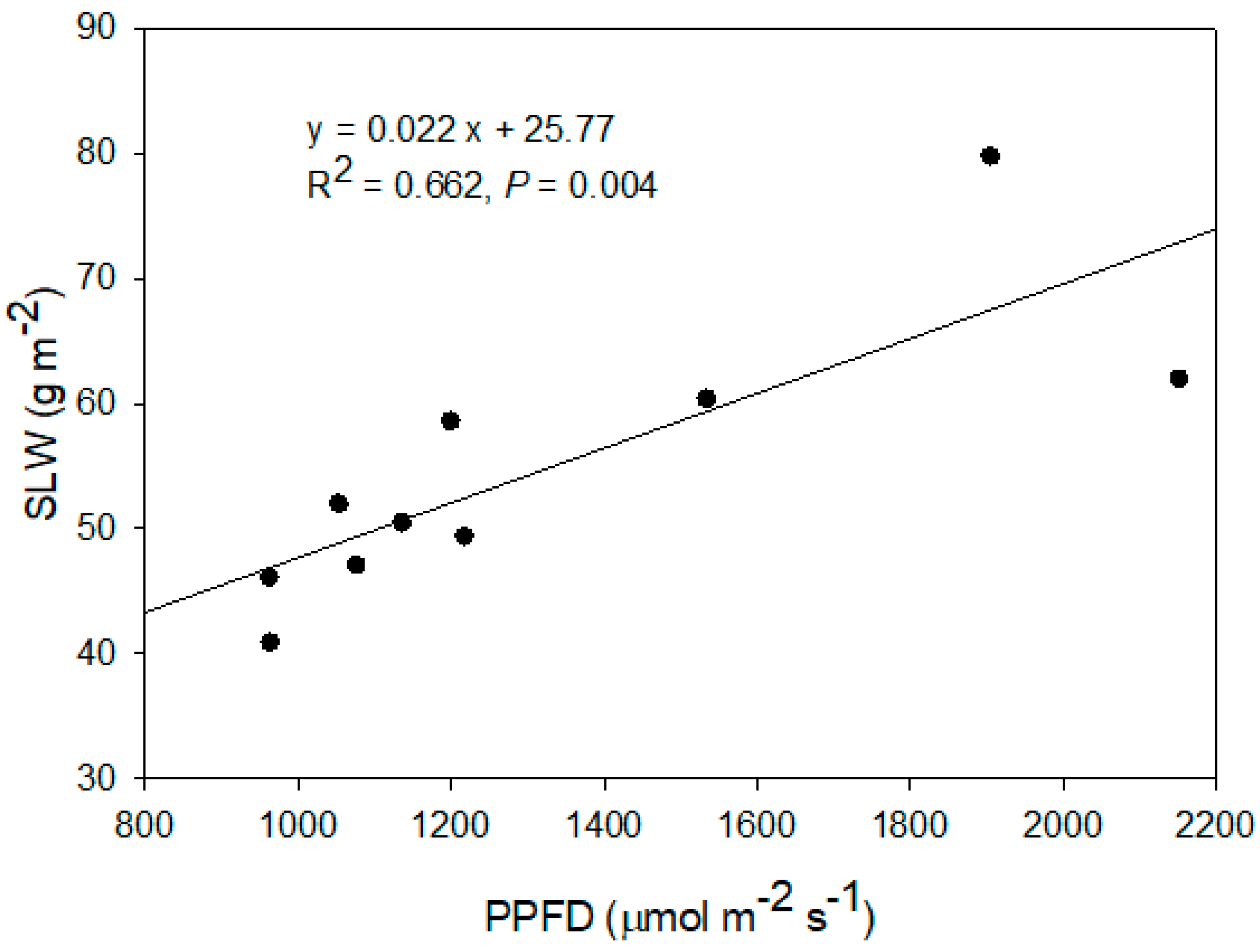
| Year | Shade Nets | Above-Ground | Leaf Area | Specific Leaf Weight | CI | Normalized CI |
|---|---|---|---|---|---|---|
| Dry Weight (g·Plant−1) | (cm2·Leaf−1) | (g·m−2) | ||||
| Black | 75.2 | 14.7 | 40.9 | 47.2 ab z | 1.33 | |
| 2019 | Red | 70.3 | 15.6 | 50.5 | 51.2 ab | 1.19 |
| Silver | 62.5 | 16.6 | 47.1 | 54.7 a | 1.22 | |
| White | 80.2 | 14.2 | 58.6 | 54.2 a | 0.96 | |
| Unshaded | 65.2 | 13.1 | 79.8 | 42.7 b | 0.8 | |
| p-Value | 0.669 | 0.398 | 0.068 | 0.011 | 0.197 | |
| Black | 123.9 | 24.4 a | 46.1 | 38.5 | 0.88 | |
| Red | 125.6 | 24.1 a | 52 | 33.8 | 0.66 | |
| 2021 | Silver | 125.2 | 24.4 a | 49.4 | 39.1 | 0.83 |
| White | 102.8 | 21.8 a | 60.4 | 41.8 | 0.66 | |
| Unshaded | 107.5 | 16.2 b | 62 | 40.3 | 0.7 | |
| p-Value | 0.295 | <0.001 | 0.115 | 0.299 | 0.328 |
| Shade Nets | Chl a | Chl b | Car | Chl (a + b) |
|---|---|---|---|---|
| µg−1·mL−1 | µg−1·mL−1 | µg−1·mL−1 | µg−1·mL−1 | |
| Black | 7.68 a z | 3.07 a | 1.95 | 10.75 a |
| Red | 6.79 ab | 2.79 ab | 1.75 | 9.58 ab |
| Silver | 7.36 a | 2.90 ab | 1.9 | 10.26 ab |
| White | 6.89 ab | 2.69 ab | 1.83 | 9.59 ab |
| Unshaded | 6.35 b | 2.57 b | 1.76 | 8.92 b |
| p-Value | 0.0017 | 0.054 | 0.13 | 0.003 |
| Macronutrients (%) | Micronutrients (mg−1·kg−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shade Nets | N | P | K | Ca | Mg | S | B | Cu | Fe | Mn | Zn |
| Black | 4.36 | 0.36 | 3.85 | 1.5 | 0.45 | 0.56 | 38 | 62 | 105 a z | 49 | 47 |
| Red | 4.39 | 0.36 | 3.91 | 1.39 | 0.42 | 0.57 | 34 | 46 | 94 b | 47 | 43 |
| Silver | 4.41 | 0.34 | 3.83 | 1.46 | 0.46 | 0.55 | 33 | 44 | 96 ab | 46 | 40 |
| White | 4.15 | 0.36 | 3.87 | 1.48 | 0.46 | 0.57 | 35 | 45 | 91 b | 45 | 41 |
| Unshaded | 4.09 | 0.37 | 3.68 | 1.43 | 0.43 | 0.55 | 41 | 56 | 88 b | 50 | 44 |
| p-Value | 0.1101 | 0.898 | 0.754 | 0.822 | 0.819 | 0.961 | 0.584 | 0.573 | 0.0029 | 0.514 | 0.104 |
| Macronutrients (%) | Micronutrients (mg−1·kg−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shade Nets | N | P | K | Ca | Mg | S | B | Cu | Fe | Mn | Zn |
| Black | 2.78 ab z | 0.5 | 2.8 | 0.87 | 0.31 | 0.35 | 33 | 12 | 148 | 46 ab | 66 |
| Red | 2.84 ab | 0.48 | 2.76 | 0.85 | 0.29 | 0.35 | 33 | 13 | 127 | 44 ab | 89 |
| Silver | 2.88 a | 0.48 | 2.94 | 0.79 | 0.31 | 0.36 | 31 | 13 | 136 | 47 a | 67 |
| White | 2.71 b | 0.54 | 2.85 | 0.85 | 0.3 | 0.36 | 35 | 13 | 139 | 44 ab | 70 |
| Unshaded | 2.90 a | 0.5 | 2.74 | 0.77 | 0.3 | 0.36 | 34 | 13 | 159 | 42 b | 83 |
| p-Value | 0.013 | 0.497 | 0.511 | 0.44 | 0.744 | 0.76 | 0.348 | 0.881 | 0.933 | 0.037 | 0.833 |
| An | Ci | E | ETR | gs | LeafT | PPFD | PhiPS2 | WUE | |
|---|---|---|---|---|---|---|---|---|---|
| Shade Nets | (µmol·m−2·s−1) | (µmol·mol−1) | (mmol·m−2·s−1) | (µmol·m−2·s−1) | (mol·m−2·s−1) | °C | (µmol·m−2·s−1) | (µmol·mmol−1) | |
| Black | 21.9 | 295.8 | 9.8 b z | 137.9 | 0.48 b | 32.5 b | 963.2 d | 0.34 a | 2.4 |
| Red | 24.9 | 295.7 | 10.4 ab | 148.9 | 0.46 b | 33.8 a | 1135.6 bc | 0.30 a | 2.2 |
| Silver | 23.7 | 300.2 | 9.8 b | 135.9 | 0.46 b | 33.5 a | 1075.6 c | 0.29 a | 2.2 |
| White | 25.6 | 304.3 | 11.6 a | 154.2 | 0.61 a | 32.9 ab | 1198.8 b | 0.30 a | 2.2 |
| Unshaded | 23.9 | 302 | 12.1 a | 146.9 | 0.54 ab | 33.8 a | 1905 a | 0.18 b | 2 |
| p-Value | 0.226 | 0.829 | 0.0003 | 0.362 | 0.019 | 0.003 | <0.0001 | <0.0001 | 0.118 |
| Marketable | Non-Marketable (Anthracnose and BER) y | Total | |||||
|---|---|---|---|---|---|---|---|
| Shade Nets | N | Wt. | Individual Fruit Wt. | N | Wt. | N | Wt. |
| (g/Plant) | (g) | (g/Plant) | (g/Plant) | ||||
| Black | 14.7 | 371 | 26.2 a z | 0.15 | 3.8 | 14.9 | 374.8 |
| Red | 14.9 | 320 | 21.9 ab | 0.28 | 6.1 | 15.2 | 326.1 |
| Silver | 16.1 | 363 | 22.3 ab | 0.11 | 2.9 | 16.2 | 365.9 |
| White | 19.1 | 423 | 21.8 ab | 0.10 | 1.9 | 19.2 | 424.9 |
| Unshaded | 17.3 | 303 | 17.5 b | 0.04 | 0.9 | 17.3 | 303.9 |
| p-Value | 0.587 | 0.426 | 0.0153 | 0.328 | 0.269 | 0.633 | 0.448 |
| Shade Nets | Large | Medium | Total Marketable | Individual Fruit | Total Yield | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Wt. | No. | Wt. | No. | Wt. | Wt. | No. | Wt. | |
| (per Plant) | (g/Plant) | (per Plant) | (g/Plant) | (per Plant) | (g/Plant) | (g) | (per Plant) | (g/Plant) | |
| Black | 3.5 | 116.4 | 6.4 b z | 151.5 b | 10.0 | 267.9 | 26.5 | 19.8 | 410.0 |
| Red | 2.9 | 98.6 | 7.4 ab | 181.5 ab | 10.5 | 284.0 | 26.3 | 21.4 | 454.3 |
| Silver | 3.5 | 113.3 | 6.3 b | 154.3 b | 10.0 | 270.4 | 26.9 | 20.7 | 445.1 |
| White | 3.1 | 102.5 | 8.1 ab | 198.6 ab | 11.2 | 301.1 | 26.3 | 27.7 | 568.9 |
| Unshaded | 3.3 | 101.4 | 11.0 a | 264.7 a | 14.4 | 366.1 | 24.3 | 29.4 | 635.1 |
| p-Value | 0.98 | 0.985 | 0.024 | 0.043 | 0.303 | 0.571 | 0.062 | 0.073 | 0.13 |
| Shade Nets | Small | Anthracnose | Weevil Infested | Total Non-Marketable Fruit | ||||
|---|---|---|---|---|---|---|---|---|
| No. | Wt. (g/Plant) | No. | Wt. (g/Plant) | No. | Wt. (g/Plant) | No. | Wt. (g/Plant) | |
| Black | 4.4 bz | 83.7 b | 0.33 | 9.4 | 5.1 | 49.0 | 9.8 | 142.2 |
| Red | 7.0 ab | 127.6 ab | 0.49 | 12.4 | 3.6 | 34.3 | 11.1 | 174.2 |
| Silver | 6.8 ab | 128.5 ab | 0.43 | 9.9 | 3.7 | 38.6 | 11.0 | 177.5 |
| White | 11.4 a | 204.7 a | 0.76 | 17.9 | 4.3 | 45.3 | 16.5 | 267.8 |
| Unshaded | 12.6 a | 234.7 a | 0.39 | 7.9 | 2.0 | 26.3 | 15.0 | 268.9 |
| p-Value | 0.007 | 0.008 | 0.489 | 0.667 | 0.824 | 0.927 | 0.832 | 0.914 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashyal, M.; Coolong, T.W.; Díaz-Pérez, J.C. Shade Nets Increase Plant Growth but Not Fruit Yield in Organic Jalapeño Pepper (Capsicum annuum L.). Agriculture 2025, 15, 1757. https://doi.org/10.3390/agriculture15161757
Bashyal M, Coolong TW, Díaz-Pérez JC. Shade Nets Increase Plant Growth but Not Fruit Yield in Organic Jalapeño Pepper (Capsicum annuum L.). Agriculture. 2025; 15(16):1757. https://doi.org/10.3390/agriculture15161757
Chicago/Turabian StyleBashyal, Mamata, Timothy W. Coolong, and Juan Carlos Díaz-Pérez. 2025. "Shade Nets Increase Plant Growth but Not Fruit Yield in Organic Jalapeño Pepper (Capsicum annuum L.)" Agriculture 15, no. 16: 1757. https://doi.org/10.3390/agriculture15161757
APA StyleBashyal, M., Coolong, T. W., & Díaz-Pérez, J. C. (2025). Shade Nets Increase Plant Growth but Not Fruit Yield in Organic Jalapeño Pepper (Capsicum annuum L.). Agriculture, 15(16), 1757. https://doi.org/10.3390/agriculture15161757








