Investigation of Relationship Between Drought Stress Resilience and Some Wrky Transcription Factor Genes in Some Kiwi (Actinidia deliciosa) Cultivars
Abstract
1. Introduction
2. Material and Methods
2.1. Initation Stage
2.2. In Vitro Drought Stress in Micropropagation Stage
2.3. In Vitro Drought Stress at the Rooting Stage
2.4. In Vitro Intense Drought Stress Experiment
2.5. RNA Extractions and cDNA Synthesis
2.6. qRT-PCR Reactions
3. Results
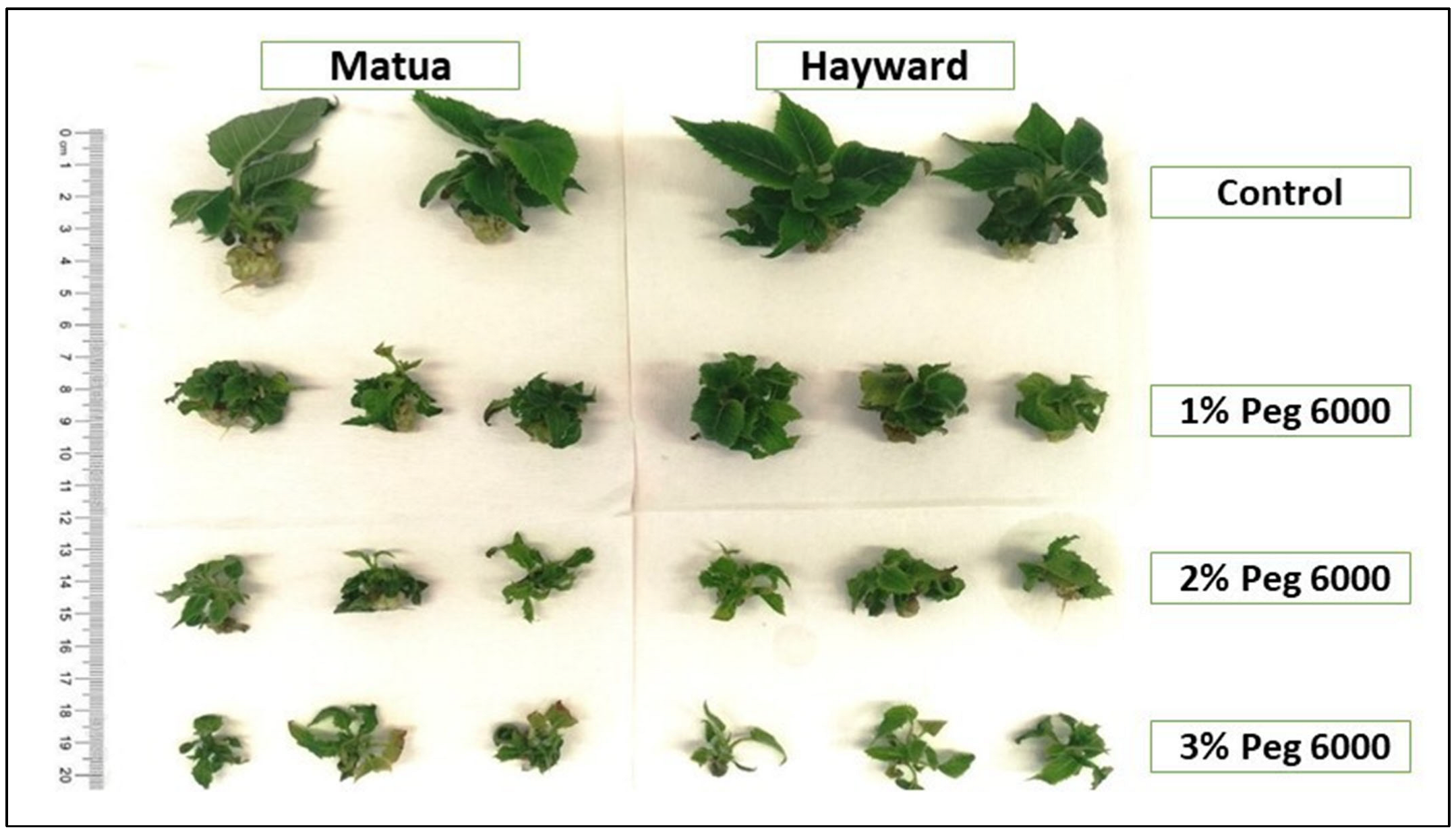
3.1. In Vitro Drought Stress Responses of Plants at the Micropropagation Stage
3.2. Rooting Stage and In Vitro Drought Stress Responses of Plants
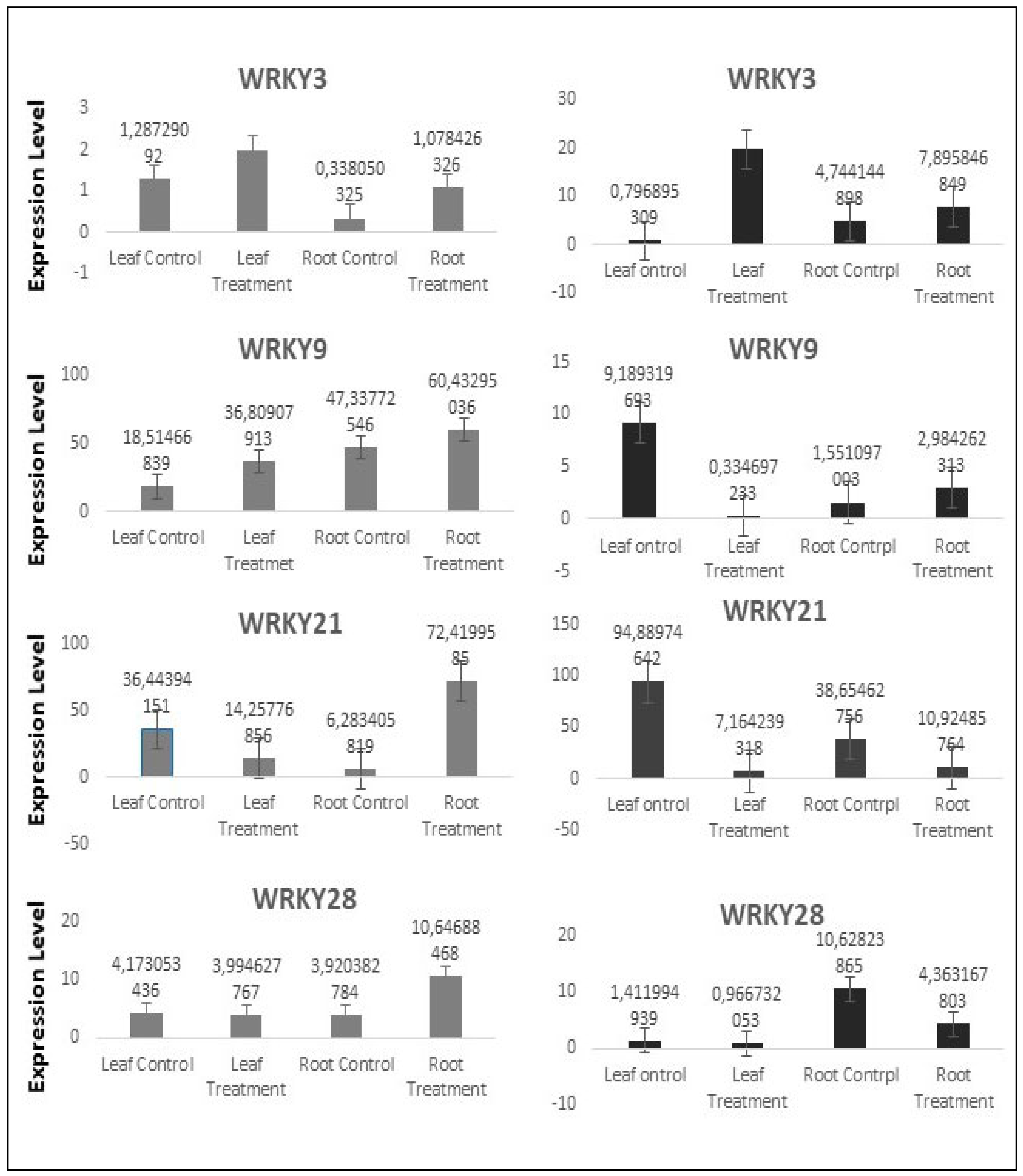
3.3. Gene Expression Level of WRKY TFs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guroo, I.; Wani, S.A.; Wani, S.M.; Ahmad, M.; Mir, S.A.; Masoodi, F.A. A Review of Production and Processing of Kiwifruit. J. Food Process. Technol. 2017, 8, 699. [Google Scholar]
- Şahin, G. KİVİ (Actinidia Deliciosa) Yetiştiriciliği Ve Türkiye Zirai Hayatındaki Yeri. Bartın Üniversitesi Edeb. Fakültesi Derg. 2019, 4, 3–32. (In Turkish) [Google Scholar]
- Drummond, L. Chapter Three—The Composition and Nutritional Value of Kiwifruit. Adv. Food Nutr. Res. 2013, 68, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.C.M.; Carr, A.C.; Pullar, J.M.; Ve Bozonet, S.M. The Bioavailability of Vitamin C From Kiwifruit. Adv. Food Nutr. Res. 2013, 68, 125–147. [Google Scholar]
- Ward, C.; Courtney, D. Kiwifruit: Taking Its Place in The Global Fruit Bowl. Adv. Food Nutr. Res. 2013, 68, 1–14. [Google Scholar] [PubMed]
- Uzundumlu, A.S.; Bilgin, K.; Kurtoğlu, S.; Ertek, N. Kivi Yetiştiriciliğinde Karşılaşılan Temel Sorunların Faktör Ve Probit Analizleri İle Belirlenmesi: Rize İli Örneği. İşletme Ekon. Ve Yönetim Araştırmaları Derg. 2018, 1, 71–92. (In Turkish) [Google Scholar]
- Debersaques, F.; Mekers, O. Growth and Production of Kiwifruit and Kiwiberry. In Soils Plant Growth Crop Production; Eolss Publishers Company Limited: Abu Dhabi, United Arab Emirates, 2010; Volume 2, p. 8. [Google Scholar]
- Greco, M.; Chiappetta, A.; Bruno, L.; Bitonti, M.B. In Posidonia Oceanica Cadmium induces Changes in DNA Methylation and Chromatin Patterning. J. Exp. Bot. 2012, 63, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and Biotic Stress Combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and Drought Stress Signal Transduction in Plants. Annu. Rev. Plant Biol. 2002, 53, 247. [Google Scholar] [CrossRef]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in Wheat. Sci. World J. 2013, 1, 610721. [Google Scholar] [CrossRef]
- Szegletes, Z.; Erdei, L.; Tari, I.; Cseuz, L. Accumulation of Osmoprotectants in Wheat Cultivars of Different Drought Tolerance. Cereal Res. Commun. 2000, 28, 403–410. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Mittler, R. The Combined Effect of Drought Stress and Heat Shock on Gene Expression in Tobacco. Plant Physiol. 2000, 130, 1143–1151. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding Plant Responses to Drought—From Genes to The Whole Plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Denby, K.; Gehring, C. Engineering Drought and Salinity tolerance in Plants: Lessons from Genome-Wide Expression Profiling in Arabidopsis. Trends Biotechnol. 2005, 23, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Bota, J.; Cifre, J.; Mariano Escalona, J.; Galmés, J.; Gulías, J.; Medrano, H. Understanding down-regulation of photosynthesis under water stress: Future prospects and searching for physiological tools for irrigation management. Ann. Appl. Biol. 2004, 144, 273–283. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Pandey, S.P.; Somssich, I.E. The role of WRKY transcription factors in plant immunity. Plant Physiol. 2009, 150, 1648–1655. [Google Scholar] [CrossRef]
- Latchman, D.S. Transcription factors: An overview. Int. J. Biochem. Cell 1997, 29, 1305–1312. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kigawa, T.; Seki, M.; Shinozaki, K.; Yokoyama, S. DNA-binding domains of plant-specific transcription factors: Structure, function, and evolution. Trends Plant Sci. 2013, 18, 267–276. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Deng, P.; Chen, L.; Wang, X.; Ma, H.; Hu, W.; He, G. A wheat WRKY transcription factor TaWRKY10 confers tolerance to multiple abiotic stresses in transgenic tobacco. PLoS ONE 2013, 8, e65120. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, F.; Li, M.; Liang, D.; Zou, J. Physiological responses of kiwifruit plants to exogenous ABA under drought conditions. Plant Growth Regul. 2011, 64, 63–74. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, Q.; Duan, X.; Zhang, Z.; Li, D. Effects of PEG-induced drought stress on regulation of indole alkaloid biosynthesis in Catharanthus roseus. J. Plant Interact. 2017, 12, 87–91. [Google Scholar] [CrossRef]
- Meher Shivakrishna, P.; Ashok Reddy, K.; Manohar Rao, D. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 2018, 25, 285–289. [Google Scholar] [CrossRef]
- Teng, K.; Li, J.; Liu, L.; Han, Y.; Du, Y.; Zhang, J.; Sun, H.; Zhao, Q. Exogenous ABA induces drought tolerance in upland rice: The role of chloroplast and ABA biosynthesis-related gene expression on photosystem II during PEG stress. Acta Physiol. Plant. 2014, 36–38, 2219–2227. [Google Scholar] [CrossRef]
- Zarei, A.; Zamani, Z.; Mousavi, A.; Fatahi, R.; Alavijeh, M.K.; Dehsara, B.; Salami, S.A. An effective protocol for isolation of high-quality RNA from pomegranate seeds. Asian Aust. J. Plant Sci. Biotechnol. 2012, 6, 32–37. [Google Scholar]
- Zhang, Y.; Chen, Q.; Lan, J.; Luo, Y.; Wang, X.; Chen, Q.; Sun, B.; Wang, Y.; Gong, R.; Tang, H. Effects of Drought Stress and Rehydration on Physiological Parameters and Proline Metabolism in Kiwifruit Seedling. Int. J. Agric. Biol. 2018, 20, 2891–2896. [Google Scholar]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Luo, X. Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Kovalikova, Z.; Jiroutova, P.; Toman, J.; Dobrovolna, D.; Drbohlavova, L. Physiological responses of apple and cherry in vitro culture under different levels of drought stress. Agronomy 2020, 10, 1689. [Google Scholar] [CrossRef]
- Sale, P.R. Kiwifruit Culture; Williams, D.A., Ward, V.R., Eds.; Government Printer: Wellington, New Zealand, 1985; pp. 14–18. [Google Scholar]
- Savé, R.; Adillón, J. Comparison between plant water relations of in vitro plants and rooted cuttings of kiwifruit. In International Symposium on Kiwifruit Mount; Acta Horticulturae: Maunganui, New Zealand, 1987; Volume 282, pp. 193–198. [Google Scholar]
- Mahiwal, S.; Pahuja, S.; Pandey, G.K. Structural-functional relationship of WRKY transcription factors: Unfolding the role of WRKY in plants. Int. J. Biol. Macromol. 2013, 257, 128769. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Han, M.; Lee, S.-K.; Cho, J.-I.; Ryoo, N.; Heu, S.; Lee, Y.-H.; Bhoo, S.H.; Wang, G.-L.; Hahn, T.-R. A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep. 2006, 25, 836–847. [Google Scholar] [CrossRef]
- Lai, Z.; Vinod, K.; Zheng, Z.; Fan, B.; Chen, Z. Roles of ArabidopsisWRKY3 and WRKY4 Transcription Factors in Plant Responses to Pathogens. BMC Plant Biol. 2008, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Lan, X.; Zhou, J.; Gao, K.; Zhong, C.; Xie, J. Dioscorea composita WRKY3 positively regulates salt-stress tolerance in transgenic Arabidopsis thaliana. J. Plant Physiol. 2022, 269, 153592. [Google Scholar] [CrossRef]
- Guo, R.; Yu, F.; Gao, Z.; An, H.; Cao, X.; Guo, X. GhWRKY3, a novel cotton (Gossypium hirsutum L.) WRKY gene, is involved in diverse stress responses. Mol. Biol. Rep. 2011, 38, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Muhovski, Y.; Žižková, E.; Dobrev, P.I.; Gharbi, E.; Franco-Zorrilla, J.M.; Lopez-Vidriero, I.; Solano, R.; Clippe, A.; Errachid, A. The Solanum lycopersicum WRKY3 transcription factor SlWRKY3 is involved in salt stress tolerance in tomato. Front. Plant Sci. 2017, 8, 1343. [Google Scholar] [CrossRef] [PubMed]
- Skibbe, M.; Qu, N.; Galis, I.; Baldwin, I.T. Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. Plant Cell 2008, 20, 1984–2000. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Vishal, B.; Ho, W.J.; Lok, F.C.J.; Lee, F.S.M.; Kumar, P.P. Regulation of a cytochrome P450 gene CYP94B1 by WRKY33 transcription factor controls apoplastic barrier formation in roots to confer salt tolerance. Plant Physiol. 2020, 184, 2199–2215. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Vishal, B.; Bhal, A.; Kumar, P.P. WRKY9 transcription factor regulates cytochrome P450 genes CYP94B3 and CYP86B1, leading to increased root suberin and salt tolerance in Arabidopsis. Physiol. Plant. 2021, 172, 1673–1687. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, Y.; Shan, D.; Shi, K.; Wang, L.; Li, Q.; Kong, J. Md WRKY 9 overexpression confers intensive dwarfing in the M26 rootstock of apple by directly inhibiting brassinosteroid synthetase Md DWF 4 expression. New Phytol. 2018, 217, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.M.; McAtee, P.A.; Waite, C.N.; Boldingh, H.L.; McGhie, T.K. Apple dwarfing rootstocks exhibit an imbalance in carbohydrate allocation and reduced cell growth and metabolism. Hortic. Res. 2017, 4, 17009. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Holst, K.; Pörs, Y.; Guivarc’h, A.; Mustroph, A.; Chriqui, D.; Grimm, B.; Schmülling, T. Cytokinin deficiency causes distinct changes of sink and source parameters in tobacco shoots and roots. J. Exp. Bot. 2008, 59, 2659–2672. [Google Scholar] [CrossRef]
- Adachi, H.; Nakano, T.; Miyagawa, N.; Ishihama, N.; Yoshioka, M.; Katou, Y.; Yaeno, T.; Shirasu, K.; Yoshioka, H. WRKY transcription factors phosphorylated by MAPK regulate a plant immune NADPH oxidase in Nicotiana benthamiana. Plant Cell 2015, 27, 2645–2663. [Google Scholar] [CrossRef]
- Phukan, U.J.; Jeena, G.S.; Shukla, R.K. WRKY transcription factors: Molecular regulation and stress responses in plants. Front. Plant science 2016, 7, 760. [Google Scholar] [CrossRef]
- Zhao, K.X.; Chu, S.S.; Zhang, X.D.; Wang, L.P.; Rono, J.K.; Yang, Z.M. AtWRKY21 negatively regulates tolerance to osmotic stress in Arabidopsis. Environ. Exp. Bot. 2020, 169, 103920. [Google Scholar] [CrossRef]
- Bakshi, M.; Oelmüller, R. WRKY transcription factors: Jack of many trades in plants. Plant Signal. Behav. 2014, 9, e27700. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Tang, M.; Zhu, J.; Shu, M.; Wen, H.; Zhu, J.; Wei, C. Alternative splicing of CsWRKY21 positively regulates cold response in tea plant. Plant Physiol. Biochem. 2024, 208, 108473. [Google Scholar] [CrossRef]
- Ou, X.; Seemann, J.R.; Neuman, D.; Shen, Q.J. A WRKY gene from creosote bush encodes an activator of the abscisic acid signaling pathway. J. Biol. Chem. 2004, 279, 55770–55779. [Google Scholar]
- Wang, W.; Li, T.; Chen, Q.; Deng, B.; Deng, L.; Zeng, K. Transcription Factor CsWRKY65 Participates in the Establishment of Disease Resistance of Citrus Fruits to Penicillium digitatum. J. Agric. Food Chem. 2021, 69, 5671–5682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gu, M.; Liang, R.; Shi, X.; Chen, L.; Hu, X.; Wang, S.; Dai, X.; Qu, H.; Li, H.; et al. OsWRKY21 and OsWRKY108 function redundantly to promote phosphate accumulation through maintaining the constitutive expression of OsPHT1;1 under phosphate-replete conditions. New Phytol. 2021, 229, 1598–1614. [Google Scholar] [CrossRef]
- Babitha, K.C.; Ramu, S.V.; Pruthvi, V.; Mahesh, P.; Nataraja, K.N.; Udayakumar, M. Co-expression of at bHLH17 and at WRKY 28 confers resistance to abiotic stress in Arabidopsis. Transgenic Res. 2013, 22, 327–341. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, F.; Wang, Z.; Zhuo, C.; Hu, K.; Li, X.; Wen, J.; Yi, B.; Shen, J.; Ma, C. Transcription factor WRKY28 curbs WRKY33-mediated resistance to Sclerotinia sclerotiorum in Brassica napus. Plant Physiol. 2022, 190, 2757–2774. [Google Scholar] [CrossRef]
- Cao, Q.; Huang, L.; Li, J.; Qu, P.; Tao, P.; Crabbe, M.J.C.; Zhang, T.; Qiao, Q. Integrated transcriptome and methylome analyses reveal the molecular regulation of drought stress in wild strawberry (Fragaria nilgerrensis). BMC Plant Biol. 2022, 22, 613. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Wang, W.; Liu, J.; Zhu, C.; Zhong, Y.; Zhang, H.; Liu, X.; Yin, X. Transcription Factors Acerf74/75 Respond to Waterlogging Stress and Trigger Alcoholic Fermentation-Related Genes in Kiwifruit. Plant Sci. 2022, 314, 111115. [Google Scholar] [CrossRef]
- Chen, L.; Song, Y.; Li, S.; Zhang, L.; Zou, C.; Yu, D. The role of WRKY transcription factors in plant abiotic stresses. Biochim. et Biophys. Acta BBA-Gene Regul. Mech. 2012, 1819, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.-Q.; Tan, X.-L.; Shan, W.; Kuang, J.-F.; Lu, W.-J.; Chen, J.-Y. BrWRKY65, a WRKY transcription factor, is involved in regulating three leaf senescence-associated genes in Chinese flowering cabbage. Int. J. Mol. Sci. 2017, 18, 1228. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.; Wang, C.-T.; Yu, T.-F.; Wang, D.-M.; Li, M.; Zhao, D.; Li, X.-T.; Fu, J.-D.; Xu, Z.-S.; Song, X.-Y. Overexpression of ZmWRKY65 transcription factor from maize confers stress resistances in transgenic Arabidopsis. Sci. Rep. 2021, 11, 4024. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Guo, J.; Qiao, Q.; Guo, X.; Ma, Y. The WRKY transcription factor PlWRKY65 enhances the resistance of Paeonia lactiflora (herbaceous peony) to Alternaria tenuissima. Hortic. Res. 2020, 7, 57. [Google Scholar] [CrossRef]
- Guo, D.; Qin, G. EXB1/WRKY71 transcription factor regulates both shoot branching and responses to abiotic stresses. Plant Signal. Behav. 2016, 11, e1150404. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhang, J.; Wang, X.; Han, X.; Wei, B.; Wang, J.; Li, B.; Yu, H.; Huang, Q.; Gu, H. The WRKY transcription factor WRKY71/EXB1 controls shoot branching by transcriptionally regulating RAX genes in Arabidopsis. Plant Cell 2015, 27, 3112–3127. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Qi, Y.; Xu, J.; Dai, X.; Chen, J.; Dong, C.; Xiang, F. Arabidopsis WRKY71 regulates ethylene-mediated leaf senescence by directly activating EIN2, ORE1 and ACS2 genes. Plant J. 2021, 107, 1819–1836. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Liu, D.; Huang, M.; Ma, J.; Li, Z.; Li, M.; Sui, S. CpWRKY71, a WRKY transcription factor gene of Wintersweet (Chimonanthus praecox), promotes flowering and leaf senescence in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 5325. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, Y.; Wang, B.; Yu, S.; Dai, H.; Li, H.; Zhang, Z.; Zhang, J. Woodland strawberry WRKY71 acts as a promoter of flowering via a transcriptional regulatory cascade. Hortic. Res. 2020, 7, 137. [Google Scholar] [CrossRef]
- Xu, Q.; Feng, W.J.; Peng, H.R.; Ni, Z.F.; Sun, Q.X. TaWRKY71, a WRKY transcription factor from wheat, enhances tolerance to abiotic stress in transgenic Arabidopsis thaliana. Cereal Res. Commun. 2014, 42, 47–57. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, L.; Chen, J.; Liu, Z.; Park, C.-M.; Xiang, F. WRKY71 acts antagonistically against salt-delayed flowering in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 414–422. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, Z.-L.; Zou, X.; Huang, J.; Ruas, P.; Thompson, D.; Shen, Q.J. Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol. 2005, 137, 176–189. [Google Scholar] [CrossRef]
- Jia, X.; Feng, H.; Bu, Y.; Ji, N.; Lyu, Y.; Zhao, S. Comparative transcriptome and weighted gene co-expression network analysis identify key transcription factors of Rosa chinensis ‘Old Blush’after exposure to a gradual drought stress followed by recovery. Front. Genet. 2021, 12, 690264. [Google Scholar] [CrossRef]


| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| WRKY 3 | AGCGGGTCTAATGGTTCAAA | GATGCTGATTGGTTGTTTCTGA |
| WRKY 9 | AGCAGAAGCAGCAGCAAAT C | TCCAAAGTTGCTCCAGTGTG |
| WRKY 21 | TGACGCAGTCCGTGAACC | GGACTTGGGAAGCTGAGGAG |
| WRKY 28 | CCAAGTGCAACGTGAAGAA G | TTTTCGAGAGTAGGGACGATG |
| WRKY 41 | CCTTCTCCTTCCCTTCGACT | AATGATCTCGGTGAGGTCAGA |
| WRKY47 | ACCTTGGTGTTGGCATCAG | GCGGCCGAATAGTACATATCA |
| WRKY 65 | CAGAACCGCCTACCTCCTC | CCGAGGTAGTGGAAGCAGAA |
| WRKY71 | GTGGTGATGGCGGTAAGAA | CCTTTCTTCCTCGGCTTGTT |
| 18S rRNA | GTCGTAACAAGGTTTCCGTAGGT | CAAAGGGAAGAAAGAGTAGGGTT |
| Cultivars | PEG 6000 (%) | Plant Height | Multiplication Rate | Number of Leaves | Fresh Weight | Dry Weight |
|---|---|---|---|---|---|---|
| Hayward | 0 | 2.25 a | 3.07 a | 14.00 a | 2.63 a | 0.26 a |
| 1 | 1.51 b | 2.90 a | 10.50 b | 1.68 b | 0.16 b | |
| 2 | 1.22 c | 2.17 b | 9.93 b | 1.10 c | 0.11 c | |
| 3 | 1.19 c | 1.70 b | 7.00 c | 0.85 d | 0.07 d |
| Cultivar | PEG 6000 (%) | Plant Height | Multiplication Rate | Number of Leaves | Fresh Weight | Dry Weight |
|---|---|---|---|---|---|---|
| Matua | 0 | 2.46 a | 3.30 a | 14.70 a | 3.02 a | 0.32 a |
| 1 | 1.78 b | 3.10 a | 12.37 a | 1.77 b | 0.18 b | |
| 2 | 1.34 c | 2.13 b | 9.67 a | 1.18 c | 0.10 c | |
| 3 | 1.28 c | 1.87 b | 9.10 a | 1.01 c | 0.09 c |
| Cultivar | PEG 6000 (%) | Plant Height | Number of Root | Root Length | Number of Leaf | Fresh Weight | Dry Weight |
|---|---|---|---|---|---|---|---|
| Hayward | 0 | 4.83 a | 10.78 a | 11.78 a | 10.61 a | 3.44 a | 0.40 a |
| 1 | 0.94 b | 1.11 b | 1.36 b | 9.55 a | 0.44 b | 0.13 b | |
| 2 | 0.63 bc | 0 | 0 | 3.91 b | 0 | 0 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cultivar | PEG 6000 (%) | Plant Height | Mumber of Root | Root Length | Mumber of Leaf | Fresh Weight | Dry Weight |
|---|---|---|---|---|---|---|---|
| Matua | 0 | 4.50 a | 4.55 a | 6.41 a | 13.44 a | 2.33 a | 0.32 a |
| 1 | 1.17 b | 0.75 b | 0.94 b | 9.06 a | 0.98 b | 0.15 b | |
| 2 | 0.77 b | 0.18 b | 0.19 b | 4.64 b | 0.32 c | 0.03 c | |
| 3 | 0.84 b | 0 | 0 | 8.67 ab | 0.38 bc | 0.07 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Açar, E.; Erol, M.H.; Aka Kaçar, Y. Investigation of Relationship Between Drought Stress Resilience and Some Wrky Transcription Factor Genes in Some Kiwi (Actinidia deliciosa) Cultivars. Agriculture 2025, 15, 1733. https://doi.org/10.3390/agriculture15161733
Açar E, Erol MH, Aka Kaçar Y. Investigation of Relationship Between Drought Stress Resilience and Some Wrky Transcription Factor Genes in Some Kiwi (Actinidia deliciosa) Cultivars. Agriculture. 2025; 15(16):1733. https://doi.org/10.3390/agriculture15161733
Chicago/Turabian StyleAçar, Emine, Mansur Hakan Erol, and Yıldız Aka Kaçar. 2025. "Investigation of Relationship Between Drought Stress Resilience and Some Wrky Transcription Factor Genes in Some Kiwi (Actinidia deliciosa) Cultivars" Agriculture 15, no. 16: 1733. https://doi.org/10.3390/agriculture15161733
APA StyleAçar, E., Erol, M. H., & Aka Kaçar, Y. (2025). Investigation of Relationship Between Drought Stress Resilience and Some Wrky Transcription Factor Genes in Some Kiwi (Actinidia deliciosa) Cultivars. Agriculture, 15(16), 1733. https://doi.org/10.3390/agriculture15161733






