Abstract
Soil contamination by heavy metals represents a critical environmental challenge, demanding reliable assessment methods. While biotoxicity assays are widely employed, the selection of sensitive biomarkers for heavy-metal contamination remains poorly defined. This study systematically assessed the sensitivity of biological indicators by analyzing 17 peer-reviewed studies (2003–2024) from various databases. The results revealed significant changes in the physiological and biochemical indicators of soil organisms exposed to heavy metals. Specifically, compared to control groups, the experimental groups showed 180%, 150%, and 145% catalase (CAT), peroxidase (POD), and malondialdehyde (MDA) concentrations, respectively. Meta-regression analysis indicated that biomarker responses are shaped by metal type, concentration, exposure duration, soil organism species, and soil variables. Cadmium exposure significantly increased CAT activity (+2.26), SOD activity (+3.46), POD activity (+3.44), and MDA content (+2.80). While CAT activity exhibited significant publication bias, POD and MDA remain promising biomarkers, with applicability varying across species and environmental conditions. This study presents a decision framework for biomarker selection based on metal speciation and soil properties, aiming to standardize ecological risk assessments and strengthen regulatory monitoring of heavy-metal impacts on soil health.
1. Introduction
Soil heavy-metal contamination has become a major environmental concern in recent years. Heavy metals in soil originate from diverse sources, such as pesticides, fertilizers, vehicle emissions, coal combustion, wastewater discharges, mining activities, and irrigation practices [1]. Soil organisms take up heavy metals via essential physiological processes, and these metals can rapidly impair their reproductive functions while inducing the production of reactive oxygen species (ROS) [2]. Excessive free radical generation triggers oxidative stress, causing irreversible damage to cells. This disrupts normal metabolic processes, ultimately leading to abnormal growth and death [3,4]. Given the characteristics of heavy-metal pollution, including its wide range of pollutants, long duration, hidden nature, and non-biodegradability, it is crucial to apply appropriate methodologies for assessing soil heavy-metal contamination.
Current techniques for assessing soil heavy-metal contamination fall into three categories: direct detection, toxicity testing, and biomarker analysis. Direct detection methods, including chemical and physical techniques, can accurately quantify heavy-metal ions in real samples and boast broad applicability and high compatibility. However, these methods are not suitable for characterizing the ecotoxicity of contaminants as perceived by living organisms [5,6]. The two most widely used toxicity testing techniques are acute and subacute toxicity tests [7,8]. Their advantages include strong relevance and ease of operation. That said, the contaminant concentrations used in such tests are often far higher than those commonly found in the natural environment, making it difficult to determine the toxicological effects of low-dose contaminants in the real environment.
Direct detection and toxicity testing fail to meet the demands of practical application. Consequently, biomarker analysis has increasingly become a research focus as a more sensitive method of contamination assessment [9]. While interconnected, biomarkers and bioindicators differ significantly. Biomarkers are defined as biochemical and physiological changes in organisms upon exposure to contaminants [10]. In contrast, a bioindicator is a quantifiable trait associated with biochemical, physiological, toxicological, or ecological processes, and is linked to effects across multiple levels, including the organism, population, community, or ecosystem [11]. Thus, biomarkers are better suited for the early detection and mechanistic prediction of environmental pollution, whereas bioindicators are better suited for comprehensive ecosystem-level assessments. To evaluate contamination levels and associated ecological risks, biomarker analysis integrates toxicity tests with direct detection methods. This is accomplished by monitoring changes at the physiological [12], biochemical [13], reproductive [14], and molecular levels [15], as well as selecting organisms directly exposed to polluted environments for exposure assessment. Compared to the first two techniques, biomarkers more accurately characterize the toxicity of pollutants to organisms [16,17]. They can act as early indicators of pollution at low concentrations, a role crucial for ecological protection. Since toxic effects first manifest at the subcellular level [18], biomarkers can serve as indicators of heavy-metal exposure, especially in low-dose scenarios.
Nevertheless, it is important to note that biomarker levels can be influenced by various factors, including environmental and biotic conditions [19], which may compromise the reliability of biomarker-based results. In addition, the bioaccumulation of heavy metals varies significantly by heavy-metal type. As noted by Ghemari et al. [20] in their study of heavy-metal pollution in the vicinity of the Gabes–Ghannouch industrial complex, the bioaccumulation of heavy metals varies between different types of metal. In particular, cadmium (Cd), zinc (Zn), and copper (Cu) accumulate much more than Pb in Porcellionides pruinosus. Furthermore, differing sensitivities of various biomarkers to heavy metals may introduce unreliability into an overall assessment of contamination. Ugbaja et al. [21] collected snail samples near a cement factory to analyze biomarkers, finding that glutathione-S-transferase (GST), arylesterase, and glutathione (GSH) activities in snail tissues correlated negatively with heavy-metal levels, whereas lactic acid dehydrogenase and malondialdehyde (MDA) showed positive correlations. Clearly, for accurate pollution assessment, biomarkers should be widely accessible, easy to sample, and sufficiently sensitive to heavy-metal contamination. In toxicity tests, soils are categorized as artificial or native. As noted in OECD guideline No. 207 [22], artificial soils may underestimate the toxicity of pollutants in real environmental conditions [23]. In contrast, native soils sourced from unpolluted environments retain their intrinsic properties, allowing for accurate predictions of metal bioavailability and preservation of natural organism responses [24]. Thus, soil type must be carefully considered in heavy-metal toxicity experiments.
Despite the utility of existing technologies for contamination assessment, methodological limitations remain, particularly for low levels of heavy-metal exposure. This study aims to identify sensitive biomarkers in soil organisms for heavy-metal exposure and to investigate the factors influencing these biomarkers. It is based on the existing literature on soil organisms exposed to heavy metals. The primary objectives of this meta-analysis are as follows: (1) to evaluate the potential of soil organisms as sources of biomarkers; (2) to examine how heavy-metal species, exposure duration, concentration, and soil organism species affect biomarker responses; and (3) to identify biomarkers suitable for assessing real-world soil heavy-metal pollution.
2. Materials and Methods
2.1. Search Strategy
We conducted a comprehensive literature search across the Web of Science, CNKI, Wanfang, and Wipro databases to retrieve studies on soil organisms under heavy-metal stress. In addition, relevant references from included studies were retrospectively screened to ensure comprehensiveness. The primary search strategy combined the following terms: (“environmental biomarkers” OR “bioindicator” OR “biological indicator” OR “biochemical marker” OR “biologic marker” OR “biological marker” OR “biomarker”) AND (“soil heavy metal stress” OR “soil heavy metal” OR “soil heavy metal contamination” OR “soil heavy metal pollution” OR “soil pollution by heavy metals”). The literature selection process was conducted independently by two reviewers, with discrepancies resolved through discussion with a third reviewer.
2.2. Inclusion and Exclusion Criteria
Original studies were included in this systematic review and meta-analysis if they met the following criteria: (i) publications in either Chinese or English; (ii) examining the effects of heavy metals on the physiological and biochemical indices of soil organisms, including soil animals; (iii) providing complete data (either in graphical or tabular form), including key information such as experimental and control group doses, treatment conditions, and means with standard deviations of relevant parameters; and (iv) published between 2003 and 2024.
Records were excluded if they met any of the following criteria: (i) abstract-only studies with full texts; (ii) reviews, commentaries, systematic reviews, or conference proceedings; (iii) studies lacking data on the physiological and biochemical indices necessary for this analysis; (iv) studies involving nanometals, mixed heavy metals, or combinations with other contaminants; and (v) duplicate reports, low-quality studies, or studies with insufficient information on confounders, rendering them unusable.
2.3. Data Extraction
Two researchers independently conducted literature searches, extracted data, and cross-checked the results. Disagreements were resolved through discussion or consultation with a third researcher. The screening process involved an initial review of titles to exclude irrelevant articles. Relevant studies were then further evaluated by reviewing abstracts and full texts to determine their eligibility for inclusion. When necessary, authors of the original studies were contacted via email or telephone to obtain missing or critical information relevant to this review. The extracted information included: (i) basic study details such as title, first author, and year of publication; (ii) heavy-metal types and concentrations, exposure duration, and selected soil organisms; (iii) soil variables, including soil type, pH, and organic carbon content; and (iv) extracted outcome data, including sample sizes, mean values with associated variability metrics (standard deviations, standard errors, or 95% confidence intervals), and all predefined outcome indicators and effect measures of interest.
2.4. Statistical Analysis
Statistical analyses were conducted using StataMP17. Means, standard deviations (SDs), and sample sizes (n) for both the experimental and control groups were extracted from the included studies. For studies that did not report standard deviations, conversions were performed using
- (i)
- Conversion of standard error (SE) to SD:
SE: standard error for the mean;
n: sample sizes;
: square root of sample sizes.
- (ii)
- Conversion of 95% confidence interval (CI) to SD:
When 95% CI is reported as [L, U],
where
L/U: lower/upper confidence limits;
n: sample sizes;
is the Z-score corresponding to the desired confidence level.
For large samples (n > 60), Z_(0.025) = 1.96 may be a substitute for the t-value.
To assess the effect size, the standardized mean difference (Hedges’ g) was chosen as the metric for analysis. Hedges’ g is calculated using the following formula:
where d is the uncorrected standardized mean difference given by
and is the pooled standard deviation, calculated as
where X1 and X2 are the means of the experimental and control groups, S1 and S2 are their respective standard deviations, and n1 and n2 are the sample sizes of the two groups. The correction factor J is given by
where are the degrees of freedom.
A random effects model was employed to account for potential heterogeneity between studies. Heterogeneity was assessed via Cochran’s Q test and the I2 statistic, with I2 values exceeding 50%, indicating substantial heterogeneity.
Given the high level of heterogeneity observed in the results, meta-regression analysis was used to further explore the factors influencing heavy-metal toxicity to soil fauna. The meta-regression model can be expressed as follows:
where
is the dependent variable (physiological or biochemical indicators of soil animals). For study i,
is the intercept;
, , , and are the coefficients for the covariates;
is the type of heavy metal;
is the exposure duration;
is the heavy-metal concentration;
is the soil biological species;
is the error term.
Regression coefficients and associated factors were analyzed to comprehensively assess the variables influencing differences in heavy-metal toxicity to soil organisms. A significance level of p < 0.05 was considered statistically significant for all analyses.
Publication bias refers to the influence of research findings on the likelihood of a study being published. Egger’s test was used to assess publication bias, with the presence of bias determined by the significance of the intercept in the regression equation, with a significance level set at α = 0.05. A p-value for the intercept of less than 0.05 indicates significant publication bias. For outcome indicators identified as having publication bias, the Trim and Fill method was applied for data correction. This method recalculates the overall effect by estimating the number and results of the unreported (missing) studies based on the existing data. All analyses were performed using StataIC15.
To assess the robustness of the results, sensitivity analyses were conducted using a leave-one-out approach. In this method, one study at a time was systematically excluded to evaluate the effect of each individual study on the overall effect size and heterogeneity. The results of these analyses were used to determine whether any single study disproportionately influenced the overall conclusions.
3. Results
3.1. Search Results
As shown in the flow chart, a total of 7702 records were obtained. Of these, 29 were from CNKI, 159 from Wanfang, 8 from Wipro, and 7506 from the Web of Science (Figure 1). After removing duplicates, 7673 records were retained. Following the exclusion of irrelevant records through screening title and abstract, 432 articles underwent full-text assessment for eligibility. Subsequent full-text review of these articles resulted in 17 being included in the qualitative analysis (see Supplementary Materials: Table S1).
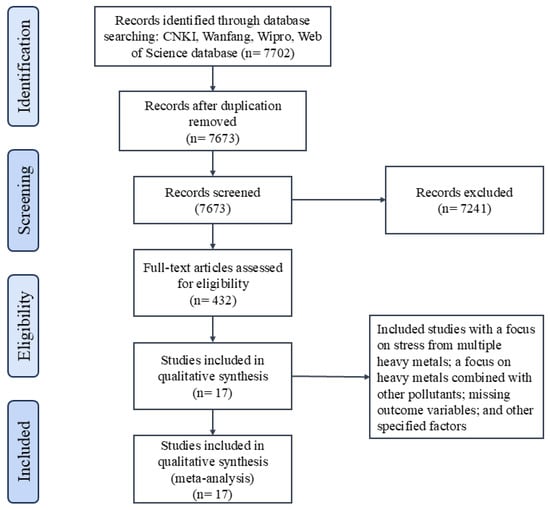
Figure 1.
Flow diagram of the literature search and selection process.
3.2. Detailed Characteristics of Included Studies
A total of 17 original studies were included in the qualitative review, with the basic characteristics of all included studies summarized in the table. They observed several biomarker indicators, including lethal concentration 50 (LC50), catalase (CAT), superoxide dismutase (SOD), peroxidase (POD), total protein (TP), MDA, and GSH (Table 1).

Table 1.
Key characteristics of the included studies.
3.3. Results of Meta-Analysis
Overall, heavy-metal treatments significantly increased the activities of antioxidant enzymes and MDA levels in soil organisms, indicating a pronounced oxidative stress response. However, the correlations between TP levels and heavy-metal treatments and GSH levels and heavy-metal treatments are weak. These results suggest that heavy-metal exposure stimulates the production of oxidative-stress-related enzymes in soil organisms. Specifically, CAT activity, POD activity, and MDA level in the experimental group increased by 80%, 50%, and 45%, respectively, compared to the control group (Figure 2A,C and Figure 3B). In contrast, heavy-metal stress had no significant overall effect on TP or GSH levels (Figure 3A,C). Furthermore, meta-regression analyses revealed that the concentration and duration of heavy-metal exposure also influenced the magnitude of biomarker responses. Regression models were established for biomarkers including CAT, SOD, POD, TP, MDA, and GSH using physiological and biochemical indicators of soil organisms as dependent variables, and heavy-metal exposure concentration and exposure duration as covariates. The results showed that CAT activity exhibited a significant positive correlation with heavy-metal exposure concentration, while SOD activity, TP content, and MDA level correlated positively with exposure duration. Prolonged exposure further increased their activities, highlighting the cumulative effects of heavy-metal stress over time (Figure 4).
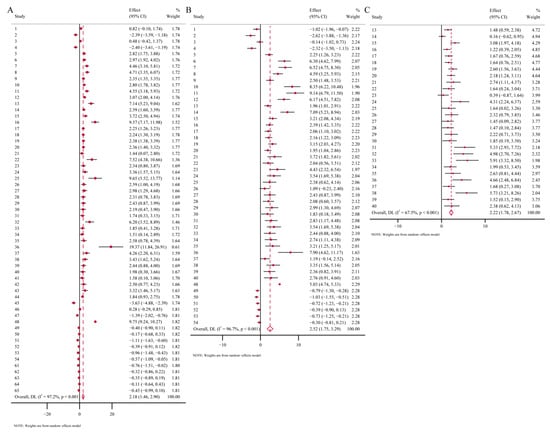
Figure 2.
Mean standardized difference (Hedges’ g with 95% CI) in biomarkers under heavy-metal stress. A negative Hedges’ g value indicates that heavy-metal stress promotes biomarker production, while a positive value indicates inhibition. (A) CAT; (B) SOD; (C) POD.
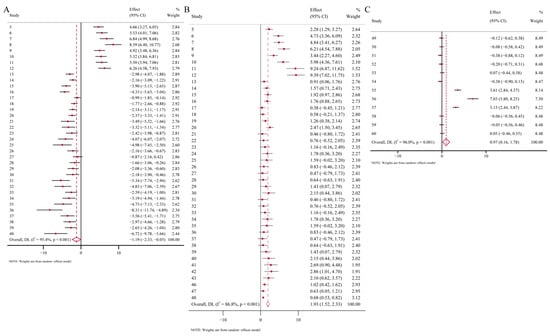
Figure 3.
Mean standardized difference (Hedges’ g with 95% CI) in biomarkers under heavy-metal stress. A negative Hedges’ g value indicates that heavy-metal stress promotes biomarker production, while a positive value indicates inhibition. (A), TP; (B), MDA; (C), GSH.
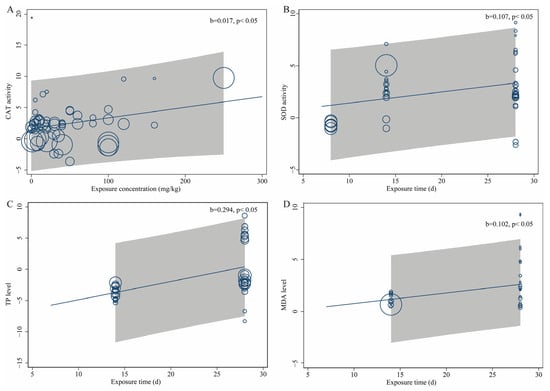
Figure 4.
Meta-regression bubble plot. (A) Correlation between CAT activity and heavy-metal concentration; (B) correlation between SOD activity and exposure time; (C) correlation between TP level and exposure time; (D) correlation between MDA level and exposure time. Each bubble represents an individual study, with bubble size proportional to the study’s weight in the analysis. The solid regression line indicates the predicted trend from the weighted meta-regression analysis. The gray-shaded areas represent the 95% confidence intervals.
The toxicity of different heavy metals to soil organisms exhibited considerable variation. Regression models for biomarkers such as CAT, SOD, POD, TP, MDA, and GSH were constructed using physiological and biochemical indicators of soil organisms as dependent variables and heavy-metal species as covariates. Meta-regression results showed that, in general, soil organisms were more sensitive to Cd than to Sb, Zn, Ag, and As (Figure 5). Additionally, based on the statistical and toxicological significance of the regression coefficients, the higher activity of oxidative-stress-related enzymes indicates more severe oxidative stress in the organisms and greater toxicity of the heavy metals.
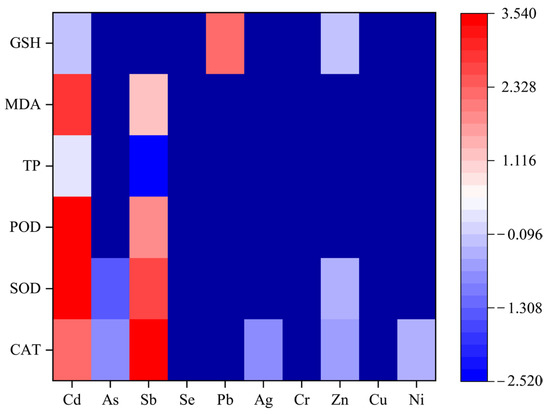
Figure 5.
Regression coefficients for the effect of heavy-metal types on soil organism biomarkers.
Discrepancies were observed in the sensitivity of different soil organism species to heavy metals. Soil organism species were included as covariates, and separate regression models were established for each physiological and biochemical index of soil organisms. The results showed the following order of heavy-metal tolerance among soil organisms: Enchytraeus albidus > Porcellionides pruinosus > Eisenia fetida > Eisenia andrei > Aporrectodea jassyensis (Figure 6). In general, E. albidus and P. pruinosus showed greater tolerance to heavy metals; their physiological and biochemical indices showed minimal response and slight changes. In contrast, the high sensitivity of E. fetida, E. andrei, and A. jassyensis to heavy metals suggests these three species may play a key role in assessing soil biological integrity under heavy-metal pollution.
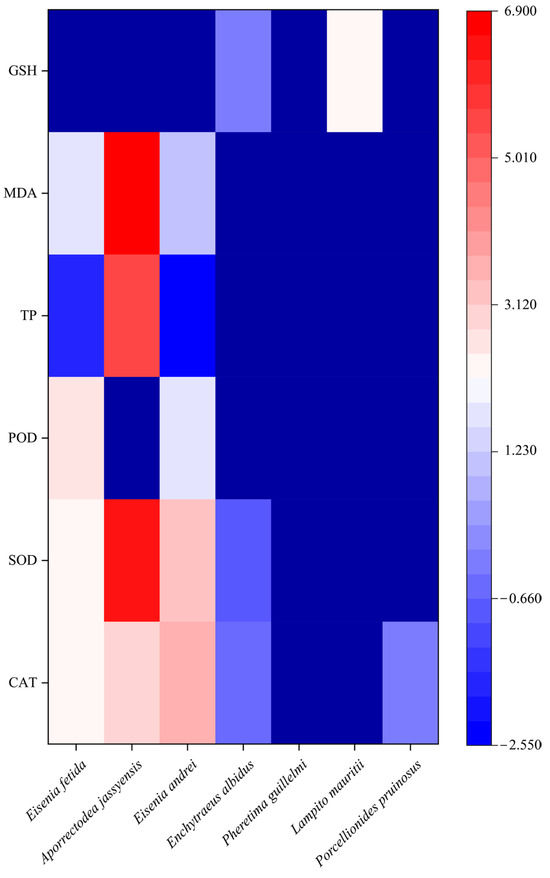
Figure 6.
Regression coefficients for the effect of soil organism species on soil organism biomarkers.
Soil variables significantly regulate enzyme activity and oxidative stress levels. For the experiment, soils were categorized into artificial and native types. In native soils, the activities of antioxidant enzymes such as CAT and SOD were higher, while GSH content was lower. All indicators (CAT, SOD, POD, TP, MDA) showed positive correlations with increasing pH, with TP being particularly sensitive to pH changes. Conversely, SOD activity and MDA content were reduced in soils with high organic carbon, while TP appeared to accumulate as organic carbon levels increased (Table 2).

Table 2.
Regression coefficients for the effect of soil variables on soil organism biomarkers.
3.4. Leave-One-out Sensitivity Analysis
Sensitivity analyses were performed by excluding each study individually to evaluate the impact of each individual study on the overall effect. As shown in Supplementary Tables S2–S7, no statistically significant differences in the effect estimates were observed after excluding any individual study.
3.5. Publication Bias
To assess potential publication bias, Egger’s regression tests were conducted, which revealed significant asymmetry in the effect sizes for CAT, POD, MDA, and GSH (p < 0.05). In contrast, no evidence of bias was observed for SOD and TP (p > 0.05). For indicators with asymmetry, trim-and-fill analysis incorporated hypothetical missing studies, demonstrating that the statistical significance of effects of POD (Figure S14), MDA (Figure S15), and GSH (Figure S16) remained unchanged after adjustment. This confirms the robustness of these results.
4. Discussion
Heavy-metal contamination significantly alters the biological environment of soils, highlighting the critical need for the accurate assessment of biotoxicity in such contexts. Previous research on soil heavy-metal biomarkers has identified several challenges related to their application and screening. First, the lack of standardization in biotoxicity testing techniques limits the applicability and comparability of individual studies in practical scenarios. Second, the insufficient sensitivity of certain biomarkers to heavy-metal exposure raises concerns about their efficacy. Third, the influence of confounding factors on biomarker responses may compromise the reliability of results and the overall quality of environmental assessments. To provide a reference framework for selecting soil heavy-metal biomarkers, this study systematically reviewed and analyzed research on biomarkers of soil heavy-metal contamination published between 2003 and 2024.
The results indicate that soil heavy-metal contamination significantly affects the physiological and biochemical markers of soil organisms, supporting the potential of these markers as reliable biomarkers for monitoring soil health. Organisms in the experimental group under heavy-metal stress showed significant increases in the activities of CAT, SOD, and POD, as well as MDA levels. However, no statistically significant effects on TP level or GSH levels were observed. The antioxidant systems, including enzymatic antioxidants and non-enzymatic antioxidants, play a critical role in defending against oxidative stress [25]. Enzymatic antioxidants are often regarded as the primary line of defense in such systems due to their efficacy in scavenging ROS. CAT, a heme-containing tetrameric enzyme, plays a pivotal role in removing hydrogen peroxide (H2O2) [26]. Under heavy-metal stress, CAT activity shows significant variability [27]. Plants possess three types of SOD isoenzymes, classified by their metal cofactors; these enzymes specifically catalyze the disproportionation of superoxide radicals into H2O2 and oxygen (O2) [28]. POD catalyzes the oxidation of phenolic and amine compounds in the presence of H2O2, thereby effectively removing both H2O2 and the toxicity of these compounds [29]. When cells are subjected to biotic or abiotic stress, free radicals induce lipoperoxidation in membranes and proteins [30]. As a major end product, MDA serves as an indicator of free radical production and subsequent tissue damage. Antioxidant markers are commonly used in the assessment of environmental pollution [31,32], as antioxidant activity directly correlates with pollutant concentration. Proteins are the primary organic components of organismal cells, and free amino acids also function as key osmotic effectors [33]. Under heavy-metal stress, the levels of free amino acids increase significantly. In addition, proteins can reduce heavy-metal biotoxicity through binding to metals. Glutathione, an important non-enzymatic antioxidant, scavenges excess ROS and H2O2 via the ascorbate-glutathione pathway [34].
However, the correlations between TP and GSH levels and heavy-metal stress were significantly weaker than those of the aforementioned antioxidant enzymes in the actual test. The weak correlation between GSH levels and heavy-metal stress may stem from several factors. One significant reason is the compensatory mechanisms within organisms. During oxidative stress induced by heavy metals, GSH is not typically the primary antioxidant in the initial response. Instead, enzymes like SOD and CAT act as the first line of defense, directly eliminating ROS [35]. GSH tends to be involved later in the process, which may explain its weaker correlation with heavy-metal stress, especially in the early stages. Another contributing factor is the limited availability of precursors required for GSH synthesis. GSH is synthesized from glutamate and cysteine, with cysteine being produced by γ-glutamylcysteine synthetase (GCS) or ligase (GCL) [36]. Insufficient sulfur supply in organisms further restricts GSH synthesis, hindering the ability to produce adequate GSH and weakening responses to heavy-metal-induced oxidative stress. Furthermore, GSH synthesis is subject to negative feedback regulation. As intracellular GSH levels increase, the synthesis process is inhibited. In the necrotic acid–glutathione pathway, GSH synthesis is activated only after GSH1 translation, and excess GSH accumulation creates a reducing environment that inhibits GSH synthesis and reduces GSH1 activity [37].
The weak correlation between TP levels and heavy-metal stress may also result from multiple factors. High concentrations of heavy metals can disrupt ribosomal structures or inhibit the activity of translation-related enzymes, reducing protein synthesis [38]. Furthermore, heavy metals can activate the ubiquitin–proteasome system or induce autophagy, accelerating the degradation of damaged proteins, such as oxidized or misfolded proteins. Under heavy-metal stress, metal ions tend to bind to cysteine residues in proteins, causing molecular cross-linking. Additionally, ROS generated by oxidative stress can cause protein carbonylation and tyrosine cross-linking [39]. Cross-linking between protein molecules forms insoluble macromolecular aggregates, which are difficult to detect using conventional TP assays, increasing potential result inaccuracies. Thus, further consideration of whole-organism cellular mechanisms of oxidative stress is needed to identify more specific biomarkers.
Meta-regression results indicated that heavy-metal type and concentration, exposure duration, soil organism species, and soil variables were sources of heterogeneity. In this study, using soil organisms as a covariate, heavy-metal toxicity was ranked as Cd > Sb > Zn > Ag > As. Different heavy metals exhibit unique modes of action and toxic effects on organisms. Patricia et al. [40] reported that heavy-metal toxicity to earthworms followed the order Pb > Cr > Zn > Cu, consistent with our findings. While Cu and Zn are essential for biological growth (organisms continuously uptake and transport these metals), environmental pollutants like Cd and Pb become harmful once they accumulate to certain concentrations. According to Šrut et al. [41], Cd alters the activity of DNA methyltransferases (DNMTs) and other DNA-methylation-related enzymes, increasing genomic DNA methylation. Pb toxicity primarily manifests as interference with structural protein synthesis, activation of specific enzymes, disruption of calcium homeostasis, inhibition of trace element uptake, and alteration of cellular redox environments [42]. Additionally, meta-regression showed that when heavy metals were used as covariates for CAT, the regression coefficients were 2.2561 (Cd), −0.8338 (As), 3.5219 (Sb), −0.8776 (Ag), −0.6373 (Zn), and −0.3055 (Ni). Studies on Cd and Sb may be more likely to report positive outcomes, while research showing negative or weak effects for As or Zn may be underpublished. Thus, heavy-metal type is a critical factor explaining variability in CAT activity, though differences in effect sizes may reflect both true biological effects and publication bias.
Dose–response relationships are increasingly used in ecotoxicological research to quantitatively and qualitatively characterize changes in physiological and biochemical responses to varying contaminant exposure levels. The specific dose–response curves of biomarkers vary by biomarker type. Our results showed that CAT activity in soil organisms was promoted under the same heavy-metal concentration, while other biomarkers exhibited inhibitory effects. Yang et al. [32] investigated the effects of different Cd concentrations on earthworms, finding increased SOD and CAT activities and MDA levels. These observations emphasize the need to thoroughly evaluate dose–toxicity relationships when selecting heavy-metal biomarkers; appropriate biomarker selection should consider heavy-metal concentration ranges and biomarker tolerance ranges. Contaminant concentration and exposure duration are critical factors influencing biotoxicity. Numerous studies show that organismal sensitivity increases with prolonged exposure. Consistent with time-dependent toxicity effects, our study found significant correlations between prolonged exposure and increased SOD activity, TP content, and MDA levels. Time-dependent toxicity also highlights the temporal dynamics of toxicant responses in organisms and their modes of action.
Furthermore, the key indicators of oxidative stress in earthworms vary with heavy-metal exposure duration. As shown by Gao et al. [43] and Ning et al. [31], MDA levels in earthworms exposed to contaminated soil increased significantly with longer exposure times. In practical environmental assessments, determining how long-term exposure alters biomarkers is particularly important.
Different species exhibit differences in ecological niches and ecotypes, which may affect contaminant uptake and translocation, thereby influencing the magnitude of biomarker responses to pollutants. Aporrectodea jassyensis (an endogeic earthworm) and Eisenia fetida (an epigeic earthworm) represent distinct eco-physiological groups. While E. fetida is widely used in ecotoxicity tests, its sensitivity to contaminants remains debated. Ahmadpour et al. [44] found that the endogeic A. jassyensis shows stronger biomarker responses than E. fetida, suggesting it may serve as a more sensitive indicator of environmental pollution. Fisker et al. [45] reached similar conclusions, noting that the epigeic Dendrobaena octaedra was the only species surviving in highly polluted areas near pollution sources, confirming higher heavy-metal resistance in epigeic earthworms. However, further research is needed to validate these interspecific differences.
Soil serves as a habitat for soil organisms, and its physicochemical properties significantly influence them. In our analysis, soils were categorized as artificial or native. Native soils possess characteristics lacking in artificial soils, including intrinsic microbial communities and oxidative stress factors. Liu et al. [46] demonstrated that soil microorganisms can convert Pb into more stable forms through metabolic activities, while soil biota can enhance microbial activity by absorbing heavy metals—highlighting the complexity of interactions between soil biota and microorganisms under heavy-metal stress.
Soil pH affects heavy-metal bioavailability and oxidative stress processes, thereby influencing metal toxicity. A lower pH typically reduces the soil adsorption of metal ions, increasing free metal ions and thus enhancing soil metal bioavailability [47]. CAT, which uses iron porphyrin as a cofactor [48], is more stable under slightly alkaline conditions in experiments, facilitating the maintenance of iron center stability.
Soil organic carbon content plays a crucial role in heavy-metal bioaccumulation and toxicity in soil organisms, involving multi-level responses. Irizar et al. [49] found that low soil organic matter reduces Cd accumulation in soil biota; however, nutritional limitations from low organic matter may weaken earthworms’ resistance to cadmium toxicity. Our results showed a strong positive correlation between TP content and organic carbon, supporting this observation. Conversely, the negative correlation between CAT, SOD, and organic carbon content necessitates further investigation into whether nutritional limitations under low organic carbon conditions exacerbate oxidative stress in soil organisms.
This study primarily focused on earthworms as model organisms, consistent with international standards that commonly use earthworms for standard soil ecotoxicity tests [50]. This aligns with our observation that earthworms constituted a significant proportion of test subjects in selected articles. While earthworms are valuable ecological indicators due to their sensitivity to environmental changes, conclusions drawn from earthworm data must be validated across other taxa, as biomarker sensitivity thresholds vary significantly among organisms with different physiological characteristics. The preponderance of earthworm studies in the literature stems from two factors: most published articles focus on earthworms, with fewer studies on other soil organisms (e.g., springtails, spiders); additionally, our search criteria required oxidative-stress-related biomarkers (CAT, SOD, POD, MDA, TP, GSH), but these are often unmeasured or incompletely reported in studies on other soil organisms, with key data (means, standard deviations, sample sizes) frequently missing. Thus, the limited taxonomic diversity in our findings restricts their generalizability to other species or ecological systems. To address this gap, we summarized additional oxidative-stress-related biomarkers identified in various organisms (Table S8). These biomarkers—including glutathione peroxidase (GPx), glutathione reductase (GR), and metallothionein (MT) [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65]—could provide valuable insights into biological responses to heavy-metal exposure. Future research should include a broader range of species from diverse taxonomic groups to enhance these findings’ applicability across ecosystems and deepen understanding of the extensive ecological impacts of soil heavy-metal contamination.
This systematic review had several limitations: (i) the findings may be constrained by incomplete data, as some original data were unavailable in the literature; (ii) studies on other soil organisms (e.g., soil microbes) were excluded, focusing solely on soil fauna; (iii) the reliability of conclusions was somewhat compromised due to variability in outcome indicators across included studies and the limited number of studies available for certain indicators.
5. Conclusions
The available data suggest that using soil organisms as biomarkers for assessing soil heavy-metal contamination has limited feasibility. This limitation mainly stems from the variability in biomarker responses, which is influenced by factors including heavy-metal type and concentration, exposure duration, soil organism species, and soil variables. Three key findings emerge: (i) SOD exhibits a particularly strong response to Cd exposure, confirming its role as a sensitive early indicator of oxidative stress; (ii) MDA reliably reflects lipid peroxidation damage, with consistent results even after statistical adjustment; and (iii) POD activity shows dose-dependent increases, confirming its role in antioxidant defense. For practical applications, it is crucial to carefully select biomarkers that are not only widely accessible and easy to sample but also sufficiently sensitive to detect heavy-metal contamination. Such considerations are critical for ensuring accurate and effective environmental assessments. In conclusion, despite the challenges in using soil organisms as biomarkers for heavy-metal contamination assessment, these biomarkers remain highly promising for practical applications.
Supplementary Materials
The following supporting information is available for downloaded at https://www.mdpi.com/article/10.3390/agriculture15161728/s1. Figure S1: Correlation between CAT activity and exposure time; Figure S2: Correlation between SOD activity and heavy-metal concentration; Figure S3: Correlation between POD activity and heavy-metal concentration; Figure S4: Correlation between POD activity and exposure time; Figure S5: Correlation between TP level and heavy-metal concentration; Figure S6: Correlation between MDA level and heavy-metal concentration; Figure S7: Correlation between GSH level and heavy-metal concentration; Figure S8: Correlation between GSH level and exposure time; Figure S9: Forest plot of LC50 values; Figure S10. Forest plot of CAT activity effect sizes after trim-and-fill adjustment for publication bias; Figure S11. Forest plot of POD activity effect sizes after trim-and-fill adjustment for publication bias; Figure S12. Forest plot of MDA level effect sizes after trim-and-fill adjustment for publication bias; Figure S13. Forest plot of GSH level effect sizes after trim-and-fill adjustment for publication bias; Figure S14. Trim-and-fill adjusted funnel plot for POD activity. Circles show original studies; squares indicate imputed studies; Figure S15. Trim-and-fill adjusted funnel plot for MDA activity. Circles show original studies; squares indicate imputed studies; Figure S16. Trim-and-fill adjusted funnel plot for GSH activity; Table S1: Concentration of heavy metals used for biomarker assessment; Table S2: Sensitivity analysis for CAT activity: sequential exclusion of individual studies; Table S3: Sensitivity analysis for SOD activity: sequential exclusion of individual studies; Table S4: Sensitivity analysis for POD activity: sequential exclusion of individual studies; Table S5: Sensitivity analysis for TP level: sequential exclusion of individual studies; Table S6: Sensitivity analysis for MDA level: sequential exclusion of individual studies; Table S7: Sensitivity analysis for GSH level: sequential exclusion of individual studies; Table S8. Additional biomarkers for assessing the biological toxicity effects of heavy metal.
Author Contributions
Y.L.: Writing—Original Draft, Visualization, Software, Data Curation, Conceptualization. Z.L.: Visualization, Software, Data Curation. L.L.: Software, Data Curation. S.Z.: Supervision, Data Curation. W.Z.: Data Curation. Y.S.: Supervision, Conceptualization, Methodology, Writing—Review and Editing, Funding Acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key Research and Development Program of China (2022YFC3701302) and natural Science Foundation of Guangdong Province, China (grant No. 2023A1515011052).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.U.; Nazir, S.; Irshad, R.; Tahir, K.; ur Rehman, K.; Islam, R.U.; Wahab, Z. Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles. J. Mol. Liq. 2021, 321, 114455. [Google Scholar] [CrossRef]
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species-sources, functions, oxidative damage. Pol. Merkur. Lek. Organ. Pol. Tow. Lek. 2020, 48, 124–127. [Google Scholar]
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. Trends Anal. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Liu, X. Detection of heavy metal ions by ratiometric photoelectric sensor. J. Agric. Food Chem. 2020, 70, 11468–11480. [Google Scholar] [CrossRef] [PubMed]
- Adnan, N.A.; Halmi, M.I.E.; Abd Gani, S.S.; Zaidan, U.H.; Abd Shukor, M.Y. Comparison of joint effect of acute and chronic toxicity for combined assessment of heavy metals on Photobacterium sp. NAA-MIE. Int. J. Environ. Res. Public Health 2021, 18, 6644. [Google Scholar] [CrossRef]
- Derrick, A.; Yohana, M.A.; Yudong, Z.; Gongyu, L.; Tan, B.; Zhang, S. Understanding the detrimental effects of heavy metal pollution in shrimp farming and treatment methods–a review. Ann. Anim. Sci. 2025, 25, 35–56. [Google Scholar] [CrossRef]
- Samanta, P.; Im, H.; Na, J.; Jung, J. Ecological risk assessment of a contaminated stream using multi-level integrated biomarker response in Carassius auratus. Environ. Pollut. 2018, 233, 429–438. [Google Scholar] [CrossRef]
- McCarthy, J.F.; Shugart, L.R. Biomarkers of Environmental Contamination. Lewis Publishers: Boca Raton, FL, USA, 1990; pp. 3–16. [Google Scholar]
- McCarty, L.S.; Power, M.; Munkittrick, K.R. Bioindicators versus biomarkers in ecological risk assessment. Hum. Ecol. Risk Assess. 2002, 8, 159–164. [Google Scholar] [CrossRef]
- Quina, A.S.; Durão, A.F.; Muñoz-Muñoz, F.; Ventura, J.; da Luz Mathias, M. Population effects of heavy metal pollution in wild Algerian mice (Mus spretus). Ecotoxicol. Environ. Saf. 2019, 171, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Swaleh, S.B.; Banday, U.Z.; Asadi, M.A.; Usmani, N. Biochemical profile and gene expression of Clarias gariepinus as a signature of heavy metal stress. Environ. Pollut. 2020, 264, 114693. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.G.; Wanjari, U.R.; Renu, K.; Vellingiri, B.; Gopalakrishnan, A.V. Heavy metal and metalloid-induced reproductive toxicity. Environ. Toxicol. Pharmacol. 2022, 92, 103859. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, J.; Cheng, Z.; Zhang, K.; Gu, H.; Feng, J.; Liu, Y. Excessive heavy metal enrichment disturbs liver functions through the gut microbe in the great Himalayan leaf-nosed bat (Hipposideros armiger). Ecotoxicol. Environ. Saf. 2024, 282, 116758. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, N.; Wang, H. Liquid chromatography-mass spectrometry for analysis of DNA damages induced by environmental exposure. Trends Anal. Chem. 2019, 120, 115645. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, Z.; Xiao, K.; Zeng, L.; Wang, J.; Gabrielsen, G.W. Antarctic Adélie penguin feathers as bio-indicators of geographic and temporal variations in heavy metal concentrations in their habitats. Ecotoxicol. Environ. Saf. 2020, 206, 111135. [Google Scholar] [CrossRef]
- Fatima, R.A.; Ahmad, M. Certain antioxidant enzymes of Allium cepa as biomarkers for the detection of toxic heavy metals in wastewater. Sci. Total Environ. 2005, 346, 256–273. [Google Scholar] [CrossRef]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Ghemari, C.; Waterlot, C.; Ayari, A.; Douay, F.; Nasri-Ammar, K. Bioaccumulation of heavy metals in the terrestrial isopod Porcellionides pruinosus in the vicinity of Gabes-Ghannouch industrial complex. Hum. Ecol. Risk Assess. 2020, 26, 1270–1284. [Google Scholar] [CrossRef]
- Ugbaja, R.N.; Enilolobo, M.A.; James, A.S.; Akinhanmi, T.F.; Akamo, A.J.; Babayemi, D.O.; Ademuyiwa, O. Bioaccumulation of heavy metals, lipid profiles, and antioxidant status of snails (Achatina achatina) around cement factory vicinities. Toxicol. Ind. Health 2020, 36, 863–875. [Google Scholar] [CrossRef]
- Chemicals, D. OECD Guideline for Testing of Chemicals; OECD: Paris, France, 2005; pp. 1–13. [Google Scholar]
- Zhu, L.; Li, B.; Wu, R.; Li, W.; Wang, J.; Zhu, L. Acute toxicity, oxidative stress and DNA damage of chlorpyrifos to earthworms (Eisenia fetida): The difference between artificial and natural soils. Chemosphere 2020, 255, 126982. [Google Scholar] [CrossRef]
- Wu, Y.; Qi, L.; Wang, B.; Medley, P.; Drake, J.; Vernon, J.; Chen, G. Assess long-term As, Pb and Cr contamination and uptake by Eriocaulon decangulare in the Apalachicola National Forest. Sci. Total Environ. 2022, 838, 156040. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Yan, D. Chromium bioaccumulation and its impacts on plants: An overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Das, S.K.; Patra, J.K.; Thatoi, H. Antioxidative response to abiotic and biotic stresses in mangrove plants: A review. Int. Rev. Hydrobiol. 2016, 101, 3–19. [Google Scholar] [CrossRef]
- Xikeranmu, Z.; Abdunasir, M.; Ma, J.; Tusong, K.; Liu, X. Characterization of two copper/zinc superoxide dismutases (Cu/Zn-SODs) from the desert beetle Microdera punctipennis and their activities in protecting E. coli cells against cold. Cryobiology 2019, 87, 15–27. [Google Scholar] [CrossRef]
- Twala, P.P.; Mitema, A.; Baburam, C.; Feto, N.A. Breakthroughs in the discovery and use of different peroxidase isoforms of microbial origin. AIMS Microbiol. 2020, 6, 330. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ghorbani, A.; Li, Y.; Pehlivan, N.; Barker, J.; Ding, Y.; Zargar, M. Responsible mechanisms for the restriction of heavy metal toxicity in plants via the co-foliar spraying of nanoparticles. Agronomy 2023, 13, 1748. [Google Scholar] [CrossRef]
- Ning, Y.; Liu, L.; Rong, G.; Cao, X.; Li, J.; Su, Y.; Zhou, D. Study on the influential biochemical indices of Cd (II) on Eisenia fetida in oxidative stress by principal component analysis in the natural soil. Environ. Sci. Pollut. Res. Int. 2018, 25, 4268–4278. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, Z.; Shu, W.; Zhu, T. Evaluation of soil antimony stress on the biological health status of earthworm Eisenia andrei using Biomarker Response Index. J. Soils Sediments 2022, 22, 1999–2008. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Liu, Y.; Gao, X. Effects of sudden drop in salinity on osmotic pressure regulation and antioxidant defense mechanism of Scapharca subcrenata. Front. Physiol. 2020, 11, 884. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [PubMed]
- Prokić, M.D.; Radovanović, T.B.; Gavrić, J.P.; Faggio, C. Ecotoxicological effects of microplastics: Examination of biomarkers, current state and future perspectives. Trends Anal. Chem. 2019, 111, 37–46. [Google Scholar] [CrossRef]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. The key role of GSH in keeping the redox balance in mammalian cells: Mechanisms and significance of GSH in detoxification via formation of conjugates. Antioxidants 2023, 12, 1953. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- de Souza, I.D.; de Andrade, A.S.; Dalmolin, R.J.S. Lead-interacting proteins and their implication in lead poisoning. Crit. Rev. Toxicol. 2018, 48, 375–386. [Google Scholar] [CrossRef]
- Saricay, Y.; Wierenga, P.A.; de Vries, R. Changes in protein conformation and surface hydrophobicity upon peroxidase-catalyzed cross-linking of apo-α-lactalbumin. J. Agric. Food Chem. 2014, 62, 9345–9352. [Google Scholar] [CrossRef]
- Patricia, C.S.; Nerea, G.V.; Erik, U.; Elena, S.M.; Eider, B.; Manu, S. Responses to silver nanoparticles and silver nitrate in a battery of biomarkers measured in coelomocytes and in target tissues of Eisenia fetida earthworms. Ecotoxicol. Environ. Saf. 2017, 141, 57–63. [Google Scholar] [CrossRef]
- Šrut, M.; Drechsel, V.; Höckner, M. Low levels of Cd induce persisting epigenetic modifications and acclimation mechanisms in the earthworm Lumbricus terrestris. PLoS ONE 2017, 12, e0176047. [Google Scholar] [CrossRef]
- Li, N.; Xu, P.; Jing, W.X.; Hwang, J.S.; Wang, L. Toxic effects of Pb2+ entering sperm through Ca2+ channels in the freshwater crab Sinopotamon henanense. Aquat. Toxicol. 2017, 192, 24–29. [Google Scholar] [CrossRef]
- Gao, C.; Xu, J.; Li, J.; Liu, Z. Biological responses in the earthworm Eisenia fetida exposed to soils near a typical lead acid battery plant. Soil Sediment. Contam. 2016, 25, 573–585. [Google Scholar]
- Ahmadpour, M.; Wang, W.; Sinkakarimi, M.H.; Ahmadpour, M.; Hosseini, S.H. Joint toxicity of cadmium and fenpyroximate on two earthworms: Interspecific differences, subcellular partitioning and biomarker responses. Chemosphere 2023, 337, 139329. [Google Scholar] [CrossRef] [PubMed]
- Fisker, K.V.; Holmstrup, M.; Sørensen, J.G. Variation in metallothionein gene expression is associated with adaptation to copper in the earthworm Dendrobaena octaedra. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 157, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ling, S.; Zhan, X.; Lin, Z.; Zhang, W.; Lin, K. Interaction effects and mechanism of Pb pollution and soil microorganism in the presence of earthworm. Chemosphere 2017, 173, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Chen, Z.; Li, Y.; Ding, K.; Liu, W.; Liu, Y.; Qiu, R. Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China. J. Hazard. Mater. 2020, 400, 123289. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Irizar, A.; Rodríguez, M.P.; Izquierdo, A.; Cancio, I.; Marigómez, I.; Soto, M. Effects of soil organic matter content on cadmium toxicity in Eisenia fetida: Implications for the use of biomarkers and standard toxicity tests. Arch. Environ. Contam. Toxicol. 2015, 68, 181–192. [Google Scholar] [CrossRef]
- Hirano, T.; Tamae, K. Earthworms and soil pollutants. Sensors 2011, 11, 11157–11167. [Google Scholar] [CrossRef]
- Markad, V.L.; Gaupale, T.C.; Bhargava, S.; Kodam, K.M.; Ghole, V.S. Biomarker responses in the earthworm, Dichogaster curgensis exposed to fly ash polluted soils. Ecotoxicol. Environ. Saf. 2015, 118, 62–70. [Google Scholar] [CrossRef]
- Vranković, J.; Janković-Tomanić, M.; Vukov, T. Comparative assessment of biomarker response to tissue metal concentrations in urban populations of the land snail Helix pomatia (Pulmonata: Helicidae). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 245, 110448. [Google Scholar] [CrossRef]
- El-Shenawy, N.S.; Mohammadden, A.; Al-Fahmie, Z.H. Using the enzymatic and non-enzymatic antioxidant defense system of the land snail Eobania vermiculata as biomarkers of terrestrial heavy metal pollution. Ecotoxicol. Environ. Saf. 2012, 84, 347–354. [Google Scholar] [CrossRef]
- Panday, R.; Bhatt, P.S.; Bhattarai, T.; Shakya, K.; Sreerama, L. Aldehyde dehydrogenase expression in Metaphire posthuma as a bioindicator to monitor heavy metal pollution in soil. BMC Res. Notes 2016, 9, 491. [Google Scholar] [CrossRef]
- Dedeke, G.A.; Owagboriaye, F.O.; Adebambo, A.O.; Ademolu, K.O. Earthworm metallothionein production as biomarker of heavy metal pollution in abattoir soil. Appl. Soil Ecol. 2016, 104, 42–47. [Google Scholar] [CrossRef]
- Hønsi, T.G.; Stubberud, H.E.; Andersen, S.; Stenersen, J. Lysosomal fragility in earthworms (Eisenia veneta) exposed to heavy metal contaminated soils from two abandoned pyrite ore mines in Southern Norway. Water Air Soil Pollut. 2003, 142, 27–37. [Google Scholar] [CrossRef]
- Calisi, A.; Zaccarelli, N.; Lionetto, M.G.; Schettino, T. Integrated biomarker analysis in the earthworm Lumbricus terrestris: Application to the monitoring of soil heavy metal pollution. Chemosphere 2013, 90, 2637–2644. [Google Scholar] [CrossRef]
- Jin, M.; Sun, Y.; Chai, B.; Wang, M.; Wu, Y.; Wu, W.; Fu, L. Combined toxicity of decabromodiphenyl ethane and Pb on earthworms (Eisenia fetida) based on multiple biomarker responses. J. Soils Sediments 2025, 25, 1897–1910. [Google Scholar] [CrossRef]
- Cao, X.; Bi, R.; Song, Y. Toxic responses of cytochrome P450 sub-enzyme activities to heavy metals exposure in soil and correlation with their bioaccumulation in Eisenia fetida. Ecotoxicol. Environ. Saf. 2017, 144, 158–165. [Google Scholar] [CrossRef]
- Markad, V.L.; Kodam, K.M.; Ghole, V.S. Effect of fly ash on biochemical responses and DNA damage in earthworm, Dichogaster curgensis. Hazard. Mater. 2012, 215, 191–198. [Google Scholar] [CrossRef]
- Larba, R.; Soltani, N. Use of the land snail Helix aspersa for monitoring heavy metal soil contamination in Northeast Algeria. Environ. Monit. Assess. 2014, 186, 4987–4995. [Google Scholar] [CrossRef]
- Bennour, A.; Habes, D.; Soltani, N. Assessment of soil quality in Annaba Area (Northeast Algeria) using the earthworm Lumbricus terrestris: Bioindicative stress responses and heavy metal contamination. Fresen Environ. Bull. 2020, 29, 9635–9643. [Google Scholar]
- Butt, A.; Aziz, N. Bioaccumulation of heavy metals mixture and its effect on detoxification enzymes of wolf spider, Pardosa oakleyi. J. Anim. Plant Sci. 2016, 26, 1507–1515. [Google Scholar]
- Jiang, Y.; Chen, J.; Wu, Y.; Wang, Q.; Li, H. Sublethal toxicity endpoints of heavy metals to the nematode Caenorhabditis elegans. PLoS ONE 2016, 11, e0148014. [Google Scholar] [CrossRef]
- Song, S.; Han, Y.; Zhang, Y.; Ma, H.; Zhang, L.; Huo, J.; Gao, M. Protective role of citric acid against oxidative stress induced by heavy metals in Caenorhabditis elegans. Environ. Sci. Pollut. Res. 2019, 26, 36820–36831. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
