Dynamic Effects of Exogenous and Epiphytic Pediococcus pentosaceus on Quality and Bacterial Community Succession of Silage Mulberry Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Growth Ability and Acid Production Capacity
2.2. Silage Preparation
2.3. Analyses of Chemical Composition
2.4. Analyses of Fermentation Quality
2.5. Sequencing-Based Bacterial Community Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Ability and Acid Production Capacity of Epiphytic and Exogenous P. pentosaceus
3.2. Chemical Compositions of Silage Mulberry Leaves
3.3. Fermentation Quality of Silage Mulberry Leaves
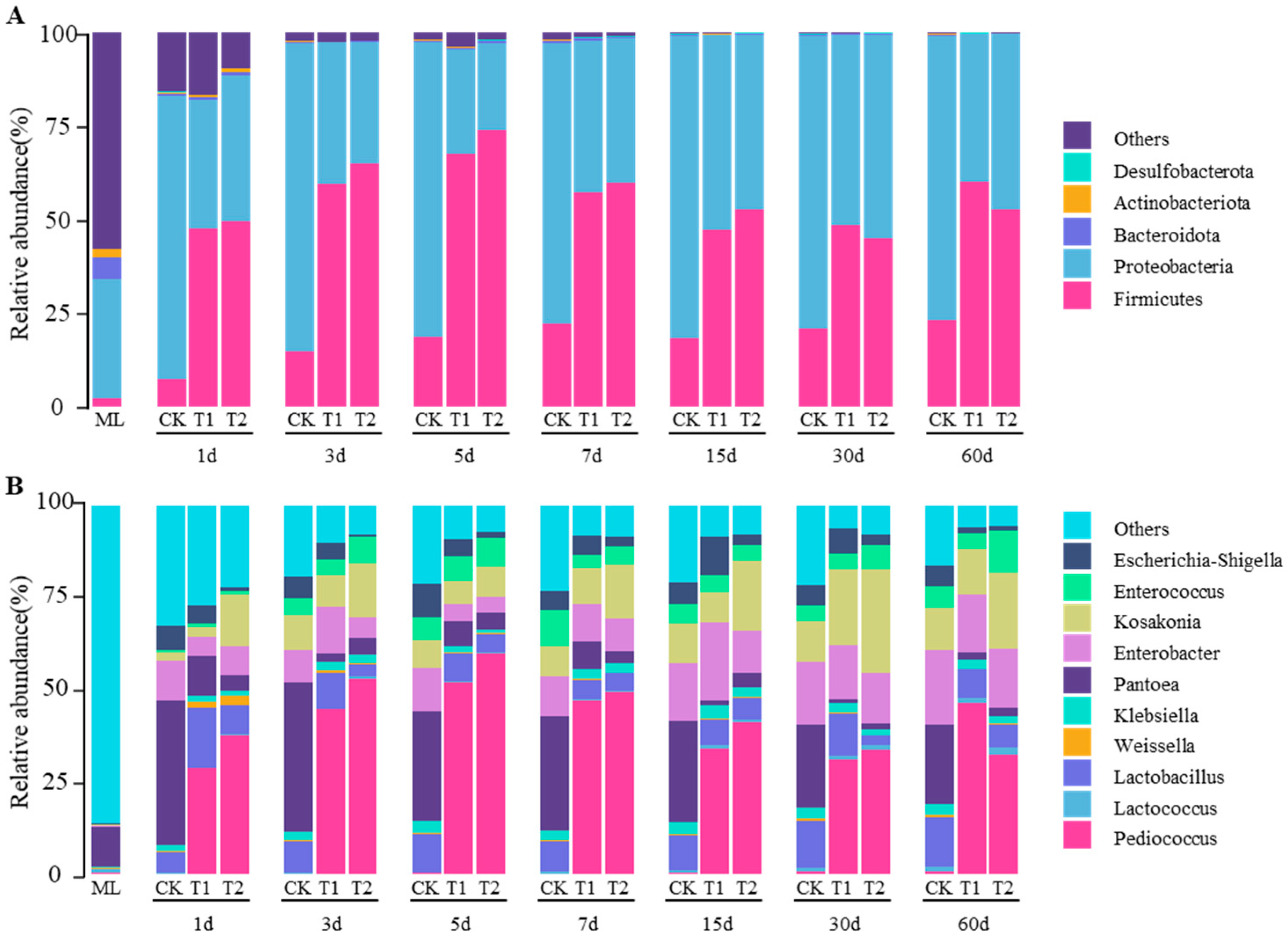
3.4. Effects of P. pentosaceus from Different Sources on the Bacterial Community of Silage Mulberry Leaves
3.5. Redundancy Analysis Between Fermentation Characteristics and Microbial Community
4. Discussion
4.1. The Influence of Different Sources of P. pentosaceus on the Chemical Composition of Silage Mulberry Leaves
4.2. Effect of Different Sources of P. pentosaceus on the Fermentation Quality of Mulberry Leaves
4.3. Bacterial Diversity of Fermentated Mulberry Leaves with P. pentosaceus from Different Sources
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A
| Items | Estimated Value |
|---|---|
| Dry matter (% FM) | 33.32 ± 0.14 |
| Crude protein (% DM) | 17.43 ± 0.01 |
| Neutral detergent fiber (% DM) | 27.76 ± 0.02 |
| Acid detergent fiber (% DM) | 16.87 ± 0.02 |
| Water-soluble carbohydrates (% DM) | 12.15 ± 0.04 |
| NH3-N (g/kg TN) | 4.00 ± 0.01 |
References
- Du, Z.; Yamasaki, S.; Oya, T.; Cai, Y. Cellulase-lactic acid bacteria synergy action regulates silage fermentation of woody plant. Biotechnol. Biofuels Bioprod. 2023, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kapoor, R.; Thathola, A.; Srivastava, R.P. Nutritional quality of leaves of some genotypes of mulberry (Morus alba). Int. J. Food Sci. Nutr. 2006, 57, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Luo, H. Effects of mulberry leaf silage on antioxidant and immunomodulatory activity and rumen bacterial community of lambs. BMC Microbiol. 2021, 21, 250. [Google Scholar] [CrossRef]
- Ma, G.; Chai, X.; Hou, G.; Zhao, F.; Meng, Q. Phytochemistry, bioactivities and future prospects of mulberry leaves: A review. Food Chem. 2022, 372, 131335. [Google Scholar] [CrossRef]
- Venkataramanan, R.; Sreekumar, C.; Anilkumar, R.; Selvaraj, P.; Vidhya, N.M.; Mathagowder, I. Effect of jaggery on the quality and intake levels of maize silage. Trop. Anim. Health Prod. 2010, 42, 1027–1029. [Google Scholar] [CrossRef]
- Deng, M.; Chi, Z.; Ling, D.; Yang, Z.; Yu, G.; Yu, G.; Xu, X.; Guo, Y.; Liu, D. Effects of fermented Broussonetia papyrifera feed on growth performance, nutrient apparent digestibility, serum biochemical indexes of weaned piglets. Chin. J. Anim. Nutr. 2021, 33, 165–174. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Li, X.; MacAdam, J.W.; Zhang, Y. Interaction between plants and epiphytic lactic acid bacteria that affect plant silage fermentation. Front. Microbiol. 2023, 14, 1164904. [Google Scholar] [CrossRef]
- Jiang, S.; Cai, L.; Lv, L.; Li, L. Pediococcus pentosaceus, a future additive or probiotic candidate. Microb. Cell Factories 2021, 20, 45. [Google Scholar] [CrossRef]
- Vadopalas, L.; Ruzauskas, M.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bartkevics, V.; Pugajeva, I.; Reinolds, I.; Badaras, S.; et al. Combination of antimicrobial starters for feed fermentation: Influence on piglet feces microbiota and health and growth performance, including mycotoxin biotransformation in vivo. Front. Vet. Sci. 2020, 7, 528990. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Kim, D.; Soundharrajan, I.; Park, H.S.; Jung, J.S.; Yang, S.H.; Choi, K.C. Low-carbohydrate tolerant LAB strains identified from rumen fluid: Investigation of probiotic activity and legume silage fermentation. Microorganisms 2020, 8, 1044. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.M.; Ke, W.C.; Zhang, P.; Li, F.H.; Guo, X.S. Characteristics of Pediococcus pentosaceus Q6 isolated from Elymus nutans growing on the Tibetan Plateau and its application for silage preparation at low temperature. J. Appl. Microbiol. 2019, 126, 40–48. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; et al. Safety and efficacy of a feed additive consisting of Pediococcus pentosaceus DSM 32292 for all animal species (Marigot Ltd t/a Celtic Sea Minerals). EFSA J. 2022, 20, e07426. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; et al. Safety and efficacy of a feed additive consisting of Pediococcus pentosaceus IMI 507025 for all animal species (ALL-TECHNOLOGY (IRELAND) LIMITED [Alltech Ireland]). EFSA J. 2021, 19, e06702. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Dusemund, B.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Assessment of the application for renewal of the authorisation of Pediococcus pentosaceus DSM 16244 as a feed additive for all animal species. EFSA J. 2020, 18, e06166. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Li, M.; Fan, X.; Chen, Y.; Sun, H.; Xie, Y.; Zheng, Y.; Chen, C.; Li, P. Effects of epiphytic and exogenous lactic acid bacteria on fermentation quality and microbial community compositions of paper mulberry silage. Front. Microbiol. 2022, 13, 973500. [Google Scholar] [CrossRef]
- Liu, C.N. Comparative Analysis of Different Sources of Lactobacillus plantarum Genome and Carbohydrate Utilizatio. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2021. [Google Scholar]
- Fugaban, J.I.I.; Vazquez Bucheli, J.E.; Park, Y.J.; Suh, D.H.; Jung, E.S.; Franco, B.D.G.M.; Ivanova, I.V.; Holzapfel, W.H.; Todorov, S.D. Antimicrobial properties of Pediococcus acidilactici and Pediococcus pentosaceus isolated from silage. J. Appl. Microbiol. 2022, 132, 311–330. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, G.; Zhang, Z.; Tang, Y.; Zhang, Y.; Chen, L. Bacillus velezensis promotes the proliferation of lactic acid bacteria and influences the fermentation quality of whole-plant corn silage. Front. Plant Sci. 2024, 15, 1285582. [Google Scholar] [CrossRef]
- Sun, W.T.; Huang, Y.; Wu, C.R.; Peng, C.; Zheng, Y.L.; Chen, C.; Hao, J. Addition of lactic acid bacteria can promote the quality and feeding value of Broussonetia papyrifera (Paper Mulberry) Silage. Fermentation 2022, 8, 25. [Google Scholar] [CrossRef]
- Berbert Queiroz, D.M.; Melo, E.C. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Rockville, MA, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Murphy, R.P. A method for the extraction of plant samples and the determination of total soluble carbohydrates. J. Sci. Food Agr. 1958, 9, 714–717. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Wei, R.; Ding, Y.; Gao, F.; Zhang, L.; Wang, L.; Li, H.; Wang, H. Community succession of the grape epidermis microbes of cabernet sauvignon (Vitis vinifera L.) from different regions in China during fruit development. Int. J. Food Microbiol. 2022, 362, 109475. [Google Scholar] [CrossRef]
- Jia, H.; Huang, L.; Liu, L.; Sun, R.; Zhang, Z.; Chen, G. Comparative study on the quality of rice straw silage of different varieties. Acta Agrestia Sin. 2022, 30, 1310–1318. [Google Scholar] [CrossRef]
- Su, R.; Ke, W.; Usman, S.; Bai, J.; Akhavan Kharazian, Z.; Guo, X. Dry matter content and inoculant alter the metabolome and bacterial community of alfalfa ensiled at high temperature. Appl. Microbiol. Biotechnol. 2023, 107, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dai, S.; Liang, L.; Sun, W.; Peng, C.; Chen, C.; Hao, J. Effects of different additives on fermentation quality and microbial diversity of paper mulberry silage. Chin. J. Anim. Nutr. 2021, 33, 1607–1617. [Google Scholar] [CrossRef]
- Li, Y.; Du, S.; Sun, L.; Cheng, Q.; Hao, J.; Lu, Q.; Ge, G.; Wang, Z.; Jia, Y. Effects of lactic acid bacteria and molasses additives on dynamic fermentation quality and microbial community of native grass silage. Front. Microbiol. 2022, 13, 830121. [Google Scholar] [CrossRef] [PubMed]
- Nuryana, I.; Andriani, A.; Lisdiyanti, P.; Yopi, Y. Analysis of organic acids produced by lactic acid bacteria. IOP Conf. Ser. Earth Environ. Sci. 2019, 251, 012054. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Zhao, J.; Dong, Z.; Dong, D.; Shao, T. Dynamics of the bacterial communities and predicted functional profiles in wilted alfalfa silage. J. Appl. Microbiol. 2022, 132, 2613–2624. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Zhu, M.; Wang, Y.; Zhou, T.; Wan, F.; Zhang, Y.; Chen, L. Isolation of Bacillus velezensis from silage and its effect on aerobic stability and in vitro methane production of whole-plant corn silage. Agriculture 2024, 14, 830. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Cai, X.; Zhang, Y. Isolation of lactobacillus producing conjugated linoleic acid in Qinghai area and its effect on quality of whole corn silage. Pratacultural Sci. 2019, 36, 243–251. [Google Scholar] [CrossRef]
- Yahaya, M.S.; Kawai, M.; Takahashi, J.; Matsuoka, S. The effects of different moisture content and ensiling time on silo degradation of structural carbohydrate of orchardgrass. Asian Australas. J. Anim. Sci. 2002, 101, 127–133. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.; Tao, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef]
- Kung, L.J.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Ding, Z.; Ke, W.; Xu, D.; Wang, M.; Huang, W.; Zhang, Y.; Liu, F.; Guo, X. Different lactic acid bacteria and their combinations regulated the fermentation process of ensiled alfalfa: Ensiling characteristics, dynamics of bacterial community and their functional shifts. Microb. Biotechnol. 2021, 14, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wu, H.; Li, L.; He, J.; Hu, Z.; Yang, X.; Xie, X. Effects of applying cellulase and starch on the fermentation characteristics and microbial communities of Napier grass (Pennisetum purpureum Schum.) silage. J. Anim. Sci. Technol. 2021, 63, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.J.; Dong, Z.H.; Li, J.F.; Shao, T. Microbial community dynamics and their contributions to organic acid production during the early stage of the ensiling of Napier grass (Pennisetum purpureum). Grass Forage Sci. 2020, 75, 37–44. [Google Scholar] [CrossRef]
- Pereira, O.G.; Rocha, K.D.; Ferreira, C.L.L.F. Chemical composition, characterization, and population of microorganisms on elephantgrass “Cameroon” (Pennisetum purpureum, Schum) and its silages. Rev. Bras. Zootec. 2007, 36, 1742–1750. [Google Scholar] [CrossRef]
- Driehuis, F.; Wilkinson, J.M.; Jiang, Y.; Ogunade, I.; Adesogan, A.T. Silage review: Animal and human health risks from silage. J. Dairy Sci. 2018, 101, 4093–4110. [Google Scholar] [CrossRef]
- Jia, T.; Yun, Y.; Yu, Z. Propionic acid and sodium benzoate affected biogenic amine formation, microbial community, and quality of oat silage. Front. Microbiol. 2021, 12, 750920. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, G.; Ke, W.; Pan, Y.; Hou, M.; Chang, C.; Sa, D.; Lv, M.; Liu, Y.; Lu, Q. Effect of endogenous sodium and potassium ions in plants on the quality of alfalfa silage and bacterial community stability during fermentation. Front. Plant Sci. 2023, 14, 1295114. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, O.C.M.; Ogunade, I.M.; Weinberg, Z.; Adesogan, A.T. Silage review: Foodborne pathogens in silage and their mitigation by silage additives. J. Dairy Sci. 2018, 5, 4132–4142. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.G.; Cheng, H.J.; Liu, D.; Wei, C.; An, W.J.; Wang, Y.F.; Sun, H.T.; Song, E.L. Treatment of whole-plant corn silage with lactic acid bacteria and organic acid enhances quality by elevating acid content, reducing pH, and inhibiting undesirable microorganisms. Front. Microbiol. 2020, 11, 593088. [Google Scholar] [CrossRef]
- Xu, D.; Ding, W.; Ke, W.; Li, F.; Zhang, P.; Guo, X. Modulation of metabolome and bacterial community in whole crop corn silage by inoculating homofermentative Lactobacillus plantarum and heterofermentative Lactobacillus buchneri. Front. Microbiol. 2019, 9, 3299. [Google Scholar] [CrossRef]
- He, L.; Chen, N.; Lv, H.; Wang, C.; Zhou, W.; Chen, X.; Zhang, Q. Gallic acid influencing fermentation quality, nitrogen distribution and bacterial community of high-moisture mulberry leaves and stylo silage. Bioresour. Technol. 2020, 295, 122255. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, P.; Zou, X.; Chen, X.; Zhang, Q. Influence of pyroligneous acid on fermentation parameters, CO2 production and bacterial communities of rice straw and stylo silage. Front. Microbiol. 2021, 12, 701434. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Na, N.; Li, X.; Li, Z.; Wang, C.; Wu, X.; Xiao, Y.; Yin, G.; Liu, S.; Liu, Z.; et al. Impact of packing density on the bacterial community, fermentation, and in vitro digestibility of whole-crop barley silage. Agriculture 2021, 11, 672. [Google Scholar] [CrossRef]
- Xiong, Y.; Xu, J.; Guo, L.; Chen, F.; Jiang, D.; Lin, Y.; Guo, C.; Li, X.; Chen, Y.; Ni, K.; et al. Exploring the Effects of different bacteria additives on fermentation quality, microbial community and in vitro gas production of forage oat silage. Animals 2022, 12, 1122. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, X.; Zheng, M.; Chen, D.; Chen, X. Altering microbial communities: A possible way of lactic acid bacteria inoculants changing smell of silage. Anim. Feed. Sci. Technol. 2021, 279, 114998. [Google Scholar] [CrossRef]
- Biswas, I.; Mohapatra, P.D. Paraburkholderia tropica PKI7 and Kosakonia arachidis PKI8: Two newly reported tannase producing bacteria isolated from forest soil and study of their tannase producing potentiality. Not. Sci. Biol. 2023, 15, 11379. [Google Scholar] [CrossRef]
- Maqsood, M.; Anam Saeed, R.; Sahar, A.; Khan, M.I. Mulberry plant as a source of functional food with therapeutic and nutritional applications: A review. J. Food Biochem. 2022, 46, e14263. [Google Scholar] [CrossRef] [PubMed]




| Index | Treatment | Ensiling Days | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 15 | 30 | 60 | ||
| DM (% FM) | CK | 32.55 ± 0.08 a | 32.38 ± 0.26 a | 31.49 ± 0.01 Bb | 30.81 ± 0.06 Bc | 30.49 ± 0.18 Bd | 29.91 ± 0.56 Cd | 29.09 ± 0.12 Ce |
| T1 | 32.83 ± 0.16 a | 32.51 ± 0.23 a | 32.18 ± 0.08 Ab | 31.57 ± 0.12 Ac | 31.05 ± 0.25 Ad | 30.79 ± 0.04 Bde | 30.63 ± 0.09 Be | |
| T2 | 32.77 ± 0.03 a | 32.54 ± 0.11 ab | 32.34 ± 0.26 Ab | 31.68 ± 0.09 Ac | 31.33 ± 0.1 Acd | 31.02 ± 0.13 Ad | 31.05 ± 0.28 Ad | |
| CP (% DM) | CK | 16.98 ± 0.28 a | 16.56 ± 0.13 Ba | 15.93 ± 0.14 Cb | 15.60 ± 0.11 Cbc | 15.23 ± 0.16 Ccd | 15.06 ± 0.16 Cd | 14.48 ± 0.17 Ce |
| T1 | 17.04 ± 0.22 a | 16.62 ± 0.14 Bb | 16.30 ± 0.13 Bbc | 16.13 ± 0.10 Bc | 15.73 ± 0.11 Bd | 15.52 ± 0.10 Bd | 15.49 ± 0.22 Bd | |
| T2 | 17.27 ± 0.14 a | 17.06 ± 0.15 Aab | 16.77 ± 0.19 Abc | 16.53 ± 0.14 Ac | 16.11 ± 0.17 Acd | 16.11 ± 0.17 Ad | 16.17 ± 0.13 Ad | |
| NDF (% DM) | CK | 24.29 ± 0.37 a | 23.65 ± 0.35 Ab | 22.67 ± 0.46 Ac | 21.98 ± 0.20 Acd | 21.66 ± 0.43 Ad | 21.56 ± 0.11 Ad | 21.41 ± 0.12 Ad |
| T1 | 24.11 ± 0.36 a | 23.13 ± 0.36 Ab | 21.90 ± 0.35 Ac | 21.28 ± 0.33 Bcd | 20.77 ± 0.27 Bd | 20.56 ± 0.20 Bd | 20.57 ± 0.21 Bd | |
| T2 | 23.67 ± 0.31 a | 22.37 ± 0.22 Bb | 21.14 ± 0.29 Bcd | 20.58 ± 0.41 Cd | 19.87 ± 0.28 Cd | 19.72 ± 0.34 Cde | 19.61 ± 0.16 Ce | |
| ADF (% DM) | CK | 16.54 ± 0.17 a | 16.21 ± 0.16 ab | 15.81 ± 0.15 Ab | 15.66 ± 0.19 Abc | 15.35 ± 0.25 Acd | 15.13 ± 0.17 Ad | 15.07 ± 0.25 Ad |
| T1 | 16.52 ± 0.32 a | 16.13 ± 0.20 ab | 15.43 ± 0.21 Bb | 15.10 ± 0.25 Bb | 14.71 ± 0.25 Bc | 14.41 ± 0.25 Bc | 14.26 ± 0.15 Bc | |
| T2 | 16.50 ± 0.24 a | 15.93 ± 0.20 b | 15.17 ± 0.18 Bc | 14.85 ± 0.20 Bcd | 14.64 ± 0.31 Bd | 14.44 ± 0.29 Bd | 14.33 ± 0.33 Bd | |
| WSCs (% DM) | CK | 11.23 ± 0.29 Aa | 10.43 ± 0.18 Ab | 9.95 ± 0.10 Ac | 9.29 ± 0.19 Ad | 8.58 ± 0.28 Ae | 7.22 ± 0.13 Af | 6.71 ± 0.22 Ag |
| T1 | 10.64 ± 0.37 Ba | 9.12 ± 0.26 Bb | 7.28 ± 0.12 Bc | 6.39 ± 0.13 Bd | 5.88 ± 0.12 Be | 5.64 ± 0.13 Be | 5.57 ± 0.13 Be | |
| T2 | 10.34 ± 0.26 Ba | 8.76 ± 0.15 Bb | 6.87 ± 0.20 Cc | 5.68 ± 0.15 Cd | 5.39 ± 0.14 Cde | 5.20 ± 0.15 Ce | 5.11 ± 0.11 Ce | |
| Item | Treatment | Ensiling Days | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 15 | 30 | 60 | ||
| LA (g/kg DM) | CK | 9.37 ± 1.05 Cc | 9.9 ± 0.29 Cc | 8.56 ± 0.33 Cc | 9.45 ± 1.01 Cc | 13.41 ± 1.33 Bb | 15.33 ± 0.93 Cb | 18.78 ± 0.96 Ba |
| T1 | 22.06 ± 1.44 Bf | 27.73 ± 1.13 Be | 32.7 ± 0.61 Bd | 35.48 ± 0.48 Bc | 41.33 ± 1.28 Ab | 44.85 ± 0.66 Aa | 45.05 ± 1.89 Aa | |
| T2 | 24.92 ± 0.96 Af | 33.67 ± 0.97 Ae | 36.58 ± 0.35 Ad | 41.2 ± 0.73 Ac | 43.53 ± 0.59 Ac | 46.52 ± 0.65 Ab | 47.63 ± 0.75 Aa | |
| AA (g/kg DM) | CK | 3.16 ± 0.14 Be | 3.4 ± 0.16 Ce | 5.55 ± 0.01 Cd | 10.31 ± 0.40 Cc | 17.2 ± 1.05 Cb | 19.66 ± 0.83 Ca | 20.08 ± 0.89 Ca |
| T1 | 12.28 ± 0.86 Ae | 16.41 ± 0.38 Ac | 15.91 ± 0.76 Ac | 23 ± 0.77 Ab | 23.76 ± 1.23 Ab | 26.05 ± 0.37 Aa | 26.56 ± 0.41 Aa | |
| T2 | 11.99 ± 1.64 Ae | 15.02 ± 0.47 Bd | 13.5 ± 0.55 Bd | 18.92 ± 0.28 Bc | 22.06 ± 0.74 Bb | 23.81 ± 0.77 Ba | 25.35 ± 0.45 Ba | |
| PA (g/kg DM) | CK | ND | ND | ND | 0.53 ± 0.02 c | 0.91 ± 0.05 b | 1.14 ± 0.07 a | 1.18 ± 0.03 a |
| T1 | ND | ND | ND | ND | ND | ND | ND | |
| T2 | ND | ND | ND | ND | ND | ND | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Shu, G.; Zhu, Z.; Li, Y.; Fang, Z.; Chen, L.; Wan, F.; Zhang, Y.; Xiao, D.; Chen, L. Dynamic Effects of Exogenous and Epiphytic Pediococcus pentosaceus on Quality and Bacterial Community Succession of Silage Mulberry Leaves. Agriculture 2025, 15, 1726. https://doi.org/10.3390/agriculture15161726
Zhang C, Shu G, Zhu Z, Li Y, Fang Z, Chen L, Wan F, Zhang Y, Xiao D, Chen L. Dynamic Effects of Exogenous and Epiphytic Pediococcus pentosaceus on Quality and Bacterial Community Succession of Silage Mulberry Leaves. Agriculture. 2025; 15(16):1726. https://doi.org/10.3390/agriculture15161726
Chicago/Turabian StyleZhang, Chen, Gangqin Shu, Zhigang Zhu, Yusen Li, Zhenyu Fang, Liyuan Chen, Fachun Wan, Yunhua Zhang, Dingfu Xiao, and Lijuan Chen. 2025. "Dynamic Effects of Exogenous and Epiphytic Pediococcus pentosaceus on Quality and Bacterial Community Succession of Silage Mulberry Leaves" Agriculture 15, no. 16: 1726. https://doi.org/10.3390/agriculture15161726
APA StyleZhang, C., Shu, G., Zhu, Z., Li, Y., Fang, Z., Chen, L., Wan, F., Zhang, Y., Xiao, D., & Chen, L. (2025). Dynamic Effects of Exogenous and Epiphytic Pediococcus pentosaceus on Quality and Bacterial Community Succession of Silage Mulberry Leaves. Agriculture, 15(16), 1726. https://doi.org/10.3390/agriculture15161726





