Application of Non-Destructive Technology in Plant Disease Detection: Review
Abstract
1. Introduction
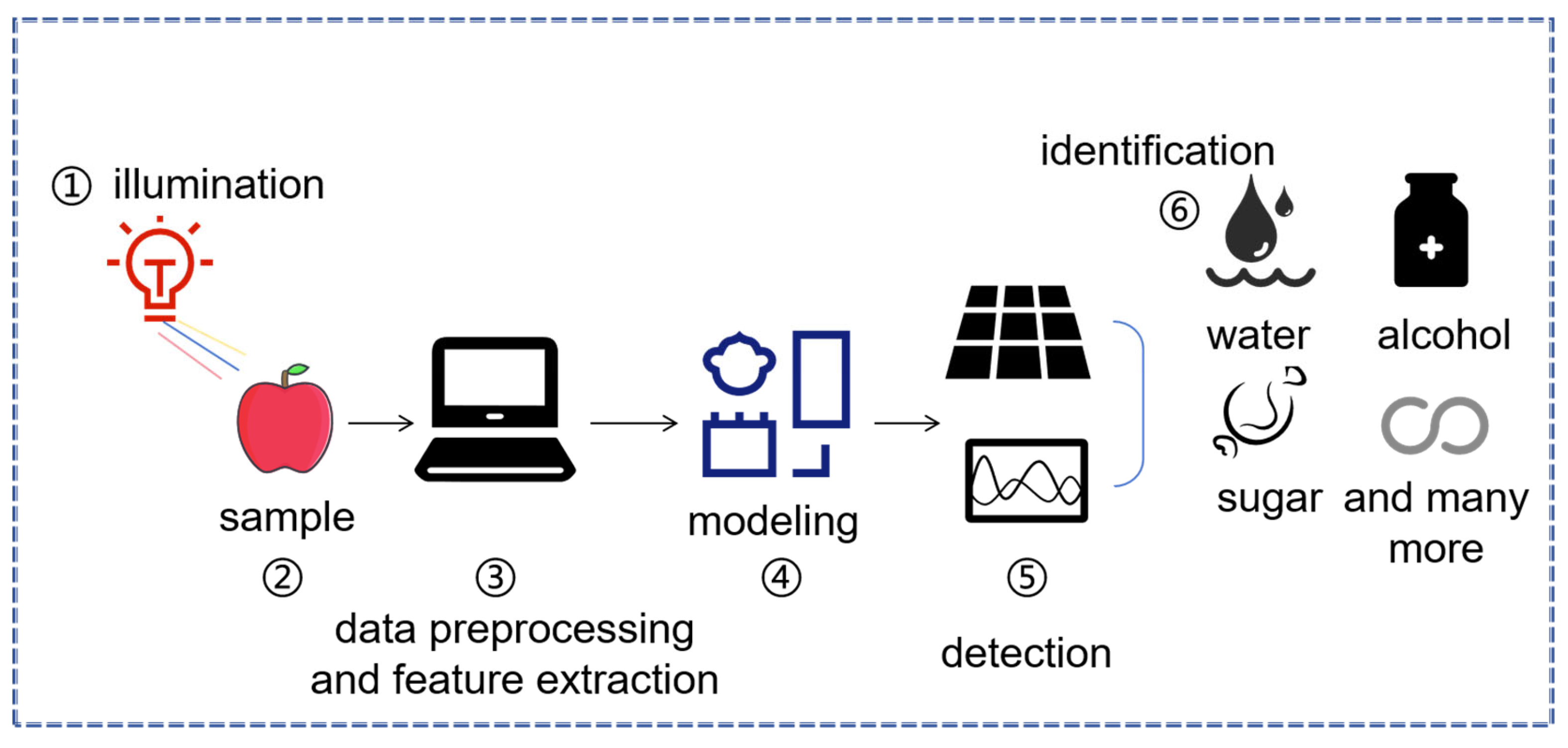
2. The Application of Spectroscopic Technology in Plant Disease Detection
2.1. Near-Infrared Spectroscopy
2.2. Raman Spectroscopy
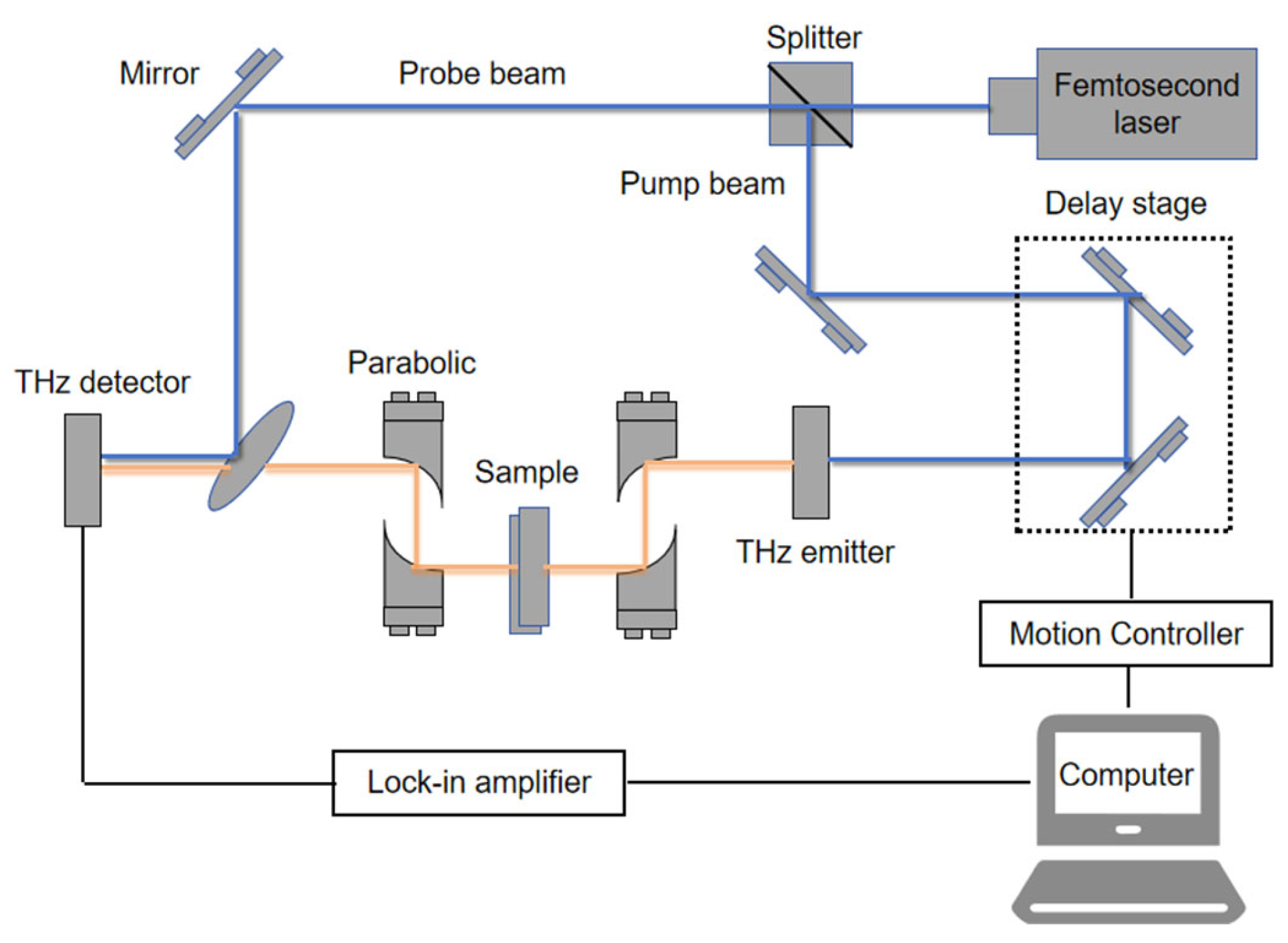
2.3. Terahertz Spectroscopy
3. The Use of Imaging Technology in Plant Disease Detection
3.1. Hyperspectral Imaging
3.2. Digital Imaging
3.3. Thermal Imaging
4. The Future Development Direction of Non-Destructive Detection Technology in Plant Disease
4.1. Limitations of Existing Non-Destructive Detection Technology
4.2. Future Development Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, L.; Li, H.; Li, C.; Wang, A.; Yang, Y.; Wang, P. Hyperspectral sensing of plant diseases: Principle and methods. Agronomy 2022, 12, 1451. [Google Scholar] [CrossRef]
- Zuo, X.; Chu, J.; Shen, J.; Sun, J. Multi-granularity feature aggregation with self-attention and spatial reasoning for fine-grained crop disease classification. Agriculture 2022, 12, 1499. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Q.Y.; Zhao, Q.H.; Dhanasekaran, S.; Ahima, J.; Zhang, X.Y.; Zhou, S.Q.; Droby, S.; Zhang, H.Y. Aureobasidium pullulans S-2 reduced the disease incidence of tomato by influencing the postharvest microbiome during storage. Postharvest Biol. Technol. 2022, 185, 111809. [Google Scholar] [CrossRef]
- Andriani, A.; Meyliana, A.; Susanto, W.E. Certainty factors in expert system to diagnose disease of chili plants. In Proceedings of the 6th International Conference on Cyber and IT Service Management (CITSM), Parapat, Indonesia, 7–9 August 2018. [Google Scholar] [CrossRef]
- Dewanto, S.; Lukas, J. Expert system for diagnosis pest and disease in fruit plants. In Proceedings of the International Conference on Advances Science and Contemporary Engineering (ICASCE), Jakarta, Indonesia, 23–24 October 2013. [Google Scholar] [CrossRef]
- Schaad, N.W.; Frederick, R.D. Real-time PCR and its application for rapid plant disease diagnostics. Can. J. Plant Pathol. 2002, 24, 250–258. [Google Scholar] [CrossRef]
- Li, M.; Hong, X.; Qiu, X.C.; Yang, C.Q.; Mao, Y.H.; Li, Y.; Liu, Z.J.; Du, D.L. Ultrasensitive monitoring strategy of PCR-like levels for zearalenone contamination based DNA barcode. J. Sci. Food Agric. 2021, 101, 4490–4497. [Google Scholar] [CrossRef]
- Brady, C.R.; Noll, L.W.; Saleh, A.A.; Little, C.R. Disease severity and microsclerotium properties of the sorghum sooty stripe pathogen, Ramulispora sorghi. Plant Dis. 2011, 95, 853–859. [Google Scholar] [CrossRef][Green Version]
- Cagliari, D.; Dias, N.P.; Galdeano, D.M.; Dos Santos, E.A.; Smagghe, G.; Zotti, M.J. Management of pest insects and plant diseases by non-transformative RNAi. Front. Plant Sci. 2019, 10, 1319. [Google Scholar] [CrossRef]
- Kuo, Y.W.; Falk, B.W. RNA interference approaches for plant disease control. Biotechniques 2020, 69, 469–477. [Google Scholar] [CrossRef]
- Yu, P.; Ong, K.; Ueckert, J.; Gu, M.M. Biochar and Trichoderma reduce containerized poinsettia root rot caused by Pythium aphanidermatum. HortScience 2023, 58, 846–854. [Google Scholar] [CrossRef]
- Shi, J.; Hu, X.; Zou, X.; Zhao, J.; Zhang, W.; Holmes, M.; Huang, X.; Zhu, Y.; Li, Z.; Shen, T.; et al. A rapid and nondestructive method to determine the distribution map of protein, carbohydrate and sialic acid on edible bird’s nest by hyper-spectral imaging and chemometrics. Food Chem. 2017, 229, 235–241. [Google Scholar] [CrossRef]
- Huang, X.Y.; Lv, R.Q.; Wang, S.; Aheto, J.H.; Dai, C.X. Integration of computer vision and colorimetric sensor array for nondestructive detection of mango quality. J. Food Process Eng. 2018, 41, e12873. [Google Scholar] [CrossRef]
- Wu, X.; Liang, X.; Wang, Y.; Wu, B.; Sun, J. Non-destructive techniques for the analysis and evaluation of meat quality and safety: A review. Foods 2022, 11, 3713. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Sun, X.; Pan, W.X.; Kutsanedzie, F.; Zhao, J.W.; Chen, Q.S. Feasibility study on nondestructively sensing meat’s freshness using light scattering imaging technique. Meat Sci. 2016, 119, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.W.; Song, D.J.; Lee, K.H.; Kim, M.J.; Kim, M.S.; Kim, K.S.; Ae, C.M. Deep learning algorithm development for early detection of botrytis cinerea infected strawberry fruit using hyperspectral fluorescence imaging. Postharvest Biol. Technol. 2024, 214, 112918. [Google Scholar] [CrossRef]
- Weng, S.; Hu, X.; Wang, J.; Tang, L.; Li, P.; Zheng, S.; Zheng, L.; Huang, L.; Xin, Z. Advanced application of Raman spectroscopy and surface-enhanced Raman spectroscopy in plant disease diagnostics: A review. J. Agric. Food Chem. 2021, 69, 2950–2964. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, G.; Pan, Y.; Yang, X.; Chen, L.; Zhao, C. A review of advanced technologies and development for hyperspectral-based plant disease detection in the past three decades. Remote Sens. 2020, 12, 3188. [Google Scholar] [CrossRef]
- Horigane, T.; Yamada, S.; Kimura-Suda, H.; Nishio, E.; Kawahara, S.; Mckeon, T. The use of spectroscopic analysis in component profiling of cereals. Comp. Biochem. Phys. A 2005, 141, S281. [Google Scholar]
- Qi, H.; Luo, J.; Wu, X.; Zhang, C. Application of nondestructive techniques for peach (Prunus persica) quality inspection: A review. J. Food Sci. 2024, 89, 6863–6887. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Wu, B.; Sun, J. Classification of fritillaria using a portable near-infrared spectrometer and fuzzy generalized singular value decomposition. Ind. Crops Prod. 2024, 218, 119032. [Google Scholar] [CrossRef]
- Li, Q.; Wu, X.; Zheng, J.; Wu, B.; Jian, H.; Sun, C.; Tang, Y. Determination of pork meat storage time using near-infrared spectroscopy combined with fuzzy clustering algorithms. Foods 2022, 11, 2101. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, X.; He, C.; Wu, B.; Zhang, S.; Sun, J. Near-infrared spectroscopy combined with fuzzy improved direct linear discriminant analysis for nondestructive discrimination of Chrysanthemum tea varieties. Foods 2024, 13, 1439. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.M.; Huang, W.Q.; Peng, Y.K.; Chen, Q.S.; Ouyang, Q.; Zhao, J.W. Color compensation and comparison of shortwave near infrared and long wave near infrared spectroscopy for determination of soluble solids content of ‘Fuji’ apple. Postharvest Biol. Technol. 2016, 115, 81–90. [Google Scholar] [CrossRef]
- Li, Y.; Pan, T.; Li, H.; Chen, S. Non-invasive quality analysis of thawed tuna using near infrared spectroscopy with baseline correction. J. Food Process Eng. 2020, 43, e13445. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, H.; Wu, B.; Fu, H. Determination of apple varieties by near infrared reflectance spectroscopy coupled with improved possibilistic Gath–Geva clustering algorithm. J. Food Process. Preserv. 2020, 44, e14561. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, X.; Wu, B.; Zhou, H. Detection of apple varieties by near-infrared reflectance spectroscopy coupled with SPSO-PFCM. J. Food Process Eng. 2022, 45, e13993. [Google Scholar] [CrossRef]
- Arslan, M.; Zou, X.B.; Tahir, H.E.; Hu, X.T.; Rakha, A.; Zareef, M.; Seweh, E.A.; Basheer, S. Nir spectroscopy coupled chemometric algorithms for rapid antioxidants activity assessment of Chinese dates (Zizyphus jujuba Mill.). Int. J. Food Eng. 2019, 15, 20180148. [Google Scholar] [CrossRef]
- Tahir, H.E.; Zou, X.B.; Shi, J.Y.; Mariod, A.A.; Wiliam, T. Rapid determination of antioxidant compounds and antioxidant activity of Sudanese karkade (Hibiscus sabdariffa L.) using near infrared spectroscopy. Food Anal. Methods 2016, 9, 1228–1236. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Maja, J.M.; Ehsani, R. Visible-near infrared spectroscopy for detection of huanglongbing in citrus orchards. Comput. Electron. Agric. 2011, 77, 127–134. [Google Scholar] [CrossRef]
- Lucas Domingos Da Silva, A.; Alves Filho, E.G.; Silva, L.M.A.; Carlos Huertas Tavares, O.; Gervasio Pereira, M.; De Campos, T.; Manoel Da Silva, L. Near infrared spectroscopy to rapid assess the rubber tree clone and the influence of maturation and disease at the leaves. Microchem. J. 2021, 168, 106478. [Google Scholar] [CrossRef]
- Barthel, D.; Dordevic, N.; Fischnaller, S.; Kerschbamer, C.; Messner, M.; Eisenstecken, D.; Robatscher, P.; Janik, K. Detection of apple proliferation disease in Malus × domestica by near infrared reflectance analysis of leaves. Spectrochim. Acta Part. A 2021, 263, 120178. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, H.; Xie, K.; Jiang, H.; Xie, Q.; Sa, J. Pretreatment and combined method based on near infrared spectroscopy. Laser Optoelectron. Prog. 2021, 58, 1617001. [Google Scholar] [CrossRef]
- Fearer, C.J.; Conrad, A.O.; Marra, R.E.; Georskey, C.; Villari, C.; Slot, J.; Bonello, P. A combined approach for early in-field detection of beech leaf disease using near-infrared spectroscopy and machine learning. Front. For. Glob. Change 2022, 5, 934545. [Google Scholar] [CrossRef]
- Ghanei Ghooshkhaneh, N.; Golzarian, M.R.; Mollazade, K. VIS-NIR spectroscopy for detection of citrus core rot caused by Alternaria alternata. Food Control 2023, 144, 109320. [Google Scholar] [CrossRef]
- Sirakov, I.; Velichkova, K.; Dinev, T.; Slavcheva-Sirakova, D.; Valkova, E.; Yorgov, D.; Veleva, P.; Atanasov, V.; Atanassova, S. Detection of fungal diseases in lettuce by VIR-NIR spectroscopy in aquaponics. Microorganisms 2023, 11, 2348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, J.; Tian, Y.; Wu, X.H.; Dai, C.X.; Li, B. Spectral classification of lettuce cadmium stress based on information fusion and VISSA-GOA-SVM algorithm. J. Food Process Eng. 2019, 42, e13085. [Google Scholar] [CrossRef]
- Shen, L.F.; Jia, S.Q.; Guo, T.T.; Wu, W.J.; Yan, Y.L.; An, D. Study of feature extraction methods for maize’s near infrared spectra in biomimetic pattern recognition. Spectrosc. Spectr. Anal. 2012, 32, 939–943. [Google Scholar] [CrossRef]
- Seehanam, P.; Sonthiya, K.; Maniwara, P.; Theanjumpol, P.; Ruangwong, O.; Nakano, K.; Ohashi, S.; Kramchote, S.; Suwor, P. Ability of near infrared spectroscopy to detect anthracnose disease early in mango after harvest. Hortic. Environ. Biotechnol. 2024, 65, 581–591. [Google Scholar] [CrossRef]
- Fernandez-Cabanas, V.M.; Borrero, C.; Cozzolino, D.; Aviles, M. Feasibility of near infrared spectroscopy for estimating suppressiveness of carnation (Dianthus cariophyllus L.) fusarium wilt in different plant growth media. Spectrochim. Acta Part A 2022, 280, 121528. [Google Scholar] [CrossRef]
- Shi, J.Y.; Wang, Y.Y.; Li, Z.H.; Huang, X.W.; Shen, T.T.; Zou, X.B. Characterization of invisible symptoms caused by early phosphorus deficiency in cucumber plants using near-infrared hyperspectral imaging technology. Spectrochim. Acta A 2022, 267, 120540. [Google Scholar] [CrossRef]
- Li, M.D.; Chen, Q.W.; Huang, P.L.; Zhou, J.; Gong, W.K. A review of classification algorithms for data mining. In Proceedings of the 2nd International Conference on Mechanical, Electronic and Engineering Technology (MEET), Xi’an, China, 19–20 January 2019. [Google Scholar]
- Ong, P.; Jian, J.; Li, X.; Zou, C.; Yin, J.; Ma, G. New approach for sugarcane disease recognition through visible and near-infrared spectroscopy and a modified wavelength selection method using machine learning models. Spectrochim. Acta Part A 2023, 302, 123037. [Google Scholar] [CrossRef]
- Ying, L.; Kun, M.; Xinyu, Z.; Qifu, Y.; Jiaquan, W.; Shuangyan, Y. Identification of disease type of tobacco leaves based on near infrared spectroscopy and convolutional neural network. J. Braz. Chem. Soc. 2024, 35, e20230181. [Google Scholar] [CrossRef]
- Zahir, S.; Omar, A.F.; Jamlos, M.F.; Azmi, M.a.M.; Muncan, J. A review of visible and near-infrared (VIS-NIR) spectroscopy application in plant stress detection. Sens. Actuators A Phys. 2022, 338, 113468. [Google Scholar] [CrossRef]
- Mohd Hilmi Tan, M.I.S.; Jamlos, M.F.; Omar, A.F.; Dzaharudin, F.; Chalermwisutkul, S.; Akkaraekthalin, P. Ganoderma boninense disease detection by near-infrared spectroscopy classification: A review. Sensors 2021, 21, 3052. [Google Scholar] [CrossRef]
- Xiong, Y.; Mccarthy, C.; Humpal, J.; Percy, C. Near-infrared spectroscopy and deep neural networks for early common root rot detection in wheat from multi-season trials. Agron. J. 2024, 116, 2370–2390. [Google Scholar] [CrossRef]
- Hu, T.; Sun, Q.; Fernandes, F.a.N. Research progress on detection of apple watercore based on visible and near-infrared spectroscopy. J. Food Process. Preserv. 2025, 2025, 4394346. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Z.; Deng, J.; Ding, Z.; Chen, Q. Quantitative detection of heavy metal cd in vegetable oils: A nondestructive method based on Raman spectroscopy combined with chemometrics. J. Food Sci. 2024, 89, 8054–8065. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, F.; Wang, Y.; Hu, L.; Yang, N.; Mao, H. A method for capture and detection of crop airborne disease spores based on microfluidic chips and micro Raman spectroscopy. Foods 2022, 11, 3462. [Google Scholar] [CrossRef]
- Xue, Y.; Jiang, H. Monitoring of chlorpyrifos residues in corn oil based on Raman spectral deep-learning model. Foods 2023, 12, 2402. [Google Scholar] [CrossRef]
- Jiang, H.; He, Y.; Xu, W.; Chen, Q. Quantitative detection of acid value during edible oil storage by Raman spectroscopy: Comparison of the optimization effects of boss and VCPA algorithms on the characteristic Raman spectra of edible oils. Food Anal. Methods 2021, 14, 1826–1835. [Google Scholar] [CrossRef]
- Guo, Z.M.; Wang, M.M.; Wu, J.Z.; Tao, F.F.; Chen, Q.S.; Wang, Q.Y.; Ouyang, Q.; Shi, J.Y.; Zou, X.B. Quantitative assessment of zearalenone in maize using multivariate algorithms coupled to Raman spectroscopy. Food Chem. 2019, 286, 282–288. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, H.; Huang, H.; Tan, Z.; Hou, C.; Zhuang, J.; Tang, Y. Raman spectroscopy and its application in fruit quality detection. Agriculture 2025, 15, 195. [Google Scholar] [CrossRef]
- Wang, J.J.; Chen, Q.S.; Belwal, T.; Lin, X.Y.; Luo, Z.S. Insights into chemometric algorithms for quality attributes and hazards detection in foodstuffs using Raman/surface enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2476–2507. [Google Scholar] [CrossRef]
- Duan, N.; Chang, B.Y.; Zhang, H.; Wang, Z.P.; Wu, S.J. Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int. J. Food Microbiol. 2016, 218, 38–43. [Google Scholar] [CrossRef]
- Li, H.H.; Chen, Q.S.; Ouyang, Q.; Zhao, J.W. Fabricating a novel Raman spectroscopy-based aptasensor for rapidly sensing Salmonella typhimurium. Food Anal. Methods 2017, 10, 3032–3041. [Google Scholar] [CrossRef]
- Andersen, M.E.; Andersen, U.; Wiking, L.; Rasmussen, J.T.; Corredig, M.; Gregersen, S.B. The exploration of milk fat crystallization in milk fat globules by confocal Raman microscopy. Food Struct. 2024, 40, 100372. [Google Scholar] [CrossRef]
- Mosca, S.; Lin, Q.Q.; Stokes, R.; Bharucha, T.; Gangadharan, B.; Clarke, R.; Fernandez, L.G.; Deats, M.; Walsby-Tickle, J.; Arman, B.Y.; et al. Innovative method for rapid detection of falsified COVID-19 vaccines through unopened vials using handheld Spatially Offset Raman Spectroscopy (SORS). Vaccine 2023, 41, 6960–6968. [Google Scholar] [CrossRef]
- Liu, Z.F.; Huang, M.; Zhu, Q.B.; Zhao, X.; Yan, S.Q. Spatially offset Raman spectroscopy analysis technology and application in food subsurface detection. Spectrosc. Spectr. Anal. 2024, 44, 1201–1208. [Google Scholar]
- Tahir, H.E.; Zou, X.B.; Li, Z.H.; Shi, J.Y.; Xiaodong, Z.D.; Sheng, W.; Mariod, A.A. Rapid prediction of phenolic compounds and antioxidant activity of Sudanese honey using Raman and Fourier transform infrared (FT-IR) spectroscopy. Food Chem. 2017, 226, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Mandrile, L.; Rotunno, S.; Miozzi, L.; Vaira, A.M.; Giovannozzi, A.M.; Rossi, A.M.; Noris, E. Nondestructive Raman spectroscopy as a tool for early detection and discrimination of the infection of tomato plants by two economically important viruses. Anal. Chem. 2019, 91, 9025–9031. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, L.; Pant, S.; Irey, M.; Mandadi, K.; Kurouski, D. Detection and identification of canker and blight on orange trees using a hand-held Raman spectrometer. J. Raman Spectrosc. 2019, 50, 1875–1880. [Google Scholar] [CrossRef]
- Farber, C.; Bennett, J.S.; Dou, T.; Abugalyon, Y.; Humpal, D.; Sanchez, L.; Toomey, K.; Kolomiets, M.; Kurouski, D. Raman-based diagnostics of stalk rot disease of maize caused by Colletotrichum graminicola. Front. Plant Sci. 2021, 12, 722898. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jiang, H.; Chen, Q. High precisive prediction of aflatoxin b(1) in pressing peanut oil using Raman spectra combined with multivariate data analysis. Foods 2022, 11, 1565. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C. Applications of Raman spectroscopy in herbal medicine. Appl. Spectrosc. Rev. 2016, 51, 1–11. [Google Scholar] [CrossRef]
- Zhu, S.S.; Zhong, Q.S.; Yan, D.H.; Yan, Z.J.; Chen, S.; Yao, Y.D.; Zhou, C. Rapid and nondestructive identification of the geographical origin of ophiopogonis radix by Raman spectroscopy and multivariate statistical analysis. Anal. Lett. 2024, 57, 3149–3163. [Google Scholar] [CrossRef]
- Saletnik, A.; Saletnik, B.; Zagula, G.; Puchalski, C. Raman spectroscopy for plant disease detection in next-generation agriculture. Sustainability 2024, 16, 5474. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, Z.; Mao, H.; Gao, H.; Zuo, Z. A lettuce moisture detection method based on terahertz time-domain spectroscopy. Ciênc. Rural 2022, 52, e20210002. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, S.; Han, K.; Song, X.; Zhang, W.; Ye, Y.; Ren, X. Development of a novel metal grating and its applications of terahertz spectroscopic detection of CuSO4 in fruit. Food Anal. Methods 2021, 14, 1590–1599. [Google Scholar] [CrossRef]
- Lu, Y.; Asante, E.A.; Duan, H.; Hu, Y. Quantitative assessment of cold injury in tea plants by terahertz spectroscopy method. Agronomy 2023, 13, 1376. [Google Scholar] [CrossRef]
- Gao, Y.; Peng, Z.; Yang, H.; Zhang, X.; Zhao, Y.; Hou, Z.; Su, B.; Li, K.; Zhang, C. Terahertz spectroscopy study of oridonin and ponicidin in the anticancer Chinese herbal medicine Rabdosia rubescens. Front. Plant Sci. 2024, 15, 1460123. [Google Scholar] [CrossRef]
- Fu, X.J.; Liu, Y.J.; Chen, Q.; Fu, Y.; Cui, T.J. Applications of terahertz spectroscopy in the detection and recognition of substances. Front. Phys. 2022, 10, 869537. [Google Scholar] [CrossRef]
- Zhu, C.X.; Liu, D.; Li, Y.Y.; Chen, T.; You, T.Y. Label-free ratiometric homogeneous electrochemical aptasensor based on hybridization chain reaction for facile and rapid detection of aflatoxin b1 in cereal crops. Food Chem. 2022, 373, 131443. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.-H.; Yang, T.-J.; Liang, Y.-T.; Ge, H.-Y.; Shen, E.-B. Comparative assessments between conventional and promising technologies for wheat aging or mold detection. Cereal Res. Commun. 2021, 49, 511–519. [Google Scholar] [CrossRef]
- Zahid, A.; Abbas, H.T.; Ren, A.; Zoha, A.; Heidari, H.; Shah, S.A.; Imran, M.A.; Alomainy, A.; Abbasi, Q.H. Machine learning driven non-invasive approach of water content estimation in living plant leaves using terahertz waves. Plant Methods 2019, 15, 138. [Google Scholar] [CrossRef]
- Gu, H.; Wang, S.; Hu, S.; Wu, X.; Li, Q.; Zhang, R.; Zhang, J.; Zhang, W.; Peng, Y. Identification of Panax notoginseng origin using terahertz precision spectroscopy and neural network algorithm. Talanta 2024, 274, 125968. [Google Scholar] [CrossRef]
- Bin, L.; Qiu, W.; Chao-Hui, Z.; Zhao-Yang, H.; Hai, Y.; Jun, L.; Yan-De, L. Research on anthracnose grade of Camellia oleifera based on the combined LIBS and THz technology. Plant Methods 2022, 18, 52. [Google Scholar] [CrossRef]
- Ong, P.; Jian, J.; Li, X.; Zou, C.; Yin, J.; Ma, G. Sugarcane disease recognition through visible and near-infrared spectroscopy using deep learning assisted continuous wavelet transform-based spectrogram. Spectrochim. Acta Part A 2025, 324, 125001. [Google Scholar] [CrossRef]
- Liu, Z.; Le, D.; Zhang, T.; Lai, Q.; Zhang, J.; Li, B.; Song, Y.; Chen, N. Detection of apple moldy core disease by fusing vibration and VIS/NIR spectroscopy data with dual-input MLP-transformer. J. Food Eng. 2024, 382, 112219. [Google Scholar] [CrossRef]
- Jiang, X.G.; Ge, K.; Li, B.; Ouyang, A.G.; Liu, Y.D.; Jiang, N.; Liu, H.F. Non-destructive detection of apple fungal infection based on VIS/NIR transmission spectroscopy. J. Food Compos. Anal. 2024, 133, 106469. [Google Scholar] [CrossRef]
- Jiang, X.G.; Ge, K.; Liu, Z.; Chen, N.; Ouyang, A.G.; Liu, Y.D.; Huang, Y.Y.; Li, J.H.; Hu, M.M. Non-destructive online detection of early moldy core apples based on VIS/NIR transmission spectroscopy. Chem. Biol. Technol. Agric. 2024, 11, 63. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Huang, Z.; Li, G.; Zhang, Z.; He, X.; Du, H.; Wang, M.; Li, Z. Early diagnosis of Cladosporium fulvum in greenhouse tomato plants based on visible/near-infrared (VIS/NIR) and near-infrared (NIR) data fusion. Sci. Rep. 2024, 14, 20176. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiang, D.; Yang, S.; Wang, X.; Li, C. Fusarium wilt of banana latency and onset detection based on visible/near infrared spectral technology. Agronomy 2024, 14, 2994. [Google Scholar] [CrossRef]
- Jin, X.; Gu, S.; Rao, Y.; Xiong, J.; Zhang, H.; Zhang, X.; Liu, L. An innovative fusion feature method of spectrum and visual image for diagnosing ‘Akizuki’ pear cork spot disorder. J. Food Compos. Anal. 2024, 127, 105963. [Google Scholar] [CrossRef]
- Tan, H.Z.; Liu, Y.; Tang, H.; Fan, W.; Jiang, L.W.; Li, P. Accurate discrimination of mold-damaged Citri Reticulatae Pericarpium using partial least-squares discriminant analysis and selected wavelengths. Foods 2024, 13, 3856. [Google Scholar] [CrossRef]
- Sahabi, H.; Baradaran-Motie, J. Detection of mite infested saffron plants using aerial imaging and machine learning classifier. Span. J. Agric. Res. 2025, 22, 20452. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Chi, H.Y.; Kim, M.K.; Kim, J.S.; Lee, S.H.; Chung, H. Feasibility study for detection of turnip yellow mosaic virus (TYMV) infection of Chinese cabbage plants using Raman spectroscopy. Plant Pathol. J. 2013, 29, 105–109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Farber, C.; Kurouski, D. Detection and identification of plant pathogens on maize kernels with a hand-held Raman spectrometer. Anal. Chem. 2018, 90, 3009–3012. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.; Joshi, R.; Juarez, I.; Bennett, J.S.; Holman, A.P.; Kolomiets, M.; Kurouski, D. Non-invasive identification of combined salinity stress and stalk rot disease caused by Colletotrichum graminicola in maize using Raman spectroscopy. Sci. Rep. 2023, 13, 7661. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Tan, Y.; Fan, W.; Song, S.; Ji, H.; Li, B. Raman spectroscopic analysis of paddy rice infected by three pests and diseases common in northeast Asia. J. Phys. Conf. Ser. 2019, 1324, 012050. [Google Scholar] [CrossRef]
- Lin, Y.J.; Lin, H.K.; Lin, Y.H. Construction of raman spectroscopic fingerprints for the detection of fusarium wilt of banana in Taiwan. PLoS ONE 2020, 15, e0230330. [Google Scholar] [CrossRef]
- Kim, S.; Hong, S.H.; Kim, J.H.; Oh, M.K.; Eom, T.J.; Park, Y.H.; Shin, G.H.; Yim, S.Y. Early on-site detection of strawberry anthracnose using portable Raman spectroscopy. Spectrochim. Acta Part. A 2023, 303, 123150. [Google Scholar] [CrossRef]
- Vallejo-Perez, M.R.; Sosa-Herrera, J.A.; Navarro-Contreras, H.R.; Alvarez-Preciado, L.G.; Rodriguez-Vazquez, A.G.; Lara-Avila, J.P. Raman spectroscopy and machine-learning for early detection of bacterial canker of tomato: The asymptomatic disease condition. Plants 2021, 10, 1542. [Google Scholar] [CrossRef]
- Orecchio, C.; Sacco Botto, C.; Alladio, E.; D’errico, C.; Vincenti, M.; Noris, E. Non-invasive and early detection of tomato spotted wilt virus infection in tomato plants using a hand-held Raman spectrometer and machine learning modelling. Plant Stress 2025, 15, 100732. [Google Scholar] [CrossRef]
- Ji, X.; Xue, J.; Shi, J.; Wang, W.; Zhang, X.; Wang, Z.; Lu, W.; Liu, J.; Fu, Y.V.; Xu, N.; et al. Noninvasive raman spectroscopy for the detection of rice bacterial leaf blight and bacterial leaf streak. Talanta 2025, 282, 126962. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, F.V.; Pagano, M.; Tredicucci, A.; Bitossi, M.; Paoletti, R.; Barzanti, G.P.; Benvenuti, C.; Roversi, P.F.; Toncelli, A. Detection of fungal infections in chestnuts: A terahertz imaging-based approach. Food Control 2021, 123, 107700. [Google Scholar] [CrossRef]
- Penkov, N.V.; Goltyaev, M.V.; Astashev, M.E.; Serov, D.A.; Moskovskiy, M.N.; Khort, D.O.; Gudkov, S.V. The application of terahertz time-domain spectroscopy to identification of potato late blight and fusariosis. Pathogens 2021, 10, 1336. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhou, Z.; Zhang, Y.; Wang, X. Detection method for tomato leaf mildew based on hyperspectral fusion terahertz technology. Foods 2023, 12, 535. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Chen, K.; Han, C.; Guan, H.; Wang, Y.; Zhao, Y. Feasibility and potential of terahertz spectral and imaging technology for Apple Valsa canker detection: A preliminary investigation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2025, 327, 125308. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.Y.S.; Zhang, N.; Ngo, A.C.Y.; Tanadi, J.; Khoo, E.H.; Zhu, Q.; Ke, L. Non-invasive early monitoring plant health using terahertz spectroscopy. J. Mater. Sci. Mater. Electron. 2024, 35, 1346. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, H.; Zhang, X.; Liu, Y.; Du, X. A rapid detection method for tomato gray mold spores in greenhouse based on microfluidic chip enrichment and lens-less diffraction image processing. Foods 2021, 10, 3011. [Google Scholar] [CrossRef]
- Xu, M.; Sun, J.; Zhou, X.; Tang, N.; Shen, J.; Wu, X. Research on nondestructive identification of grape varieties based on EEMD-DWT and hyperspectral image. J. Food Sci. 2021, 86, 2011–2023. [Google Scholar] [CrossRef]
- Li, W.Z.; Fazli, S.; Maharjan, S.; El-Askary, H. Deciphering water quality and algal dynamics in clear lake through hyperspectral analysis using emit data. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Athens, Greece, 7–12 July 2024. [Google Scholar] [CrossRef]
- Kim, G.; Baek, I.; Stocker, M.D.; Smith, J.E.; Van Tassell, A.L.; Qin, J.W.; Chan, D.E.; Pachepsky, Y.; Kim, M.S. Hyperspectral imaging from a multipurpose floating platform to estimate chlorophyll-a concentrations in irrigation pond water. Remote Sens. 2020, 12, 2070. [Google Scholar] [CrossRef]
- Peyghambari, S.; Zhang, Y. Hyperspectral remote sensing in lithological mapping, mineral exploration, and environmental geology: An updated review. J. Appl. Remote Sens. 2021, 15, 031501. [Google Scholar] [CrossRef]
- Xu, M.; Sun, J.; Cheng, J.; Yao, K.; Wu, X.; Zhou, X. Non-destructive prediction of total soluble solids and titratable acidity in Kyoho grape using hyperspectral imaging and deep learning algorithm. J. Food Sci. Technol. 2022, 58, 9–21. [Google Scholar] [CrossRef]
- Zhong, Y.; Sun, J.; Yao, K.; Cheng, J.; Du, X. Detection of rice (with husk) moisture content based on hyperspectral imaging technology combined with MSLPP–ESMA–SVR model. J. Food Saf. 2024, 44, e13112. [Google Scholar] [CrossRef]
- Dai, C.; Sun, J.; Huang, X.; Zhang, X.; Tian, X.; Wang, W.; Sun, J.; Luan, Y. Application of hyperspectral imaging as a nondestructive technology for identifying tomato maturity and quantitatively predicting lycopene content. Foods 2023, 12, 2957. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Li, J.; Wu, X.; Dai, C. Quantitative analysis of cadmium content in tomato leaves based on hyperspectral image and feature selection. Appl. Eng. Agric. 2018, 34, 789–798. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, J.; Zhang, Y.; Tian, Y.; Yao, K.; Xu, M. Visualization of heavy metal cadmium in lettuce leaves based on wavelet support vector machine regression model and visible-near infrared hyperspectral imaging. J. Food Process Eng. 2021, 44, e13897. [Google Scholar] [CrossRef]
- Paulus, S.; Mahlein, A.K. Technical workflows for hyperspectral plant image assessment and processing on the greenhouse and laboratory scale. GigaScience 2020, 9, giaa090. [Google Scholar] [CrossRef]
- Chen, S.; Lu, X.; Fang, H.; Perumal Anand, B.; Li, R.; Feng, L.; Wang, M.; Liu, Y. Early surveillance of rice bakanae disease using deep learning and hyperspectral imaging. aBIOTECH 2024, 5, 281–297. [Google Scholar] [CrossRef]
- Liu, W.T.; Xie, Z.R.; Du, J.; Li, Y.H.; Long, Y.B.; Lan, Y.B.; Liu, T.Y.; Sun, S.; Zhao, J. Early detection of pine wilt disease based on UAV reconstructed hyperspectral image. Front. Plant Sci. 2024, 15, 1453761. [Google Scholar] [CrossRef]
- Lu, B.; Jun, S.; Ning, Y.; Xiaohong, W.; Xin, Z. Identification of tea white star disease and anthrax based on hyperspectral image information. J. Food Process Eng. 2020, 44, e13584. [Google Scholar] [CrossRef]
- Mei, G.; Li, R.; Mei, X.; Chen, R.; Fan, Y.; Cheng, J.; Feng, Z.; Tao, T.; Zhao, Q.; Zhao, P.; et al. A VSURF-CA based hyperspectral disease index estimation model of wheat stripe rust. Sci. Agric. Sin. 2024, 57, 484–499. [Google Scholar] [CrossRef]
- Watt, M.S.; Poblete, T.; De Silva, D.; Estarija, H.J.C.; Hartley, R.J.L.; Leonardo, E.M.C.; Massam, P.; Buddenbaum, H.; Zarco-Tejada, P.J. Prediction of the severity of Dothistroma needle blight in radiata pine using plant based traits and narrow band indices derived from UAV hyperspectral imagery. Agric. For. Meteorol. 2023, 330, 109294. [Google Scholar] [CrossRef]
- Zerdoner, M.; Gabriëls, S.; Arens, P.; Visser, R.G.F.; Mishra, P. Detection of botrytis cinerea severity in rose petals using hyperspectral imaging for plant breeding applications. Comput. Electron. Agric. 2025, 233, 110210. [Google Scholar] [CrossRef]
- Leucker, M.; Wahabzada, M.; Kersting, K.; Peter, M.; Beyer, W.; Steiner, U.; Mahlein, A.K.; Oerke, E.C. Hyperspectral imaging reveals the effect of sugar beet quantitative trait loci on Cercospora leaf spot resistance. Funct. Plant Biol. 2017, 44, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Zhang, Z.; Wang, Z.; Liu, B.; Liu, C.; Huang, C.; Dong, S.; Pu, X.; Wan, F. Plant image recognition with deep learning: A review. Comput. Electron. Agric. 2023, 212, 108072. [Google Scholar] [CrossRef]
- Batchuluun, G.; Nam, S.H.; Park, K.R. Deep learning-based plant-image classification using a small training dataset. Mathematics 2022, 10, 3091. [Google Scholar] [CrossRef]
- Leong, D.P.; Joseph, P.G.; Yusuf, S.; Mbiostat. Imaging asymptomatic individuals for coronary disease. JACC-Cardiovasc. Imaging 2017, 10, 318–320. [Google Scholar] [CrossRef]
- Xia, D.H.; Song, S.; Tao, L.; Qin, Z.; Wu, Z.; Gao, Z.; Wang, J.; Hu, W.; Behnamian, Y.; Luo, J.L. Review-material degradation assessed by digital image processing: Fundamentals, progresses, and challenges. J. Mater. Sci. Technol. 2020, 53, 146–162. [Google Scholar] [CrossRef]
- Hu, T.; Wang, W.; Gu, J.; Xia, Z.; Zhang, J.; Wang, B. Research on apple object detection and localization method based on improved YOLOX and RGB-D images. Agronomy 2023, 13, 1816. [Google Scholar] [CrossRef]
- Kior, A.; Yudina, L.; Zolin, Y.; Sukhov, V.; Sukhova, E. RGB imaging as a tool for remote sensing of characteristics of terrestrial plants: A review. Plants 2024, 13, 1262. [Google Scholar] [CrossRef]
- Khirade, S.D.; Patil, A.B. Plant disease detection using image processing. In Proceedings of the 2015 International Conference on Computing Communication Control and Automation, Pune, India, 26–27 February 2015; pp. 768–771. [Google Scholar] [CrossRef]
- Zhao, S.; Peng, Y.; Liu, J.; Wu, S. Tomato leaf disease diagnosis based on improved convolution neural network by attention module. Agriculture 2021, 11, 651. [Google Scholar] [CrossRef]
- Moupojou, E.; Tagne, A.; Retraint, F.; Tadonkemwa, A.; Wilfried, D.; Tapamo, H.; Nkenlifack, M. Fieldplant: A dataset of field plant images for plant disease detection and classification with deep learning. IEEE Access 2023, 11, 35398–35410. [Google Scholar] [CrossRef]
- Zilberman, A.; Ben Asher, J.; Kopeika, N.S.; Reshef, Y. Application of remote sensing for detecting plant disease using color and morphological features. In Proceedings of the Conference on Remote Sensing for Agriculture, Ecosystems, and Hydrology XXI held at SPIE Remote Sensing, Strasbourg, France, 9–11 September 2019. [Google Scholar] [CrossRef]
- Dang, M.; Wang, H.X.; Li, Y.F.; Nguyen, T.H.; Tightiz, L.; Xuan-Mung, N.; Nguyen, T.N. Computer vision for plant disease recognition: A comprehensive review. Bot. Rev. 2024, 90, 251–311. [Google Scholar] [CrossRef]
- Wilson, A.N.; Gupta, K.A.; Koduru, B.H.; Kumar, A.; Jha, A.; Cenkeramaddi, L.R. Recent advances in thermal imaging and its applications using machine learning: A review. IEEE Sens. J. 2023, 23, 3395–3407. [Google Scholar] [CrossRef]
- Brzezinski, R.Y.; Levin-Kotler, L.; Rabin, N.; Ovadia-Blechman, Z.; Zimmer, Y.; Sternfeld, A.; Finchelman, J.M.; Unis, R.; Lewis, N.; Tepper-Shaihov, O.; et al. Automated thermal imaging for the detection of fatty liver disease. Sci. Rep. 2020, 10, 15532. [Google Scholar] [CrossRef]
- Li, Y.H.; Sun, X.G.; Lian, J.H.; Liu, S. Research on heat conduction performance of carbon-fibre fabric based on infrared thermal imaging technology. In Proceedings of the International Symposium on Knowledge Acquisition and Modeling, Wuhan, China, 21–22 December 2008. [Google Scholar] [CrossRef]
- Batchuluun, G.; Nam, S.H.; Park, K.R. Deep learning-based plant classification and crop disease classification by thermal camera. J. King Saud Univ.-Comput. Inf. Sci. 2022, 34, 10474–10486. [Google Scholar] [CrossRef]
- Pathmanaban, P.; Gnanavel, B.K.; Anandan, S.S. Guava fruit (Psidium guajava) damage and disease detection using deep convolutional neural networks and thermal imaging. Imaging Sci. J. 2023, 70, 102–116. [Google Scholar] [CrossRef]
- Yang, N.; Yuan, M.; Wang, P.; Zhang, R.; Sun, J.; Mao, H. Tea diseases detection based on fast infrared thermal image processing technology. J. Sci. Food Agric. 2019, 99, 3459–3466. [Google Scholar] [CrossRef]
- Feng, Z.; Song, L.; Zhang, S.; Jing, Y.; Duan, J.; He, L.; Yin, F.; Feng, W. Wheat powdery mildew monitoring based on information fusion of multi-spectral and thermal infrared images acquired with an unmanned aerial vehicle. Sci. Agric. Sin. 2022, 55, 890–906. [Google Scholar] [CrossRef]
- Singh, R.N.; Krishnan, P.; Singh, V.K.; Banerjee, K. Application of thermal and visible imaging to estimate stripe rust disease severity in wheat using supervised image classification methods. Ecol. Inf. 2022, 71, 101774. [Google Scholar] [CrossRef]
- Francesconi, S.; Harfouche, A.; Maesano, M.; Balestra, G.M. UAV-based thermal, RGB imaging and gene expression analysis allowed detection of fusarium head blight and gave new insights into the physiological responses to the disease in durum wheat. Front. Plant Sci. 2021, 12, 628575. [Google Scholar] [CrossRef]
- Singh, R.N.; Krishnan, P.; Singh, V.K.; Das, B. Estimation of yellow rust severity in wheat using visible and thermal imaging coupled with machine learning models. Geocarto Int. 2023, 38, 2160831. [Google Scholar] [CrossRef]
- Xie, C.Q.; Shao, Y.N.; Li, X.L.; He, Y. Detection of early blight and late blight diseases on tomato leaves using hyperspectral imaging. Sci. Rep. 2015, 5, 16564. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-H.; Chung, W.-C.; Liao, J.-Y.; Chung, C.-L.; Kuo, Y.-F.; Lin, T.-T. Strawberry foliar anthracnose assessment by hyperspectral imaging. Comput. Electron. Agric. 2016, 122, 1–9. [Google Scholar] [CrossRef]
- Moghadam, P.; Ward, D.; Goan, E.; Jayawardena, S.; Sikka, P.; Hernandez, E. Plant disease detection using hyperspectral imaging. In Proceedings of the International Conference on Digital Image Computing-Techniques and Applications (DICTA), Sydney, Australia, 29 November–1 December 2017. [Google Scholar] [CrossRef]
- Morel, J.; Jay, S.; Feret, J.B.; Bakache, A.; Bendoula, R.; Carreel, F.; Gorretta, N. Exploring the potential of PROCOSINE and close-range hyperspectral imaging to study the effects of fungal diseases on leaf physiology. Sci. Rep. 2018, 8, 15933. [Google Scholar] [CrossRef]
- Förster, A.; Behley, J.; Behmann, J.; Roscher, R. Hyperspectral plant disease forecasting using generative adversarial networks. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Yokohama, Japan, 2 August 2019. [Google Scholar] [CrossRef]
- Jiang, D.Y.; Chang, Q.R.; Zhang, Z.J.; Liu, Y.F.; Zhang, Y.; Zheng, Z.K. Monitoring the degree of mosaic disease in apple leaves using hyperspectral images. Remote Sens. 2023, 15, 2504. [Google Scholar] [CrossRef]
- Guo, A.; Huang, W.; Dong, Y.; Ye, H.; Ma, H.; Liu, B.; Wu, W.; Ren, Y.; Ruan, C.; Geng, Y. Wheat yellow rust detection using UAV-based hyperspectral technology. Remote Sens. 2021, 13, 123. [Google Scholar] [CrossRef]
- Xie, Y.; Plett, D.; Evans, M.; Garrard, T.; Butt, M.; Clarke, K.; Liu, H. Hyperspectral imaging detects biological stress of wheat for early diagnosis of crown rot disease. Comput. Electron. Agric. 2024, 217, 108571. [Google Scholar] [CrossRef]
- Chen, S.T.; Ouyang, Y.C.; Shih, M.S.; Liu, T.S.; Chang, C. Fusarium wilt detection in phalaenopsis through integrated hyperspectral imaging and deep learning techniques. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Athens, Greece, 7–12 July 2024. [Google Scholar] [CrossRef]
- Raza, S.E.; Prince, G.; Clarkson, J.P.; Rajpoot, N.M. Automatic detection of diseased tomato plants using thermal and stereo visible light images. PLoS ONE 2015, 10, e0123262. [Google Scholar] [CrossRef]
- Ma, J.; Du, K.; Zheng, F.; Zhang, L.; Gong, Z.; Sun, Z. A recognition method for cucumber diseases using leaf symptom images based on deep convolutional neural network. Comput. Electron. Agric. 2018, 154, 18–24. [Google Scholar] [CrossRef]
- Yu, Y. Research on Precise Real-Time Detection of Corn Leaf Blight Based on Improved Convolutional Neural Network; Tongfang Knowledge Network (Beijing) Technology Co., Ltd.: Beijing, China, 2021. [Google Scholar] [CrossRef]
- Dong, X.Y.; Wang, Q.; Huang, Q.D.; Ge, Q.L.; Zhao, K.J.; Wu, X.C.; Wu, X.; Lei, L.; Hao, G.F. PDDD-PreTrain: A series of commonly used pre-trained models support image-based plant disease diagnosis. Plant Phenomics 2023, 5, 0054. [Google Scholar] [CrossRef] [PubMed]
- Pawar, R.; Jadhav, A. Pomogranite disease detection and classification. In Proceedings of the IEEE International Conference on Power, Control, Signals and Instrumentation Engineering (IEEE ICPCSI), Chennai, India, 21–22 September 2017. [Google Scholar] [CrossRef]
- Cohen, B.; Edan, Y.; Levi, A.; Alchanatis, V. Early detection of grapevine (Vitis vinifera) downy mildew (Peronospora) and diurnal variations using thermal imaging. Sensors 2022, 22, 3585. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, I.; Phadikar, S.; Majumder, K.; Mukherjee, H.; Sau, A. A novel plant disease prediction model based on thermal images using modified deep convolutional neural network. Precis. Agric. 2022, 24, 23–39. [Google Scholar] [CrossRef]
- Ha, S.T.T.; Kim, Y.T.; In, B.C. Early detection of botrytis cinerea infection in cut roses using thermal imaging. Plants 2023, 12, 4087. [Google Scholar] [CrossRef]
- Farokhzad, S.; Modaress Motlagh, A.; Ahmadi Moghaddam, P.; Jalali Honarmand, S.; Kheiralipour, K. A machine learning system to identify progress level of dry rot disease in potato tuber based on digital thermal image processing. Sci. Rep. 2024, 14, 1995. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Sun, H.; An, L.; Zhao, R.; Liu, M.; Tang, W.; Li, M.; Yan, X.; Ma, Y.; et al. Exploring multi-features in UAV based optical and thermal infrared images to estimate disease severity of wheat powdery mildew. Comput. Electron. Agric. 2024, 225, 109285. [Google Scholar] [CrossRef]
- Tuğrul, K.M. Early detection of sugar beet Cercospora leaf spot disease using machine learning-assisted thermal image processing method. Sugar Tech 2025, 27, 54–964. [Google Scholar] [CrossRef]
- Correa Da Silva, P.E.; Almeida, J. An edge computing-based solution for real-time leaf disease classification using thermal imaging. IEEE Geosci. Remote Sens. Lett. 2025, 22, 7000105. [Google Scholar] [CrossRef]
- Hashim, I.C.; Shariff, A.R.M.; Bejo, S.K.; Muharam, F.M.; Ahmad, K.; Hashim, H. Application of thermal imaging for plant disease detection. In Proceedings of the 10th Institution-of-Geospatial-and-Remote-Sensing-Malaysia (IGRSM) International Conference and Exhibition on Geospatial and Remote Sensing, Kuala Lumpur, Malaysia, 20–21 October 2020. [Google Scholar] [CrossRef]


| Techniques | Samples | Applications | Algorithms | Equipment | Reference |
|---|---|---|---|---|---|
| Near-infrared spectroscopy | Sugar Cane | Sugarcane disease recognition | CNN, CWT | UV2600 | [79] |
| Apple | Detection of apple moldy core disease | DMLPT, PLS-DA, SVM, ELM | QE65pro | [80] | |
| Apple | Detection of apple fungal infection | LDA, KNN, RF | QE65pro | [81] | |
| Apple | Detection of early moldy core apples | SVM, ELM, KNN | QE65pro | [82] | |
| Tomato | Diagnosis of Cladosporium fulvum in greenhouse tomato plants | PCA, RBF, BP, SVM | NIR system of Headwall Photonics Company, Bolton, MA, USA | [83] | |
| Banana | Detection of the incubation period and onset period of banana wilt disease | FDA, ELM, 1D-CNN | Uspectral-RIT-2.7.0 | [84] | |
| ‘Akizuki’ pear | Diagnosing ‘Akizuki’ pear cork spot disorder | SVM, RF | NIR-S-G1 | [85] | |
| Citri Reticulatae Pericarpium | Discrimination of mold-damaged Citri Reticulatae Pericarpium | PLS-DA, MSC | i-Spec Plus | [86] | |
| Saffron plants | Detection of mite-infested saffron plants | SVM, RBF | Hyspim, Sweden | [87] | |
| Raman spectroscopy | Chinese cabbage | Detection of turnip yellow mosaic virus (TYMV) infection | PCA, LDA | Distributed Raman Microscope (Kaiser Optical Inc., Ann Arbor, MI, USA) | [88] |
| Maize Kernels | Detection and identification of plant pathogens | OPLS-DA | Handheld Rigaku Progeny ResQ Spectrometer | [89] | |
| Maize | Identification of combined salinity stress and stalk rot disease | SNV, PLS-DA | Handheld Resolve Agilent Spectrometer | [90] | |
| Paddy rice | Analysis of paddy rice infected by three pests and diseases | PLSDA | TriVista 555CRS Laser Raman Spectrometer | [91] | |
| Banana | Detection of Fusarium wilt | MDIP, IPDP | Portable QE65 Pro Raman Spectrometer System | [92] | |
| Strawberry | Early on-site detection of strawberry anthracnose | PCA, LDA | Portable XPE85-NIR Spectrometer | [93] | |
| Tomato | Early detection of bacterial canker of tomato | PCA, LDA, MLP | Horiba XploRA ONETM Confocal Microscopy Spectrometer | [94] | |
| Tomato | Early detection of tomato spotted wilt virus infection | ML, PLS-DA | Handheld Bruker BRAVO Spectrometer | [95] | |
| Rice | Detection of rice bacterial leaf blight and bacterial leaf streak | CNN, SVM, RF, PCA | Portable Raman spectrometer (produced by Ocean Optics of the United States, Largo, FL, USA) | [96] | |
| Terahertz spectroscopy | Chestnut | Detection of fungal infections in chestnuts | Birnbaum-Saunders | THz camera (Tera-1024 32 × 32, Terasense, San Jose, CA, USA) | [97] |
| Potato | Identification of potato late blight and fusariosis | RT-PCR | Terahertz time-domain spectrometer (TPS Spectra 3000, Teraview, UK) | [98] | |
| Tomato | Detection method for tomato leaf mildew | PCA, BPNN | TS7400 Terahertz Time Domain Spectroscopy Measurement System | [99] | |
| Apple | Apple Valsa canker detection | MSC, SG | CCT-1800 Terahertz Time-Domain Imaging System | [100] | |
| Plant leaf | Non-invasive early monitoring of plant health | CNN | TERA K15 Terahertz Time Domain Spectroscopy System | [101] |
| Techniques | Samples | Applications | Algorithms | Equipment | Reference |
|---|---|---|---|---|---|
| Hyperspectral imaging | Tomato | Detection of early blight and late blight diseases | ELM, SPA | Imaging spectrometer (V10E-QE, Specim, Finland) | [141] |
| Strawberry | Strawberry foliar anthracnose assessment | SAM, SDA, PLS | VNIR A series hyperspectral camera(Headwall HyperspecTM, Bolton, MS, USA) | [142] | |
| Capsicum | Plant disease detection | SVM, RBF | VNIR A series and SWIR M series hyperspectral cameras | [143] | |
| Banana | The effects of fungal diseases | LDA | HySpex VNIR-1600 Hyperspectral Camera | [144] | |
| Hordeum vulgare | Plant disease forecasting | GAN | Hyper-spectral microscope | [145] | |
| Apple Leaves | Monitoring the degree of mosaic disease | SPA, CWT, PLSR | SOC-710 Portable Hyperspectral Instrument (Surface Optics Corp, San Diego, CA, USA) | [146] | |
| Wheat | Wheat yellow rust detection | PLSR | High-spectrum imaging sensor (UHD 185) | [147] | |
| Wheat | Early diagnosis of crown rot disease | SVM, LDA | FX10 camera and short-wave infrared camera | [148] | |
| Phalaenopsis | Fusarium wilt detection in Phalaenopsis | 2D-CNN, CBAM-E | Hyper-spectral sensor | [149] | |
| Digital imaging | Tomato | Detection of diseased tomato plants | SGM, SVM | Visible light imaging camera (Canon Powershot S100) | [150] |
| Cucumber | A recognition method for cucumber diseases | DCNN, SGDM | Nikon Coolpix S3100 Digital Camera | [151] | |
| Corn | Detection of corn leaf blight | SSD, GIoU | Camera | [152] | |
| Rice, Wheat, Tomato, Pepper, Cucumber, Squash, Corn | Plant disease diagnosis | ResNet50 | Camera, locator | [153] | |
| Pomegranate | Pomegranate disease detection and classification | K-propagation | Camera | [154] | |
| Thermal imaging | Grapevine | Early detection of grapevine downy mildew | SVM | Thermal imager (model FLIR SC655) | [155] |
| Rice Plants | Plant disease prediction | CNN | FLIR C2 Camera | [156] | |
| Rose | Detection of Botrytis cinerea infection in cut roses | LSD | Infrared thermal imager (T530) | [157] | |
| Potato | Identification of progress level of dry rot disease | SVM | Infrared thermal imager (model G120, NEC Avio, Tokyo, Japan) | [158] | |
| Wheat | Estimation of disease severity of wheat powdery mildew | RFE | Altum Camera (MicaSense USA, Inc., Raleigh, NC, USA) | [159] | |
| Sugar Beet | Early detection of sugar beet Cercospora leaf spot disease | SVM, KNN | High-resolution thermal imaging camera | [160] | |
| Persea americana, Malpighia emarginata, Myrciaria glazioviana | Real-time leaf disease classification | InceptionV3, MobileNetV1, VGG-16 | Infiray T3C Thermal Imaging Camera | [161] | |
| Cucumber, Sweet Potato, Wheat, Peanut, Oil Palm | Plant disease detection | PCA, SVM | Portable thermal imager | [162] |
| Techniques | Cost | Portability | Depth of Penetration | Humidity Sensitivity |
|---|---|---|---|---|
| Near-infrared spectroscopy | Lower, relatively inexpensive equipment and low operation cost, suitable for large-scale application | Higher, portable devices (such as handheld spectrometers) can be used in field sites | Medium, can obtain information on internal structure and composition of plant tissues, but with limited penetration depth | Higher, spectral information is susceptible to humidity, which may cause data fluctuations and affect detection accuracy |
| Raman spectroscopy | Medium, moderate cost for ordinary equipment, enhanced technologies (such as SERS) may be more expensive | Higher, handheld devices can be used for on-site detection | Shallow, mainly detects molecular vibration information on the surface or shallow layers of samples | Medium, humidity may have some impact on detection, but the degree of influence is relatively small |
| Terahertz Spectroscopy | Higher, expensive equipment limits its wide application | Lower, equipment is large in size and has poor portability, currently mainly used in laboratories | Deeper, can penetrate many non-conductive materials and obtain deeper information on plant tissues | Higher, terahertz waves are susceptible to humidity during propagation and detection performance may decline in high-humidity environments |
| Hyperspectral imaging | Higher, expensive equipment and high data processing and storage costs | Lower, equipment is usually large, although it can be combined with drones, overall portability is still limited, more used for laboratory fine analysis | Medium, can simultaneously obtain image and spectral information, with moderate penetration depth for plant tissues | Higher, data collection is affected by environmental factors such as light and weather, humidity may also have some impact |
| Digital Imaging | Lower, equipment (such as ordinary cameras, cameras on drones) has low cost, easy to obtain | Higher, equipment has good portability, can be collected on-site using smartphones, cameras, or drones | Shallow, mainly obtains color, texture, etc., features of plant surfaces | Lower, humidity has a relatively small impact on digital imaging |
| Thermal Imaging | Medium, moderate equipment cost, handheld devices and devices that can be mounted on drones | Higher, can be monitored on-site using handheld cameras or thermal imaging sensors mounted on drones | Shallow, mainly detects temperature changes on the surface of plants to identify diseases | Lower, humidity has a relatively small impact on thermal imaging |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Sun, J.; Wu, Z.; Jia, Y.; Dai, C. Application of Non-Destructive Technology in Plant Disease Detection: Review. Agriculture 2025, 15, 1670. https://doi.org/10.3390/agriculture15151670
Wang Y, Sun J, Wu Z, Jia Y, Dai C. Application of Non-Destructive Technology in Plant Disease Detection: Review. Agriculture. 2025; 15(15):1670. https://doi.org/10.3390/agriculture15151670
Chicago/Turabian StyleWang, Yanping, Jun Sun, Zhaoqi Wu, Yilin Jia, and Chunxia Dai. 2025. "Application of Non-Destructive Technology in Plant Disease Detection: Review" Agriculture 15, no. 15: 1670. https://doi.org/10.3390/agriculture15151670
APA StyleWang, Y., Sun, J., Wu, Z., Jia, Y., & Dai, C. (2025). Application of Non-Destructive Technology in Plant Disease Detection: Review. Agriculture, 15(15), 1670. https://doi.org/10.3390/agriculture15151670






