Current Context of Cannabis sativa Cultivation and Parameters Influencing Its Development
Abstract
1. Introduction
2. Biology and Applications of C. sativa
3. Influence of Light Quality, Intensity, and Photoperiod on C. sativa Cultivation
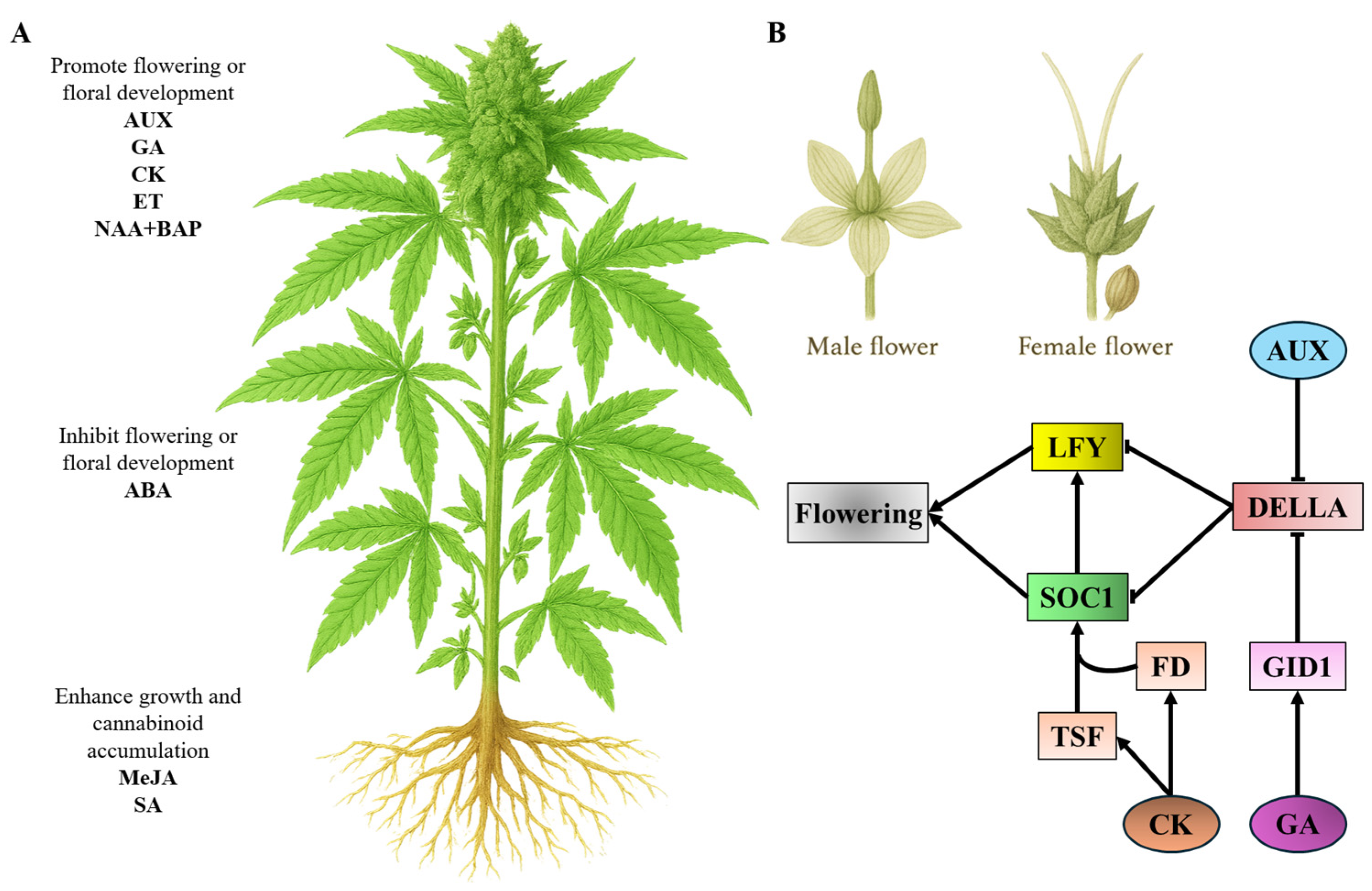
4. Influence of Phytohormones on Floral Development and Cannabinoid Production in C. sativa
5. Integrated Cultivation Methods for C. sativa Balancing Production Quality
6. Final Considerations and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| C. sativa | Cannabis sativa |
| THC | Δ9-tetrahydrocannabinol |
| CBD | Cannabidiol |
| ECS | Endocannabinoid System |
| CRC | Colorectal Cancer |
| CBDA | Cannabidiolic Acid |
| THCA | Tetrahydrocannabinolic Acid |
| CBGA | Cannabigerolic Acid |
| CBG | Cannabigerol |
| CBCA | Cannabichromenic Acid |
| CO2 | Carbon Dioxide |
| PAR | Photosynthetically Active Radiation |
| UVA | Ultraviolet-A |
| ROS | Reactive Oxygen Species |
| LUE | Light Use Efficiency |
| FR | Far-Red |
| R:FR | Red to Far-Red |
| PPFD | Photosynthetic Photon Flux Density |
| UV | Ultraviolet |
| LED | Light-Emitting Diode |
| CsPRR37 | Cannabis sativa Pseudo-Response Regulator 37 |
| FT | FLOWERING LOCUS T |
| CsFT1 | Cannabis sativa FLOWERING LOCUS T gene |
| ABA | Abscisic Acid |
| AUXs | Auxins |
| GAs | Gibberellins |
| CKs | Cytokinins |
| ET | Ethylene |
| SAs | Salicylates |
| JAs | Jasmonates |
| PGRs | Plant Growth Regulators |
| NAA | Naphthalene Acetic Acid |
| BAP | 6-Benzylaminopurine |
| SaA | Salicylic Acid |
| MeJA | Methyl Jasmonate |
| GABA | γ-Aminobutyric Acid |
| SOC1 | Suppressor of Overexpression of Constans1 |
| UV-B | Ultraviolet-B |
| HID | High-Intensity Discharge |
| MH | Metal Halide |
| HPS | High-Pressure Sodium |
References
- El Oihabi, M.; Soultana, M.; Ammari, M.; Ben Allal, L.; Fakih Lanjri, A. Diversity and variability of bioactive compounds in Cannabis sativa: Effects on therapeutic and environmental uses and orientations for future research. Case Stud. Chem. Environ. Eng. 2024, 9, 100732. [Google Scholar] [CrossRef]
- Pollio, A. The Name of Cannabis: A Short Guide for Nonbotanists. Cannabis Cannabinoid Res. 2016, 1, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Lamark, J.B. Encyclopédie Méthodique. Botanique; Panckoucke: Paris, France, 1783. [Google Scholar]
- Janischevsky, D.E. A form of hemp in wild areas of southeastern Russia. NG Cern. Univ. 1924, 2, 3–17. [Google Scholar]
- Small, E.; Cronquist, A. A Practical and Natural Taxonomy for Cannabis. Taxon 1976, 25, 405–435. [Google Scholar] [CrossRef]
- Small, E. Evolution and Classification of Cannabis sativa (Marijuana, Hemp) in Relation to Human Utilization. Bot. Rev. 2015, 81, 189–294. [Google Scholar] [CrossRef]
- Trancoso, I.; De Souza, G.A.R.; Dos Santos, P.R.; Dos Santos, K.D.; De Miranda, R.M.D.S.N.; Da Silva, A.L.P.M.; Santos, D.Z.; García-Tejero, I.F.; Campostrini, E. Cannabis sativa L. Crop Management and Abiotic Factors That Affect Phytocannabinoid Production. Agronomy 2022, 12, 1492. [Google Scholar] [CrossRef]
- Wani, K.A.; Andrabi, S.J.; Manzoor, J.; Qadir, H.; Jan, K. Cultivation of Cannabis: Medicinal, Social, and Legal Aspects. In Cannabis sativa Cultivation, Production, and Applications in Pharmaceuticals and Cosmetics; IGI Global Scientific Publishing: Hershey, PA, USA, 2023; pp. 43–51. [Google Scholar]
- Rupasinghe, H.P.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef] [PubMed]
- Saloner, A.; Bernstein, N. Nitrogen Source Matters: High NH4/NO3 Ratio Reduces Cannabinoids, Terpenoids, and Yield in Medical Cannabis. Front. Plant Sci. 2022, 13, 830224. [Google Scholar] [CrossRef]
- Cockson, P.; Schroeder-Moreno, M.; Veazie, P.; Barajas, G.; Logan, D.; Davis, M.; Whipker, B.E. Impact of Phosphorus on Cannabis sativa Reproduction, Cannabinoids, and Terpenes. Appl. Sci. 2020, 10, 7875. [Google Scholar] [CrossRef]
- Shiponi, S.; Bernstein, N. Response of medical cannabis (Cannabis sativa L.) genotypes to P supply under long photoperiod: Functional phenotyping and the ionome. Ind. Crops Prod. 2021, 161, 113154. [Google Scholar] [CrossRef]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, Phenolics, Terpenes and Alkaloids of Cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef]
- Oriola, A.O.; Kar, P.; Oyedeji, A.O. Cannabis sativa as an Herbal Ingredient: Problems and Prospects. Molecules 2024, 29, 3605. [Google Scholar] [CrossRef] [PubMed]
- Fordjour, E.; Manful, C.F.; Sey, A.A.; Javed, R.; Pham, T.H.; Thomas, R.; Cheema, M. Cannabis: A multifaceted plant with endless potentials. Front. Pharmacol. 2023, 14, 1200269. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef]
- Grosso, A.F. Cannabis: From plant condemned by prejudice to one of the greatest therapeutic options of the century. J. Hum. Growth Dev. 2020, 30, 94–97. [Google Scholar] [CrossRef]
- Pratt, M.; Stevens, A.; Thuku, M.; Butler, C.; Skidmore, B.; Wieland, L.S.; Clemons, M.; Kanji, S.; Hutton, B. Benefits and harms of medical cannabis: A scoping review of systematic reviews. Syst. Rev. 2019, 8, 320. [Google Scholar] [CrossRef]
- Citti, C.; Braghiroli, D.; Vandelli, M.A.; Cannazza, G. Pharmaceutical and biomedical analysis of cannabinoids: A critical review. J. Pharm. Biomed. Anal. 2018, 147, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Vučković, S.; Srebro, D.; Vujović, K.; Vučetić, C.; Prostran, M. Cannabinoids and Pain: New Insights from Old Molecules. Front. Pharmacol. 2018, 9, 1259. [Google Scholar] [CrossRef]
- Liktor-Busa, E.; Keresztes, A.; LaVigne, J.; Streicher, J.; Largent-Milnes, T. Analgesic Potential of Terpenes Derived from Cannabis sativa. Pharmacol. Rev. 2021, 73, 98–126. [Google Scholar] [CrossRef]
- McDougall, J.J.; McKenna, M.K. Anti-Inflammatory and Analgesic Properties of the Cannabis Terpene Myrcene in Rat Adjuvant Monoarthritis. Int. J. Mol. Sci. 2022, 23, 7891. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Silva-Reis, R.; Silva, A.M.S.; Oliveira, P.A.; Cardoso, S.M. Antitumor Effects of Cannabis sativa Bioactive Compounds on Colorectal Carcinogenesis. Biomolecules 2023, 13, 764. [Google Scholar] [CrossRef]
- De Medeiros Dantas, J.M.; Chastel, C.F.; Wolfaardt, F.J.; Ghislain, T.; Lavoie, J.-M. Cannabis-based biofuels in a biorefinery approach. Ind. Crops Prod. 2023, 204, 117225. [Google Scholar] [CrossRef]
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The biosynthesis of the cannabinoids. J. Cannabis Res. 2021, 3, 7. [Google Scholar] [CrossRef]
- Krejčí, Z.; Šantavý, F. The isolation of further substances from the leaves of Indian hemp (Cannabis sativa L., var. indica). Acta Univ. Palacki. Olomuc. Fac. Med. 1955, 6, 59–66. [Google Scholar]
- DeCarcer, G.A.; Kagia, J.; Morrissey, K.; McCann, M.; Tomares, N.; Alvarado, I.; McCoy, J.J.; Watkins, E. The Global Cannabis Report; New Frontier Data: Washington, DC, USA, 2021. [Google Scholar]
- Prohibition Partners. The Global Cannabis Report: 5th Edition (Updated); PP Intelligence Ltd.: London, UK, 2024. [Google Scholar]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Front. Plant Sci. 2019, 9, 1969. [Google Scholar] [CrossRef]
- Naeem, M.Y.; Corbo, F.; Crupi, P.; Clodoveo, M.L. Hemp: An Alternative Source for Various Industries and an Emerging Tool for Functional Food and Pharmaceutical Sectors. Processes 2023, 11, 718. [Google Scholar] [CrossRef]
- Bertoli, A.; Tozzi, S.; Pistelli, L.; Angelini, L.G. Fibre hemp inflorescences: From crop-residues to essential oil production. Ind. Crops Prod. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- Strzelczyk, M.; Lochynska, M.; Chudy, M. Systematics and Botanical Characteristics of Industrial Hemp Cannabis sativa L. J. Nat. Fibers 2022, 19, 5804–5826. [Google Scholar] [CrossRef]
- Sorrentino, G. Introduction to emerging industrial applications of cannabis (Cannabis sativa L.). Rend. Lincei Sci. Fis. E Nat. 2021, 32, 233–243. [Google Scholar] [CrossRef]
- Yano, H.; Fu, W. Hemp: A Sustainable Plant with High Industrial Value in Food Processing. Foods 2023, 12, 651. [Google Scholar] [CrossRef]
- Kaur, G.; Kander, R. The Sustainability of Industrial Hemp: A Literature Review of Its Economic, Environmental, and Social Sustainability. Sustainability 2023, 15, 6457. [Google Scholar] [CrossRef]
- Tang, L.; Fan, C.; Yuan, H.; Wu, G.; Sun, J.; Zhang, S. The Effect of Rotational Cropping of Industrial Hemp (Cannabis sativa L.) on Rhizosphere Soil Microbial Communities. Agronomy 2022, 12, 2293. [Google Scholar] [CrossRef]
- Visković, J.; Zheljazkov, V.D.; Sikora, V.; Noller, J.; Latković, D.; Ocamb, C.M.; Koren, A. Industrial Hemp (Cannabis sativa L.) Agronomy and Utilization: A Review. Agronomy 2023, 13, 931. [Google Scholar] [CrossRef]
- Kok, C.J.; Coenen, G.C.M.; de Heij, A. The effect of fibre hemp (Cannabis sativa L.) on selected soil-borne pathogens. J. Int. Hemp Assoc. 1994, 1, 6–9. [Google Scholar]
- Flajšman, M.; Košmelj, K.; Grčman, H.; Ačko, D.K.; Zupan, M. Industrial hemp (Cannabis sativa L.)—A valuable alternative crop for growing in agricultural soils contaminated with heavy metals. Environ. Sci. Pollut. Res. 2023, 30, 115414–115429. [Google Scholar] [CrossRef] [PubMed]
- Charkowski, E. Hemp ‘Eats’ Chernobyl Waste. Available online: https://rediscoverhemp.thisfemmedaddy.com/inspire/hemp-eats-chernobyl-waste-offers-hope-for-hanford/ (accessed on 18 February 2022).
- Golia, E.E.; Bethanis, J.; Ntinopoulos, N.; Kaffe, G.-G.; Komnou, A.A.; Vasilou, C. Investigating the potential of heavy metal accumulation from hemp. The use of industrial hemp (Cannabis sativa L.) for phytoremediation of heavily and moderated polluted soils. Sustain. Chem. Pharm. 2023, 31, 100961. [Google Scholar] [CrossRef]
- Kraszkiewicz, A.; Kachel, M.; Parafiniuk, S.; Zając, G.; Niedziółka, I.; Sprawka, M. Assessment of the Possibility of Using Hemp Biomass (Cannabis sativa L.) for Energy Purposes: A Case Study. Appl. Sci. 2019, 9, 4437. [Google Scholar] [CrossRef]
- Schilling, J.M.; Hughes, C.G.; Wallace, M.S.; Sexton, M.; Backonja, M.; Moeller-Bertram, T. Cannabidiol as a Treatment for Chronic Pain: A Survey of Patients’ Perspectives and Attitudes. J. Pain Res. 2021, 14, 1241–1250. [Google Scholar] [CrossRef]
- Johnson, N. American Weed: A History of Cannabis Cultivation in the United States. EchoGéo 2019, 48, 1–22. [Google Scholar] [CrossRef]
- Tanney, C.A.S.; Backer, R.; Geitmann, A.; Smith, D.L. Cannabis Glandular Trichomes: A Cellular Metabolite Factory. Front. Plant Sci. 2021, 12, 721986. [Google Scholar] [CrossRef]
- Cermeño, Z.S. Construcción de Invernaderos; MundiPrensa: Madrid, Spain, 2005. [Google Scholar]
- Ahrens, A.; Llewellyn, D.; Zheng, Y. Is Twelve Hours Really the Optimum Photoperiod for Promoting Flowering in Indoor-Grown Cultivars of Cannabis sativa? Plants 2023, 12, 2605. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; Khan, I.A.; Elsohly, M.A. Photosynthetic response of Cannabis sativa L. to variations in photosynthetic photon flux densities, temperature and CO2 conditions. Physiol. Mol. Biol. Plants 2008, 14, 299–306. [Google Scholar] [CrossRef]
- Magagnini, G.; Grassi, G.; Kotiranta, S. The Effect of Light Spectrum on the Morphology and Cannabinoid Content of Cannabis sativa L. Med. Cannabis Cannabinoids 2018, 1, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Reichel, P.; Munz, S.; Hartung, J.; Präger, A.; Kotiranta, S.; Burgel, L.; Schober, T.; Graeff-Hönninger, S. Impact of Three Different Light Spectra on the Yield, Morphology and Growth Trajectory of Three Different Cannabis sativa L. Strains. Plants 2021, 10, 1866. [Google Scholar] [CrossRef]
- Rodriguez-Morrison, V.; Llewellyn, D.; Zheng, Y. Cannabis Yield, Potency, and Leaf Photosynthesis Respond Differently to Increasing Light Levels in an Indoor Environment. Front. Plant Sci. 2021, 12, 646020. [Google Scholar] [CrossRef]
- Park, S.-H.; Pauli, C.S.; Gostin, E.L.; Staples, S.K.; Seifried, D.; Kinney, C.; Vanden Heuvel, B.D. Effects of short-term environmental stresses on the onset of cannabinoid production in young immature flowers of industrial hemp (Cannabis sativa L.). J. Cannabis Res. 2022, 4, 1. [Google Scholar] [CrossRef]
- Morgan, W.; Singh, J.; Kesheimer, K.; Davis, J.; Sanz-Saez, A. Severe drought significantly reduces floral hemp (Cannabis sativa L.) yield and cannabinoid content but moderate drought does not. Environ. Exp. Bot. 2024, 219, 105649. [Google Scholar] [CrossRef]
- Dilena, E.; Close, D.C.; Hunt, I.; Garland, S.M. Investigating how nitrogen nutrition and pruning impacts on CBD and THC concentration and plant biomass of Cannabis sativa. Sci. Rep. 2023, 13, 19533. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F.M. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E.; Ottosen, C.-O. Spectral Effects of Artificial Light on Plant Physiology and Secondary Metabolism: A Review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Azcón-Bieto, J.; Talón, M. Fundamentos de Fisiología Vegetal; McGraw-Hill—Interamericana de España, S.L.: Madrid, Spain, 2000. [Google Scholar]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants response to light stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review: Effects of light on vegetable phytochemicals. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Light matters: Effect of light spectra on cannabinoid profile and plant development of medical cannabis (Cannabis sativa L.). Ind. Crops Prod. 2021, 164, 113351. [Google Scholar] [CrossRef]
- Tan, T.; Li, S.; Fan, Y.; Wang, Z.; Ali Raza, M.; Shafiq, I.; Wang, B.; Wu, X.; Yong, T.; Wang, X.; et al. Far-red light: A regulator of plant morphology and photosynthetic capacity. Crop J. 2022, 10, 300–309. [Google Scholar] [CrossRef]
- Paik, I.; Huq, E. Plant photoreceptors: Multi-functional sensory proteins and their signaling networks. Semin. Cell Dev. Biol. 2019, 92, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Sae-Tang, W.; Heuvelink, E.; Kohlen, W.; Argyri, E.; Nicole, C.C.S.; Marcelis, L.F.M. Effect of far-red and blue light on rooting in medicinal cannabis cuttings and related changes in endogenous auxin and carbohydrates. Sci. Hortic. 2024, 325, 112614. [Google Scholar] [CrossRef]
- Chen, S.; Marcelis, L.F.M.; Offringa, R.; Kohlen, W.; Heuvelink, E. Far-red light-enhanced apical dominance stimulates flower and fruit abortion in sweet pepper. Plant Physiol. 2024, 195, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Kinoshita, T.; Matsumoto, M.; Nakayama, K.I.; Doi, M.; Shimazaki, K. Blue light-induced autophosphorylation of phototropin is a primary step for signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 5626–5631. [Google Scholar] [CrossRef]
- Suetsugu, N.; Wada, M. Evolution of the Cp-Actin-based Motility System of Chloroplasts in Green Plants. Front. Plant Sci. 2016, 7, 561. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.-G. Meeting the Global Food Demand of the Future by Engineering Crop Photosynthesis and Yield Potential. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Schoepke, T. Cultivo de Marihuana: Manual Básico de Agricultura. 2014. Available online: https://docs.wixstatic.com/ugd/e7ce82_8dff8abf99c4456294a94b71a177bcf0.pdf (accessed on 12 April 2025).
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of growth under different light spectra on the subsequent high light tolerance in rose plants. AoB PLANTS 2018, 10, ply052. [Google Scholar] [CrossRef]
- Li, T.; Heuvelink, E.; Dueck, T.A.; Janse, J.; Gort, G.; Marcelis, L.F.M. Enhancement of crop photosynthesis by diffuse light: Quantifying the contributing factors. Ann. Bot. 2014, 114, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Ryu, B.R.; Rahman, M.H.; Rana, M.S.; Cheong, E.J.; Wang, M.-H.; Lim, J.-D.; Hossain, M.A.; Lim, Y.-S. Cannabinoid accumulation in hemp depends on ROS generation and interlinked with morpho-physiological acclimation and plasticity under indoor LED environment. Front. Plant Sci. 2022, 13, 984410. [Google Scholar] [CrossRef]
- Jiang, C.; Johkan, M.; Maruo, T.; Hohjo, M.; Tsukagoshi, S.; Ebihara, M.; Nakaminami, A. Effect of supplemental far-red light with blue and red LED lamps on leaf photosynthesis, stomatal regulation and plant development of protected cultivated tomato. Acta Hortic. 2018, 1227, 533–540. [Google Scholar] [CrossRef]
- Liu, J.; Van Iersel, M.W. Far-red Photons Increase Light Capture but Have Lower Photosynthetic Capacity Than Red Photons. J. Am. Soc. Hortic. Sci. 2023, 148, 253–265. [Google Scholar] [CrossRef]
- Jin, W.; Urbina, J.L.; Heuvelink, E.; Marcelis, L.F.M. Adding Far-Red to Red-Blue Light-Emitting Diode Light Promotes Yield of Lettuce at Different Planting Densities. Front. Plant Sci. 2021, 11, 609977. [Google Scholar] [CrossRef]
- Fleischer, W.E. From the Laboratory of Plan—Physiology; Cornell University: Ithaca, NY, USA, 1935. [Google Scholar]
- Ji, Y.; Ocaña, D.N.; Choe, D.; Larsen, D.H.; Marcelis, L.F.M.; Heuvelink, E. Far-red radiation stimulates dry mass partitioning to fruits by increasing fruit sink strength in tomato. New Phytol. 2020, 228, 1914–1925. [Google Scholar] [CrossRef]
- Pawłowska, B.; Szewczyk-Taranek, B.; Dziedzic, E.; Żupnik, M. Rooting response under LED systems in Rosa canina in vitro cultures. Acta Hortic. 2017, 1155, 519–524. [Google Scholar] [CrossRef]
- Christiaens, A.; Gobin, B.; Van Huylenbroeck, J.; Van Labeke, M.-C. Adventitious rooting of Chrysanthemum is stimulated by a low red:far-red ratio. J. Plant Physiol. 2019, 236, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Peçanha, D.A.; Moro Peña, J.Á.; Freitas, M.S.M.; Chourak, Y.; Urrestarazu, M. Effect of light spectra on stem cutting rooting and lavender growth. Acta Sci. Agron. 2023, 45, e58864. [Google Scholar] [CrossRef]
- Wang, J.-F.; Zhang, T.-L.; Fu, B.-J. A measure of spatial stratified heterogeneity. Ecol. Indic. 2016, 67, 250–256. [Google Scholar] [CrossRef]
- Lund, J.B.; Blom, T.J.; Aaslyng, J.M. End-of-day Lighting with Different Red/Far-red Ratios Using Light-emitting Diodes Affects Plant Growth of Chrysanthemum × morifolium Ramat. ‘Coral Charm.’ HortScience 2007, 42, 1609–1611. [Google Scholar] [CrossRef]
- Eaves, J.; Eaves, S.; Morphy, C.; Murray, C. The relationship between light intensity, cannabis yields, and profitability. Agron. J. 2020, 112, 1466–1470. [Google Scholar] [CrossRef]
- Llewellyn, D.; Golem, S.; Foley, E.; Dinka, S.; Jones, A.M.P.; Zheng, Y. Indoor grown cannabis yield increased proportionally with light intensity, but ultraviolet radiation did not affect yield or cannabinoid content. Front. Plant Sci. 2022, 13, 974018. [Google Scholar] [CrossRef]
- Li, M.; Roman, M.; Yuan, J.; Rehman, M.; Liu, L. Varying light intensity can alter metabolic profile and cannabispiradienone content of industrial hemp. Ind. Crops Prod. 2023, 202, 117031. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Rajesh, M.; Horváth, B.; Bátkai, S.; Park, O.; Tanchian, G.; Gao, R.Y.; Patel, V.; Wink, D.A.; Liaudet, L.; et al. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radic. Biol. Med. 2011, 50, 1368–1381. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.; Ahmadi, S.; De Costa, F.; Li, N.; Kerman, K. Attenuation of Oxidative Stress by Cannabinoids and Cannabis Extracts in Differentiated Neuronal Cells. Pharmaceuticals 2020, 13, 328. [Google Scholar] [CrossRef]
- Kim, H.-H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light Supplementation for Enhanced Lettuce Growth under Red- and Blue-light-emitting Diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal red:blue ratio in led lighting for nutraceutical indoor horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Jenkins, M.W.; Livesay, C.B. Photosynthetic Performance and Potency of Cannabis sativa L. Grown under LED and HPS Illumination. Agric. Sci. 2021, 12, 293–304. [Google Scholar] [CrossRef]
- Carranza-Ramírez, J.E.; Borda, A.M.; Moreno-Fonseca, L.P. LED light modifies plant architecture, physiological parameters and cannabinoid content in three varieties of Cannabis sativa L. S. Afr. J. Bot. 2025, 176, 231–240. [Google Scholar] [CrossRef]
- Kotiranta, S.; Pihlava, J.-M.; Kotilainen, T.; Palonen, P. The morphology, inflorescence yield, and secondary metabolite accumulation in hemp type Cannabis sativa can be influenced by the R:FR ratio or the amount of short wavelength radiation in a spectrum. Ind. Crops Prod. 2024, 208, 117772. [Google Scholar] [CrossRef]
- Holweg, M.M.S.F.; Kaiser, E.; Kappers, I.F.; Heuvelink, E.; Marcelis, L.F.M. The role of red and white light in optimizing growth and accumulation of plant specialized metabolites at two light intensities in medical cannabis (Cannabis sativa L.). Front. Plant Sci. 2024, 15, 1393803. [Google Scholar] [CrossRef]
- Takeda, F.; Newell, M. A Method for Increasing Fall Flowering in Short-day `Carmine’ Strawberry. HortScience 2006, 41, 480–481. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Substituting Far-Red for Traditionally Defined Photosynthetic Photons Results in Equal Canopy Quantum Yield for CO2 Fixation and Increased Photon Capture During Long-Term Studies: Implications for Re-Defining PAR. Front. Plant Sci. 2020, 11, 581156. [Google Scholar] [CrossRef] [PubMed]
- Kotiranta, S.; Sarka, A.; Kotilainen, T.; Palonen, P. Decreasing R:FR ratio in a grow light spectrum increases inflorescence yield but decreases plant specialized metabolite concentrations in Cannabis sativa. Environ. Exp. Bot. 2025, 229, 106059. [Google Scholar] [CrossRef]
- Peterswald, T.J.; Mieog, J.C.; Azman Halimi, R.; Magner, N.J.; Trebilco, A.; Kretzschmar, T.; Purdy, S.J. Moving Away from 12:12; the Effect of Different Photoperiods on Biomass Yield and Cannabinoids in Medicinal Cannabis. Plants 2023, 12, 1061. [Google Scholar] [CrossRef]
- Chazaux, M.; Schiphorst, C.; Lazzari, G.; Caffarri, S. Precise estimation of chlorophyll a, b and carotenoid content by deconvolution of the absorption spectrum and new simultaneous equations for Chl determination. Plant J. 2022, 109, 1630–1648. [Google Scholar] [CrossRef]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.; Samaniego, I.; López, D.; Viera, W.; Mejía, P.; Jaramillo, P.; Viteri, P.; Gaona, P. Yield and content of cannabidiol (CBD) and tetrahydrocannabinol (THC) in medicinal cannabis (Cannabis sativa) grown in the Ecuadorian highlands. Manglar 2024, 21, 107–113. [Google Scholar] [CrossRef]
- Amrein, P.; Rinner, S.; Pittorino, T.; Espel, J.; Schmidmayr, D. Influence of Light Spectra on the Production of Cannabinoids. Med. Cannabis Cannabinoids 2020, 3, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.S.; Yun, C.M. Dynamic spectrum lighting impact on plant morphology and cannabinoid profile of medical and recreational cannabis—A novel leapfrog strategy towards shaping the future of horticulture lighting. Ind. Crops Prod. 2023, 199, 116799. [Google Scholar] [CrossRef]
- Folta, K.M.; Carvalho, S.D. Photoreceptors and Control of Horticultural Plant Traits. HortScience 2015, 50, 1274–1280. [Google Scholar] [CrossRef]
- Pocock, T. Light-emitting Diodes and the Modulation of Specialty Crops: Light Sensing and Signaling Networks in Plants. HortScience 2015, 50, 1281–1284. [Google Scholar] [CrossRef]
- Thoma, F.; Somborn-Schulz, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Effects of Light on Secondary Metabolites in Selected Leafy Greens: A Review. Front. Plant Sci. 2020, 11, 497. [Google Scholar] [CrossRef]
- Meng, Q.; Boldt, J.; Runkle, E.S. Blue Radiation Interacts with Green Radiation to Influence Growth and Predominantly Controls Quality Attributes of Lettuce. J. Am. Soc. Hortic. Sci. 2020, 145, 75–87. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue Light added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef]
- Hawley, D.; Graham, T.; Stasiak, M.; Dixon, M. Improving Cannabis Bud Quality and Yield with Subcanopy Lighting. HortScience 2018, 53, 1593–1599. [Google Scholar] [CrossRef]
- Hall, W.; Stjepanović, D.; Caulkins, J.; Lynskey, M.; Leung, J.; Campbell, G.; Degenhardt, L. Public health implications of legalising the production and sale of cannabis for medicinal and recreational use. Lancet 2019, 394, 1580–1590. [Google Scholar] [CrossRef]
- Maliakal, S.K.; McDonnell, K.; Dudley, S.A.; Schmitt, J. Effects of Red to Far-Red Ratio and Plant Density on Biomass Allocation and Gas Exchange in Impatiens capensis. Int. J. Plant Sci. 1999, 160, 723–733. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; Van Ieperen, W.; Harbinson, J.; Van Der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of Continuous or End-of-Day Far-Red Light on Tomato Plant Growth, Morphology, Light Absorption, and Fruit Production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, E.; Ji, Y.; Kerstens, T.; Lai, X.; Deelen, S.; De Beer, E.; Millenaar, F.; Marcelis, L.F.M.; Heuvelink, E. Duration, not timing during the photoperiod, of far-red application determines the yield increase in tomato. Sci. Hortic. 2024, 338, 113553. [Google Scholar] [CrossRef]
- Sarlikioti, V.; De Visser, P.H.B.; Buck-Sorlin, G.H.; Marcelis, L.F.M. How plant architecture affects light absorption and photosynthesis in tomato: Towards an ideotype for plant architecture using a functional–structural plant model. Ann. Bot. 2011, 108, 1065–1073. [Google Scholar] [CrossRef]
- Takenaka, A. Effects of leaf blade narrowness and petiole length on the light capture efficiency of a shoot. Ecol. Res. 1994, 9, 109–114. [Google Scholar] [CrossRef]
- Casal, J.J. Photoreceptor Signaling Networks in Plant Responses to Shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta BBA—Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Moher, M.; Jones, M.; Zheng, Y. Photoperiodic Response of In Vitro Cannabis sativa Plants. HortScience 2021, 56, 108–113. [Google Scholar] [CrossRef]
- Cervantes, J. The Cannabis Encyclopedia: The Definitive Guide to Cultivation & Consumption of Medical Marijuana; Van Patten Publishing: Vancouver, WA, USA, 2015. [Google Scholar]
- Dang, M.; Arachchige, N.M.; Campbell, L.G. Optimizing Photoperiod Switch to Maximize Floral Biomass and Cannabinoid Yield in Cannabis sativa L. A Meta-Analytic Quantile Regression Approach. Front. Plant Sci. 2022, 12, 797425. [Google Scholar] [CrossRef] [PubMed]
- Morello, V.; Brousseau, V.D.; Wu, N.; Wu, B.-S.; MacPherson, S.; Lefsrud, M. Light Quality Impacts Vertical Growth Rate, Phytochemical Yield and Cannabinoid Production Efficiency in Cannabis sativa. Plants 2022, 11, 2982. [Google Scholar] [CrossRef]
- Ahrens, A.; Llewellyn, D.; Zheng, Y. Longer Photoperiod Substantially Increases Indoor-Grown Cannabis’ Yield and Quality: A Study of Two High-THC Cultivars Grown under 12 h vs. 13 h Days. Plants 2024, 13, 433. [Google Scholar] [CrossRef]
- Choi, H.; Back, S.; Kim, G.W.; Lee, K.; Venkatesh, J.; Lee, H.B.; Kwon, J.-K.; Kang, B.-C. Development of a speed breeding protocol with flowering gene investigation in pepper (Capsicum annuum). Front. Plant Sci. 2023, 14, 1151765. [Google Scholar] [CrossRef]
- Gao, C.; Xin, P.; Cheng, C.; Tang, Q.; Chen, P.; Wang, C.; Zang, G.; Zhao, L. Diversity Analysis in Cannabis sativa Based on Large-Scale Development of Expressed Sequence Tag-Derived Simple Sequence Repeat Markers. PLoS ONE 2014, 9, e110638. [Google Scholar] [CrossRef]
- Lynch, R.C.; Vergara, D.; Tittes, S.; White, K.; Schwartz, C.J.; Gibbs, M.J.; Ruthenburg, T.C.; deCesare, K.; Land, D.P.; Kane, N.C. Genomic and Chemical Diversity in Cannabis. Crit. Rev. Plant Sci. 2016, 35, 349–363. [Google Scholar] [CrossRef]
- Sawler, J.; Stout, J.M.; Gardner, K.M.; Hudson, D.; Vidmar, J.; Butler, L.; Page, J.E.; Myles, S. The Genetic Structure of Marijuana and Hemp. PLoS ONE 2015, 10, e0133292. [Google Scholar] [CrossRef]
- Watson, A.; Ghosh, S.; Williams, M.J.; Cuddy, W.S.; Simmonds, J.; Rey, M.-D.; Asyraf Md Hatta, M.; Hinchliffe, A.; Steed, A.; Reynolds, D.; et al. Speed breeding is a powerful tool to accelerate crop research and breeding. Nat. Plants 2018, 4, 23–29. [Google Scholar] [CrossRef]
- Schilling, S.; Melzer, R.; Dowling, C.A.; Shi, J.; Muldoon, S.; McCabe, P.F. A protocol for rapid generation cycling (speed breeding) of hemp (Cannabis sativa) for research and agriculture. Plant J. 2023, 113, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P.; et al. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 2018, 13, 2944–2963. [Google Scholar] [CrossRef]
- Segrestin, J.; Bernard-Verdier, M.; Violle, C.; Richarte, J.; Navas, M.; Garnier, E. When is the best time to flower and disperse? A comparative analysis of plant reproductive phenology in the Mediterranean. Funct. Ecol. 2018, 32, 1770–1783. [Google Scholar] [CrossRef]
- Stack, G.M.; Toth, J.A.; Carlson, C.H.; Cala, A.R.; Marrero-González, M.I.; Wilk, R.L.; Gentner, D.R.; Crawford, J.L.; Philippe, G.; Rose, J.K.C.; et al. Season-long characterization of high-cannabinoid hemp (Cannabis sativa L.) reveals variation in cannabinoid accumulation, flowering time, and disease resistance. GCB Bioenergy 2021, 13, 546–561. [Google Scholar] [CrossRef]
- Dong, H.; Clark, L.V.; Jin, X.; Anzoua, K.; Bagmet, L.; Chebukin, P.; Dzyubenko, E.; Dzyubenko, N.; Ghimire, B.K.; Heo, K.; et al. Managing flowering time in Miscanthus and sugarcane to facilitate intra- and intergeneric crosses. PLoS ONE 2021, 16, e0240390. [Google Scholar] [CrossRef] [PubMed]
- Stetter, M.G.; Zeitler, L.; Steinhaus, A.; Kroener, K.; Biljecki, M.; Schmid, K.J. Crossing Methods and Cultivation Conditions for Rapid Production of Segregating Populations in Three Grain Amaranth Species. Front. Plant Sci. 2016, 7, 816. [Google Scholar] [CrossRef] [PubMed]
- Ferwerda, F.P. Methods to synchronize the flowering time of the components in crossing plots for the production of hybrid seed corn. Euphytica 1953, 2, 127–134. [Google Scholar] [CrossRef]
- Dowling, C.A.; Shi, J.; Toth, J.A.; Quade, M.A.; Smart, L.B.; McCabe, P.F.; Schilling, S.; Melzer, R. A FLOWERING LOCUS T ortholog is associated with photoperiod-insensitive flowering in hemp (Cannabis sativa L.). Plant J. 2024, 119, 383–403. [Google Scholar] [CrossRef]
- Leckie, K.M.; Sawler, J.; Kapos, P.; MacKenzie, J.O.; Giles, I.; Baynes, K.; Lo, J.; Baute, G.J.; Celedon, J.M. Loss of daylength sensitivity by splice site mutation in Cannabis pseudo-response regulator. Plant J. 2024, 1186, 2020–2036. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, S.; Jose-Santhi, J.; Kalia, D.; Singh, R.K. Hormones regulate the flowering process in saffron differently depending on the developmental stage. Front. Plant Sci. 2023, 14, 1107172. [Google Scholar] [CrossRef]
- Burgel, L.; Hartung, J.; Schibano, D.; Graeff-Hönninger, S. Impact of Different Phytohormones on Morphology, Yield and Cannabinoid Content of Cannabis sativa L. Plants 2020, 9, 725. [Google Scholar] [CrossRef]
- Garrido, J.; Rico, S.; Corral, C.; Sánchez, C.; Vidal, N.; Martínez-Quesada, J.J.; Ferreiro-Vera, C. Exogenous application of stress-related signaling molecules affect growth and cannabinoid accumulation in medical cannabis (Cannabis sativa L.). Front. Plant Sci. 2022, 13, 1082554. [Google Scholar] [CrossRef]
- Lorensen, M.D.B.B.; Hayat, S.Y.; Wellner, N.; Bjarnholt, N.; Janfelt, C. Leaves of Cannabis sativa and their trichomes studied by DESI and MALDI mass spectrometry imaging for their contents of cannabinoids and flavonoids. Phytochem. Anal. 2023, 34, 269–279. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Plackett, A.R.G.; Wilson, Z.A. Gibberellins and Plant Reproduction. In Annual Plant Reviews Online; Roberts, J.A., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 323–358. [Google Scholar]
- Lee, J.; Oh, M.; Park, H.; Lee, I. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates LEAFY. Plant J. 2008, 55, 832–843. [Google Scholar] [CrossRef]
- Song, Y.H.; Lee, I.; Lee, S.Y.; Imaizumi, T.; Hong, J.C. CONSTANS and ASYMMETRIC LEAVES 1 complex is involved in the induction of FLOWERING LOCUS T in photoperiodic flowering in Arabidopsis. Plant J. 2012, 69, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Klasfeld, S.; Jeong, C.W.; Jin, R.; Goto, K.; Yamaguchi, N.; Wagner, D. TERMINAL FLOWER 1-FD complex target genes and competition with FLOWERING LOCUS T. Nat. Commun. 2020, 11, 5118. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, A.; Narváez-Zapata, J.A.; Salvador-Figueroa, M. Genes Involved in the Transition and Floral Sexual Differentiation of Jatropha curcas L. Plant Mol. Biol. Rep. 2024, 42, 201–217. [Google Scholar] [CrossRef]
- Alter, H.; Sade, Y.; Sood, A.; Carmeli-Weissberg, M.; Shaya, F.; Kamenetsky-Goldstein, R.; Bernstein, N.; Spitzer-Rimon, B. Inflorescence development in female cannabis plants is mediated by photoperiod and gibberellin. Hortic. Res. 2024, 11, uhae245. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.F.F.; Silva, E.M.; Correa, J.P.O.; Vicente, M.H.; Jiang, N.; Notini, M.M.; Junior, A.C.; De Jesus, F.A.; Castilho, P.; Carrera, E.; et al. Tomato floral induction and flower development are orchestrated by the interplay between gibberellin and two unrelated micro RNA -controlled modules. New Phytol. 2019, 221, 1328–1344. [Google Scholar] [CrossRef]
- King, R.W.; Ben-Tal, Y. A Florigenic Effect of Sucrose in Fuchsia hybrida Is Blocked by Gibberellin-Induced Assimilate Competition. Plant Physiol. 2001, 125, 488–496. [Google Scholar] [CrossRef]
- Reinecke, D.M.; Wickramarathna, A.D.; Ozga, J.A.; Kurepin, L.V.; Jin, A.L.; Good, A.G.; Pharis, R.P. Gibberellin 3-oxidase Gene Expression Patterns Influence Gibberellin Biosynthesis, Growth, and Development in Pea. Plant Physiol. 2013, 163, 929–945. [Google Scholar] [CrossRef]
- Flajšman, M.; Slapnik, M.; Murovec, J. Production of Feminized Seeds of High CBD Cannabis sativa L. by Manipulation of Sex Expression and Its Application to Breeding. Front. Plant Sci. 2021, 12, 718092. [Google Scholar] [CrossRef]
- Baiton, A. Novel Strategies for Sustainable Rapid Breeding of Cannabis sativa L. Master’s thesis, University of Guelph, Guelph, ON, Canada, 2024. [Google Scholar]
- Gruda, N. Impact of Environmental Factors on Product Quality of Greenhouse Vegetables for Fresh Consumption. Crit. Rev. Plant Sci. 2005, 24, 227–247. [Google Scholar] [CrossRef]
- Summers, H.M.; Sproul, E.; Quinn, J.C. The greenhouse gas emissions of indoor cannabis production in the United States. Nat. Sustain. 2021, 4, 644–650. [Google Scholar] [CrossRef]
- Namdar, D.; Charuvi, D.; Ajjampura, V.; Mazuz, M.; Ion, A.; Kamara, I.; Koltai, H. LED lighting affects the composition and biological activity of Cannabis sativa secondary metabolites. Ind. Crops Prod. 2019, 132, 177–185. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bassal, A.; Leonardi, C.; Giuffrida, F.; Colla, G. Vegetable quality as affected by genetic, agronomic and environmental factors. J. Food Agric. Environ. 2012, 10, 680–688. [Google Scholar]
- Nitz, G.M.; Grubmüller, E.; Schnitzler, W.H. Differential Flavonoid Response to PAR and UV-B Light in Chive (Allium schoenoprasum L.). Acta Hortic. 2004, 659, 825–830. [Google Scholar] [CrossRef]
- Bafort, F.; Libault, A.; Maron, E.; Kohnen, S.; Ancion, N.; Jijakli, M.H. Operational Costs and Analysis of Agronomic Characteristics on Cannabidiol and Cannabigerol Hemp (Cannabis sativa L.) in Hydroponic Soilless Greenhouse and Field Cultivation. Horticulturae 2024, 10, 1271. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Hernández, A.; Ferreiro-Vera, C.; Zuazo, V.H.D.; García, J.H.; Sánchez-Carnerero, C.; Casano, S. Yield of new hemp varieties for medical purposes under semi-arid Mediterranean environment conditions. Comun. Sci. 2020, 11, e3264. [Google Scholar] [CrossRef]
- Charles, A.P.R.; Gu, Z.; Archer, R.; Auwarter, C.; Hatterman-Valenti, H.; Rao, J.; Chen, B. Effect of High-Tunnel and Open-Field Production on the Yield, Cannabinoids, and Volatile Profiles in Industrial Hemp (Cannabis sativa L.) Inflorescence. J. Agric. Food Chem. 2024, 72, 12975–12987. [Google Scholar] [CrossRef]
- García-Tejero, I.F.; Durán Zuazo, V.H.; Sánchez-Carnenero, C.; Hernández, A.; Ferreiro-Vera, C.; Casano, S. Seeking suitable agronomical practices for industrial hemp (Cannabis sativa L.) cultivation for biomedical applications. Ind. Crops Prod. 2019, 139, 111524. [Google Scholar] [CrossRef]
- Lecholocholo, N.; Shoko, T.; Manhivi, V.E.; Maboko, M.M.; Akinola, S.A.; Sivakumar, D. Influence of different rootstocks on quality and volatile constituents of cantaloupe and honeydew melons (Cucumis melo. L) grown in high tunnels. Food Chem. 2022, 393, 133388. [Google Scholar] [CrossRef]
- Patel, H.; Taghavi, T.; Samtani, J.B. Fruit Quality of Several Strawberry Cultivars during the Harvest Season under High Tunnel and Open Field Environments. Horticulturae 2023, 9, 1084. [Google Scholar] [CrossRef]
- Jameel, M.M.; Khairie Khessro, M.; Jawad AL-Bayati, H. Effect Traditional Greenhouse (High Tunnel) in the Characteristics of Growth and Yield of the Cucumber. Plant Arch. 2021, 21, 781–784. [Google Scholar] [CrossRef]
- Zheng, Y.; Llewellyn, D. Lighting and CO2 in cannabis production. In Handbook of Cannabis Production in Controlled Environments; CRC Press: Boca Raton, FL, USA, 2022; pp. 163–188. [Google Scholar]
- Caplan, D.; Dixon, M.; Zheng, Y. Optimal Rate of Organic Fertilizer during the Vegetative-stage for Cannabis Grown in Two Coir-based Substrates. HortScience 2017, 52, 1307–1312. [Google Scholar] [CrossRef]
- Reichel, P.; Munz, S.; Hartung, J.; Kotiranta, S.; Graeff-Hönninger, S. Impacts of Different Light Spectra on CBD, CBDA and Terpene Concentrations in Relation to the Flower Positions of Different Cannabis sativa L. Strains. Plants 2022, 11, 2695. [Google Scholar] [CrossRef]
- Hazekamp, A. An evaluation of the quality of medicinal grade cannabis in the Netherlands. Cannabinoids 2006, 1, 1–9. [Google Scholar]
- Kowal, M.A.; Hazekamp, A.; Grotenhermen, F. Review on clinical studies with cannabis and cannabinoids 2010–2014. Cannabinoids 2016, 11, 1–18. [Google Scholar]
- Burgie, E.S.; Bussell, A.N.; Walker, J.M.; Dubiel, K.; Vierstra, R.D. Crystal structure of the photosensing module from a red/far-red light-absorbing plant phytochrome. Proc. Natl. Acad. Sci. USA 2014, 111, 10179–10184. [Google Scholar] [CrossRef] [PubMed]
- Galvão, V.C.; Fankhauser, C. Sensing the light environment in plants: Photoreceptors and early signaling steps. Curr. Opin. Neurobiol. 2015, 34, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Krahmer, J.; Ganpudi, A.; Abbas, A.; Romanowski, A.; Halliday, K.J. Phytochrome, Carbon Sensing, Metabolism, and Plant Growth Plasticity. Plant Physiol. 2018, 176, 1039–1048. [Google Scholar] [CrossRef]
- Morrow, R.C. LED Lighting in Horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Mills, E. Energy-intensive indoor cultivation drives the cannabis industry’s expanding carbon footprint. One Earth 2025, 8, 101179. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Too Dense or Not Too Dense: Higher Planting Density Reduces Cannabinoid Uniformity but Increases Yield/Area in Drug-Type Medical Cannabis. Front. Plant Sci. 2022, 13, 713481. [Google Scholar] [CrossRef]
- Benevenute, S.d.S.; Freeman, J.H.; Yang, R. How do pinching and plant density affect industrial hemp produced for cannabinoids in open field conditions? Agron. J. 2021, 114, 618–626. [Google Scholar] [CrossRef]
- Feeney, M.; Punja, Z.K. Tissue culture and Agrobacterium-mediated transformation of hemp (Cannabis sativa L.). Vitr. Cell. Dev. Biol.-Plant 2003, 39, 578–585. [Google Scholar] [CrossRef]
- Lata, H.; Chandra, S.; Khan, I.; ElSohly, M.A. Thidiazuron-induced high-frequency direct shoot organogenesis of Cannabis sativa L. Vitr. Cell. Dev. Biol.-Plant 2009, 45, 12–19. [Google Scholar] [CrossRef]
- Piunno, K.F.; Golenia, G.; Boudko, E.A.; Downey, C.; Jones, A.M.P. Regeneration of shoots from immature and mature inflorescences of Cannabis sativa. Can. J. Plant Sci. 2019, 99, 556–559. [Google Scholar] [CrossRef]
- Mobini, S.H.; Lulsdorf, M.; Warkentin, T.D.; Vandenberg, A. Plant growth regulators improve in vitro flowering and rapid generation advancement in lentil and faba bean. Vitr. Cell. Dev. Biol.-Plant 2015, 51, 71–79. [Google Scholar] [CrossRef]
- Adams, S.R.; Valdés, V.M.; Fuller, D. The effects of day and night temperature on Chrysanthemum morifolium: Investigating the safe limits for temperature integration. J. Hortic. Sci. Biotechnol. 2009, 84, 604–608. [Google Scholar] [CrossRef]
- Hall, J.; Bhattarai, S.P.; Midmore, D.J. The Effects of Photoperiod on Phenological Development and Yields of Industrial Hemp. J. Nat. Fibers 2014, 11, 87–106. [Google Scholar] [CrossRef]
- Croser, J.S.; Pazos-Navarro, M.; Bennett, R.G.; Tschirren, S.; Edwards, K.; Erskine, W.; Creasy, R.; Ribalta, F.M. Time to flowering of temperate pulses in vivo and generation turnover in vivo–in vitro of narrow-leaf lupin accelerated by low red to far-red ratio and high intensity in the far-red region. Plant Cell Tissue Organ Cult. 2016, 127, 591–599. [Google Scholar] [CrossRef]

| Light Parameter | Effect on Growth | Effect on Cannabinoid/Terpene Synthesis | References |
|---|---|---|---|
| Blue Light (400–500 nm) | Promotes compact growth, leaf expansion | Increases THC and CBD content | [51,92,93,94] |
| Red Light (600–700 nm) | Enhances stem elongation, flowering | Modulates flowering time, may affect cannabinoids | [75,76,95,96] |
| Far-Red Light (700–750 nm) | Influences flowering and shade avoidance | Alters secondary metabolite profiles | [63,84,97,98,99] |
| Light Intensity | Higher intensity increases photosynthesis | Enhances biomass and cannabinoid yield | [85,86,87,88,89,95,96,100,101] |
| Photoperiod | Controls flowering induction | Affects timing and levels of cannabinoid accumulation | [41,49,99,102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saragoça, A.; Silva, A.C.; Varanda, C.M.R.; Materatski, P.; Ortega, A.; Cordeiro, A.I.; Telo da Gama, J. Current Context of Cannabis sativa Cultivation and Parameters Influencing Its Development. Agriculture 2025, 15, 1635. https://doi.org/10.3390/agriculture15151635
Saragoça A, Silva AC, Varanda CMR, Materatski P, Ortega A, Cordeiro AI, Telo da Gama J. Current Context of Cannabis sativa Cultivation and Parameters Influencing Its Development. Agriculture. 2025; 15(15):1635. https://doi.org/10.3390/agriculture15151635
Chicago/Turabian StyleSaragoça, Andreia, Ana Cláudia Silva, Carla M. R. Varanda, Patrick Materatski, Alfonso Ortega, Ana Isabel Cordeiro, and José Telo da Gama. 2025. "Current Context of Cannabis sativa Cultivation and Parameters Influencing Its Development" Agriculture 15, no. 15: 1635. https://doi.org/10.3390/agriculture15151635
APA StyleSaragoça, A., Silva, A. C., Varanda, C. M. R., Materatski, P., Ortega, A., Cordeiro, A. I., & Telo da Gama, J. (2025). Current Context of Cannabis sativa Cultivation and Parameters Influencing Its Development. Agriculture, 15(15), 1635. https://doi.org/10.3390/agriculture15151635








