Abstract
Soil pH strongly influences phosphorus (P) availability and, consequently, plant response to P fertilization. This study aimed to assess how soil pH affects P availability, uptake, and fertilizer use efficiency in maize (Zea mays L.) grown under controlled conditions. A pot experiment was conducted using three soil pHKCl levels (4.2, 5.2, and 6.4) and five P application doses (0, 0.5, 1, 1.5, and 2 g P pot−1). Each pot contained 10 kg of soil. Results showed that soil P concentration after harvest increased with both P dose and pH, with the highest values recorded at pH 6.4. Maize grain and straw yields responded differently to P fertilization depending on pH. At pH 5.2, the highest grain yield and agronomic efficiency (AE) were observed at the 0.5 g P dose, while higher doses led to yield reductions. At pH 4.2, P fertilization significantly increased both grain yield and P uptake, but excessive doses reduced yields. In contrast, at pH 6.4, yield increased steadily with rising P doses, though AE and apparent phosphorus recovery (APR) were lowest. The highest APR was observed at pH 4.2 and the lowest at pH 6.4. Overall, the results suggest that optimal maize response to P fertilization occurs near pH 5.2, where both yield and efficiency indices peak.
1. Introduction
Modern agriculture is inconceivable without the application of phosphorus (P) fertilizers, as achieving satisfactory crop yields is not feasible without soil supplementation of this essential nutrient [1]. However, excessive or improper application of P can have significant adverse effects on the environment. Aquatic ecosystems are particularly at risk, as nutrient overload, primarily nitrogen (N) and phosphorus, can lead to eutrophication. These elements may leach from fertilizers into groundwater through infiltration or enter surface waters via runoff, eventually reaching large water bodies such as lakes, rivers, and seas [2,3]. Elevated concentrations of N and P stimulate excessive algal growth, which depletes dissolved oxygen and leads to the death of fish and other aquatic organisms.
In Europe, the Baltic Sea is particularly vulnerable to nutrient pollution, and the surrounding countries have undertaken efforts to mitigate its progressive degradation. One approach to reducing this environmental pressure is to revise agricultural phosphorus management practices. There is a pressing need to develop new fertilization guidelines that align not only with crop productivity goals, but also with environmental sustainability [4,5]. Past recommendations focused primarily on maximizing yields, which is no longer sufficient in the face of environmental challenges. Phosphorus application rates must now meet crop demands without posing a threat to groundwater or surface water quality. Therefore, a better understanding of plant P uptake, P losses through leaching, and fertilizer P use efficiency is essential.
Phosphorus losses through leaching are especially pronounced in coarse-textured sandy soils. These soils are characterized by rapid infiltration rates and a low capacity to retain water and nutrients [6]. The large pore spaces between soil particles allow water to move quickly through the unsaturated zone and reach the groundwater [7]. In Poland, which is one of the four largest exporters of riverine water to the Baltic Sea, agricultural land is predominantly situated on coarse-textured sandy soils [8]. Moreover, Polish farmland accounts for over 50% of the agricultural area within the entire Baltic catchment [9]. These two factors contribute to the disproportionately high share of phosphorus emissions from Polish agriculture to the Baltic Sea [10,11]. Therefore, research into optimized P application rates and P use efficiency should be regarded as a priority, as any surplus P not absorbed by plants or retained by the soil poses a potential risk to water quality.
Phosphorus uptake by plants depends largely on soil pH. It is commonly accepted that soil pH is one of the main factors influencing phosphorus availability to plants [12,13]. According to the traditional view, phosphorus in acidic soils is bound by aluminum (Al) and iron (Fe), while in alkaline soils, it is immobilized by calcium (Ca). The highest phosphorus solubility—and, thus, its availability to plants—is assumed to occur at pHCaCl2 between 6.5 and 7.0, depending on soil type and plant species [12,14,15]. However, not all researchers agree with this opinion. Barrow [16] and Barrow et al. [17] argue that this view is incorrect and suggest that the optimal pHCaCl2 for phosphorus availability is slightly above 5, rather than close to 7.
Fertilizer P use efficiency can be evaluated using several agronomic indices that describe the relationship between the amount of applied P and plant biomass production [18,19]. The most commonly used indices include agronomic efficiency (AE), apparent P recovery (APR), agrophysiological efficiency (APE), physiological efficiency (PE), and utilization efficiency (UE). These indices are calculated based on plant yield and phosphorus uptake. For cereal crops, AE is the grain yield per unit of P applied, APR is the phosphorus uptake per unit of P applied, APE is the grain yield per unit of P uptake, and PE is the combined yield of grain and straw per unit of P uptake. Utilization efficiency (UE) is calculated as the product of PE and APR [20,21].
Due to the ongoing discussion in the literature about the effect of soil pH on phosphorus availability, we decided to investigate how fertilizer P use efficiency in sandy soil depends on pH. Sandy soil was selected because of its large presence in the Baltic Sea catchment area and its high permeability, which increases the risk of phosphorus leaching. A pot experiment was used to reduce the variability of soil conditions and avoid weather-related risks, such as too little or too much rainfall. Although field experiments allow for the testing of realistic phosphorus rates, they can be affected by field heterogeneity and unpredictable weather, which has become more frequent. Pot experiments under controlled conditions eliminate these problems and allow for the testing of a large number of treatments [17].
The aim of our study was to assess the impact of three soil pH levels and different phosphorus application rates on AE and APR in sandy soil under pot experiment conditions. The results, supplemented by field studies, will be helpful in developing new phosphorus fertilization strategies that support environmental protection.
2. Materials and Methods
2.1. Pot Experiment
In 2024, a pot experiment was conducted in a greenhouse at the Experimental Station of the Institute of Soil Science and Plant Cultivation, located in Jelcz-Laskowice near Wroclaw, Poland. Mitcherlich-type pots with a capacity of 10 kg of soil were used. The pots were filled with sandy soil characterized by a very low phosphorus concentration that was collected from an agricultural field near the greenhouse. After collection, the soil was heaped, thoroughly homogenized, and sieved to remove impurities such as stones and roots. The basic properties of the soil are presented in Table 1.

Table 1.
Initial properties of soil used in the experiment.
The experiment included 15 treatments: 5 phosphorus doses (0, 0.5, 1, 1.5, and 2 g P pot−1) at 3 soil pH levels (4.2, 5.2, 6.4), each in 4 replications (a total of 60 pots). The experiment was arranged in a completely randomized design.
The three target pH levels of the experimental soil were achieved by (1) adding 2.5 g pot−1 of elemental sulfur, (2) no amendment, preserving the soil’s native pH, and (3) adding 10 g pot−1 of calcium carbonate (CaCO3). Sulfur and CaCO3 were incorporated during pot preparation and thoroughly mixed with the soil. After a 1.5-month incubation period, three distinct pH levels were established, as shown in Table 2. The exchangeable Ca and AlMehlich 3 contents at increasing pH levels were 600, 590, and 870 mg kg−1, and 534, 523, and 506 mg kg−1, respectively.

Table 2.
Soil pHKCl after 1.5 months of incubation (mean of 4 replications ± SE).
After pH stabilization, P was applied according to the experimental design, along with background fertilization of N, K, and Mg. Soil from each pot was removed, mixed thoroughly with fertilizers, and returned to the pots. P was added as NaH2PO4·2H2O, N at 1 g pot−1 as NH4NO3 (plus 1 g pot−1 as top dressing), K at 2.5 g pot−1 as KCl, and Mg at 0.5 g pot−1 as MgSO4·7H2O (Table 3).

Table 3.
Dates of agrotechnical treatments.
The test plant—maize (Zea mays L., hybrid Pionier 8604)—was sown two weeks after fertilizer application at a rate of 5 seeds per pot. Three weeks after emergence, seedlings were thinned to 2 plants per pot. Soil moisture was maintained at 70% of field water capacity (FWC). No insect, pest, or disease infestation was observed throughout the growing period. Maize was harvested at full maturity. Plants were cut 5 cm above ground level, separated into stalks and ears, and dried at 65 °C until constant weight was reached. Ears were threshed manually, and grain and straw yields were determined for each pot. Additionally, samples of grain, straw, and soil were collected from each pot. Plant material was finely ground, while soil samples were dried at room temperature, ground in a mortar, and passed through a 2 mm sieve.
2.2. Chemical Analysis
P content in plant material was determined using continuous flow analysis (CFA) with spectrophotometric detection. For soil samples, the Enger–Riehm (DL) method was employed, using 0.02 M calcium lactate as the extractant, followed by determination via phosphomolybdenum blue spectrophotometry [22]. Just before the experiment was established, soil pH was measured potentiometrically in 1 mol dm−3 KCl [23], total organic carbon (TOC) by the Tiurin method with potassium dichromate [24], texture by the aerometric method [25], K using the Enger–Riehm method [26], and Mg by the Schachtschabel method [27]. Exchangeable Ca in the soil was extracted with 1 mol dm−3 ammonium acetate and measured by FAAS. S-SO4 was extracted using ammonium acetate + acetic acid and measured by ICP-OES. Al in the soil was determined using the Mehlich 3 method: extraction with 0.2 M CH3COOH + 0.25 M NH4NO3 + 0.015 M NH4F + 0.013 M HNO3 + 0.001 M EDTA followed by measurement using FAAS.
All chemical analyses of soil and plant material were conducted at the Central Chemical Laboratory of the Institute of Soil Science and Plant Cultivation–State Research Institute in Pulawy, accredited by the Polish Centre for Accreditation (certificate no. AB 339, in accordance with PN-EN ISO/IEC 17025) [28].
2.3. Calculation of Harvest Index, Agronomic Efficiency, and Apparent Recovery
Harvest index (HI) was calculated as follows.
HI = grain yield/(grain yield + straw yield) × 100
To assess the effectiveness of applied P doses, two agronomic indices proposed by Fageria [20] were used and calculated as follows:
where YP—grain yield in P-fertilized treatment in g pot−1, Y0—grain yield in unfertilized treatment (control) in g pot−1, Pdose—dose of P in g pot−1:
where UP—P uptake (grain + straw) in P-fertilized treatment in mg pot−1, P uptake (grain + straw) in unfertilized treatment (control) in mg pot−1, Pdose—dose of P in mg pot−1.
Agronomic efficiency (AE) = YP − Y0/Pdose
Apparent recovery (APR) = UP − U0/Pdose × 100
2.4. Statistical Analyses
A two-way ANOVA was used to assess the effects of pH level and P dose on yield components, harvest index (HI), P content in plant and soil, P uptake, and agronomic indices. Statistical analyses were conducted using Statgraphics Centurion XVI (Version 16.2.04, StatPoint Inc., Warrenton, VA, USA). For data with significant differences identified by ANOVA, multiple comparisons among groups were made with a Tukey HSD post-hoc test (p < 0.05).
3. Results
3.1. Phosphorus Concentration in Soil
After maize harvest, P concentration in soil from control treatments ranged from 24 to 30 mg·kg−1, which is considered very low (Table 4). In P-fertilized treatments, soil P content increased consistently with increasing P dose.

Table 4.
Mean (±SE) of P concentration in soil after harvest (mg kg−1).
At P doses of 0.5 and 1 g pot−1, no significant differences in soil P concentration were observed between pH 4.2 and pH 5.2, with values reaching 43 and 60 mg·kg−1, respectively. In contrast, at pH 6.4, significantly higher concentrations were recorded: 55 mg·kg−1 for the 0.5 g dose and 85 mg·kg−1 for the 1 g dose.
At higher doses (1.5 and 2 g pot−1), soil P concentrations varied significantly across pH levels. For the 1.5 g dose, concentrations reached 80, 97, and 125 mg·kg−1 at pH 4.2, 5.2, and 6.4, respectively. At the 2 g dose, values increased further to 96, 121, and 165 mg·kg−1.
3.2. Yields and Harvest Index
Grain yield in control treatments (no P fertilization) varied significantly with soil pH (Figure 1). At pH 4.2, yield reached 30.4 g pot−1, while at pH 5.2, it was more than twice as high. In contrast, at pH 6.4, grain yield was over 20% lower than at pH 5.2, indicating that pH 5.2 provided the most favorable growth conditions for maize.
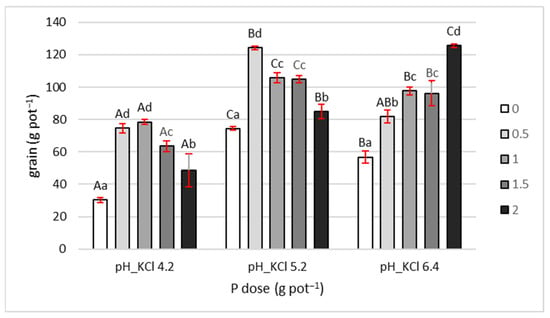
Figure 1.
Grain yield. Values are expressed as means ± SE. Different lowercase letters indicate statistically significant differences between P doses within the same pH level; uppercase letters indicate differences between pH levels (Tukey’s HSD test, p < 0.05).
P fertilization affected grain yield depending on the applied dose and soil pH. Under very acidic conditions (pH 4.2), doses of 0.5 and 1 g pot−1 increased yield by approximately 150% compared to the control, with no difference between the two. However, higher doses (1.5 and 2 g pot−1) led to a gradual yield decline. The 2 g dose resulted in approximately 65% lower yield compared to the 1 g dose.
At pH 5.2, the highest yield was observed with the 0.5 g dose, which was 67% higher than the control. Further increases in P dose led to yield reductions relative to 0.5 g. The decreases for 1 and 1.5 g doses were both 15%, while the 2 g dose caused a 30% reduction.
At pH 6.4, grain yield increased consistently with increasing P dose. Compared to the control, yield increases were 45%, 72%, 69%, and 120% for the 0.5, 1, 1.5, and 2 g pot−1 doses, respectively.
Straw yield showed a slightly different pattern than grain yield (Figure 2). In control treatments (without P), no reduction was observed at pH 6.4 compared to pH 5.2, as was the case for grain yield. Instead, a consistent increase was noted with rising soil pH. At pH 4.2, straw yield was 28.5 g pot−1, increasing by 45% and 75% at pH 5.2 and 6.4, respectively, relative to pH 4.2.
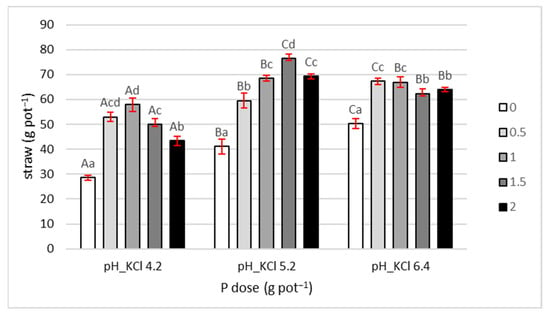
Figure 2.
Straw yield. Values are expressed as means ± SE. Different lowercase letters indicate statistically significant differences between P doses within the same pH level; uppercase letters indicate differences between pH levels (Tukey’s HSD test, p < 0.05).
The response of straw yield to increasing P doses also differed from that of grain. At pH 4.2, the highest straw yields were recorded in treatments with 0.5 and 1 g pot−1, with no significant difference between them. Yield in these treatments was, on average, 95% higher than in the control. The next two P doses reduced straw yield, with the 2 g dose causing a 25% decrease relative to the 1 g dose.
At pH 5.2, straw yield increased with the first three P doses (0.5, 1, and 1.5 g pot−1) by 45%, 66%, and 86%, respectively, compared to the control without P. Notably, the highest yield was obtained with the 1.5 g dose, while the maximum P dose (2 g) reduced yield to the level observed at 1 g.
At pH 6.4, differences between P doses were smaller than at lower pH. The highest yields were recorded at 0.5 and 1 g pot−1, 34% higher than the control. The two highest doses (1.5 and 2 g) caused a slight reduction of 6% compared to the previous two.
The harvest index (HI), defined as the percentage share of grain yield in total maize biomass, indicated the P dose at which the most favorable grain-to-straw ratio was achieved (Table 5). At pH 4.2, the best results were observed for doses of 0.5 and 1 g pot−1, with HI values of 58%. In contrast, doses of 1.5 and 2 g resulted in lower HI values of 56% and 53%, respectively.

Table 5.
Harvest index in %.
At pH 5.2, the highest HI (68%) was recorded for the 0.5 g dose, while higher doses decreased the index to 61%, 58%, and 53%, respectively.
A different response to P fertilization was observed at pH 6.4. As the dose increased from 0.5 to 2 g pot−1, HI rose from 55% to 66%. A statistically significant difference was found only between the 0.5 g dose and the 1.5 and 2 g doses.
3.3. Phosphorus Content in Plants
The concentration of P in maize grain from control treatments varied significantly depending on soil pH (Table 6). The highest value was recorded at pH 6.4 (3.1 g kg−1), while lower levels were observed at pH 4.2 (2.5 g kg−1) and 5.2 (2.3 g kg−1).

Table 6.
Mean (±SE) of P concentration in grain (g kg−1 d.m.).
Significant changes were observed following P application. At pH 4.2, the 1 g per pot dose increased grain P by 24% compared to the control. The 1.5 and 2 g doses raised it by 44%. Similarly, at pH 5.2, the 1, 1.5, and 2 g doses resulted in increases of 14%, 39%, and 55%, respectively. In contrast, at pH 6.4, P application reduced grain P compared to the control. Reductions of 8% were noted at 0.5, 1, and 1.5 g doses, and 13% at the 2 g dose.
P levels in straw, as in grain, depended on soil pH and the P dose (Table 7). In control treatments, the highest value was found at pH 6.4 (1.1 g kg−1), followed by pH 4.2 (1.0 g kg−1) and pH 5.2 (0.9 g kg−1). Fertilization influenced these values. At pH 4.2, all doses except 0.5 g significantly increased straw P compared to the control and the previous dose. The highest level (4.0 g·kg−1) was recorded at the maximum P rate (2 g·pot−1), which was four times higher than in straw from the control treatment.

Table 7.
Mean (±SE) of P concentration in straw (g kg−1 d.m.).
At pH 5.2, only the 2 g dose increased P in straw compared to the control (by 21%). The 0.5 and 1 g doses resulted in lower levels than the control, while 1.5 g produced similar values.
At pH 6.4, P application generally reduced P in straw. Decreases of 26%, 19%, and 7% were observed with 0.5, 1, and 1.5 g doses, respectively; the 2 g dose led to a 33% decrease.
3.4. Phosphorus Uptake by Plants
Phosphorus uptake by maize in control treatments varied depending on soil pH (Table 8). The highest P uptake was recorded at pH 6.4 (231 mg pot−1), followed closely by pH 5.2 (207 mg pot−1). In contrast, P uptake at pH 4.2 was twice as low (99 mg pot−1) compared to soils with a higher pH.

Table 8.
Mean (±SE) of P uptake by plants (mg pot−1).
P application increased its uptake, with the most pronounced effect observed at the lowest soil pH. At pH 4.2, plants absorbed 1.3 to 2.5 times more P from fertilized treatments than from the unfertilized control proportionally to the applied dose.
At pH 5.2, the application of 0.5 and 1 g P increased uptake by approximately 40% compared to the control, while doses of 1.5 and 2 g resulted in increases of 95% and 112%, respectively.
The lowest impact of P application on uptake was observed at pH 6.4. The 0.5 g P dose increased uptake by 24%, doses of 1 and 1.5 g by 45%, and the 2 g dose by 66% compared to the control.
3.5. Phosphorus Use Efficiency
Agronomic efficiency (AE), defined as grain yield per unit of applied P fertilizer, decreased with increasing P dose (Figure 3). At all three soil pH levels, the 0.5 g pot−1 dose showed the highest AE among the tested treatments. However, the efficiency of this dose varied depending on soil pH.
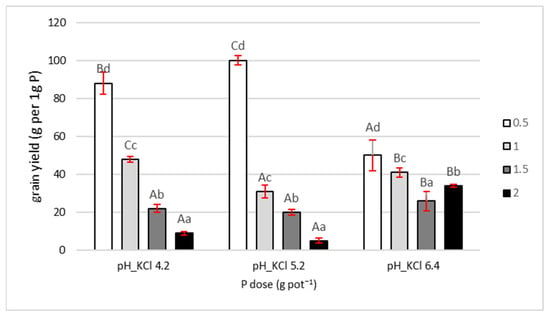
Figure 3.
Agronomic effectivity of phosphorus application. Values are expressed as means ± SE. Different lowercase letters indicate statistically significant differences between P doses within the same pH level; uppercase letters indicate differences between pH levels (Tukey’s HSD test, p < 0.05).
The highest AE for the 0.5 g dose was recorded at pH 5.2, reaching 100 g of grain per 1 g of applied P. At the same pH, the decline in AE with increasing P rates was the most pronounced: AE for the 1, 1.5, and 2 g pot−1 doses was 68%, 80%, and 95% lower, respectively, than for the 0.5 g dose.
At pH 4.2, the 0.5 g dose resulted in an AE of 88 g grain per 1 g of applied P, while higher doses led to reductions in AE of 46%, 75%, and 90%, respectively, compared to the lowest dose.
At pH 6.4, AE showed a different pattern than at the other two pH levels. For the 0.5 g dose, AE was substantially lower, amounting to only 50 g grain per 1 g of applied P. Moreover, the differences in AE between P doses were smallest at this pH. Compared to the 0.5 g dose, AE for the 1, 1.5, and 2 g treatments was 19%, 36%, and 32% lower, respectively.
During the growing season, the plant absorbs P both from the soil and from the fertilizer. Apparent phosphorus recovery (APR) indicates how much of the applied P was taken up by the plant, expressed as a percentage of the applied dose. In our study, maize absorbed the most P from the fertilizer at pH 4.2, less at pH 5.2, and the least at pH 6.4 (Figure 4). The mean APR values, calculated across four P doses, were 19%, 13%, and 9% for pH 4.2, 5.2, and 6.4, respectively. As expected, the highest APR was observed at the lowest P dose (0.5 g pot−1) at all three pH levels. Moreover, APR variability was greatest at pH 4.2 and lowest at pH 6.4.
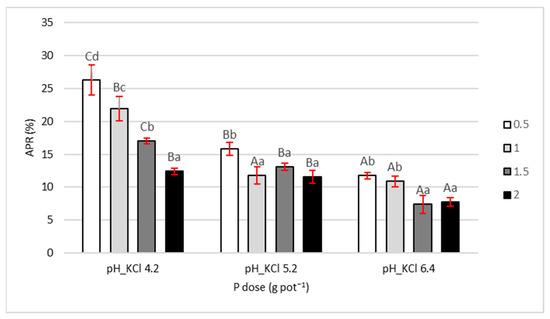
Figure 4.
Apparent phosphorus recovery APR. Values are expressed as means ± SE. Different lowercase letters indicate statistically significant differences between P doses within the same pH level; uppercase letters indicate differences between pH levels (Tukey’s HSD test, p < 0.05).
4. Discussion
Research on fertilizer phosphorus use efficiency is essential for developing rational strategies for managing this nutrient, aiming to balance food production needs with environmental protection. The importance of such studies also results from the high global prices of phosphate rock, which raise the cost of phosphorus fertilizers.
The literature provides numerous indices for assessing fertilizer use efficiency. Syers et al. [19] describe two methods for calculating these indices. According to the balance method, the amount of P taken up by plants from the unfertilized control is not subtracted. This approach is typically applied when evaluating fertilizer P use efficiency in crop rotations. As a result, efficiency indices may reach up to 50%, whereas direct measurements using 32 P-labelled phosphate show that P uptake by plants rarely exceeds 25% of the applied dose [19]. The second method, known as the difference method, involves subtracting the P uptake from the control. It is commonly used in pot experiments and generally yields lower efficiency indices than the balance method.
The aim of the pot experiment was to assess the efficiency of freshly applied phosphorus (P) as affected by soil pH, which directly influences P solubility and plant availability. According to Bedassa [29], in neutral and alkaline soils, calcium phosphates are the dominant P compounds, and their solubility is mainly controlled by the Ca/PO4 ratio. In these soils, increased Ca2+ activity reduces phosphate solubility. In contrast, in neutral and acidic soils, phosphate adsorption and desorption occur primarily on the surfaces of Al and Fe oxides and hydroxides. Phosphorus bound to aluminum (P-Al) represents the most labile form, supplying P to plants and enabling its redistribution within the soil profile. Phosphorus is also present in organic compounds, which undergo microbial mineralization to plant-available forms. Besides phosphatases, which directly release P from organic matter into the soil solution, sulfur—particularly in the form of SO42−—is relevant. Sulfur promotes the development of S-oxidizing microorganisms and enhances the general microbial activity involved in organic phosphorus mineralization. However, the soil used in our study had a low organic matter content; therefore, even if sulfur promoted P release from organic forms, its effect on the efficiency of mineral P fertilization was likely negligible.
Fertilizer P use efficiency was evaluated based on agronomic efficiency (AE) and apparent recovery efficiency (APR), both calculated relative to the unfertilized control.
Phosphorus fertilization efficiency is influenced not only by soil pH, native soil P content, and other soil-related factors, but also by climatic and weather conditions, as well as the crop species [19]. Maize was used as the test plant in our study.
It can be assumed that, in our study, maize grain yield obtained in the control treatments without P indicated the availability of soil P to plants at different pH levels, despite the fact that available PDL in the soil after harvest did not show differences between treatments. This may indicate certain limitations of the DL method for determining the content of plant-available forms of P in soil [5].
The lowest yield was observed at pHKCl 4.2 and the highest at pHKCl 5.2, with a decline at pHKCl 6.4. It appears that the lowest maize yield at pH 4.2 resulted from the combined effects of available P forms and toxic Al in the soil. According to Rahman et al. [30], toxic forms of Al3+ dominate, especially below pH 4.5.
Baquy et al. [31] reported the highest maize biomass at pHH2O 6.0, which corresponds well with our result at pHKCl 5.2, as KCl pH values are typically 0.73–1.00 units lower than those measured in H2O [32,33]. These findings suggest that optimal soil P availability for maize occurs around pHKCl 5.0.
According to textbook knowledge, maximum phosphorus availability for plants occurs at pHCaCl2 around 4.5 due to minimal P fixation by Fe and Al, and at pHCaCl2 around 6.5 due to low binding by Al and Ca [12,14,15]. However, Barrow et al. [17], examining P availability at seven pHCaCl2 levels ranging from 3.99 to 7.26, contradicted this by demonstrating that plant growth was optimal at pHCaCl2 5.5 and poorest at neutral pH. The authors attributed the decline in plant growth at higher pH to reduced P availability and slower P uptake. The optimal pHCaCl2 level identified in their study aligns with the pHKCl 5.2 observed in our experiment, after conversion using the equation provided by the European Soil Data Centre [34].
In our study, fertilizer P use efficiency for a given dose was likely related to the amount of soluble P available in the soil without P fertilization and the degree of phosphorus deficiency limiting plant growth. Baquy et al. [31] reported that soluble P concentrations increased with rising soil pHH2O from 4.5 to 6.0, which corresponds to approximately pHKCl from 3.5 to 5.0. They also observed a significant negative correlation between soluble P and P uptake efficiency (total P uptake relative to the applied dose), with r = –0.659 **.
In our study, the strongest plant response to the lowest phosphorus dose was observed at pHKCl 4.2 (a 150% increase in grain yield). It was likely the result of toxic Al binding by fertilizer P and an increase in soil-available P, which, at pH 4.2, was insufficient for proper plant growth.
At pHKCl 4.2, the lowest P dose also resulted in the highest values for other parameters, including HI (58%), AE (88 g grain per g P applied), and APR (26%) compared to higher doses. Increasing the P dose did not enhance yield and even reduced it. HI also declined from 58% to 53% with increasing P doses. Doubling the dose reduced AE by 45%, while further increasing the dose lowered AE by 75% and 90%, respectively. APR dropped from 26% to 22%, 17%, and 12%.
At pHKCl 5.2, the response to the lowest P dose was weaker than at pHKCl 4.2, resulting in a 67% grain yield increase relative to the control without P. Nevertheless, the absolute yield was highest at pHKCl 5.2, comparable to that observed with the maximum P dose at pH 6.4. This treatment also produced the highest HI (68%) and APR (100 g g) among all combinations. Although AE was higher than in the corresponding treatment at pHKCl 4.2, APR was substantially lower (16%). This indicates that, at pHKCl 5.2, plants relied primarily on native soil P, which was more available than at pHKCl 4.2.
The application of higher P doses at pHKCl 5.2 led to a decline in grain yield. Although yield declines following additional P doses were less pronounced than at pH 4.2, the reduction in AE was markedly greater. AE decreased by 69% at the second P dose compared to the lowest dose, and by 80% and 95% at the third and fourth doses, respectively. APR declined from 16% to 12% with increasing P doses.
At pHKCl 6.4, plant response to increasing P doses differed from that observed at pHKCl 4.2 and 5.2. Grain yield increased by 44% compared to the control after application of the lowest P dose, accompanied by the lowest HI (55%). At the same time, AE (50 g per 1 g P) and APR (12%) were the lowest compared to the same P dose at pHKCl 4.2 and 5.2. Unlike at pHKCl 4.2 and 5.2, increasing P doses did not reduce yields but led to further increases. The fourfold P dose resulted in the highest grain yield, equal to that obtained with the lowest P dose at pHKCl 5.2, representing a 120% increase relative to the control. With increasing P dose, HI increased from 55% to 66%. However, as at pHKCl 4.2 and 5.2, P use efficiency indices declined. AE decreased by 18%, 48%, and 32%, respectively, compared to the lowest dose. APR decreased from 12% to 11%, 7%, and 8%, respectively. This suggests that, at pHKCl 6.4, plants relied more on soil P than on fertilizer P compared to pHKCl 5.2. This is difficult to explain in light of the theory proposed by Barrow et al. [17], which states that P availability declines with increasing soil pH. It is possible that the plant response to P fertilization at pHKCl 6.4 resulted from the fact that, as reported by Bedassa [29], in neutral soils, P is bound not only by Al and Fe, but also by Ca, affecting the sorption–desorption dynamics differently than in soils with pH 4.2 and 5.2, where these processes are mainly controlled by Al and Fe oxides and hydroxides. According to Barrow et al. [17], the optimal pHCaCl2 for P availability is much lower than the commonly cited value of 7.0. The optimal pH for plant growth depends on the interaction of two factors: the decrease in P uptake rate with increasing pH and the increase in Al toxicity with decreasing pH. According to the conversion provided by the European Soil Data Centre [34], pHCaCl2 7.0 corresponds approximately to our pHKCl 6.4.
In general, our study showed that fertilizer P use efficiency for maize decreased with increasing P dose and was lowest at pHKCl 6.4. Syers et al. [19], referencing Johnston and Richards [35], also demonstrated that recovery of added P declines with increasing application doses. At low soil POlsen, APR dropped from 43% to 24% as P doses increased; at much higher soil POlsen, APR remained low (3–4%) regardless of the dose. Similarly, Ibrahim et al. [36], in field experiments with maize, found that APR decreased with increasing P rates at a given soil POlsen level.
5. Conclusions
- 1.
- The best growth of maize was observed at pHKCl 5.2 compared to pHKCl 4.2 and 6.4. At pHKCl 5.2, the availability of soil P appeared to be greatest, as indicated by yields in control treatments without P application.
- 2.
- The response of maize to P application varied with soil pH and the applied rate of P. Across all pH levels, the highest agronomic efficiency (AE) was obtained at the lowest P rate. Notably, AE at this rate was greatest at pHKCl 5.2.
- 3.
- Increasing the P rate to two, three, and four times the lowest rate resulted in a significant and progressive decline in AE. The most pronounced decrease occurred at pHKCl 5.2 in contrast to the more moderate reductions at pHKCl 4.2 and especially at pHKCl 6.4.
- 4.
- Maize took up P from both soil reserves and the applied fertilizer. Apparent phosphorus recovery (APR) was generally highest at pHKCl 4.2, followed by pHKCl 5.2, and lowest at pHKCl 6.4. As with AE, APR values were highest at the lowest P rate and declined with increasing application rates. The greatest variability in APR was observed at pHKCl 4.2 and the lowest at pHKCl 6.4.
- 5.
- At pHKCl 4.2 and 5.2, the highest absolute grain yield and the highest proportion of grain in total aboveground maize biomass (HI) were recorded at the lowest P rate. This rate also resulted in the highest values of AE and APR. However, at pHKCl 6.4, maize showed a different response to P application than at pHKCl 4.2 and 5.2. The highest grain yield and HI were observed at the maximum P rate, although AE at this rate was lower than that obtained at the lowest and double rates. Nevertheless, AE for the highest P rate at pHKCl 6.4 was greater than that observed for the same rate at pHKCl 4.2 and 5.2.
- 6.
- The response of maize to P fertilization at pHKCl 6.4 is not entirely consistent with the commonly accepted theory of P availability across different pH ranges. Further research is needed to better understand the mechanisms underlying this response.
Author Contributions
Conceptualization, J.K., E.S.-G., and J.B.; methodology, J.K. and E.S.-G.; formal analysis, J.B.; investigation, J.K. and J.B.; data curation, J.K. and J.B.; writing—original draft preparation, J.K. and J.B.; writing—review and editing, J.K. and E.S.-G.; supervision, E.S.-G.; project administration, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
Work was funded by the Polish Ministry of Agriculture and Rural Development under the 2.10 Scientific Research Program of the Institute of Soil Science and Plant Cultivation—State Research Institute in Pulawy.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cordell, D.; Drangert, J.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Bartosova, A.; Capell, R.; Olesen, J.E.; Jabloun, M.; Refsgaard, J.C.; Donnelly, C.; Arheimer, B. Future socioeconomic conditions may have a larger impact than climate change on nutrient loads to the Baltic Sea. Ambio 2019, 48, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, L.M.; Gustafsson, B. Waterborne Nitrogen and Phosphorus Inputs and Water Flow to the Baltic Sea 1995–2018. HELCOM Baltic Sea Environmental Fact Sheet 2020. Available online: https://helcom.fi/wp-content/uploads/2024/10/BSEFS-on-Waterborne-nitrogen-and-phosphorus-inputs-and-water-flow-to-the-Baltic-Sea-1995-2022.pdf (accessed on 2 June 2025).
- Iho, A.; Valve, H.; Ekholm, P.; Uusitalo, R.; Lehtoranta, J.; Soinne, H.; Salminen, J. Efficient protection of the Baltic Sea needs a revision of phosphorus metric. Ambio 2023, 52, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Korzeniowska, J.; Stanislawska-Glubiak, E. Evaluation of the Egner–Riehm DL and Mehlich 3 Tests for the Determination of Phosphorus: The Influence of Soil Properties on Extraction Efficiency and Test Conversion. Agronomy 2024, 14, 2921. [Google Scholar] [CrossRef]
- Bockheim, J.G.; Hartemink, A.E.; Huang, J. Distribution and properties of sandy soils in the conterminous USA–A conceptual thickness model, and taxonomic analysis. Catena 2020, 195, 104746. [Google Scholar] [CrossRef]
- de Holanda, S.F.; Vargas, L.K.; Granada, C.E. Challenges for sustainable production in sandy soils: A review. Environ. Dev. Sustain. 2025, 27, 53–66. [Google Scholar] [CrossRef]
- Krasowicz, S.; Oleszek, W.; Horabik, J.; Debicki, R.; Jankowiak, J.; Stuczynski, T.; Jadczyszyn, J. Racjonalne gospodarowanie środowiskiem glebowym Polski (Rational management of the soil environment in Poland). Pol. J. Agron. 2011, 7, 43–58. [Google Scholar]
- Pastuszak, M.; Igras, J. Temporal and Spatial Differences in Emission of Nitrogen and Phosphorus from Polish Territory to the Baltic Sea; National Marine Fisheries Research Institute: Gdynia/Pulawy, Poland, 2012. [Google Scholar]
- Matej-Lukowicz, K.; Wojciechowska, E.; Nawrot, N.; Dzierzbicka-Głowacka, L.A. Seasonal contributions of nutrients from small urban and agricultural watersheds in northern Poland. PeerJ 2020, 8, e8381. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, S.; Pazikowska-Sapota, G.; Dembska, G.; Dzierzbicka-Glowacka, L.A.; Juszkowska, D.; Majewska, Z.; Galer-Tatarowicz, K. Risk of phosphorus losses in surface runoff from agricultural land in the Baltic Commune of Puck in the light of assessment performed on the basis of DPS indicator. PeerJ 2020, 8, e8396. [Google Scholar] [CrossRef] [PubMed]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers; Macmillan Publishing Company: New York, NY, USA, 1985. [Google Scholar]
- Price, G. Australian Soil Fertility Manual, 3rd ed.; CSIRO Pub: Collingwood, Australia, 2006. [Google Scholar]
- Barrow, N.J. The effects of pH on phosphate uptake from the soil. Plant Soil 2017, 410, 401–410. [Google Scholar] [CrossRef]
- Barrow, N.J.; Debnath, A.; Sen, A. Measurement of the effects of pH on phosphate availability. Plant Soil 2020, 454, 217–224. [Google Scholar] [CrossRef]
- Blair, G. Nutrient Efficiency—What Do We Really Mean? In Genetic Aspects of Plant Mineral Nutrition; Springer: Dordrecht, The Netherlands, 1993; pp. 205–213. [Google Scholar]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of soil and fertilizer phosphorus use. FAO Fertil. Plant Nutr. Bull. 2008, 18, 5–50. [Google Scholar]
- Fageria, N.K. The Use of Nutrients in Crop Plants, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: London, UK, 2009. [Google Scholar]
- de Oliveira, A.K.S.; Soares, E.B.; dos Santos, M.G.; Lins, H.A.; de Freitas Souza, M.; dos Santos Coêlho, E.; de Araújo Rangel Lopes, W. Efficiency of phosphorus use in sunflower. Agronomy 2022, 12, 1558. [Google Scholar] [CrossRef]
- PN-R-04023:1996; Agrochemical Soil Analyse–Determination of Assimilated Phosphorus Contents. Committee for Standardization: Warsaw, Poland, 1996. (In Polish)
- PN-EN ISO 10390:2022-09; Soil, Treated Biowaste and Sludge—Determination of pH. Polish Committee for Standardization: Warsaw, Poland, 2022. (In Polish)
- PN-ISO 14235:2003; Soil Quality: Determination of Organic Carbon in Soil by Sulfochromic Oxidation. Polish Committee for Standardization: Warsaw, Poland, 2003. (In Polish)
- PN-R-04033:1998; Soil and Mineral Soil Materials—Particle Size Distribution on Soil Classes. Polish Committee for Standardization: Warsaw, Poland, 1998. (In Polish)
- PN-R-04022:1996; Agrochemical Soil Analyse—Determination of Assimilated Potassium Content. Committee for Standardization: Warsaw, Poland, 1996. (In Polish)
- PN-R-04020:1994; Agrochemical Soil Analyse—Determination of Assimilated Magnesium Content. Committee for Standardization: Warsaw, Poland, 1994. (In Polish)
- PN-EN ISO/IEC 17025:2018-02; General Requierments for the Competence of Testing and Calibration Laboratories. Polish Committee for Standarization: Warsaw, Poland, 2018. (In Polish)
- Bedassa, M. Fractionation and distribution of phosphorus in acid soils: Review. Int. J. Hortic. Food Sci. 2023, 5, 64–70. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, S.H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.W. Importance of mineral nutrition for mitigating aluminum toxicity in plants on acidic soils: Current status and opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef] [PubMed]
- Baquy, M.; Li, J.Y.; Nkoh, J.N.; Biswash, M.R.; Xu, R.K. Determining critical soil pH for phosphorus uptake efficiency in an acidic Ultisol for maize. Egypt. J. Soil Sci. 2024, 64, 1525–1536. [Google Scholar] [CrossRef]
- Kome, G.K.; Enang, R.K.; Yerima, B.P.K.; Lontsi, M.G.R. Models relating soil pH measurements in H2O, KCl and CaCl2 for volcanic ash soils of Cameroon. Geoderma Reg. 2018, 14, e00185. [Google Scholar] [CrossRef]
- Sadovski, A.N. Study on pH in water and potassium chloride for Bulgarian soils. Eurasian J. Soil Sci. 2019, 8, 11–16. [Google Scholar] [CrossRef]
- Soil pH in Europe. European Commission, Joint Research Centre (JRC). 2010. Available online: https://esdac.jrc.ec.europa.eu/Library/Data/PH/Documents/pH_Pub.pdf (accessed on 5 July 2025).
- Johnston, A.E.; Richards, I.R. Effectiveness of different precipitated phosphates as phosphorus sources for plants. Soil Use Manag. 2003, 19, 45–49. [Google Scholar] [CrossRef]
- Ibrahim, K.; Wang, Q.; Wang, L.; Zhang, W.; Peng, C.; Zhang, S. Determining the optimum level of soil Olsen phosphorus and phosphorus fertilizer application for high phosphorus-use efficiency in Zea mays L. in black soil. Sustainability 2021, 13, 5983. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).