Evaluation of Olive Mill Waste Compost as a Sustainable Alternative to Conventional Fertilizers in Wheat Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Experimental Design
2.2. Soil Analysis
2.3. Plant Analysis
2.4. Greenhouse Gas Measurements
2.5. Statistical Analysis
3. Results
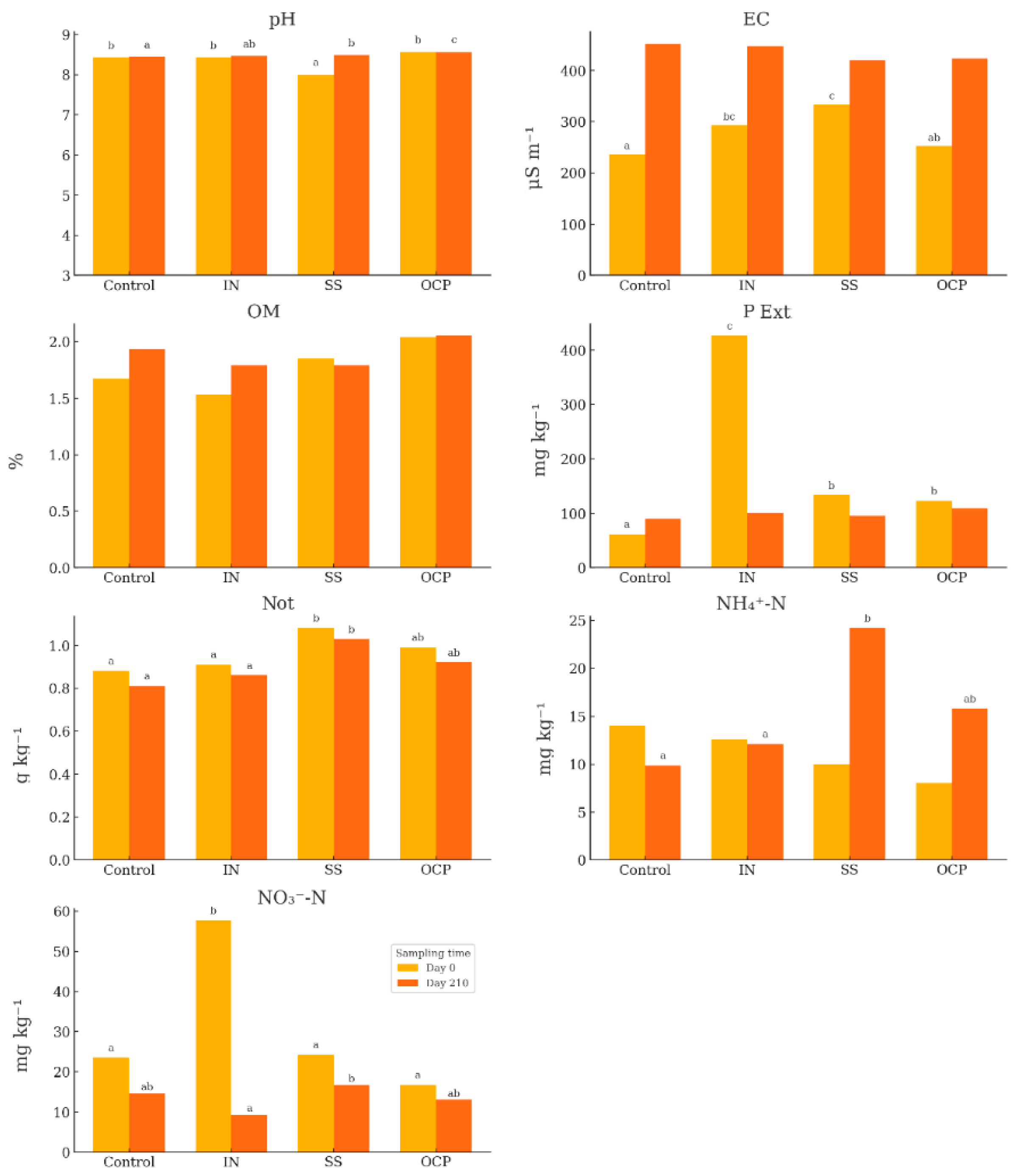
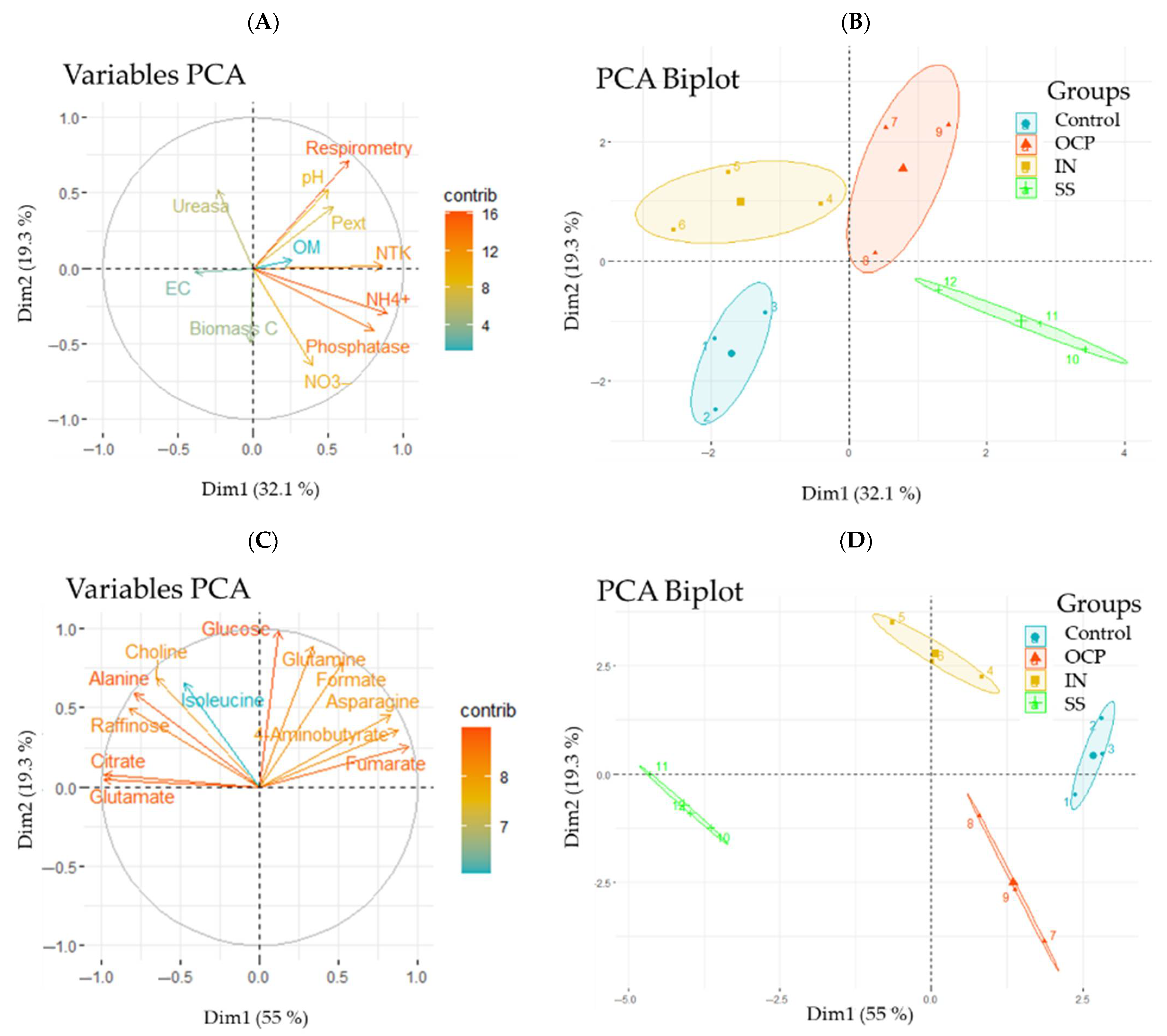
3.1. Soil Parameters
3.2. Yield and Grain Quality
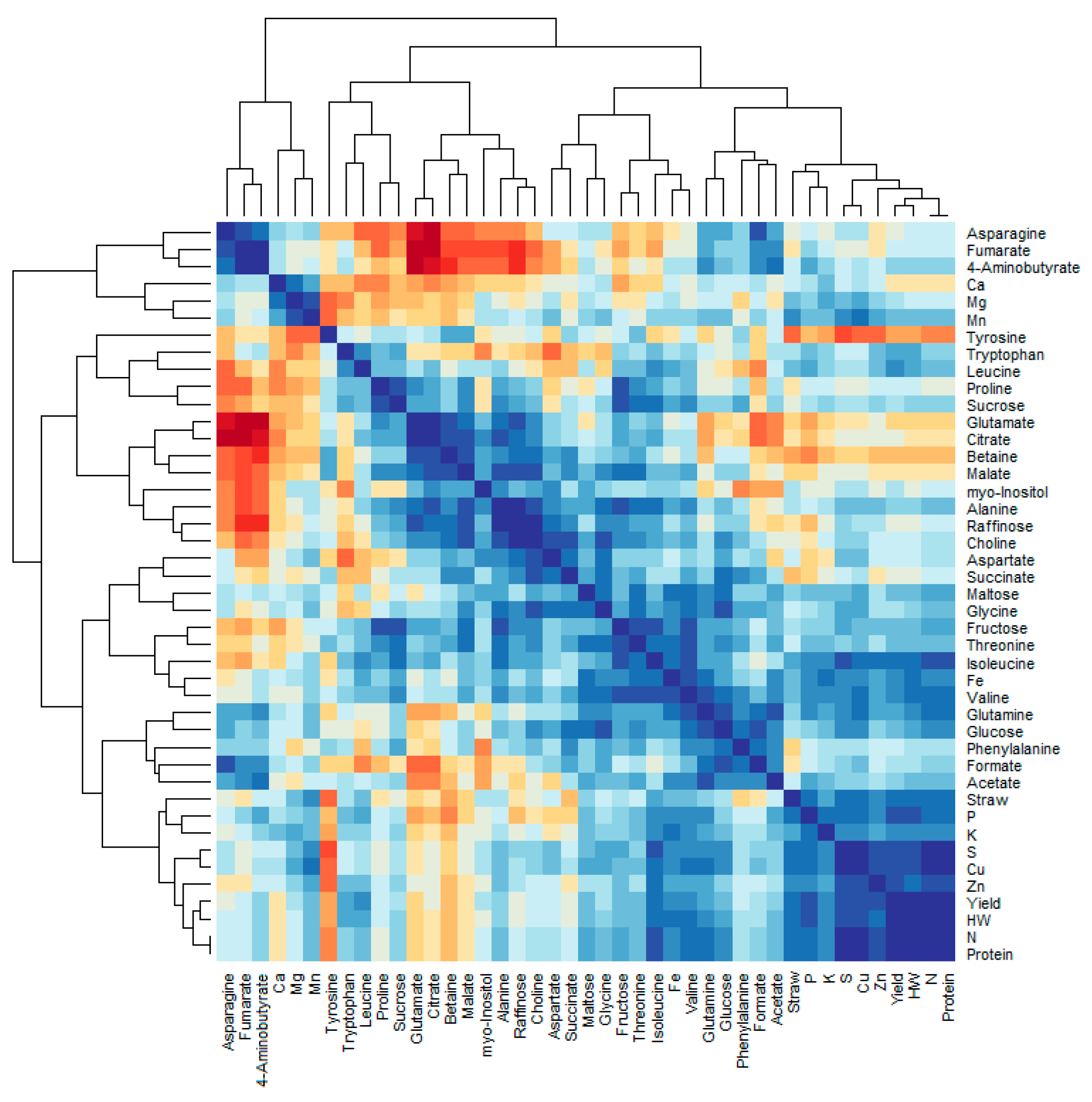
3.3. Metabolomics
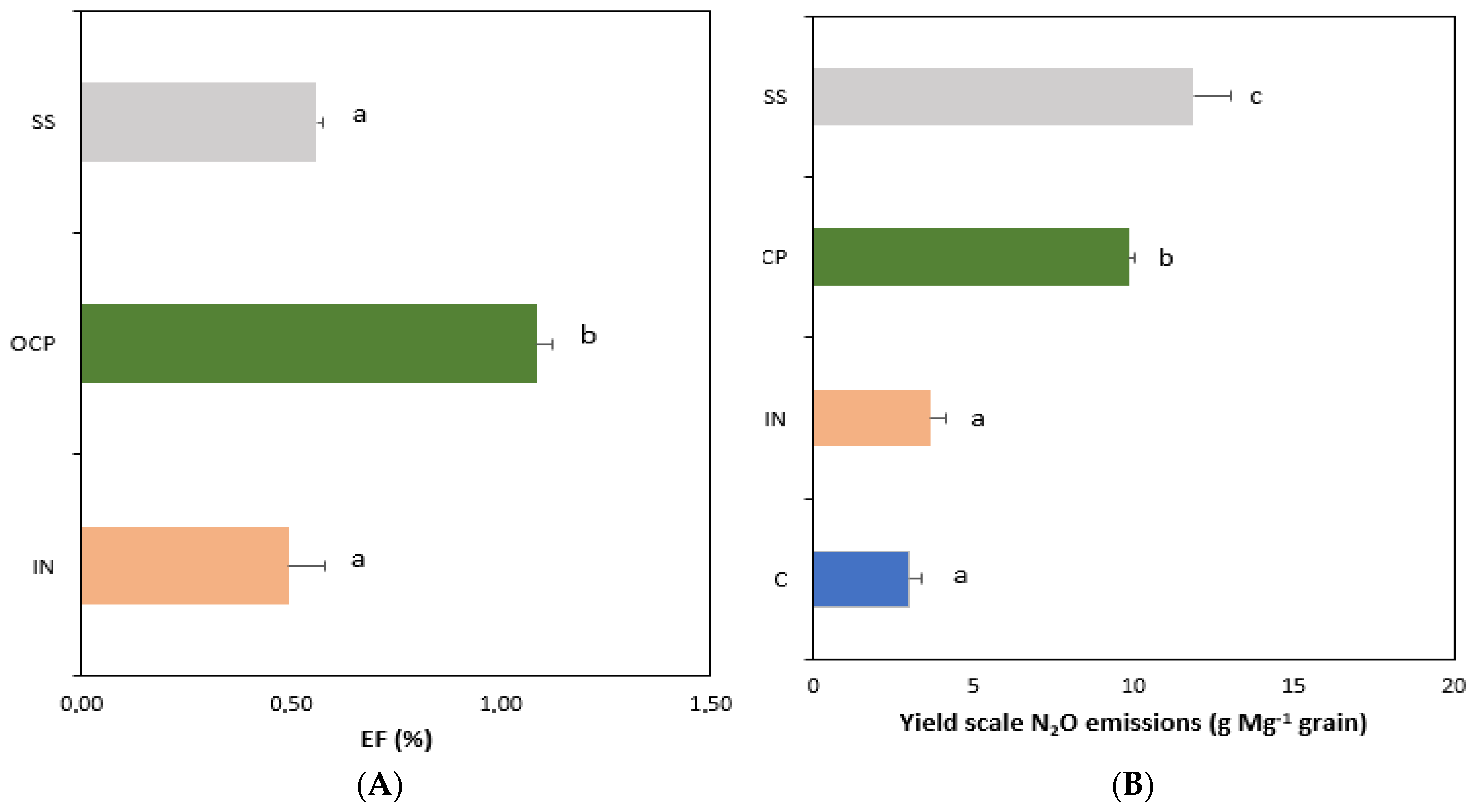
3.4. Soil GHG Fluxes and Cumulative Emissions
4. Discussion
4.1. Effects of Fertilization on Soil Properties
4.2. Effects of Fertilization on Yield
4.3. Effects of Fertilization on Metabolomic, Sugar and Amino Acid Profiles
4.4. Effects of Fertilization on Soil GHG Fluxes and Cumulative Emissions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fertilizers Europe. Facts & Figures. June 2023. Available online: https://www.fertilizerseurope.com/publications/fertilizers-industry-facts-and-figures-2023-edition/ (accessed on 15 July 2025).
- Grijalva-Contreras, R.L.; Robles-Contreras, F.; Macías-Duarte, R.; Santillano-Cázares, J.; Núñez-Ramírez, F. Nitrógeno en Trigo y su Efecto en el Rendimiento y en la Concentración de Nitratos y Potasio en el Extracto Celular de Tallo (ECT). Acta Univ. 2016, 26, 48–54. [Google Scholar][Green Version]
- Konopka, I.; Tańska, M.; Faron, A.; Stępień, A.; Wojtkowiak, K. Comparison of the Phenolic Compounds, Carotenoids and Tocochromanols Content in Wheat Grain under Organic and Mineral Fertilization Regimes. Molecules 2012, 17, 12341–12356. [Google Scholar] [CrossRef] [PubMed]
- Saia, S.; Fragasso, M.; De Vita, P.; Beleggia, R. Metabolomics Provides Valuable Insight for the Study of Durum Wheat: A Review. J. Agric. Food Chem. 2019, 67, 3069–3085. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Zhou, J.; Deng, X.; Zhu, G.; Cao, H.; Wang, Z.; Yan, Y. Metabolite Profiling of the Response to High-Nitrogen Fertilizer During Grain Development of Bread Wheat (Triticum aestivum L.). J. Cereal Sci. 2016, 69, 85–94. [Google Scholar] [CrossRef]
- Shewry, P.; Rakszegi, M.; Lovegrove, A.; Amos, D.; Corol, D.I.; Tawfike, A.; Mikó, P.; Ward, J.L. Effects of Organic and Conventional Crop Nutrition on Profiles of Polar Metabolites in Grain of Wheat. J. Agric. Food Chem. 2018, 66, 5346–5351. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2013, the Physical Science Basis. 2013. Available online: http://www.ipcc.ch/report/ar5/wg1/ (accessed on 15 July 2025).
- Sporchia, F.; Caro, D. Exploring the Potential of Circular Solutions to Replace Inorganic Fertilizers in the European Union. Sci. Total Environ. 2023, 892, 164636. [Google Scholar] [CrossRef] [PubMed]
- European Commission. The European Green Deal; COM. 640 final; European Commission: Brussels, Belgium, 2019.
- European Commission. A Farm to Fork Strategy for a Fair, Healthy and Environmentally Friendly Food System; COM/2020/381 final; European Commission: Brussels, Belgium, 2020.
- International Olive Council. Statistics. 2025. Available online: https://www.internationaloliveoil.org/what-we-do/statistics/ (accessed on 15 July 2025).
- Pinho, I.A.; Lopes, D.V.; Martins, R.C.; Quina, M.J. Phytotoxicity Assessment of Olive Mill Solid Wastes and the Influence of Phenolic Compounds. Chemosphere 2017, 185, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Romero, C.; de los Santos, B.; de Castro, A.; García, A.; Romero, F.; Brenes, M. Antimicrobial Activity of Olive Solutions from Stored Alpeorujo against Plant Pathogenic Microorganisms. J. Agric. Food Chem. 2011, 59, 6927–6932. [Google Scholar] [CrossRef] [PubMed]
- García-Randez, A.; Marks, E.A.N.; Pérez-Murcia, M.D.; Orden, L.; Andreu-Rodriguez, J.; Sabater, E.M.; Cháfer, M.T.; Moral, R. Is the Direct Soil Application of Two-Phase Olive Mill Waste (Alperujo) Compatible with Soil Quality Protection? Agronomy 2023, 13, 2585. [Google Scholar] [CrossRef]
- Sáez, J.A.; Pérez-Murcia, M.D.; Vico, A.; Martínez-Gallardo, M.R.; Andreu-Rodríguez, F.J.; López, M.J.; Bustamante, M.A.; Sanchez-Hernandez, J.C.; Moreno, J.; Moral, R. Olive Mill Wastewater-Evaporation Ponds Long Term Stored: Integrated Assessment of In Situ Bioremediation Strategies Based on Composting and Vermicomposting. J. Hazard. Mater. 2021, 402, 123481. [Google Scholar] [CrossRef] [PubMed]
- Monetta, P.; Sanchez-Montilla, R.; Bosch-Rubia, G.; Rizzo, P.F.; Crespo, D.; Gouiric, S.; Herrera, M.V. Co-composting alperujo with other agroindustrial residues as sustainable practice for its recycling. Acta Hortic. 2014, 1057, 709–716. [Google Scholar] [CrossRef]
- García-Rández, A.; Orden, L.; Marks, E.A.N.; Andreu-Rodríguez, J.; Franco-Luesma, S.; Martínez Sabater, E.; Sáez-Tovar, J.A.; Pérez-Murcia, M.D.; Agulló, E.; Bustamante, M.A.; et al. Monitoring of greenhouse gas emissions and compost quality during olive mill waste co-composting at industrial scale: The effect of N and C sources. J. Waste Manag. 2025, 193, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Cucci, G.; Lacolla, G.; Caranfa, G. Use of Composted Olive Waste as Soil Conditioner and Its Effects on the Soil. Int. J. Agric. Res. 2013, 8, 149–157. [Google Scholar] [CrossRef]
- Papandrea, S.F.; Cataldo, M.F.; Palma, A.; Gallucci, F.; Zimbalatti, G.; Proto, A.R. Pelletization of Compost from Different Mixtures with the Addition of Exhausted Extinguishing Powders. Agronomy 2021, 11, 1357. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Yap, Y.J.; Ling, T.C.; Tao, Y.; Show, P.L. Densification of Food Waste Compost: Effects of Moisture Content and Dairy Powder Waste Additives on Pellet Quality. Process Saf. Environ. Prot. 2018, 116, 780–786. [Google Scholar] [CrossRef]
- Pampuro, N.; Bagagiolo, G.; Priarone, P.C.; Cavallo, E. Effects of Pelletizing Pressure and the Addition of Woody Bulking Agents on the Physical and Mechanical Properties of Pellets Made from Composted Pig Solid Fraction. Powder Technol. 2017, 311, 112–119. [Google Scholar] [CrossRef]
- Usman, K.; Khan, S.; Ghulam, S.; Khan, M.; Khan, N.; Khan, M.; Khalil, S. Sewage Sludge: An Important Biological Resource for Sustainable Agriculture and Its Environmental Implications. Am. J. Plant Sci. 2012, 3, 1708–1721. [Google Scholar] [CrossRef]
- Jamil, M.; Qasim, M.; Umar, M.; Rehman, K. Impact of Organic Wastes (Sewage Sludge) on the Yield of Wheat (Triticum aestivum L.) in a Calcareous Soil. Int. J. Agric. Biol. 2004, 6, 465–467. [Google Scholar]
- Brown, S.; Leonard, P. Recycling of Organic Carbon in Organic Wastes Is Also Important for C Sequestration in Soils. Biocycle 2004, 45, 25–29. [Google Scholar]
- Zhou, Z.; Zhang, S.; Jiang, N.; Xiu, W.; Zhao, J.; Yang, D. Effects of Organic Fertilizer Incorporation Practices on Crop Yield, Soil Quality, and Soil Fauna Feeding Activity in the Wheat-Maize Rotation System. Front. Environ. Sci. 2022, 10, 1058071. [Google Scholar] [CrossRef]
- Álvaro-Fuentes, J.; Cantero-Martínez, C.; López, M.V.; Arrúe, J.L. Soil Carbon Dioxide Fluxes Following Tillage in Semiarid Mediterranean Agroecosystems. Soil Tillage Res. 2007, 96, 331–341. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2022; p. 401. [Google Scholar]
- Paredes, C.; Pérez-Murcia, M.D.; Pérez-Espinosa, A.; Bustamante, M.A.; Moreno-Caselles, J. Recycling of Two-Phase Olive-Mill Cake “Alperujo” by Co-Composting with Animal Manures. Commun. Soil Sci. Plant Anal. 2015, 46, 238–247. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 548. [Google Scholar]
- Bustamante, M.A.; Paredes, C.; Moral, R.; Moreno-Caselles, J.; Perez-Murcia, M.D.; Perez-Espinosa, A.; Bernal, M.P. Co-Composting of Distillery and Winery Wastes with Sewage Sludge. Water Sci. Technol. 2007, 56, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, J.; Bremmer, J.M. A Rapid and Precise Method for Routine Determination of Organic Carbon in Soil. Commun. Soil Sci. Plant Anal. 1989, 19, 1467–1476. [Google Scholar] [CrossRef]
- Bremmer, J.M.; Britembeck, G.A. A Simple Method for Determination of Ammonium in Semimicro-Kjeldahl Analysis of Soils and Plant Materials Using a Block Digester. Commun. Soil Sci. Plant Anal. 1983, 14, 905–913. [Google Scholar] [CrossRef]
- Bremmer, J.M.; Keeney, D.R. Steam Distillation Methods for Determination of Ammonium Nitrate and Nitrite. Anal. Chim. Acta 1965, 32, 485–495. [Google Scholar] [CrossRef]
- Olsen, S.; Cole, C.; Watanabe, F.; Dean, L. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular 939; US Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Hao, X.; Ball, B.C.; Culley, J.L.B.; Carter, M.R.; Parkin, G.W. Soil density and porosity. In Soil Sampling and Methods of Analysis; Canadian Society of Soil Science: Vancouver, BC, Canada, 2008; pp. 743–759. [Google Scholar]
- Nannipieri, P.; Ceccanti, B.; Cervelli, S.; Matarese, E. Extraction of Phosphatase, Urease, Protease, Organic Carbon, and Nitrogen from Soil. Soil Sci. Soc. Am. J. 1980, 44, 1011–1016. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Domsch, K.H. A Physiological Method for the Quantitative Measurement of Microbial Biomass in Soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Anderson, J.P.E. Soil Respiration. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 831–871. [Google Scholar] [CrossRef]
- Wringley, C.; Batey, I. Assessing Grain Quality. In Food Science, Technology and Nutrition; Cauvain, S.P., Ed.; Woodhead Publishing: Cambridge, MA, USA, 2003; pp. 71–96. [Google Scholar] [CrossRef]
- Marshall, D.R.; Ellison, F.W.; Mares, D.J. Effects of Grain Shape and Size on Milling Yields in Wheat. I. Theoretical Analysis Based on Simple Geometric Models. Aust. J. Agric. Res. 1984, 35, 619–630. [Google Scholar] [CrossRef]
- López-Bellido, L.; López-Bellido, J.; Redondo, R. Nitrogen Efficiency in Wheat under Rainfed Mediterranean Conditions as Affected by Split Nitrogen Application. Field Crops Res. 2005, 94, 86–97. [Google Scholar] [CrossRef]
- Van der Sar, S.; Kim, H.K.; Meissner, A.; Verpoorte, R.; Choi, Y.H. Nuclear Magnetic Resonance Spectroscopy for Plant Metabolite Profiling. In The Handbook of Plant Metabolomics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 57–76. [Google Scholar]
- Alfosea-Simón, M.; Simón-Grao, S.; Zavala-Gonzalez, E.A.; Navarro-Morillo, I.; Martínez-Nicolás, J.J.; Alfosea-Simón, F.J.; García-Sánchez, F. Ionomic, Metabolic and Hormonal Characterization of the Phenological Phases of Different Tomato Genotypes Using Omics Tools. Sci. Hortic. 2022, 293, 110697. [Google Scholar] [CrossRef]
- Franco-Luesma, S.; Lafuente, V.; Alonso-Ayuso, M.; Bielsa, A.; Kouchami-Sardoo, I.; Arrué, J.L.; Álvaro-Fuentes, J. Maize Diversification and Nitrogen Fertilization Effects on Soil Nitrous Oxide Emissions in Irrigated Mediterranean Conditions. Front. Environ. Sci. 2022, 10, 914851. [Google Scholar] [CrossRef]
- Menéndez, S.; Merino, P.; Pinto, M.; González-Murua, C.; Estavillo, J.M. 3,4-Dimethylpyrazol Phosphate Effect on Nitrous Oxide, Nitric Oxide, Ammonia, and Carbon Dioxide Emissions from Grasslands. J. Environ. Qual. 2006, 35, 973–981. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Chapter 7. The Earth’s Energy Budget, Climate Feedbacks and Climate Sensitivity. In Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2021; p. 132. [Google Scholar]
- Yao, Z.; Guo, H.; Wang, Y.; Zhan, Y.; Zhang, T.; Wang, R.; Zheng, X.; Butterbach-Bahl, K. A Global Meta-Analysis of Yield-Scaled N2O Emissions and Its Mitigation Efforts for Maize, Wheat, and Rice. Glob. Change Biol. 2024, 30, e17177. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, E.; Lassaleta, L.; Sanz-Cobena, A.; Garnier, J.; Vallejo, A. The Potential of Organic Fertilizers and Water Management to Reduce N2O Emissions in Mediterranean Climate Cropping Systems: A Review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; González, L.; Tablada, M.; Robledo, C.W. InfoStat, versión 2020; Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2020; Available online: http://www.infostat.com.ar (accessed on 15 July 2025).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P. Vegan: Community Ecology Package, version 2.6-2; The R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 July 2025).
- Martínez-Sabater, E.; Pérez-Murcia, M.D.; Andreu-Rodríguez, F.J.; Orden, L.; Agulló, E.; Sáez-Tovar, J.; Martínez-Tome, J.; Bustamante, M.Á.; Moral, R. Enhancing Sustainability in Intensive Dill Cropping: Comparative Effects of Biobased Fertilizers vs. Inorganic Commodities on Greenhouse Gas Emissions, Crop Yield, and Soil Properties. Agronomy 2022, 12, 2124. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Mondini, C.; de Nobili, M.; Leita, L.; Roig, A. Land application of biosolids: Soil response to different stabilization degree or treated organic matter. Waste Manag. 2004, 24, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Orden, L.; Iocoli, G.A.; Bustamante, M.Á.; Moral, R.; Rodríguez, R.A. Nutrient Release Dynamics in Argentinean Pampean Soils Amended with Composts under Laboratory Conditions. Agronomy 2022, 12, 795. [Google Scholar] [CrossRef]
- Antil, R.S.; Bar-Tal, A.; Fine, P.; Hadas, A. Predicting nitrogen and carbon mineralization of composted manure and sewage sludge in soil. Compost Sci. Util. 2011, 19, 33–43. [Google Scholar] [CrossRef]
- Bernal, M.; Alburquerque, J.; Moral, R. Moral Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, D. Macroscale Chemical Properties and X-Ray Absorption Spectroscopy of Soil Phosphorus. In Global Change and Forest Soils; Elsevier: Amsterdam, The Netherlands, 2010; Volume 34, pp. 313–356. [Google Scholar]
- Yan, Z.; Chen, S.; Dari, B.; Sihi, D.; Chen, Q. Phosphorus transformation response to soil properties changes induced by manure application in a calcareous soil. Geoderma 2018, 322, 163–171. [Google Scholar] [CrossRef]
- Lizcano-Toledo, R.; Reyes-Martín, M.P.; Celi, L.; Fernández-Ondoño, E. Phosphorus Dynamics in the Soil–Plant–Environment Relationship in Cropping Systems: A Review. Appl. Sci. 2021, 11, 11133. [Google Scholar] [CrossRef]
- Weihrauch, C.; Opp, C. Ecologically relevant phosphorus pools in soils and their dynamics: The story so far. Geoderma 2018, 325, 183–194. [Google Scholar] [CrossRef]
- Lei, X.; Shen, Y.; Zhao, J.; Huang, J.; Wang, H.; Yu, Y.; Xiao, C. Root Exudates Mediate the Processes of Soil Organic Carbon Input and Efflux. Plants 2023, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; Boukhari, M.E.; Mphatso, C.; Zeroual, Y.; Lyamlouli, K. Conversion of waste into organo-mineral fertilizers: Current technological trends and prospects. Rev. Environ. Sci. Biotechnol. 2022, 21, 425–446. [Google Scholar] [CrossRef]
- Smith, G.H.; Chaney, K.; Murray, C.; Le, S.M. The effect of organo-mineral fertilizer applications on the yield of winter wheat, spring barley, forage maize and grass cut for silage. J. Environ. Prot. 2015, 6, 103–109. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Tong, L.; Lv, Y. Sustainable Agriculture Practices: Utilizing Composted Sludge Fertilizer for Improved Crop Yield and Soil Health. Agronomy 2024, 14, 756. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.L. Application of Different Organic Wastes on Soil Properties and Wheat Yield. Agron. J. 2007, 99, 1597–1606. [Google Scholar] [CrossRef]
- Fernández, J.M.; Plaza, C.; García-Gil, J.C.; Polo, A. Biochemical properties and barley yield in a semiarid Mediterranean soil amended with two kinds of sewage sludge. Appl. Soil Ecol. 2009, 42, 18–24. [Google Scholar] [CrossRef]
- Koutroubas, S.D.; Antoniadis, V.; Fotiadis, S.; Damalas, C.A. Growth, grain yield and nitrogen use efficiency of Mediterranean wheat in soils amended with municipal sewage sludge. Nutr. Cycl. Agroecosyst. 2014, 100, 227–243. [Google Scholar] [CrossRef]
- Antolín, M.C.; Pascual, I.; García, C.; Polo, A.; Sánchez-Díaz, M. Growth, yield and solute content of barley in soils treated with sewage sludge under semiarid Mediterranean conditions. Field Crops Res. 2005, 94, 224–237. [Google Scholar] [CrossRef]
- Motta, S.R.; Maggiore, T. Evaluation of nitrogen management in maize cultivation grows on soil amended with sewage sludge and urea. Eur. J. Agron. 2013, 45, 59–67. [Google Scholar] [CrossRef]
- Deeks, L.K.; Chaney, K.; Murray, C.; Sakrabani, R.; Gedara, S.; Le, M.S.; Tyrrel, S.; Pawlett, M.; Read, R.; Smith, G.H. A new sludge-derived organo-mineral fertilizer gives similar crop yields as conventional fertilizers. Agron. Sustain. Dev. 2013, 33, 539–549. [Google Scholar] [CrossRef]
- Saha, B.K.; Rose, M.T.; Van Zwieten, L.; Wong, V.N.L.L.; Patti, A.F. Slow release brown coal-urea fertilizer potentially influences greenhouse gas emissions, nitrogen use efficiency, and sweet corn yield in Oxisol. ACS Agric. Sci. Technol. 2021, 1, 469–478. [Google Scholar] [CrossRef]
- Sui, B.; Feng, X.; Tian, G.; Hu, X.; Shen, Q.; Guo, S. Optimizing Nitrogen Supply Increases Rice Yield and Nitrogen Use Efficiency by Regulating Yield Formation Factors. Field Crops Res. 2013, 150, 99–107. [Google Scholar] [CrossRef]
- Colomb, B.; Debaeke, P.; Jouany, C.; Nolot, J.M. Phosphorus management in low stockless cropping systems: Crop and soil responses to contrasting P regimes in a 36-year experiment in southern France. Eur. J. Agron. 2007, 26, 154–165. [Google Scholar] [CrossRef]
- Duan, Y.; Xu, M.; Gao, S.; Yang, X.; Huang, S.; Liu, H.; Wang, B. Nitrogen use efficiency in a wheat–corn cropping system from 15 years of manure and fertilizer applications. Field Crops Res. 2014, 157, 47–56. [Google Scholar] [CrossRef]
- Dhillon, J.; Torres, G.; Driver, E.; Figuereido, B.; Raun, W.R. World Phosphorus Use Efficiency in Cereal Crops. Agron. J. 2017, 109, 1670–1677. [Google Scholar] [CrossRef]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of soil and fertilizer phosphorus use: Reconciling changing concepts of soil phosphorus behaviour with agronomic information. In FAO Fertilizer and Plant Nutrition Bulletin; FAO: Rome, Italy, 2008; Volume 18. [Google Scholar]
- Xin, X.; Qin, S.; Zhang, J.; Zhu, A.; Yang, W.; Zhang, X. Yield, phosphorus use efficiency and balance response to substituting long-term chemical fertilizer use with organic manure in a wheat-maize system. Field Crops Res. 2017, 208, 27–33. [Google Scholar] [CrossRef]
- Jones, C.; Olson-rutz, K.; Dinkins, C.P. Nutrient Uptake Timing by Crops; Montana State University: Bozeman, MT, USA, 2015. [Google Scholar]
- Mnthambala, F.; Tilley, E.; Tyrrel, S.; Sakrabani, R. Effect of Various Organic Fertilisers on Phosphorus Mineralisation, Use Efficiency and Maize Yield. Resources 2022, 11, 86. [Google Scholar] [CrossRef]
- Vita, F.; Giuntoli, B.; Arena, S.; Quaranta, F.; Bertolino, E.; Lucarotti, V.; Guglieminetti, L.; Alessio, M.; Scaloni, A.; Alpi, A. Effects of different nitrogen fertilizers on two wheat cultivars: An integrated approach. Plant Direct. 2018, 2, e00089. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.M.; Sun, Y.Y.; Ye, X.Y.; Li, Z.G. Signaling Role of Glutamate in Plants. Front. Plant Sci. 2020, 10, 1743. [Google Scholar] [CrossRef] [PubMed]
- Bonte, A.; Neuweger, H.; Goesmann, A.; Thonar, C.; Mader, P.; Langenkamper, G.; Niehaus, K. Metabolite profiling on wheat grain to enable a distinction of samples from organic and conventional farming systems. J. Sci. Food Agric. 2014, 94, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, D.; Wang, C.; Zhao, H.; Zhu, Y.; Guo, T. Responses of Amino Acid Composition to Nitrogen Application in High-and Low-Protein Wheat Cultivars at Two Planting Environments. Crop Sci. 2016, 56, 1277–1287. [Google Scholar] [CrossRef]
- Isaychev, V.; Andreev, N.; Mudarisov, F. IOP Conf. Ser. Earth Environ. Sci. 2021, 937, 022130. [Google Scholar]
- Zörb, C.; Langenkämper, G.; Betsche, T.; Niehaus, K.; Barsch, A. Metabolite Profiling of Wheat Grains (Triticum aestivum L.) from Organic and Conventional Agriculture. Agric. Food Chem. 2006, 54, 8301–8306. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Millar, A.H. The plant mitochondrial transportome: Balancing metabolic demands with energetic constraints. Trends Plant Sci. 2016, 21, 662–676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fernie, A.R. The Role of TCA Cycle Enzymes in Plants. Adv. Biol. 2023, 7, e2200238. [Google Scholar] [CrossRef] [PubMed]
- Chia, D.W.; Yoder, T.J.; Reiter, W.D.; Gibson, S.I. Fumaric acid: An overlooked form of fixed carbon in Arabidopsis and other plant species. Planta 2000, 211, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Shi, J.; Shi, F.; Xu, H.; He, K.; Wang, Z. Aphid fecundity and defenses in wheat exposed to a combination of heat and drought stress. J. Exp. Bot. 2020, 9, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Aytenew, M.; Wolancho, G.B. Effects of Organic Amendments on Soil Fertility and Environmental Quality: A Review. J. Plant Sci. 2020, 8, 112–119. [Google Scholar] [CrossRef]
- Li, S.; Li, T.; Kim, W.D.; Kitaoka, M.; Yoshida, S.; Nakajima, M.; Kobayashi, H. Characterization of raffinose synthase from rice (Oryza sativa L. var. Nipponbare). Biotechnol. Lett. 2007, 29, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Mejide, A.; Cárdenas, L.M.; Sánchez-Martín, L.; Vallejo, A. Carbon dioxide and methane fluxes from a barley field amended with organic fertilizers under Mediterranean climatic conditions. Plant Soil 2010, 328, 353–367. [Google Scholar] [CrossRef]
- Chadwick, D.R.; Van der Weerden, T.; Martinez, J.; Pain, B.F. Nitrogen transformation and losses from pig slurry applications to a natural soil filter system (solepur process) in Britany, France. J. Agric. Eng. Res. 1998, 68, 85–95. [Google Scholar] [CrossRef]
- Sánchez-Navarro, V.; Shahrokh, V.; Martínez-Martínez, S.; Acosta, J.A.; Almagro, M.; Martínez-Mena, M.; Boix-Fayos, C.; Díaz-Pereira, E.; Zornoza, R. Perennial alley cropping contributes to decrease soil CO2 and N2O emissions and increase soil carbon sequestration in a Mediterranean almond orchard. Sci. Total Environ. 2022, 845, 157225. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.E.; Kharecha, P.; Sato, M.; Tselioudis, G.; Kelly, J.; Bauer, S.E.; Pokela, A. Global Warming Has Accelerated: Are the United Nations and the Public Well-Informed? Environ. Sci. Policy Sustain. Dev. 2025, 67, 6–44. [Google Scholar] [CrossRef]
- Kravchenko, I.K. Microbial Oxidation of Atmospheric Methane in Natural and Agricultural Upland Soils. In Agro-Environmental Sustainability; Singh, J., Seneviratne, G., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Sun, B.F.; Zhao, H.; Lü, Y.Z.; Lu, F.; Wang, X.K. The effects of nitrogen fertilizer application on methane and nitrous oxide emission/uptake in Chinese croplands. J. Integr. Agric. 2016, 15, 440–450. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous oxide emissionsfrom soils: How well do we understand theprocesses and their controls? Philos. Trans. R. Soc. 2013, 368, 20130122. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Xia, L.; Lam, S.K.; Wang, E.; Zhang, Y.; Mosier, A.; Chen, D.A. Global Synthesis of Soil Denitrification: Driving Factors and Mitigation Strategies. Agric. Ecosyst. Environ. 2022, 327, 107850. [Google Scholar] [CrossRef]
- Vico, A.; Sáez, J.A.; Pérez-Murcia, M.D.; Martinez-Tomé, J.; Andreu-Rodríguez, J.; Agulló, E.; Bustamante, M.A.; Sanz-Cobena, A.; Moral, R. Production of spinach in intensive Mediterranean horticultural systems can be sustained by organic-based fertilizers without yield penalties and with low environmental impacts. Agric. Syst. 2020, 178, 102765. [Google Scholar] [CrossRef]
- Charles, A.; Rochette, P.; Whalen, J.K.; Angers, D.A.; Chantigny, M.H.; Bertrand, N. Global nitrous oxide emission factors from agricultural soils after addition of organic amendments: A meta-analysis. Agric. Ecosyst. Environ. 2017, 236, 88–98. [Google Scholar] [CrossRef]
- Lombardi, B.; Orden, L.; Varela, P.; Garay, M.; Iocoli, G.A.; Montenegro, A.; Sáez-Tovar, J.; Bustamante, M.Á.; Juliarena, M.P.; Moral, R. Is Dairy Effluent an Alternative for Maize Crop Fertigation in Semiarid Regions? An Approach to Agronomic and Environmental Effects. Animals 2022, 12, 2025. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, M.L.; Velthof, G.L.; Mondini, C.; Sinicco, T.; Van Groeningen, J.W. Nitrous oxide and carbon dioxide emissions during initial decomposition of animal by-products applied as fertilisers to soils. Geoderma 2010, 157, 235–242. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Aguilera, E.; Sanz-Cobena, A.; Adams, D.C.; Abalos, D.; Barton, L.; Ryalsh, R.; Silver, W.L.; Alfaro, M.A.; Pappa, V.A.; et al. Direct nitrous oxide emissions in Mediterranean climate cropping systems: Emission factors based on a meta-analysis of available measurement data. Agric. Ecosyst. Environ. 2017, 238, 25–35. [Google Scholar] [CrossRef]
| Treatment | Nutrient Rate (kg ha−1) | Fertilizer Rate (kg ha−1) | |||
|---|---|---|---|---|---|
| P | N | P | N | ||
| Control 1 | C | 0 | 0 | 0 | 0 |
| Inorganic (MAP + Urea) | IN | 50 | 150 | 188 | 326 |
| Sewage Sludge | SS | 60 | 150 | 7500 | |
| Organic Pelletized Compost | OCP | 40 | 150 | 6000 | |
| Nutrient a | SS | OCP |
|---|---|---|
| pH | 6.56 ± 0.43 | 9.05 ± 1.61 |
| EC (dS m−1) | 5.68 ± 0.87 | 3.51 ± 2.34 |
| OM (%) | 52.4 ± 0.01 | 79.6 ± 1.17 |
| TOC (g kg−1) | 292 ± 1.02 | 386 ± 0.79 |
| TN (g kg−1) | 46.2 ± 0.96 | 25.4 ± 0.49 |
| NH4+-N (mg kg−1) | 26.3 ± 2.22 | 54.7 ± 0.09 |
| NO3−-N (mg kg−1) | 8.20 ± 0.05 | 25.5 ± 10.1 |
| P (g kg−1) | 8.03 ± 10.9 | 6.56 ± 0.28 |
| K (g kg−1) | 2.51 ± 1.02 | 26.9 ± 1.49 |
| Na (g kg−1) | 1.20 ± 5.57 | 1.12 ± 0.65 |
| S (g kg−1) | 14.0 ± 2.3 | 3.68 ± 0.01 |
| Ca (g kg−1) | 44.6 ± 7.8 | 38.2 ± 0.6 |
| Mg (g kg−1) | 5.84 ± 4.23 | 4.40 ± 1.20 |
| Fe (g kg−1) | 12062 ± 12 | 2212 ± 0 |
| Mn (mg kg−1) | 240 ± 2 | 294 ± 2 |
| Cu (mg kg−1) | 166 ± 3 | 96.9 ± 0.5 |
| Zn (mg kg−1) | 743 ± 2 | 235 ± 0 |
| Treatment | Respirometry (mg CO2-C kg soil−1) | Biomass C (mg C kg soil−1) | Urease (μmol NH4+ g soil−1) | Phosphatase (μmoles p-nitrophenol g suelo−1) |
|---|---|---|---|---|
| Control | 5.88 a | 962 | 0.68 a | 0.36 a |
| IN | 9.21 b | 997 | 0.79 ab | 2.02 a |
| SS | 11.2 b | 923 | 0.88 c | 6.51 b |
| OCP | 9.94 b | 709 | 0.83 b | 0.78 a |
| F-ANOVA | 7.42 * | 1.21 ns | 6.10 * | 17.6 *** |
| Treatment | Grain Yield (kg ha−1) | Straw (kg ha−1) | TGW (g) | HW (kg hL−1) | Protein (%) |
|---|---|---|---|---|---|
| Control | 6429 a | 4660 a | 49.4 | 75.4 a | 7.6 a |
| IN | 8618 c | 6283 b | 50.2 | 79.4 c | 10.6 c |
| SS | 6704 a | 5030 a | 48.8 | 75.6 ab | 7.7 b |
| OCP | 7268 b | 5310 ab | 49.9 | 76.5 b | 8.0 b |
| F-ANOVA | 78.7 *** | 4.3 * | 0.3 ns | 39.7 *** | 157 *** |
| Treatment | N Uptake (kg N ha−1) | NUE (%) | P Uptake (kg P ha−1) | PUE (%) | K Uptake (kg K ha−1) | KUE (%) |
|---|---|---|---|---|---|---|
| Control | 84.5 a | - | 21.2 a | - | 29.4 a | - |
| IN | 158 c | 49.2 c | 30.1 b | 18.8 c | 42.6 b | - |
| SS | 89.5 a | 1.45 a | 22.4 a | 1.4 a | 31.2 a | 5.4 |
| OCP | 101 b | 10.8 b | 24.1 a | 10.3 b | 32.9 a | 2.8 |
| F-ANOVA | 328 *** | 706 *** | 16.3 * | 47 *** | 22.6 *** | 7 ns |
| Treatment | g N2O-N m−2 | kg CH4-C m−2 | kg CO2-C m−2 | CO2eq (kg m−2) |
|---|---|---|---|---|
| Control | 1.47 a | 0.31 a | 5869 a | 109 a |
| IN | 3.30 b | 0.50 a | 6354 a | 914 b |
| SS | 6.25 d | 2.83 b | 9059 b | 1784 d |
| OCP | 5.48 c | -0.60 a | 7901 b | 1481 c |
| F-ANOVA | 305 *** | 7.46 * | 12.1 ** | 210 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Rández, A.; Sánchez Méndez, S.; Orden, L.; Andreu-Rodríguez, F.J.; Mira-Urios, M.Á.; Sáez-Tovar, J.A.; Martínez-Sabater, E.; Bustamante, M.Á.; Pérez-Murcia, M.D.; Moral, R. Evaluation of Olive Mill Waste Compost as a Sustainable Alternative to Conventional Fertilizers in Wheat Cultivation. Agriculture 2025, 15, 1543. https://doi.org/10.3390/agriculture15141543
García-Rández A, Sánchez Méndez S, Orden L, Andreu-Rodríguez FJ, Mira-Urios MÁ, Sáez-Tovar JA, Martínez-Sabater E, Bustamante MÁ, Pérez-Murcia MD, Moral R. Evaluation of Olive Mill Waste Compost as a Sustainable Alternative to Conventional Fertilizers in Wheat Cultivation. Agriculture. 2025; 15(14):1543. https://doi.org/10.3390/agriculture15141543
Chicago/Turabian StyleGarcía-Rández, Ana, Silvia Sánchez Méndez, Luciano Orden, Francisco Javier Andreu-Rodríguez, Miguel Ángel Mira-Urios, José A. Sáez-Tovar, Encarnación Martínez-Sabater, María Ángeles Bustamante, María Dolores Pérez-Murcia, and Raúl Moral. 2025. "Evaluation of Olive Mill Waste Compost as a Sustainable Alternative to Conventional Fertilizers in Wheat Cultivation" Agriculture 15, no. 14: 1543. https://doi.org/10.3390/agriculture15141543
APA StyleGarcía-Rández, A., Sánchez Méndez, S., Orden, L., Andreu-Rodríguez, F. J., Mira-Urios, M. Á., Sáez-Tovar, J. A., Martínez-Sabater, E., Bustamante, M. Á., Pérez-Murcia, M. D., & Moral, R. (2025). Evaluation of Olive Mill Waste Compost as a Sustainable Alternative to Conventional Fertilizers in Wheat Cultivation. Agriculture, 15(14), 1543. https://doi.org/10.3390/agriculture15141543







