Transcriptomics and Metabolomics Reveal the Dwarfing Mechanism of Pepper Plants Under Ultraviolet Radiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Light Treatment Design
2.2. Growth Measurement
2.2.1. Plant Morphology and Growth Characteristics
2.2.2. Photosynthetic Pigment Content
2.2.3. RNA-Seq and Data Processing
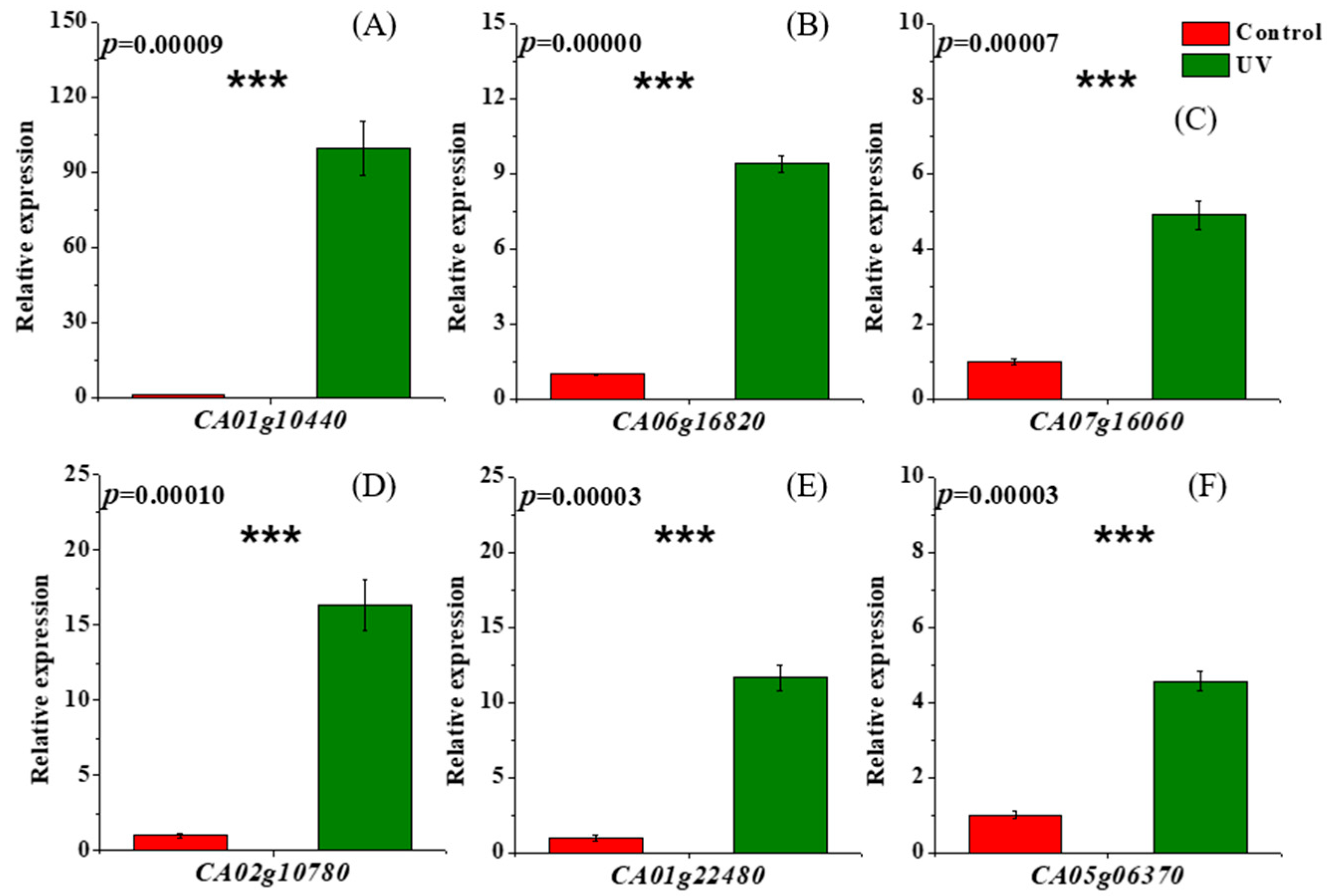
2.2.4. qRT-PCR Analysis
2.2.5. Metabolomics Analysis of Gibberellins
2.3. Statistical Analysis
3. Results
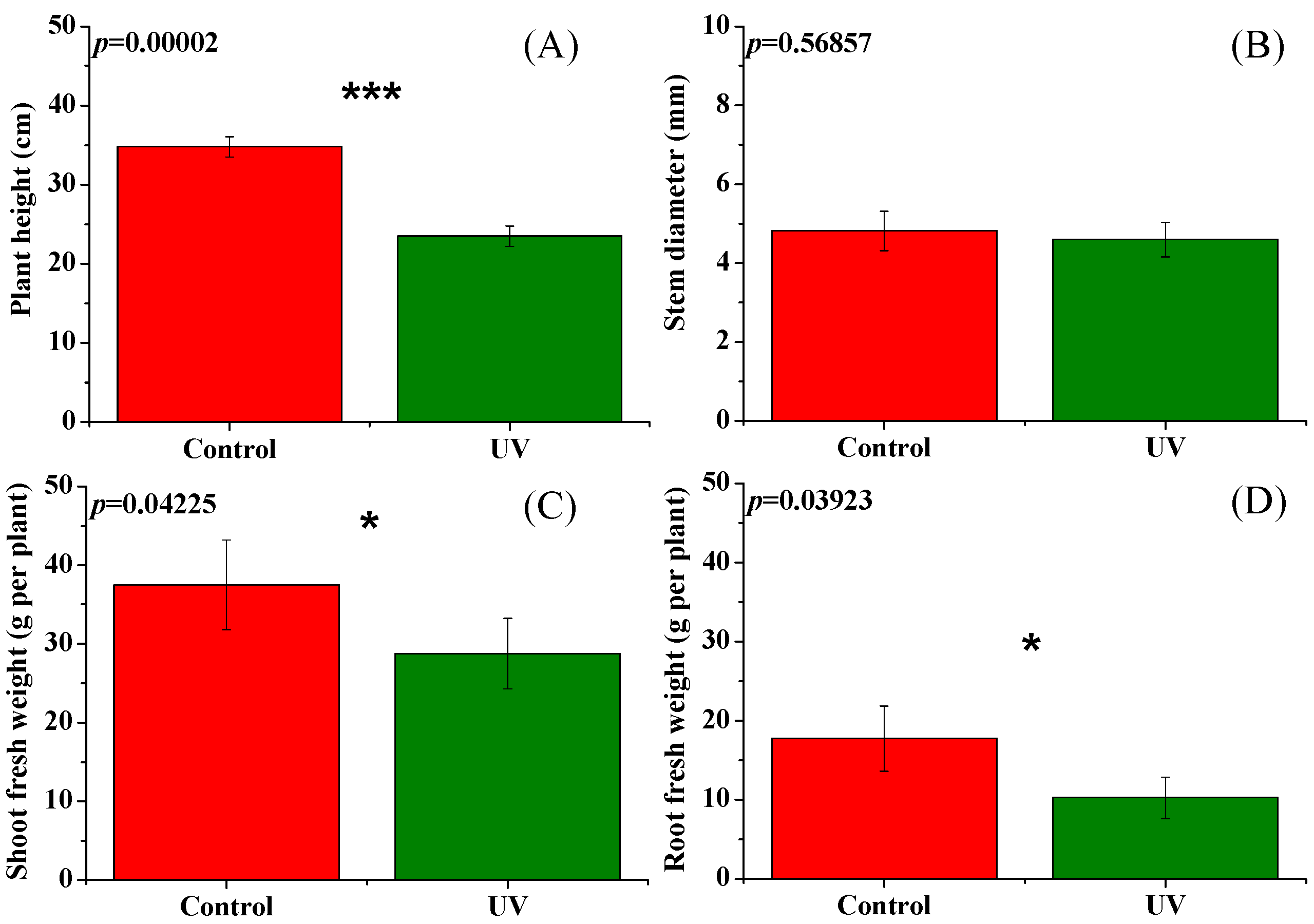
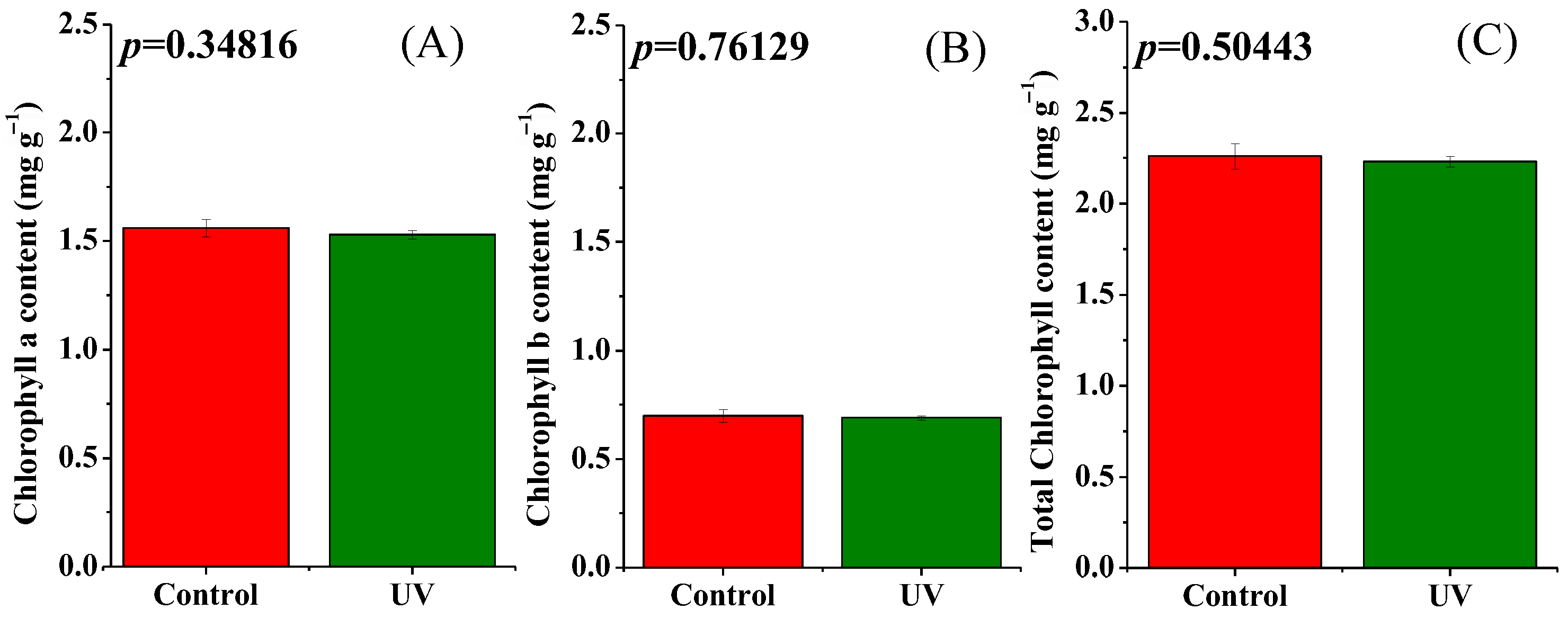
3.1. Plant Morphology, Photosynthetic Pigment Content, and Biomass Accumulation of Pepper Plants as Affected by UV-B Radiation
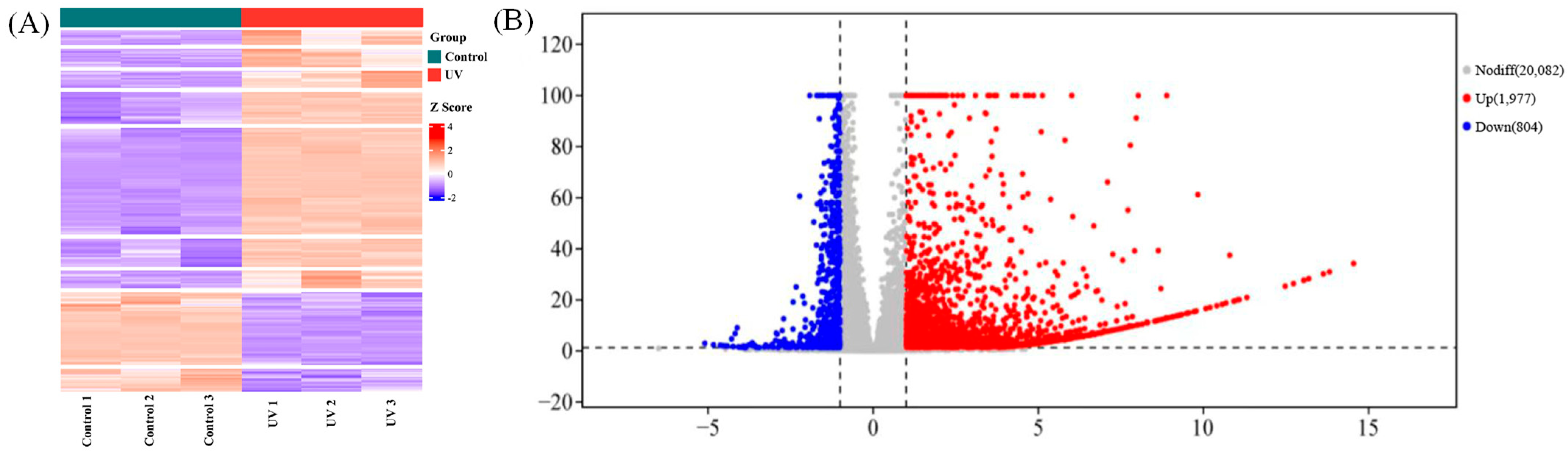
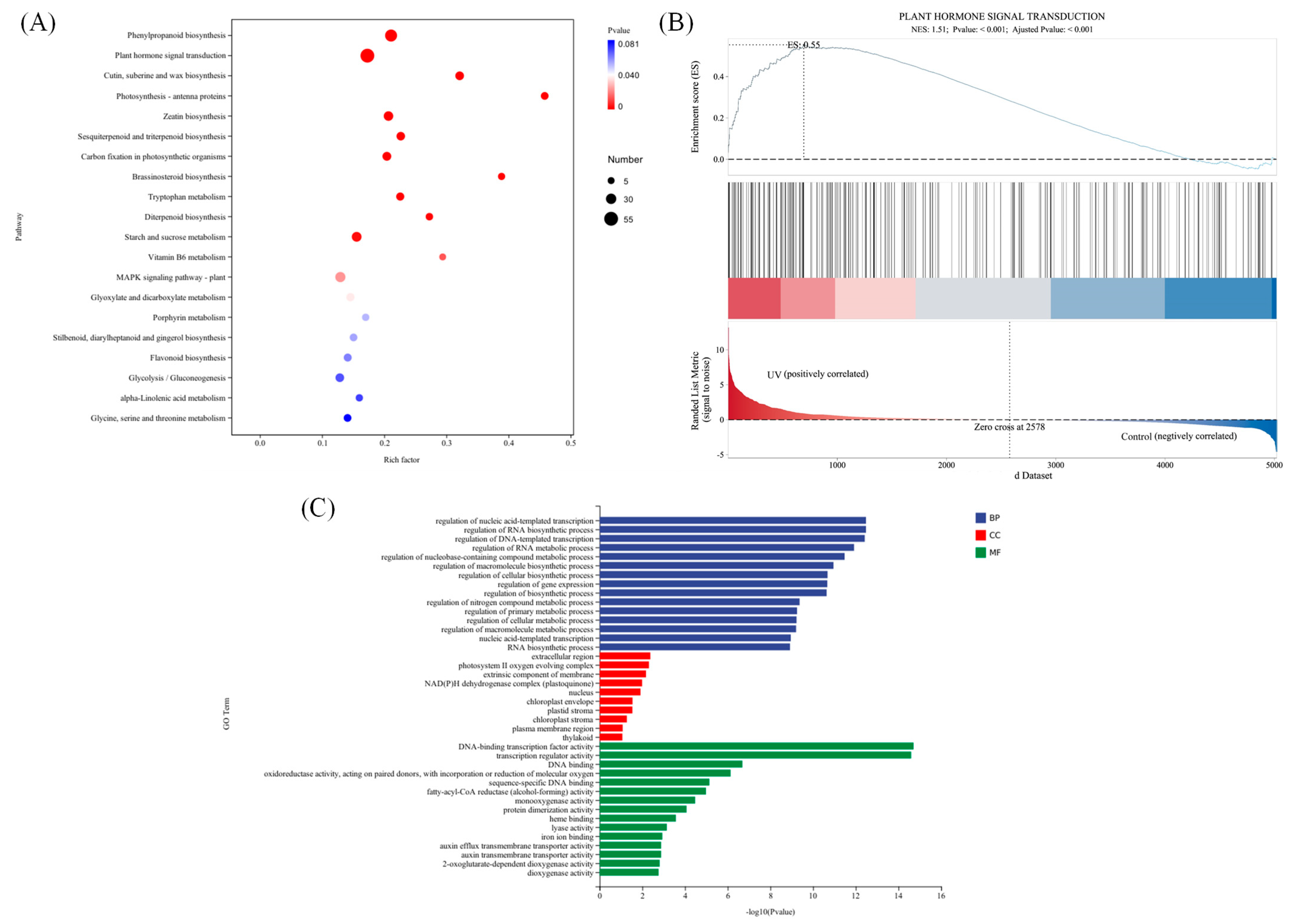
3.2. Transcriptome Sequencing, Clustering, and Function Enrichment
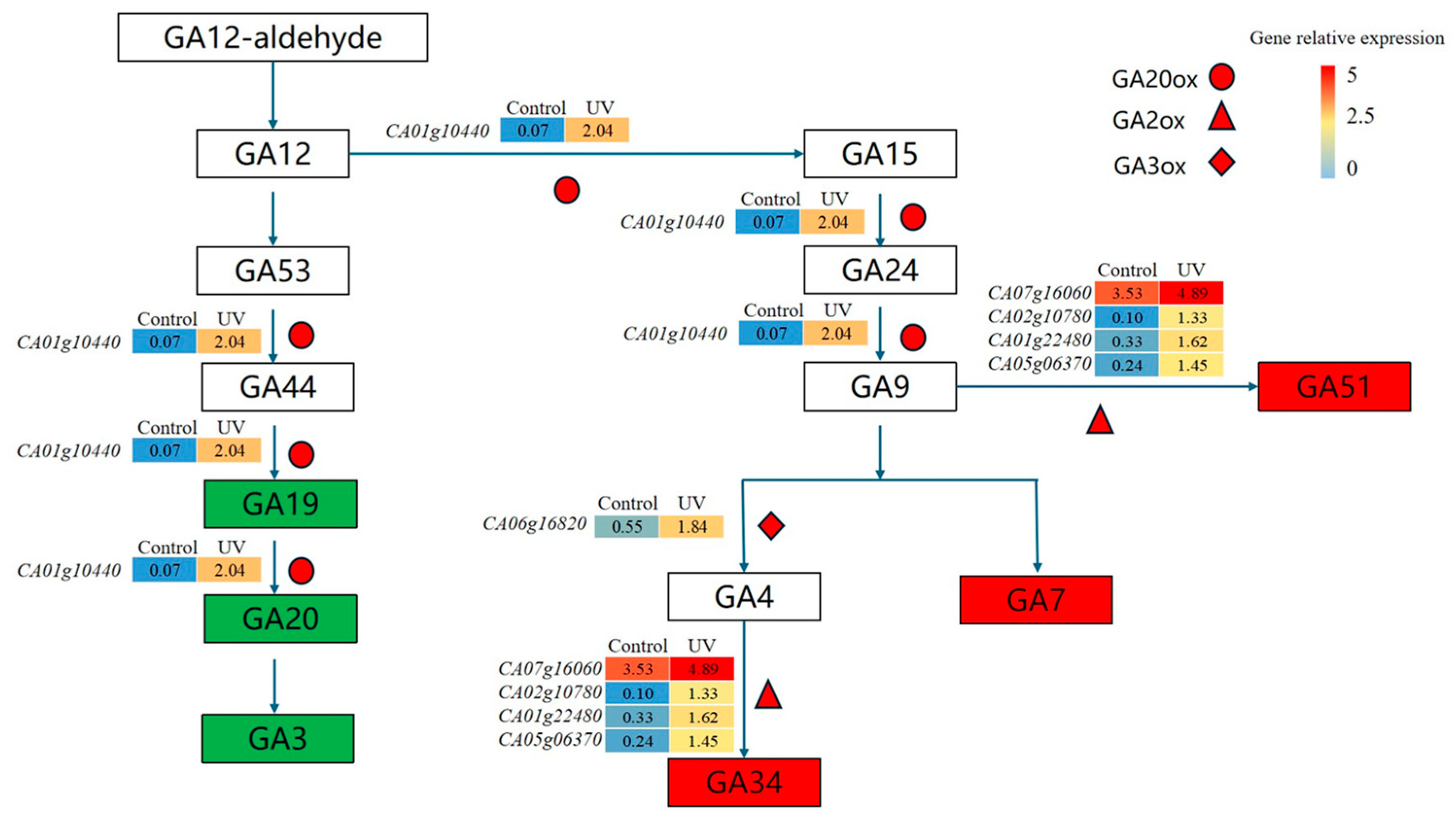
3.3. Analysis of the Gibberellin Metabolic Pathway of Pepper Plants Exposed to UV Radiation
4. Discussion
4.1. Effects of UV-B Radiation on the Leaf Morphology, Pigment Content, and Biomass of Pepper Plants
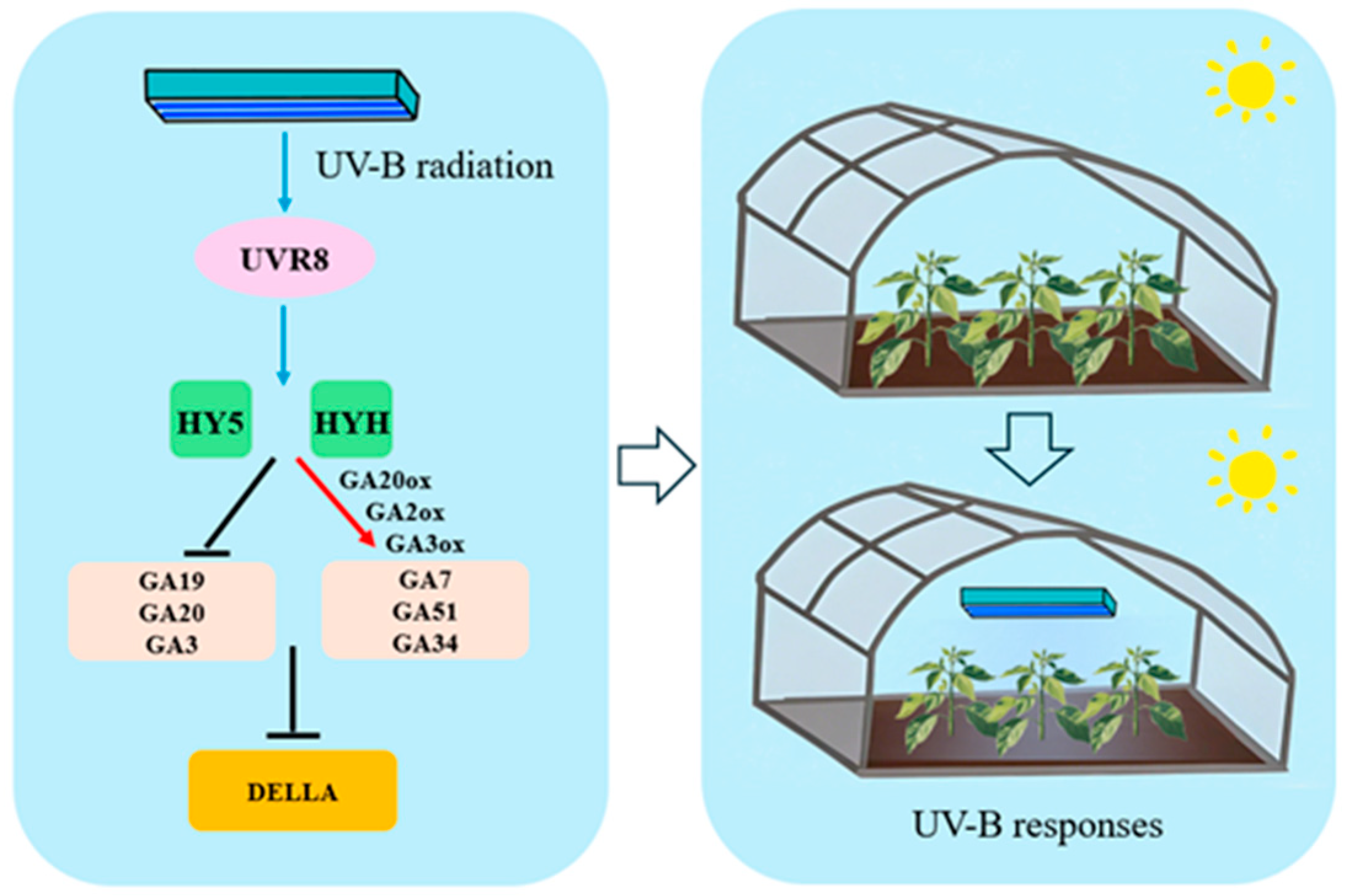
4.2. Transcriptomics and Metabolomics Reveal the Changes in the Plant Height of Pepper Plants Under UV Radiation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Sun, X.; Battino, M.; Wei, X.; Shi, J.; Zhao, L.; Liu, S.; Xiao, J.; Shi, B.; Zou, X. A comparative overview on chili pepper (capsicum genus) and sichuan pepper (zanthoxylum genus): From pungent spices to pharma-foods. Trends Food Sci. Technol. 2021, 117, 148–162. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: www.fao.org/faostat/zh/#data/QCL (accessed on 20 March 2025).
- Lemarie, S.; Guérin, V.; Jouault, A.; Proost, K.; Cordier, S.; Guignard, G.; Demotes-Mainard, S.; Bertheloot, J.; Sakr, S.; Peilleron, F. Melon and potato crops productivity under a new generation of optically active greenhouse films. Acta Hortic. 2019, 1296, 517–526. [Google Scholar] [CrossRef]
- Kim, M.; Lee, Y.-B.; Choi, K.-Y. Effects of greenhouse temperatures and solar irradiance levels on the monthly production of summer-cultivated Paprika. Hortic. Sci. Technol. 2024, 42, 339–349. [Google Scholar] [CrossRef]
- Šalagovič, J.; Vanhees, D.; Verboven, P.; Holsteens, K.; Verlinden, B.; Huysmans, M.; Van de Poel, B.; Nicolaï, B. Microclimate monitoring in commercial tomato (Solanum lycopersicum L.) greenhouse production and its effect on plant growth, yield and fruit quality. Front. Hortic. 2024, 3, 1425285. [Google Scholar] [CrossRef]
- Weiss, J.; Gruda, N.S. Novel breeding techniques and strategies for enhancing greenhouse vegetable product quality. Agronomy 2025, 15, 207. [Google Scholar] [CrossRef]
- Kharshiing, E.V.; Lyngdoh, M.O.I.; Vardhana, L.; Ramyani, B.; Sahoo, L. Manipulation of light environment for optimising photoreceptor activity towards enhancing plant traits of agronomic and horticultural importance in crops. J. Hortic. Sci. Biotechnol. 2022, 97, 535–551. [Google Scholar] [CrossRef]
- Lemarié, S.; Proost, K.; Quellec, A.-S.; Cordier, S.; Guignard, G.; Guérin, V.; Sakr, S.; Peilleron, F. The innovative ‘LIGHT CASCADE®’ sunlight photoconversion greenhouse films enhance horticultural crops production in Europe. Acta Hortic. 2023, 1377, 275–282. [Google Scholar] [CrossRef]
- Lin, T.; Maier, C.R.; Liang, W.; Klause, N.; He, J.; Tissue, D.T.; Lan, Y.-C.; Sethuvenkatraman, S.; Goldsworthy, M.; Chen, Z.-H. A light-blocking greenhouse film differentially impacts climate control energy use and capsicum production. Front. Energy Res. 2024, 12, 1360536. [Google Scholar] [CrossRef]
- Mu, Z.; Bo, Y.; Xu, J.; Song, K.; Dong, B.; Wang, J.; Shu, S.; Wang, Y.; Guo, S. Development and application of greenhouse light environment simulation technology based on light path tracing. Comput. Electron. Agric. 2024, 218, 108652. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Zeng, B.; Jiang, Q.; Wang, S.; Li, X. Growth and transcriptomics analysis of Michelia macclurei Dandy plantlets with different LED quality treatments. Phyton-Int. J. Exp. Bot. 2023, 92, 2891–2906. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, S.; Yu, D. The role of light quality in regulating early seedling development. Plants 2023, 12, 2746. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Sun, H.; Xu, X.; Yang, J.; Wang, G.; Wei, Z.; Xu, T.; Yin, J. Study on the method and mechanism of seedling picking for pepper (Capsicum annuum L.) plug seedlings. Agriculture 2024, 14, 11. [Google Scholar] [CrossRef]
- 14Si, C.; Lin, Y.; Luo, S.; Yu, Y.; Liu, R.; Naz, M.; Dai, Z. Effects of LED light quality combinations on growth and leaf colour of tissue culture-generated plantlets in Sedum rubrotinctum. Hortic. Sci. Technol. 2024, 42, 53–67. [Google Scholar]
- Bi, X.; Xu, H.; Yang, C.; Zhang, H.; Li, W.; Su, W.; Zheng, M.; Lei, B. Investigating the influence of varied ratios of red and far-red light on lettuce (Lactuca sativa): Effects on growth, photosynthetic characteristics and chlorophyll fluorescence. Front. Plant Sci. 2024, 15, 1430241. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Sun, Q.; Zheng, Y.; Xu, Y.; Liu, B.; Li, Q. Effects of red and blue light on the growth, photosynthesis, and subsequent growth under fluctuating light of cucumber seedlings. Plants 2024, 13, 1668. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Chowdhury, M.K.; Mazumder, S.R.; Solaiman, A.H.M.; Tasnim, Z.; Arefin, S.M.A.; Uddain, J. Evaluation of growth and nutritional profile of microgreens of different crops under various LEDs light spectrums. J. Appl. Hortic. 2024, 26, 231–236. [Google Scholar] [CrossRef]
- Shen, Y.; Han, X.; Wang, H.; Shen, J.; Sun, L.; Fan, K.; Wang, Y.; Ding, S.; Song, D.; Ding, Z. Full-length transcriptome sheds light into the molecular mechanism of tea leaf yellowing induced by red light. Sci. Rep. 2024, 14, 29901. [Google Scholar] [CrossRef] [PubMed]
- Mickens, M.A.; Skoog, E.J.; Reese, L.E.; Barnwell, P.L.; Spencer, L.E.; Massa, G.D.; Wheeler, R.M. A strategic approach for investigating light recipes for ‘Outredgeous’ red romaine lettuce using white and monochromatic LEDs. Life Sci. Space Res. 2018, 19, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A.; Kearney, E.E.; Hastings, A.P.; Ramsey, T.E. Attenuation of the jasmonate burst, plant defensive traits, and resistance to specialist monarch caterpillars on shaded common milkweed (Asclepias syriaca). J. Chem. Ecol. 2012, 38, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Yoshida, H.; Hikosaka, S.; Goto, E. Effect of red and blue light versus white light on fruit biomass radiation-use efficiency in dwarf tomatoes. Front. Plant Sci. 2024, 15, 1393918. [Google Scholar] [CrossRef] [PubMed]
- Heijde, M.; Ulm, R. UV-B photoreceptor-mediated signalling in plants. Trends Plant Sci. 2012, 17, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sun, W.; Ma, Z.; Guo, C.; Chen, J.; Wu, Q.; Wang, X.; Chen, H. Integrated network analyses identify MYB4R1 neofunctionalization in the UV-B adaptation of Tartary buckwheat. Plant Commun. 2022, 3, 100414. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.H.A.; Rodrigues, A.A.; Resende, E.C.; da Silva, F.B.; Farnese, F.D.S.; Silva, L.J.; Rosa, M.; Reis, M.N.O.; Bessa, L.A.; de Oliveira, T.C.; et al. Light means power: Harnessing light spectrum and UV-B to enhance photosynthesis and rutin levels in microtomato plants. Front. Plant Sci. 2023, 14, 1261174. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Calzada, T.; Qian, M.; Strid, Å.; Neugart, S.; Schreiner, M.; Torres-Pacheco, I.; Guevara-González, R.G. Effect of UV-B radiation on morphology, phenolic compound production, gene expression, and subsequent drought stress responses in chili pepper (Capsicum annuum L.). Plant Physiol. Biochem. 2019, 134, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Chen, Y.; Qian, C.; Ren, H.; Liang, X.; Tao, W.; Chen, Y.; Wang, J.; Dong, Y.; Han, J. Nuclear accumulation of rice UV-B photoreceptors is UV-B-and OsCOP1-independent for UV-B responses. Nat. Commun. 2024, 15, 6396. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, Y.; Xu, C.; Mei, X. Editorial: Contribution of phenylpropanoid metabolism to plant development and stress responses. Front. Plant Sci. 2024, 15, 1456913. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, M.M.; Robberecht, R.; Billings, W. A steep latitudinal gradient of solar ultraviolet-B radiation in the arctic-alpine life zone. Ecology 1980, 61, 600–611. [Google Scholar] [CrossRef]
- Qian, G.; Ran, X.; Zhou, C.; Deng, D.; Zhang, P.; Guo, Y.; Luo, J.; Zhou, X.; Xie, H.; Cai, M. Systemic lupus erythematosus patients in the low-latitude plateau of China: Altitudinal influences. Lupus 2014, 23, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.B.; Shekasteband, R.; Hutton, S.F.; Lee, T.G. A mutant allele of the flowering promoting factor 1 gene at the tomato BRACHYTIC locus reduces plant height with high quality fruit. Plant Direct. 2022, 6, e422. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Jang, Y.H.; Kim, E.G.; Hur, S.S.; Kim, K.M. Quantitative Trait Loci Mapping Identified Candidate Genes Involved in Plant Height Regulation in Rice. Int. J. Mol. Sci. 2023, 24, 16895. [Google Scholar] [CrossRef] [PubMed]
- Bhat, J.A.; Karikari, B.; Adeboye, K.A.; Ganie, S.A.; Barmukh, R.; Hu, D.; Varshney, R.K.; Yu, D. Identification of superior haplotypes in a diverse natural population for breeding desirable plant height in soybean. Theor. Appl. Genet. 2022, 135, 2407–2422. [Google Scholar] [CrossRef] [PubMed]
- Bhujbal, S.K.; Rai, A.N.; Joshi-Saha, A. Dwarfs standing tall: Breeding towards the ‘Yellow revolution’ through insights into plant height regulation. Plant Mol. Biol. 2025, 115, 34. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Mo, M.; Gao, Y.; Ma, H.; Xiang, D.; Ma, G.; Mao, H. Effects of new compounds into substrates on seedling qualities for efficient transplanting. Agronomy 2022, 12, 983. [Google Scholar] [CrossRef]
- Stubbs, C.J.; Kunduru, B.; Bokros, N.; Verges, V.; Porter, J.; Cook, D.D.; DeBolt, S.; McMahan, C.; Sekhon, R.S.; Robertson, D.J. Moving toward short stature maize: The effect of plant height on maize stalk lodging resistance. Field Crops Res. 2023, 300, 109008. [Google Scholar] [CrossRef]
- Niu, Y.; Han, W.; Zhang, H.; Zhang, L.; Chen, H. Estimating maize plant height using a crop surface model constructed from UAV RGB images. Biosyst. Eng. 2024, 241, 56–67. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Xing, J.; Yu, H.; Zhang, R.; Chen, Y.; Zhang, D.; Yin, P.; Tian, X.; Wang, Q.; et al. Introducing selective agrochemical manipulation of gibberellin metabolism into a cereal crop. Nat. Plants 2020, 6, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Azhdarpoor, A. The application of plant growth regulators to improve phytoremediation of contaminated soils: A review. Chemosphere 2019, 220, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Urbanova, T.; Tarkowská, D.; Strnad, M.; Hedden, P. Gibberellins-Terpenoid plant hormones: Biological importance and chemical analysis. Collect. Czech. Chem. Commun. 2012, 76, 1669–1686. [Google Scholar] [CrossRef]
- Sarwar, R.; Zhu, K.M.; Jiang, T.; Ding, P.; Gao, Y.; Tan, X.L. DELLAs directed gibberellins responses orchestrate crop development: A brief review. Crop Sci. 2023, 63, 1–28. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yu, R.; Fan, L.-M.; Wei, N.; Chen, H.; Deng, X.W. DELLA-mediated PIF degradation contributes to coordination of light and gibberellin signalling in Arabidopsis. Nat. Commun. 2016, 7, 11868. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Chen, Z.; Liu, Q.; Mao, W.; Chen, Y.; Tian, W.; Liu, Y.; Han, J.; Ouyang, X.; Huang, X. Coordinated Transcriptional regulation by the UV-B photoreceptor and multiple transcription factors for plant UV-B responses. Mol. Plant 2020, 13, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.; Wellburn, A.R. Determination of total carotenoids and chlorophylls a and b of leaf in different solvents. Biochem. Soc. Trans. 1985, 11, 591–592. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Wu, D.; Duan, C.; Yan, X.; Du, Y.; Zou, J.; Guan, Y. Spatial profiling of gibberellins in a single leaf based on microscale matrix solid-phase dispersion and precolumn derivatization coupled with ultraperformance liquid chromatography-tandem mass spectrometry. Anal. Chem. 2017, 89, 9537–9543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hao, Y.-H.; Ding, J.; Xu, S.-N.; Yuan, B.-F.; Feng, Y.-Q. One-pot preparation of a mixed-mode organic-silica hybrid monolithic capillary column and its application in determination of endogenous gibberellins in plant tissues. J. Chromatogr. A. 2015, 1416, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, Z.; Liu, C.; Li, J.; Xu, W.; Chen, Y. Quantification of near-attomole gibberellins in floral organs dissected from a single Arabidopsis thaliana flower. Plant J. 2017, 91, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, Q.; Wang, Z.; Li, J.; Liu, S.; Chang, R.; Chen, H.; Liu, G. Integrated analysis of transcriptomics and metabolomics of peach under cold stress. Front. Plant Sci. 2023, 14, 1153902. [Google Scholar] [CrossRef] [PubMed]
- Hang, T.; Lu, N.; Takagaki, M.; Mao, H. Leaf area model based on thermal effectiveness and photosynthetically active radiation in lettuce grown in mini-plant factories under different light cycles. Sci. Hortic. 2019, 252, 113–120. [Google Scholar] [CrossRef]
- Lazzarin, M.; Meisenburg, M.; Meijer, D.; van Ieperen, W.; Marcelis, L.F.M.; Kappers, I.F.; van der Krol, A.R.; van Loon, J.J.A.; Dicke, M. LEDs make it resilient: Effects on plant growth and defense. Trends Plant Sci. 2021, 26, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Leschevin, M.; Ksas, B.; Baltenweck, R.; Hugueney, P.; Caffarri, S.; Havaux, M. Photosystem rearrangements, photosynthetic efficiency, and plant growth in far red-enriched light. Plant J. 2024, 120, 2536–2552. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Shibuya, T.; Watanabe, T.; Kato, K.; Kanayama, Y. Effect of light quality on metabolomic, ionomic, and transcriptomic profiles in tomato fruit. Int. J. Mol. Sci. 2022, 23, 13288. [Google Scholar] [CrossRef] [PubMed]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gleason, S.M.; Hao, G.; Hua, L.; He, P.; Goldstein, G.; Ye, Q. Hydraulic traits are coordinated with maximum plant height at the global scale. Sci. Adv. 2019, 5, eaav1332. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Zhang, H.; Deng, H.; Zhang, Z.; Qiu, F.; Yang, F. Maize COMPACT PLANT 3 regulates plant architecture and facilitates high-density planting. Plant Cell. 2025, 37, koaf029. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wang, J.; Zhang, X.; Wang, C.; Li, G.; Wan, J.S.H.; Du, D. Warming significantly inhibited the competitive advantage of native plants in interspecific competition under phosphorus deposition. Plant Soil. 2023, 486, 503–518. [Google Scholar] [CrossRef]
- Guo, F.; Hou, L.; Ma, C.; Li, G.; Lin, R.; Zhao, Y.; Wang, X. Comparative transcriptome analysis of the peanut semi-dwarf mutant 1 reveals regulatory mechanism involved in plant height. Gene 2021, 791, 145722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Zou, J.; Bian, Z.; Yang, Q.; Li, T. UVA radiation promotes tomato growth through morphological adaptation leading to increased light interception. Environ. Exp. Bot. 2020, 176, 104073. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Aphalo, P.J.; Zhang, Y.; Cheng, R.; Li, T. Ultraviolet-A1 radiation induced a more favorable light-intercepting leaf-area display than blue light and promoted plant growth. Plant Cell Environ. 2024, 47, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Hernández, D.I.; González-García, Y.; Olivares-Sáenz, E.; Juárez-Maldonado, A. Seedling priming with UV-A radiation induces positive responses in tomato and bell pepper plants under water stress. Sci. Hortic. 2024, 332, 113235. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, N.; Na, X.; Li, K.; Pu, M.; Sun, H.; Song, Y.; Peng, T.; Fei, P.; Li, J.; et al. UV-B stress reshapes root-associated microbial communities and networks, driven by host plant resistance. Soil Biol. Biochem. 2025, 205, 109767. [Google Scholar] [CrossRef]
- Li, W.; Zhang, C.; Ma, T.; Li, W. Estimation of summer maize biomass based on a crop growth model. Emir. J. Food Agric. 2021, 33, 742–750. [Google Scholar] [CrossRef]
- Song, J.; Zhang, R.; Yang, F.; Wang, J.; Cai, W.; Zhang, Y. Nocturnal LED supplemental lighting improves quality of tomato seedlings by increasing biomass accumulation in a controlled environment. Agronomy 2024, 14, 1888. [Google Scholar] [CrossRef]
- Xiong, F.S.; Day, T.A. Effect of solar ultraviolet-B radiation during springtime ozone depletion on photosynthesis and biomass production of Antarctic vascular plants. Plant Physiol. 2001, 125, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Dong, Y.; Huang, X. Plant responses to UV-B radiation: Signaling, acclimation and stress tolerance. Stress Biol. 2022, 2, 51. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, D.; Pandey, A.; Agrawal, M.; Agrawal, S.B. Photosynthetic, biochemical and secondary metabolite changes in a medicinal plant Chlorophytum borivillianum (Safed musli) against low and high doses of UV-B radiation. Photochem. Photobiol. 2023, 99, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Skowron, E.; Trojak, M.; Pacak, I.; Wezigowska, P.; Szymkiewicz, J. Enhancing the quality of indoor-grown basil microgreens with low-dose UV-B or UV-C light supplementation. Int. J. Mol. Sci. 2025, 26, 2352. [Google Scholar] [CrossRef] [PubMed]
- Tilbrook, K.; Arongaus, A.B.; Binkert, M.; Heijde, M.; Yin, R.; Ulm, R. The UVR8 UV-B photoreceptor: Perception, signaling and response. Arab. Book Am. Soc. Plant Biol. 2013, 11, e0164. [Google Scholar] [CrossRef] [PubMed]
- Rasool, G.; Guo, X.; Wang, Z.; Hassan, M.; Aleem, M.; Javed, Q.; Chen, S. Effect of buried straw layer coupled with fertigation on florescence and yield parameters of Chinese cabbage under greenhouse environment. J. Soil Sci. Plant Nutr. 2020, 20, 598–609. [Google Scholar] [CrossRef]
- Elsayed, S.; El-Hendawy, S.; Elsherbiny, O.; Okasha, A.M.; Elmetwalli, A.H.; Elwakeel, A.E.; Memon, M.S.; Ibrahim, M.E.M.; Ibrahim, H.H. Estimating chlorophyll content, production, and quality of sugar beet under various nitrogen levels using machine learning models and novel spectral indices. Agronomy 2023, 13, 2743. [Google Scholar] [CrossRef]
- León-Chan, R.G.; López-Meyer, M.; Osuna-Enciso, T.; Sañudo-Barajas, J.A.; Heredia, J.B.; León-Félix, J. Low temperature and ultraviolet-B radiation affect chlorophyll content and induce the accumulation of UV-B-absorbing and antioxidant compounds in bell pepper (Capsicum annuum) plants. Environ. Exp. Bot. 2017, 139, 143–151. [Google Scholar] [CrossRef]
- Yue, J.; Zhang, D.; Chen, G.; Shen, X. ApGA20ox1, a key gibberellin biosynthesis gene, regulates somatic embryogenesis and plant height in Agapanthus praecox. Sci. Hortic. 2023, 312, 111846. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Liu, W.; Mu, X.; Xu, Y.; Gu, Z.; Hong, B.; Zhao, X. Diversity of gibberellins contributes to different flowering ecotypes in chrysanthemum. Sci. Hortic. 2024, 338, 113702. [Google Scholar] [CrossRef]
- Hao, P.; Jian, C.; Hao, C.; Liu, S.; Hou, J.; Liu, H.; Liu, H.; Zhang, X.; Zhao, H.; Li, T. Coordination of miR319–TaPCF8 with TaSPL14 orchestrates auxin signaling and biosynthesis to regulate plant height in common wheat. J. Integr. Plant Biol. 2024, 66, 2362–2378. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.J.C.; Arruda, R.S.; Pimenta, T.M.; Souza, G.A.; Teixeira, L.S.; Araújo, W.L.; Zsogon, A.; Ribeiro, D.M. Ethylene modulates peanut (Arachis hypogaea L.) growth and yield in response to planting depth. Field Crops Res. 2025, 322, 109751. [Google Scholar] [CrossRef]
- Muniandi, S.K.M.; Hossain, M.A.; Abdullah, M.P.; Ab Shukor, N.A. Gibberellic acid (GA3) affects growth and development of some selected kenaf (Hibiscus cannabinus L.) cultivars. Ind. Crops Prod. 2018, 118, 180–187. [Google Scholar] [CrossRef]
- Othman, Y.A.; Leskovar, D.I. Foliar application of gibberellic acid improves yield and head phenolic compounds in globe artichoke. Sci. Hortic. 2022, 301, 111115. [Google Scholar] [CrossRef]
- Chandel, A.; Thakur, M.; Rakwal, A.; Chauhan, S.; Bhargava, B. Exogenous applications of gibberellic acid modulate the growth, flowering and longevity of calla lily. Heliyon 2023, 9, e16319. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Shu, A.; Wang, Y.; Geng, G. Effects of foliar spraying gibberellin and brassicin physiological characteristics of sugar beet. J. Eng. Heilongjiang Univ. 2022, 13, 91–96. [Google Scholar]
- Shohat, H.; Cheriker, H.; Kilambi, H.V.; Illouz Eliaz, N.; Blum, S.; Amsellem, Z.; Tarkowská, D.; Aharoni, A.; Eshed, Y.; Weiss, D. Inhibition of gibberellin accumulation by water deficiency promotes fast and long-term ‘drought avoidance’ responses in tomato. New Phytol. 2021, 232, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Hu, J.; Chen, H.; Cai, W. SMAX1 interacts with DELLA protein to inhibit seed germination under weak light conditions via gibberellin biosynthesis in Arabidopsis. Cell Rep. 2023, 42, 112740. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Ogawa, M.; Kuwahara, A.; Hanada, A.; Kamiya, Y.; Yamaguchi, S. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell. 2004, 16, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huang, S.; Lu, Q.; Li, S.; Zhao, S.; Zheng, X.; Zhou, Q.; Zhang, W.; Li, J.; Wang, L.; et al. UV-B irradiation-activated E3 ligase GmILPA1 modulates gibberellin catabolism to increase plant height in soybean. Nat. Commun. 2023, 14, 6262. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Peng, S.; Xian, Z.; Lin, D.; Hu, G.; Yang, L.; Ren, M.; Li, Z. Overexpression of a tomato miR171 target gene SlGRAS24 impacts multiple agronomical traits via regulating gibberellin and auxin homeostasis. Plant Biotechnol. J. 2017, 15, 472–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, J.; Lu, W.; Deng, D. Gibberellin in plant height control: Old player, new story. Plant Cell Rep. 2017, 36, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Ninkuu, V.; Aluko, O.O.; Yan, J.; Zeng, H.; Liu, G.; Zhao, J.; Li, H.; Chen, S.; Dakora, F.D. Phenylpropanoids metabolism: Recent insight into stress tolerance and plant development cues. Front. Plant Sci. 2025, 16, 1571825. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gu, X.; Jiang, Y.; Wang, L.; Xiao, N.; Chen, Y.; Jin, B.; Wang, L.; Li, W. UV-B promotes flavonoid biosynthesis in Ginkgo biloba by inducing the GbHY5-GbMYB1-GbFLS module. Hortic. Res. 2023, 10, uhad118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, H.; Song, W.; Zheng, L. Pre-harvest UVB irradiation enhances the phenolic and flavonoid content, and antioxidant activity of green- and red-leaf lettuce cultivars. Horticulturae 2023, 9, 695. [Google Scholar] [CrossRef]
- Song, Y.; Ma, B.; Guo, Q.; Zhou, L.; Lv, C.; Liu, X.; Wang, J.; Zhou, X.; Zhang, C. UV-B induces the expression of flavonoid biosynthetic pathways in blueberry (Vaccinium corymbosum) calli. Front. Plant Sci. 2022, 13, 1079087. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Guan, Y.; Zhang, D.; Tang, X.; Chang, Y. Integrated mRNA and miRNA transcriptome analysis suggests a regulatory network for UV–B-controlled terpenoid synthesis in fragrant woodfern (Dryopteris fragrans). Int. J. Mol. Sci. 2022, 23, 5708. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Di, T.; Chen, X.; Zheng, T.; Sun, W.; Yang, M.; Zhou, M.; Shen, Z.; Chen, H.; Su, N. CbMYB108 Integrates the Regulation of Diterpene Biosynthesis and Trichome Development in Conyza blini against UV-B. Plant Cell Environ. 2024, 47, 1300–1318. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Feng, S.Y.; Yang, Q.H.; Bhetariya, P.J.; Gong, K.; Cui, C.L.; Song, J.; Ping, X.R.; Pei, Q.Y.; Yu, T.; et al. Integration of the metabolome and transcriptome reveals the metabolites and genes related to nutritional and medicinal value in Coriandrum sativum. J. Integr. Agric. 2021, 20, 1807–1818. [Google Scholar] [CrossRef]
- He, J.; Xin, P.; Ma, X.; Chu, J.; Wang, G. Gibberellin metabolism in flowering plants: An update and perspectives. Front. Plant Sci. 2020, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.; Ali, S.S.; Gao, L.; Ni, X.; Li, X.; Wu, Y.; Jiang, J. Identification and expression analysis of Sorghum bicolor gibberellin oxidase genes with varied gibberellin levels involved in regulation of stem biomass. Ind. Crops Prod. 2020, 145, 111951. [Google Scholar] [CrossRef]
- Camut, L.; Gallova, B.; Jilli, L.; Sirlin-Josserand, M.; Carrera, E.; Sakvarelidze-Achard, L.; Ruffel, S.; Krouk, G.; Thomas, S.G.; Hedden, P.; et al. Nitrate signaling promotes plant growth by upregulating gibberellin biosynthesis and destabilization of DELLA proteins. Curr. Biol. 2021, 31, 4971–4982.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, T.; Zhang, C.; Zheng, W.; Li, J.; Jiang, W.; Xiao, C.; Wei, D.; Yang, C.; Xu, R.; et al. Gibberellin disturbs the balance of endogenesis hormones and inhibits adventitious root development of Pseudostellaria heterophylla through regulating gene expression related to hormone synthesis. Saudi J. Biol. Sci. 2021, 28, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yin, S.; Tu, Y.; Mei, H.; Wang, Y.; Yang, Y. SlCAND1, encoding cullin-associated Nedd8-dissociated protein 1, regulates plant height, flowering time, seed germination, and root architecture in tomato. Plant Mol. Biol. 2020, 102, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Sponsel, V.M.; Hoad, G.; Beeley, L. The biological activities of some new gibberellins (GAs) in six plant bioassays. Planta 1977, 135, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hou, M.; Liu, L.; Wu, S.; Shen, Y.; Ishiyama, K.; Kobayashi, M.; McCarty, D.R.; Tan, B.-C. The maize DWARF1 encodes a gibberellin 3-oxidase and is dual localized to the nucleus and cytosol. Plant Physiol. 2014, 166, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kaiser, E.; Aphalo, P.J.; Marcelis, L.F.M.; Li, T. Plant responses to UV-A1 radiation are genotype and background irradiance dependent. Environ. Exp. Bot. 2024, 219, 105621. [Google Scholar] [CrossRef]
- Salazar-Cerezoa, S.; Martínez-Montiel, N.; García-Sáncheza, J.; Pérez-y-Terrón, R.; Martínez-Contreras, R.D. Gibberellin biosynthesis and metabolism: A convergent route for plants, fungi and bacteria. Microbiol. Res. 2018, 208, 85–98. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Yan, Z.; Ding, X.; Shen, H.; Liu, Q.; Song, J.; Liang, Y.; Lu, N.; Tang, L. Transcriptomics and Metabolomics Reveal the Dwarfing Mechanism of Pepper Plants Under Ultraviolet Radiation. Agriculture 2025, 15, 1535. https://doi.org/10.3390/agriculture15141535
Zhang Z, Yan Z, Ding X, Shen H, Liu Q, Song J, Liang Y, Lu N, Tang L. Transcriptomics and Metabolomics Reveal the Dwarfing Mechanism of Pepper Plants Under Ultraviolet Radiation. Agriculture. 2025; 15(14):1535. https://doi.org/10.3390/agriculture15141535
Chicago/Turabian StyleZhang, Zejin, Zhengnan Yan, Xiangyu Ding, Haoxu Shen, Qi Liu, Jinxiu Song, Ying Liang, Na Lu, and Li Tang. 2025. "Transcriptomics and Metabolomics Reveal the Dwarfing Mechanism of Pepper Plants Under Ultraviolet Radiation" Agriculture 15, no. 14: 1535. https://doi.org/10.3390/agriculture15141535
APA StyleZhang, Z., Yan, Z., Ding, X., Shen, H., Liu, Q., Song, J., Liang, Y., Lu, N., & Tang, L. (2025). Transcriptomics and Metabolomics Reveal the Dwarfing Mechanism of Pepper Plants Under Ultraviolet Radiation. Agriculture, 15(14), 1535. https://doi.org/10.3390/agriculture15141535





