Is the Cultivation of Dictyophora indusiata with Grass-Based Substrates an Efficacious and Sustainable Approach for Enhancing the Understory Soil Environment?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design Methodology
2.2. Cultivation of D. indusiata with Forest Ecosystems
2.3. Analysis of Soil Fertility and Enzyme Activity During D. indusiata Cultivation
2.4. Soil DNA Extraction and 16S rRNA Amplicon Profiling
2.5. Targeted Metabolomic Analysis of Soil Organic Acids
2.6. Data Analysis
3. Results
3.1. Assessment of Soil Fertility and Enzyme Activity Variations During the Cultivation of D. indusiata
3.2. Analysis of the 16S RNA Diversity of Soil Bacteria for D. indusiata Cultivation
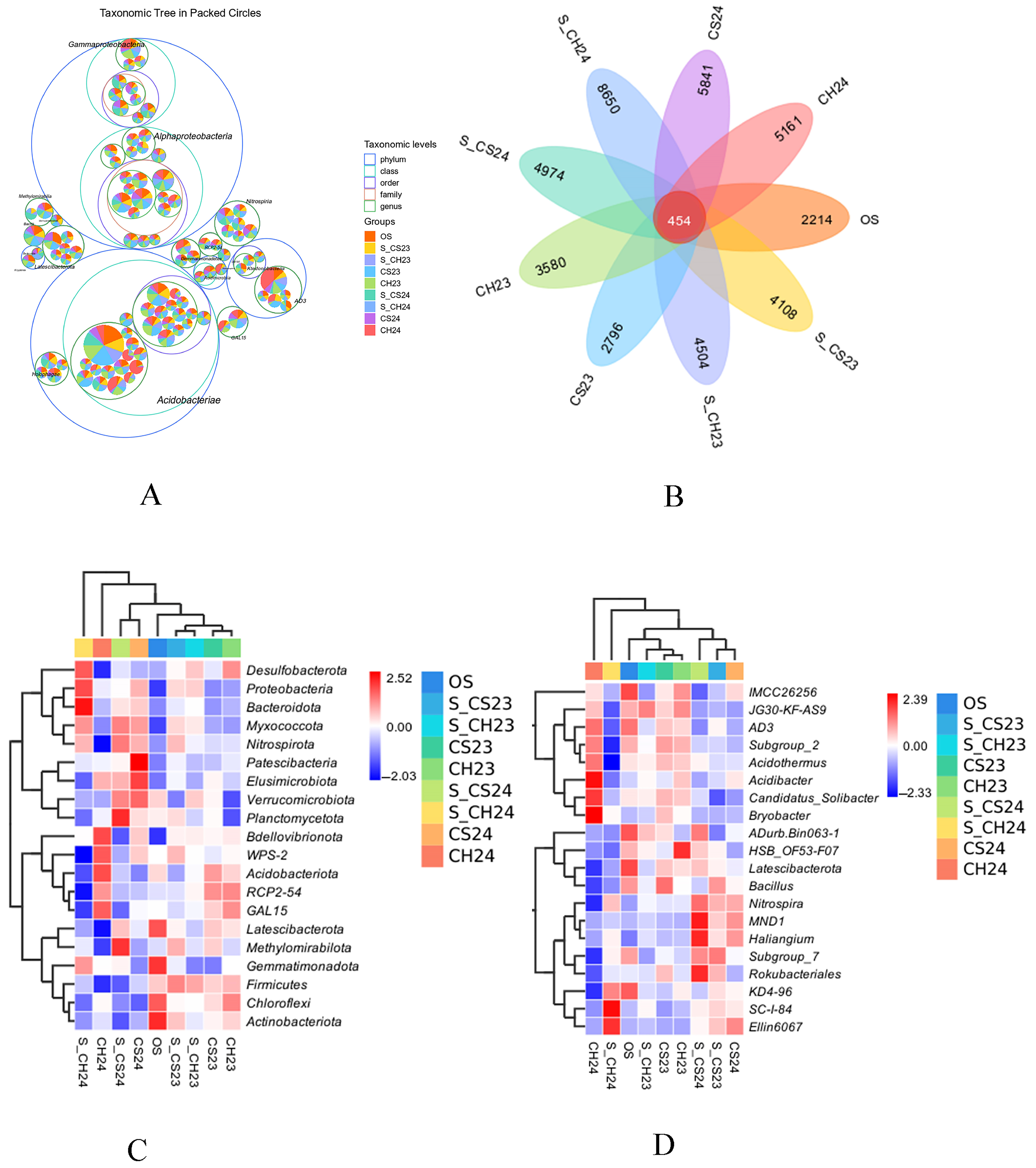
3.2.1. Changes in the Characteristics of Soil Bacterial Diversity
3.2.2. Analysis of the Composition and Structure of the Soil Bacterial Communities
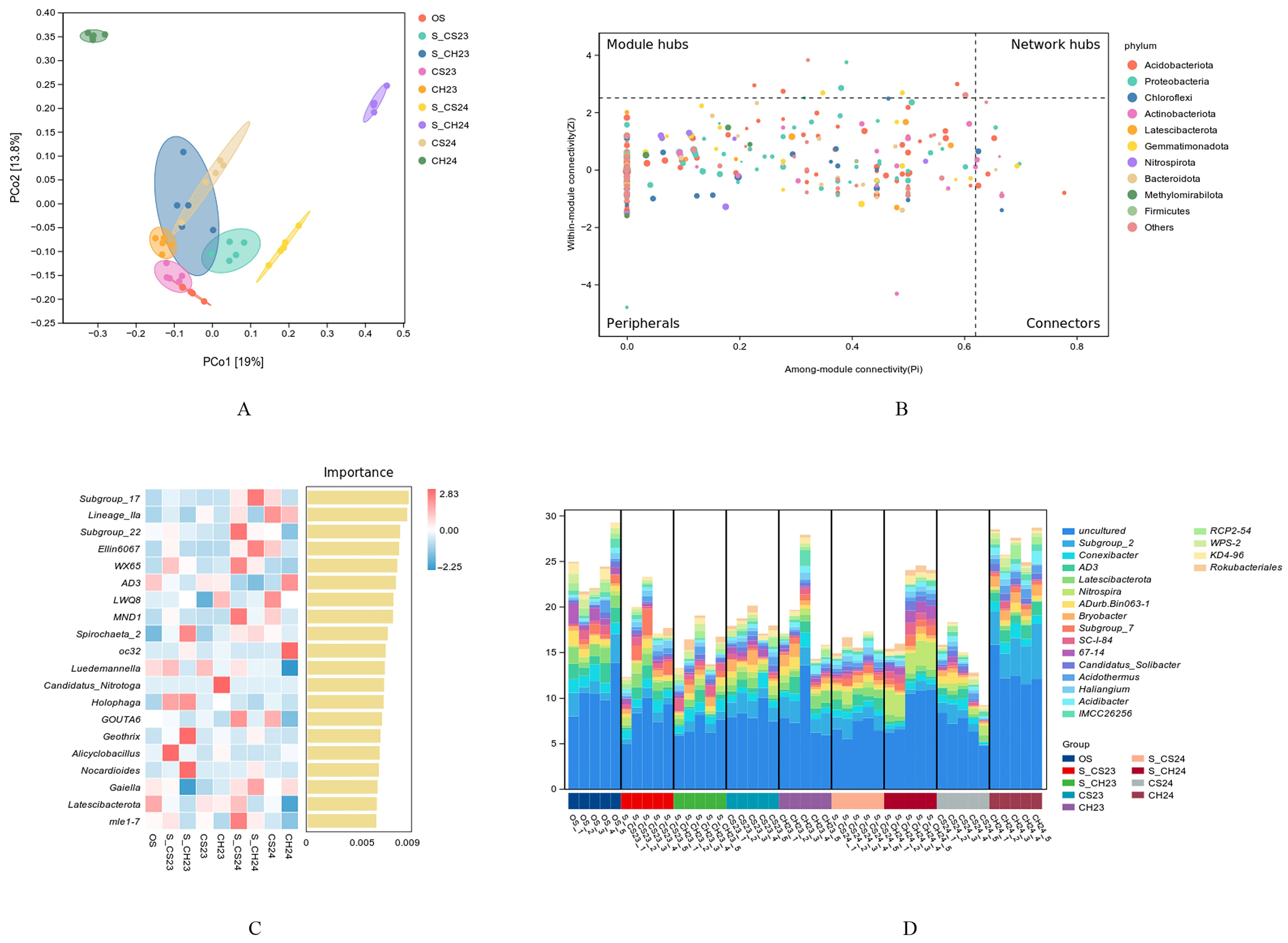
3.2.3. Molecular Ecological Network Structure of the Soil Bacterial Communities
3.2.4. Functional and Metabolic Characteristics of Soil Bacterial Communities
3.3. Targeted Metabolic Analysis of Organic Acids in D. indusiata Cultivation Soil
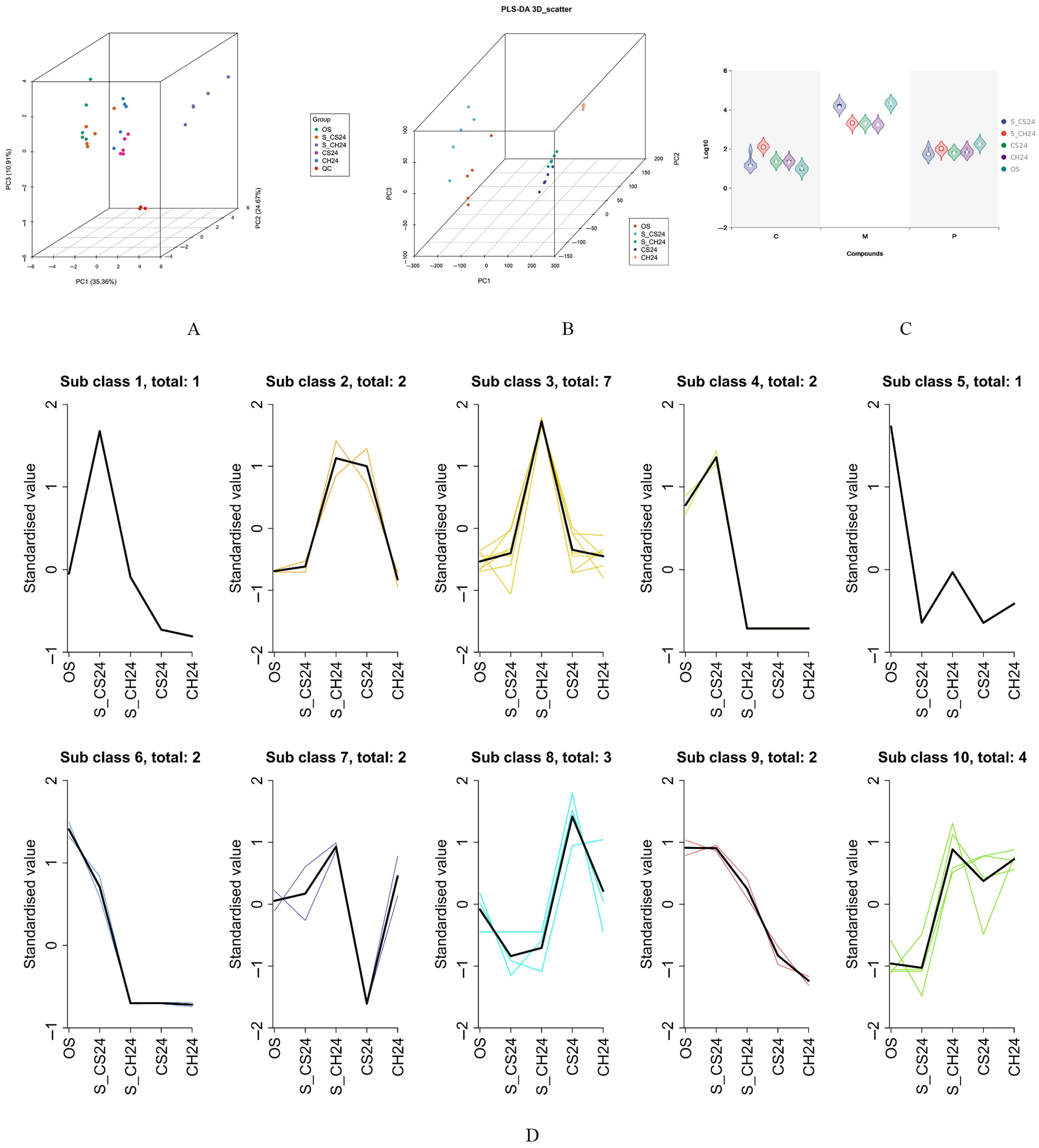
3.3.1. Sample Quality Control by LC–ESI–MS/MS
3.3.2. Multivariate Statistical Analysis
3.3.3. Analysis of Differentially Abundant Metabolites
3.4. Metabolite and Microbial Enrichment Analysis
3.5. KEGG Enrichment Analysis of Soil Bacterial Diversity and Differentially Abundant Metabolites
4. Discussion
4.1. Enhancing Soil Quality Through Edible Mushroom Cultivation in the Forest Understory
4.2. The Impact of Edible Mushroom Cultivation in Forest Understory Conditions on Soil Organic Acids
4.3. Impact of Edible Mushroom Cultivation in Forest Understory Conditions on Soil Microorganisms
4.4. Insights from Developing an Understory Economy for Agricultural Adjustment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Huang, L.; Wu, D.; Li, J.; Lei, J.; Fu, M.; Zhang, Q.; Qin, W. Catabolism of Dictyophora indusiata polysaccharide and its impacts on gut microbial composition during in vitro digestion and microbial fermentation. Foods 2023, 12, 1909. [Google Scholar] [CrossRef]
- Zhang, Y.; Xun, H.; Gao, Q.; Qi, F.; Sun, J.; Tang, F. Chemical constituents of the mushroom Dictyophora indusiata and their anti-inflammatory activities. Molecules 2023, 28, 2760. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Aliya, S.; Xin, Y. Anti-obesity effect of Dictyophora indusiata mushroom polysaccharide (DIP) in high fat diet-induced obesity via regulating inflammatory cascades and intestinal microbiome. Front. Endocrinol. 2020, 19, 558874. [Google Scholar] [CrossRef] [PubMed]
- Romanello, M.; Walawender, M.; Hsu, S.C.; Moskeland, A.; Palmeiro-Silva, Y.; Scamman, D.; Ali, Z.; Ameli, N.; Angelova, D.; Ayeb-Karlsson, S.; et al. The 2024 report of the Lancet Countdown on health and climate change: Facing record-breaking threats from delayed action. Lancet 2024, 404, 1847–1896. [Google Scholar] [CrossRef] [PubMed]
- Winkel, G.; Sotirov, M.; Moseley, C. Forest environmental frontiers around the globe: Old patterns and new trends in forest governance. Ambio 2021, 50, 2129–2137. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, B.; Hua, X.; Gao, R.; Wang, Y.; Zhang, Z.; Zhang, Y.; Mei, J.; Huang, Y.; Huang, Y.; et al. A near-complete genome assembly of the allotetrapolyploid Cenchrus fungigraminus (JUJUNCAO) provides insights into its evolution and C4 photosynthesis. Plant Commun. 2023, 4, 100633. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Yu, S.; Luo, Z.; Su, D.; Zheng, D.; Zhou, H.; Zhu, J.; Lin, X.; Luo, H.; et al. Long-Term Benefits of Cenchrus fungigraminus Residual Roots Improved the Quality and Microbial Diversity of Rhizosphere Sandy Soil through Cellulose Degradation in the Ulan Buh Desert, Northwest China. Plants 2024, 13, 708. [Google Scholar] [CrossRef]
- Song, S.; Zhou, H.; Luo, Y.; Yu, S.; Su, D.; Zheng, D.; Zhang, Z.; Luo, Z.; Liu, B.; Lin, Z.; et al. Effects of waterlogging at different duration on growth and physiological characteristics of Cenchrus fungigraminus. Environ. Exp. Bot. 2025, 231, 106096. [Google Scholar] [CrossRef]
- Lin, B.; Liu, J.; Zhang, X.; Weng, C.; Lin, X. The Flora Compositions of Nitrogen-Fixing Bacteria and the Differential Expression of nifH Gene in Pennisetum giganteum z.x.lin Roots. BioMed Res. Int. 2021, 23, 5568845. [Google Scholar] [CrossRef]
- Li, J.; Zhou, B.; Li, T.; Lin, H.; Lin, Z.; Lu, G.; Liu, Y.; Lin, B.; Lin, D. Isolation of rhizobacteria from the Cenchrus fungigraminus rhizosphere and characterization of their nitrogen-fixing performance and potential role in plant growth promotion. Plant Soil 2023, 489, 405–421. [Google Scholar] [CrossRef]
- Li, J.; Lei, Y.; Qin, Z.; Liu, J.; Rensing, C.; Lin, Z.; Lin, D. Effects of seafood mushroom spent substrate solid-state fermentation combined with PGPR as a microbial fertilizer on the soil environment and growth promotion of Cenchrus fungigraminus. J. Soil Sci. Plant Nutr. 2024, 24, 1261–1277. [Google Scholar] [CrossRef]
- Paredes, C.; Medina, E.; Moral, R.; Pérez-Murcia, M.; Moreno-Caselles, J.; Angeles Bustamante, M.; Cecilia, J. Characterization of the different organic matter fractions of spent mushroom substrate. Commun. Soil Sci. Plant Anal. 2009, 40, 150–161. [Google Scholar] [CrossRef]
- Ma, X.; Yan, S.; Wang, M. Spent mushroom substrate: A review on present and future of green applications. J. Environ. Manag. 2025, 373, 123970. [Google Scholar] [CrossRef] [PubMed]
- Ström, L.; Owen, A.; Godbold, D.; Jones, D. Organic acid behaviour in a calcareous soil: Sorption reactions and biodegradation rates. Soil Boil. Biochem. 2001, 23, 2021–2036. [Google Scholar] [CrossRef]
- Jones, D. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Jones, B.; Haynes, R.; Phillips, I. Effect of amendment of bauxite processing sand with organic materials on its chemical, physical and microbial properties. J. Environ. Manag. 2010, 91, 2281–2288. [Google Scholar] [CrossRef]
- Alonso, S.; Rendueles, M.; Díaz, M. Microbial production of specialty organic acids from renewable and waste materials. Crit. Rev. Biotechnol. 2015, 35, 497–513. [Google Scholar] [CrossRef]
- Lei, Y.; Jiang, F.; Di, X.; Lin, D.; Ling, P.; Zhang, Y.; Sun, Y.; Lin, Z.; Li, J. Cultivating Dictyophora indusiata with Cenchrus fungigraminus and seafood mushroom spent substrate enhanced soil nutrient accumulation and microbial community stabilisation. Environ. Technol. Innov. 2025, 38, 104200. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Arunrat, N.; Sereenonchai, S.; Hatano, R. Effects of fire on soil organic carbon, soil total nitrogen, and soil properties under rotational shifting cultivation in northern Thailand. J. Environ. Manag. 2022, 302, 113978. [Google Scholar] [CrossRef]
- Muhammad, I.; Lv, J.; Yang, L.; Ahmad, S.; Farooq, S.; Zeeshan, M.; Zhou, X. Low irrigation water minimizes the nitrate nitrogen losses without compromising the soil fertility, enzymatic activities and maize growth. BMC Plant Biol. 2022, 22, 159. [Google Scholar] [CrossRef]
- Niepsch, D.; Clarke, L.; Tzoulas, K.; Cavan, G. Distinguishing atmospheric nitrogen compounds (nitrate and ammonium) in lichen biomonitoring studies. Environ. Sci.-Proc. Imp. 2021, 23, 2021–2036. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Lv, J.; Jiang, L.; Geng, P.; Cao, D.; Wang, Y. Changes of soil dissolved organic matter and its relationship with microbial community along the Hailuogou Glacier Forefield chronosequence. Environ. Sci. Technol. 2023, 57, 4027–4038. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Sheng, J.; Li, G.; Ma, J.; Wang, X.; Jiang, C.; Zhang, Z. Black soldier fly larvae vermicompost alters soil biochemistry and bacterial community composition. Appl. Microbiol. Biotechnol. 2022, 106, 4315–4328. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, F.; Zhu, Y.; Zhang, X.; Li, Z.; Ji, J.; Wang, G.; Guan, C. Laccase as a useful assistant for maize to accelerate the phenanthrene degradation in soil. Environ. Sci. Pollut. Res. Int. 2024, 31, 4848–4863. [Google Scholar] [CrossRef]
- Vipotnik, Z.; Michelin, M.; Tavares, T. Development of a packed bed reactor for the removal of aromatic hydrocarbons from soil using laccase/mediator feeding system. Microbiol. Res. 2021, 245, 126687. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Callahan, B.; Mcmurdie, P.; Rosen, M.; Han, A.; Johnson, A.; Holmes, S. Dada2: High-resolution sample inference from illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Price, M.; Dehal, P.; Arkin, A. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef]
- Bokulich, N.; Kaehler, B.; Ram, R.; Matthew, D.; Evan, B.; Rob, K.; Huttley, G.; Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with qiime 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Kõljalg, U.; Nilsson, R.; Abarenkov, K.; Tedersoo, L.; Taylor, A.; Bahram, M.; Bates, S.; Bruns, T.; Bengtsson-Palme, J.; Callaghan, T.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Li, L.; Qin, L.; Huang, Y.; Ye, Y.; Wang, M.; Wang, Y.; Xu, Y.; Luo, F.; Mei, H. Targeted metabolomics reveals impact of application on accumulation of amino acids, flavonoids and phytohormones in tea shoots under soil nutrition deficiency stress. Forests 2022, 13, 1629. [Google Scholar] [CrossRef]
- Hou, M.; Gao, D.; Chen, W.; Jiang, W.; Yu, D.; Li, X. UHPLC-QTOF-MS-Based Targeted Metabolomics Provides Novel Insights into the Accumulative Mechanism of Soil Types on the Bioactive Components of Salvia miltiorrhiza. Molecules 2024, 29, 4016. [Google Scholar] [CrossRef]
- Song, J.; Yan, Y.; Wang, X.; Li, X.; Chen, Y.; Lu, L.; Li, W. Characterization of fatty acids, amino acids and organic acids in three colored quinoas based on untargeted and targeted metabolomics. LWT 2021, 140, 110690. [Google Scholar] [CrossRef]
- Bray, J.; Curtis, J. An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Kruskal, J. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 1964, 29, 1–27. [Google Scholar] [CrossRef]
- Ramette, A. Multivariate analyses in microbial ecology. FEMS Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.; Huse, S.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef]
- Zgadzaj, R.; Garridooter, R.; Jensen, D.; Koprivova, A.; Schulzelefert, P.; Radutoiu, S. Root nodule symbiosis in Lotus japonicus drives the establishment of distinctive rhizosphere, root, and nodule bacterial communities. Proc. Natl. Acad. Sci. USA 2016, 113, E7996–E8005. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 23. [Google Scholar]
- Deng, Y.; Jiang, Y.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Strumińska-Parulska, D.; Olszewski, G.; Moniakowska, A.; Zhang, J.; Falandysz, J. Bolete mushroom Boletus bainiugan from Yunnan as a reflection of the geographical distribution of 210Po, 210Pb and uranium (234U, 235U, 238U) radionuclides, their intake rates and effective exposure doses. Chemosphere 2020, 253, 126585. [Google Scholar] [CrossRef] [PubMed]
- Hanć, A.; Fernandes, A.R.; Falandysz, J.; Zhang, J. Mercury and selenium in developing and mature fruiting bodies of Amanita muscaria. Environ. Sci. Pollut. Res. Int. 2021, 28, 60145–60153. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, T.; Sakamoto, F.; Kozai, N.; Nanba, K.; Neda, H.; Sasaki, Y.; Niizato, T.; Watanabe, N.; Kozaki, T. Role of filamentous fungi in migration of radioactive cesium in the Fukushima forest soil environment. Environ. Sci.-Proc. Imp. 2019, 21, 1164–1173. [Google Scholar] [CrossRef]
- Krasińska, G.; Falandysz, J. Mercury in Orange Birch Bolete Leccinum versipelle and soil substratum: Bioconcentration by mushroom and probable dietary intake by consumers. Environ. Sci. Pollut. Res. Int. 2016, 23, 860–869. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Krishnaa, D.; Balakrishnan, G.; Manickam, M.; Abdulkader, A.; Dharumadurai, D. Association of bacterial communities with Psychedelic mushroom and soil as revealed in 16S rRNA gene sequencing. Appl. Biochem. Biotechnol. 2024, 196, 2566–2590. [Google Scholar] [CrossRef]
- Sui, X.; Li, M.; Frey, B.; Wang, M.; Weng, X.; Wang, X.; Chen, F.; Li, X.; Du, Z.; Yang, L.; et al. Climax forest has a higher soil bacterial diversity but lower soil nutrient contents than degraded forests in temperate northern China. Ecol. Evol. 2022, 12, e9535. [Google Scholar] [CrossRef]
- Pang, W.; Zhang, P.; Zhang, Y.; Zhang, X.; Huang, Y.; Zhang, T.; Liu, B. The ectomycorrhizal fungi and soil bacterial communities of the five typical tree species in the Junzifeng National Nature Reserve, Southeast China. Plants 2023, 12, 3853. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, H.; Li, Q.; Gao, N.; Yao, Y.; Xu, H. Combined remediation of Cd-phenanthrene co-contaminated soil by Pleurotus cornucopiae and Bacillus thuringiensis FQ1 and the antioxidant responses in Pleurotus cornucopiae. Ecotoxicol. Environ. Saf. 2015, 120, 386–393. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.; Tong, B.; Wang, D.; Tian, X.; Liu, J.; Chen, J.; Xiao, X.; Wang, S. Rhizobacterial compositions and their relationships with soil properties and medicinal bioactive ingredients in Cinnamomum migao. Front. Microbiol. 2023, 14, 1078886. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S.; Kaur, I.; Sodhi, H.; Choudhary, R.; Ercisli, S.; Fidan, H.; Dasci, E.; Ullah, R.; Bari, A. Purification, kinetic characterization of thermostable multicopper oxidase from the oyster mushroom and its versatility for greener agro-pulp bio bleaching in the paper industry. Cell Mol. Biol. 2024, 70, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Dennis, P.; Owen, A.; Van Hees, P. Organic acids behavior in soils-misconceptions and knowledge gaps. Plant Soil 2003, 248, 31–41. [Google Scholar] [CrossRef]
- Luo, Z.K. Big soil benefits from buried straw. Nat. Geosci. 2025, 18, 570–571. [Google Scholar] [CrossRef]
- Peña, A. A comprehensive review of recent research concerning the role of low molecular weight organic acids on the fate of organic pollutants in soil. J. Hazard. Mater. 2022, 434, 128875. [Google Scholar] [CrossRef]
- Ru, R.; Li, X.; Hunag, X.; Gao, F.; Wang, J.; Li, B.; Zhang, Z. Effect of substrate of edible mushroom on continuously cropping obstacle of Rehmannia glutinosa. China J. Chin. Mater. Med. 2014, 39, 3036–3041. [Google Scholar]
- Ma, H.; Li, X.; Hou, S.; Peng, D.; Wang, Y.; Xu, F.; Xu, H. The activation and extraction systems using organic acids and Lentinus edodes to remediate cadmium contaminated soil. Environ. Pollut. 2019, 255 Pt 2, 113252. [Google Scholar] [CrossRef]
- Ng, P.; Ferrarese, M.; Huber, D.; Ravagnani, A.; Ferrarese-Filho, O. Canola (Brassica napus L.) seed germination influenced by cinnamic and benzoic acids and derivatives: Effects on peroxidase. Seed Sci. Technol. 2003, 31, 39–46. [Google Scholar] [CrossRef]
- Politycka, B.; Mielcarz, B. Involvement of ethylene in growth inhibition of cucumber roots by ferulic and p-coumaric acids. Allelopathy J. 2007, 19, 451–460. [Google Scholar]
- Soares, A.; de Lourdes Lucio Ferrarese, M.; de Cássia Siqueira-Soares, R.; Marchiosi, R.; Finger-Teixeira, A.; Ferrarese-Filho, O. The allelochemical L-DOPA increases melanin production and reduces reactive oxygen species in soybean roots. J. Chem. Ecol. 2011, 37, 891–898. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, J.; Fang, Y.; Xu, X.; Wang, F.; Tang, Y.; Yin, D.; Cookson, A.; Zhu, W.; Mao, S.; et al. Straw-based compost cultivation disproportionally contributes to the environmental persistence of antibiotic resistance from raw cattle manure to organic vegetables. Microbiol. Res. 2024, 278, 127540. [Google Scholar] [CrossRef]
- Xing, R.; Yan, H.; Gao, Q.; Zhang, F.; Wang, J.; Chen, S. Microbial communities inhabiting the fairy ring of Floccularia luteovirens and isolation of potential mycorrhiza helper bacteria. J. Basic Microbiol. 2018, 58, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, Y.; Shen, M.; Dong, S.; Zhang, F.; Gao, Q.; He, P.; Shen, G.; Yang, J.; Wang, Z.; et al. Soil conditioner affects tobacco rhizosphere soil microecology. Microb. Ecol. 2023, 86, 460–473. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, R.; He, T.; Sun, Y.; Wu, M.; Xue, Y.; Meng, F.; Wang, J. Disentangling the impact of straw incorporation on soil microbial communities: Enhanced network complexity and ecological stochasticity. Sci. Total Environ. 2023, 863, 160918. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, P.; Zhou, W.; Liu, H.; Cheng, K.; Xiao, X.; Tang, H.; Yi, Z. Response characteristics of soil Cd availability to microbes in paddy soil with long-term fertilization and its impact on Cd uptake in rice. Sci. Total Environ. 2024, 957, 177680. [Google Scholar] [CrossRef]
- Su, P.; Gao, P.; Sun, W.; Gao, W.; Xu, F.; Wang, Q.; Xiao, E.; Soleimani, M.; Sun, X. Keystone taxa and functional analysis in arsenic and antimony co-contaminated rice terraces. Environ. Sci. Pollut. Res. Int. 2022, 29, 61236–61246. [Google Scholar] [CrossRef]
- Bergkemper, F.; Schöler, A.; Engel, M.; Lang, F.; Krüger, J.; Schloter, M.; Schulz, S. Phosphorus depletion in forest soils shapes bacterial communities towards phosphorus recycling systems. Environ. Microbiol. 2016, 18, 1988–2000. [Google Scholar] [CrossRef]
- Magingo, F.; Oriyo, N.; Kivaisi, A.; Danell, E. Cultivation of Oudemansiella tanzanica nom. prov. on agricultural solid wastes in Tanzania. Mycologia 2004, 96, 197–204. [Google Scholar] [CrossRef]
- Pecina, V.; Valtera, M.; Drápela, K.; Novotný, R.; Vahalík, P.; Komendová, R.; Brtnický, M.; Juřička, D. Influence of beech and spruce on potentially toxic elements-related health risk of edible mushrooms growing on unpolluted forest soils. Sci. Rep. 2022, 12, 5407. [Google Scholar] [CrossRef]


| Year | Treatment | pH | TN (mg/kg) | NH4+-N (μg/g) | NO3−-N (μg/g) | SOM (mg/g) | SOC (mg/g) | SCAT (μmol/d/g) | SL (U/g) |
|---|---|---|---|---|---|---|---|---|---|
| 2023 | OS | 4.28 | 997.76 ± 41.84 c | 1.47 ± 0.42 d | 11.17 ± 4.79 cd | 20.77 ± 1.14 cd | 12.05 ± 0.66 cd | 26.93 ± 5.95 d | 0.16 ± 0.10 de |
| S-CS23 | 3.80 | 880.65 ± 22.97 e | 0.70 ± 0.15 d | 19.68 ± 3.95 a | 17.93 ± 0.50 ef | 10.40 ± 0.29 ef | 24.53 ± 2.42 d | 0.13 ± 0.03 e | |
| S-CH23 | 4.21 | 859.22 ± 50.07 e | 1.61 ± 0.48 d | 18.24 ± 1.25 a | 17.92 ± 1.32 ef | 10.40 ± 0.77 ef | 27.96 ± 6.51 d | 0.32 ± 0.23 d | |
| CS23 | 4.07 | 854.33 ± 27.32 e | 1.41 ± 0.47 d | 10.89 ± 1.29 cde | 17.71 ± 0.78 ef | 10.27 ± 0.45 ef | 26.07 ± 3.01 d | 0.20 ± 0.10 de | |
| CH23 | 4.34 | 919.54 ± 48.72 e | 1.81 ± 0.36 d | 13.62 ± 1.49 bc | 19.12 ± 1.10 de | 11.09 ± 0.64 de | 31.15 ± 6.24 d | 0.21 ± 0.21 de | |
| 2024 | S-CS24 | 4.36 | 1075.66 ± 23.08 b | 27.00 ± 1.79 a | 16.41 ± 0.63 ab | 27.84 ± 2.39 b | 16.15 ± 1.39 b | 45.26 ± 1.60 bc | 0.53 ± 0.04 c |
| S-CH24 | 5.06 | 1917.52 ± 107.07 a | 19.64 ± 2.01 b | 10.28 ± 0.92 cde | 33.71 ± 2.18 a | 19.55 ± 1.26 a | 38.72 ± 2.49 a | 1.80 ± 0.10 a | |
| CS24 | 5.54 | 924.52 ± 36.90 de | 15.64 ± 0.44 c | 7.56 ± 0.87 e | 16.48 ± 0.76 f | 9.56 ± 0.44 f | 53.97 ± 9.29 a | 0.79 ± 0.08 b | |
| CH24 | 5.00 | 988.14 ± 33.52 cd | 14.96 ± 1.22 c | 8.54 ± 2.66 de | 21.78 ± 1.92 c | 12.63 ± 1.11 c | 49.89 ± 5.79 ab | 0.84 ± 0.09 b |
| Year | Treatment | Richness | Coverage | Evenness | Diversity | |
|---|---|---|---|---|---|---|
| Chao1 | Faith_pd | Goods_Coverage | Pielou_e | Shannon | ||
| 2023 | OS | 2157.55 ± 59.35 e | 172.70 ± 26.64 c | 0.99 ± 0.00 a | 0.90 ± 0.00 c | 9.96 ± 0.03 f |
| S-CS23 | 2909.35 ± 184.34 c | 213.33 ± 54.89 bc | 0.99 ± 0.00 ab | 0.92 ± 0.00 b | 10.55 ± 0.09 cd | |
| S-CH23 | 2838.99 ± 54.34 c | 239.70 ± 40.99 bc | 0.99 ± 0.00 ab | 0.92 ± 0.01 b | 10.47 ± 0.08 d | |
| CS23 | 2460.40 ± 262.42 de | 234.93 ± 37.98 bc | 0.99 ± 0.00 ab | 0.90 ± 0.01 c | 10.09 ± 0.22 ef | |
| CH23 | 2739.72 ± 308.66 cd | 227.44 ± 48.98 bc | 0.99 ± 0.00 b | 0.90 ± 0.00 c | 10.28 ± 0.15 e | |
| 2024 | S-CS24 | 3432.09 ± 199.63 b | 274.99 ± 75.86 b | 0.99 ± 0.00 c | 0.92 ± 0.00 b | 10.69 ± 10.13 bc |
| S-CH24 | 4088.98 ± 312.64 a | 345.01 ± 51.11 a | 0.98 ± 0.00 d | 0.93 ± 0.00 a | 11.08 ± 0.11 a | |
| CS24 | 3396.84 ± 419.78 b | 232.49 ± 21.62 bc | 0.99 ± 0.00 c | 0.92 ± 0.01 b | 10.76 ± 0.27 b | |
| CH24 | 2989.73 ± 137.91 c | 251.11 ± 52.43 b | 0.99 ± 0.00 c | 0.89 ± 0.01 d | 10.23 ± 0.01 e | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Jiang, F.; Di, X.; Lai, Q.; Feng, D.; Zeng, Y.; Lei, Y.; Yin, Y.; Lin, B.; He, X.; et al. Is the Cultivation of Dictyophora indusiata with Grass-Based Substrates an Efficacious and Sustainable Approach for Enhancing the Understory Soil Environment? Agriculture 2025, 15, 1533. https://doi.org/10.3390/agriculture15141533
Li J, Jiang F, Di X, Lai Q, Feng D, Zeng Y, Lei Y, Yin Y, Lin B, He X, et al. Is the Cultivation of Dictyophora indusiata with Grass-Based Substrates an Efficacious and Sustainable Approach for Enhancing the Understory Soil Environment? Agriculture. 2025; 15(14):1533. https://doi.org/10.3390/agriculture15141533
Chicago/Turabian StyleLi, Jing, Fengju Jiang, Xiaoyue Di, Qi Lai, Dongwei Feng, Yi Zeng, Yufang Lei, Yijia Yin, Biaosheng Lin, Xiuling He, and et al. 2025. "Is the Cultivation of Dictyophora indusiata with Grass-Based Substrates an Efficacious and Sustainable Approach for Enhancing the Understory Soil Environment?" Agriculture 15, no. 14: 1533. https://doi.org/10.3390/agriculture15141533
APA StyleLi, J., Jiang, F., Di, X., Lai, Q., Feng, D., Zeng, Y., Lei, Y., Yin, Y., Lin, B., He, X., Liu, P., Lin, Z., Lin, X., & Lin, D. (2025). Is the Cultivation of Dictyophora indusiata with Grass-Based Substrates an Efficacious and Sustainable Approach for Enhancing the Understory Soil Environment? Agriculture, 15(14), 1533. https://doi.org/10.3390/agriculture15141533








