Path Mechanism and Field Practice Effect of Green Agricultural Production on the Soil Organic Carbon Dynamics and Greenhouse Gas Emission Intensity in Farmland Ecosystems
Abstract
1. Introduction
2. Materials and Methods
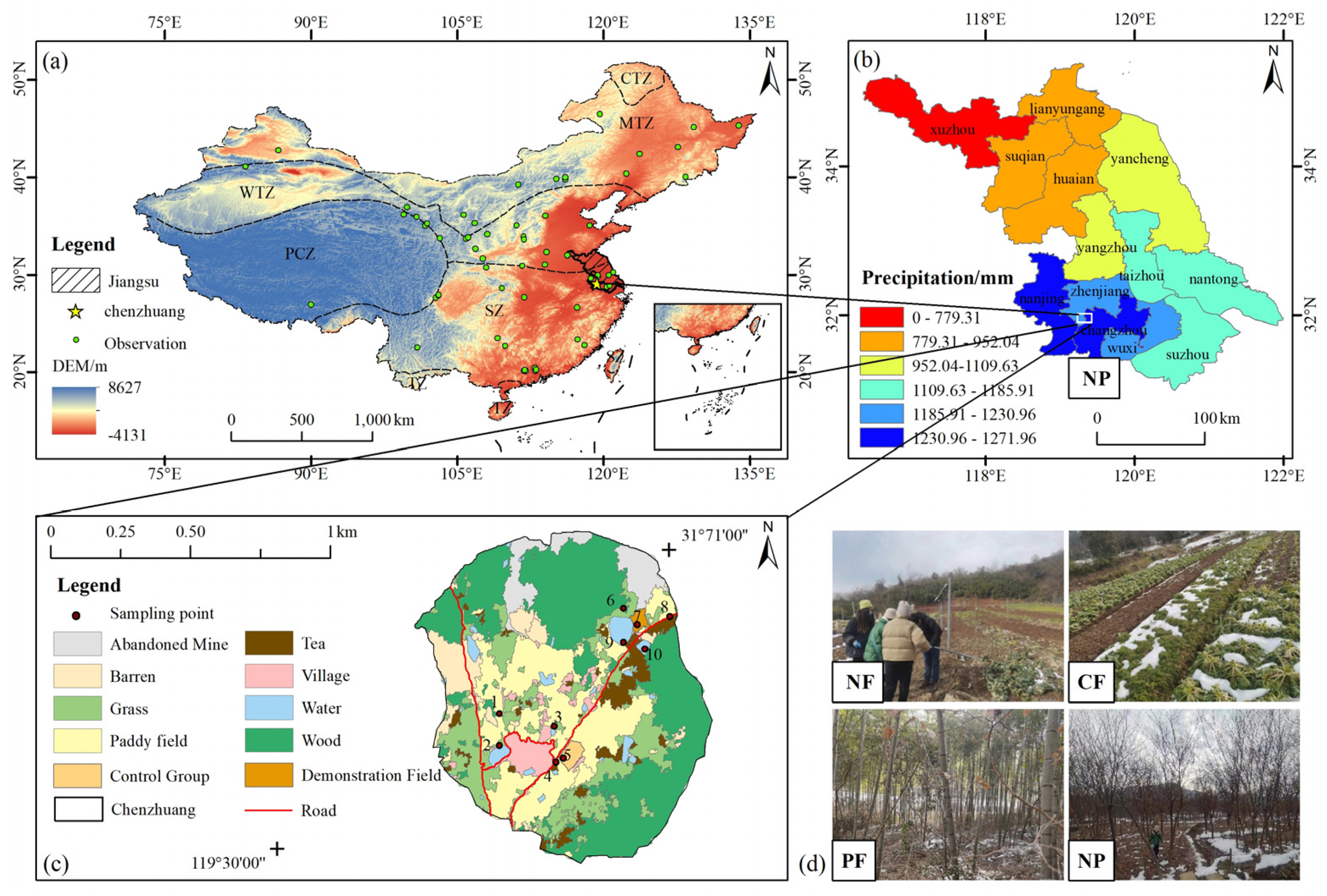
2.1. Study Area
2.2. Meta Analysis
2.2.1. Literature Searching
2.2.2. Database Establishment
2.2.3. Microbiological Informatics Analysis
2.2.4. Construction of Structural Equation Model
2.3. Field Experiment
2.3.1. Experimental Design
2.3.2. Sample Collection and Index Determination
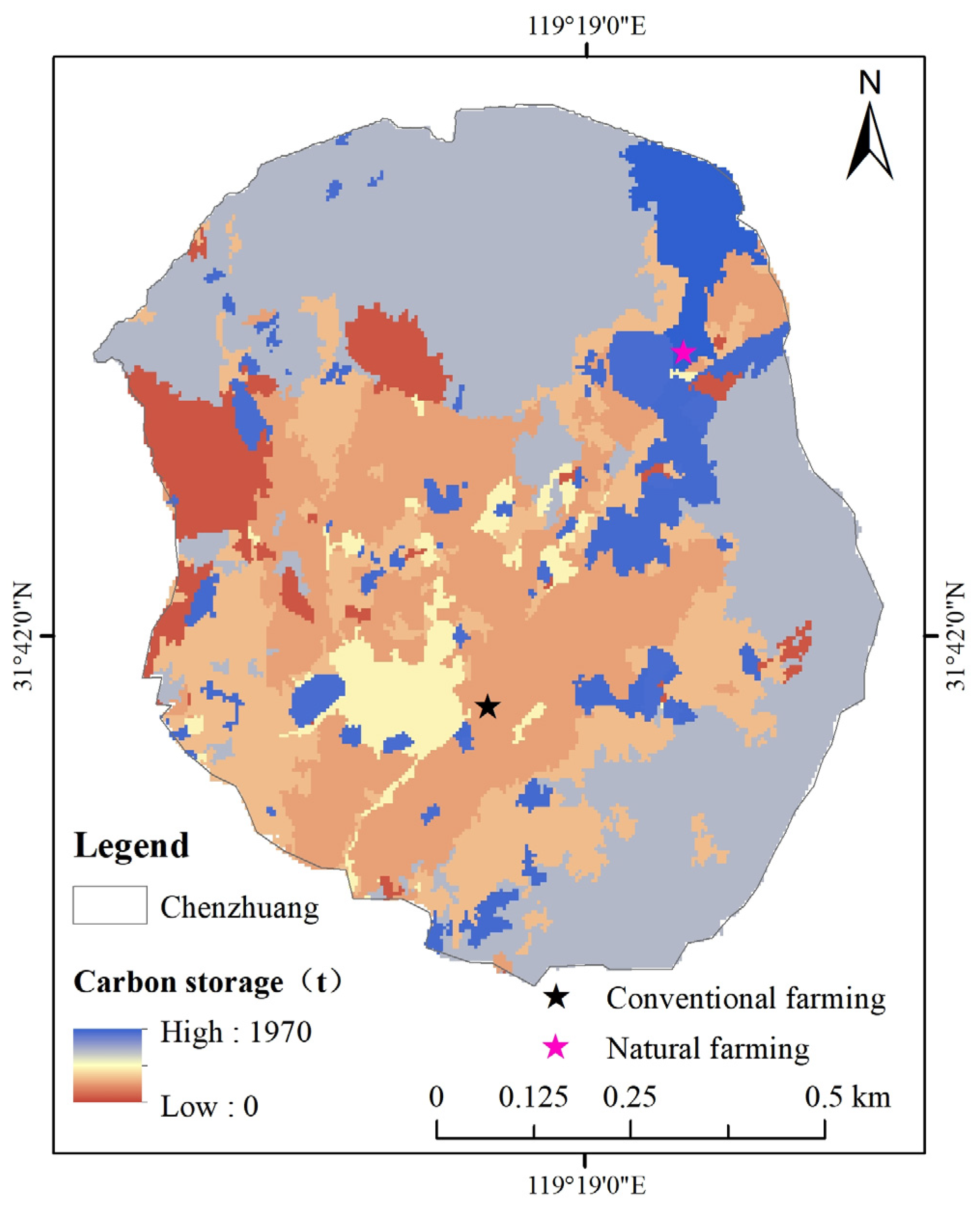
2.3.3. Estimation of Soil Carbon Storage
3. Results
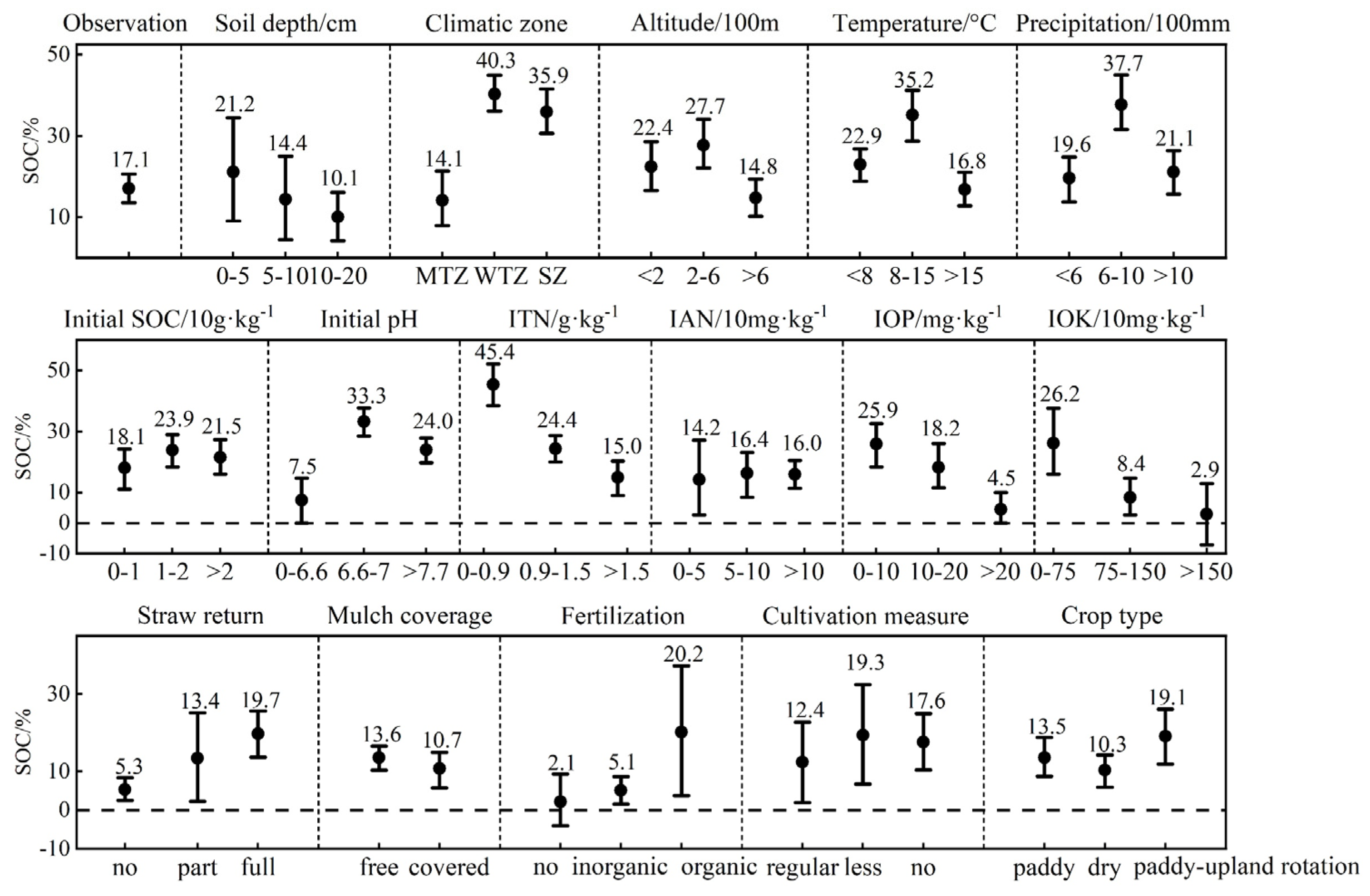
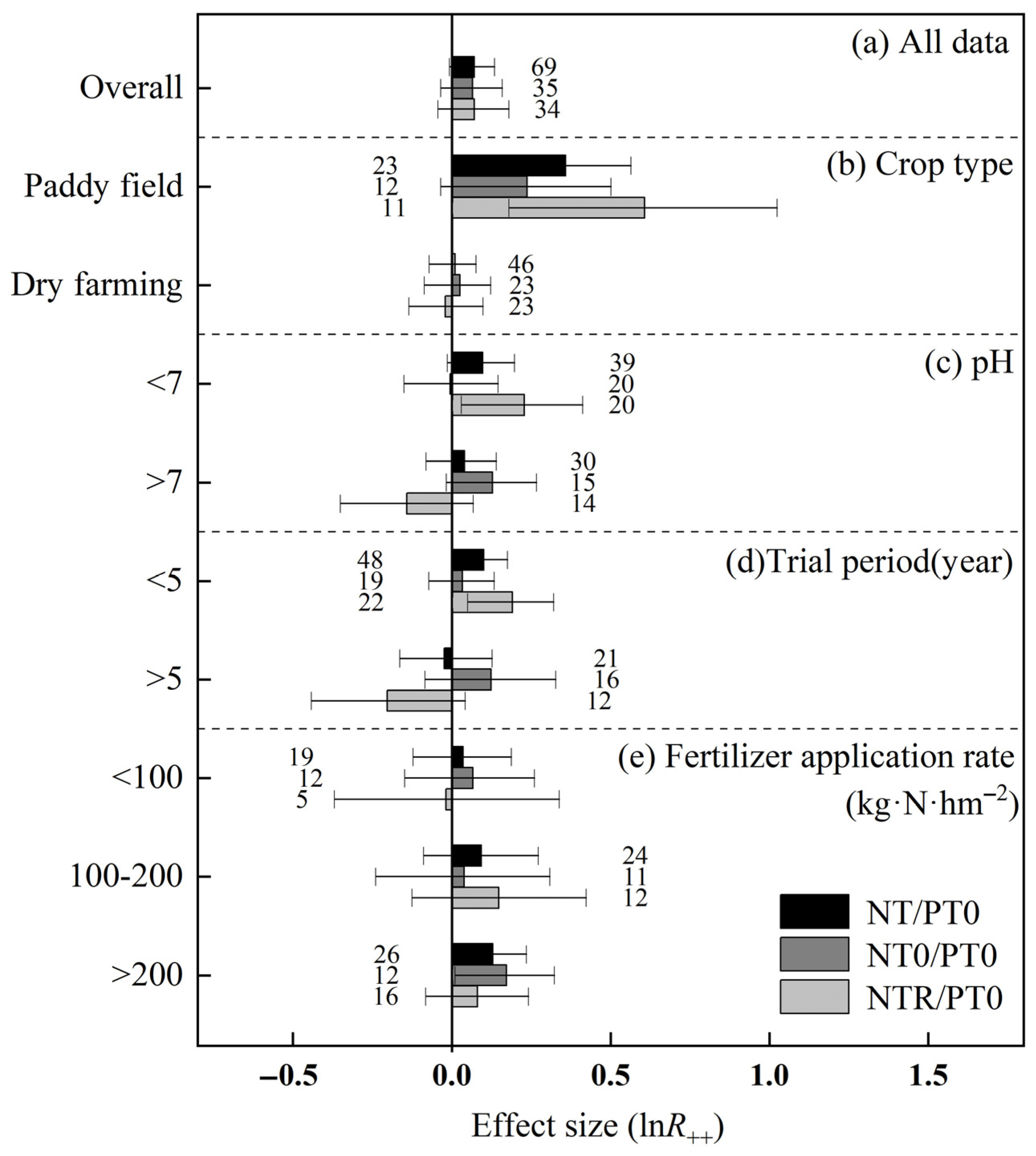
3.1. Carbon Budget Differences Obtained from META
3.1.1. Soil Organic Carbon Content
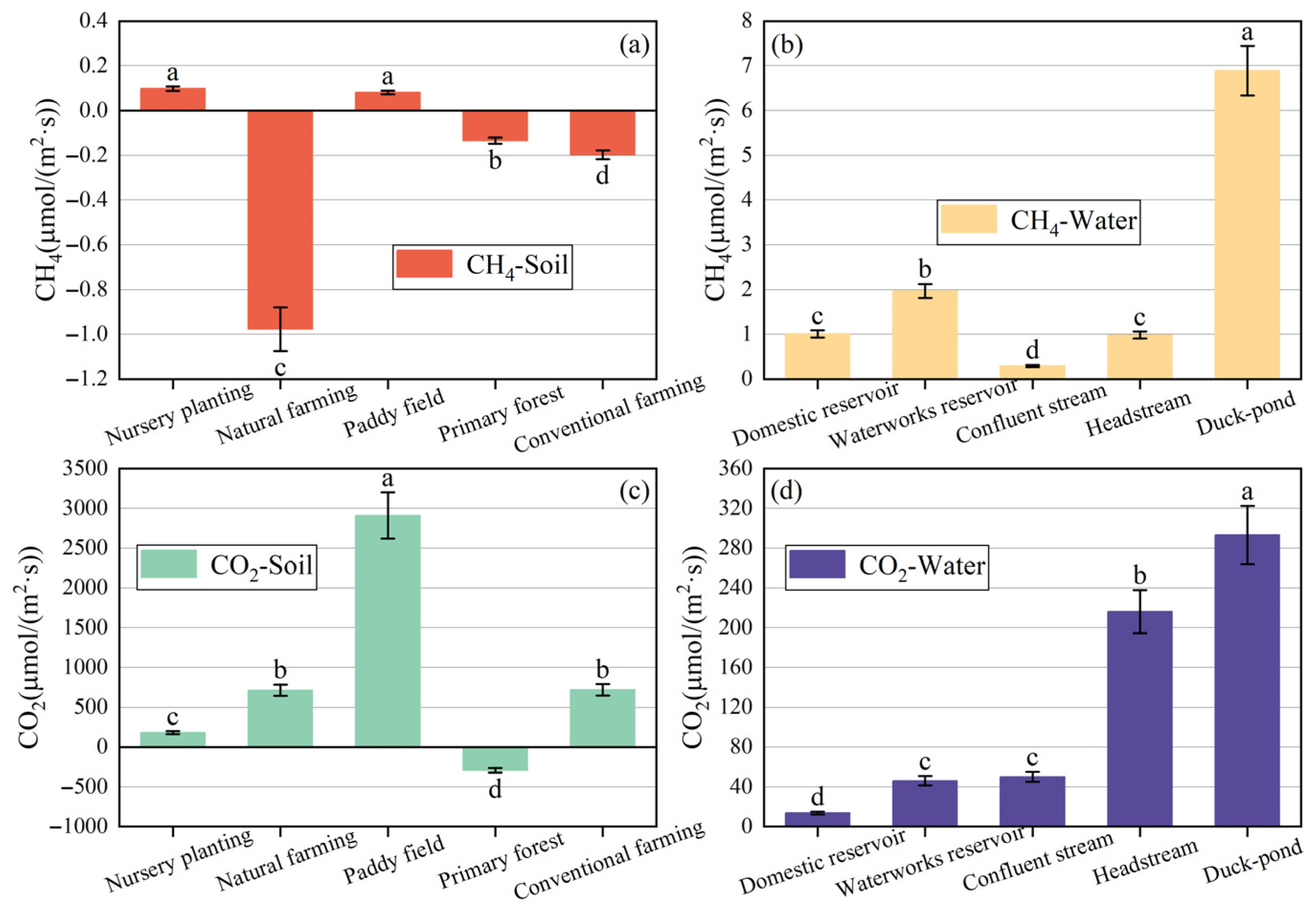
3.1.2. Greenhouse Gas Emissions
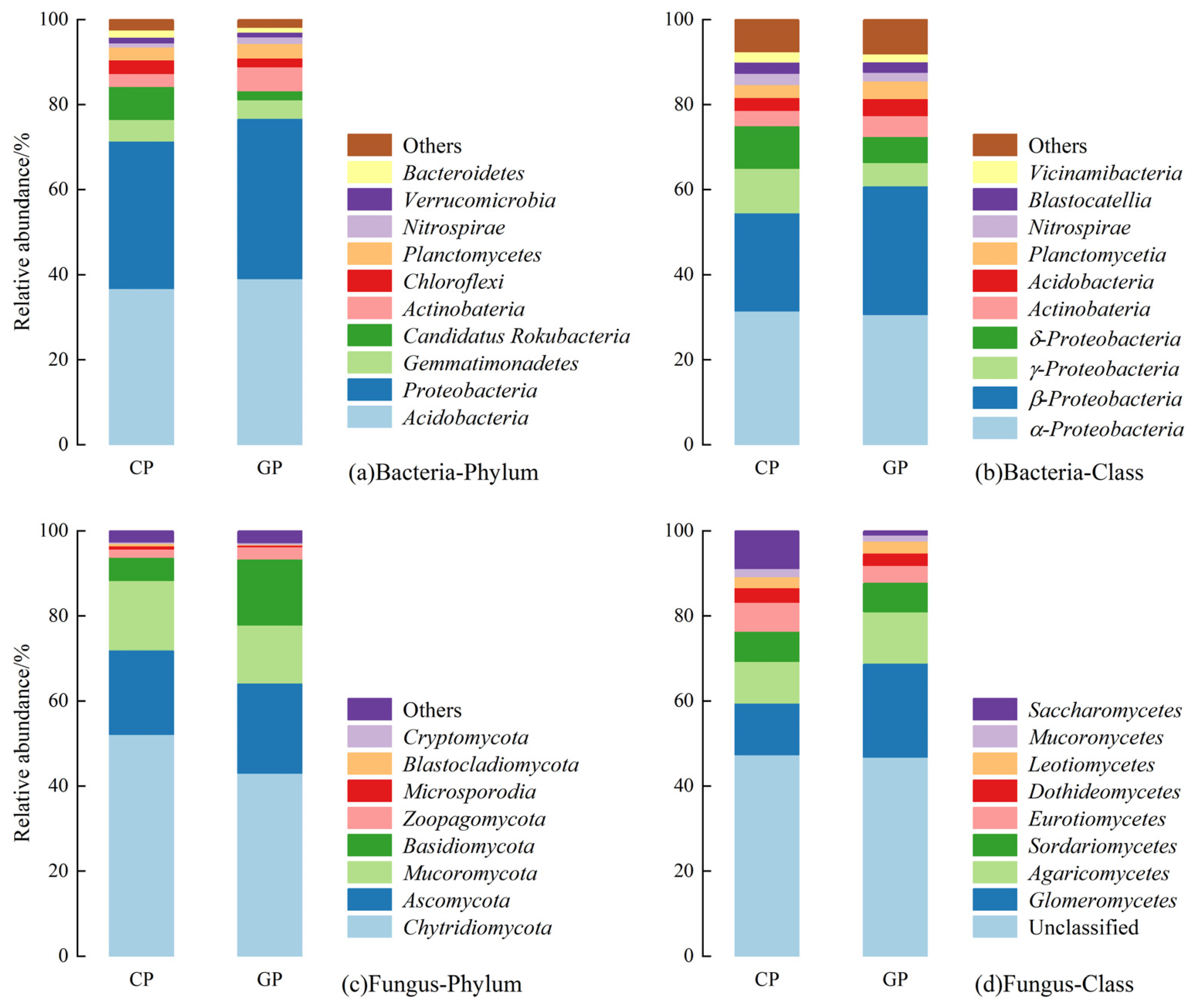
3.2. Soil Microbial Changes Under Different Agricultural Management Practices
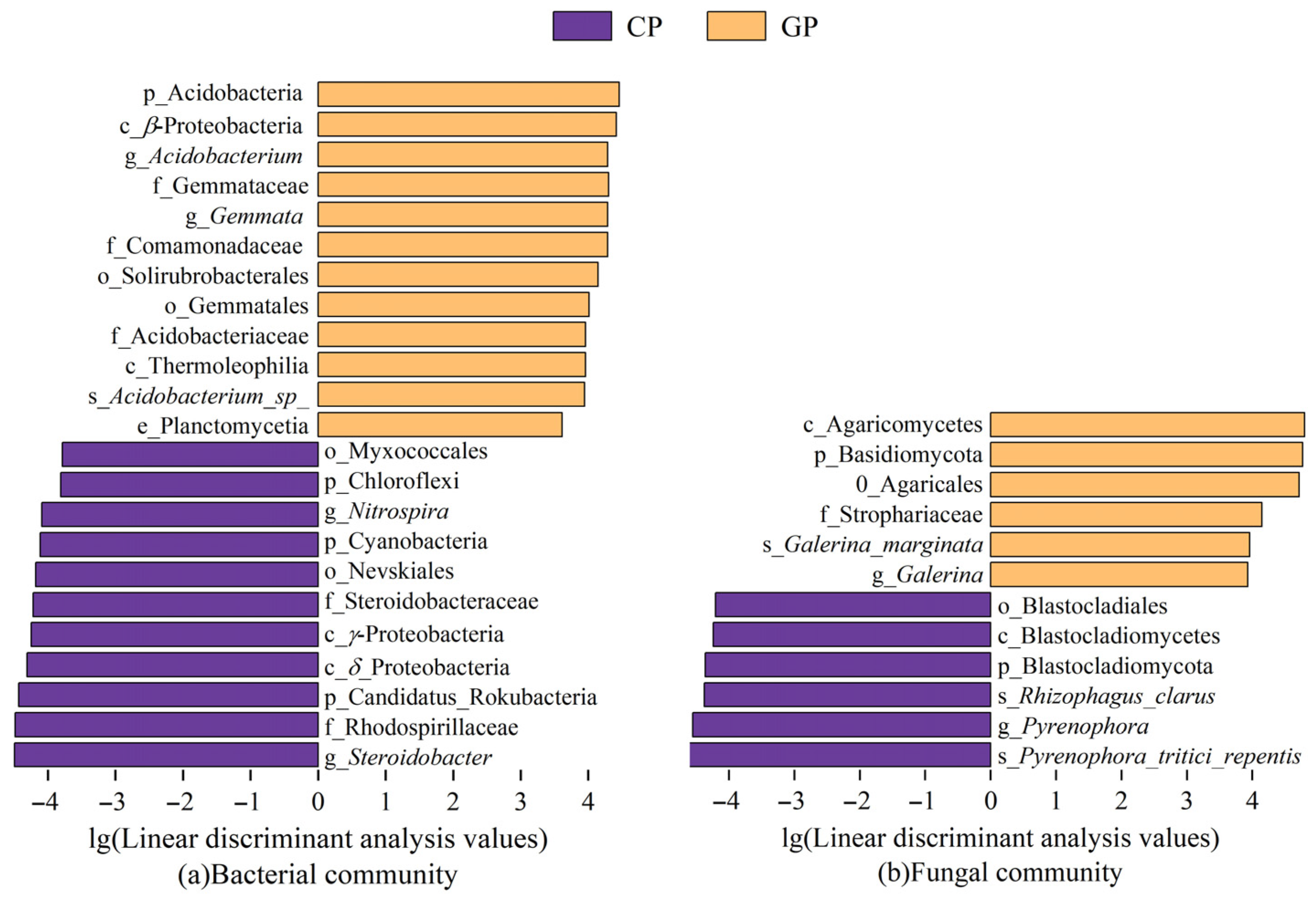
3.2.1. Microbial Community Structure
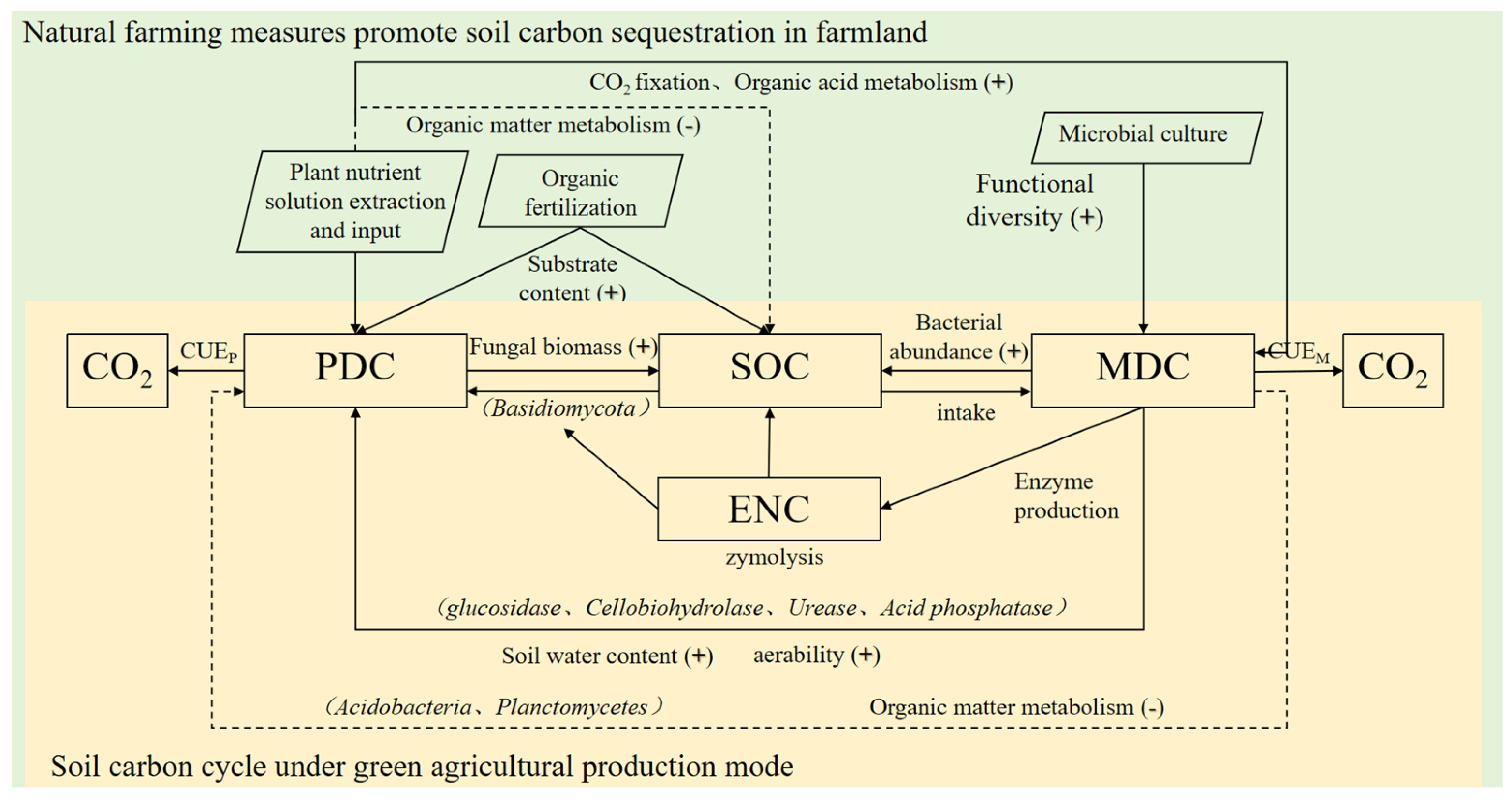
3.2.2. Interaction Among Plant–Microorganism–Soil Carbon
3.3. Carbon Flux Dynamics in the Field Experimental Area
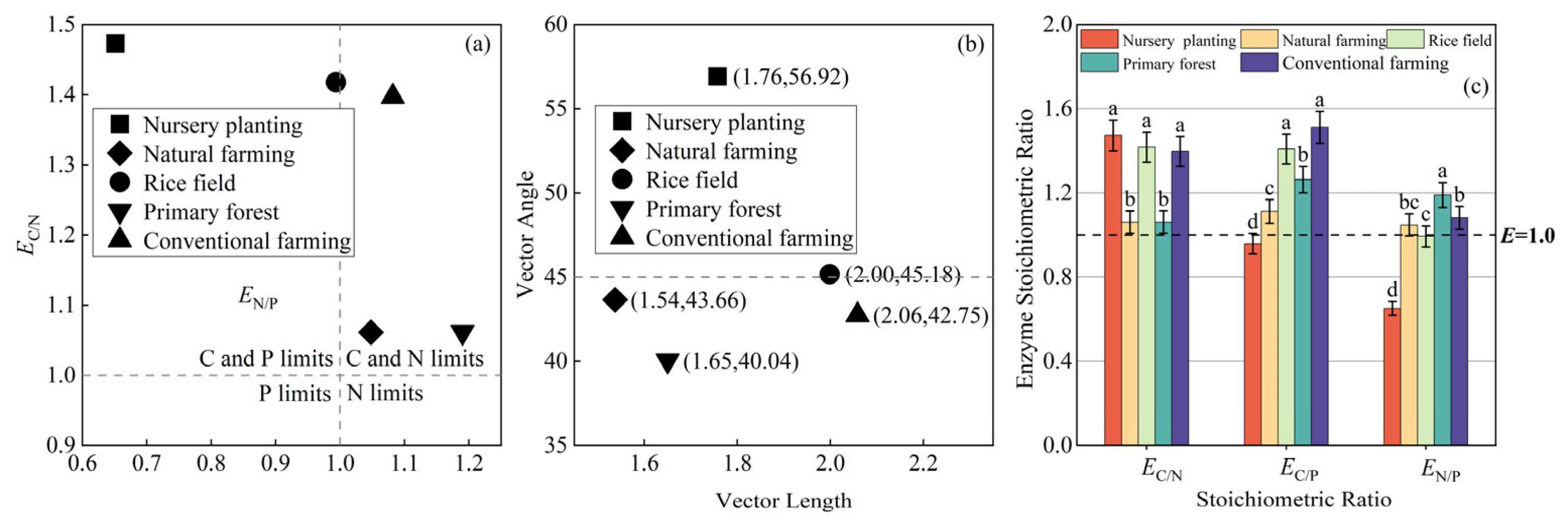
3.3.1. Soil Enzyme Activity
3.3.2. Greenhouse Gas Emission Flux
3.3.3. SOC Distribution Patterns
4. Discussion
4.1. Positive Responses of SOC Increment, CH4 Absorption and CO2 Reduction to Green Agricultural Production Practices
4.2. Green Agricultural Production Promotes Soil Organic Carbon Sinks by Regulating the Structure and Metabolism of MICROORGANISMS
4.3. Innovative Suggestions for Integrating Green Agricultural Production into Local Areas
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Slessarev, E.W.; Mayer, A.; Kelly, C.; Georgiou, K.; Pett-Ridge, J.; Nuccio, E.E. Initial soil organic carbon stocks govern changes in soil carbon: Reality or artifact? Glob. Change Biol. 2023, 29, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, Y.; Yang, Z.; Zhu, Y.; Han, Y. Mapping soil organic carbon in low-relief farmlands based on stratified heterogeneous relationship. Remote Sens. 2022, 14, 3575. [Google Scholar] [CrossRef]
- Liu, X.; Li, S.; Wang, S.; Bian, Z.; Zhou, W.; Wang, C. Effects of farmland landscape pattern on spatial distribution of soil organic carbon in Lower Liaohe Plain of northeastern China. Ecol. Indic. 2022, 145, 109652. [Google Scholar] [CrossRef]
- Fan, X.; Chen, X.; Chen, T.; Liu, X.; Song, Y.; Tan, S.; Chen, Y.; Yan, P.; Wang, X. Effects of substituting synthetic nitrogen with organic amendments on crop yield, net greenhouse gas emissions and carbon footprint: A global meta-analysis. Field Crops Res. 2023, 301, 109035. [Google Scholar] [CrossRef]
- Li, B.; Song, H.; Cao, W.; Wang, Y.; Chen, J.; Guo, J. Responses of soil organic carbon stock to animal manure application: A new global synthesis integrating the impacts of agricultural managements and environmental conditions. Glob. Change Biol. 2021, 27, 5356–5367. [Google Scholar] [CrossRef]
- Sinitambirivoutin, M.; Milne, E.; Schiettecatte, L.-S.; Tzamtzis, I.; Dionisio, D.; Henry, M.; Brierley, I.; Salvatore, M.; Bernoux, M. An updated IPCC major soil types map derived from the harmonized world soil database v2. 0. Catena 2024, 244, 108258. [Google Scholar] [CrossRef]
- Berhane, M.; Xu, M.; Liang, Z.; Shi, J.; Wei, G.; Tian, X. Effects of long-term straw return on soil organic carbon storage and sequestration rate in North China upland crops: A meta-analysis. Glob. Change Biol. 2020, 26, 2686–2701. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, B.; Das, R.; Nath, D.J.; Dutta, S.; Borah, M.; Talukdar, L.; Patgiri, D.K.; Pathak, K.; Valente, D.; Petrosillo, I. Long-term management of rice agroecosystem towards climate change mitigation. Ecol. Indic. 2024, 160, 111876. [Google Scholar] [CrossRef]
- Ma, X.; Yuan, H. Ecological footprint and carrying capacity of agricultural water-land-energy nexus in China. Ecol. Indic. 2024, 168, 112786. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Z.; Weng, Z.; Sun, K.; Zhang, Y.; Liu, Q.; Yang, Y.; Li, Y.; Wang, Z.; Luo, Y. Formation of soil organic carbon pool is regulated by the structure of dissolved organic matter and microbial carbon pump efficacy: A decadal study comparing different carbon management strategies. Glob. Change Biol. 2023, 29, 5445–5459. [Google Scholar] [CrossRef]
- Hao, X.; Ma, X.; Zhou, B.; Li, Y. Effect of different long-term fertilization practices on carbon sequestration in black soil. J. Soil Water Conserv. 2016, 5, 316–321. [Google Scholar]
- Jin, B.; Cui, C.; Wen, L.; Shi, R.; Zhao, M. Regional differences and convergence of agricultural carbon efficiency in China: Embodying carbon sink effect. Ecol. Indic. 2024, 169, 112929. [Google Scholar] [CrossRef]
- Yin, X.; Peñuelas, J.; Sardans, J.; Xu, X.; Chen, Y.; Fang, Y.; Wu, L.; Singh, B.P.; Tavakkoli, E.; Wang, W. Effects of nitrogen-enriched biochar on rice growth and yield, iron dynamics, and soil carbon storage and emissions: A tool to improve sustainable rice cultivation. Environ. Pollut. 2021, 287, 117565. [Google Scholar] [CrossRef]
- Li, H.; Lin, L.; Peng, Y.; Hao, Y.; Li, Z.; Li, J.; Yu, M.; Li, X.; Lu, Y.; Gu, W. Biochar’s dual role in greenhouse gas emissions: Nitrogen fertilization dependency and mitigation potential. Sci. Total Environ. 2024, 917, 170293. [Google Scholar] [CrossRef]
- Liu, J.; Fang, L.; Qiu, T.; Chen, J.; Wang, H.; Liu, M.; Yi, J.; Zhang, H.; Wang, C.; Sardans, J. Crop residue return achieves environmental mitigation and enhances grain yield: A global meta-analysis. Agron. Sustain. Dev. 2023, 43, 78. [Google Scholar] [CrossRef]
- Dong, L.; Zha, J.; Shen, M.; Wang, H.; Shi, L.; Tao, Y.; Zhou, X.; Lu, C. Effect of long-term straw returning on soil organic carbon fractions composition in rice-wheat rotation ecosystem. J. Agric. Sci. Technol. 2022, 24, 166–175. [Google Scholar]
- von Fromm, S.F.; Hoyt, A.M.; Sierra, C.A.; Georgiou, K.; Doetterl, S.; Trumbore, S.E. Controls and relationships of soil organic carbon abundance and persistence vary across pedo-climatic regions. Glob. Change Biol. 2024, 30, e17320. [Google Scholar] [CrossRef]
- Lessmann, M.; Ros, G.H.; Young, M.D.; de Vries, W. Global variation in soil carbon sequestration potential through improved cropland management. Glob. Change Biol. 2022, 28, 1162–1177. [Google Scholar] [CrossRef]
- Wang, S.; Tian, L.; Zhou, Y. Butterfly diversity and seasonal dynamics in Chenzhuang, Jurong City, Jiangsu Province. J. Jiangsu For. Sci. Technol. 2021, 48, 26–32. [Google Scholar]
- Li, G.; Tang, X.; Hou, Q.; Li, T.; Xie, H.; Lu, Z.; Zhang, T.; Liao, Y.; Wen, X. Response of soil organic carbon fractions to legume incorporation into cropping system and the factors affecting it: A global meta-analysis. Agric. Ecosyst. Environ. 2023, 342, 108231. [Google Scholar] [CrossRef]
- Zhu, X.; Jackson, R.D.; DeLucia, E.H.; Tiedje, J.M.; Liang, C. The soil microbial carbon pump: From conceptual insights to empirical assessments. Glob. Change Biol. 2020, 26, 6032–6039. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhu, M.; Zhang, X.; Guo, Y.; Yang, Y.; Yun, C.; Wu, Y.; Wang, Q.; Wang, W.; Wang, H. Northeastern China shelterbelt-farmland glomalin differences depend on geo-climates, soil depth, and microbial interaction: Carbon sequestration, nutrient retention and implication. Appl. Soil Ecol. 2023, 191, 105068. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Zhao, Y.; Dong, W.; Liu, E. Effects of film mulching on soil microbial carbon source metabolism in dry-farmland. Acta Ecol. Sin. 2022, 42, 9213–9225. [Google Scholar]
- Chen, L.; Sun, S.; Zhou, Y.; Zhang, B.; Peng, Y.; Zhuo, Y.; Ai, W.; Gao, C.; Wu, B.; Liu, D.; et al. Straw and straw biochar differently affect fractions of soil organic carbon and microorganisms in farmland soil under different water regimes. Environ. Technol. Innov. 2023, 32, 103412. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Cui, S.; Liang, G.; Zhang, Q. Microbial-derived carbon components are critical for enhancing soil organic carbon in no-tillage croplands: A global perspective. Soil Tillage Res. 2021, 205, 104758. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef]
- Ma, L.; Niu, W.; Li, G.; Du, Y.; Sun, J.; Siddique, K.H. Linking microbial carbon-degrading potential to organic carbon sequestration in fertilized soils: Insights from metagenomics. Land Degrad. Dev. 2023, 34, 6021–6035. [Google Scholar] [CrossRef]
- Ippolito, T.; Balkovič, J.; Skalsky, R.; Folberth, C.; Krisztin, T.; Neff, J. Predicting spatiotemporal soil organic carbon responses to management using EPIC-IIASA meta-models. J. Environ. Manag. 2023, 344, 118532. [Google Scholar] [CrossRef]
- Lin, B.; Cheng, J.; Duan, H.; Liu, W.; Dang, Y.P.; Zhao, X.; Zhang, H. Optimizing straw and nitrogen fertilizer resources for low-carbon sustainable agriculture. Resour. Conserv. Recycl. 2024, 209, 107743. [Google Scholar] [CrossRef]
- Ren, T.; Ukalska-Jaruga, A.; Smreczak, B.; Cai, A. Dissolved organic carbon in cropland soils: A global meta-analysis of management effects. Agric. Ecosyst. Environ. 2024, 371, 109080. [Google Scholar] [CrossRef]
- Sun, Q.; Gu, X.; Wang, Y.; Gao, H.; Wang, X.; Chen, X.; Sun, S. Impact of straw return on greenhouse gas emissions from maize fields in China: Meta-analysis. Front. Plant Sci. 2025, 16, 1493357. [Google Scholar] [CrossRef]
- Liu, X.; Tan, S.; Song, X.; Wu, X.; Zhao, G.; Li, S.; Liang, G. Response of soil organic carbon content to crop rotation and its controls: A global synthesis. Agric. Ecosyst. Environ. 2022, 335, 108017. [Google Scholar] [CrossRef]
- Bregaglio, S.; Mongiano, G.; Ferrara, R.M.; Ginaldi, F.; Lagomarsino, A.; Rana, G. Which are the most favourable conditions for reducing soil CO2 emissions with no-tillage? Results from a meta-analysis. Int. Soil Water Conserv. Res. 2022, 10, 497–506. [Google Scholar] [CrossRef]
- Li, N.; Zhao, Y.; Han, J.; Yang, Q.; Liang, J.; Liu, X.; Wang, Y.; Huang, Z. Impacts of future climate change on rice yield based on crop model simulation—A meta-analysis. Sci. Total Environ. 2024, 949, 175038. [Google Scholar] [CrossRef]
- Mao, L.; Zhang, H.; Yang, Z.; Li, Y.; Shen, Y. Site-specific effects of fertilizer on hay and grain yields of oats: Evidence from large-scale field experiments. J. Sci. Food Agric. 2025, 105, 2429–2439. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Cotrufo, M.F. Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Kuhn, N.J.; Zhao, R.; Cao, L. Net effects of conservation agriculture principles on sustainable land use: A synthesis. Glob. Change Biol. 2021, 27, 6321–6330. [Google Scholar] [CrossRef]
- Ding, W.; Gao, H.; Qi, Z.; Sun, L.; Zheng, C.; Huang, J.; Filipović, V.; He, H. Enhancing soil ecological stoichiometry and orchard yield through ground cover management: A meta-analysis across China. Agric. Ecosyst. Environ. 2025, 384, 109556. [Google Scholar] [CrossRef]
- Huang, M.; Gu, C.; Bai, Y. Effect of fertilization on methane and nitrous oxide emissions and global warming potential on agricultural land in China: A meta-analysis. Agriculture 2023, 14, 34. [Google Scholar] [CrossRef]
- Zhou, S.; Li, X.; Zhang, P.; Lu, G.; Zhang, X.; Zhang, H.; Zhang, F. Carbon Sequestration Capacity after Ecological Restoration of Open-Pit Mines: A Case Study in Yangtze River Basin, Jurong City, Jiangsu Province. Sustainability 2024, 16, 8149. [Google Scholar] [CrossRef]
- Jha, S.K.; Mishra, V.K.; Damodaran, T.; Singh, A.K.; Sharma, P.C.; Sharma, D.K.; Ojha, D.; Singh, Y.P. Conservation tillage and waste crop residue based zinc fortification in rice and wheat under reclaimed sodic soils: Progress toward nutrient circularity and sustainability. J. Agric. Food Chem. 2023, 71, 2853–2863. [Google Scholar] [CrossRef] [PubMed]
- Mastiholi, A.B.; Sowmya, B.; Maheswarappa, H.; Gondi, S.P.; Shantappa, T.; Rudresh, D.; Gopali, J. Organic and natural farming improve microbial diversity and dehydrogenase activity in clusterbean-tomato cropping sequence. Arch. Agron. Soil Sci. 2023, 69, 3705–3716. [Google Scholar] [CrossRef]
- Chen, W.; Chen, C.; Gao, J. Towards rural neo-endogenous development driven by knowledge. Acta Geogr. Sin. 2024, 80, 259–271. [Google Scholar]
- Gen, P.; Chen, W.; Yang, J.; Yang, L. Obstacles and paths for local implantation of rural ecological innovation technologies: A case of implementing natural agricultural technology in Chenzhuang Village. Resour. Sci. 2020, 42, 1298–1310. [Google Scholar]
- Tatsumi, C.; Lin, J.; Ishiguro, M.; Uchida, Y. Natural farming diversifies resource-utilisation patterns and increases network complexity in soil microbial community of paddy fields. Agric. Ecosyst. Environ. 2023, 356, 108618. [Google Scholar] [CrossRef]
- Wang, F.; Li, B.; Tian, S.; Zheng, D.; Ge, Q. Updated Scheme for Eco-Geographical Regionalization in China. Acta Geogr. Sin. 2024, 79, 3–16. [Google Scholar]
- Zhang, X.-Y.; Zhang, G.-B.; Yang, J.; Jing, M.A.; Hua, X.U.; Zu-Cong, C.A.I. Straw application altered CH4 emission, concentration and 13C-isotopic signature of dissolved CH4 in a rice field. Pedosphere 2012, 22, 13–21. [Google Scholar] [CrossRef]
- Payen, F.T.; Sykes, A.; Aitkenhead, M.; Alexander, P.; Moran, D.; MacLeod, M. Soil organic carbon sequestration rates in vineyard agroecosystems under different soil management practices: A meta-analysis. J. Clean. Prod. 2021, 290, 125736. [Google Scholar] [CrossRef]
- Lima, J.; Manning, T.; Rutherford, K.; Baima, E.; Dewhurst, R.; Walsh, P.; Roehe, R. Taxonomic annotation of 16S rRNA sequences of pig intestinal samples using MG-RAST and QIIME2 generated different microbiota compositions. J. Microbiol. Methods 2021, 186, 106235. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Liu, Y.; Liu, H.; Zhang, Z.; Gao, Q.; Wang, Y.; Feng, Y.; Yang, G.; Ren, C. The contributions of soil biochemical characteristics and soil organic carbon (SOC) structure to SOC mineralization rate during forest succession on the Loess Plateau, China. Land Degrad. Dev. 2022, 33, 3375–3386. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Shen, T.; Cheng, W.; Yan, Z.; Yu, L. Influence of soil bacteria and carbonic anhydrase on karstification intensity and regulatory factors in a typical karst area. Geoderma 2018, 313, 17–24. [Google Scholar] [CrossRef]
- van der Wal, A.; de Boer, W.; Smant, W.; van Veen, J. Initial decay of woody fragments in soil is influenced by size, vertical position, nitrogen availability and soil origin. Plant Soil 2007, 301, 189–201. [Google Scholar] [CrossRef]
- Chang, F.; He, S.; Dang, C. Assisted selection of biomarkers by linear discriminant analysis effect size (LEfSe) in microbiome data. J. Vis. Exp. 2022, 16, e61715. [Google Scholar]
- Angelini, M.E.; Heuvelink, G.; Kempen, B. Multivariate mapping of soil with structural equation modelling. Eur. J. Soil Sci. 2017, 68, 575–591. [Google Scholar] [CrossRef]
- Dar, A.A.; Parthasarathy, N. Ecological drivers of soil carbon in Kashmir Himalayan forests: Application of machine learning combined with structural equation modelling. J. Environ. Manag. 2023, 330, 117147. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, T.; Guo, X.; Xia, L.; Lu, C.; Wang, C. Plus-InVEST Study of the Chengdu-Chongqing urban agglomeration’s land-use change and carbon storage. Land 2022, 11, 1617. [Google Scholar] [CrossRef]
- Gong, K.; Huang, Z.; Qu, M.; He, Z.; Chen, J.; Wang, Z.; Yu, Q.; Feng, H.; He, J. Influences of climate change on carbon and water fluxes of the ecosystem in the Qinling Mountains of China. Ecol. Indic. 2024, 166, 112504. [Google Scholar] [CrossRef]
- Saiya-Cork, K.; Sinsabaugh, R.; Zak, D. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Qin, L.; Freeman, C.; Jia, X.; Zhang, Z.; Liu, B.; Zhang, S.; Jiang, M. Microbial enzyme activity and stoichiometry signal the effects of agricultural intervention on nutrient cycling in peatlands. Ecol. Indic. 2021, 122, 107242. [Google Scholar] [CrossRef]
- Borase, D.; Nath, C.; Hazra, K.; Senthilkumar, M.; Singh, S.; Praharaj, C.; Singh, U.; Kumar, N. Long-term impact of diversified crop rotations and nutrient management practices on soil microbial functions and soil enzymes activity. Ecol. Indic. 2020, 114, 106322. [Google Scholar] [CrossRef]
- Alam, S.A.; Starr, M.; Clark, B.J. Tree biomass and soil organic carbon densities across the Sudanese woodland savannah: A regional carbon sequestration study. J. Arid Environ. 2013, 89, 67–76. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, L.; Zheng, H.; Chen, J. Spatiotemporal dynamics of carbon storage in response to urbanization: A case study in the Su-Xi-Chang region, China. Processes 2019, 7, 836. [Google Scholar] [CrossRef]
- Jones, P.G.; Thornton, P.K. Representative soil profiles for the Harmonized World Soil Database at different spatial resolutions for agricultural modelling applications. Agric. Syst. 2015, 139, 93–99. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, L.; Huang, J. Integrating the SD-CLUE-S and InVEST models into assessment of oasis carbon storage in northwestern China. PLoS ONE 2017, 12, e0172494. [Google Scholar] [CrossRef]
- Wang, N.; Guo, Z.; Shang, D.; Li, K. Carbon trading price forecasting in digitalization social change era using an explainable machine learning approach: The case of China as emerging country evidence. Technol. Forecast. Soc. Change 2024, 200, 123178. [Google Scholar] [CrossRef]
- Min, K.; Lynch, L.; Zheng, T.; Chen, F.; Liang, C. Factors driving microbial biomass and necromass relationships display ecosystem-dependent responses. Eur. J. Soil Sci. 2024, 75, e13555. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Wang, L.; Tang, Y.; Chen, J.; Fu, X.; Le, Y.; Wu, J. Variability of soil organic carbon reservation capability between coastal salt marsh and riverside freshwater wetland in Chongming Dongtan and its microbial mechanism. J. Environ. Sci. 2012, 24, 1053–1063. [Google Scholar] [CrossRef]
- Qin, J.; Liu, H.; Zhao, J.; Wang, H.; Zhang, H.; Yang, D.; Zhang, N. The roles of bacteria in soil organic carbon accumulation under nitrogen deposition in stipa baicalensis steppe. Microorganisms 2020, 8, 326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, T.; Wang, Y.; Tang, M. Exogenous arbuscular mycorrhizal fungi increase soil organic carbon and change microbial community in poplar rhizosphere. Plant Soil Environ. 2019, 65, 152. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Rong, L.; Duan, X. Land-use driven changes in soil microbial community composition and soil fertility in the dry-Hot Valley region of southwestern China. Microorganisms 2022, 10, 956. [Google Scholar] [CrossRef]
- Hu, Y.; Cong, M.; Chen, M.; Abulaizi, M.; Yu, G.; Yang, Z.; Zhu, X.; Jia, H. Soil bacteria mediate organic carbon stability during alpine wetland biogeomorphic succession in the arid region of Central Asia. Plant Soil 2024, 497, 429–447. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Zhang, Y.; Lai, Z.; She, W.; Bai, Y.; Feng, W.; Qin, S. Microbial communities and their genetic repertoire mediate the decomposition of soil organic carbon pools in revegetation shrublands in a desert in northern China. Eur. J. Soil Sci. 2020, 71, 93–105. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, W.; Xu, H.; Xia, Y.; Gong, Q.; Xu, Z.; Zhang, N.; Xun, W.; Shen, Q.; Zhang, R. A novel decomposer-exploiter interaction framework of plant residue microbial decomposition. Genome Biol. 2025, 26, 20. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.-M.; Jayawardena, R.S.; Luangharn, T.; Zeng, X.-Y.; Li, C.-J.-Y.; Bao, S.-X.; Ba, H.; Zhou, D.-Q.; Tang, S.-M.; Hyde, K.D. Species diversity of fungal pathogens on cultivated mushrooms: A case study on morels (Morchella, Pezizales). Fungal Divers. 2024, 125, 157–220. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.; Wang, B.; Wang, Y.; Liang, C.; An, S.; Soromotin, A.; Kuzyakov, Y. Increasing contribution of microbial residues to soil organic carbon in grassland restoration chronosequence. Soil Biol. Biochem. 2022, 170, 108688. [Google Scholar] [CrossRef]
- Bharti, P.; Das, A.; Kumar, S.; Rakshit, R. Assessment of Soil Specific Enzyme Activities in Aggregates Size Fractions: A Case Study from Subtropical Agro-ecosystem. Eurasian Soil Sci. 2024, 57, 646–656. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Liu, Y.; Wang, J. Effects of long-term crop cultivation on soil organic carbon in China’s farmland: A meta-analysis. Acta Pedol. Sin. 2024, 61, 1628–1638. [Google Scholar]
- Zhang, S.; Ren, T.; Fang, Y.; Zhao, J.; Zhu, J.; Cong, W.-f.; Lambers, H.; Lu, J. Enhancing soil labile organic matter through oilseed rape-rice rotation and straw returning in paddy-upland systems. Plant Soil 2025, 1–18. [Google Scholar] [CrossRef]
- Feng, S.; Tan, S.; Zhang, A.; Zhang, Q.; Pan, G.; Qu, F.; Smith, P.; Li, L.; Zhang, X. Effect of household land management on cropland topsoil organic carbon storage at plot scale in a red earth soil area of South China. J. Agric. Sci. 2011, 149, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, J.; Sun, W.; Gao, Y.; Yu, Z.; Dong, Y.; Li, P. Temporal and Spatial Variations in Soil Elemental Stoichiometry Coupled with Alterations in Agricultural Land Use Types in the Taihu Lake Basin. Agriculture 2023, 13, 484. [Google Scholar] [CrossRef]
- Shen, Q.; Niu, J.; Liu, Q.; Liao, D.; Du, T. A resilience-based approach for water resources management over a typical agricultural region in Northwest China under water-energy-food nexus. Ecol. Indic. 2022, 144, 109562. [Google Scholar] [CrossRef]
- Lockwell, J.; Guidi, W.; Labrecque, M. Soil carbon sequestration potential of willows in short-rotation coppice established on abandoned farm lands. Plant Soil 2012, 360, 299–318. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, Y.; Zong, L.; Zhang, W.; Xu, M.; Liu, L. Simulation and prediction of soil organic carbon dynamics in Jiangsu Province based on model and GIS techniques. Sci. Agric. Sin. 2003, 36, 1312–1317. [Google Scholar]
- Chen, Y.; Shen, J.; Liu, L.; Sun, Y.; Pan, Y.; Lin, W. Preservation of organic matter in aqueous deposits and soils across the Mars-analog qaidam basin, NW China: Implications for biosignature detection on Mars. J. Geophys. Res. Planets 2022, 127, e2022JE007418. [Google Scholar] [CrossRef]
- Ma, M.; Qu, Y.; Lyu, J.; Zhang, X.; Su, Z.; Gao, H.; Yang, X.; Chen, X.; Jiang, T.; Zhang, J. The 2022 extreme drought in the Yangtze River Basin: Characteristics, causes and response strategies. River 2022, 1, 162–171. [Google Scholar] [CrossRef]
- Gao, M.; Xu, H.; Ma, M.; Gao, G.; Chen, X.; Chen, J.; Liu, X.; Qi, L. Global intercountry croplands’ greenhouse gas emissions differences and their potential drivers from economic levels perspective. Ecol. Indic. 2024, 167, 112635. [Google Scholar] [CrossRef]
- Beni, C.; Neri, U.; Papetti, P.; Altimari, A. Natural horticultural systems in organic farming as a tool for resilience: Improvement of economic performance and prevention of soil erosion. Agroecol. Sustain. Food Syst. 2021, 45, 1375–1398. [Google Scholar] [CrossRef]
- Tian, J.; Dungait, J.A.; Hou, R.; Deng, Y.; Hartley, I.P.; Yang, Y.; Kuzyakov, Y.; Zhang, F.; Cotrufo, M.F.; Zhou, J. Microbially mediated mechanisms underlie soil carbon accrual by conservation agriculture under decade-long warming. Nat. Commun. 2024, 15, 377. [Google Scholar] [CrossRef]
- Wang, X.; Yu, G.; Kuzyakov, Y.; Yin, B.; Kappler, A.; Liu, C. Contribution of fungal biomass to persistent soil carbon across natural ecosystems. Sci. China Earth Sci. 2025, 68, 444–456. [Google Scholar] [CrossRef]
- Liang, Y.; Wei, L.; Wang, S.; Hu, C.; Xiao, M.; Zhu, Z.; Deng, Y.; Wu, X.; Kuzyakov, Y.; Chen, J. Long-term fertilization suppresses rice pathogens by microbial volatile compounds. J. Environ. Manag. 2023, 336, 117722. [Google Scholar] [CrossRef]
- Qian, Y.; Sun, J.; Hillier, J.; Sheng, J.; Guo, Z.; Zhu, P.; Cheng, K.; Pan, G.; Li, Y.; Wang, X. Green manure rotation and application increase rice yield and soil carbon in the Yangtze River valley of China. Pedosphere 2023, 33, 589–599. [Google Scholar]
- Tripathy, S.; Biswas, S.; Singh, P.; Ghose, T.; Purakayastha, T.; Ahmed, N.; Pandey, R.; Das, T.; Ramakrishnan, B. Soil quality, resilience, and crop productivity under 32-year-old long-term rice-rice system in acidic alfisol of Assam. J. Soil Sci. Plant Nutr. 2023, 23, 5333–5344. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, C.; Zhang, L.; Zhang, C.; Lv, Y.; Nie, L.; Zhang, X.; Li, B.; Cao, W.; Li, M. Effects of Chinese milk vetch returning on soil properties, microbial community, and rice yield in paddy soil. Sustainability 2022, 14, 16065. [Google Scholar] [CrossRef]
- Meier, E.A.; Thorburn, P.J.; Kragt, M.E.; Dumbrell, N.P.; Biggs, J.S.; Hoyle, F.C.; van Rees, H. Greenhouse gas abatement on southern Australian grains farms: Biophysical potential and financial impacts. Agric. Syst. 2017, 155, 147–157. [Google Scholar] [CrossRef]
- Sharma, R.; Sindhu, S.S.; Glick, B.R. Potassium solubilizing microorganisms as potential biofertilizer: A sustainable climate-resilient approach to improve soil fertility and crop production in agriculture. J. Plant Growth Regul. 2024, 43, 2503–2535. [Google Scholar] [CrossRef]
- Li, C.; Jia, Z.; Tang, Y.; Zhang, S.; Li, T.; Ma, S.; Nie, H.; Zhai, L.; Zhang, B.; Liu, X. Mineral-solubilizing microbial inoculants facilitate the rejuvenation of soil multifunctionality and plant growth at abandoned mine sites. Land Degrad. Dev. 2024, 35, 442–454. [Google Scholar] [CrossRef]
- Dong, S.; Xu, Y.; Li, S.; Shen, H.; Yang, M.; Xiao, J. Restoration actions associated with payment for ecosystem services promote the economic returns of alpine grasslands in China. J. Clean. Prod. 2024, 458, 142439. [Google Scholar] [CrossRef]
- Feng, X.; Wang, S. Plant influences on soil microbial carbon pump efficiency. Glob. Change Biol. 2023, 29, 3854–3856. [Google Scholar] [CrossRef]
- Tang, X.; Liu, S.; Zhou, G.; Zhang, D.; Zhou, C. Soil-atmospheric exchange of CO2, CH4, and N2O in three subtropical forest ecosystems in southern China. Glob. Change Biol. 2006, 12, 546–560. [Google Scholar] [CrossRef]
- Yang, X.; Xiong, J.; Du, T.; Ju, X.; Gan, Y.; Li, S.; Xia, L.; Shen, Y.; Pacenka, S.; Steenhuis, T.S.; et al. Diversifying crop rotation increases food production, reduces net greenhouse gas emissions and improves soil health. Nat. Commun. 2024, 15, 198. [Google Scholar] [CrossRef]
- Zhou, X.; Guan, F.; Zhang, X.; Li, C.; Zhou, Y. Response of moso bamboo growth and soil nutrient content to strip cutting. Forests 2022, 13, 1293. [Google Scholar] [CrossRef]
- Li, P.; Zhou, G.; Du, H.; Lu, D.; Mo, L.; Xu, X.; Shi, Y.; Zhou, Y. Current and potential carbon stocks in Moso bamboo forests in China. J. Environ. Manag. 2015, 156, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil organic matter and water retention. Agron. J. 2020, 112, 3265–3277. [Google Scholar] [CrossRef]
- Luo, G.; Li, L.; Friman, V.-P.; Guo, J.; Guo, S.; Shen, Q.; Ling, N. Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: A meta-analysis. Soil Biol. Biochem. 2018, 124, 105–115. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Seufert, V.; Ramankutty, N.; Foley, J.A. Comparing the yields of organic and conventional agriculture. Nature 2012, 485, 229–232. [Google Scholar] [CrossRef]





| Land Use Type | Aboveground Biomass | Subsurface Biomass | Soil Organic Matter |
|---|---|---|---|
| Plowland | 0.26 | 3.72 | 8.71 |
| Forest land | 1.96 | 5.34 | 12.76 |
| Grassland | 1.63 | 3.99 | 8.03 |
| Water area | 0.14 | 0 | 1.22 |
| Building land | 0.12 | 0 | 2.38 |
| Unused land | 0.06 | 0 | 1.74 |
| Plowland | 0.26 | 3.72 | 8.71 |
| Forest land | 1.96 | 5.34 | 12.76 |
| Totality | Paddy Field | Dry Farming | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SE | n | Mean | SE | n | Mean | SE | ||
| CH4 | PT0 | 59 | 4.55 | 0.89 | 39 | 5.10 | 0.77 | 20 | −2.58 | 0.98 |
| NT | 66 | 7.35 | 0.25 | 46 | 2.55 | 0.78 | 20 | −1.82 | 0.25 | |
| NT0 | 34 | 5.23 | 0.47 | 25 | 3.25 | 0.71 | 9 | −1.99 | 0.37 | |
| NTR | 32 | 9.25 | 0.12 | 21 | 1.22 | 0.11 | 11 | −1.20 | 0.39 | |
| CO2 | PT0 | 59 | 200.33 | 74.25 | 18 | 300.45 | 20.47 | 41 | 3.77 | 1.67 |
| NT | 69 | 168.25 | 28.56 | 23 | 255.17 | 42.12 | 46 | 2.22 | 1.07 | |
| NT0 | 35 | 223.47 | 45.32 | 12 | 332.10 | 31.09 | 23 | 4.25 | 2.44 | |
| NTR | 34 | 90.22 | 28.35 | 11 | 117.20 | 30.01 | 23 | 1.79 | 1.51 | |
| Independent Variable | CH4 Emission | CH4 Absorption | CO2 Emission | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Regression Coefficient | R2 | n | Regression Coefficient | R2 | n | Regression Coefficient | R2 | ||
| NT0/PT0 | AAT | 19 | 0.1786 | 0.0265 | 10 | 0.0355 | 0.1455 | 27 | 0.1123 * | 0.2137 |
| MAP | 20 | −0.0006 | 0.1277 | 10 | 0.0005 | 0.0785 | 28 | 0.0008 ** | 0.2630 | |
| Soil pH | 21 | 0.3965 *** | 0.4205 | 8 | 0.3547 | 0.2258 | 32 | −0.0778 | 0.0167 | |
| ED | 20 | 0.1258 | 0.2351 | 10 | 0.0478 | 0.1109 | 38 | −0.0110 | 0.0026 | |
| FR | 19 | −0.0035 | 0.0778 | 10 | −0.0355 | 0.2999 | 34 | −0.2255 | 0.0085 | |
| NTR/PT0 | AAT | 10 | 0.3579 | 0.0124 | 12 | 0.1122 ** | 0.7039 | 30 | 0.1103 | 0.1271 |
| MAP | 10 | −0.0335 | 0.0874 | 12 | 0.0077 *** | 0.7852 | 30 | 0.0112 * | 0.1893 | |
| Soil pH | 23 | −0.2525 | 0.0277 | 8 | 0.8965 * | 0.6002 | 26 | −0.2598 * | 0.2149 | |
| ED | 21 | −0.0781 | 0.0087 | 12 | 0.2558 ** | 0.6025 | 33 | −0.1108 * | 0.1532 | |
| FR | 19 | 0.0007 | 0.0758 | 12 | −0.0736 ** | 0.7782 | 31 | 0.0005 | 0.0016 | |
| NT/PT0 | AAT | 32 | −0.0458 | 0.0012 | 22 | 0.1022 * | 0.2056 | 57 | 0.1394 ** | 0.1524 |
| MAP | 30 | −0.0001 | 0.0348 | 22 | 0.0078 * | 0.3221 | 58 | 0.1002 *** | 0.2068 | |
| Soil pH | 38 | 0.1878 | 0.2476 | 16 | 0.4452 * | 0.2789 | 58 | −0.1447 * | 0.1027 | |
| ED | 41 | 0.0112 | 0.0007 | 22 | 0.0889 | 0.3657 | 71 | −0.0035 | 0.0540 | |
| FR | 37 | −0.0007 | 0.0058 | 22 | −0.0115 ** | 0.2589 | 65 | −0.0009 | 0.0040 | |
| Microbial Class | Dominant Microorganism | Farming Method | |||
|---|---|---|---|---|---|
| CP | GP | p | |||
| Bacteria | Phylum | Acidobacteria | 0.37 ± 0.01 | 0.39 ± 0.01 | <0.05 * |
| Proteobacteria | 0.34 ± 0.03 | 0.39 ± 0.02 | >0.05 | ||
| Gemmatimonadetes | 0.054 ± 0.01 | 0.046 ± 0.007 | >0.05 | ||
| Class | a-Proteobacteria | 0.31 ± 0.02 | 0.30 ± 0.01 | >0.05 | |
| b-Proteobacteria | 0.23 ± 0.002 | 0.35 ± 0.01 | <0.01 ** | ||
| g-Proteobacteria | 0.10 ± 0.01 | 0.06 ± 0.01 | >0.05 | ||
| Fungus | Phylum | Chytridiomycota | 0.53 ± 0.12 | 0.47 ± 0.10 | >0.05 |
| Ascomycota | 0.20 ± 0.10 | 0.21 ± 0.08 | >0.05 | ||
| Mucoromycota | 0.16 ± 0.04 | 0.14 ± 0.03 | >0.05 | ||
| Class | Unclassified | 0.48 ± 0.03 | 0.47 ± 0.06 | >0.05 | |
| Glomeromycetes | 0.22 ± 0.09 | 0.25 ± 0.01 | <0.01 ** | ||
| Agaricomycetes | 0.04 ± 0.01 | 0.15 ± 0.09 | >0.05 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Y.; Chen, W.; He, B. Path Mechanism and Field Practice Effect of Green Agricultural Production on the Soil Organic Carbon Dynamics and Greenhouse Gas Emission Intensity in Farmland Ecosystems. Agriculture 2025, 15, 1499. https://doi.org/10.3390/agriculture15141499
Li X, Wang Y, Chen W, He B. Path Mechanism and Field Practice Effect of Green Agricultural Production on the Soil Organic Carbon Dynamics and Greenhouse Gas Emission Intensity in Farmland Ecosystems. Agriculture. 2025; 15(14):1499. https://doi.org/10.3390/agriculture15141499
Chicago/Turabian StyleLi, Xiaoqian, Yi Wang, Wen Chen, and Bin He. 2025. "Path Mechanism and Field Practice Effect of Green Agricultural Production on the Soil Organic Carbon Dynamics and Greenhouse Gas Emission Intensity in Farmland Ecosystems" Agriculture 15, no. 14: 1499. https://doi.org/10.3390/agriculture15141499
APA StyleLi, X., Wang, Y., Chen, W., & He, B. (2025). Path Mechanism and Field Practice Effect of Green Agricultural Production on the Soil Organic Carbon Dynamics and Greenhouse Gas Emission Intensity in Farmland Ecosystems. Agriculture, 15(14), 1499. https://doi.org/10.3390/agriculture15141499








