Amino Acids as Biostimulants: Effects on Growth, Chlorophyll Content, and Antioxidant Activity in Ocimum basilicum L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Conditions
2.2. Research Methods
2.3. Statistical Analysis of Research Data
3. Results and Discussion
3.1. Effect of Amino Acid Treatment on Shoot System Fresh and Dry Weight
3.2. Effect of Amino Acid Treatment on the Content of Primary Photosynthetic Pigments
3.3. Effect of Amino Acid Treatment on the Total Phenolic Content
3.4. Effect of Amino Acid Treatment on the Antioxidant Activity
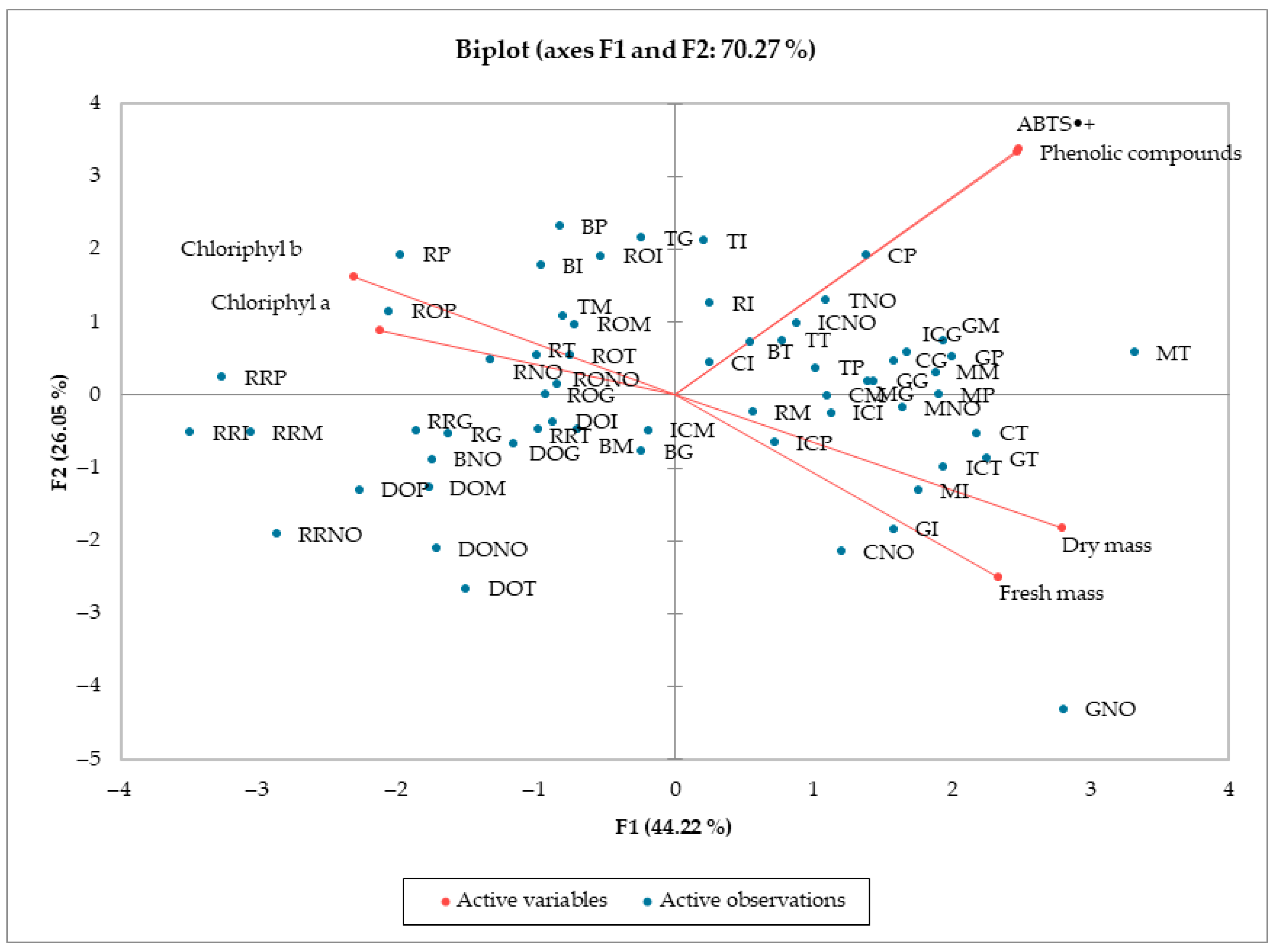
3.5. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and Crop Responses: A Review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Mafakheri, S.; Asghari, B. Effect of Seaweed Extract, Humic Acid and Chemical Fertilizers on Morphological, Physiological and Biochemical Characteristics of Trigonella foenum-graecum L. J. Agric. Sci. Technol. 2018, 20, 1505–1516. Available online: http://dorl.net/dor/20.1001.1.16807073.2018.20.7.2.6 (accessed on 24 March 2025).
- European Union. Regulation of the European Parliament and of the Council Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003; Regulation (EU) 2019/1009. In Official Journal of the European Union; European Union: Luxembourg, 2019. [Google Scholar]
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General Principles to Justify Plant Biostimulant Claims. Front. Plant Sci. 2019, 10, 494. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Radkowski, A.; Radkowska, I. Effect of Foliar Application of Growth Biostimulant on Quality and Nutritive Value of Meadow Sward. Ecol. Chem. Eng. A 2013, 20, 1205–1211. [Google Scholar] [CrossRef]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Górecki, H. Effect of the New Plant Growth Biostimulants Based on Amino Acids on Yield and Grain Quality of Winter Wheat. Molecules 2018, 23, 470. [Google Scholar] [CrossRef]
- Biswas, A.; Ullah, H.; Himanshu, S.K.; Praseartkul, P.; Tisarum, R.; Cha-um, S.; Datta, A. Biostimulant Enhances Growth, Herbage Yield, and Physio-Biochemical Characteristics of Sweet Basil Plants under Drought Stress. Russ. J. Plant Physiol. 2025, 72, 4. [Google Scholar] [CrossRef]
- Maach, M.; Boudouasar, K.; Akodad, M.; Skalli, A.; Moumen, A.; Baghour, M. Application of Biostimulants Improves Yield and Fruit Quality in Tomato. J. Appl. Aquac. 2021, 27, 288–293. [Google Scholar] [CrossRef]
- Alibakhshi, M.; Asadi-Gharneh, H.A. Growth and Biochemical Properties of Green Basil (Ocimum basilicum L.) Affected by Foliar Application of Biostimulants. J. Crop Nutr. Sci. 2021, 7, 34–45. [Google Scholar]
- Regina, A.; Glory, J.R.; Tulin, A.B. Micronutrients Biofortification (Zn, Fe, Cu, and Mn) Improves the Growth, Yield, and Chlorophyll Contents of Sweet Basil (Ocimum basilicum L.) Grown on a near Neutral Soil. Sci. Humanit. J. 2021, 15, 70–86. [Google Scholar] [CrossRef]
- Egata, D.F. Benefit and Use of Sweet Basil (Ocimum basilicum L.) in Ethiopia: A Review. Nutr. Food Process. 2021, 4, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Bensaid, A.; Boudard, F.; Servent, A.; Morel, S.; Portet, K.; Guzman, C.; Vitou, M.; Bichon, F.; Poucheret, P. Differential Nutrition-Health Properties of Ocimum basilicum Leaf and Stem Extracts. Foods 2022, 11, 1699. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liang, D.; Xia, H.; Pang, Y.; Xiao, Q.; Huang, Y.; Zhang, W.; Pu, C.; Wang, J.; Lv, X. Biostimulants Promote the Accumulation of Carbohydrates and Biosynthesis of Anthocyanins in ‘Yinhongli’ Plum. Front. Plant Sci. 2023, 13, 1074965. [Google Scholar] [CrossRef]
- Forsum, O.; Svennerstam, H.; Ganeteg, U.; Näsholm, T. Capacities and Constraints of Amino Acid Utilization in Arabidopsis. New Phytol. 2008, 179, 1058–1069. [Google Scholar] [CrossRef]
- Malík, M.; Velechovský, J.; Praus, L.; Janatová, A.; Kahánková, Z.; Klouček, P.; Tlustoš, P. Amino Acid Supplementation as a Biostimulant in Medical Cannabis (Cannabis sativa L.) Plant Nutrition. Front. Plant Sci. 2022, 13, 868350. [Google Scholar] [CrossRef]
- Böttcher, C.; Burbidge, C.A.; di Rienzo, V.; Boss, P.K.; Davies, C. Jasmonic Acid-isoleucine Formation in Grapevine (Vitis vinifera L.) by Two Enzymes with Distinct Transcription Profiles. J. Integr. Plant Biol. 2015, 57, 618–627. [Google Scholar] [CrossRef]
- Perveen, S.; Hussain, S.A. Methionine-Induced Changes in Growth, Glycinebetaine, Ascorbic Acid, Total Soluble Proteins and Anthocyanin Contents of Two Zea mays L. Varieties under Salt Stress. J. Anim. Plant Sci. 2021, 31, 131–142. [Google Scholar] [CrossRef]
- Hacham, Y.; Matityahu, I.; Amir, R. Transgenic Tobacco Plants Having a Higher Level of Methionine Are More Sensitive to Oxidative Stress. Physiol. Plant. 2017, 160, 242–252. [Google Scholar] [CrossRef]
- Kan, C.C.; Chung, T.Y.; Juo, Y.A.; Hsieh, M.H. Glutamine Rapidly Induces the Expression of Key Transcription Factor Genes Involved in Nitrogen and Stress Responses in Rice Roots. BMC Genom. 2015, 16, 731. [Google Scholar] [CrossRef]
- Naveed, M.; Qureshi, M.A.; Zahir, Z.A.; Hussain, M.B.; Sessitsch, A.; Mitter, B. L-Tryptophan-Dependent Biosynthesis of Indole-3-Acetic Acid (IAA) Improves Plant Growth and Colonization of Maize by Burkholderia phytofirmans PsJN. Ann. Microbiol. 2015, 65, 1381–1389. [Google Scholar] [CrossRef]
- López-Gómez, P.; Smith, E.N.; Bota, P.; Cornejo, A.; Urra, M.; Buezo, J.; Moran, J.F. Tryptophan Levels as a Marker of Auxins and Nitric Oxide Signaling. Plants 2022, 11, 1304. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.; Rengasamy, B.; Sinha, A.K. Nutrient and Water Availability Influence Rice Physiology, Root Architecture and Ionomic Balance via Auxin Signalling. Plant Cell Environ. 2025, 48, 2691–2705. [Google Scholar] [CrossRef] [PubMed]
- Feduraev, P.; Skrypnik, L.; Riabova, A.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Phenylalanine and Tyrosine as Exogenous Precursors of Wheat (Triticum aestivum L.) Secondary Metabolism through PAL-Associated Pathways. Plants 2020, 9, 476. [Google Scholar] [CrossRef]
- Patel, M.K.; Maurer, D.; Feygenberg, O.; Ovadia, A.; Elad, Y.; Oren-Shamir, M.; Alkan, N. Phenylalanine: A Promising Inducer of Fruit Resistance to Postharvest Pathogens. Foods 2020, 9, 646. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Yim, S.H.; Nam, S.H. Physiochemical, Nutritional and Functional Characterization of 10 Different Pear Cultivars (Pyrus Spp.). J. Appl. Bot. Food Qual. 2016, 89, 73–81. [Google Scholar] [CrossRef]
- Rafique, M.; Ali, A.; Naveed, M.; Abbas, T.; Al-Huqail, A.A.; Siddiqui, M.H.; Nawaz, A.; Brtnicky, M.; Holatko, J.; Kintl, A. Deciphering the Potential Role of Symbiotic Plant Microbiome and Amino Acid Application on Growth Performance of Chickpea under Field Conditions. Front. Plant Sci. 2022, 13, 852851. [Google Scholar] [CrossRef]
- Kany, M.A. Effect of Different Sources of Organic Fertilizers and Foliar Application of Some Amino Acids on Wheat Productivity and Some Soil Properties. J. Soil Sci. Agric. Eng. 2023, 14, 209–215. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Dalir, N.; Rahnemaie, R.; Ebadi, M.T. Phosphate Concentrations and Methionine Application Affect Quantitative and Qualitative Traits of Valerian (Valeriana officinalis L.) under Hydroponic Conditions. Ind. Crops Prod. 2021, 171, 113821. [Google Scholar] [CrossRef]
- Maqsood, M.F.; Shahbaz, M.; Kanwal, S.; Kaleem, M.; Shah, S.M.R.; Luqman, M.; Iftikhar, I.; Zulfiqar, U.; Tariq, A.; Naveed, S.A. Methionine Promotes the Growth and Yield of Wheat under Water Deficit Conditions by Regulating the Antioxidant Enzymes, Reactive Oxygen Species, and Ions. Life 2022, 12, 969. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous Application of Amino Acids Improves the Growth and Yield of Lettuce by Enhancing Photosynthetic Assimilation and Nutrient Availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Shekari, G.; Javanmardi, J. Application of Cysteine, Methionine and Amino Acid Containing Fertilizers to Replace Urea: The Effects on Yield and Quality of Broccoli. Adv. Crop Sci. Technol. 2017, 5, 283. [Google Scholar] [CrossRef]
- Khater, M.A.; Zaki, F.S.; Dawood, M.G.; El-Awadi, M.E.; Elsayed, A.E. Comparing Physiological Role of L-Methionine (Sulphur Containing Amino Acid) and Its Encapsulated Nano-Form on Growth and Crop Productivity of Onion (Allium cepa L.). Egypt. J. Chem. 2024, 67, 291–307. [Google Scholar] [CrossRef]
- El-Lethy, S.R.; Talaat, I.M.; Tarraf, S.A.; El Moursi, A. Impacts of Some Biostimulants on Guar (Cyamopsis tetragonoloba L.) Plants. Bull. Natl. Res. Cent. 2019, 43, 169. [Google Scholar] [CrossRef]
- Youssef, A. Influence of Some Amino Acids and Micro-Nutrients Treatments on Growth and Chemical Constituents of Echinacea purpurea Plant. J. Plant Prod. 2014, 5, 527–543. [Google Scholar] [CrossRef]
- EL-Leithy, A.S.; El-Shorbagy, M.S.; Aly, S. Effect of Zinc and Amino Acids on Growth, Yield and Chemical Constituents of Caraway (Carum carvi L.) Plants. J. Prod. Dev. 2007, 12, 347–366. [Google Scholar]
- Karnwal, A.; Dohroo, A. Effect of Maize Root Exudates on Indole-3-Acetic Acid Production by Rice Endophytic Bacteria under Influence of L-Tryptophan. F1000Research 2018, 7, 112. [Google Scholar] [CrossRef]
- Parvez, M.A.; Muhammad, F.; Ahmad, M. Effect of Auxin Precursor (L-Trypophan) on the Growth and Yield of Tomato (Lycopersicon esculentum). Pak. J. Biol. Sci. 2000, 3, 1154–1155. [Google Scholar] [CrossRef][Green Version]
- Bakhoum, G.S.; Badr, E.A.E.; Sadak, M.S.; Dawood, M.G. Improving Growth, Some Biochemical Aspects and Yield of Three Cultivars of Soybean Plant by Methionine Treatment under Sandy Soil Condition. Int. J. Environ. Res. 2019, 13, 35–43, Correction in Int. J. Environ. Res. 2019, 13, 45. [Google Scholar] [CrossRef]
- Rosianskey, Y.; Dahan, Y.; Yadav, S.; Freiman, Z.E.; Milo-Cochavi, S.; Kerem, Z.; Eyal, Y.; Flaishman, M.A. Chlorophyll Metabolism in Pollinated vs. Parthenocarpic Fig Fruits throughout Development and Ripening. J. Plant Physiol. 2016, 244, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Herbst, J.; Pang, X.; Roling, L.; Grimm, B. A Novel Tetratricopeptide-Repeat Protein, TTP1, Forms Complexes with Glutamyl-TRNA Reductase and Protochlorophyllide Oxidoreductase during Tetrapyrrole Biosynthesis. Plant Cell 2024, 75, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, A.; Sasaki, K.; Nashima, K.; Oda-Yamamizo, C.; Hirashima, M.; Sumitomo, K. Transcriptome Analysis in Petals and Leaves of Chrysanthemums with Different Chlorophyll Levels. BMC Plant Biol. 2017, 17, 202. [Google Scholar] [CrossRef] [PubMed]
- El-Awadi, M.E.; Abd El Wahed, M. Improvement the Growth and Quality of Green Onion (Allium cepa L.) Plants by Some Bioregulators in the New Reclaimed Area at Nobaria Region, Egypt. Int. J. Plant Soil Sci. 2012, 5, 114–120. [Google Scholar] [CrossRef]
- Khattab, M.; Shehata, A.; Abou El-Saadate, E.; Al-Hasni, K. Effect of Glycine, Methionine and Tryptophan on the Vegetative Growth, Flowering and Corms Production of Gladiolus Plant. Alex. Sci. Exch. J. 2016, 37, 647–659. [Google Scholar]
- Saburi, M.; Mohammad, R.; Sayed, H.; Mohammad, S.; Taghi, D. Effect of Amino Acids and Nitrogen Fixing Bacteria on Quantitative Yield and Essential Oil Content of Basil Ocimum basilicum. Agric. Sci. Dev. 2014, 3, 265–268. [Google Scholar]
- Narouei, Z.; Sedaghathoor, S.; Kaviani, B.; Ansari, M.H. Effects of Irrigation Intervals and Foliar Application of Amino Acids and Humic Acid on the Physiological Traits of Strawberries under Colored Shading Nets. J. Berry Res. 2022, 12, 187–208. [Google Scholar] [CrossRef]
- Noroozlo, Y.A.; Souri, M.K.; Delshad, M. Stimulation Effects of Foliar Applied Glycine and Glutamine Amino Acids on Lettuce Growth. Open Agric. 2019, 4, 164–172. [Google Scholar] [CrossRef]
- Asgher, M.; Sehar, Z.; Rehaman, A.; Rashid, S.; Ahmed, S.; Per, T.S.; Alyemeni, M.N.; Khan, N.A. Exogenously-Applied L-Glutamic Acid Protects Photosynthetic Functions and Enhances Arsenic Tolerance through Increased Nitrogen Assimilation and Antioxidant Capacity in Rice (Oryza sativa L.). Environ. Pollut. 2022, 301, 119008. [Google Scholar] [CrossRef]
- Nieves-Silva, E.; Sandoval-Castro, E.; Delgado-Alvarado, A.; Castañeda-Antonio, M.D.; Huerta-De la Peña, A. Nitrate Reductase and Glutamine Synthetase Enzyme Activities and Chlorophyll in Sorghum Leaves (Sorghum bicolor) in Response to Organic Fertilization. Int. J. Plant Biol. 2024, 15, 827–836. [Google Scholar] [CrossRef]
- Qian, Y.; Lynch, J.H.; Guo, L.; Rhodes, D.; Morgan, J.A.; Dudareva, N. Completion of the Cytosolic Post-Chorismate Phenylalanine Biosynthetic Pathway in Plants. Nat. Commun. 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Jin, J.S.; Kwak, Y.-S.; Hwang, G.-S. Metabolic Response of Strawberry (Fragaria x ananassa) Leaves Exposed to the Angular Leaf Spot Bacterium (Xanthomonas fragariae). J. Agric. Food Chem. 2016, 64, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Guleria, M.; Sharma, Y.; Sharma, S.; Chaudhary, A.; Sharma, R.; Kumar, P. Insights into Elicitor’s Role in Augmenting Secondary Metabolites Production and Climate Resilience in Genus Ocimum–A Globally Important Medicinal and Aromatic Crop. Ind. Crops Prod. 2023, 202, 117078. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C. Exploring Plant Tissue Culture to Improve the Production of Phenolic Compounds: A Review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Gómez-Plaza, E. Elicitors: A Tool for Improving Fruit Phenolic Content. Sci. Hortic. 2013, 3, 33–52. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Zhang, X.; Cheng, Z.; Song, Y.; Li, H.; Wang, N.; Liu, S.; Cao, Z.; Li, H. Transcriptomic and Metabolomic Analyses Reveal the Role of Phenylalanine Metabolism in the Maize Response to Stalk Rot Caused by Fusarium proliferatum. Int. J. Mol. Sci. 2024, 25, 1492. [Google Scholar] [CrossRef]
- Manela, N.; Oliva, M.; Ovadia, R.; Sikron-Persi, N.; Ayenew, B.; Fait, A.; Galili, G.; Perl, A.; Weiss, D.; Oren-Shamir, M. Phenylalanine and Tyrosine Levels Are Rate-Limiting Factors in Production of Health Promoting Metabolites in Vitis vinifera cv. Gamay Red Cell Suspension. Front. Plant Sci. 2015, 6, 538. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant Activities, Total Phenolics and Flavonoids Content in Two Varieties of Malaysia Young Ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef]
- Ulewicz-Magulska, B.; Wesolowski, M. Antioxidant Activity of Medicinal Herbs and Spices from Plants of the Lamiaceae, Apiaceae and Asteraceae Families: Chemometric Interpretation of the Data. Antioxidants 2023, 12, 2039. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Lenti, L.; Rigano, D.; Woo, S.L.; Nartea, A.; Pacetti, D.; Maggi, F.; Fiorini, D.A. Rapid Procedure for the Simultaneous Determination of Eugenol, Linalool and Fatty Acid Composition in Basil Leaves. Plants 2022, 11, 3315. [Google Scholar] [CrossRef] [PubMed]
- Basiouny, E.A.A. Comparative Study between Three Essential Oils in Terms of Their Chemical Composition and Antioxidant Activity. Plant Arch. 2023, 8, 81–91. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
| Amino Acid Treatment | Varieties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| “Rosie” | “Red Opal” | “Bordeaux” | “Dark Opal” | “Red Rubin” | “Genovese” | “Cinamon” | “Italiano Classico” | “Marseillais” | “Thai” | |
| No treatment | 4.85 ± 0.13 c | 5.82 ± 0.24 b | 6.37 ± 0.11 bc | 7.85 ± 0.81 bc | 5.99 ± 0.65 b | 18.15 ± 0.93 a | 10.41 ± 0.92 a | 8.03 ± 0.10 b | 7.71 ± 0.51 b | 9.65 ± 0.48 a |
| Isoleucine | 5.67 ± 0.56 bc | 5.14 ± 0.51 b | 4.83 ± 0.18 cd | 4.99 ± 0.43 d | 4.30 ± 0.08 c | 8.70 ± 0.12 d | 5.70 ± 0.13 c | 6.67 ± 0.13 c | 11.14 ± 0.81 a | 7.97 ± 0.85 bc |
| Methionine | 8.91 ± 0.01 a | 7.17 ± 0.67 a | 9.04 ± 0.35 a | 7.21 ± 0.02 c | 4.58 ± 0.33 c | 12.14 ± 1.68 bc | 8.50 ± 0.65 b | 6.64 ± 0.35 c | 8.02 ± 0.63 b | 6.32 ± 0.41 d |
| Glutamine | 6.05 ± 0.01 b | 5.86 ± 0.52 b | 8.90 ± 0.63 a | 9.87 ± 0.33 ab | 6.52 ± 0.23 b | 10.59 ± 0.52 cd | 8.47 ± 0.91 b | 7.14 ± 0.30 c | 11.40 ± 0.58 a | 6.71 ± 0.33 cd |
| Tryptophan | 5.87 ± 0.21 c | 5.42 ± 0.53 b | 7.89 ± 1.14 ab | 10.20 ± 0.30 a | 7.67 ± 0.35 a | 13.18 ± 0.62 b | 8.52 ± 0.56 b | 9.54 ± 0.10 a | 8.66 ± 0.59 b | 8.18 ± 0.46 abc |
| Phenylalanine | 3.13 ± 0.12 d | 3.48 ± 0.48 c | 3.20 ± 0.53 d | 6.98 ± 0.56 c | 3.83 ± 0.31 c | 8.23 ± 0.31 d | 5.14 ± 0.26 c | 5.31 ± 0.08 d | 8.33 ± 0.93 b | 8.99 ± 0.21 ab |
| Amino Acid Treatment | Varieties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| “Rosie” | “Red Opal” | “Bordeaux” | “Dark Opal” | “Red Rubin” | “Genovese” | “Cinamon” | “Italiano Classico” | “Marseillais” | “Thai” | |
| No treatment | 0.42 ± 0.01 cd | 0.42 ± 0.04 b | 0.58 ± 0.02 bc | 0.63 ± 0.03 bc | 0.52 ± 0.04 ab | 2.01 ± 0.23 a | 1.56 ± 0.18 a | 0.86 ± 0.15 ab | 0.98 ± 0.15 ab | 1.20 ± 0.24 a |
| Isoleucine | 0.52 ± 0.06 bc | 0.48 ± 0.02 b | 0.46 ± 0.09 cd | 0.55 ± 0.05 c | 0.33 ± 0.01 c | 1.36 ± 0.10 b | 0.56 ± 0.01 d | 0.69 ± 0.03 bc | 1.28 ± 0.11 a | 0.82 ± 0.03 bc |
| Methionine | 0.94 ± 0.01 a | 0.68 ± 0.04 a | 0.96 ± 0.05 a | 0.66 ± 0.01 bc | 0.47 ± 0.02 bc | 1.46 ± 0.22 b | 1.12 ± 0.24 abc | 0.74 ± 0.04 abc | 0.88 ± 0.09 b | 0.69 ± 0.04 c |
| Glutamine | 0.60 ± 0.09 b | 0.45 ± 0.04 b | 0.88 ± 0.05 a | 0.71 ± 0.02 b | 0.61 ± 0.11 a | 1.44 ± 0.03 b | 1.31 ± 0.07 ab | 0.81 ± 0.06 ab | 1.20 ± 0.07 ab | 0.63 ± 0.08 c |
| Tryptophan | 0.53 ± 0.09 bc | 0.45 ± 0.05 b | 0.65 ± 0.04 b | 0.91 ± 0.04 a | 0.64 ± 0.03 a | 1.41 ± 0.15 b | 0.96 ± 0.22 bcd | 0.97 ± 0.07 a | 1.02 ± 0.09 ab | 1.03 ± 0.09 ab |
| Phenylalanine | 0.31 ± 0.02 d | 0.34 ± 0.05 c | 0.35 ± 0.02 d | 0.63 ± 0.11 bc | 0.34 ± 0.01 c | 1.48 ± 0.16 b | 0.74 ± 0.12 cd | 0.54 ± 0.05 c | 1.09 ± 0.23 ab | 1.14 ± 0.07 ab |
| Amino Acid Treatment | Varieties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| “Rosie” | “Red Opal” | “Bordeaux” | “Dark Opal” | “Red Rubin” | “Genovese” | “Cinamon” | “Italiano Classico” | “Marseillais” | “Thai” | |
| No treatment | 2.37 ± 0.002 b | 2.30 ± 0.005 d | 2.38 ± 0.012 ab | 2.31 ± 0.005 e | 2.38 ± 0.003 e | 2.22 ± 0.001 e | 2.28 ± 0.004 c | 2.33 ± 0.001 a | 1.86 ± 0.018 d | 2.45 ± 0.003 b |
| Isoleucine | 2.29 ± 0.001 d | 2.40 ± 0.013 b | 2.40 ± 0.005 a | 2.23 ± 0.019 f | 2.44 ± 0.001 c | 2.07 ± 0.001 f | 2.14 ± 0.001 e | 1.99 ± 0.001 e | 2.35 ± 0.015 b | 2.49 ± 0.001 a |
| Methionine | 2.32 ± 0.012 c | 2.46 ± 0.003 a | 2.41 ± 0.004 a | 2.53 ± 0.001 a | 2.58 ± 0.001 a | 2.47 ± 0.001 a | 2.33 ± 0.004 b | 2.29 ± 0.001 b | 2.15 ± 0.007 c | 2.49 ± 0.011 a |
| Glutamine | 2.37 ± 0.006 b | 2.31 ± 0.008 cd | 2.32 ± 0.066 bc | 2.43 ± 0.001 c | 2.42 ± 0.001 d | 2.41 ± 0.001 b | 2.35 ± 0.003 a | 2.14 ± 0.002 c | 2.40 ± 0.002 a | 2.44 ± 0.004 c |
| Tryptophan | 2.37 ± 0.001 b | 2.32 ± 0.004 c | 2.30 ± 0.003 c | 2.39 ± 0.002 d | 2.38 ± 0.002 e | 2.32 ± 0.002 d | 2.05 ± 0.004 f | 2.02 ± 0.012 d | 1.93 ± 0.021 c | 2.38 ± 0.001 d |
| Phenylalanine | 2.52 ± 0.011 a | 2.27 ± 0.002 e | 2.39 ± 0.001 ab | 2.49 ± 0.001 b | 2.48 ± 0.010 b | 2.36 ± 0.006 c | 2.23 ± 0.003 d | 1.95 ± 0.001 f | 2.13 ± 0.011 c | 2.42 ± 0.010 c |
| Amino Acid Treatment | Varieties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| “Rosie” | “Red Opal” | “Bordeaux” | “Dark Opal” | “Red Rubin” | “Genovese” | “Cinamon” | “Italiano Classico” | “Marseillais” | “Thai” | |
| No treatment | 1.14 ± 0.001 c | 1.03 ± 0.004 e | 1.18 ± 0.016 d | 1.08 ± 0.005 d | 1.24 ± 0.013 e | 0.88 ± 0.001 d | 0.91 ± 0.002 d | 1.13 ± 0.002 a | 1.10 ± 0.004 b | 1.44 ± 0.016 b |
| Isoleucine | 0.99 ± 0.006 e | 1.29 ± 0.005 c | 1.38 ± 0.001 b | 0.98 ± 0.010 f | 1.52 ± 0.004 b | 0.76 ± 0.001 c | 0.96 ± 0.004 c | 0.78 ± 0.002 c | 0.71 ± 0.017 d | 1.52 ± 0.007 a |
| Methionine | 1.06 ± 0.001 d | 1.39 ± 0.001 b | 1.43 ± 0.003 a | 1.04 ± 0.001 e | 1.30 ± 0.012 d | 1.38 ± 0.009 c | 0.99 ± 0.003 c | 0.92 ± 0.004 b | 0.74 ± 0.003 d | 1.34 ± 0.033 c |
| Glutamine | 1.23 ± 0.018 b | 1.04 ± 0.003 e | 1.18 ± 0.028 d | 1.44 ± 0.001 a | 1.34 ± 0.005 c | 1.30 ± 0.014 a | 1.06 ± 0.018 a | 0.77 ± 0.006 c | 1.23 ± 0.005 a | 1.49 ± 0.011 a |
| Tryptophan | 1.22 ± 0.011 b | 1.07 ± 0.003 d | 1.04 ± 0.004 e | 1.21 ± 0.001 c | 1.18 ± 0.004 f | 1.01 ± 0.002 e | 0.60 ± 0.002 e | 0.72 ± 0.016 d | 0.68 ± 0.007 e | 1.19 ± 0.003 d |
| Phenylalanine | 1.39 ± 0.008 a | 1.56 ± 0.006 a | 1.23 ± 0.003 c | 1.24 ± 0.008 b | 1.56 ± 0.004 a | 0.99 ± 0.012 d | 0.91 ± 0.015 d | 0.60 ± 0.009 e | 0.86 ± 0.009 c | 1.10 ± 0.002 e |
| Amino Acid Treatment. | Varieties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| “Rosie” | “Red Opal” | “Bordeaux” | “Dark Opal” | “Red Rubin” | “Genovese” | “Cinamon” | “Italiano Classico” | “Marseillais” | “Thai” | |
| No treatment | 10.99 ± 0.23 b | 11.86 ± 0.17 a | 9.42 ± 0.23 c | 8.65 ± 0.04 c | 7.84 ± 0.17 c | 9.77 ± 0.23 c | 10.21 ± 0.05 d | 12.90 ± 0.34 a | 11.89 ± 0.50 cd | 13.31 ± 0.06 a |
| Isoleucine | 11.63 ± 0.11 ab | 12.56 ± 0.43 a | 12.30 ± 0.19 a | 10.12 ± 0.15 a | 8.93 ± 0.19 b | 10.57 ± 0.05 c | 12.53 ± 0.09 b | 11.83 ± 0.18 abc | 11.49 ± 0.10 d | 12.24 ± 0.02 bc |
| Methionine | 11.28 ± 0.35 b | 11.73 ± 0.24 ab | 10.69 ± 0.30 b | 9.59 ± 0.59 ab | 9.19 ± 0.10 b | 13.31 ± 0.18 a | 12.77 ± 0.12 b | 11.00 ± 0.12 c | 12.69 ± 0.20 b | 11.59 ± 0.46 c |
| Glutamine | 9.76 ± 0.28 c | 9.85 ± 0.58 c | 10.67 ± 0.02 b | 10.04 ± 0.44 a | 9.79 ± 0.15 a | 12.59 ± 0.13 ab | 12.63 ± 0.15 b | 12.74 ± 0.23 ab | 12.34 ± 0.04 bc | 13.02 ± 0.06 ab |
| Tryptophan | 11.06 ± 0.17 b | 10.84 ± 0.14 bc | 12.11 ± 0.16 a | 8.04 ± 0.29 c | 10.05 ± 0.12 a | 11.61 ± 0.78 b | 11.82 ± 0.09 c | 11.76 ± 0.87 bc | 13.58 ± 0.22 a | 12.15 ± 0.30 bc |
| Phenylalanine | 12.25 ± 0.18 a | 10.81 ± 0.17 bc | 12.93 ± 0.67 a | 8.82 ± 0.13 bc | 9.13 ± 0.16 b | 12.90 ± 0.21 a | 13.61 ± 0.49 a | 10.97 ± 0.08 c | 12.23 ± 0.16 bc | 13.49 ± 0.49 a |
| Amino Acid Treatment | Varieties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| “Rosie” | “Red Opal” | “Bordeaux” | “Dark Opal” | “Red Rubin” | “Genovese” | “Cinamon” | “Italiano Classico” | “Marseillais” | “Thai” | |
| No treatment | 11.64 ± 0.32 cd | 10.76 ± 0.24 d | 10.48 ± 0.39 c | 9.26 ± 0.03 c | 8.94 ± 0.74 e | 10.52 ± 1.27 b | 11.24 ± 0.30 d | 13.45 ± 0.25 a | 12.82 ± 0.30 bc | 14.08 ± 0.67 a |
| Isoleucine | 12.70 ± 0.54 ab | 13.51 ± 0.32 a | 13.00 ± 0.18 a | 11.43 ± 0.05 a | 9.41 ± 0.19 de | 11.50 ± 0.70 b | 11.71 ± 0.14 cd | 12.21 ± 0.66 b | 12.26 ± 0.23 c | 13.39 ± 0.48 ab |
| Methionine | 12.67 ± 0.13 ab | 12.75 ± 0.03 b | 11.42 ± 0.08 b | 9.86 ± 0.04 b | 9.84 ± 0.12 cd | 14.38 ± 0.02 a | 12.16 ± 0.32 c | 11.37 ± 0.15 b | 13.51 ± 0.10 b | 12.94 ± 0.31 b |
| Glutamine | 10.84 ± 0.08 d | 12.45 ± 0.16 b | 11.38 ± 0.12 b | 11.32 ± 0.07 a | 10.71 ± 0.11 bc | 13.34 ± 0.27 a | 13.70 ± 0.38 b | 13.58 ± 0.20 a | 13.63 ± 0.15 b | 14.02 ± 0.15 ab |
| Tryptophan | 12.14 ± 0.29 bc | 12.57 ± 0.08 b | 13.36 ± 0.16 a | 9.46 ± 0.23 c | 11.61 ± 0.12 a | 13.62 ± 0.03 a | 13.23 ± 0.25 b | 12.25 ± 0.16 b | 14.60 ± 0.50 a | 13.73 ± 0.07 ab |
| Phenylalanine | 13.57 ± 0.27 a | 11.83 ± 0.21 c | 13.31 ± 0.06 a | 9.91 ± 0.07 b | 10.79 ± 0.08 ab | 13.99 ± 0.12 a | 14.57 ± 0.17 a | 11.71 ± 0.26 b | 13.45 ± 0.16 b | 14.18 ± 0.59 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deveikytė, J.; Blinstrubienė, A.; Burbulis, N. Amino Acids as Biostimulants: Effects on Growth, Chlorophyll Content, and Antioxidant Activity in Ocimum basilicum L. Agriculture 2025, 15, 1496. https://doi.org/10.3390/agriculture15141496
Deveikytė J, Blinstrubienė A, Burbulis N. Amino Acids as Biostimulants: Effects on Growth, Chlorophyll Content, and Antioxidant Activity in Ocimum basilicum L. Agriculture. 2025; 15(14):1496. https://doi.org/10.3390/agriculture15141496
Chicago/Turabian StyleDeveikytė, Justina, Aušra Blinstrubienė, and Natalija Burbulis. 2025. "Amino Acids as Biostimulants: Effects on Growth, Chlorophyll Content, and Antioxidant Activity in Ocimum basilicum L." Agriculture 15, no. 14: 1496. https://doi.org/10.3390/agriculture15141496
APA StyleDeveikytė, J., Blinstrubienė, A., & Burbulis, N. (2025). Amino Acids as Biostimulants: Effects on Growth, Chlorophyll Content, and Antioxidant Activity in Ocimum basilicum L. Agriculture, 15(14), 1496. https://doi.org/10.3390/agriculture15141496






