Selenium Alleviates Low-Temperature Stress in Rice by Regulating Metabolic Networks and Functional Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Measurement of Morphological and Physiological Traits
2.3. Library Construction for Omics Analysis
2.4. Quality Control of Transcriptome Data
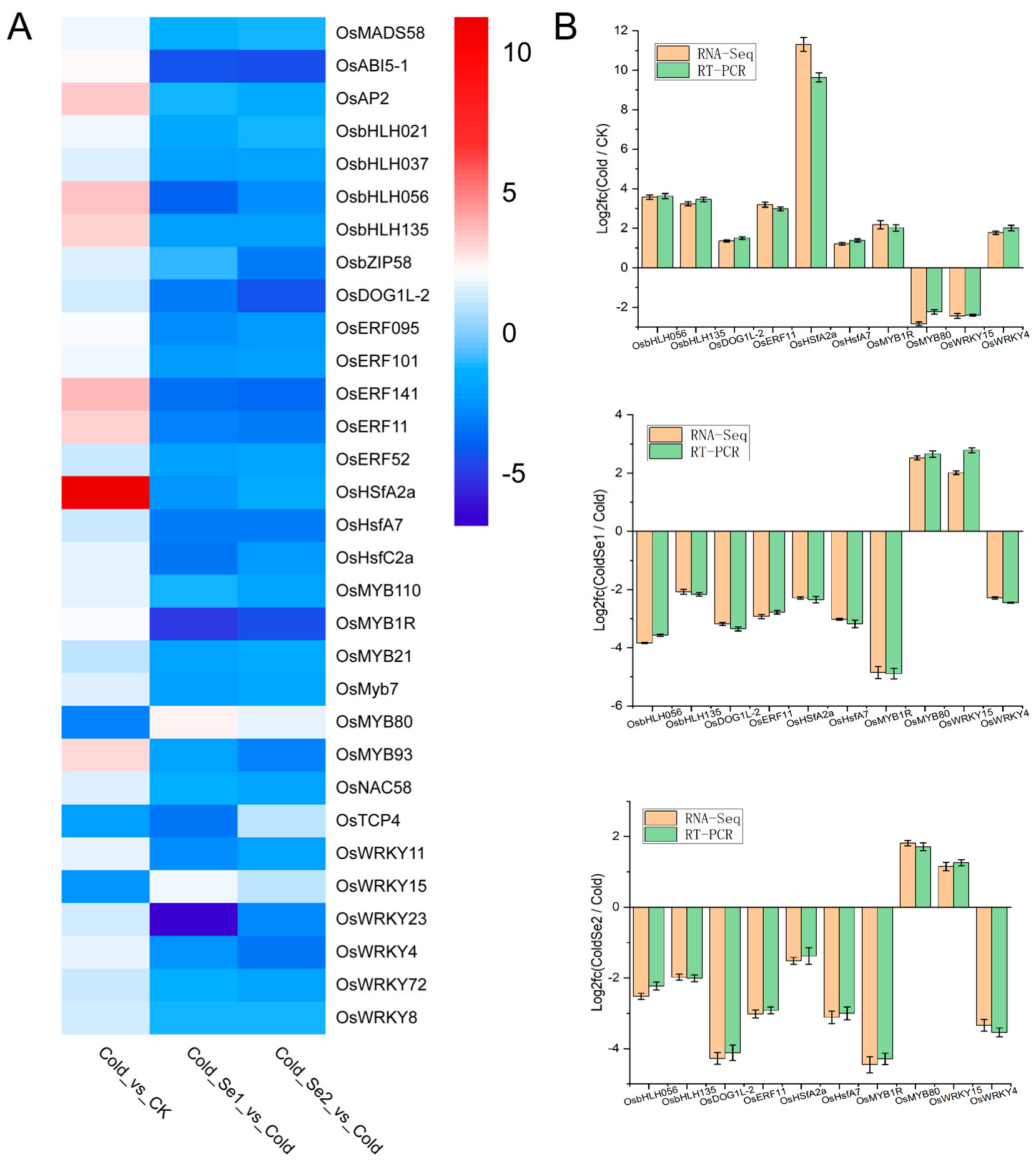
2.5. Validation of Differentially Expressed Transcription Factors Associated with Selenium Induction Using qRT-PCR
2.6. Metabolomics Analysis
2.7. Statistical Analysis
3. Results
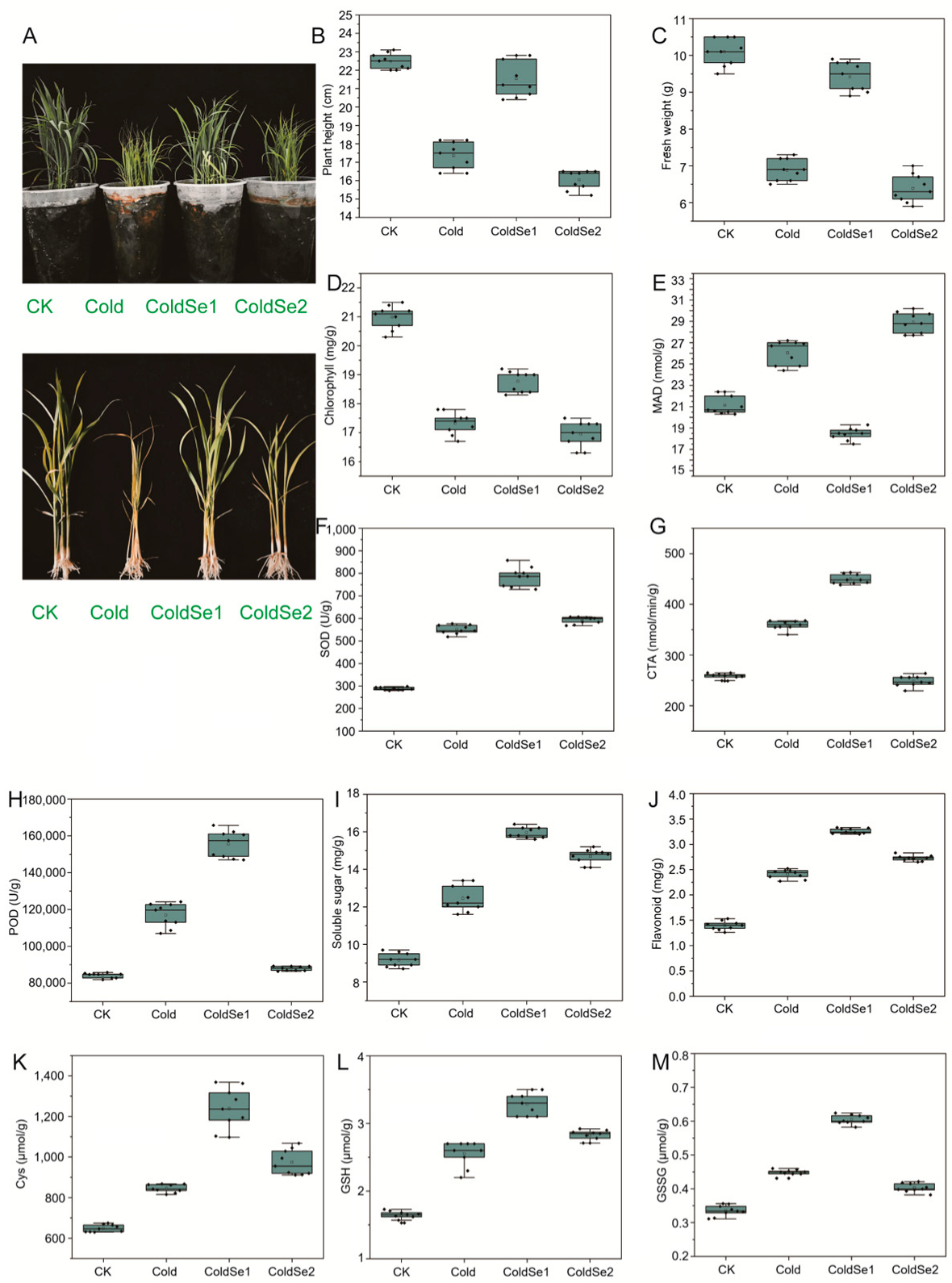
3.1. Measurement of Plant Morphological and Physiological Phenotypes
3.2. Enrichment of Differentially Expressed Functional Genes in Rice Seedlings
3.3. GO and KEGG Pathway Enrichment Analysis of DEGs in Rice Seedlings
3.4. Key Pathways of DEG Involvement in Rice Seedlings
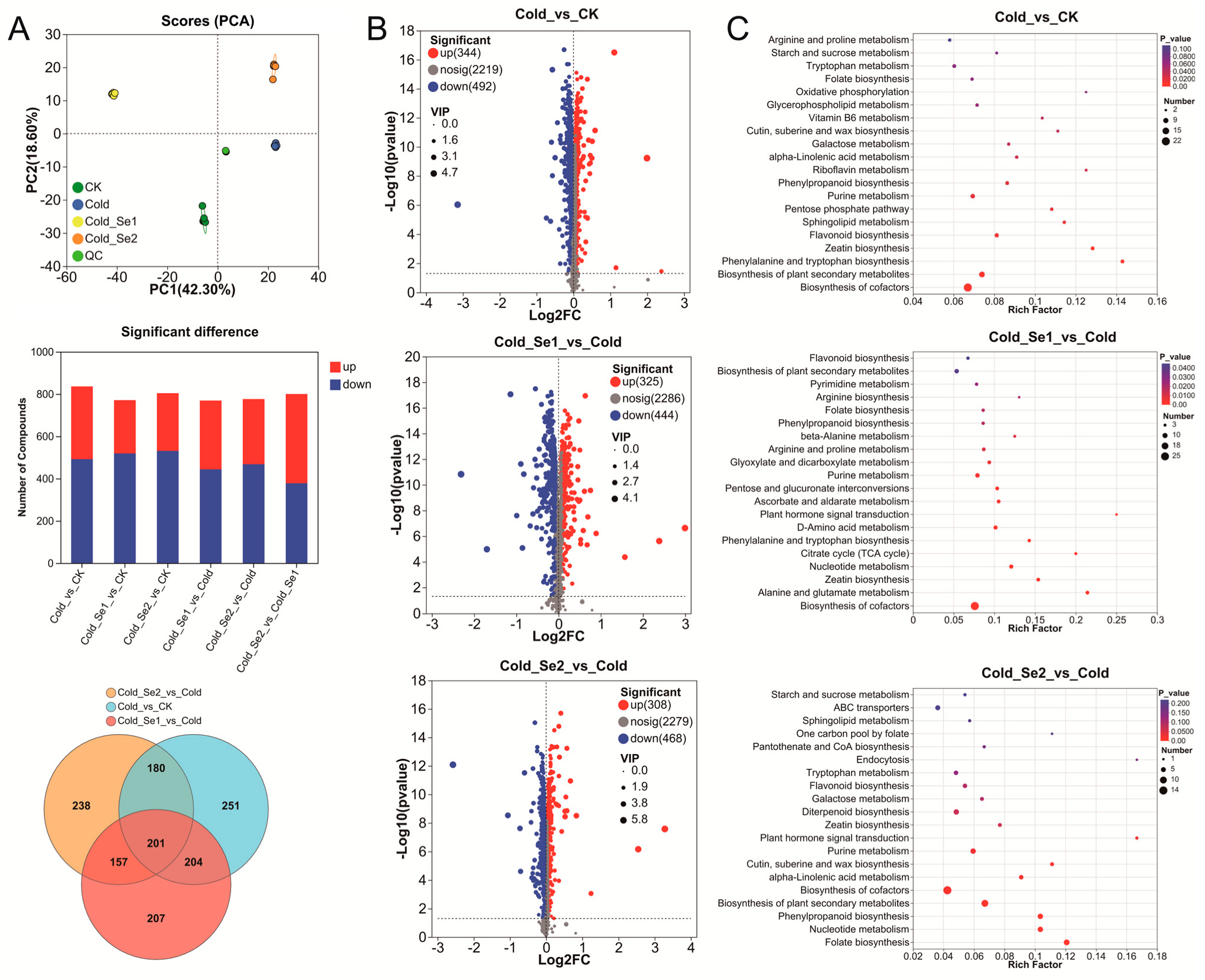
3.5. Metabolome Analysis of Rice Seedlings
3.6. Integrated Transcriptomic and Metabolomic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, S.; Wu, T.; Li, X.; Wang, S.; Hu, B. Identification of candidate genes controlling cold tolerance at the early seedling stage from Dongxiang wild rice by QTL mapping, BSA-Seq and RNA-Seq. BMC Plant Biol. 2024, 24, 649. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Chen, S.; Cheng, S.; Li, C.; Li, S.; Chen, J.; Zha, W.; Liu, K.; Xu, H.; Li, P.; et al. Transcriptome Analysis Revealed the Dynamic and Rapid Transcriptional Reprogramming Involved in Cold Stress and Related Core Genes in the Rice Seedling Stage. Int. J. Mol. Sci. 2023, 24, 1914. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, G.; Qin, H.; Guan, Y.; Wang, T.; Ni, W.; Xie, H.; Xing, Y.; Tian, G.; Lyu, M.; et al. Metabolomics combined with physiology and transcriptomics reveal key metabolic pathway responses in apple plants exposure to different selenium concentrations. J. Hazard. Mater. 2024, 464, 132953. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Sharma, S.; Kaur, S.; Nayyar, H. Selenium in agriculture: A nutrient or contaminant for crops? Arch. Agron. Soil Sci. 2014, 60, 1593–1624. [Google Scholar] [CrossRef]
- Huang, C.; Qin, N.; Sun, L.; Yu, M.; Hu, W.; Qi, Z. Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Strawberry Seedlings under Low-Temperature Stress. Int. J. Mol. Sci. 2018, 19, 1913. [Google Scholar] [CrossRef]
- De Sousa, G.F.; Silva, M.A.; De Morais, E.G.; Van Opbergen, G.A.Z.; Van Opbergen, G.G.A.; De Oliveira, R.R.; Amaral, D.; Brown, P.; Chalfun-Junior, A.; Guilherme, L.R.G. Selenium enhances chilling stress tolerance in coffee species by modulating nutrient, carbohydrates, and amino acids content. Front. Plant Sci. 2022, 13, 1000430. [Google Scholar] [CrossRef]
- Liu, K.; Li, S.; Han, J.; Zeng, X.; Ling, M.; Mao, J.; Li, Y.; Jiang, J. Effect of selenium on tea (Camellia sinensis) under low temperature: Changes in physiological and biochemical responses and quality. Environ. Exp. Bot. 2021, 188, 104475. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Cabral Gouveia, G.C.; Galindo, F.S.; Dantas Bereta Lanza, M.G.; Caroline da Rocha Silva, A.; Pereira de Brito Mateus, M.; Souza da Silva, M.; Rimoldi Tavanti, R.F.; Tavanti, T.R.; Lavres, J.; Reis, A.R.D. Selenium toxicity stress-induced phenotypical, biochemical and physiological responses in rice plants: Characterization of symptoms and plant metabolic adjustment. Ecotoxicol. Environ. Saf. 2020, 202, 110916. [Google Scholar] [CrossRef]
- Rajashekar, C.B.; Panda, M. Water stress is a component of cold acclimation process essential for inducing full freezing tolerance in strawberry. Sci. Hortic. 2014, 174, 54–59. [Google Scholar] [CrossRef]
- Huang, C.; Wang, D.; Sun, L.; Wei, L. Effects of exogenous salicylic acid on the physiological characteristics of Dendrobium officinale under chilling stress. Plant Growth Regul. 2016, 79, 199–208. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, M. Alleviation of selenium toxicity in Brassica juncea L.: Salicylic acid-mediated modulation in toxicity indicators, stress modulators, and sulfur-related gene transcripts. Protoplasma 2016, 253, 1515–1528. [Google Scholar] [CrossRef]
- Dai, H.; Jia, G.; Shan, C. Jasmonic acid-induced hydrogen peroxide activates MEK1/2 in upregulating the redox states of ascorbate and glutathione in wheat leaves. Acta Physiol. Plant. 2015, 37, 1–6. [Google Scholar] [CrossRef]
- Wang, S.; Liang, D.; Li, C.; Hao, Y.; Ma, F.; Shu, H. Influence of drought stress on the cellular ultrastructure and antioxidant system in leaves of drought-tolerant and drought-sensitive apple rootstocks. Plant Physiol. Biochem. 2012, 51, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H.Y. Selenium Promotes the Growth and Photosynthesis of Tomato Seedlings Under Salt Stress by Enhancing Chloroplast Antioxidant Defense System. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Alghanem, S.M.S.; Alhaithloul, H.A.S.; Abbas, Z.K.; Al-Balawi, S.M.; Darwish, D.B.E.; Ali, B.; Malik, T.; Javed, S. Selenium mitigates vanadium toxicity through enhanced nutrition, photosynthesis, and antioxidant defense in rice (Oryza sativa L.) seedlings. BMC Plant Biol. 2024, 24, 1071. [Google Scholar] [CrossRef]
- Huang, S.; Qin, H.; Jiang, D.; Lu, J.; Zhu, Z.; Huang, X. Bio-nano selenium fertilizer improves the yield, quality, and organic selenium content in rice. J. Food Compos. Anal. 2024, 132, 106348. [Google Scholar] [CrossRef]
- Kühlbrandt, W. Structure and Mechanisms of F-Type ATP Synthases. Annu. Rev. Biochem. 2019, 88, 515–549. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Siahpoosh, M.R.; Sanchez, D.H.; Schlereth, A.; Scofield, G.N.; Furbank, R.T. Modification of OsSUT1 gene expression modulates the salt response of rice Oryza sativa cv. Taipei 309. Plant Sci. 2012, 182, 101–111. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2021, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.; Hwang, Y.S.; Wang, B.; Kim, M.; Jeong, J.; Lee, C.G.; Hu, Q.; Han, D.; Jin, E. Comparative analyses of lipidomes and transcriptomes reveal a concerted action of multiple defensive systems against photooxidative stress in Haematococcus pluvialis. J. Exp. Bot. 2014, 65, 4317–4334. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Wei, G.; Liu, Z.; Chen, L.; Guo, H.; Li, Y.; Li, Y.; Dai, S.; Wang, J.; Li, W.; et al. Selenium and anthocyanins share the same transcription factors R2R3MYB and bHLH in wheat. Food Chem. 2021, 356, 129699. [Google Scholar] [CrossRef]
- Guo, L.; Liao, Y.; Deng, S.; Li, J.; Bu, X.; Zhu, C.; Zhang, W.; Cong, X.; Cheng, S.; Chen, Q.; et al. Genome-wide analysis of NAC transcription factors and exploration of candidate genes regulating selenium metabolism in Broussonetia papyrifera. Planta 2024, 260, 1. [Google Scholar] [CrossRef] [PubMed]
- Larabee, J.L.; Hocker, J.R.; Hanas, J.S. Mechanisms of inhibition of zinc-finger transcription factors by selenium compounds ebselen and selenite. J. Inorg. Biochem. 2009, 103, 419–426. [Google Scholar] [CrossRef]
- Liu, X.M.; Yuan, Z.G.; Rao, S.; Zhang, W.W.; Ye, J.B.; Cheng, S.Y.; Xu, F. Identification, characterization, and expression analysis of WRKY transcription factors in Cardamine violifolia reveal the key genes involved in regulating selenium accumulation. BMC Plant Biol. 2024, 24, 860. [Google Scholar] [CrossRef]
- Abdullah; Wani, K.I.; Hayat, K.; Naeem, M.; Aftab, T. Multifaceted role of selenium in plant physiology and stress resilience: A review. Plant Sci. 2025, 355, 112456. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Parrasia, S.; Vecchia, F.D. Selenium accumulation and metabolism in algae. Aquat. Toxicol. 2017, 189, 1–8. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.P.; Singh, P.K.; Awasthi, S.; Chakrabarty, D.; Trivedi, P.K.; Tripathi, R.D. Selenium ameliorates arsenic induced oxidative stress through modulation of antioxidant enzymes and thiols in rice (Oryza sativa L.). Ecotoxicology 2014, 23, 1153–1163. [Google Scholar] [CrossRef]
- Panda, A.; Fatnani, D.; Parida, A.K. Uptake, impact, adaptive mechanisms, and phytoremediation of heavy metals by plants: Role of transporters in heavy metal sequestration. Plant Physiol. Biochem. 2025, 221, 109578. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Role of Reactive Oxygen Species and Hormones in Plant Responses to Temperature Changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 2015, 3, 11. [Google Scholar] [CrossRef]
- Guo, Z.; Cai, L.; Liu, C.; Chen, Z.; Guan, S.; Ma, W.; Pan, G. Low-temperature stress affects reactive oxygen species, osmotic adjustment substances, and antioxidants in rice (Oryza sativa L.) at the reproductive stage. Sci. Rep. 2022, 12, 6224. [Google Scholar] [CrossRef] [PubMed]
- Toh, P.; Nicholson, J.L.; Vetter, A.M.; Berry, M.J.; Torres, D.J. Selenium in Bodily Homeostasis: Hypothalamus, Hormones, and Highways of Communication. Int. J. Mol. Sci. 2022, 23, 15445. [Google Scholar] [CrossRef]
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, R.C.; Arthur, J.R.; Beckett, G.J. Selenium and the regulation of cell signaling, growth, and survival: Molecular and mechanistic aspects. Antioxid. Redox Signal. 2002, 4, 339–351. [Google Scholar] [CrossRef]
- Chen, T.F.; Zheng, W.J.; Wong, Y.S.; Yang, F. Selenium-induced changes in activities of antioxidant enzymes and content of photosynthetic pigments in Spirulina platensis. J. Integr. Plant Biol. 2008, 50, 40–48. [Google Scholar] [CrossRef]
- Habibi, G. Effect of drought stress and selenium spraying on photosynthesis and antioxidant activity of spring barley. Acta Agric. Slov. 2013, 101, 31–39. [Google Scholar] [CrossRef]
- Song, J.; Xin, L.; Gao, F.; Liu, H.; Wang, X. Effects of Foliar Selenium Application on Oxidative Damage and Photosynthetic Properties of Greenhouse Tomato under Drought Stress. Plants 2024, 13, 302. [Google Scholar] [CrossRef]
- Quispe, A.P.V.; de Morais, E.G.; Benevenute, P.A.N.; de Sousa Lima, J.; Dos Santos, L.C.; Silva, M.A.; Chalfun-Júnior, A.; Marchiori, P.E.R.; Guilherme, L.R.G. Priming effect with selenium and iodine on broccoli seedlings: Activation of biochemical mechanisms to mitigate cold damages. Plant Physiol. Biochem. 2025, 223, 109876. [Google Scholar] [CrossRef] [PubMed]
- Moreno Rodriguez, M.J.; Cala Rivero, V.; Jiménez Ballesta, R. Selenium distribution in topsoils and plants of a semi-arid Mediterranean environment. Environ. Geochem. Health 2005, 27, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.; Garifullina, G.F.; Abdel-Ghany, S.; Kato, S.; Mihara, H.; Hale, K.L.; Burkhead, J.L.; Esaki, N.; Kurihara, T.; Pilon, M. Characterization of a NifS-like chloroplast protein from Arabidopsis. Implications for its role in sulfur and selenium metabolism. Plant Physiol. 2002, 130, 1309–1318. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Rinklebe, J.; Tsang, D.C.; Tack, F.M.; Abbasi, G.H.; Hussain, A.; Igalavithana, A.D.; Lee, B.C.; et al. Effects of selenium on the uptake of toxic trace elements by crop plants: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2531–2566. [Google Scholar] [CrossRef]
- Kidokoro, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 2022, 27, 922–935. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Sun, S.; Zhao, W.; Yang, X.; Mao, H.; Sheng, L.; Chen, Z. Utilizing transcriptomics and proteomics to unravel key genes and proteins of Oryza sativa seedlings mediated by selenium in response to cadmium stress. BMC Plant Biol. 2024, 24, 360. [Google Scholar] [CrossRef]
- Guo, G.; Wang, Y.; Zhang, B.; Yu, H.; Li, L.; Cao, G.; Chen, B.; Li, C.; Bu, F.; Teng, S.; et al. Comparative transcriptomic and metabolomic analysis reveals mechanisms of selenium-regulated anthocyanin synthesis in waxy maize (Zea mays L.). Front. Plant Sci. 2024, 15, 1466756. [Google Scholar] [CrossRef]
- Chongping, H.; Wenjie, H.; Junlin, L. Selenium-and Nano-Selenium-Mediated Cold-Stress Tolerance in Crop Plants. In Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement; Springer: Cham, Switzerland, 2022; pp. 173–190. [Google Scholar]
- Chu, J.; Yao, X.; Zhang, Z. Responses of wheat seedlings to exogenous selenium supply under cold stress. Biol. Trace Elem. Res. 2010, 136, 355–363. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, W.; Li, D.; Wang, H.; Yang, Y.; You, J.; Liu, H.; Ai, L.; Zhang, M. Combined transcriptome and metabolome analyses reveal the effects of selenium on the growth and quality of Lilium lancifolium. Front. Plant Sci. 2024, 15, 1399152. [Google Scholar] [CrossRef]
- Nazir, F.; Kumari, S.; Mahajan, M.; Khan, M.I.R. An explicit story of plant abiotic stress resilience: Overtone of selenium, plant hormones and other signaling molecules. Plant Soil. 2022, 486, 135–163. [Google Scholar] [CrossRef]
- Callejón-Leblic, B.; Selma-Royo, M.; Collado, M.C.; Gómez-Ariza, J.L.; Abril, N.; García-Barrera, T. Untargeted Gut Metabolomics to Delve the Interplay between Selenium Supplementation and Gut Microbiota. J. Proteome Res. 2022, 21, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Wallenberg, M.; Olm, E.; Hebert, C.; Björnstedt, M.; Fernandes, A.P. Selenium compounds are substrates for glutaredoxins: A novel pathway for selenium metabolism and a potential mechanism for selenium-mediated cytotoxicity. Biochem. J. 2010, 429, 85–93. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Yu, Q.; Chen, B.; Li, C.; Bu, F.; Li, J.; Peng, X.; Lu, Y. Selenium Alleviates Low-Temperature Stress in Rice by Regulating Metabolic Networks and Functional Genes. Agriculture 2025, 15, 1489. https://doi.org/10.3390/agriculture15141489
Liu N, Yu Q, Chen B, Li C, Bu F, Li J, Peng X, Lu Y. Selenium Alleviates Low-Temperature Stress in Rice by Regulating Metabolic Networks and Functional Genes. Agriculture. 2025; 15(14):1489. https://doi.org/10.3390/agriculture15141489
Chicago/Turabian StyleLiu, Naixin, Qingtao Yu, Baicui Chen, Chengxin Li, Fanshan Bu, Jingrui Li, Xianlong Peng, and Yuncai Lu. 2025. "Selenium Alleviates Low-Temperature Stress in Rice by Regulating Metabolic Networks and Functional Genes" Agriculture 15, no. 14: 1489. https://doi.org/10.3390/agriculture15141489
APA StyleLiu, N., Yu, Q., Chen, B., Li, C., Bu, F., Li, J., Peng, X., & Lu, Y. (2025). Selenium Alleviates Low-Temperature Stress in Rice by Regulating Metabolic Networks and Functional Genes. Agriculture, 15(14), 1489. https://doi.org/10.3390/agriculture15141489





