Toward Sustainable Broiler Production: Evaluating Microbial Protein as Supplementation for Conventional Feed Proteins
Abstract
1. Introduction
2. Protein Profile in Poultry Diets
3. Conventional Protein Sources in Broiler Diets: Nutritional Profiles and Utilization
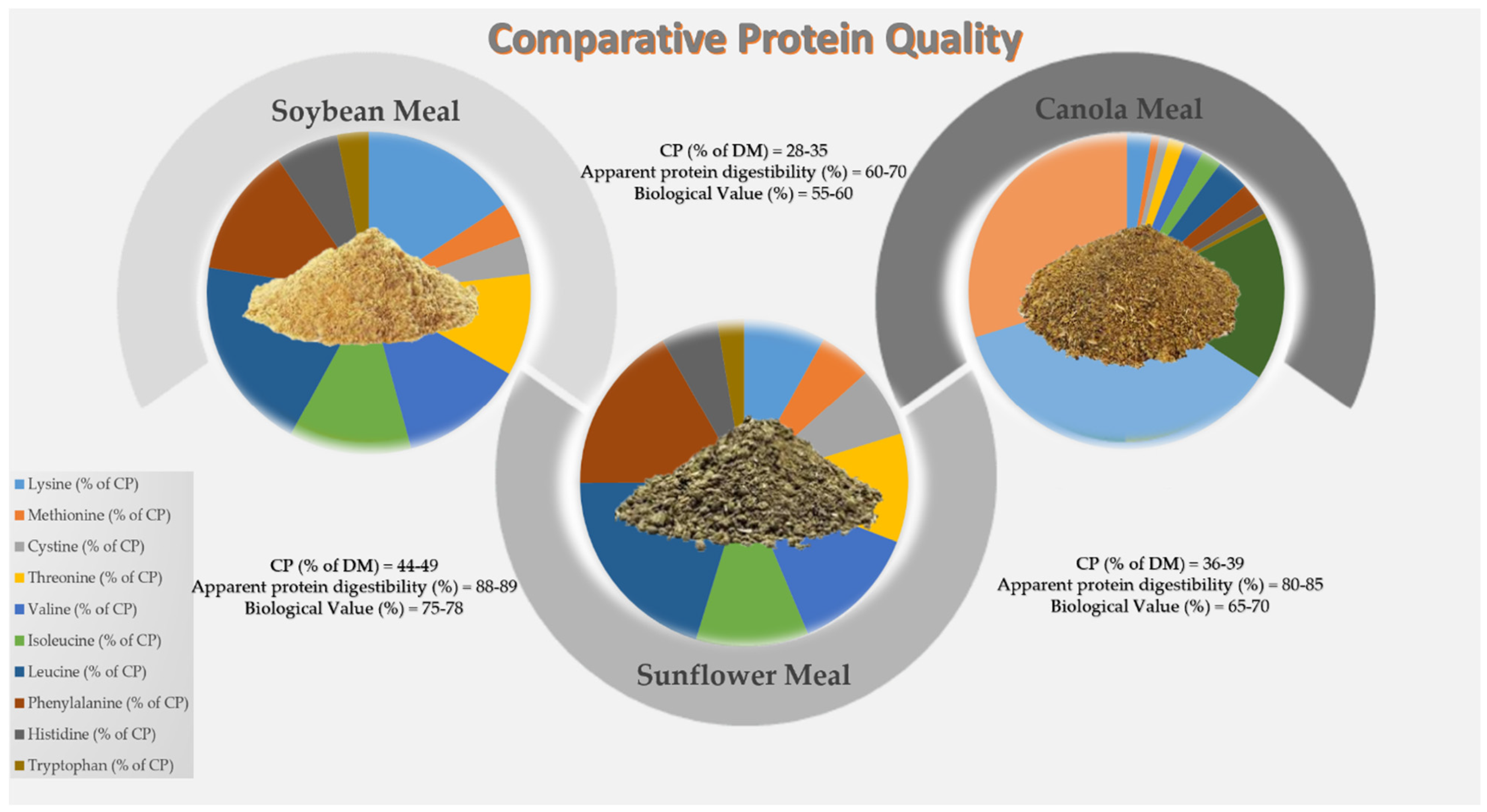
3.1. Soybean Meal
3.2. Sunflower Meal
3.3. Canola Meal
3.4. Lentils
3.5. Chickpea
4. Trends in Poultry Nutrition: Challenges and Innovations
4.1. Protein Bioproducts: Role and Application in Poultry Nutrition
4.2. Microbial Protein Bioproducts
4.2.1. Yeasts
4.2.2. Mixed Microbial Cultures
4.2.3. Brewer’s Spent Yeasts
5. Regulations and Legal Standards on the Use of Microbial Protein Sources in Animal Nutrition
- The EFSA QPS list is based at the species or group level, not the strain level.
- Some species present on the QPS list have beneficial effects, and some potential harmful effects, and this could mask pathogenic potential, especially for genera like Bacillus, Enterococcus, or even certain Lactobacillus species.
6. Assessment of the Impact of Microbial Protein Alternatives on Poultry Growth Performance and Health
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alders, R.G.; Dumas, S.E.; Rukambile, E.; Magoke, G.; Maulaga, W.; Jong, J.; Costa, R. Family Poultry: Multiple Roles, Systems, Challenges, and Options for Sustainable Contributions to Household Nutrition Security Through a Planetary Health Lens. Matern. Child Nutr. 2018, 14, e12668. [Google Scholar] [CrossRef]
- Thrane, M.; Paulsen, P.V.; Orcutt, M.W.; Krieger, T.M. Chapter 2—Soy Protein: Impacts, Production, and Applications. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 23–45. ISBN 978-0-12-802778-3. [Google Scholar]
- Nirmal, N.; Anyimadu, C.F.; Khanashyam, A.C.; Bekhit, A.E.A.; Dhar, B.K. Alternative Protein Sources: Addressing Global Food Security and Environmental Sustainability. Sustain. Dev. 2025, 33, 3958–3969. [Google Scholar] [CrossRef]
- Hallmann, A.; Rampelotto, P.H. Grand Challenges in Algae Biotechnology; Grand Challenges in Biology and Biotechnology; Springer International Publishing: Cham, Switzerland, 2020; ISBN 9783030252335. [Google Scholar]
- Astudillo, Á.; Rubilar, O.; Briceño, G.; Diez, M.C.; Schalchli, H. Advances in Agroindustrial Waste as a Substrate for Obtaining Eco-Friendly Microbial Products. Sustainability 2023, 15, 3467. [Google Scholar] [CrossRef]
- Bansfield, D. Pioneering Circular Pathways: Improved Recycling of Nutrients in Industrial Wastewater into Valueadded Products. Ph.D. Thesis, Aalto University, Espoo, Finland, 2025. [Google Scholar]
- Fremu Chollom, P.; Bede Agbo, E.; Dass Doma, U.; Julius Okojokwu, O.; Gana Yisa, A. Nutritional Value of Spent Brewers’ Yeast (Saccharomyces cerevisiae): A Potential Replacement for Soya Bean in Poultry Feed Formulation. Researcher 2017, 9, 70–74. [Google Scholar] [CrossRef]
- Nath, P.C.; Ojha, A.; Debnath, S.; Sharma, M.; Nayak, P.K.; Sridhar, K.; Inbaraj, B.S. Valorization of Food Waste as Animal Feed: A Step Towards Sustainable Food Waste Management and Circular Bioeconomy. Animals 2023, 13, 1366. [Google Scholar] [CrossRef]
- Park, Y.H.; Hamidon, F.; Rajangan, C.; Soh, K.P.; Gan, C.Y.; Lim, T.S.; Abdullah, W.N.W.; Liong, M.T. Application of Probiotics for the Production of Safe and High-Quality Poultry Meat. Korean J. Food Sci. Anim. Resour. 2016, 36, 567–576. [Google Scholar] [CrossRef]
- Hassanzadeh Seyedi, A.; Janmohammadi, H.; Mirghelenj, S.A.; Olyayee, M. Responses of Ross 308 broiler chickens to different levels of digestible valine during growth period. Anim. Sci. Res. 2019, 29, 35–47. [Google Scholar]
- Chuang, W.Y.; Hsieh, Y.C.; Lee, T.T. The Effects of Fungal Feed Additives in Animals: A Review. Animals 2020, 10, 805. [Google Scholar] [CrossRef]
- Pokludová, L.; Bureš, J. EU Policies and Regulatory Surroundings. In Antimicrobials in Livestock 1: Regulation, Science, Practice: A European Perspective; Pokludová, L., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 19–41. ISBN 978-3-030-46721-0. [Google Scholar]
- Guarino Amato, M.; Castellini, C. Adaptability Challenges for Organic Broiler Chickens: A Commentary. Animals 2022, 12, 1354. [Google Scholar] [CrossRef]
- Öztekin, S.; Anaya, K.; Yurdunuseven-Yıldız, A. Regulation of Natural Food Additives. In Natural Additives in Foods; Valencia, G.A., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 343–372. ISBN 978-3-031-17346-2. [Google Scholar]
- Mottet, A.; Tempio, G. Global Poultry Production: Current State and Future Outlook and Challenges. World’s Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). World Food and Agriculture—Statistical Yearbook 2022; Food & Agriculture Organization: Rome, Italy, 2022; ISBN 9789251369302. [Google Scholar]
- Dolberg, F. Poultry Production for Livelihood Improvement and Poverty Alleviation. In Proceedings of the Poultry in the 21st Century: Avian Influenza and Beyond, International Poultry Conference, Bangkok, Thailand, 5–7 November 2007; Volume 26. [Google Scholar]
- Magdelaine, P.; Spiess, M.P.; Valceschini, E. Poultry Meat Consumption Trends in Europe. World’s Poult. Sci. J. 2008, 64, 53–64. [Google Scholar] [CrossRef]
- Gale, F.; Dong, F. China’s Meat Consumption: Growth Potential. Available online: https://www.ers.usda.gov/publications/pub-details?pubid=106998 (accessed on 12 May 2025).
- Constantin, M.; Ignat, R.; Deaconu, E.-M.; Chiripuci, B.C. The EU Meat Market: A Focused Overview of the International Trade Flow Performance. In Proceedings of the 10th BASIQ International Conference on New Trends in Sustainable Business and Consumption, Almeria, Spain, 6–8 June 2024. [Google Scholar]
- Food and Agriculture Organization (FAO). Renewable Energy Interventions in the Wheat Landscape in Uzbekistan; FAO: Rome, Italy, 2023; ISBN 9789251380086. [Google Scholar]
- Guyomard, H.; Bouamra-Mechemache, Z.; Chatellier, V.; Delaby, L.; Détang-Dessendre, C.; Peyraud, J.-L.; Réquillart, V. Review: Why and How to Regulate Animal Production and Consumption: The Case of the European Union. Animal 2021, 15, 100283. [Google Scholar] [CrossRef]
- Boland, M.J.; Rae, A.N.; Vereijken, J.M.; Meuwissen, M.P.M.; Fischer, A.R.H.; van Boekel, M.A.J.S.; Rutherfurd, S.M.; Gruppen, H.; Moughan, P.J.; Hendriks, W.H. The Future Supply of Animal-Derived Protein for Human Consumption. Trends Food Sci. Technol. 2013, 29, 62–73. [Google Scholar] [CrossRef]
- Tudorache, M.; Ioan, C. Technological Advances and Socio-Economic Implications in the Poultry Industry—An Analysis of Current Trends in Poultry Meat. 2024. Available online: https://animalsciencejournal.usamv.ro/pdf/2024/issue_1/Art62.pdf (accessed on 12 May 2025).
- Institutul Național de Statistica. Disponibilităţile de Consum Ale Populaţiei. 2021. Available online: https://insse.ro/cms/ro/tags/disponibilitatile-de-consum-ale-populatiei (accessed on 12 May 2025).
- Caratus Stanciu, M. Research Regarding Consumers Attitude, in Relation with Poultry Meat Purchase and Consumption. Case Study Sibiu, Romania. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2020, 20, 125–130. [Google Scholar]
- Gheorghe, R.A.I.; Tapaloaga, D.; Sonea, C.; Ilie, L.I.; Tapaloaga, P.-R. Chicken Meat Production Trends in Romania—A Twelve-Year Forecast. Ann. Valahia Univ. Târgovişte. Agric. 2023, 15, 6–8. [Google Scholar] [CrossRef]
- Cercel, F.; Stroiu, M.; Alexe, P.; Ianiţchi, D. Characterization of Myofibrillar Chicken Breast Proteins for Obtain Protein Films and Biodegradable Coatings Generation. Agric. Agric. Sci. Procedia 2015, 6, 197–205. [Google Scholar] [CrossRef]
- Pingali, P.; Boiteau, J.; Choudhry, A.; Hall, A. Making Meat and Milk from Plants: A Review of Plant-Based Food for Human and Planetary Health. World Dev. 2023, 170, 106316. [Google Scholar] [CrossRef]
- de França, T.P.; Ferreira Rde, S.; Leo, R.A.R.; de Oliveira, C.H.; Dias, K.M.M.; Gomes, K.M.; Costa, L.S.; Albino, L.F.T. Effects of Carbohydrase and Phytase Enzymes Supplementation Within Low Energy Diets on Performance and Energy Utilization of Broiler Chickens. Livest. Sci. 2023, 274, 105271. [Google Scholar] [CrossRef]
- Cheng, T.K.; Hamre, M.L.; Coon, C.N. Effect of Environmental Temperature, Dietary Protein, and Energy Levels on Broiler Performance1. J. Appl. Poult. Res. 1997, 6, 1–17. [Google Scholar] [CrossRef]
- Wu, G. Dietary Requirements of Synthesizable Amino Acids by Animals: A Paradigm Shift in Protein Nutrition. J. Anim. Sci. Biotechnol. 2014, 5, 34. [Google Scholar] [CrossRef]
- Wu, G.; Li, P. The “Ideal Protein” Concept Is Not Ideal in Animal Nutrition. Exp. Biol. Med. 2022, 247, 1191–1201. [Google Scholar] [CrossRef]
- Liu, S.Y.; Macelline, S.P.; Chrystal, P.V.; Selle, P.H. Progress Towards Reduced-Crude Protein Diets for Broiler Chickens and Sustainable Chicken-Meat Production. J. Anim. Sci. Biotechnol. 2021, 12, 20. [Google Scholar] [CrossRef]
- Hudson, J.L.; Baum, J.I.; Diaz, E.C.; Børsheim, E. Dietary Protein Requirements in Children: Methods for Consideration. Nutrients 2021, 13, 1554. [Google Scholar] [CrossRef]
- de Rauglaudre, T.; Méda, B.; Fontaine, S.; Lambert, W.; Fournel, S.; Létourneau-Montminy, M.P. Meta-Analysis of the Effect of Low-Protein Diets on the Growth Performance, Nitrogen Excretion, and Fat Deposition in Broilers. Front. Anim. Sci. 2023, 4, 1214076. [Google Scholar] [CrossRef]
- Brandejs, V.; Kupcikova, L.; Tvrdon, Z.; Hampel, D.; Lichovnikova, M. Broiler Chicken Production Using Dietary Crude Protein Reduction Strategy and Free Amino Acid Supplementation. Livest. Sci. 2022, 258, 104879. [Google Scholar] [CrossRef]
- Mirzaei-Alamouti, H.; Beiranvand, A.; Abdollahi, A.; Amanlou, H.; Patra, A.K.; Aschenbach, J.R. Growth Performance, Eating Behavior, Digestibility, Blood Metabolites, and Carcass Traits in Growing-Finishing Fat-Tailed Lambs Fed Different Levels of Dietary Neutral Detergent Fiber with High Rumen Undegradable Protein. Agriculture 2021, 11, 1101. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Barkat, R.A.; Gabr, A.A.; Foda, M.A.; Noreldin, A.E.; Khafaga, A.F.; El-Sabrout, K.; et al. Potential Role of Important Nutraceuticals in Poultry Performance and Health—A Comprehensive Review. Res. Vet. Sci. 2021, 137, 9–29. [Google Scholar] [CrossRef]
- Hasan, M.S.; Crenshaw, M.A.; Liao, S.F. Dietary Lysine Affects Amino Acid Metabolism and Growth Performance, Which May Not Involve the GH/IGF-1 Axis, in Young Growing Pigs1. J. Anim. Sci. 2020, 98, skaa004. [Google Scholar] [CrossRef]
- Aviagen. Ross Broiler: Nutrition Specifications 2019. Ross. Broiler Nutr. Specif. 2019, 419, 1–10. [Google Scholar]
- Fouad, A.M.; Ruan, D.; Wang, S.; Chen, W.; Xia, W.; Zheng, C. Nutritional Requirements of Meat-Type and Egg-Type Ducks: What Do We Know? J. Anim. Sci. Biotechnol. 2018, 9, 1. [Google Scholar] [CrossRef]
- Cobb-Vantress. Cobb 500 Broiler Performance & Nutrition Supplement (2022). 2022. Available online: https://www.cobbgenetics.com/assets/Cobb-Files/2022-Cobb500-Broiler-Performance-Nutrition-Supplement.pdf (accessed on 12 May 2025).
- Langyan, S.; Khan, F.N.; Kumar, A. Advancement in Nutritional Value, Processing Methods, and Potential Applications of Pseudocereals in Dietary Food: A Review. Food Bioprocess Technol. 2024, 17, 571–590. [Google Scholar] [CrossRef]
- Dieryck, I.; Dejonghe, W.; Van Hecke, W.; Delacourt, J.; Bautil, A.; Courtin, C.M.; Vermeulen, D.; Buyse, J.; Paeshuyse, J. Toward Renewable-Based Prebiotics from Woody Biomass: Potential of Tailored Xylo-Oligosaccharides Obtained by Enzymatic Hydrolysis of Beechwood Xylan as a Prebiotic Feed Supplement for Young Broilers. Animals 2023, 13, 3511. [Google Scholar] [CrossRef]
- Zouaoui, M.; Lambert, W.; Létourneau-Montminy, M.P. Estimating Standardized Ileal Digestible Valine Requirements for Broiler Chickens Based on Two Different Meta-Analytical Selection Procedures. Anim.-Open Space 2024, 3, 100058. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Park, J.; Kim, Y.-B.; Kwon, B.-Y.; Kim, D.-H.; Song, J.-Y.; Lee, K.-W. Effects of Dietary Protein Levels on Performance, Nitrogen Excretion, and Odor Emission of Growing Pullets and Laying Hens. Poult. Sci. 2023, 102, 102798. [Google Scholar] [CrossRef]
- Oliveira, C.H.; Bernardes, R.D.; Dias, K.M.M.; Ribeiro, A.M.; Rodrigueiro, R.J.B.; Koo, B.K.; Tak, J.; Park, C.; Calderano, A.A.; Albino, L.F.T. Research Note: The Influence of Different Isoleucine: Lysine Ratios on the Growth Performance of Broiler Chickens Fed Low-Protein Diets. Poult. Sci. 2023, 102, 102270. [Google Scholar] [CrossRef]
- Millecam, J.; Khan, D.R.; Dedeurwaerder, A.; Saremi, B. Optimal Methionine plus Cystine Requirements in Diets Supplemented with L-Methionine in Starter, Grower, and Finisher Broilers. Poult. Sci. 2021, 100, 910–917. [Google Scholar] [CrossRef]
- Ciurescu, G.; Vasilachi, A.; Idriceanu, L.; Dumitru, M. Effects of Corn Replacement by Sorghum in Broiler Chickens Diets on Performance, Blood Chemistry, and Meat Quality. Ital. J. Anim. Sci. 2023, 22, 537–547. [Google Scholar] [CrossRef]
- Gheorghe, A.; Hăbeanu, M.; Lefter, N.A.; Grigore, D.M. Effect of Dietary Extruded Linseed and Walnut Meal Mixture (8:1) on Performance and Plasma Protein Profile in Weaned Piglets. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2018, 75, 121. [Google Scholar] [CrossRef]
- Koukoumaki, D.I.; Tsouko, E.; Papanikolaou, S.; Ioannou, Z.; Diamantopoulou, P.; Sarris, D. Recent Advances in the Production of Single Cell Protein from Renewable Resources and Applications. Carbon Resour. Convers. 2024, 7, 100195. [Google Scholar] [CrossRef]
- Grigore, D.M.; Ciurescu, G.; Radu, N.; Babeanu, N. Health Status, Performance and Carcass Caracteristics of Broiler Chicks Supplemented with Yeasts Bioproducts. Sci. Papers. Ser. D. Anim. Sci. 2022, 65. [Google Scholar]
- Terefe, G.; Walelgne, M.; Fekadu, D.; Kitaw, G.; Dejene, M.; Kehaliu, A.; Mekonne, B.; Habteyesus, Y. Inclusion of Sun Dried Brewer’s Spent Yeast to Improves Nutritive Value, In Vitro Digestibility and Rumen Degradability of Wheat Straw. 2022. Available online: https://www.researchgate.net/publication/363909010_Inclusion_of_sun_dried_brewer%27s_spent_yeast_to_improves_nutritive_value_in_vitro_digestibility_and_rumen_degradability_of_wheat_straw/fulltext/6334eac2ff870c55cee5459e/Inclusion-of-sun-dried-brewers-spent-yeast-to-improves-nutritive-value-in-vitro-digestibility-and-rumen-degradability-of-wheat-straw.pdf (accessed on 12 May 2025).
- Li, M.; Dong, H.; Wu, D.; Chen, H.; Qin, W.; Liu, W.; Yang, W.; Zhang, Q. Nutritional Evaluation of Whole Soybean Curd Made from Different Soybean Materials Based on Amino Acid Profiles. Food Qual. Saf. 2020, 4, 41–50. [Google Scholar] [CrossRef]
- Ciurescu, G.; Vasilachi, A.; Grosu, H. Efficacy of Microbial Phytase on Growth Performance, Carcass Traits, Bone Mineralization, and Blood Biochemistry Parameters in Broiler Turkeys Fed Raw Chickpea (Cicer arietinum L., Cv. Burnas) Diets. J. Appl. Poult. Res. 2020, 29, 171–184. [Google Scholar] [CrossRef]
- Ward, N.E. Vitamin Supplementation in Broiler Feeds and U.S. Survey on Fortification Rates; Babinszky, E.P.L., Ed.; IntechOpen: Rijeka, Croatia, 2023; p. 2. ISBN 978-1-83769-082-4. [Google Scholar]
- Liu, H.; Gishini, M.F.S.; Pope, M.; Doehring, T.; Kachroo, P.; Hildebrand, D. Comparison of the Quality of Soybean Meal and Oil by Soybean Production Origin. J. Am. Oil Chem. Soc. 2024, 101, 817–826. [Google Scholar] [CrossRef]
- Parrini, S.; Aquilani, C.; Pugliese, C.; Bozzi, R.; Sirtori, F. Soybean Replacement by Alternative Protein Sources in Pig Nutrition and Its Effect on Meat Quality. Animals 2023, 13, 494. [Google Scholar] [CrossRef]
- Grigore, D.M.; Mironeasa, S.; Ciurescu, G.; Ungureanu-Iuga, M.; Batariuc, A.; Babeanu, N.E. Carcass Yield and Meat Quality of Broiler Chicks Supplemented with Yeasts Bioproducts. Appl. Sci. 2023, 13, 1607. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Yang, G. Effects of Soybean Oligosaccharide, Stachyose, and Raffinose on Growth Performance and Cecal Microbiota in Broiler Chickens. Anim. Sci. J. 2021, 92, e13668. [Google Scholar] [CrossRef]
- Wilson, W.C.; Slingerland, M.; Baijukya, F.P.; van Zanten, H.; Oosting, S.; Giller, K.E. Integrating the Soybean-Maize-Chicken Value Chains to Attain Nutritious Diets in Tanzania. Food Secur. 2021, 13, 1595–1612. [Google Scholar] [CrossRef]
- De Oliveira, R.C.; de Souza e Silva, R.D. Increase of Agribusiness in the Brazilian Amazon: Development or Inequality? Earth 2021, 2, 1077–1100. [Google Scholar] [CrossRef]
- Puttha, R.; Venkatachalam, K.; Hanpakdeesakul, S.; Wongsa, J.; Parametthanuwat, T.; Srean, P.; Pakeechai, K.; Charoenphun, N. Exploring the Potential of Sunflowers: Agronomy, Applications, and Opportunities Within Bio-Circular-Green Economy. Horticulturae 2023, 9, 1079. [Google Scholar] [CrossRef]
- Petrenko, V.; Topalov, A.; Khudolii, L.; Honcharuk, Y.; Bondar, V. Profiling and Geographical Distribution of Seed Oil Content of Sunflower in Ukraine. Oil Crop Sci. 2023, 8, 111–120. [Google Scholar] [CrossRef]
- Tejeda, O.J.; Kim, W.K. Role of Dietary Fiber in Poultry Nutrition. Animals 2021, 11, 461. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Sunflower Meal/Cake as a Sustainable Protein Source for Global Food Demand: Towards a Zero-Hunger World. Food Hydrocoll. 2024, 147, 109329. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Alonso, E. Optimization of the Subcritical Water Treatment from Sunflower By-Product for Producing Protein and Sugar Extracts. Biomass Convers. Biorefin. 2024, 14, 1637–1650. [Google Scholar] [CrossRef]
- Demirel, C.; Kabutey, A.; Herák, D.; Hrabě, P.; Mizera, Č.; Dajbych, O. Optimizing Uniaxial Oil Extraction of Bulk Rapeseeds: Spectrophotometric and Chemical Analyses of the Extracted Oil Under Pretreatment Temperatures and Heating Intervals. Processes 2021, 9, 1755. [Google Scholar] [CrossRef]
- Biesek, J.; Kuźniacka, J.; Banaszak, M.; Kaczmarek, S.; Adamski, M.; Rutkowski, A.; Zmudzińska, A.; Perz, K.; Hejdysz, M. Growth Performance and Carcass Quality in Broiler Chickens Fed on Legume Seeds and Rapeseed Meal. Animals 2020, 10, 846. [Google Scholar] [CrossRef]
- Jabbar, A.; Tahir, M.; Naz, S.; Alrefaei, A.F.; Sultan, A.; Abdelrahaman, S.; Selvaggi, M. Enhancing Calcium and Phosphorus Utilization in Broiler Chickens Through Cholecalciferol in Sequential Calcium-Phosphorus-Deficient Diets. J. Appl. Anim. Res. 2024, 52, 2337177. [Google Scholar] [CrossRef]
- Makkar, H.; Wamatu, J.; Jones, C.; Duncan, A.; Louhaichi, M. Research and Development Work Conducted Under the Feeds and Forages Flagship of the CGIAR Research Program on Livestock. On Farm and Off-Farm Feed Utilization and Improved Management Options: A Synthesis. 2022. Available online: https://cgspace.cgiar.org/items/8f0fb599-8180-41a4-9726-f08f04e21333 (accessed on 12 May 2025).
- Mudgal, V.; Mehta, M.K.; Rane, A.S. Lentil Straw (Lens culinaris): An Alternative and Nutritious Feed Resource for Kids. Anim. Nutr. 2018, 4, 417–421. [Google Scholar] [CrossRef]
- Dhull, S.B.; Kinabo, J.; Uebersax, M.A. Nutrient Profile and Effect of Processing Methods on the Composition and Functional Properties of Lentils (Lens culinaris Medik): A Review. Legum. Sci. 2023, 5, e156. [Google Scholar] [CrossRef]
- Ciurescu, G.; Vasilachi, A.; Ropota, M.; Palade, M.; Dragomir, C. Beneficial Effects of Increasing Dietary Levels of Raw Lentil Seeds on Meat Fatty Acid and Plasma Metabolic Profile in Broiler Chickens. Indian J. Anim. Sci. 2017, 87, 1385–1390. [Google Scholar] [CrossRef]
- Liu, L.; Knight, J.D.; Lemke, R.L.; Farrell, R.E. Quantifying the Contribution of Above- and below-Ground Residues of Chickpea, Faba Bean, Lentil, Field Pea and Wheat to the Nitrogen Nutrition of a Subsequent Wheat Crop. Field Crops Res. 2024, 313, 109412. [Google Scholar] [CrossRef]
- David, L.S.; Nalle, C.L.; Abdollahi, M.R.; Ravindran, V. Feeding Value of Lupins, Field Peas, Faba Beans and Chickpeas for Poultry: An Overview. Animals 2024, 14, 619. [Google Scholar] [CrossRef]
- Idate, A.; Shah, R.; Gaikwad, V.; Kumathekar, S.; Temgire, S. A Comprehensive Review on Antinutritional Factors of Chickpea (Cicer arietinum L.). Pharma Innov. 2021, 10, 816–823. [Google Scholar] [CrossRef]
- Georgeta, C.; Vasilachi, A.; Ropotă, M. Effect of Dietary Cowpea (Vigna unguiculata [L] Walp) and Chickpea (Cicer arietinum L.) Seeds on Growth Performance, Blood Parameters and Breast Meat Fatty Acids in Broiler Chickens. Ital. J. Anim. Sci. 2022, 21, 97–105. [Google Scholar] [CrossRef]
- Levic, J.; Sredanovic, S.; Djuragic, O. Sunflower Meal Protein as a Feed for Broilers. Acta Period. Technol. 2005, 36, 3–10. [Google Scholar] [CrossRef]
- Mejicanos, G.; Sanjayan, N.; Kim, I.H.; Nyachoti, C.M. Recent Advances in Canola Meal Utilization in Swine Nutrition. J. Anim. Sci. Technol. 2016, 58, 7. [Google Scholar] [CrossRef]
- Amara, A.A.; El-Baky, N.A. Fungi as a Source of Edible Proteins and Animal Feed. J. Fungi 2023, 9, 73. [Google Scholar] [CrossRef]
- Lee, J.T.; Rochell, S.J.; Kriseldi, R.; Kim, W.K.; Mitchell, R.D. Functional Properties of Amino Acids: Improve Health Status and Sustainability. Poult. Sci. 2023, 102, 102288. [Google Scholar] [CrossRef]
- Selle, P.H.; Macelline, S.P.; Chrystal, P.V.; Liu, S.Y. A Reappraisal of Amino Acids in Broiler Chicken Nutrition. World’s Poult. Sci. J. 2023, 79, 429–447. [Google Scholar] [CrossRef]
- Taylor-Bowden, T.; Bhogoju, S.; Khwatenge, C.N.; Nahashon, S.N. The Impact of Essential Amino Acids on the Gut Microbiota of Broiler Chickens. Microorganisms 2024, 12, 693. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Sabiu, S. Interplay of Poultry–Microbiome Interactions—Influencing Factors and Microbes in Poultry Infections and Metabolic Disorders. Br. Poult. Sci. 2024, 65, 523–537. [Google Scholar] [CrossRef]
- Kishawy, A.T.; El-Wahab, R.A.A.; Eldemery, F.; Rahman, M.M.I.A.; Altuwaijri, S.; Ezz-Eldin, R.M.; Abd-Allah, E.M.; Zayed, S.; Mulla, Z.S.; El Sharkawy, R.B.; et al. Insights of Early Feeding Regime Supplemented with Glutamine and Various Levels of Omega-3 in Broiler Chickens: Growth Performance, Muscle Building, Antioxidant Capacity, Intestinal Barriers Health and Defense Against Mixed Eimeria spp Infection. Vet. Q. 2024, 44, 1–20. [Google Scholar] [CrossRef]
- Fathima, S.; Hakeem, W.G.A.l.; Shanmugasundaram, R.; Selvaraj, R.K. Effect of Arginine Supplementation on the Growth Performance, Intestinal Health, and Immune Responses of Broilers during Necrotic Enteritis Challenge. Poult. Sci. 2024, 103, 103815. [Google Scholar] [CrossRef]
- Prates, J.A.M. Heat Stress Effects on Animal Health and Performance in Monogastric Livestock: Physiological Responses, Molecular Mechanisms, and Management Interventions. Vet. Sci. 2025, 12, 429. [Google Scholar] [CrossRef]
- Zaytsoff, S.J.M.; Boras, V.F.; Uwiera, R.R.E.; Inglis, G.D. A Stress-Induced Model of Acute Necrotic Enteritis in Broiler Chickens Using Dietary Corticosterone Administration. Poult. Sci. 2022, 101, 101726. [Google Scholar] [CrossRef]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to Antibiotics for Maximizing Growth Performance and Feed Efficiency in Poultry: A Review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast protein as an easily accessible food source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Bajic, B.; Vucurovic, D.; Vasic, D.; Jevetic-Mucibabic, R.; Dodic, S. Biotechnological Production of Sustainable Microbial Protein Sources. Foods 2023, 12, 107. [Google Scholar] [CrossRef]
- Smith, V.H.; Glauber, J.W. Trade, Policy, and Food Security. Agric. Econ. 2020, 51, 159–171. [Google Scholar] [CrossRef]
- Kolawole, A.S.; Iyiola, A.O. Environmental Pollution: Threats, Impact on Biodiversity, and Protection Strategies. In Sustainable Utilization and Conservation of Africa’s Biological Resources and Environment; Izah, S.C., Ogwu, M.C., Eds.; Springer Nature: Singapore, 2023; pp. 377–409. ISBN 978-981-19-6974-4. [Google Scholar]
- Rasouli, Z.; Valverde-Pérez, B.; D’Este, M.; De Francisci, D.; Angelidaki, I. Nutrient Recovery from Industrial Wastewater as Single Cell Protein by a Co-Culture of Green Microalgae and Methanotrophs. Biochem. Eng. J. 2018, 134, 129–135. [Google Scholar] [CrossRef]
- Carranza-Méndez, R.C.; Chávez-González, M.L.; Sepúlveda-Torre, L.; Aguilar, C.N.; Govea-Salas, M.; Ramos-González, R. Production of Single Cell Protein from Orange Peel Residues by Candida Utilis. Biocatal. Agric. Biotechnol. 2022, 40, 102298. [Google Scholar] [CrossRef]
- Sunish, K.S.; Thazeem, B. Microbial Biomass. In Handbook of Biomass; Thomas, S., Hosur, M., Pasquini, D., Jose Chirayil, C., Eds.; Springer Nature: Singapore, 2023; pp. 1–24. ISBN 978-981-19-6772-6. [Google Scholar]
- Ghilardi, C.; Sanmartin Negrete, P.; Carelli, A.A.; Borroni, V. Evaluation of Olive Mill Waste as Substrate for Carotenoid Production by Rhodotorula mucilaginosa. Bioresour. Bioprocess. 2020, 7, 52. [Google Scholar] [CrossRef]
- Kaur, P.; Ghoshal, G.; Jain, A. Bio-Utilization of Fruits and Vegetables Waste to Produce β-Carotene in Solid-State Fermentation: Characterization and Antioxidant Activity. Process Biochem. 2019, 76, 155–164. [Google Scholar] [CrossRef]
- Sharma, R.; Ghoshal, G. Optimization of Carotenoids Production by Rhodotorula Mucilaginosa (MTCC-1403) Using Agro-Industrial Waste in Bioreactor: A Statistical Approach; Elsevier: Amsterdam, The Netherlands, 2020; Volume 25, ISBN 9101722779173. [Google Scholar]
- Student, M.T.; Kumar, R.R.; Omments, R.E.C.; Prajapati, A.; Blockchain, T.-A.; Ml, A.I.; Randive, P.S.N.; Chaudhari, S.; Barde, S.; Devices, E.; et al. Covariance structure analysis of health-related indicators in elderly people living at home, focusing on subjective health. Front. Neurosci. 2021, 14, 1–13. [Google Scholar]
- Sekaran, U.; Lai, L.; Ussiri, D.A.N.; Kumar, S.; Clay, S. Role of Integrated Crop-Livestock Systems in Improving Agriculture Production and Addressing Food Security—A Review. J. Agric. Food Res. 2021, 5, 100190. [Google Scholar] [CrossRef]
- Sijpestijn, G.F.; Wezel, A.; Chriki, S. Can Agroecology Help in Meeting Our 2050 Protein Requirements? Livest. Sci. 2022, 256, 104822. [Google Scholar] [CrossRef]
- Bitew, D.; Tesfaye, A.; Andualem, B. Brewing Performance Evaluation of Saccharomyces cerevisiae Strains Isolated from Ethiopian Traditional Fermented Beverages. Eur. Food Res. Technol. 2024, 250, 649–665. [Google Scholar] [CrossRef]
- Balan, I.M.; Gherman, E.D.; Gherman, R.; Brad, I.; Pascalau, R.; Popescu, G.; Trasca, T.I. Sustainable Nutrition for Increased Food Security Related to Romanian Consumers’ Behavior. Nutrients 2022, 14, 4892. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Tiwari, S.; Kumar, A.; Raman, R.K.; Kadyan, S. Review on Factors Affecting and Control of Post-Acidification in Yoghurt and Related Products. Trends Food Sci. Technol. 2021, 109, 499–512. [Google Scholar] [CrossRef]
- Huyghebaert, G.; Ducatelle, R.; Immerseel, F. Van An Update on Alternatives to Antimicrobial Growth Promoters for Broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef]
- Jones, S.W.; Karpol, A.; Friedman, S.; Maru, B.T.; Tracy, B.P. Recent Advances in Single Cell Protein Use as a Feed Ingredient in Aquaculture. Curr. Opin. Biotechnol. 2020, 61, 189–197. [Google Scholar] [CrossRef]
- Syrpas, M.; Venskutonis, P.R. Chapter 6—Algae for the Production of Bio-Based Products. In Biobased Products and Industries; Galanakis, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–243. ISBN 978-0-12-818493-6. [Google Scholar]
- Zhou, Y.-M.; Chen, Y.-P.; Guo, J.-S.; Shen, Y.; Yan, P.; Yang, J.-X. Recycling of Orange Waste for Single Cell Protein Production and the Synergistic and Antagonistic Effects on Production Quality. J. Clean. Prod. 2019, 213, 384–392. [Google Scholar] [CrossRef]
- Spalvins, K.; Ivanovs, K.; Blumberga, D. Single Cell Protein Production from Waste Biomass: Review of Various Agricultural by-Products. Agron. Res. 2018, 16, 1493–1508. [Google Scholar] [CrossRef]
- Higa, Y.; Kim, Y.S.; Altaf-Ul-Amin, M.; Huang, M.; Ono, N.; Kanaya, S. Divergence of Metabolites in Three Phylogenetically Close Monascus Species (M. pilosus, M. ruber, and M. purpureus) Based on Secondary Metabolite Biosynthetic Gene Clusters. BMC Genom. 2020, 21, 679. [Google Scholar] [CrossRef]
- Paynor, K.A.; David, E.S.; Valentino, M.J.G. Endophytic Fungi Associated with Bamboo as Possible Sources of Single Cell Protein Using Corn Cob as a Substrate. Mycosphere 2016, 7, 139–147. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef]
- Zhu, W.; He, Q.; Gao, H.; Nitayavardhana, S.; Khanal, S.K.; Xie, L. Bioconversion of Yellow Wine Wastes into Microbial Protein via Mixed Yeast-Fungus Cultures. Bioresour. Technol. 2020, 299, 122565. [Google Scholar] [CrossRef]
- Salas-Millán, J.Á.; Aguayo, E. Fermentation for Revalorisation of Fruit and Vegetable By-Products: A Sustainable Approach Towards Minimising Food Loss and Waste. Foods 2024, 13, 3680. [Google Scholar] [CrossRef]
- Aggelopoulos, T.; Katsieris, K.; Bekatorou, A.; Pandey, A.; Banat, I.M.; Koutinas, A.A. Solid State Fermentation of Food Waste Mixtures for Single Cell Protein, Aroma Volatiles and Fat Production. Food Chem. 2014, 145, 710–716. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Ajila, C.M.; Bezawada, J.; Tyagi, R.D.; Surampalli, R.Y. Food-Grade Single-Cell Protein Production, Characterization and Ultrafiltration Recovery of Residual Fermented Whey Proteins from Whey. Food Bioprod. Process. 2016, 99, 156–165. [Google Scholar] [CrossRef]
- Hashem, M.; Al-Qahtani, M.S.; Alamri, S.A.; Moustafa, Y.S.; Lyberatos, G.; Ntaikou, I. Valorizing Food Wastes: Assessment of Novel Yeast Strains for Enhanced Production of Single-Cell Protein from Wasted Date Molasses. Biomass Convers. Biorefin. 2022, 12, 4491–4502. [Google Scholar] [CrossRef]
- Kornochalert, N.; Kantachote, D.; Chaiprapat, S.; Techkarnjanaruk, S. Use of Rhodopseudomonas Palustris P1 Stimulated Growth by Fermented Pineapple Extract to Treat Latex Rubber Sheet Wastewater to Obtain Single Cell Protein. Ann. Microbiol. 2014, 64, 1021–1032. [Google Scholar] [CrossRef]
- Kurbanoglu, E.B.; Algur, O.F. Single-Cell Protein Production from Ram Horn Hydrolysate by Bacteria. Bioresour. Technol. 2002, 85, 125–129. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Mrabti, H.N.; El Hachlafi, N.; El Kamili, T.; Elbouzidi, A.; Abdallah, E.M.; Flouchi, R.; Assaggaf, H.; Qasem, A.; Zengin, G.; et al. Integrated Analysis of Antimicrobial, Antioxidant, and Phytochemical Properties of Cinnamomum verum: A Comprehensive In vitro and In silico Study. Biochem. Syst. Ecol. 2023, 110, 104700. [Google Scholar] [CrossRef]
- Wang, J.P.; Kim, J.D.; Kim, J.E.; Kim, I.H. Amino Acid Digestibility of Single Cell Protein from Corynebacterium ammoniagenes in Growing Pigs. Anim. Feed. Sci. Technol. 2013, 180, 111–114. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Tewari, A. Effect of Soda Ash Industry Effluent on Protein Content of Two Green Seaweeds. J. Hazard. Mater. 2008, 151, 559–561. [Google Scholar] [CrossRef]
- Gressler, V.; Yokoya, N.S.; Fujii, M.T.; Colepicolo, P.; Filho, J.M.; Torres, R.P.; Pinto, E. Lipid, Fatty Acid, Protein, Amino Acid and Ash Contents in Four Brazilian Red Algae Species. Food Chem. 2010, 120, 585–590. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Sukhikh, S.; Kalashnikova, O.; Ivanova, S.; Kashirskikh, E.; Prosekov, A.; Michaud, P.; Babich, O. Food Proteins: Potential Resources. Sustainability 2023, 15, 5863. [Google Scholar] [CrossRef]
- Gharib, F.A.E.L.; Osama, K.; El Sattar, A.M.A.; Ahmed, E.Z. Impact of Chlorella vulgaris, Nannochloropsis salina, and Arthrospira platensis as Bio-Stimulants on Common Bean Plant Growth, Yield and Antioxidant Capacity. Sci. Rep. 2024, 14, 1398. [Google Scholar] [CrossRef]
- Spínola, M.P.; Costa, M.M.; Prates, J.A.M. Enhancing Digestibility of Chlorella Vulgaris Biomass in Monogastric Diets: Strategies and Insights. Animals 2023, 13, 1017. [Google Scholar] [CrossRef]
- Binati, R.L.; Salvetti, E.; Bzducha-Wróbel, A.; Bašinskienė, L.; Čižeikienė, D.; Bolzonella, D.; Felis, G.E. Non-Conventional Yeasts for Food and Additives Production in a Circular Economy Perspective. FEMS Yeast Res. 2021, 21, foab052. [Google Scholar] [CrossRef]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces Marxianus: An Emerging Yeast Cell Factory for Applications in Food and Biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Wang, Q.; Zhang, F.; Zhang, S.-C.; Chi, Z.-M.; Madzak, C. Direct Conversion of Inulin into Single Cell Protein by the Engineered Yarrowia lipolytica Carrying Inulinase Gene. Process Biochem. 2011, 46, 1442–1448. [Google Scholar] [CrossRef]
- Tullio, V. Yeast Genomics and Its Applications in Biotechnological Processes: What Is Our Present and Near Future? J. Fungi 2022, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Ienczak, J.L.; de Oliveira Pereira, I.; da Silveira, J.M. Utilization of Saccharomyces cerevisiae as a Source of Natural Food Additives. In Natural Additives in Foods; Valencia, G.A., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 185–214. ISBN 978-3-031-17346-2. [Google Scholar]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces cerevisiae and Its Industrial Applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Thiviya, P.; Gamage, A.; Kapilan, R.; Merah, O.; Madhujith, T. Single Cell Protein Production Using Different Fruit Waste: A Review. Separations 2022, 9, 178. [Google Scholar] [CrossRef]
- Di Fidio, N.; Minonne, F.; Antonetti, C.; Galletti, A.M.R. Cutaneotrichosporon oleaginosus: A Versatile Whole-Cell Biocatalyst for the Production of Single-Cell Oil from Agro-Industrial Wastes. Catalysts 2021, 11, 1291. [Google Scholar] [CrossRef]
- Moraba Legodi, L.; Maureen Moganedi, K.L. Evaluation of Oleaginous Yeasts Isolated from Lignocellulosic Waste, Sugarcane Bagasse as a Potential Source for Single Cell Oil Production and Other Biochemical Metabolites. Food Sci. 2022, 1–19. [Google Scholar] [CrossRef]
- Ciurea, C.N.; Kosovski, I.-B.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and Candidiasis—Opportunism Versus Pathogenicity: A Review of the Virulence Traits. Microorganisms 2020, 8, 857. [Google Scholar] [CrossRef]
- Gottardi, D.; Siroli, L.; Vannini, L.; Patrignani, F.; Lanciotti, R. Recovery and Valorization of Agri-Food Wastes and by-Products Using the Non-Conventional Yeast Yarrowia lipolytica. Trends Food Sci. Technol. 2021, 115, 74–86. [Google Scholar] [CrossRef]
- Tesfaw, A.; Oner, E.T.; Assefa, F. Evaluating Crude Whey for Bioethanol Production Using Non-Saccharomyces Yeast, Kluyveromyces marxianus. SN Appl. Sci. 2021, 3, 42. [Google Scholar] [CrossRef]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single Cell Protein: Sources, Mechanism of Production, Nutritional Value and Its Uses in Aquaculture Nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Sakarika, M.; Candry, P.; Depoortere, M.; Ganigué, R.; Rabaey, K. Impact of Substrate and Growth Conditions on Microbial Protein Production and Composition. Bioresour. Technol. 2020, 317, 124021. [Google Scholar] [CrossRef] [PubMed]
- Myint, K.T.; Otsuka, M.; Okubo, A.; Mitsuhashi, R.; Oguro, A.; Maeda, H.; Shigeno, T.; Sato, K.; Nakajima-Kambe, T. Isolation and Identification of Flower Yeasts for the Development of Mixed Culture to Produce Single-Cell Protein from Waste Milk. Bioresour. Technol. Rep. 2020, 10, 100401. [Google Scholar] [CrossRef]
- Moravej, R.; Hammoodi Alameri, K.A.; Nowrouzi, S. The Potential of Kluyveromyces marxianus in Mixed Culture to Produce Single-Cell Proteins from Whey and Reduce the Biological Oxygen Demand. Ind. Biotechnol. 2024, 20, 236–247. [Google Scholar] [CrossRef]
- Demirgül, F.; Ömer, Ş.; Fatih, B.; Enes, D.; Sağdıç, O. Production and Characterization of Yeast Extracts Produced by Saccharomyces cerevisiae, Saccharomyces boulardii and Kluyveromyces marxianus. Prep. Biochem. Biotechnol. 2022, 52, 657–667. [Google Scholar] [CrossRef]
- Olajire, A.A. The Brewing Industry and Environmental Challenges. J. Clean. Prod. 2020, 256, 102817. [Google Scholar] [CrossRef]
- Taylor, R.P.; Jones, C.L.W.; Laing, M.; Dames, J. The Potential Use of Treated Brewery Effluent as a Water and Nutrient Source in Irrigated Crop Production. Water Resour. Ind. 2018, 19, 47–60. [Google Scholar] [CrossRef]
- Pasquet, P.L.; Villain-Gambier, M.; Trébouet, D. By-Product Valorization as a Means for the Brewing Industry to Move Toward a Circular Bioeconomy. Sustainability 2024, 16, 3472. [Google Scholar] [CrossRef]
- Simate, G.S.; Cluett, J.; Iyuke, S.E.; Musapatika, E.T.; Ndlovu, S.; Walubita, L.F.; Alvarez, A.E. The Treatment of Brewery Wastewater for Reuse: State of the Art. Desalination 2011, 273, 235–247. [Google Scholar] [CrossRef]
- Karlović, A.; Jurić, A.; Ćorić, N.; Habschied, K.; Krstanović, V.; Mastanjević, K. By-Products in the Malting and Brewing Industries-Re-Usage Possibilities. Fermentation 2020, 6, 82. [Google Scholar] [CrossRef]
- Hejna, A. More than Just a Beer—The Potential Applications of by-Products from Beer Manufacturing in Polymer Technology. Emergent Mater. 2022, 5, 765–783. [Google Scholar] [CrossRef]
- Afek, A.A.; Carly, D.Z.S.; Jong, N.E. A Field Survey to Assess the Consumption of Nkang for Standardization and Valorization in the North-West Region of Cameroon. Green Sustain. Chem. 2021, 11, 107–123. [Google Scholar] [CrossRef]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Kabir, M.F.; Ju, L.-K. On Optimization of Enzymatic Processes: Temperature Effects on Activity and Long-Term Deactivation Kinetics. Process Biochem. 2023, 130, 734–746. [Google Scholar] [CrossRef]
- Morgan, D.R.; Thomas Lane, E.; Styles, D. Crafty Marketing: An Evaluation of Distinctive Criteria for “Craft” Beer. Food Rev. Int. 2022, 38, 913–929. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.-P.; Hubinger, M.D. Spent Brewer’s Yeast as a Source of High Added Value Molecules: A Systematic Review on Its Characteristics, Processing and Potential Applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef]
- Gokulakrishnan, M.; Kumar, R.; Ferosekhan, S.; Siddaiah, G.M.; Nanda, S.; Pillai, B.R.; Swain, S.K. Bio-Utilization of Brewery Waste (Brewer’s Spent Yeast) in Global Aquafeed Production and Its Efficiency in Replacing Fishmeal: From a Sustainability Viewpoint. Aquaculture 2023, 565, 739161. [Google Scholar] [CrossRef]
- Timira, V.; Chen, X.; Zhou, P.; Wu, J.; Wang, T. Potential Use of Yeast Protein in Terms of Biorefinery, Functionality, and Sustainability in Food Industry. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13326. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C.; Park, S. Advances in the Valorization of Spent Brewer’s Yeast. Innov. Food Sci. Emerg. Technol. 2020, 62, 102350. [Google Scholar] [CrossRef]
- Zeko-Pivač, A.; Habschied, K.; Kulisic, B.; Barkow, I.; Tišma, M. Valorization of Spent Brewer’s Yeast for the Production of High-Value Products, Materials, and Biofuels and Environmental Application. Fermentation 2023, 9, 208. [Google Scholar] [CrossRef]
- Lohan, N.; Sharma, S.C. Use of Yeast in the Welfare of Human and Their Applications. In Role of Microbes in Sustainable Development: Human Health and Diseases; Sobti, R.C., Kuhad, R.C., Lal, R., Rishi, P., Eds.; Springer Nature: Singapore, 2023; pp. 653–665. ISBN 978-981-99-3126-2. [Google Scholar]
- Cheli, F.; Gallo, R.; Battaglia, D.; Dell’Orto, V. EU Legislation on Feed Related Issues: An Update. Ital. J. Anim. Sci. 2013, 12, 295–312. [Google Scholar] [CrossRef]
- Meyer, A.H. Risk Analysis in Accordance with Article 6, Regulation (EC) No. 178/2002. Eur. Food Feed. Law Rev. 2006, 1, 146–153. [Google Scholar]
- Kavanagh, G.; Pentieva, K.; Kennedy, J.; Moran, C.A. The Impact of Regulation (EC) 767/2009 on the Practice of Feed Advertising in Europe. J. Appl. Anim. Nutr. 2012, 1, e4. [Google Scholar] [CrossRef]
- European, P.; Europene, C.U.; European, A.P.; European, D.P.; Oficial, J.; European, D.P.; Consiliului, D. Jurnalul Oficial al Comunităților Europene. 2002. Available online: https://eur-lex.europa.eu/oj/direct-access.html?locale=ro (accessed on 12 May 2025).
- Ardizzone, M.; Paoletti, C.; Waigmann, E. Explanatory Note on the Selection of Forage Material Suitable for the Risk Assessment of GM Feed of Plant Origin. EFSA Support. Publ. 2018, 15, 1366E. [Google Scholar] [CrossRef][Green Version]
- Leuschner, R.G.K.; Robinson, T.P.; Hugas, M.; Cocconcelli, P.S.; Richard-Forget, F.; Klein, G.; Licht, T.R.; Nguyen-The, C.; Querol, A.; Richardson, M.; et al. Qualified Presumption of Safety (QPS): A Generic Risk Assessment Approach for Biological Agents Notified to the European Food Safety Authority (EFSA). Trends Food Sci. Technol. 2010, 21, 425–435. [Google Scholar] [CrossRef]
- Varzakas, T.; Smaoui, S. Global Food Security and Sustainability Issues: The Road to 2030 from Nutrition and Sustainable Healthy Diets to Food Systems Change. Foods 2024, 13, 306. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 741/2003 of 28 April 2003 Amending Regulation (EC) No 1555/96 as Regards the Trigger Levels for Additional Duties on Cherries, Other Than Sour Cherries. 2003. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:106:0014:0015:EN:PDF (accessed on 12 May 2025).
- European Commission No 68/2013 of 16 January 2013 on the Catalogue of Feed Materials Text with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/reg/2013/68/oj/eng (accessed on 12 May 2025).
- Ciurescu, G.; Dumitru, M.; Gheorghe, A. Use of Brewer’s Yeast (Saccharomyces cerevisiae) in Broiler Feeds to Replace Corn Gluten Meal with or Without Probiotic Additives. Arch. Zootech. 2021, 24, 66–83. [Google Scholar] [CrossRef]
- Roy, B.C.; Ray, B.C. Potentiality of Saccharomyces cerevisiae in Replacing Antibiotic Growth Promoters on Growth, Gut Microbiology, Histology, and Serum Antibody Titers of Commercial Broilers. J. Appl. Poult. Res. 2023, 32, 100352. [Google Scholar] [CrossRef]
- Maoba, S.; Ogbuewu, I.P.; Oguttu, J.W.; Mbajiorgu, C.A. Haematological Profiles of Indigenous Boschveld Chickens on Probiotic-Yeast (Saccharomyces cerevisiae) Supplementation. Comp. Clin. Path. 2021, 30, 293–299. [Google Scholar] [CrossRef]
- Lin, J.; Comi, M.; Vera, P.; Alessandro, A.; Qiu, K.; Wang, J.; Wu, S.; Qi, G.; Zhang, H. Effects of Saccharomyces cerevisiae Hydrolysate on Growth Performance, Immunity Function, and Intestinal Health in Broilers. Poult. Sci. 2023, 102, 102237. [Google Scholar] [CrossRef]
- Attia, Y.A.; Basiouni, S.; Abdulsalam, N.M.; Bovera, F.; Aboshok, A.A.; Shehata, A.A.; Hafez, H.M. Alternative to Antibiotic Growth Promoters: Beneficial Effects of Saccharomyces cerevisiae and/or Lactobacillus acidophilus Supplementation on the Growth Performance and Sustainability of Broilers’ Production. Front. Vet. Sci. 2023, 10, 1259426. [Google Scholar] [CrossRef] [PubMed]
- Ahiwe, E.; Kadurumba, O.; Princewill, O.; Ejiofor, I.; Iwuji, T.; Odoemelam, V.; Etuk, I.; Emenalom, O. Performance, Haematology, and Blood Chemistry of Broiler Chickens Fed Diets Having Reduced Energy and Protein with or Without Baker’s Yeast (Saccharomyces cerevisiae). Comp. Clin. Path. 2024, 33, 577–584. [Google Scholar] [CrossRef]
- Alagbe, E.O.; Schulze, H.; Adeola, O. Growth Performance, Nutrient Digestibility, Intestinal Morphology, Cecal Mucosal Cytokines and Serum Antioxidant Responses of Broiler Chickens to Dietary Enzymatically Treated Yeast and Coccidia Challenge. J. Anim. Sci. Biotechnol. 2023, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Ababor, S.; Tamiru, M.; Alkhtib, A.; Wamatu, J.; Kuyu, C.G.; Teka, T.A.; Terefe, L.A.; Burton, E. The Use of Biologically Converted Agricultural Byproducts in Chicken Nutrition. Sustainability 2023, 15, 14562. [Google Scholar] [CrossRef]

| Item | SBM | SFM | CM | Lentils | Chickpea |
|---|---|---|---|---|---|
| CP% | 44–49 | 34–44 | 36.5 | 26.7 | 26.1 |
| ME [MJ/kg] | 20.1 | 12.7 | 18.6 | 12.7 | 12.5 |
| Amino acid content % | |||||
| Lysine | 2.74 | 1.18 | 2.13 | 1.81 | 1.84 |
| Methionine | 0.6 | 0.72 | 0.7 | 0.79 | 1.14 |
| Cysteine | 0.63 | 0.55 | 0.82 | 0.15 | |
| Threonine | 1.72 | 1.21 | 1.54 | 1.01 | 1.22 |
| Tryptophan | 0.59 | 0.45 | 0.48 | - | - |
| Arginine | 3.28 | 2.68 | 2.38 | 2.01 | 1.78 |
| Glycine | 1.86 | 1.92 | 1.77 | 0.92 | 0.73 |
| Serine | 2.25 | 1.40 | 1.44 | 1.25 | 0.97 |
| Histidine | 1.17 | 0.82 | 1.22 | - | 2 |
| Isoleucine | 2.13 | 1.47 | 1.25 | 0.91 | 1.2 |
| Leucine | 3.4 | 2.12 | 2.22 | 1.92 | 1.83 |
| Phenylalanine | 2.22 | 1.50 | 1.46 | 1.24 | 1.39 |
| Tyrosine | 1.62 | 0.81 | 0.9 | 0.63 | 0.71 |
| Valine | 2.19 | 1.78 | 1.78 | 1.05 | 1.15 |
| References | [80] | [80] | [81] | [75] | [79] |
| Birds’ Health Status | Potential Effects on Digestibility | Protein Metabolism | References |
|---|---|---|---|
| Boilers with homeostatic status | Efficient digestion and nutrient absorption with optimal enzymatic activity and balanced gut flora. | High protein synthesis rates, efficient amino acid utilization, normal nitrogen retention. | [85] |
| Presence of coccidiosis infection | Could present damaged intestinal lining, reduced nutrient absorption and impaired enzyme secretion. | Increased protein catabolism, reduced growth performance, poor feed conversion. | [86] |
| Broilers with necrotic enteritis | Might present inflammation of gut mucosa, with decreased villus height and impaired digestion. | Increased muscle breakdown lowered protein deposition, elevated plasma uric acid levels. | [87] |
| Broilers infected with Clostridium perfringens | Disruption of gut microbiota with increased digesta viscosity and reduced digestibility. | Altered nitrogen metabolism impaired amino acid absorption. | [88] |
| Forage mycotoxin exposure | It might inhibit the digestive enzymes’ activities and damage the intestinal epithelium surface. | Inhibition of protein synthesis, increased liver stress decreased nitrogen retention. | [89] |
| Broilers suffering heat stress | Could alter the gut permeability, with direct effects of reducing feed intake; also enzyme denaturation. | Increased protein breakdown, reduced protein accretion, impaired muscle development. | [90] |
| Subclinical inflammation or stress on broilers | Present as mild reduction in nutrient digestibility with subtle shifts in gut microbiota. | Redirection of amino acids toward immune response, reduced growth rate. | [91] |
| Use of antibiotics or probiotics in broilers’ nutrition | Might improve nutrient digestibility through the stabilization of gut flora. | Enhanced protein metabolism, improved nitrogen utilization and feed efficiency | [92] |
| Microorganism | Microorganism Nutrient Substrate | References |
|---|---|---|
| Fungi | ||
| Aspergillus oryzae | organic | [111] |
| Aspergillus ochraceus | organic | [112] |
| Cladosporium cladosporioides | organic | [93] |
| Monascus ruber | organic | [113] |
| Penicillium citrinum | organic | [114] |
| Yeast | ||
| Saccharomyces cerevisiae | organic | [115] |
| Candida utilis | organic | [116] |
| Kefir sp. | organic | [117] |
| Kluyveromyces marxianus | organic | [118,119] |
| Yarrowia lipolytica | organic | |
| Hanseniaspora uvarum | organic | [120] |
| Bacteria | ||
| Rhodopseudomonas blastica | mix | [121] |
| Escherichia coli | organic | [122] |
| Bacillus subtilis sp. | organic | [123] |
| Bacillus cereus | organic | [122] |
| Corynebacterium ammoniagenes | organic | [124] |
| Algae | ||
| Ulva fasciata | mix | [125] |
| Laurencia intricate | mix | [126] |
| Chlorella sorokiniana | mix | [127] |
| Arthrospira platensis | mix | [128] |
| Chlorella sp. | mix | [129] |
| References | Level (% in Diet) | Body Weight Gain (g/bird) | Feed Conversion Ratio | Immune Markers/Blood Markers | Other Effects/Remarks |
|---|---|---|---|---|---|
| [172] | SC: 0, 25, and 50%, to replace corn gluten meal (CGM) | 25% had comparable body weight (BWG), feed intake (FI), and feed conversion ratio (FCR) to the birds fed only CGM. | - | Improved gut morphology | |
| [173] | SC: 20 g/100 kg feed | Significant improvement in body weight, body weight gain, feed efficiency, and carcass yield compared to that of the control. | Antibody titer against Newcastle disease (ND) and infectious bronchitis disease (IBD) was significantly increased in PB and AGP groups. | Optimal performance, improved blood profile | |
| [53] | SC: 0, 0.6, 1 and 1.3 g/kg feed | Having growth-promoting and product-quality-enhancing benefits, when fed up till 0.6 g/kg feed. | - | Yeast supplementation could synergically enhance meat quality attributes and might positively modulate the consumer’s preference, increasing meat moisture, lightness, redness, and decreasing the browning index. | |
| [174] | SC: 2.5, 5.0, 7.5, 10.0 and 12.5 g/kg feed | - | When fed with SC: 7.5 or 10.0 g/kg feed, had improved (p < 0.05) packed cell volume (PCV), hemoglobin (Hb), mean cell hemoglobin (MCH) and mean cell volume (MCV) compared to birds. | Balanced and improved blood profile. | |
| [175] | SC: 500 mg/kg in starter and grower phase, and 250 mg/kg in finisher phase | SC promoted growth during d 15 to 28 (p < 0.05) | Significantly increased the jejunum villus height (VH) and the ratio of villus height to crypt depth (VCR) of jejunum, and decreased the crypt depth (CD) of ileum (p < 0.05). | Stronger performance and overall heath | |
| [175,176] | SC: at a 1 g/kg diet and 2 g/Kg feed | SC group resulted in a better (p < 0.05) feed conversion rate (FCR) than the control group | Meat and liver cholesterol, as well as the cholesterol-to-lipid ratio of meat and liver, were significantly decreased (p < 0.05) in SC. | Boost in growth and health performance | |
| [176] | SC and Lactobacillus acidophillus | Mixed supplementation group resulted in a better (p < 0.05) feed conversion rate (FCR) than the control group | ↑ Lactobacillus spp., ↓ E. coli GUT populations. | Improved the gut flora, enhanced gut health | |
| [177] | Baker’s yeast: 0, 0.20%, 0.40% dietary feed | No or lowest supplementation affected negatively (p < 0.05) the average daily feed intake (ADFI), final live weight (FLW), average daily weight gain (ADWG), and FCR compared to those in the group supplemented with 0.40% feed. | ↑ PCV, ↑ serum albumin. | There were no significant (p > 0.05) differences in white blood cell, red blood cell, hemoglobin, lymphocyte, heterophils, H/L ratio, platelets, mean corpuscular volume, mean corpuscular hemoglobin concentration, and mean corpuscular hemoglobin values across the dietary groups. | |
| [178] | 0, 1, or 2 g/kg feed | - | Increased (p < 0.05) the ileal villus height-to-crypt depth ratio, and ileal goblet cell density in broiler chickens. | SC enhanced nutrient utilization and augmented intestinal development in broiler chickens. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigore, D.-M.; Mircea, M.-L.; Pogurschi, E.N. Toward Sustainable Broiler Production: Evaluating Microbial Protein as Supplementation for Conventional Feed Proteins. Agriculture 2025, 15, 1486. https://doi.org/10.3390/agriculture15141486
Grigore D-M, Mircea M-L, Pogurschi EN. Toward Sustainable Broiler Production: Evaluating Microbial Protein as Supplementation for Conventional Feed Proteins. Agriculture. 2025; 15(14):1486. https://doi.org/10.3390/agriculture15141486
Chicago/Turabian StyleGrigore, Daniela-Mihaela, Maria-Luiza Mircea, and Elena Narcisa Pogurschi. 2025. "Toward Sustainable Broiler Production: Evaluating Microbial Protein as Supplementation for Conventional Feed Proteins" Agriculture 15, no. 14: 1486. https://doi.org/10.3390/agriculture15141486
APA StyleGrigore, D.-M., Mircea, M.-L., & Pogurschi, E. N. (2025). Toward Sustainable Broiler Production: Evaluating Microbial Protein as Supplementation for Conventional Feed Proteins. Agriculture, 15(14), 1486. https://doi.org/10.3390/agriculture15141486








