Abstract
Nano-titanium dioxide (nano-TiO2) can alleviate oxidative damage in plants subjected to abiotic stress, interfere with related gene expression, and change metabolite content. Polylactic acid (PLA) microplastics can inhibit plant growth, induce oxidative stress in plant cells, and alter the biophysical properties of rhizosphere soil. In this study, untargeted metabolomics (LC-MS) and RNA-seq sequencing were performed on radish root cells exposed to nano-TiO2 and PLA. The results showed that nano-TiO2 alleviated the growth inhibition of radish roots induced by PLA. Nano-TiO2 alleviated PLA-induced oxidative stress, and the activities of SOD and POD were decreased by 28.6% and 36.0%, respectively. A total of 1673 differentially expressed genes (DEGs, 844 upregulated genes, and 829 downregulated genes) were detected by transcriptome analysis. Metabolomics analysis showed that 5041 differential metabolites were involved; they mainly include terpenoids, fatty acids, alkaloids, shikimic acid, and phenylpropionic acid. Among them, phenylpropanoid biosynthesis as well as flavone and flavonol biosynthesis were the key metabolic pathways. This study demonstrates that nano-TiO2 mitigates PLA phytotoxicity in radish via transcriptional and metabolic reprogramming of phenylpropanoid biosynthesis. These findings provide important references for enhancing crop resilience against pollutants and underscore the need for ecological risk assessment of co-existing novel pollutants in agriculture.
1. Introduction
Nanomaterials have three distinctive effects: the surface effect, the small size effect, and the macroscopic quantum tunneling effect [1]. Due to the excellent properties of nanomaterials, they are mixed with fertilizers or pesticides and directly applied to crops, or the nanoparticles are absorbed by crops in the waste treatment process [2]. Nano-TiO2 is among the most prevalent nanoparticles [3]. It exhibits excellent photocatalytic performance and ultraviolet protection ability, which makes it widely used in photocatalysis, atmospheric protection, antibacterial sterilization, cosmetics, and food preservation [4].
Plastics are widely used in every aspect of daily life owing to their affordability, durability, light weight, and malleability [5]. By 2025, it is anticipated that 11 billion tons of plastic waste will remain in diverse environments [6]. Plastics, when exposed to environmental factors such as ultraviolet radiation and mechanical abrasion, gradually degrade into microplastics with diameters smaller than 5 mm. Microplastics are emerging as one of the pollution sources in various environments [7]. They have been detected in plants, animals, and even in humans [8]. Due to their small diameter and light weight, microplastics can readily accumulate in ecosystems. In agriculture, due to long-term residues of mulch, sewage irrigation, application of agricultural sludge, use of organic fertilizer, and atmospheric deposition, microplastics can enter plants through the root system and aboveground parts [9]. Once inside plants, they may induce oxidative stress, disrupt photosynthesis, hinder nutrient uptake, and modify gene expression [10]. Polylactic acid (PLA) is a kind of polyester polymer synthesized by polymerization of lactic acid. It is one of the common types of plastics.
In complex agricultural ecosystems, the combination of nanoparticles and microplastics may have certain effects on plants. For instance, the combined toxic effects of microplastics and nano-zinc oxide on lettuce germination are antagonistic [11]. Nano-TiO2 alleviates the toxicity of microplastics on maize [12]. Microplastics exacerbate the toxicity of nano-iron oxide on lettuce [13]. These findings suggest that the combined effects of nanoparticles and microplastics on plants are affected by various factors, including plant species, particle type, and environmental conditions. Thus, researching the combined effects of nanoparticles and microplastics on plants is a crucial issue that demands immediate attention. At present, there are few studies on the toxicity of nano-TiO2 and PLA on crops. This emphasizes the necessity of studying composite pollution as a new environmental paradigm, especially regarding different microplastic–pollutant combinations [14].
Radish (Raphanus sativus L.) belongs to the cruciferous family and is widely cultivated in our country as an important agricultural vegetable crop. Radish has developed a rhizome and can be used as an ecological evaluation plant according to seed germination and root and sprout growth [15].
This study used radish as the test plant to explore its response mechanisms to combined exposure to nano-TiO2, PLA, and nano-TiO2 + PLA composites. Through transcriptomics and metabolomics analysis, differentially expressed genes (DEGs) and metabolites (DEM) were identified in key metabolic pathways under composite pollution. By concentrating on molecular-level alterations, this study aims to clarify the mechanisms through which nano-TiO2 and PLA, either individually or in combination, affect radish growth and stress adaptation. These findings offer crucial references for improving crop resilience to pollutants and highlight the necessity of ecological risk assessment for co-existing novel pollutants in agriculture.
2. Materials and Methods
2.1. Experimental Design
Radish seeds (Raphanus sativus L.) were obtained from the Runda Agricultural Science and Technology Development Center (Shenyang, China). The rutile nano-TiO2 particles (size 30 nm, purity > 99.8%) and PLA (size 60 μm, purity > 99.8%) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). The nano-TiO2 used in this study was the anatase type. Under the electron microscope, the size and particle diameter of nano-TiO2 and PLA were basically consistent with the data provided by the company (SEM, Hitachi SU3500, Tokyo, Japan). SEM images showed that the approximately 60 μm PLA particles were irregularly spherical with a rough surface, implying that the particles had a large specific surface area. This experiment used three treatments (nano-TiO2, PLA, nano-TiO2 + PLA) and one blank control group (CK) (Supplementary Figure S1).
2.2. Cultivation of Radish
Flotation was used to screen for plump radish seeds. The screened seeds were soaked in a 5% hydrogen peroxide solution for 3 min. Subsequently, they were rinsed three times with sterile water and placed on dry filter paper. Two layers of dry filter paper were placed at the bottom of a sterile Petri dish. Using disinfected tweezers, 25 radish seeds were evenly distributed at the bottom of each Petri dish. A total of 5 mL of sterile water was added to the blank group. A total of 5 mL nano-TiO2 or PLA was added to each petri dish of the single experimental group. A total of 5 mL nano-TiO2 and PLA mixture was added to each petri dish of the composite contaminant experimental group. The specific experimental concentrations are detailed in Table 1. Sterile water was used as the solvent to prepare the above solutions. Prior to use, the solutions were placed in an ultrasonic water bath until the solutes were evenly distributed. A sealing membrane was used to quickly cover and seal the petri dishes; then, they were placed in a constant-temperature incubator at 25 °C and kept in the dark for 4 days (17 October 2024).

Table 1.
Chemical concentration settings for the experimental group.
2.3. Determination of Morphological Indexes in Radish
Germination index of radish seeds: After 4 d incubation, the germination rate of seeds was determined. The formula is as follows:
Morphological indexes of radish seedlings: Radish seedlings were cultured for 4 d. Seedlings were carefully clamped in glass petri dishes with tweezers. Root length and shoot length were manually measured with a scale with an accuracy of 1 mm.
2.4. Antioxidant Enzyme Activities in Radish
A 0.1 g root sample of radish seedlings in a 0 °C refrigerator was removed, and 1 mL of extract was added. The extract was configured as described in the kit instructions. After grinding in a mortar on ice for 3 min until the sample was completely dissolved, the samples were introduced into a 2 mL centrifuge tube. After centrifugation at 8000× g for 10 min at 4 °C, the supernatant was transferred into a 2 mL centrifuge tube and placed on ice until measured. SOD activity was measured using the photochemical method described by Spitz (1989) [16], CAT activity was measured using Johansson’s UV (240 nm) absorption method (1988) [17], and POD activity was measured using Doerge’s (1997) method [18] (24 November 2024).
2.5. Transcriptome Sequencing and Data Analysis
We selected the blank control group, 150 mg/L nano-TiO2, 150 mg/L PLA, and 150 mg/L +150 mg/L nano-TiO2 + PLA for transcriptome and metabolome analysis based on the conclusion of changes in “growth indicators” and “physiological and biochemical levels”. According to the manufacturer’s instructions, total RNA was isolated and purified from a fresh root (1–2 cm) using TRIzol (Thermofisher, Waltham, MA, USA). The amount and purity of total RNA were quality controlled using a NanoDrop ND-1000 (NanoDrop, Wilmington, DE, USA). After that, the integrity of the RNA was examined by a Bioanalyzer 2100 (Agilent, CA, USA); concentration > 50 ng/μL, RIN value > 7.0, total RNA > 1 μg. Bipartite sequencing was performed on the illumina Novaseq platform of BIOTREE (Shanghai, China) Biomedical Technology Co. Cutadapt was used to filter out unqualified sequences (sequencing junctions, low-quality sequences, etc.) from the raw data to obtain clean data, and Hisat2 was used to perform the reference genome comparison. Based on the results of the Hisat2 comparison, StringTie was used to reconstruct the transcripts and calculate the expression levels of all genes in each sample. Expression levels were estimated using the number of fragments per kilobase of exon per million mapped reads (FPKM) based on the length of the gene and the number of sequences. The p-value calculation was performed using a p-value calculation model based on the negative binomial distribution, where biological replicates were analyzed using DESeq2, and no biological replicates (inter-sample comparisons) were analyzed using edgeR. Multiple group comparisons were analyzed using edgeR; p-value corrections were made using BH, which in turn yielded q-values (FDR values, p.adj values). The multiplicity of difference is the division of the mean value of expression in the experimental group and the mean value of expression in the control group. Fold difference FC ≥ 2 or FC ≥ 5 (i.e., absolute value of log2FC ≤ 1) and q-value < 0.05 (|log2fc| ≥ 1 and q < 0.05) were used as threshold criteria. Genes meeting the criteria were considered differentially expressed genes (13 March 2025).
2.6. Metabolomic Measurements and Data Analysis
The samples were lyophilized and weighed in EP tubes prior to the addition of homogenization beads. Metabolites were extracted using a methanol–acetonitrile–water solvent system (2:2:1, v/v/v) at a predetermined ratio. The mixture was vigorously vortexed for 30 s, followed by mechanical homogenization (35 Hz, 4 min) using a bead mill homogenizer. To ensure complete extraction, samples underwent three consecutive cycles of ice–water bath sonication (5 min per cycle) interspersed with homogenization steps. After extraction, samples were maintained at −40 °C for 1 h to facilitate protein precipitation. A 400 μL aliquot of the supernatant was loaded onto a 0.22 μm filter plate assembled with a collection plate in a positive pressure manifold (6 psi, 120 s). The filtrate was collected and analyzed using a Vanquish UHPLC system (Thermo Fisher Scientific, Waltham, MA, USA) coupled to a high-resolution mass spectrometer. Chromatographic separation was achieved using insert column details and gradient conditions as appropriate. Raw data were processed using SIMCA software (v18.0.1) for multivariate statistical analysis. Principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA) were performed following Pareto scaling. Model validity was assessed through permutation testing (200 iterations) with evaluation parameters R2 (goodness-of-fit) and Q2 (predictive ability). Differential metabolites were identified using combined criteria: p-value < 0.05 (Student’s t-test), variable importance in projection (VIP) > 1 from the first OPLS-DA component, and fold-change thresholds (FC < 0.5 or > 2). Metabolic pathway analysis was conducted through MetaboAnalyst 5.0 using the KEGG pathway database. Statistically significant pathways were determined by pathway impact value (PIV) > 0 combined with −log10(p) > 1, corresponding to p < 0.1 in pathway enrichment analysis (13 March 2025).
2.7. Statistical Analyses
Data were processed using IBM SPSS Statistics 27.0.1, and results are expressed as mean ± standard deviation; LSD multiple comparisons in one-way ANOVA were utilized to test the significance of differences in results between treatments: p < 0.05 was considered significant, and p < 0.01 was considered highly significant.
3. Results
3.1. Effect of Nano-TiO2 and PLA on Radish Growth
Compared with the control group, nano-TiO2 stress treatment at 5 mg/L and 150 mg/L had no significant effect on radish germination (p > 0.05), with a germination rate of nearly 100%. Similarly, both 5 mg/L and 150 mg/L PLA treatments showed comparable germination percentages (98–100%, p > 0.05). In the experimental group of radish seeds subjected to combined stress from two pollutants, the germination rate of radish seeds fluctuated between 97% and 98.5% (Supplementary Figures S2 and S3).
An amount of 5 mg/L of nano-TiO2 promoted a 73.0% increase in radish root length. An amount of 150 mg/L of nano-TiO2 promoted a 19.0% increase in radish root length. PLA with 5 mg/L increased root length by 21.5% (p < 0.05), while PLA with 150 mg/L decreased it by 35.1% (p < 0.01). In the composite experimental group, a 5 mg/L + 5 mg/L T + P combination resulted in an 8.4% increase (p > 0.05, not significant), 5 mg/L + 150 mg/L T + P caused a 36.1% increase (p < 0.01), 150 mg/L + 5 mg/L T + P led to a 39.5% increase (p < 0.01), and 150 mg/L + 150 mg/L T + P showed a 1.3% increase (p > 0.05, not significant), as shown in Figure 1a.
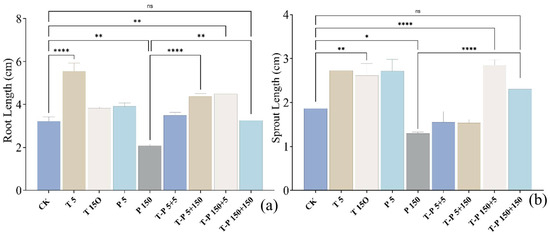
Figure 1.
Changes in radish root length (a); changes in radish sprout length (b). The bars indicate standard error. * indicates p-value ≤ 0.05, ** indicates p-value ≤ 0.01, **** indicates p-value ≤ 0.0001.
An amount of 5 mg/L of nano-TiO2 promoted a 46.0% increase in radish sprout length (p < 0.01). An amount of 150 mg/L of nano-TiO2 promoted a 40.1% increase in radish sprout length (p < 0.01). PLA with 5 mg/L increased sprout length by 45.5% (p < 0.01), while 150 mg/L caused a 30.5% reduction (p < 0.01). In the composite experimental group, a 5 mg/L + 5 mg/L T + P combination resulted in a 17.1% decrease (p < 0.05), 5 mg/L + 150 mg/L T + P caused a 17.7% decrease (p < 0.05), 150 mg/L + 5 mg/L T + P led to a 52.4% increase (p < 0.01), and 150 mg/L + 150 mg/L T + P showed a 23.5% increase (p < 0.05), as shown in Figure 1b (Supplementary Figure S4).
3.2. Effect of Nano-TiO2 and PLA on Radish Enzyme Activities
The activities of key antioxidant enzymes (CAT, POD, SOD) in radish were significantly affected by nano-TiO2 and PLA exposure, either individually or in combination. The activities of catalase (CAT, +39.3%), peroxidase (POD, +157%), and superoxide dismutase (SOD, +5.6%) of radish were significantly increased in the experimental group with 5 mg/L nano-TiO2. At 150 mg/L nano-TiO2, the enzyme levels of CAT, POD, and SOD increased by 72.2%, 201.5%, and 84.4%, respectively. At 5 mg/L PLA elevated CAT (+72.2%), POD (+38.5%), and SOD (+42.1%) activities relative to controls. At 150 mg/L, PLA provoked substantially greater enzymatic upregulation, with CAT, POD, and SOD activities increasing by 297.2%, 281.5%, and 236.3%. Combined nano-TiO2 and PLA treatments generally amplified antioxidant enzyme activities compared to controls. However, antagonistic effects emerged at higher pollutant concentrations: CAT activity decreased by 2.8% in the 5 mg/L + 150 mg/L T + P group versus PLA-alone treatment; POD level in the 150 mg/L + 150 mg/L T + P group was 28.6% lower than that in the 150 mg/L PLA group; SOD activity reduced by 12.1% and 24.5% in the 5 mg/L + 5 mg/L T + P and 5 mg/L + 150 mg/L T + P groups relative to corresponding PLA-only treatments (Figure 2).
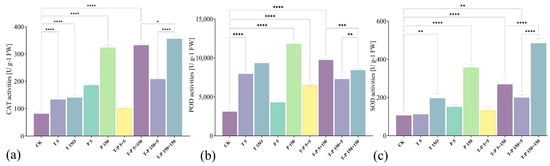
Figure 2.
CAT (a), POD (b), and SOD (c) activities’ changes under different treatments. The bars indicate standard error. * indicates p-value ≤ 0.05, ** indicates p-value ≤ 0.01, *** indicates p-value ≤ 0.001, **** indicates p-value ≤ 0.0001.
3.3. Transcriptomic Responses to PLA, Nano-TiO2, and Nano-TiO2 + PLA
To investigate the toxicological mechanisms of nano-TiO2 and PLA on radish root cells and elucidate the molecular basis for morphological and antioxidant enzyme alterations, transcriptomic analysis was performed to construct a differential gene expression library, followed by KEGG pathway enrichment (Supplementary Figure S5). KEGG pathway analysis revealed that differentially expressed genes were primarily associated with five functional categories: cellular processes, environmental information processing, genetic information processing, metabolism, and organismal systems. In the comparison between the control (CK) and 150 mg/L nano-TiO2 groups, 191 upregulated and 33 downregulated genes were identified (|log2(fold change)| > 1, q < 0.05). KEGG enrichment showed significant upregulation of genes involved in peroxisome biogenesis (6 genes), MAPK signaling (6 genes), phenylpropanoid biosynthesis (10 genes), and glyoxylate/dicarboxylate metabolism (7 genes). For the CK vs. 150 mg/L PLA comparison, 1463 differentially expressed genes were detected. Enriched pathways included plant–pathogen interactions (15 downregulated, 67 upregulated), ribosome function (131 downregulated, 2 upregulated), phytohormone signaling (29 downregulated, 49 upregulated), and starch/sucrose metabolism (4 downregulated, 14 upregulated). In the CK vs. composite 150 mg/L + 150 mg/L T + P group, 844 upregulated and 829 downregulated genes were identified (Figure 3). Key enriched pathways included phytohormone signaling, ribosome biogenesis, starch/sucrose metabolism, and plant–pathogen interactions. Specifically, ribosome-related genes showed 91 downregulated and 2 upregulated entries, while phenylalanine/tyrosine/tryptophan biosynthesis and cysteine/methionine metabolism exhibited 1 downregulated/10 upregulated and 9 downregulated/10 upregulated genes, respectively. Focusing on phenylpropanoid biosynthesis, KEGG analysis demonstrated distinct regulation across treatments: 150 mg/L nano-TiO2: 10 genes upregulated (q < 0.05); 150 mg/L PLA: 34 genes upregulated (q < 0.05); composite treatment: 21 genes upregulated and 4 downregulated (q < 0.05) (Figure 4).
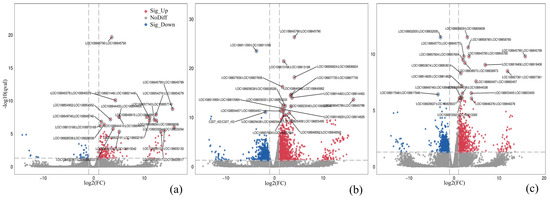
Figure 3.
Volcano plot of differentially expressed genes (DEGs) in radishes treated with nano-TiO2 (a), PLA (b), and nano-TiO2 + PLA (c). Red indicates significantly up-regulated genes, blue indicates significantly down-regulated genes, and gray indicates no significant difference. The grey dashed line represents log2(FC) = 0.
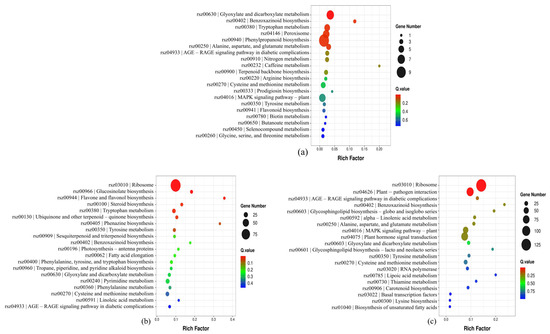
Figure 4.
KEGG pathway diagram of differentially expressed genes (DEGs) in radishes with nano-TiO2 (a), PLA (b), and nano-TiO2 + PLA (c).
3.4. Metabolomic Response After Treatment with PLA, Nano-TiO2, and Nano-TiO2 + PLA
In the metabolomic analysis, 36,325 metabolites were detected in each experimental group. Comparative analysis revealed the following: CK vs. nano-TiO2 (150 mg/L): 4425 significantly differential metabolites (q < 0.05), including 1502 upregulated and 2923 downregulated. CK vs. PLA (150 mg/L): 5694 differential metabolites (q < 0.05), with 2207 upregulated and 3487 downregulated. CK vs. composite 150 mg/L + 150 mg/L T + P: 5041 differential metabolites (q < 0.05), comprising 734 upregulated and 4370 downregulated (Figure 5). Heat map analysis indicated metabolite clustering within major metabolic pathways. Enrichment analysis relative to CK revealed primary enrichment in phenylalanine metabolism for nano-TiO2 (150 mg/L). For PLA (150 mg/L), dominant enrichment occurred in starch and sucrose metabolism, riboflavin metabolism, galactose metabolism, and phenylalanine metabolism. The composite treatment showed key enrichment in flavone/flavonol biosynthesis and riboflavin metabolism (Figure 6).
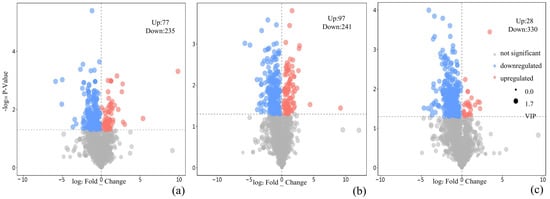
Figure 5.
Metabolite volcano plots of nano-TiO2 (a), PLA (b), and nano-TiO2 + PLA (c). The grey dashed line represents log2(FC) = 0.
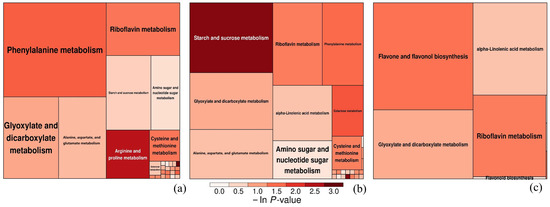
Figure 6.
Heat map of differential metabolites (dem) in radishes treated with nano-TiO2 (a), PLA (b), and nano-TiO2 + PLA (c).
In this research, the content of L-penylalanine, the initial precursor of the phenylpropanoid biosynthesis pathway, was increased. The activities of two key enzymes declined: 4-coumarate-CoA ligase (EC 6.2.1.12), which regulates Cinnamoyl-CoA, and trans-cinnamate 4-monooxygenase (EC 1.14.14.91), which regulates 4-Hydroxycinnamoyl-CoA. These two metabolites serve as the initial precursors for the flavonoid biosynthesis pathway. The activity of flavonol synthase (EC 1.14.20.6) modulates the content of Kaempferol, the initial precursor of the flavone and flavonol biosynthesis pathway. The altered activity of flavonoid 3′-monooxygenase (EC 1.14.14.82) affects Quercitrin levels, thereby influencing the contents of the metabolites Rutin and Myricetin. Changes in the activity of flavonol 3-O-glucosyltransferase (EC 2.4.1.91) impacted the levels of Kaempferol-3-O-rutinoside and Kaempferol 3-O-beta-D-sophoroside (Figure 7).
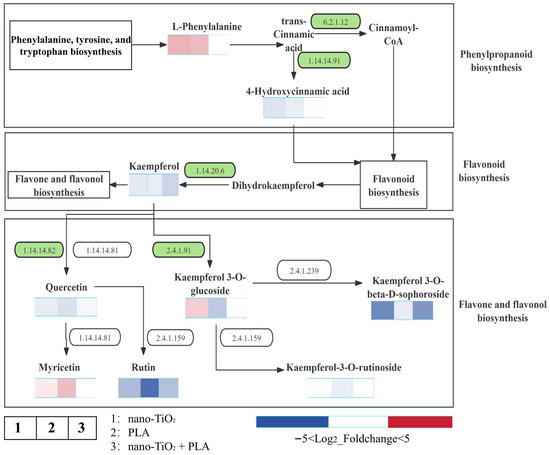
Figure 7.
Changes in metabolic pathways in radish roots under different treatments.
4. Discussion
4.1. Germination and Growth Responses: Hormetic Effects and Composite Mitigation
Composite contaminant exposure (nano-TiO2 + PLA) maintained radish seed germination between 97 and 98.5%, indicating that radish seeds were insensitive to single and composite nanoparticle exposure within the tested concentrations. In past studies, the effect of titanium dioxide nanoparticles on plants showed a concentration-dependent effect. It promotes plant growth at low concentrations and inhibits plant growth at high concentrations [19]. In this study, both 5 mg/L and 150 mg/L of titanium dioxide nanoparticles promoted elongation of radish root length and shoot length. This is in agreement with studies on chrysanthemum and rape [20,21]. Polylactic acid also showed similar effects on plants, where 5 mg/L PLA promoted radish root and shoot elongation, but 150 mg/L PLA inhibited radish root and shoot growth. In a similar study, plant height and root length of oilseed rape were promoted at low concentration (1 mg/L) and inhibited at high concentration (100 mg/L) [22]. The same trend was also shown in the study of terrestrial vascular plants [23]. In the composite experimental group, both 5 mg/L + 150 mg/L T + P and 150 mg/L + 150 mg/L T + P alleviated the PLA-induced growth inhibition of radish roots and shoots compared with 150 mg/L PLA. It has been shown that nano-TiO2 has the ability to mitigate plant damage caused by environmental stresses [24]. In similar studies, nano-TiO2 alleviated growth inhibition in water-stressed wheat and salt-stressed maize [20,25]. The mechanisms by which nano-TiO2 increases plant stress resistance include enhancing antioxidant capacity, affecting the differential expression of relevant genes, and regulating the production of relevant metabolites [12,26,27].
4.2. Antioxidant Defense Dynamics: Enzyme Activation and Composite Interactions
The first line of plant antioxidant defense is the production of the disproportionation product H2O2 from O2− catalyzed by superoxide dismutase (SOD), which is further scavenged by catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX), etc. Meanwhile, small molecule metabolites, such as ascorbate, glutathione, carotenoids, tocopherols, and phenolic compounds, are also involved in plant ROS scavenging [28]. The increase in CAT and POD in the experimental groups with 5 mg/L and 150 mg/L of nano-TiO2 indicated that nano-TiO2 induced the generation of reactive oxygen species (ROS) through photocatalysis after entering the plant cells and broke the dynamic balance of intracellular ROS. In the study of kidney beans, nano-TiO2 also enhanced the antioxidant enzyme activities in the cells of the bean [29]. An amount of 5 mg/L and 150 mg/L of PLA in the experimental group showed an increase in CAT and POD enzyme activity. This is consistent with the effect of polystyrene on faba bean, which suggests that radish counteracts PLA stress and reduces its own damage by increasing the activity of its own antioxidant system [30]. In both groups of 5 mg/L + 150 mg/L T + P and 150 mg/L + 150 mg/L T + P, CAT and POD enzyme activity decreased with the increase in the concentration of nano-TiO2 addition. Nano-TiO2 addition relieved the antioxidant enzyme system damage of PLA on radish root tip cells. In a study on corn, nano-TiO2 similarly attenuated the growth inhibition induced by polystyrene nanoplastics [12]. The changes in SOD enzyme activities were basically consistent with those of POD and CAT enzyme activities, but in the 150 mg/L + 150 mg/L T + P group, SOD enzyme activity was abnormally increased, indicating that nano-TiO2 did not play a role in alleviating PLA stress. The presumed reason for this phenomenon is that SOD, as the first line of defense, collapses in the face of stress. These changes in antioxidant enzyme activities may be related to the uptake and transport of nano-TiO2 and PLA in radish roots. When the particle size of nano-TiO2 is close to or smaller than the aperture of the cell wall of the root epidermal cells (5–20 nm), nano-TiO2 can diffuse into the root system along the exosome pathway by means of the transportation of water and ions, which has been reported in crops such as rice, wheat, rape, and cabbage [31]. Similarly, in a study on Brassica napus, microplastics could enter the xylem conduits of the roots and subsequently be carried to the petioles through transpiration tension and eventually transported from the xylem conduits of the petioles to the leaves [32]. PLA microplastics have a large surface area [33], and in this study, titanium dioxide nanoparticles may have been carried by PLA into radish roots, exerting a stress-relieving effect while mitigating the toxicity of PLA. A similar carrier role is also seen in complexes with other heavy metals such as Cu, Pb, Cr, and As, which exacerbate or mitigate the toxicity of heavy metals to plant cells [34,35,36,37]. The compounding effect of nanoparticles and microplastics on plants may depend on the physicochemical properties of the nanoparticles. The phytotoxicity of nanoparticles with high solubility of metal ions, such as nano-ZnO, is mainly associated with their release of metal ions [38], whereas for some of the refractory or poorly soluble nanoparticles, such as titanium dioxide nanoparticles and ferric iron trioxide nanoparticles, their direct contact with the cells as well as their surface photosensitizing properties have been suggested to be the main factors contributing to cytotoxicity [39]. In the study of polystyrene and nano-Fe2O3 on lettuce, nano-polystyrene interacted with nano-Fe2O3 to form heterogeneous agglomerates that promoted the leaching of iron ions, thus aggravating the toxic effects [13]. On the other hand, nano-graphene alleviated the effects of nano-polystyrene on lettuce [40]. These studies corroborate with the present study and provide a reference for solving the problem of nanoparticle and microplastic pollution.
4.3. Transcriptomic and Metabolomic Insights: Phenylpropanoid Pathway Activation Under Stress
To further verify the above conclusions, we selected four sets of experiments, CK, 150 mg/L nano-TiO2, 150 mg/L PLA, and 150 mg/L + 150 mg/L T + P, for transcriptome and metabolome analysis. Differential gene expression profiles indicate that radish root cells activate powerful mechanisms to mitigate abiotic stress. Among many pathways, we selected phenylpropanoid biosynthesis for analysis. This pathway is involved in important physiological processes such as cell division, hormone regulation, photosynthesis, nutrient mineralization, and reproduction [41], and the products of this metabolic pathway include flavonoids, hydroxycinnamic acids, hydroxycinnamic amides (HCAAs), and precursors of lignin and tannin [42]. Phenylpropanoid biosynthesis works through these products in tandem with flavonoid biosynthesis and flavone and flavonol biosynthesis. Flavonoid metabolism is an important branch of phenylpropanoid metabolism and produces the largest class of polyphenol metabolite compounds [43]. Phenolic compounds are a class of secondary metabolites with antioxidant activity in plants [44]. Polyphenols such as phenolic acids and flavonoids alleviate stress and enable plants to adapt to environmental constraints. The levels of phenolic compounds were significantly elevated in all of these plants. Under abiotic stress (e.g., drought, heavy metals, salinity, temperature extremes, UV exposure), phenylpropanoid biosynthesis triggers the accumulation of phenolic compounds and the production of ROS scavenging molecules [45]. A total of 18 metabolites, including phenolic acids, flavonoids, lignans, and isoflavones, were detected in the comparison of CK with 150 mg/L nano-TiO2. A total of 22 metabolites, including phenylpropanoids, phenolic acids, coumarins, lignans, and isoflavones, were identified in the analysis of CK with 150 mg/L PLA. A total of 15 metabolites were identified in the comparison of CK with 150 mg/L + 150 mg/L T + P, including phenolic acids, phenylpropanoids, flavonoids, and lignans. These metabolite expression patterns confirmed the changes in transcriptome gene expression. Lignin provides mechanical strength and rigidity to the cell wall and promotes the formation of xylem conduits [46]. Changes in lignin content explained the promotional effect of nano-titanium dioxide and polylactic acid on radish roots. Similar findings were found in the interaction of nano/micro polystyrene with taro. Taro roots showed doubled lignification and thickened epidermal/cortical cell walls [47]. Flavonoids (including flavonols, flavonoids, proanthocyanidins, and anthocyanidins) function as antioxidants scavenging ROS [48]. In this study, five flavonoids were detected in the titanium dioxide nano-treated group, eleven in the PLA-treated group, and six in the composite-treated group. The dynamics of these flavonoids explain that nano-TiO2 attenuated PLA-induced damage to the antioxidant enzyme system in radish root cells.
5. Conclusions
This study systematically revealed the toxic effects of nano-titanium dioxide (nano-TiO2) and polylactic acid (PLA) microplastic composite pollution on radishes. In single experiments, nano-TiO2 (5/150 mg/L) promoted the growth of radish roots and sprouts. PLA exhibited a “low-promoting, high-inhibiting” phenomenon (5 mg/L promoted growth, while 150 mg/L inhibited it). In composite experiments, 5 mg/L + 150 and 150 mg/L + 150 mg/L T + P significantly alleviated the growth inhibition of roots and sprouts caused by high concentrations of PLA. Single nano-TiO2 or PLA induced ROS production to activate antioxidant enzymes such as CAT and POD. In composite experiments, nano-TiO2 reduced the abnormal increase in antioxidant enzyme activity caused by PLA and alleviated oxidative damage by activating the phenylpropanoid metabolic pathway (enhanced accumulation of lignin precursors to strengthen cell wall structure and increased flavonoid metabolites), forming an “enzyme–metabolite” synergistic defense system. This confirms that nano-TiO2 exerts a “detoxification” effect by regulating antioxidant defense and metabolic pathways. This study elucidates the mechanism by which nano-TiO2 alleviates PLA phytotoxicity, providing new insights for the remediation of composite pollution in farmland. Future research should establish precise risk assessment strategies based on the solubility differences of nanoparticles and further investigate the migration patterns of composite pollutants in ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture15141478/s1, Figure S1: SEM images show the surface morphology of nano-TiO2/PLA MPs; Figure S2: The germination rate of radish seeds; Figure S3: The growth conditions of radishes at different concentrations; Figure S4: The length graphs of the roots and sprouts of radish seedlings under different pollution conditions; Figure S5: Bar charts showing differentially expressed genes (DEGs) in radishes treated with nano-TiO2 (a), PLA (b), and nano-TiO2+PLA (c).
Author Contributions
Conceptualization, L.J.; methodology, W.L.; software, W.L. and Y.Z.; validation, Z.L. and L.G.; formal analysis, W.L. and Y.Y.; investigation, W.L. and Z.Y.; resources, W.F.; data curation, W.L.; writing—original draft preparation, L.J. and W.L.; writing—review and editing, L.J.; visualization, C.G.; supervision, W.F.; project administration, L.J.; funding acquisition, L.J. and W.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Liaoning Provincial Universities Basic Research funds special fund (LJ202410166039, LJ202410166041), Liaoning Provincial University Students’ Innovation Training Program Project (S202410166006).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Safdar, M.; Kim, W.; Park, S.; Gwon, Y.; Kim, Y.; Kim, J. Engineering plants with carbon nanotubes: A sustainable agriculture approach. J. Nanobiotechnol. 2022, 20, 275. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.; Venkatachalam, P.; Sahi, S.; Sharma, N. Reprint of: Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol. 2016, 107, 147–163. [Google Scholar] [CrossRef]
- Shi, X.; Li, Z.; Chen, W. Fate of TiO2 nanoparticles entering sewage treatment plants and bioaccumulation in fish in the receiving streams. Nanoimpact 2016, 3, 96–103. [Google Scholar] [CrossRef]
- Yin, Z.F.; Wu, L.; Yang, H.G. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844–4858. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources, 1st ed.; IUCN: Gland, Switzerland, 2017; p. 5. [Google Scholar] [CrossRef]
- Brahney, J.; Hallerud, M.; Heim, E. Plastic rain in protected areas of the United States. Science 2020, 368, 1257–1260. [Google Scholar] [CrossRef]
- Tang, K. Microplastics in agricultural soils in China: Sources, impacts and solutions. Environ. Pollut. 2023, 322, 121–235. [Google Scholar] [CrossRef]
- Tang, K.; Li, R.; Li, Z.; Wang, D. Health risk of human exposure to microplastics: A review. Environ. Chem. Lett. 2024, 22, 1155–1183. [Google Scholar] [CrossRef]
- Yang, L.; Luo, L.; Cai, W. Changes in carbohydrate metabolism and soil microorganisms under the stress of polyamide and polyethylene nanoplastics during rice (Oryza sativa L.) growth. Sci. Total Environ. 2024, 912, 169–183. [Google Scholar] [CrossRef]
- Khan, A.; Li, G. Micro/nanoplastics: Critical review of their impacts on plants, interactions with other contaminants (antibiotics, heavy metals, and polycyclic aromatic hydrocarbons), and management strategies. Sci. Total Environ. 2024, 912, 169420. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, B.; Zhang, F.; Wang, Z. Combined effects of micro/nanoplastics and ZnO nanoparticles on lactuca sativa seedlings under varied lighting. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2025, 296, 110233. [Google Scholar] [CrossRef]
- Yang, X.; Feng, K.; Wang, G.; Zhang, S.; Zhao, J.; Yuan, X.; Ren, J. Titanium dioxide nanoparticles alleviates polystyrene nanoplastics induced growth inhibition by modulating carbon and nitrogen metabolism via melatonin signaling in maize. Nanobiotechnology 2024, 22, 262. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Bai, X.; Weng, Y.; Kang, M.; Huang, Y.; Li, F.; Chen, Y. Phytotoxicity of binary nanoparticles and humic acid on (Lactuca sativa L). Environ. Sci. Process Impacts 2022, 24, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Issac, M.N.; Kandasubramanian, B. Effect of microplastics in water and aquatic systems. Environ. Sci. Pollut. 2021, 28, 19544–19562. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Ben, H.; Wang, L.; Fan, T.; Xie, X.; Shi, Y.; Li, B.; Chai, A. First Report of Radish Tuber Black Heart Rot Caused by Pectobacterium parvum in China. Plant Dis. 2025, 107, 2839. [Google Scholar] [CrossRef]
- Spitz, D.R.; Oberley, L.W. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989, 179, 8–18. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.A. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Doerge, D.R.; Divi, R.L. Identification of the colored guaiacol oxidation product produced by peroxidases. Anal. Biochem. 1997, 250, 10–17. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Environmental hormesis of non-specific and specific adaptive mechanisms in plants. Sci. Total Environ. 2022, 804, 150059. [Google Scholar] [CrossRef]
- Jaberzadeh, A.; Moaveni, P. Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Not. Bot. Hort. Agrobot. Cluj. 2013, 41, 201. [Google Scholar] [CrossRef]
- Sehrish, A.K.; Ahmad, S.; Alomrani, S.O.; Ahmad, A.; Al-Ghanim, K.A.; Alshehri, M.A.; Tauqeer, A.; Ali, S.; Sarker, P.K. Nutrient strengthening and lead alleviation in (Brassica Napus L.) by foliar ZnO and TiO2-NPs modulating antioxidant system, improving photosynthetic efficiency and reducing lead uptake. Sci. Rep. 2024, 14, 19437. [Google Scholar] [CrossRef]
- Li, Q.; Yan, J.; Li, Y.; Liu, Y.; Andom, O.; Li, Z. Microplastics alter cadmium accumulation in different soil-plant systems: Revealing the crucial roles of soil bacteria and metabolism. J. Hazard. Mater. 2024, 474, 134768. [Google Scholar] [CrossRef] [PubMed]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant (Lepidium sativum). Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Kumar, D.; Dhankher, O.P.; Tripathi, R.D.; Seth, C.S. Titanium dioxide nanoparticles potentially regulate the mechanism (s) for photosynthetic attributes, genotoxicity, antioxidants defense machinery, and phytochelatins synthesis in relation to hexavalent chromium toxicity in (Helianthus annuus L). J.Hazard. Mater. 2023, 454, 131418. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Latif, S.; Saeed, F. Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud. Univ. Sci. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Amini, S.; Maali-Amiri, R.; Mohammadi, R. cDNA-AFLP analysis of transcripts induced in chickpea plants by TiO2 nanoparticles during cold stress. Plant Physiol. Biochem. 2017, 111, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.D.; Liu, N.; Wang, W. Photosynthesis and related metabolic mechanism of promoted rice (Oryza sativa L.) growth by TiO2 nanoparticles. Front. Environ. Sci. Eng. 2020, 14, 103. [Google Scholar] [CrossRef]
- Chibani, K.; Gherli, H.; Fan, M. The role of blue light in plant stress responses: Modulation through photoreceptors and antioxidant mechanisms. Front. Plant Sci. 2025, 16, 1554281. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Galavi, M.; Ramroudi, M. Effect of TiO2 nanoparticles on antioxidant enzymes activity and biochemical biomarkers in pinto bean (Phaseolus vulgaris L.). J. Mol. Biol. Res. 2015, 6, 58. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, H.; Liao, Y.; Ye, Z.; Li, M.; Klobučar, G. Ecotoxicity and genotoxicity of polystyrene microplastics on higher plant (Vicia faba). Environ. Pollut. 2019, 250, 831–838. [Google Scholar] [CrossRef]
- Larue, C.; Laurette, J.; Herlin-Boime, N. Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): Influence of diameter and crystal phase. Sci. Total Environ. 2012, 431, 197–208. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, M.; Li, R.; Yang, L.; Liu, P.; Shi, Q. Transport Dynamics and Physiological Responses of Polystyrene Nanoplastics in Pakchoi: Implications for Food Safety and Environmental Health. J. Agric. Food Chem. 2025, 73, 10923–10933. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, Z.; Ji, K.; Hu, Z.; Xi, Y.; Xiang, X. Enhanced copper adsorption by polyamide and polylactic acid microplastics: The role of biofilm development and chemical aging. Environ. Res. 2025, 282, 122040. [Google Scholar] [CrossRef]
- Gong, K.; Zhang, Q.; Shao, X.; Wu, Y.; Qiao, Z.; Qiu, L.; Zhang, W.; Peng, C. Microplastics alter Cr accumulation and fruit quality in Cr(VI) contaminated soil-cucumber system during the lifecycle: Insight from rhizosphere bacteria and root metabolism. Sci. Total Environ. 2024, 912, 168792. [Google Scholar] [CrossRef]
- Tian, X.; Weixie, L.; Wang, S.; Zhang, Y.; Xiang, Q.; Yu, X.; Zhao, K.; Zhang, L.; Penttinen, P.; Gu, Y. Effect of polylactic acid microplastics and lead on the growth and physiological characteristics of buckwheat. Chemosphere 2023, 337, 139356. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yan, C.; Shang, J.; Zeng, Z.; Huo, Y.; Li, D.; Xu, Y.; Xian, X.; Li, Y.; Gao, X.; et al. Microplastic mediated arsenic toxicity involves differential bioavailability of arsenic and modulated uptake in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2025, 300, 118448. [Google Scholar] [CrossRef]
- Arruda, S.C.; Silva, A.L.; Galazzi, R.M. Nanoparticles applied toplant science: A review. Talanta 2015, 131, 693–705. [Google Scholar] [CrossRef]
- Conway, J.R.; Beaulieu, A.L.; Beaulieu, N.L. Environmental stresses increase photosynthetic disruption by metal oxide nanomaterials ina soil-grown plant. ACS Nano 2015, 9, 11737–11749. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, F.; Wang, Z. Impacts of Micro/Nanoplastics Combined with Graphene Oxide on Lactuca sativa Seeds: Insights into Seedling Growth, Oxidative Stress, and Antioxidant Gene Expression. Plants 2024, 3, 3466. [Google Scholar] [CrossRef]
- Wan, R.; Wang, H.; Hui, T.; Yang, L.; Wang, X.; Cao, Y.; An, W.; Zhang, X.; Zhao, J.; Wang, Y.; et al. Morphological, physiological, and transcriptomic insights into response the of (Lycium barbarum L) seedlings to low-nitrogen stress. Genomics 2025, 117, 111065. [Google Scholar] [CrossRef]
- Gray, J.; Caparrós-Ruiz, D.; Grotewold, E. Grass phenylpropanoids: Regulate before using! Plant Sci. 2012, 184, 112–120. [Google Scholar] [CrossRef]
- Tohge, T.; Watanabe, M.; Hoefgen, R.; Fernie, A.R. Theevolution of phenylpropanoid metabolism in the green lineage. Crit.Rev. Biochem. Mol. Biol. 2013, 48, 123–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Du, W.; Peralta-Videa, J. Metabolomics reveals howcucumber (Cucumis sativus) reprograms metabolites to cope with silver ions and silver nanoparticle-induced oxidative stress. Environ. Sci. Technol. 2018, 52, 8016–8026. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From biosynthesisto health benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dong, Q.; Ge, S.; He, X.; Verdier, J.; Li, D.; Zhao, J. Metabolic engineering of proanthocyanidin production by repressingthe isoflavone pathways and redirecting anthocyanidin precursor flux in legume. Plant Biotechnol. J. 2016, 14, 1604–1618. [Google Scholar] [CrossRef]
- Xu, G.; Li, X.; Zhu, T.; Wang, F.; Yin, J. When Nano and Microplastics Meet Taro (Colocasia esculenta) Roots: Their Size-Dependent Adsorption, Penetration, and Promotion on Secondary Wall Reinforcement. Environ. Sci. Technol. 2025, 59, 8345–8356. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Saito, K. Integrated metabolomics forabiotic stress responses in plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).