Regulatory Mechanisms of Medium-Term Crop Rotation on Soil Organic Carbon Storage in Red Soils at the Aggregate Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sampling
2.3. Laboratory Analysis
2.4. Data Analysis
3. Results
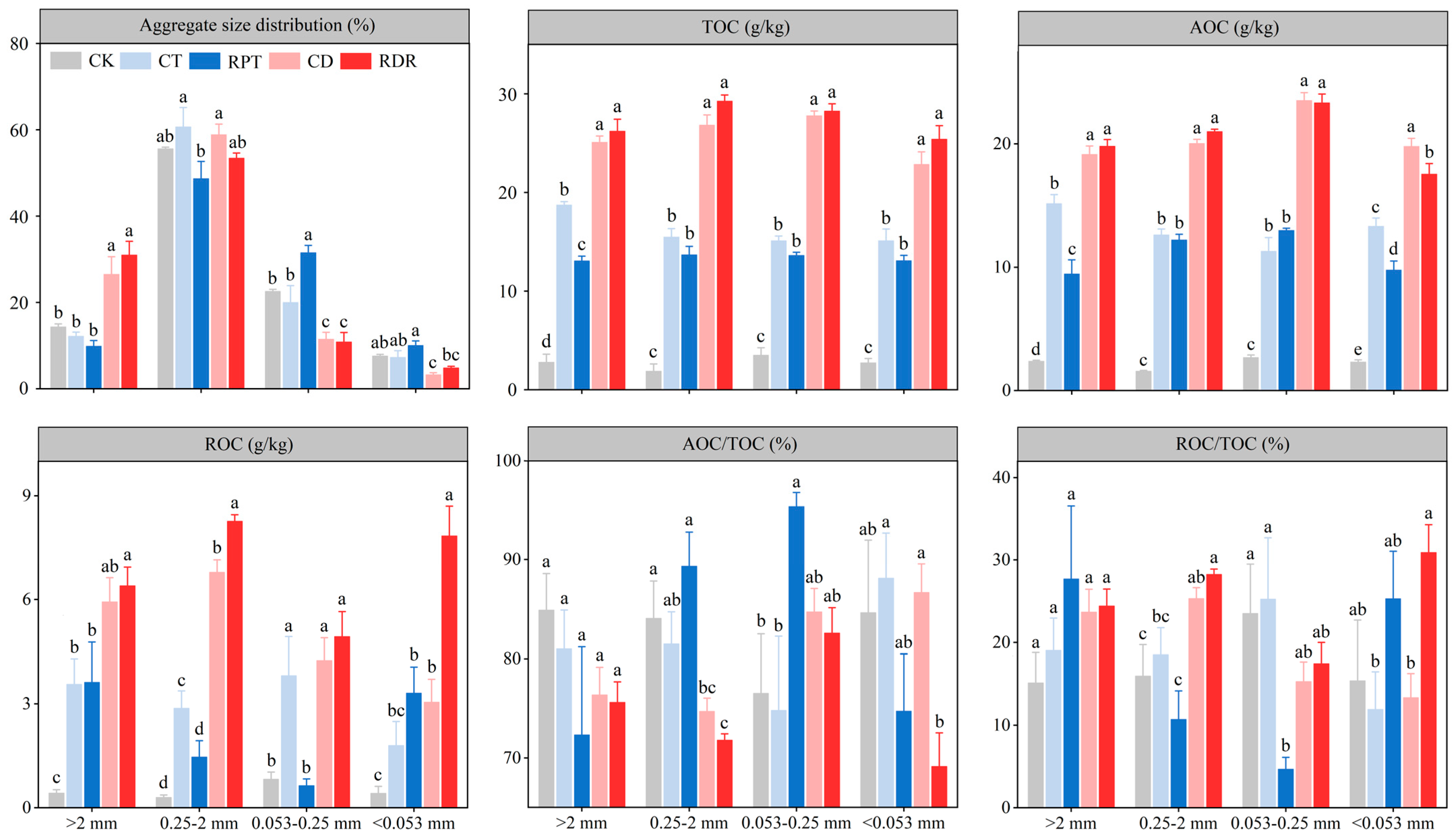
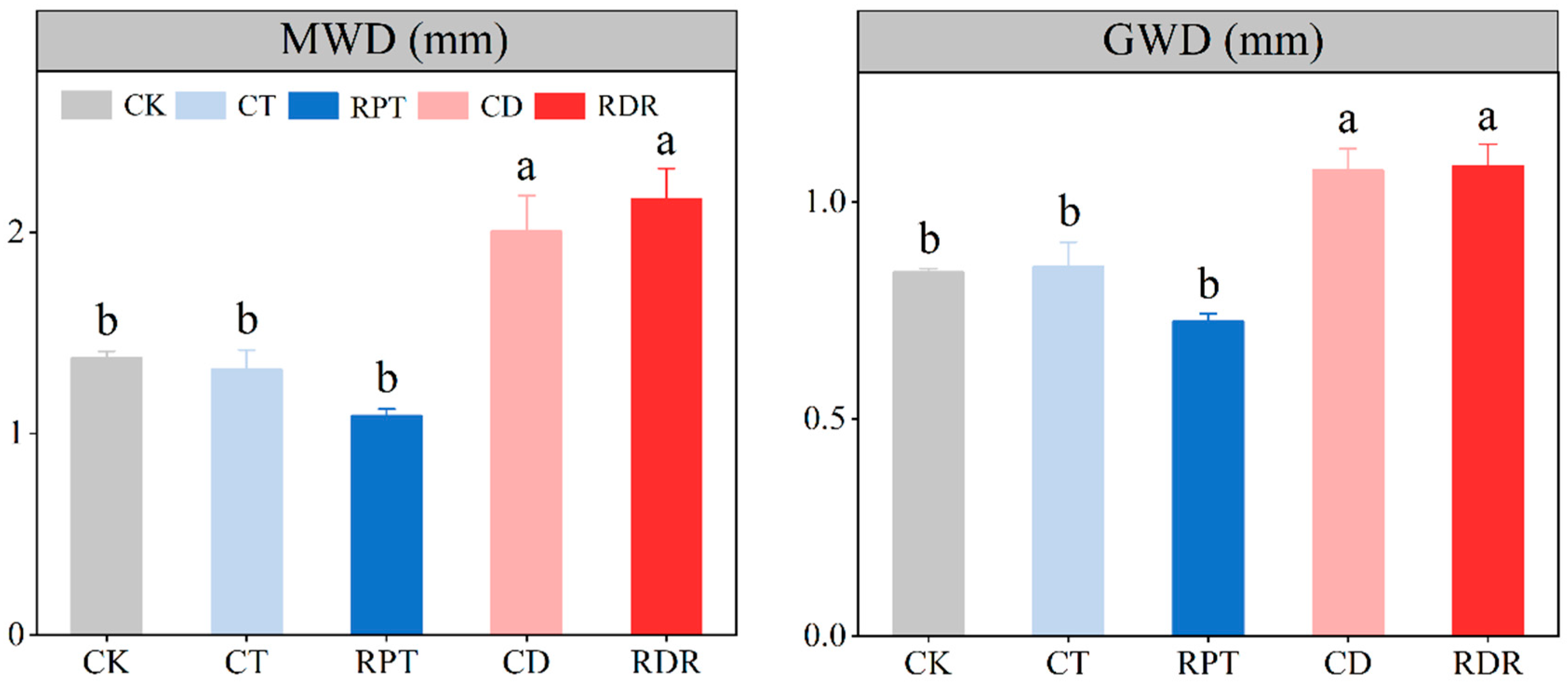
3.1. Soil Aggregate Size Distribution and Stability
3.2. Soil Organic Carbon in the Aggregate Fractions
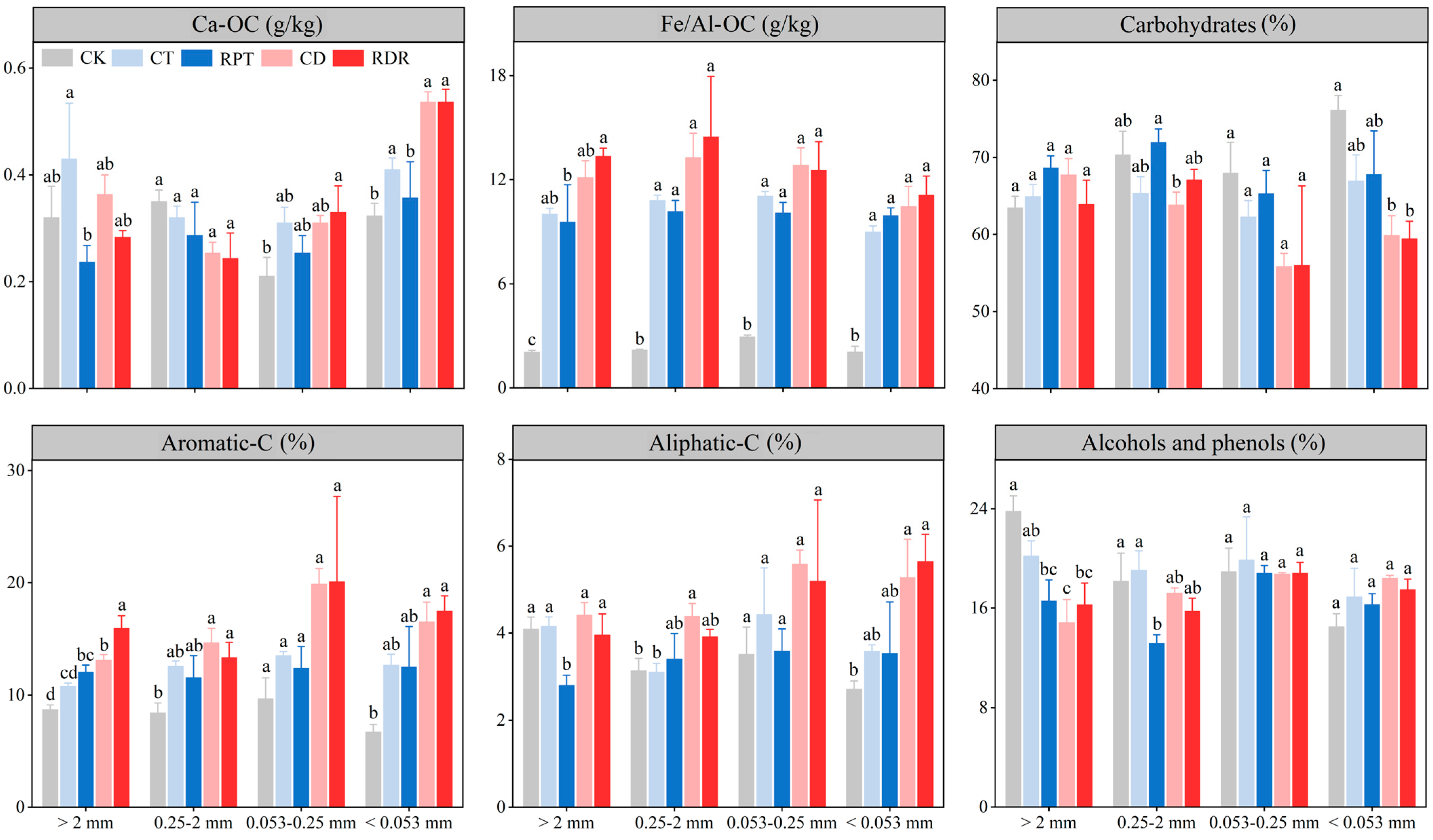
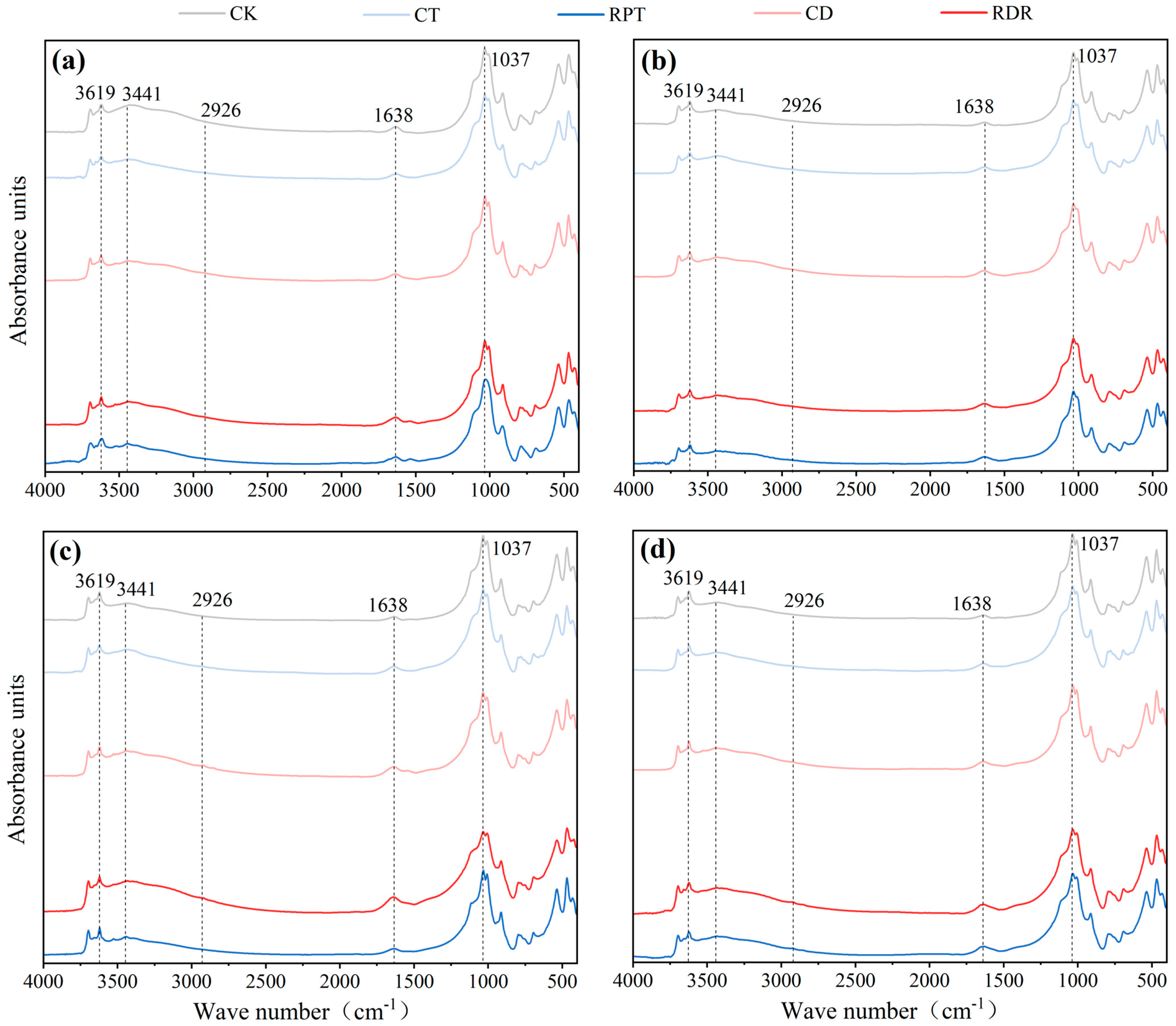
3.3. Soil Chemical-Bound Organic Carbon and Carbon Functional Groups in the Aggregate Fractions
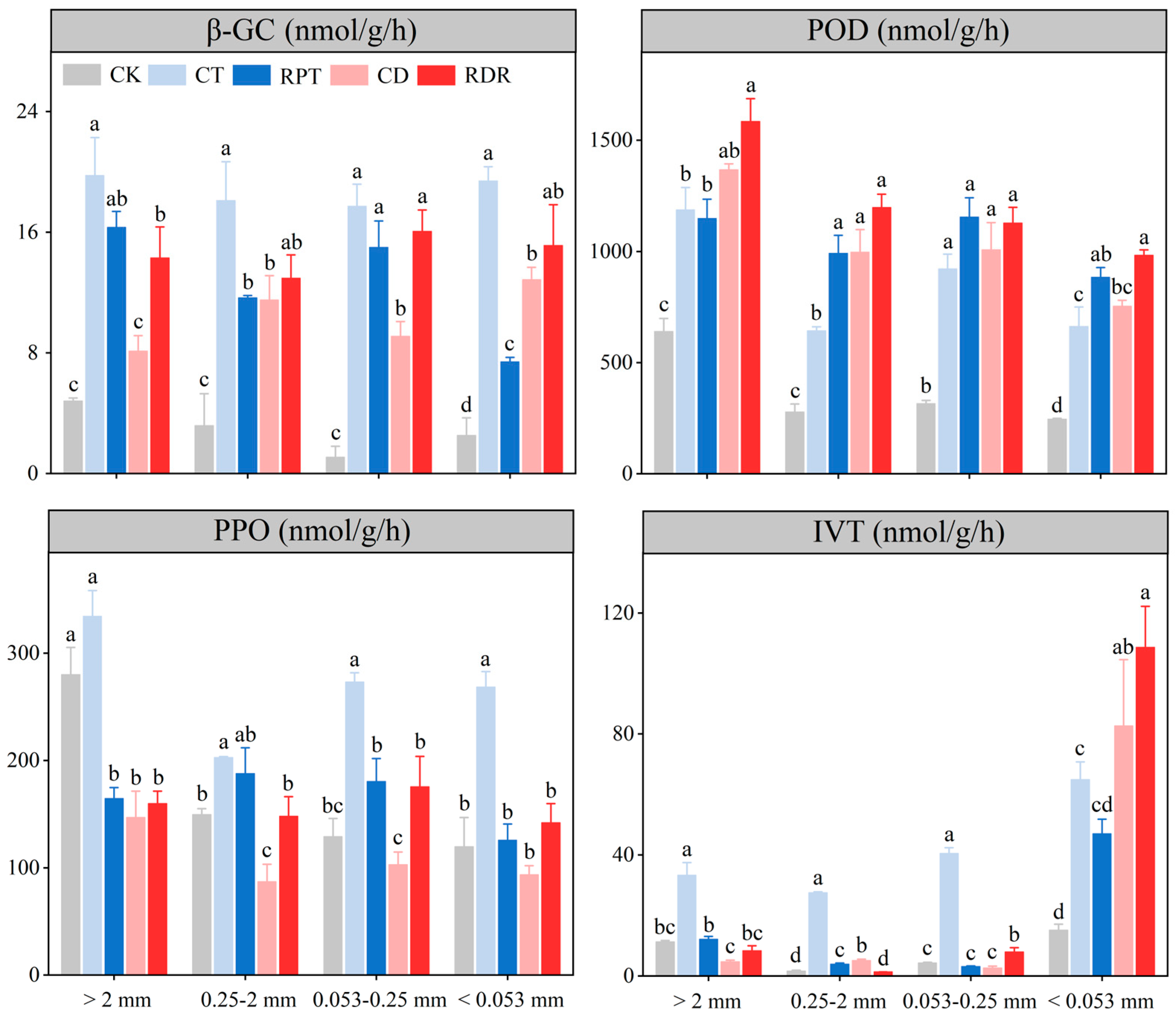
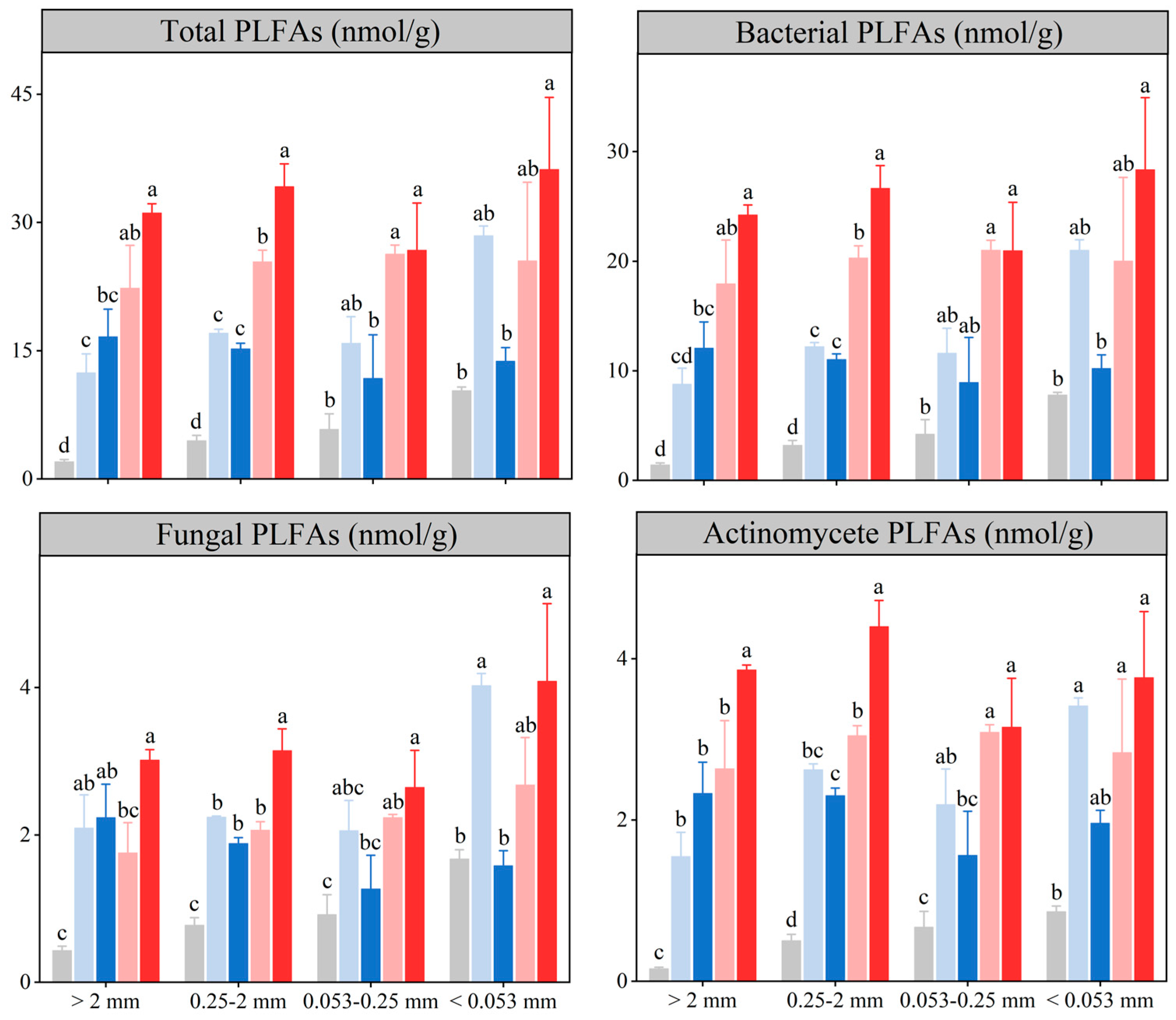
3.4. Soil Microbial Properties in the Aggregate Fractions
3.5. Relationships of Soil Organic Carbon with Aggregate Size Distribution and Biochemical Properties at the Aggregate Level
4. Discussion
4.1. Effects of Medium-Term Crop Rotation on Soil Aggregate Size Distribution and Stability
4.2. Effects of Medium-Term Crop Rotation on Soil Organic Carbon in the Aggregate Fractions
4.3. Effects of Medium-Term Crop Rotation on Soil Chemical-Bound Organic Carbon and Carbon Functional Groups in the Aggregate Fractions
4.4. Effects of Medium-Term Crop Rotation on Soil Microbial Properties in the Aggregate Fractions
4.5. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zang, H.; Blagodatskaya, E.; Wen, Y.; Xu, X.; Dyckmans, J.; Kuzyakov, Y. Carbon sequestration and turnover in soil under the energy crop Miscanthus: Repeated 13C natural abundance approach and literature synthesis. GCB Bioenergy 2018, 10, 262–271. [Google Scholar] [CrossRef]
- Cooper, J.; Greenberg, I.; Ludwig, B.; Hippich, L.; Fischer, D.; Glaser, B.; Kaiser, M. Effect of biochar and compost on soil properties and organic matter in aggregate size fractions under field conditions. Agric. Ecosyst. Environ. 2020, 295, 106882. [Google Scholar] [CrossRef]
- Sokol, N.W.; Whalen, E.D.; Jilling, A.; Kallenbach, C.; Pett-Ridge, J.; Georgiou, K. Global distribution, formation and fate of mineral-associated soil organic matter under a changing climate: A trait-based perspective. Funct. Ecol. 2022, 36, 1411–1429. [Google Scholar] [CrossRef]
- Kan, Z.; Ma, S.; Liu, Q.; Liu, B.; Virk, A.; Qi, J.; Zhao, X.; Zhang, H. Carbon sequestration and mineralization in soil aggregates under long-term conservation tillage in the North China Plain. Catena 2020, 188, 104428. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Liang, G.; Zheng, F.; Zhang, M.; Li, S. A global meta-analysis of the impacts of no-tillage on soil aggregation and aggregate-associated organic carbon. Land Degrad. Dev. 2021, 32, 5292–5305. [Google Scholar] [CrossRef]
- Lal, R. Carbon cycling in global drylands. Curr. Clim. Change Rep. 2019, 5, 221–232. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, J.S.; O’connell, C.; Ray, D.K.; West, P.C. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Jansson, J.K.; Hofmockel, K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 181, 35–46. [Google Scholar] [CrossRef]
- Lal, R. Sequestering carbon and increasing productivity by conservation agriculture. J. Soil Water Conserv. 2015, 70, 55A–62A. [Google Scholar] [CrossRef]
- Piazza, G.; Pellegrino, E.; Moscatelli, M.C.; Ercoli, L. Long-term conservation tillage and nitrogen fertilization effects on soil aggregate distribution, nutrient stocks and enzymatic activities in bulk soil and occluded microaggregates. Soil Till. Res. 2020, 196, 104482. [Google Scholar] [CrossRef]
- Hati, K.M.; Jha, P.; Dalal, R.C.; Jayaraman, S.; Dang, Y.P.; Kopittke, P.M.; Kirchhof, G.; Menzies, N.W. 50 years of continuous no-tillage, stubble retention and nitrogen fertilization enhanced macro-aggregate formation and stabilisation in a Vertisol. Soil Till. Res. 2021, 214, 105163. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Beillouin, D.; Lambers, H.; Yang, Y.; Smith, P.; Zeng, Z.; Olesen, J.E.; Zang, H. Global systematic review with meta-analysis reveals yield advantage of legume-based rotations and its drivers. Nat. Commun. 2022, 13, 4926. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Cooper, K.; Klaustermeier, A.; Awale, R.; Cihacek, L.J. Does crop species diversity influence soil carbon and nitrogen pools? Agron. J. 2016, 108, 427–432. [Google Scholar] [CrossRef]
- Liu, X.; Peng, C.; Ge, Z.; Xie, N.; Zhang, W.; Li, S.; Wang, J. Subsoiling tillage with straw incorporation improves soil microbial community characteristics in the whole cultivated layers: A one-year study. Soil Till. Res. 2022, 215, 105188. [Google Scholar] [CrossRef]
- Zhang, K.; Maltais-Landry, G.; Liao, H. How soil biota regulate C cycling and soil C pools in diversified crop rotations. Soil Biol. Biochem. 2021, 156, 108219. [Google Scholar] [CrossRef]
- Zhang, W.; Surigaoge, S.; Yang, H.; Yu, R.; Wu, J.; Xing, Y.; Chen, Y.; Li, L. Diversified cropping systems with complementary root growth strategies improve crop adaptation to and remediation of hostile soils. Plant Soil 2024, 502, 7–30. [Google Scholar] [CrossRef]
- Trivedi, P.; Rochester, I.J.; Trivedi, C.; Van Nostrand, J.D.; Zhou, J.; Karunaratne, S.; Anderson, I.C.; Singh, B.K. Soil aggregate size mediates the impacts of cropping regimes on soil carbon and microbial communities. Soil Biol. Biochem. 2015, 91, 169–181. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, Z.; Chen, J.; Yao, P.; Zhao, N.; He, W.; Li, Y.; Zhang, S.; Zhai, B.; Wang, Z.; et al. Improving soil aggregation, aggregate-associated C and N, and enzyme activities by green manure crops in the Loess Plateau of China. Eur. J. Soil Sci. 2019, 70, 1267–1279. [Google Scholar]
- Yan, Z.; Zhou, J.; Nie, J.; Yang, Y.; Zhao, J.; Zeng, Z.; Marshall, M.R.; Peixoto, L.; Zang, H. Do cropping system and fertilization rate change water-stable aggregates associated carbon and nitrogen storage. Environ. Sci. Pollut. Res. 2021, 28, 65862–65871. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, S.; Xue, S.; Hu, Y.; Wang, X. Long-term tillage and cropping systems affect soil organic carbon components and mineralization in aggregates in semiarid regions. Soil Till. Res. 2023, 231, 105742. [Google Scholar] [CrossRef]
- Morugán-Coronado, A.; Pérez-Rodríguez, P.; Insolia, E.; Soto-Gómez, D.; Fernández-Calviño, D.; Zornoza, R. The impact of crop diversification, tillage and fertilization type on soil total microbial, fungal and bacterial abundance: A worldwide meta-analysis of agricultural sites. Agric. Ecosyst. Environ. 2022, 329, 107867. [Google Scholar] [CrossRef]
- Li, T.; Li, G.; Lu, Z.; Zhao, D.; Li, Y.; Wang, Z.; Wen, X.; Liao, Y. Crop diversification increases soil extracellular enzyme activities under no tillage: A global meta-analysis. Soil Till. Res. 2024, 235, 105870. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Z.; Wang, D.; Li, C.; Cao, C. Effects of short-term conservation management practices on soil organic carbon fractions and microbial community composition under a rice-wheat rotation system. Biol. Fertil. Soils 2015, 51, 65–75. [Google Scholar] [CrossRef]
- Oliveira, M.; Barré, P.; Trindade, H.; Virto, I. Different efficiencies of grain legumes in crop rotations to improve soil aggregation and organic carbon in the short-term in a sandy Cambisol. Soil Till. Res. 2019, 186, 23–35. [Google Scholar] [CrossRef]
- Mahecha, M.D.; Reichstein, M.; Carvalhais, N.; Lasslop, G.; Lange, H.; Seneviratne, S.I.; Vargas, R.; Ammann, C.; Arain, M.A.; Cescatti, A.; et al. Global convergence in the temperature sensitivity of respiration at ecosystem level. Science 2010, 329, 838–840. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Mao, X.; Yu, W.; Xiao, L.; Wang, M.; Zhang, S.; Zheng, J.; Zhou, H.; Luo, L.; Chang, J.; et al. A field incubation approach to evaluate the depth dependence of soil biogeochemical responses to climate change. Glob. Change Biol. 2022, 29, 909–920. [Google Scholar] [CrossRef]
- Fujisaki, K.; Chevallier, T.; Chapuis-Lardy, L.; Albrecht, A.; Razafimbelo, T.; Masse, D.; Ndour, Y.B.; Chotte, J.L. Soil carbon stock changes in tropical croplands are mainly driven by carbon inputs: A synthesis. Agric. Ecosyst. Environ. 2018, 259, 147–158. [Google Scholar] [CrossRef]
- Sarker, J.R.; Singh, B.P.; Cowie, A.L.; Fang, Y.; Collins, D.; Badgery, W.; Dalal, R.C. Agricultural management practices impacted carbon and nutrient concentrations in soil aggregates, with minimal influence on aggregate stability and total carbon and nutrient stocks in contrasting soils. Soil Till. Res. 2018, 178, 209–223. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Kan, Z.; Liu, W.; Liu, W.; Lal, R.; Dang, Y.; Zhao, X.; Zhang, H. Mechanisms of soil organic carbon stability and its response to no-till: A global synthesis and perspective. Glob. Change Biol. 2021, 28, 693–710. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Morris, E.K.; Morris, D.J.P.; Vogt, S.; Gleber, S.C.; Bigalke, M.; Wilcke, W.; Rillig, M.C. Visualizing the dynamics of soil aggregation as affected by arbuscular mycorrhizal fungi. ISME J. 2019, 13, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Chaplot, V.; Cooper, M. Soil aggregate stability to predict organic carbon outputs from soils. Geoderma 2015, 243–244, 205–213. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Faust, J.C.; Tessin, A.; Fisher, B.J.; Zindorf, M.; Papadaki, S.; Hendry, K.R.; Doyle, K.A.; März, C. Millennial scale persistence of organic carbon bound to iron in Arctic marine sediments. Nat. Commun. 2021, 12, 275. [Google Scholar] [CrossRef]
- Franks, M.; Duncan, E.; King, K.; Vázquez-Ortega, A. Role of Fe- and Mn-(oxy)hydroxides on carbon and nutrient dynamics in agricultural soils: A chemical sequential extraction approach. Chem. Geol. 2021, 561, 120035. [Google Scholar] [CrossRef]
- Xu, Z.; Tsang, D.C.W. Mineral-mediated stability of organic carbon in soil and relevant interaction mechanisms. Eco Environ. Health 2024, 3, 59–76. [Google Scholar] [CrossRef]
- Miltner, A.; Bombach, P.; Schmidt-Brücken, B.; Kästner, M. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 2012, 111, 41–55. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Wichern, F. Alive and kicking: Why dormant soil microorganisms matter. Soil Biol. Biochem. 2018, 116, 419–430. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Soil Survey Staff Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; USDA Soil Conservation Service: Washington, DC, USA, 1999. [Google Scholar]
- Zhang, W.; Xu, A.; Zhang, R.; Ji, H. Review of soil classification and revision of China soil classification system. J. Integr. Agric. 2014, 47, 3214–3230. [Google Scholar]
- Cambardella, C.; Elliott, E. Carbon and nitrogen distribution in aggregates from cultivated and native grassland soils. Soil Sci. Soc. Am. J. 1993, 57, 1071–1076. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis: Part 2 Chemical and Microbial Properties; Agronomy Society of America, Agronomy Monograph: Madison, WI, USA, 1982; Volume 9. [Google Scholar]
- Xu, J.; Hou, H.; Yuan, K. Studies on organo-mineral complexes in soil Vlll. Sodium sulphate as extractant to separate calcium-bound organo-mineral complexes in soil. Acta Pedol. Sin. 1998, 35, 468–474. [Google Scholar]
- Calderón, F.J.; Mikha, M.M.; Vigil, M.F.; Nielsen, D.C.; Benjamin, J.G.; Reeves, J.B., III. Diffuse-reflectance mid-infrared spectral properties of soils under alternative crop rotations in a semi-arid climate. Commun. Soil Sci. Plant Anal. 2011, 42, 2143–2159. [Google Scholar] [CrossRef]
- Demyan, M.S.; Rasche, F.; Schulz, E.; Breulmann, M.; Müller, T.; Cadisch, G. Use of specific peaks obtained by diffuse reflectance Fourier transform mid-infrared spectroscopy to study the composition of organic matter in a Haplic Chernozem. Eur. J. Soil Sci. 2012, 63, 189–199. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998, 35, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, B.; Hao, X.; Zhang, J. Effects of residue incorporation and plant growth on soil labile organic carbon and microbial function and community composition under two soil moisture levels. Environ. Sci. Pollut. Res. 2017, 24, 18849–18859. [Google Scholar] [CrossRef]
- Peng, S.; Eissenstat, D.M.; Graham, J.H.; Williams, K.; Hodge, N.C. Growth depression in mycorrhizal citrus at high phosphorus supply. Plant Physiol. 1993, 101, 1063–1071. [Google Scholar] [CrossRef]
- Pan, F.; Li, Y.; Chapman, S.J.; Yao, H. Effect of rice straw application on microbial community and activity in paddy soil under different water status. Environ. Sci. Pollut. Res. 2016, 23, 5941–5948. [Google Scholar] [CrossRef]
- Moore-Kucera, J.; Dick, R.P. PLFA profiling of microbial community structure and seasonal shifts in soils of a douglas fir chronosequence. Microb. Ecol. 2008, 55, 500–511. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.C. Aggregate Stability and Size Distribution. In Methods of Soil Analysis: Part 1, 2nd ed.; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 837–871. [Google Scholar]
- Tenenhaus, M.; Esposito, V.V.; Chatelin, Y.M.; Lauro, C. PLS path modeling. Comput. Stat. Data Anal. 2005, 48, 159–205. [Google Scholar] [CrossRef]
- Gupta, V.V.S.R.; Germida, J.J. Soil aggregation: Influence on microbial biomass and implications for biological processes. Soil Biol. Biochem. 2015, 80, A3–A9. [Google Scholar] [CrossRef]
- Zhang, W.; Munkholm, L.J.; Liu, X.; An, T.; Xu, Y.; Ge, Z.; Xie, N.; Li, A.; Dong, Y.; Peng, C.; et al. Soil aggregate microstructure and microbial community structure mediate soil organic carbon accumulation: Evidence from one-year field experiment. Geoderma 2023, 430, 116324. [Google Scholar] [CrossRef]
- Zhang, M.; Song, X.; Wu, X.; Zheng, F.; Li, S.; Zhuang, Y.; Mane, X.; Degrvé, A. Microbial regulation of aggregate stability and carbon sequestration under long-term conservation tillage and nitrogen application. Sustain. Prod. Consump. 2024, 44, 74–86. [Google Scholar] [CrossRef]
- Wang, H.; Gao, J.; Yuan, W.; Li, Y.; Gao, Y. Spatially heterogeneous characteristics of surface soil particles around nebkhas in the Gobi Desert. Chin. J. Plant Ecol. 2013, 37, 464–473. [Google Scholar] [CrossRef]
- He, J.; Shi, W.; Liu, F.; Ma, Q.; Yang, Y.; Yang, J.; Cui, H.; Chen, Y. Effects of Fenlong cultivation on soil key physical properties in arid areas. Agric. Res. Arid Areas 2023, 3, 195–201. [Google Scholar]
- Mao, N.; Li, X.; Wei, X.; Shao, M. The effects of roots and earthworms on aggregate size distribution and their associated carbon under contrasting soil types and soil moisture conditions. Catena 2024, 246, 108434. [Google Scholar] [CrossRef]
- Qiu, L.; Wei, X.; Gao, J.; Zhang, X. Dynamics of soil aggregate-associated organic carbon along an afforestation chronosequence. Plant Soil 2015, 391, 237–251. [Google Scholar] [CrossRef]
- Jiang, X.; Xie, D. Combining ridge with no-tillage in lowland rice-base cropping system: Long-term effect on soil and rice yield. Pedosphere 2009, 19, 515–522. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, J.; Yang, L.; Gunin, A.; Yang, Y.; Peixoto, L.; Zeng, Z.; Zang, H.; Kuzyakov, Y. Diversified cropping systems benefit soil carbon and nitrogen stocks by increasing aggregate stability: Results of three fractionation methods. Sci. Total Environ. 2022, 824, 153878. [Google Scholar] [CrossRef] [PubMed]
- Kurganova, I.; de Gerenyu, V.L.; Six, J.; Kuzyakov, Y. Carbon cost of collective farming collapse in Russia. Glob. Change Biol. 2014, 20, 938–947. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Dong, L.; Wang, K.; Liu, Y.; Hai, X.; Pan, Y.; Lv, W.; Wang, X.; Shangguan, Z.; et al. Plant productivity and microbial composition drive soil carbon and nitrogen sequestrations following cropland abandonment. Sci. Total Environ. 2020, 744, 140802. [Google Scholar] [CrossRef]
- Deng, L.; Shangguan, Z.; Sweeney, S. Changes in soil carbon and nitrogen following land abandonment of farmland on the Loess Plateau, China. PLoS ONE 2013, 8, e71923. [Google Scholar] [CrossRef]
- Wu, C.; Deng, L.; Huang, C.; Chen, Y.; Peng, C. Effects of vegetation restoration on soil nutrients, plant diversity, and its spatiotemporal heterogeneity in a desert–oasis ecotone. Land Degrad. Dev. 2021, 32, 670–683. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Xu, W.; Wang, Y.; Wan, H.; Chen, D.; Tang, Z.; Tang, X.; Zhou, G.; Xie, Z.; et al. Plant diversity enhances productivity and soil carbon storage. Proc. Natl. Acad. Sci. USA 2018, 115, 4027–4032. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.K.; Li, X.; Xie, J.; Homyak, P.M.; Ukonmaanaho, L.; Yang, Z.; Liu, X.; Ruan, C.; Yang, Y. Root-microbial interaction accelerates soil nitrogen depletion but not soil carbon after increasing litter inputs to a coniferous forest. Plant Soil 2019, 444, 153–164. [Google Scholar] [CrossRef]
- Mayer, S.; Kühnel, A.; Burmeister, J.; Kögel-Knabner, I.; Wiesmeier, M. Controlling factors of organic carbon stocks in agricultural topsoils and subsoils of Bavaria. Soil Till. Res. 2019, 192, 22–32. [Google Scholar] [CrossRef]
- Qiao, Y.; Miao, S.; Han, X.; Yue, S.; Tang, C. Improving soil nutrient availability increases carbon rhizodeposition under maize and soybean in Mollisols. Sci. Total Environ. 2017, 603, 416–424. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Shi, L.; Marshall, M.R.; Kuzyakov, Y.; Blagodatskaya, E.; Zang, H. Strong priming of soil organic matter induced by frequent input of labile carbon. Soil Biol. Biochem. 2021, 152, 108069. [Google Scholar] [CrossRef]
- Mooshammer, M.; Grandy, A.S.; Calder’on, F.; Culman, S.; Deen, B.; Drijber, R.A.; Dunfield, K.; Jin, V.L.; Lehman, R.M.; Osborne, S.L.; et al. Microbial feedbacks on soil organic matter dynamics underlying the legacy effect of diversified cropping systems. Soil Biol. Biochem. 2022, 167, 108584. [Google Scholar] [CrossRef]
- Wright, A.L.; Hons, F.M.; Lemon, R.G.; McFarland, M.L.; Nichols, R.L. Microbial activity and soil C sequestration for reduced and conventional tillage cotton. Appl. Soil Ecol. 2008, 38, 168–173. [Google Scholar] [CrossRef]
- Gentsch, N.; Boy, J.; Batalla, J.D.K.; Heuermann, D.; von Wiren, N.; Schweneker, D.; Feuerstein, U.; Gross, J.; Bauer, B.; Reinhold-Hurek, B.; et al. Catch crop diversity increases rhizosphere carbon input and soil microbial biomass. Biol. Fert. Soils 2020, 56, 943–957. [Google Scholar] [CrossRef]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Change 2015, 5, 588–595. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Z.c; Chen, L.; Yang, Z.; Liu, J. Facilitated destabilization of physicochemically protected soil organic matter by root derived low-molecular-weight organic acids. J. Soils Sediments 2022, 22, 1677–1686. [Google Scholar] [CrossRef]
- Ding, Y.; Ye, Q.; Liu, M.; Shi, Z.; Liang, Y. Reductive release of Fe mineral-associated organic matter accelerated by oxalic acid. Sci. Total Environ. 2021, 763, 142937. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, G. Contribution of natural and agricultural activities on Fe dynamics: Insights from Fe isotope in soils under different land-use types. Agric. Ecosyst. Environ. 2023, 358, 108705. [Google Scholar] [CrossRef]
- Wang, A.; Zha, T.; Zhang, Z. Variations in soil organic carbon storage and stability with vegetation restoration stages on the Loess Plateau of China. Catena 2023, 228, 107142. [Google Scholar] [CrossRef]
- Dong, L.; Hcu, W.; Wang, D.; Zhang, H.; Wu, J.; Liao, Y.; Li, J.; Shangguan, Z.; Deng, L. Effect of vegetation restoration on soil iron-associated carbon dynamics: Insights from different soil textures. J. Geophys. Res. Biogeosci. 2024, 129, e2024JG008278. [Google Scholar] [CrossRef]
- Du, Y.; Ramirez, C.E.; Jaffé, R. Fractionation of dissolved organic matter by co-precipitation with iron: Effects of composition. Environ. Process. 2018, 5, 5–21. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Keiluweit, M.; Yang, Y.; Yang, Y.; Jin, J.; Sun, H.; Wu, F.; Xing, B. Mobilization of ferrihydrite-associated organic carbon during Fe reduction: Adsorption versus coprecipitation. Chem. Geol. 2019, 503, 61–68. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, M.; Yang, G.; Li, T.; Li, Q.; Liu, S.; Li, J. Stability and molecular fractionation of ferrihydrite-bound organic carbon during iron reduction by dissolved sulfide. Chem. Geol. 2022, 594, 120774. [Google Scholar] [CrossRef]
- Bao, Y.; Bolan, N.S.; Lai, J.; Wang, Y.; Jin, X.; Kirkham, M.B.; Xu, X.; Fang, Z.; Zhang, Y.; Wang, H. Interactions between organic matter and Fe(hydr)oxides and their influences on immobilization and remobilization of metal(loid)s: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 4016–4037. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Qu, C.; Chen, W.; Chen, C.; Cai, P.; Huang, Q. Diverse regulations on the accumulation of fungal and bacterial necromass in cropland soils. Geoderma 2022, 410, 115675. [Google Scholar] [CrossRef]
- Song, X.; Wang, P.; Van Zwieten, L.; Bolan, N.; Wang, H.; Li, X.; Cheng, K.; Yang, Y.; Wang, M.; Liu, T.; et al. Towards a better understanding of the role of Fe cycling in soil for carbon stabilization and degradation. Carbon Res. 2022, 1, 5. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lam, H.; Nguyen, H.T.; Siddique, K.H.M.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Hayes, F.J.; Borden, K.A.; Buchanan, S.W.; Gordon, A.M.; Isaac, M.E.; Thevathasan, N.V. Integrating nitrogen fixing structures into above-and belowground functional trait spectra in soy (Glycine max). Plant Soil 2019, 440, 53–69. [Google Scholar] [CrossRef]
- Gou, X.; Reich, P.B.; Qiu, L.; Shao, M.; Wei, G.; Wang, J.; Wei, X. Leguminous plants significantly increase soil nitrogen cycling across global climates and ecosystem types. Glob. Change Biol. 2023, 29, 4028–4043. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, J.; Liu, C.; Jia, R.; Mgang, K.Z.; Yang, L.; Yang, Y.; Peixoto, L.; Zang, H.; Zeng, Z. Legume-based crop diversification reinforces soil health and carbon storage driven by microbial biomass and aggregates. Soil Till. Res. 2023, 234, 105848. [Google Scholar] [CrossRef]
- Zhang, B.; He, H.; Ding, X.; Zhang, X.; Zhang, X.; Yang, X.; Filley, T.R. Soil microbial community dynamics over a maize (Zea mays L.) growing season under conventional- and no-tillage practices in a rainfed agroecosystem. Soil Till. Res. 2012, 124, 153–160. [Google Scholar] [CrossRef]
- Li, Y.; Song, D.; Liang, S.; Dang, P.; Qin, X.; Liao, Y.; Siddique, K.H.M. Effect of no-tillage on soil bacterial and fungal community diversity: A meta-analysis. Soil Till. Res. 2020, 204, 104721. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Panchal, P.; Preece, C.; Peñuelas, J.; Giri, J. Soil carbon sequestration by root exudates. Trends Plant Sci. 2022, 27, 749–757. [Google Scholar] [CrossRef]
- Wei, L.; Ge, T.; Zhu, Z.; Ye, R.; Peñuelas, J.; Li, Y.; Lynn, T.M.; Jones, D.L.; Wu, J.; Kuzyakov, Y. Paddy soils have a much higher microbial biomass content than upland soils: A review of the origin, mechanisms, and drivers. Agric. Ecosyst. Environ. 2022, 326, 107798. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Tu, C.; Hoyt, G.D.; DeForest, J.L.; Hu, S. Long-term no-tillage and organic input management enhanced the diversity and stability of soil microbial community. Sci. Total Environ. 2017, 609, 341–347. [Google Scholar] [CrossRef]


| Sample ID | Seasonal Gap | Preceding Gap | Average Annual Fertilizer Application (kg/ha/year) | pH | OC (g/kg) | TN (g/kg) | Particle Composition (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P2O5 | K2O | Manure | Sand | Clay | Silt | ||||||
| CK | Weed | Weed | 0 | 0 | 0 | 0 | 5.7 | 5.9 | 0.4 | 25.2 | 58.5 | 16.3 |
| CT | Fallow | Tobacco | 212.5 | 109.8 | 563 | 0 | 5.4 | 21.6 | 1.6 | 42.3 | 52.5 | 5.2 |
| RPT | Pea | Tobacco | 210 | 99.8 | 504.8 | 0 | 4.7 | 16.5 | 1.1 | 12.5 | 59.8 | 27.7 |
| CD | Fallow | Dasheen | 180 | 180 | 180 | 400 | 4.1 | 28.5 | 2.1 | 21.5 | 67.7 | 10.8 |
| RDR | Ryegrass | Dasheen | 180 | 180 | 180 | 400 | 4.7 | 32.0 | 2.0 | 18.3 | 55.5 | 26.5 |
| Enzyme | EC Number | Substrate | Reaction Principle | Measured Product | Detection Wavelength |
|---|---|---|---|---|---|
| β-glucosidase | 3.2.1.21 | p-Nitrophenyl-β-D-glucopyranoside | Hydrolysis: pNPG → p-Nitrophenol + Glucose | p-Nitrophenol | 405 |
| Peroxidase | 1.11.1.7 | Pyrogallol + H2O2 | H2O2-dependent oxidation: Pyrogallol → Purpurogallin | Purpurogallin chromophore | 470 |
| Polyphenol oxidase | 1.10.3.1 | Pyrogallol/L-DOPA | Oxidation: Pyrogallol → Purpurogallin (O2-dependent) | Purpurogallin chromophore | 430 |
| Invertase | 3.2.1.26 | Sucrose (8% w/v) | Hydrolysis: Sucrose → Glucose + Fructose | Reducing sugars | 540 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gou, X.; Wang, X.; Wang, X.; Cai, Y.; Li, B.; Zhang, Y.; Han, L. Regulatory Mechanisms of Medium-Term Crop Rotation on Soil Organic Carbon Storage in Red Soils at the Aggregate Level. Agriculture 2025, 15, 1460. https://doi.org/10.3390/agriculture15141460
Gou X, Wang X, Wang X, Cai Y, Li B, Zhang Y, Han L. Regulatory Mechanisms of Medium-Term Crop Rotation on Soil Organic Carbon Storage in Red Soils at the Aggregate Level. Agriculture. 2025; 15(14):1460. https://doi.org/10.3390/agriculture15141460
Chicago/Turabian StyleGou, Xiaomei, Xiangning Wang, Xuemei Wang, Yan Cai, Bing Li, Yi Zhang, and Lihong Han. 2025. "Regulatory Mechanisms of Medium-Term Crop Rotation on Soil Organic Carbon Storage in Red Soils at the Aggregate Level" Agriculture 15, no. 14: 1460. https://doi.org/10.3390/agriculture15141460
APA StyleGou, X., Wang, X., Wang, X., Cai, Y., Li, B., Zhang, Y., & Han, L. (2025). Regulatory Mechanisms of Medium-Term Crop Rotation on Soil Organic Carbon Storage in Red Soils at the Aggregate Level. Agriculture, 15(14), 1460. https://doi.org/10.3390/agriculture15141460






