Abstract
Cultivated pasture grasses contribute forage to more than 40% of cattle produced in 11 southern states in the USA. In recent years the increasing intoxication of cattle feeding on pasture grasses raised serious concerns about their palatability. While molecular and metagenomics techniques have revealed the great diversity of microbial composition and functional richness of the grass endosphere microbiome, meta-sequencing techniques enable us to gain a bird’s-eye view of all plant-associated microbiomes as a ‘holobiont’. Plant holobionts provide a more comprehensive approach where one can define the functions of microbial communities and feedback between the core and satellite microbiomes of a targeted host. In the near future we will be able to tailor our grasses and their endosphere microbiomes through the host-directed selection of a ‘modular microbiome’, leading to ‘plant enhanced holobionts’ as a microbiome-driven solution to managing the intoxication of pasture grasses in livestock. The present review aims to understand the potential co-relation between the endosphere microbiome community composition and mycotoxin production in forage grasses in the southern United States.
1. Introduction
There are more than 100 native grasses widely cultivated across the southern United States, most commonly found in Florida, North/South Carolina, Georgia, Mississippi, Louisiana, Oklahoma, Texas, Tennessee, Arkansas, and Alabama. About 16 exotic invasive grasses were also introduced from Africa during 16th century, mostly as forage [1]. Many of these grasses in the southern US are warm-season and vegetative in nature, and many have been widely planted across different niches. The most popular warm-season grasses are Bahia (Paspalum notatum), Centipede (Eremochloa ophiuroides), Star grass (Cynodon nlemfuensis), St. Augustine (Stenotaphrum secundatum), Bermuda (Cynodon dactylon), Limpo (Hemarthria altissima), Signal grass (Brachiaria decumbens), and Zoysia (Zoysia japonica) grasses, which fall into the Category-II classification of FLEPPC (The Florida Exotic Pest Plant Council). However, in recent studies, elevated mycotoxin levels in several species of forage grasses has been observed, which poses serious health issues in livestock and other herbivores [2,3,4]. Several studies suggested the presence of microbial endophytes and their associated mycotoxins in many of these popular forage grass species [2,3,4,5]. Interestingly, both the community of microbial associations and the mycotoxin concentrations suggested a correlation between each other, depending on seasonal and environmental factors [2,6]. The associated endophytic microbial community of these grasses provides beneficial characteristics to the plant; however, naturally occurring filamentous fungi microbes produce toxic secondary metabolites such as aflatoxin, citrinin, fumonisins, ochratoxin A, patulin, trichothecenes, zearalenone, etc., causing livestock intoxication. Toxins produced by these endophytes are in part responsible for the poisonings of mammalian grazers and plant resistance to insect herbivores [2,3,4]. There are several microbial communities associated with grasses that are either directly or indirectly associated with the toxin production. A plethora of microbial inhabitants of grasses were identified as the most common producers of mycotoxins such as Balansia, Myriogenospora, Atkinsonella, Claviceps, Alternaria, Fusarium, Aspergillus, Penicillium, Neotyphodium, and Epichloë. Not only fungi produce toxins; some bacterial strains like Bacillus, Pseudomonas, Paenibacillus, Micrococcus, Staphylococcus, Pantoea, and Acinetobacter also produce toxins [5,6,7], and many of them, if not all, are known to cause serious toxicosis in cattle. Fescue toxicosis, caused by the fungal endophyte Sphacelia typhina, is the best studied system to exemplify this [8,9].
There are several recent studies available, where researchers investigated and surveyed the presence of toxins in forage grasses and their toxic effects on grazing mammals (Table 1). For example, a recent survey by Gott et al. [10] indicated that common Bermuda grass (Cynodon dactylon) pastures contain 61% of Zearalenone (ZEA), a very potent estrogenic mycotoxin in south Florida. ZEA concentration was higher during winter because of plant dormancy, which makes it more vulnerable to Fusarium infection, leading to elevated ZEA production. Under normal summer conditions, the ZEA concentration dropped below 250 ppb [11,12]. However, Gallo et al. (2015) [4] observed no negative effect on the livestock, even though the concentration of ZEA was found to be higher than the recommended limit of 250 ppb. It is now a widely accepted fact that the production of mycotoxins is regulated by several key factors that include host species, species of causal agents, and other environmental factors [4]. According to Gott et al. [3], a mycotoxin survey of southern USA pastures identified the potential for high levels of contamination in several grasses with ZEN, type-A trichothecenes (T-2 toxin and HT-2 toxin), and type B trichothecenes, such as deoxynivalenol (vomitoxin), nivalenol, and fusarenon X, which causes perennial ryegrass staggers and paspalum staggers in livestock.

Table 1.
The identification of dominant endosphere microbiomes in grass species of the southern United States.
This review aims to discuss the recent advances in understanding the structure and functions of microbial communities of grasses in general, and warm-season grasses in particular. Endophyte–grass interactions and the crosstalk between plant and microbial community dynamics have recently been linked in with regard to the toxigenic nature of grasses. This work tries to understand ‘plant-holobionts’ metagenomics and their ecological functions at the nested level of hierarchy, which might enable the exploration of microbiome-driven solutions for the management of pasture grass toxicity to livestock.
2. Grass–Endophyte Microbial Community Dynamics
2.1. Composition, Distribution, and Diversity of Endosphere Microbiome
The endosphere microbiome is a highly diverse compartment of the ‘plant-holobiont’ and is affected significantly by eco-location, soil type, and host genotype [17,18,19]. While there are many reports on the endosphere microbiome of non-grass hosts, relatively fewer reports exist on the microbial community associated with the grasses [20,21,22,23,24,25,26,27]. The grass endophytes have been studied mostly as commensalistic symbionts, latent pathogens and/or saprotrophs, or mutualistic partners [28]; however, non-mycorrhizal root-associated microbes and Dark Septate Endophytes (DSE) have also been studied very frequently in arid, semiarid, and temperate grassland ecosystems of Southern America and Europe [29,30,31,32,33]. There has been an increased focus on the grass endosphere of Asian grasslands, especially in China [34,35,36,37], and studies have indicated the presence of a core member of the microbial community regardless of grass type, compartmentalization, or edaphic factors [38,39,40] (Table 2). Ugarelli et al. [41] studied two common seagrasses of Florida Thalassia testudinum (turtle grass) and Syringodium filliforme (manatee grass) to determine the microbiome composition of their Phyllosphere and Rhizosphere. A comparative analysis of the microbial community of leaves and rhizomes shows about 47 percent similarity between them, suggesting that a core microbiome is present in all species of seagrasses [42]. Ghimire et al. [43] studied the fungal communities of switch grass (Panicum virgatum) and found comparatively higher species diversity in shoot tissue than in communities from the root tissue. Gagne-Bourgue et al. [23] found that the bacterial community of switch grass contained Microbacterium testaceum, Curtobacterium flaccumfaciens, Bacillus subtilis, Pseudomonas fluorescens, Sphingomonas parapaucimobilis, Serratia sp., and Pantoea ananatis as dominant bacterial species. The study also re-inoculated the roots of switch grass seedlings with these endophytes singly and in combination, which established their ability to migrate to the upper aerial parts of the plant. It has also been observed that a high abundance of taxa from the order Hypocreales (to which mutualistic, clavicipitaceous endophytes of grasses belong) was found in 64% in shoots throughout the growing season. These studies suggested that the microbial community of grasses was not only distinct in different sample types (seeds/aerial parts), but also very much distinct from the surrounding areas. The microbial community structure of Panicum grass, a close relative of switch grass species from Texas, was observed to have a relatively large core endophytic microbiome dominated by the ubiquitously root-colonizing bacterial genera Streptomyces, Pseudomonas, and Bradyrhizobium [44]. This study concluded that sampling sites may impact the rhizosphere community, but the endosphere community remained the same, and the core microbial assembly of phyllosphere microbiota remained consistent across the growing season in Panicum sp. [45]. Several studies have noted that agricultural and grassland management practices also influence the endosphere microbial communities, such as one study that investigated the effect of fertilizer application on fungal endophyte communities and their life strategies in the aerial tissues of three agriculturally important grass species (Dactylis glomerata, Festuca rubra, and Lolium perenne) over two consecutive years [46,47], which found that the effects of grassland management practices on endophyte communities differed not only among grass species, but also between sampling years. Contrary to changes in community composition, the ecological life strategies did not differ among the grass species. The rapid changes in grassland ecosystem due to the domination of non-native exotic species over native species can also influence microbial community composition [40]. The toxic nature of pasture grasses is credited to either the grass itself or the associated fungi and bacteria which colonizes it [48]. Tall fescue and perennial ryegrass toxicosis are classic examples of grass-associated endophytes, which are also associated with livestock intoxication in the United States [3,4,10,49,50]; however, not all endophytes are associated with livestock toxicosis. In Texas, annual ryegrass forage yields were enhanced when the plants were infected with a Neotyphodium endophyte, but no adverse effects on animals consuming the infected plants were reported [51]. There are several other endophyte associations with turf grasses which clearly provide biological protection to their hosts, without causing livestock toxicosis [52]. As with the genetic variation in plant germplasm, the genetic variation in endophytes is also potentially beneficial [53]; however, there are several reports demonstrated that the fungal association of pasture grasses causes toxicosis [54].
It is noticed that native and exotic plant-dominated areas had significantly different fungal community compositions and structures, but not diversity. This conversion of native to exotic C4-dominated grasslands more strongly impacts fungal than bacterial community structure and composition [40]. Brachiaria decumbens, also known as signal grass, is another southern grazing forage grass, widely grown in southern Florida for its high forage quality and ability to thrive in warmer climates. Steroidal saponins such as dichotomin, protodioscin, and dioscin, found in signal grasses, are related to secondary hepatogenous photosensitization, which is a very common symptom in livestock that graze on them. Steroidal saponins are birefringent crystals that blocks the bile ducts, resulting in elevated liver enzymes, associated with intoxicated animals [55]. B. decumbens causes hepatogenous photosensitization in grazing animals, and affected animals develop photophobia and severe dermatitis. It was suggested earlier that the photosensitization outbreaks seen in cattle grazing on Brachiaria decumbens pastures may have been caused by the mycotoxin sporidesmin rather than by intrinsic factors in the grass; however, later convincing evidence was found that confirms that steroidal saponins such as dichotomin, protodioscin, and dioscin were primarily involved. Sporidesmin and other hepatotoxic mycotoxins could certainly have a synergistic effect [56].

Table 2.
The representative references that determined grass mycotoxins in the southern United States.
Table 2.
The representative references that determined grass mycotoxins in the southern United States.
| Plant Species | Type | Locations | Compartments | Dominant Microbial Communities | Refs. |
|---|---|---|---|---|---|
| Bahia Grass (Paspalum notatum) | Warm season | Northwest Florida | Leaves and inflorescence | Sordariomycetes, Glomeromycetes, Dothideomycetes, and Agaricomycetes Penicillium spp., Fusarium sp. | [12,32] |
| Ryegrass (Lolium perenne L.) | Cool season | Southern USA, Australia, and New Zealand | The base, leaves, and inflorescence | E. festucae var. lolii or Neotyphodium lolii, L. perenne | [57,58] |
| Switch Grass (Panicum virgatum) | Warm Season | Central North America, Texas, Minnesota, and Oklahoma | The shoot and root tissues | Alternaria, Epicoccum, Phoma, Phaeosphaeria, and Stagonospora | [23,24,26,43] |
| Centipede Grass (Eremochloa ophiuroides) | Warm Season | Southeastern US and Hawaii | The shoot and root tissues | Neotyphodium coenophialum | [10,51] |
| Star Grass (Cynodon nlemfuensis) | Warm Season | Florida and Texas | Aerial parts | Sarocladium sp., Penicillium spp., Aspergillus, and Cladosporium spp. | [3,4] |
2.2. Mycotoxin Production in Grasses: The Role of Associated Microbes
Grasses have several mechanisms that may deter grazing, including the fungal induction of toxins, hosting toxin-producing endophytes, and enhanced uptake of silicon, which alleviates the toxic effects caused by abiotic stresses, e.g., salt stress, drought, and heavy metals [56,59]. Grasses tolerate herbivory through the rapid re-growth of plant tissues and do not rely on chemical defense strategies [60]. Fungal symbiosis can alter the plant’s relation to herbivory through the synthesis of herbivore-deterring mycotoxins [61].
However, this grass–fungus interaction depends on several factors, such as the genotype of the endophyte and the grass [62], and several abiotic factors, including plant nutrition [63], temperature [64], and drought conditions [65]. Microbial endophytes associated with grasses were initially identified as serious problems because of their toxicity to livestock; however in later years they have also been recognized for their beneficial attributes to their host plants [66]. Many crop plants including wheat, rice, and maize have been extensively studied for their associated microbes and their function in growth promotion, nutrient scavenging, nitrogen fixation, and pathogen antagonism [67,68]. However, not all microbial associations with host grasses are advantageous to the host plant, as some associations, like that with the endophyte Epichloë festucae ver. lolii, cause toxicity to grazers by producing lolitrem B, ergovaline, and piramine [69]. However another strain of Epichloë AR1 produces neither lolitrem B nor ergovaline, but instead produces peramine, which provides resistance against Argentine Stem Weevil [70]. Strains of Epichloë also produces epoxy-janthitrems [71], which is an indole diterpene found only in perennial ryegrass infected with the strain AR37 [72]. Epichloë endophytes were discovered in tall fescue pastures in several parts of the USA; these are responsible for the vasoconstriction of blood vessels, leading to debilitating animal health and welfare [73,74]. The genome sequences of these endophytes show that despite having a gene cluster encoding toxic mycotoxins, they still require host genomic machinery for significant expression of the alkaloid [75,76,77]. There are several stimuli which can trigger the expression of toxic metabolites in endophytes, such as a limitation of food sources and the presence of competition with other organisms, alongside various environmental stresses, and, sometimes, inter-microbial interactions and potential cross signal-transduction between microbes, which also induces silent gene clusters and increases the expression of toxic metabolites [78,79]. The seasonal influence on the toxicity of these endophytes has also been observed in several studies [63,64,80]. Livestock toxicity is mostly restricted to the summer months, when the bioactivity thresholds for the herbivores recorded the maximum value [81,82,83]. Interestingly, one-year-old infected grasses seem to be non-toxic for herbivores, i.e., the younger the plant, the less toxicity it contains, due to low alkaloid production. Low alkaloid concentrations in young plants indicate that alkaloid biosynthesis may be costly, and nutrient resources are used mainly for primary metabolism such as plant and endophyte growth, rather than secondary metabolite synthesis [84].
2.3. Ideation of Microbial Manipulation to Reduce Toxin Production
Microbial community composition can be directly involved in toxin production and regulation [85,86,87]. It was observed that Acremonium zeae, a fungal endophyte of maize, has been shown to limit Aspergillus flavus colonization and thus reduce aflotoxin contamination in maize [88]. This endophyte also inhibits the growth of F. verticillioides; since F. verticillioides and A. zeae are endophytes of maize, interactions between the two may offer interesting insights into competitive traits that influence toxin production in their host. Interestingly, there are also inter-kingdom interactions between fungal–bacterial microbes which control the expression of toxic metabolites in their host. These specific interactions typically between an antagonist and a mycotoxin producer have been examined in laboratory culture or in greenhouse experiments using sterilized soil. However, testing if these microbial interactions can produce similar results under natural environmental conditions is needed. Surprisingly, the presence of specific endophytes and the difference between endophytic strains can affect some plant properties more than genetic variation intrinsic to the host [89]. The presence of endophytes can also impact on the ability of the plant to enhance its nutrient uptake ability. Some endophytic interactions can enhance plant nutrient uptake and overall health, while others can negatively impact it. These endophytes increase plant nutrient uptake and induce plant resistance to pathogens, osmotic stress, heavy metals, xenobiotic contaminants, and other forms of abiotic stress by producing phyto-siderphores, chelating agents, and nutrient solubilizations [90].
2.4. Impact of Elevated Mycotoxin Production on Herbivory and Livestock
Merely producing mycotoxins does not ensure a grass will be toxic to herbivores; a certain threshold concentration of alkaloid in the plant is needed to confer herbivore toxicity. While the larval mortality of the Argentine Stem Weevil increased when levels of peramine concentration exceeded from 2 μg g−1 [80,91], ergovaline caused symptoms in vertebrates at concentration ranges between 0.3 and 0.8 μg g−1, and lolitrem B caused symptoms at concentrations above 1.8 μg g−1 [81,82]. Similarly, a high concentration of the tremorgens paspalitrems and paspaline-like indole-diterpene was associated with low concentrations of ergine and erganovine observed in the seed-heads of mature Bermuda grass (Cynodon dactylon) infected with C. cynodontis in California, Oklahoma, and Texas [82]. Studies estimate possible intoxication risks due to the production of toxic mycotoxin through monitoring fungal load and infection rates. The greater concern with pasture grasses is that toxicity and poisoning is intermittent and highly unpredictable, unlike poisonous weed contamination of pastures. Factors associated with grasses prime the host for elevated mycotoxin production, leading to achieving a threshold for livestock toxicity in each ecosystem [60,63,64]. A study of 13 grasses, analyzed for infection rates and alkaloid production in Germany, showed that the start gene for producing ergovaline is largely absent in perennial ryegrasses in Germany, and that the substance is not produced [92]. This information confirms the hypothesis that the presence or absence of different genes both from the endophyte and the host are required to produce mycotoxins. This may be a strategy for seed developers seeking to harness the benefits of fungal infection while avoiding the negative impacts on grazing livestock. However, this does not apply to lolitrem B, another vertebrate poison found in perennial ryegrass, which is present in toxic concentrations. Multiple mycotoxins can be produced by a single endophytic fungi within the grasses, and some of them may be non-toxic for ruminants due to their low concentrations. Livestock toxicity is also associated with toxin metabolism in ruminants, such as that of zearalenone, T-2 toxin, diacetoxyscirpenol, and deoxynivalenol, which are metabolized by whole rumen fluid, whereas aflatoxin B1 and ochratoxin A are not [93], thus a threshold concentration of toxicity of a given toxin in ruminants may be affected by several factors. In addition, the selective recruitment of microbial community from the rhizo-interactome may also lead to the suppression of toxic gene expression induced by the endophytes within the host [94].
Another very important aspect of mycotoxin production is on-farm microbial propagation practices, which includes composting, AMF, and indigenous microorganisms. In many instances these practices do not follow the safety precautions, quality control, and growth kinetics monitoring and evaluations [94,95]. On-farm microbial propagation on various occasions have contributed to toxicosis by spreading pathogenic microbes and their toxins, and the best way to mitigate this is to maintain hygiene, reduce chemical fertilizers, and reduce heavy use of antibiotics. There is a need to motivate farmers to learn and adopt sustainable agricultural practices that support beneficial plant-associated microbiomes [96].
3. How Do Microbial Communities Modulate Mycotoxin Levels in Grasses?
3.1. Role of Endosphere Consortium in Mycotoxin Production
The endosphere consortium is an integral part of a plant and a major group within plant holobionts. The endosphere community is unique in that the constituent microbes are selectively chosen by the plant and are a constitute part of the rhizo-interactome (see Figure 1). Toxigenic endophytes in grasses are also part of that endosphere continuum determined by the rhizosphere/rhizoplane community. The entry and establishment of endophytes within the plants is also a crucial factor, as microbes are vertically transmitted through seeds, in addition to stomata, from where both bacteria and fungi can enter into the plant [95]. The fungus provides its hyphal surface as a niche for bacterial growth, which is an endobacterial–mycorrhizal association [96,97,98]; however, many fungal species can co-occur and impact each other for growth and mycotoxin production [99,100,101,102]. The co-occurrence of Aspergillus flavus and Fusarium verticillioides was studied, and no positive correlation was found between Aspergillus flavus and Fusarium verticillioides in fungal growth in maize, while a significant and positive correlation was observed between their relative mycotoxin levels [103]. The dynamics of multiple mycotoxins and the co-occurrence of fungi can also be investigated in the field during the growing season, considering fungal dynamics, to predict the effect on mycotoxin production [104,105].

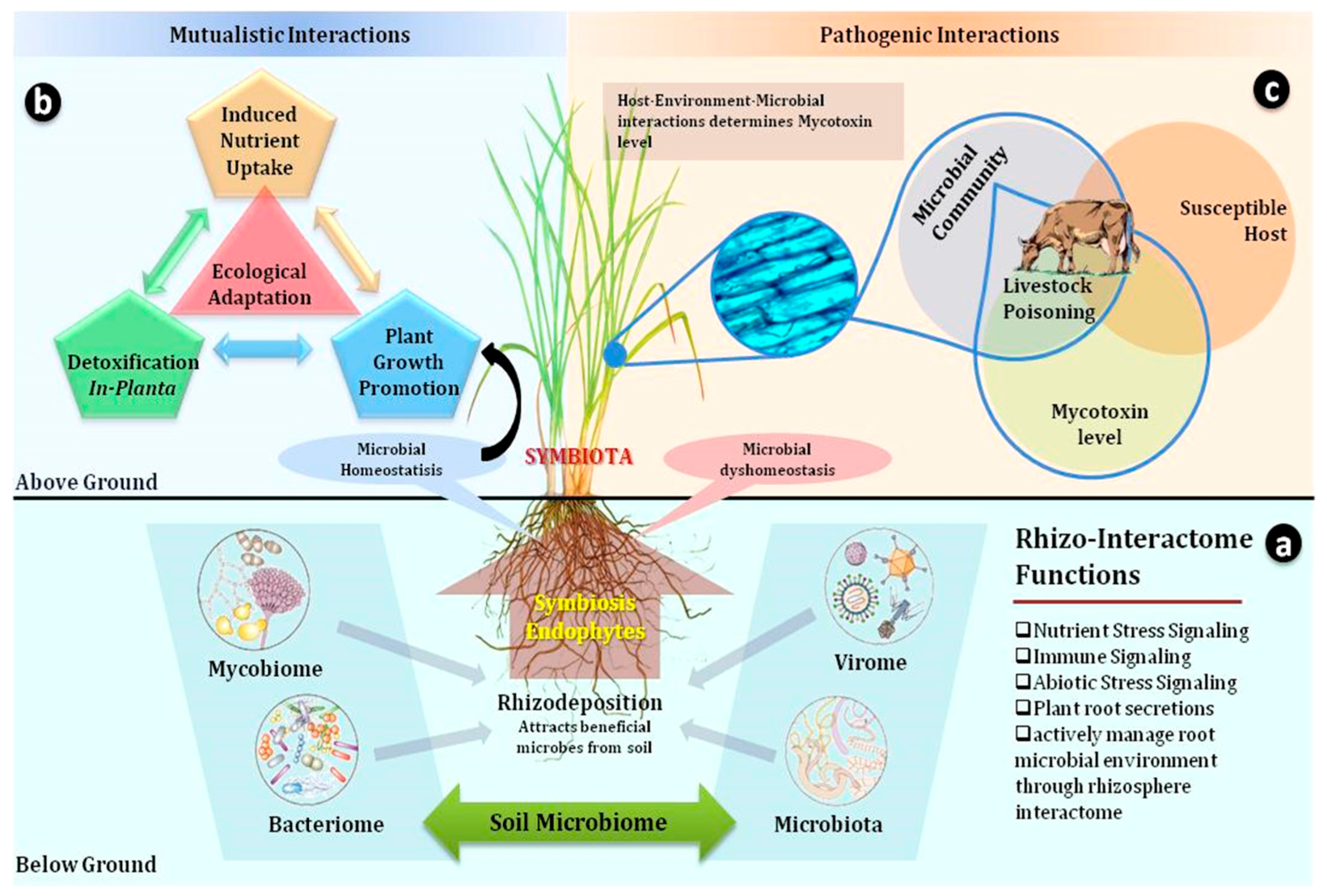
Figure 1.
Grass Holobionts; The grass–endophyte–livestock continuum involves a nested level of interwoven interactions leading to a comprehensive ‘Grass-Interactome’. (a) The major component of this interactome is the ‘Rhizo-interactome’ which helps the root to select beneficial microbes from their surrounding microbiota, mycobiome, virome, and bacteriome, and these processes are modulated by the host rhizodeposition, which attracts beneficial microbes. Once symbiota are established, they function in two directions, either to maintain microbial homeostasis or dyshomeostasis. (b) When the symbiota establish a microbial homeostasis, it facilitates a beneficial mutualistic interaction which supports the host in ecological adaptation through growth promotion, induces nutrient uptake, and allows for the in-planta detoxification of mycotoxins. (c) The establishment of toxigenic microbial symbiosis causes a microbial dyshomeostasis, which leads to livestock toxicosis. The toxigenic microbial association is dependent on host susceptibility and endophyte genotype combinations, and on several occasions, the extent of toxicity has been associated with specific toxins produced by grass–endophyte associations (symbiota).
3.2. Host Genotype and Host Selectivity in Toxin Production
Microbial toxins are classified in two categories: host-selective or -specific, and host nonspecific. The host-selective toxins (HSTs) cause toxicity to the host, which harbors the toxigenic fungi, in contrast to nonspecific toxins, which can be toxic to a wide range of susceptible plants regardless of the host or non-host of the toxigenic fungi. In the case of grass–endophyte interactions, however, host benefit and symbiotic dependence are asymmetric in nature [61,63,84,86,87]. Since the fitness and distribution of a fungus largely depends on host fitness [106], seemingly, any advantage to the host plant due to mutualistic cooperation also benefits the fungus. In contrast, reciprocal benefits such as enhanced growth [107], biotic and abiotic stress tolerance, and increased competitive abilities further support the concept of grass–endophyte mutualism [108,109,110]. Plant genotype, taxonomic identity, and plant chemical properties can affect the microbial community and diversity, and a highly diverse microbial population can create a beneficial interactome for the host plant [111,112,113,114]. In certain endophytes, switching their lifestyle to a pathogenic state is also dependent on the host genotype and local abiotic stress factors [115]. Although non-pathogenic endophytes cause no disease symptoms, they can influence the phenotype and epigenome of their host plant [116]. The mycotoxin level in grasses is linked to increased genetic heterogeneity in both the host grass and the endophyte and interactions between variable genotypes [84]. Symbiont genotype also influences the persistence of grass–endophyte symbiosis and plant community composition [117].
4. Biological Control Strategies for Mycotoxigenic Microorganisms
4.1. Microbe-Mediated Regulation of Toxin Production
Bacteria and fungi produce various secondary metabolites, including toxins, some of which are lethal to animals and human beings; however their natural functions are unknown [118]. Studies were performed to correlate microbe interaction with community-level antagonistic warfare in plant–microbiota interactions [119,120]; they found that microbial communities of the rhizo-interactome can be altered by introducing selected microbial strains that enhance the performance and growth of host plant holobionts. Microbial communication appears to regulate epigenetic modifiers that in turn control mycotoxin biosynthesis. For grass–endophyte interactions, the composition and concentration of toxic mycotoxins vary among endophytic microbial species and genotypes [13,14,121]; in contrast, the host genotype can convert the lifestyle of symbiotic microbes into pathogens [115]. Wild host grasses are more variable in their toxic alkaloid production compared to domesticated agronomic grasses [122,123], despite the fact that the number of non-toxic endophyte-infected grasses exceeds that of toxic ones [51]. The genetic basis and origin of genes encoding toxin synthesis are clustered and co-regulated and largely remain unexplored. Some researchers have proposed that horizontal gene transfer or horizontal chromosome transfer are mechanisms for microbes to acquire toxin-production genes. Comparative genomic analysis can help to dissect the biosynthetic pathways involved in toxin production. The synthetic machinery is thought to be regulated by factors that govern the quantity and quality of the produced toxin [124]. Howitz and Sinclair [125] proposed that homologous gene clusters present in hosts and symbionts may get activated by specific stress-induced molecules from the host or symbiont. For example, endophytes of Papaver somniferum in the genera Acinetobactor sp. and Marmoricola sp. upregulate the genes involved in the biosynthesis of benzylisoquinoline alkaloid [126,127], while the terpenoid-indol alkaloid pathway genes are induced in the presence of endophytic Curvularia sp. and Choanephora infundibulifera in Catharanthus roseus [128,129]. The biosynthesis of Pyrrolizidine alkaloid (PA), which is an essential class of metabolite for defense against herbivores, is dependent on the nodulation of Bradyrhizobium [130]. Thus, the hypothesis is strongly supported by these studies that suggest that the presence of specific endophytes may regulate the synthetic machinery of toxin-producing genes. Fungal endophytes also interact with pathogenic strains and reduce the production of toxins, such as trichothecenes and zearalenone, in maize [124]. Another very important aspect is the changes in the nutritional value of forage grasses, which can be significantly impacted by any biological manipulation. It has been noticed that the introduction of certain microbes could help plants mitigate the aftermath of bio-manipulation. For example, phosphorus fertilization sometime increases the insect’s activities, but this effect is mitigated in plants that host certain endophyte strains, which suppress insect activities [131]. These studies clearly indicate that endophytic symbionts have a wide range of check points in the biosynthesis of toxins in host plants that might help to develop a predictive framework for the effective management of toxicosis.
4.2. Microbial Detoxification of Produced Toxins
Grass–endophyte associations have played a vital role in chemical transformation of mycotoxin within their host [132,133,134,135]. Mycotoxins can be considered vital players in altering the microbial community within the host plant [118]; many studies show that endophytes help the host plant in herbicide tolerance by degrading it in-planta [136]. Similarly, plant-associated bacteria have been identified that can degrade and thereby detoxify atrazine or glyphosate herbicides [137,138]. Endophytes could also influence tolerance to herbicides by inducing the innate stress tolerance through the ethylene (ET)/jasmonic acid (JA)/salicylic acid (SA) pathways [136]. For example, Neotyphodium occultans, an endophyte of Lolium rigidum, showed resistance to diclofop-methyl in response to high stress tolerance [139]. Many fungal endophytes of grasses exhibit reactive oxygen species scavenging activity and enhanced antioxidant content [140], which might protect plants from the downstream toxicity induced by herbicides [141]. Most of the toxicity associated with toxigenic endophytes requires host machinery to express the fungal genes responsible for toxin production. For example, plant signaling is required to induce the expression of fungal gene clusters for lolitrem B biosynthesis in E. festucae var. lolii., and they are thus highly expressed in-planta but few or undetectable in culture-grown fungal mycelia [142]. Similar observations also were noticed in lolines and epoxy-janthitrems of Epichloë sp. that produce little or no compound when grown ex-planta. In contrast, some detoxification genes are expressed in plants to limit pre-harvest mycotoxin production and to protect crop plants from the phytotoxic effects of mycotoxins [135]. These studies provide greater insight in the understanding of endophyte–host interactions in the orchestration of toxin production, simultaneously providing keys to explore and engineer such interactions for the effective mitigation of toxicosis.
4.3. Eliciting Systemic Resistance by Colonization
Plant-associated microbes induced systemic resistance (ISR) through their colonization of the host via SA, JA, and ET pathways [143,144,145]. However, some microbial inductions of systemic resistance are totally independent of these pathways, such as the endophyte Fusarium oxysporum f. sp. lycopersici in tomato plant, which shows endophyte-mediated resistance that is independent of SA, JA, and ET pathways and differs from the classical induced resistance response [145,146]. Recent plant gene expression and microRNA (miRNA) studies suggest that the expression and modulation of gene cascades of the pathways are strongly dependent on endophytes [68]. There are potential genomic markers for the regulation of the expression of genes involved in inducing ISR and the endophytic life cycle [147,148,149]. In a study on the bacterial endophyte Azospirillum sp. B510, gene expression analysis indicated that ET signaling is required for endophyte-mediated ISR in rice [150]. However, during mutualistic interactions, late induction of the SA/JA/ET signaling pathways prevents the microbe from overriding the host plant [151]. Endophytes mediated ISR upon insect herbivory, including hypersensitive response (HR)-type reactions leading to the production of defense-related compounds like flavonoids, lignin, and other secondary metabolites that produce effective defenses against a wide range of insect herbivores, and is regulated through JA/ET and SA pathways [152,153,154]. Among these compounds, camalexin and glucosinolates have important roles in plant defenses against leaf-chewing and sap-sucking insect herbivores [152,155]. Thus, the resistance response induced by the symbiosis of plants–endophytes is systemic.
5. Recent Novel Approaches to Cope with Elevated Toxin Levels
5.1. Microbial Manipulation to Reduce Toxin Production
The rhizosphere microbiome plays a vital role in the natural selection of beneficial microbes for the host plant, which in turn influences host competitiveness, health, and productivity [156,157,158]. However, modern domesticated plants are losing their ability to recruit their own rhizosphere microbiome compared to their wild relatives [159,160], which poses serious consequences to the host in its capability to fight with biotic and abiotic stresses [161,162]. The host-associated microbial community provides tools to the host plant for fighting with disease resistance [163], growth, abiotic stress tolerance [164], nutrient acquisition [165], flowering time, and biomass production [166]. Several factors that influence this highly complex microbial community might reverse it and be deleterious for the host, such as agriculture management practices [167,168,169], grazers, and browsers [170,171]. Different host plants have different levels of tolerance to biotic and abiotic stresses, whereas in other cases, their associated microbiome enables them to overcome these conditions [172,173]; thus, it could be useful to transfer these supporting microbiotas into the plants that lack them, improving their ability to survive under stresses [156,174]. The host-specific microbiome of Bermuda grass (Cynodon dactylon), for example, was inoculated into wheat, causing a 5-day delay in the onset of drought symptoms in wheat seedlings and demonstrating that the host phenotype (‘Phenome’) can be a selective marker with which to engineer microbiomes [175]. The selective adaptation of beneficial endophytes might be another approach that can be used to engineer pasture grasses that can reduce livestock toxicosis [176]. The microbiome manipulation either ends as a perturbation or results in successful microbiome engineering, depending on several factors (Figure 2), including the recruitment of a core microbiome and the successful assembly of a ‘modular microbiome’.

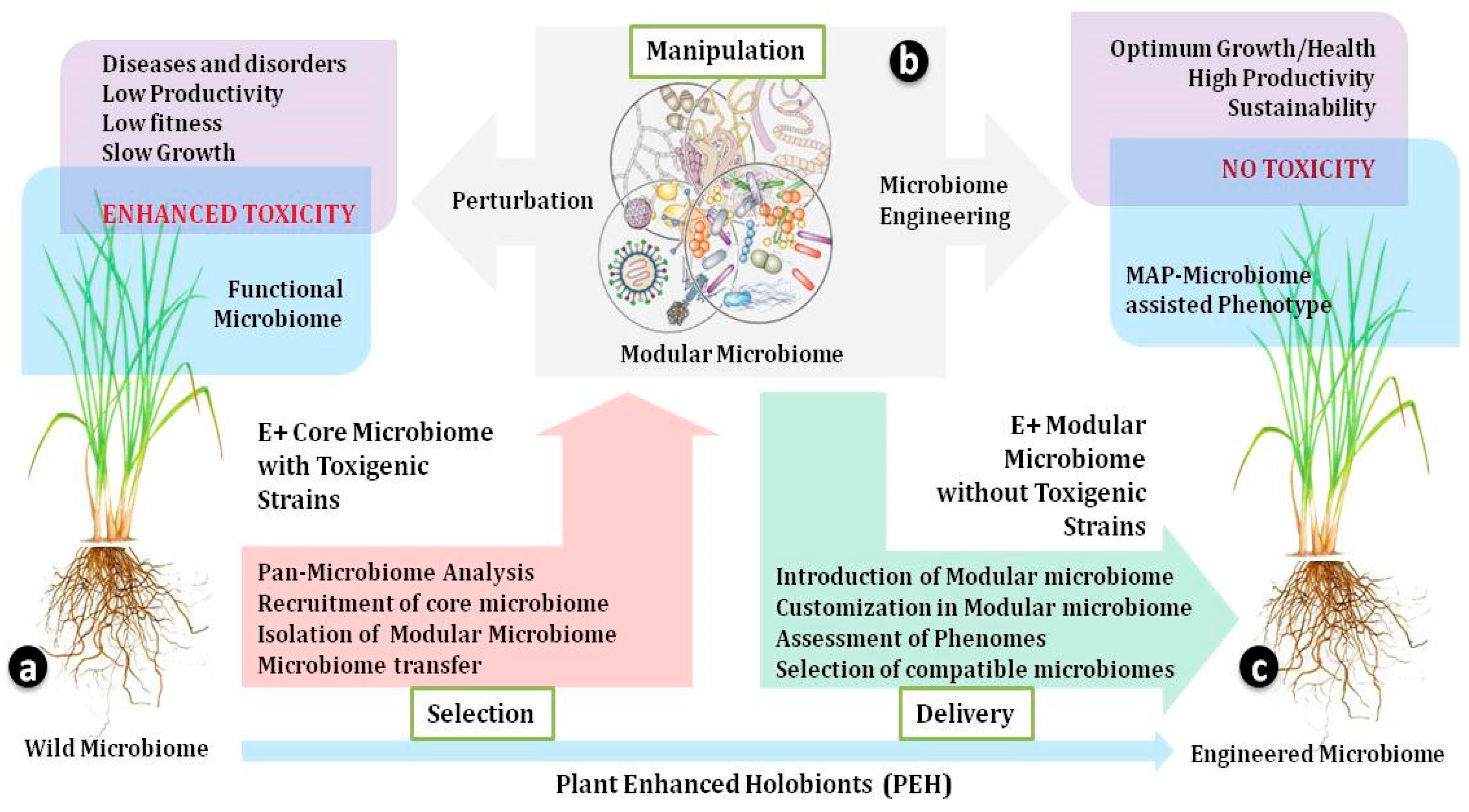
Figure 2.
Plant-Enhanced Holobionts: Grass endosphere holobionts play fundamental roles in shaping and structuring microbial networks in favor of their host. The endosphere microbiome can be engineered for through selection, manipulation, and delivery of a ‘modular microbiome’, preventing its host from reaching dysbiosis. (a) Selection: The analysis of the host pan-microbiome can help in the identification and recruitment of candidate strains, leading to the establishment of a modular microbiome ready for transfer into another host. The core microbiome of the donor host contains an endosphere (E+) functional microbiome, including toxigenic strains responsible for low productivity, fitness, and growth in the host with enhanced toxicity. (b) Manipulation: A modular microbiome is a new sub-set of the microbiome that lacks toxigenic strains and is manipulated through the artificial selection of replacement strains. The inter-microbial interactions within the plants and microbial networks for holobionts define the fate of modular microbiome introduction. Microbial manipulation may take its own course of action, becoming established in the host and exerting a beneficial impact on the host, comprising successful microbiome engineering; otherwise, it may take the route of perturbation and lead to deleterious impacts. (c) Delivery: Once the modular microbiome is ready, it gets transferred to the recipient host and displays phenomic variability due to MAPs (microbiome-assisted phenotypes), responsible for optimal growth, high productivity, and an absence of toxicity. Thus, successful plant-enhanced holobiont effort requires attention to both host–microbe and microbe–microbe interaction within the host to establish a modular microbiome in the future.
5.2. Engineered Microorganisms for Detoxification of Toxins
Grass–endophyte associations can be highly toxic for livestock due to the production of mycotoxins belonging to alkaloids and diterpenes, affecting the forage quality and agronomic value of the host plant [57,177]. Thus, to avoid livestock toxicosis without losing the benefits of symbiosis, employing endophyte–grass combinations, which are safer for livestock, has become a recent thrust area [7,50,178,179]. Many such combinations of endophytes–hosts–grasses have been developed and successfully commercialized [58]. For instance, the Epichloë occultans endophyte is an asexual hybrid with active loline biosynthetic pathways which produce N1-acetylloline, N1-acetylnorloline, N1formylloline, and N1-methylloline alkaloids, and contain indole-diterpene genes [55,57]. Large-scale screening of Epichloë was performed to identify animal-friendly strains suitable for agricultural uses, which revealed a relation between microsatellite-based haplotypes and loline alkaloid levels [121]. For example, an endophyte strain that lacks or produces a very low amount of lolitrem B and ergovaline, such as AR1 and NEA2, has been selected and introduced in perennial ryegrass and successfully commercialized [180]. Thus, the genetic modification of endophytes with beneficial traits may be used to confer additional valuable attributes to the host, following inoculation with a suitably altered endophyte [181]. The genes and genetic cascade of alkaloid biosynthesis provide obvious targets for such modifications; for example, ergovaline production has been eliminated from an Epichloë–grass association through the ablation of a peptide synthetase gene, lps, which is essential for ergopeptine biosynthesis [182]. Similarly, a single, multifunctional fungal gene, perA, is required for peramine biosynthesis, so a functional copy of the perA gene was introduced into recipient endophyte genomes by Agrobacterium tumefaciens-mediated transformation, which controlled peramine levels in-planta [181,182]. Thus, the potential for the use of genome editing techniques to produce reduced toxin production under the control of a native promoter, based on the effective expression of truncated gene copies of targeted genesis, is an exciting area of research.
5.3. Microbiome-Engineered Plants for Reduced Production of Toxins
With recent advances in next-generation sequencing techniques, and a better understanding of the grass–endophyte association, it is possible to predict host-associated microbiome genome functions [69,183,184,185]. Recent reviews provide greater insight into the use of rhizosphere and endosphere communities for host fitness and microbiome-driven strategies for the sustainable management of host performance under biotic and abiotic stresses [13,15,16,156,186]. Livestock toxicosis associated with grass–endophyte interactions has been a serious problem in the USA, with an estimated loss of about USD 1 billion annually. Understanding the causes of toxicosis, while using endophyte-enhanced grasses as forage, is important [69]. Previous studies have used genetic transformation technology to specifically knock out the production of mycotoxins that cause livestock toxicosis: for example, the elimination of ergovaline from a grass–Neotyphodium endophyte symbiosis by the genetic modification of the endophyte. For the grass–Neotyphodium endophyte, a knockout analysis was performed to establish the function of a peptide synthetase required for ergovaline production, demonstrating that ergovaline can be eliminated from a Neotyphodium grass symbiosis by the genetic modification of the endophyte [182]. The gene cloning of alkaloid biosynthetic pathways and their potential manipulation can provide vulnerable targets in plant–fungus associations via knockout and antisense strategies. Besides genetic manipulation, host-mediated microbiome engineering has recently become a new tool to fight livestock toxicosis in grass–endophyte symbiosis. By utilizing a host-centric selection approach, the microbiota is selected at the community level using artificial selection to alter the microbiome through both ecological and evolutionary processes; this approach is known as the ‘Modular microbiome’ concept [187]. It has been achieved in wheat, wherein six rounds of artificial selection of beneficial microbiota was performed at the community level in Bermuda grass (Cynodon spp.), and then this was introduced into wheat, leading to a 5-day delay in the onset of drought tolerance [175]. This is a classic example of a plant microbiome being engineered to achieve a desired trait: plant adaptation to water. The concept of ‘Modular Microbiome’ is gaining attention, where synthetic microbial consortia are engineered and manipulated by integrating host and microbial traits called microbiome-associated phenotypes (MAPs). These MAPs, having a genetic basis, provide a means for both natural and artificial selection for plant-enhanced holobionts (PEHs) [183,184,185]. Plant-enhanced holobionts involve inherent protocols for host-centric selection, and the manipulation and delivery of a ‘modular microbiome’, giving its host the ability to survive biotic and abiotic stresses. The selection, manipulation, and delivery triangle is the core framework for a new PEH initiative- (a) Selection: The analysis of host pan-microbiome can help in the identification and recruitment of candidate strains, leading to the establishment of a modular microbiome ready for transfer into another host. The core microbiome of the donor host contains an endosphere (E+) functional microbiome, including toxigenic strains, responsible for the low productivity, fitness, and growth of the host, with enhanced toxicity. (b) Manipulation: A modular microbiome is a new microbiome sub-set that lacks toxigenic strains and is manipulated through artificial selection of replacement strains. Inter-microbial interactions within the plants and microbial networks for holobionts define the fate of modular microbiome introduction. Microbial manipulation may take its own course of action, either establishing itself in the host and exerting beneficial impacts on it, resulting in successful microbiome engineering, or taking the route of perturbation and leading to deleterious impacts. (c) Delivery: Once the modular microbiome is ready, it gets transferred to the recipient host and displays phenomics variability due to MAPs (microbiome assisted phenotypes), which are responsible for optimal growth, high productivity, and an absence of toxicity (Figure 2). Thus, a successful plant-enhanced holobiont requires attention to both host–microbe and microbe–microbe interactions within the host to establish a modular microbiome in the future.
6. Conclusions
The grass–endophyte–livestock continuum and its complex beneficial associations and ecological functions are very important aspects of the management of forage livestock toxicosis. Mammalian toxicosis from grasses has gained attention due to the need for a better understanding of grass–endophyte associations at nested levels of hierarchy, which will further contribute to the sustainability and resiliency of these important grass-based agro-ecosystems. With the increasing importance of both the economic and environmental sustainability of grass–endophyte symbioses, it is imperative that we consider a holistic approach to managing the diversity and complexity of microbial interactions with each other and with their hosts. The recent advancements in plant genomes and the microbial genes of their associated microbiota have led to a new unit of selection, ‘the holobiont’, which not only helps plants to remain fit, but also offers critical genetic variability, allowing them to develop high-yielding, disease-tolerant, or drought-resistant varieties, with relatively lower livestock intoxication. With a gradual advancement and understanding of grass–endophyte interactions, the concept of a ‘plant enhanced holobionts’ approach to the selective adaptation of beneficial traits can be tested to engineer pasture grasses that can reduce livestock toxicosis. A better understanding of lifestyle characteristics can also provide clues for engineering the microbial community for sustainable grassland pasture grass management. Thus, a new avenue of research is open for exploration that could revolutionize the very idea of ‘microbiome manipulation’.
Author Contributions
Conceptualization, V.C.V. and I.K.; writing—original draft preparation, V.C.V.; writing—review and editing, V.C.V. and I.K.; supervision, I.K.; project administration, I.K.; funding acquisition, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by Agriculture and Food Research Initiative Competitive, Grant no. 2019-67013-29107, from the USDA National Institute of Food and Agriculture, and the Florida Cattle Enhancement Fund 2019-2020, Subcontract No. 25.
Data Availability Statement
No original data were produced for this manuscript; this is a review article based on the literature from 2015 to 2023.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FLEPPC. List of Invasive Plant Species. Florida Exotic Pest Plant Council. 2019. Available online: https://www.floridainvasives.org/ (accessed on 25 June 2025).
- Blount, A.R. Presence of grass endophytes and mycotoxins in Florida pastures? In Proceedings of the 68th Annual Florida Beef Cattle Short Course Proceedings. Connecting the Dots, Calf to Carcass, Gainesville, FL, USA, 8–10 May 2019. [Google Scholar]
- Gott, P.; Stam, A.; Johns, A.; Hendel, E.; Mendoza, S.; Hofstetter-Schahs, U.; Bell, B.; Murugesan, G. 424 Mycotoxin survey of southern US pasture grasses. J. Anim. Sci. 2018, 96, 209. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on mycotoxin issues in ruminants: Occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef] [PubMed]
- Wemheuer, F.; Wemheuer, B.; Daniel, R.; Vidal, S. Deciphering bacterial and fungal endophyte communities in leaves of two maple trees with green islands. Sci. Rep. 2019, 9, 14183. [Google Scholar] [CrossRef]
- Tidke, S.A.; Kiran, S.; Giridhar, P.; Gokare, R.A. Current understanding and future perspectives of endophytic microbes vis-a-vis production of secondary metabolites. In Endophytes and Secondary Metabolites. Reference Series in Phytochemistry; Jha, S., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Card, S.; Johnson, L.; Teasdale, S.; Caradus, J. Deciphering endophyte behaviour: The link between endophyte biology and efficacious biological control agents. FEMS Microb. Ecol. 2016, 92, 114. [Google Scholar] [CrossRef]
- Bacon, C.W.; Porter, J.K.; Robbins, J.D.; Luttrell, E.S. Epichloe typhina from toxic tall fescue grasses. Appl. Environ. Microbiol. 1977, 34, 576–581. [Google Scholar] [CrossRef]
- Saikkonen, K.; Lehtonen, P.; Helander, M.; Koricheva, J.; Faeth, S.H. Model systems in ecology: Dissecting the endophyte-grass literature. Trends Plant Sci. 2006, 11, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Gott, P.N.; Stam, A.; Johns, A.; Miller, B.G.; Bell, B.; Jenkins, T.; Murugesan, T.R. Mycotoxin survey of common bermuda grass in south-central Florida. J. Anim. Sci. 2017, 95, 19–20. [Google Scholar] [CrossRef]
- Whitlow, L.W.; Hagler, W.M., Jr. Mold and Mycotoxin Issues in Dairy Cattle: Effects, Prevention and Treatment. eXtension. 2017. Available online: https://dairy-cattle.extension.org/mold-and-mycotoxin-issues-in-dairy-cattle-effects-prevention-and-treatment/ (accessed on 11 July 2018).
- Beule, L.; Chen, K.H.; Hsu, C.M.; Mackowiak, C.; Dubeux, J.C.B.; Blount, A.; Liao, H.L. Soil bacterial and fungal communities of six bahiagrass cultivars. Peer J. 2019, 7, 7014. [Google Scholar] [CrossRef]
- Bony, S.; Pichon, N.; Ravel, C.; Durix, A.; Balfourier, C.; Guillaumin, J.J. The relationship between myotoxin synthesis in fungal endophytes of Lolium perenne. New Phytol. 2001, 152, 125–137. [Google Scholar] [CrossRef]
- Chen, K.; Marcón, F.; Duringer, J.; Blount, A.; Mackowiak, C.; Liao, H. Leaf Mycobiome and Mycotoxin Profile of Warm-Season Grasses Structured by Plant Species, Geography, and Apparent Black-Stroma Fungal Structure. Appl. Environ. Microbiol. 2022, 88, e00942-22. [Google Scholar] [CrossRef]
- Afzal, F.; Li, H.; Gul, A.; Subhani, A.; Ali, A.; Mujeeb-Kazi, A.; Ogbonnaya, F.; Trethowan, R.; Xia, X.; He, Z.; et al. Genome-wide analyses reveal footprints of divergent selection and drought adaptive traits in synthetic-derived wheats. G3 Genes Genomes Genet. 2019, 9, 1957–1973. [Google Scholar] [CrossRef]
- Skladanka, J.; Adam, V.; Dolezal, P.; Nedelnik, J.; Kizek, R.; Linduskova, H.; Mejia, J.E.A.; Nawrath, A. How do grass species, season and ensiling influence mycotoxin content in forage? Int. J. Environ. Res. Public. Health 2013, 10, 6084–6095. [Google Scholar] [CrossRef]
- Bever, J.D.; Platt, T.G.; Morton, E.R. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbiol. 2012, 66, 265–283. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Trimblay, J.; Engelbrektson, A.; Kunin, V.; Glavina Del Rio, T.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Wagner, M.; Lundberg, D.; del Rio, T.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 2016, 7, 12151. [Google Scholar] [CrossRef]
- Allgaier, M.; Reddy, A.; Park, J.I.; Ivanova, N.; D’haeseleer, P.; Lowry, S.; Sapra, R.; Hazen, T.C.; Simmons, B.A.; Hugenholtz, P. Targeted discovery of glycoside hydrolases from a switch grass-adapted compost community. PLoS ONE 2010, 5, 8812. [Google Scholar] [CrossRef]
- Gladden, J.M.; Allgaier, M.; Miller, C.S.; Hazen, T.C.; Vander Gheynst, J.S.; Hugenholtz, P.; Simmons, B.A.; Singer, S.W. Glycoside hydrolase activities of thermophilic bacterial consortia adapted to switch grass. Appl. Environ. Microbiol. 2011, 77, 5804–5812. [Google Scholar] [CrossRef]
- D’haeseleer, P.; Gladden, J.M.; Allgaier, M.; Chain, P.S.G.; Tringe, S.G.; Malfatti, S.A.; Aldrich, J.T.; Nicora, C.D.; Robinson, E.W.; Paša-Tolić, L.; et al. Proteogenomic analysis of a thermophilic bacterial consortium adapted to deconstruct switch grass. PLoS ONE 2013, 8, 68465. [Google Scholar]
- Gagne-Bourgue, F.; Aliferis, K.A.; Seguin, P.; Rani, M.; Samson, R.; Jabaji, S. Isolation and characterization of indigenous endophytic bacteria associated with leaves of switch grass (Panicum virgatum L.) cultivars. J. Appl. Microbiol. 2013, 114, 836–853. [Google Scholar] [CrossRef]
- Xia, Y.; Greissworth, E.; Mucci, C.; Williams, M.A.; Debolt, S. Characterization of culturable bacterial endophytes of switch grass (Panicum virgatum L.) and their capacity to influence plant growth. GCB Bioenergy 2013, 5, 674–682. [Google Scholar] [CrossRef]
- Piao, H.; Lachman, M.; Malfatti, S.; Sczyrba, A.; Knierim, B.; Auer, M.; Tringe, S.G.; Mackie, R.I.; Yeoman, C.J.; Hess, M. Temporal dynamics of fibrolytic and methanogenic rumen microorganisms during in-situ incubation of switch grass determined by 16S rRNA gene profiling. Front. Microbiol. 2014, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Guo, L.; Niu, M.; Miao, F.; Jiao, S.; Hu, T.; Long, M. Ecological diversity and co-occurrence patterns of bacterial community through soil profile in response to long-term switch grass cultivation. Sci. Rep. 2017, 7, 3608. [Google Scholar]
- Chen, H.; Yang, Z.K.; Yip, D.; Morris, R.H.; Lebreux, S.J.; Cregger, M.A.; Klingerman, D.M.; Hui, D.; Hettich, R.L.; Wilhelm, S.W.; et al. One-time nitrogen fertilization shifts switch grass soil microbiomes within a context of larger spatial and temporal variation. PLoS ONE 2019, 14, 0211310. [Google Scholar]
- Yakti, W.; Andrade-Linares, D.; Ngwene, B.; Bitterlich, M.; Kovács, G.; Franken, P. Phosphate Nutrition in Root–Fungus Interactions. In Endophytes for a Growing World; Hodkinson, T., Doohan, F., Saunders, M., Murphy, B., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 120–142. [Google Scholar]
- Kovács, G.M.; Szigetvári, C. Mycorrhizae and other root-associated fungal structures of the plants of a sandy grassland on the Great Hungarian Plain. Phyton—Annales Rei Botanicae 2002, 42, 211–223. [Google Scholar]
- Mandyam, K.; Jumpponen, A. Seeking the elusive functions of the root-colonizing dark septate endophytic fungi. Stud. Mycol. 2005, 53, 173–189. [Google Scholar] [CrossRef]
- Porras-Alfaro, A.; Herrera, J.; Odenbach, K.; Lowrey, T.; Sinsabaugh, R.L.; Natvig, D.O. A novel root fungal consortium associated with a dominant desert grass. Appl. Environ. Microbiol. 2008, 74, 2805–2813. [Google Scholar] [CrossRef]
- Sánchez Márquez, S.; Bills, G.F.; Zabalgogeazcoa, I. Diversity and structure of the fungal endophytic assemblages from two sympatric coastal grasses. Fungal Divers. 2008, 33, 87–100. [Google Scholar]
- Knapp, D.G.; Pintye, A.; Kovács, G.M. The dark side is not fastidious—Dark septate endophytic fungi of native and invasive plants of semiarid sandy areas. PLoS ONE 2012, 7, 32570. [Google Scholar] [CrossRef]
- Li, B.; He, X.; He, C.; Chen, Y.; Wang, X. Spatial dynamics of dark septate endophytes and soil factors in the rhizosphere of Ammopiptanthus mongolicus in inner mongolia. China. Symbiosis 2015, 65, 75–84. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Hou, L.; Ren, Y.; Wang, S.; Su, F. Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci. Rep. 2018, 8, 7896. [Google Scholar] [CrossRef]
- Li, X.; He, C.; He, X.L.; Su, F.; Hou, L.F.; Ren, Y.; Hou, Y. Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development. Plant Soil 2019, 439, 259–272. [Google Scholar] [CrossRef]
- Xie, L.; He, X.; Wang, K.; Hou, L.; Sun, Q. Spatial dynamics of dark septate endophytes in the roots and rhizospheres of Hedysarum scoparium in northwest China and the influence of edaphic variables. Fungal Ecol. 2017, 26, 135–143. [Google Scholar] [CrossRef]
- Khidir, H.H.; Eudy, D.M.; Porras-Alfaro, A.; Herrera, J.; Natvig, D.O.; Sinsabaugh, R.L. A general suite of fungal endophytes dominate the roots of two dominant grasses in semiarid grassland. J. Arid. Environ. 2010, 74, 35–42. [Google Scholar] [CrossRef]
- Knapp, A.K.; Fay, P.A.; Blair, J.M.; Collins, S.L.; Smith, M.D.; Carlisle, J.D.; Harper, C.W.; Danner, B.T.; Lett, M.S.; McCarron, J.K. Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science 2002, 298, 2202–2205. [Google Scholar] [CrossRef] [PubMed]
- Sielaff, A.C.; Upton, R.N.; Hofmockel, K.S.; Xu, X.; Wayne Polley, H.; Wilsey, B.J. Microbial community structure and functions differ between native and novel (exotic-dominated) grassland ecosystems in an 8-year experiment. Plant Soil 2018, 432, 359–372. [Google Scholar] [CrossRef]
- Ugarelli, K.; Laas, P.; Stingl, U. The microbial communities of leaves and roots associated with Turtle Grass (Thalassia testudinum) and Manatee Grass (Syringodium filliforme) are distinct from seawater and sediment communities, but are similar between species and sampling sites. Microorganisms 2019, 7, 4. [Google Scholar] [CrossRef]
- Cúcio, C.; Engelen, A.H.; Costa, R.; Muyzer, G. Rhizosphere microbiomes of European sea grasses are selected by the plant, but are not species specific. Front. Microbiol. 2016, 7, 440. [Google Scholar] [CrossRef]
- Ghimire, S.R.; Charlton, N.D.; Bell, J.D.; Krishnamurthy, Y.L.; Craven, K.D. Biodiversity of fungal endophyte communities inhabiting switch grass (Panicum virgatum L.) growing in the native tall grass prairie of northern Oklahoma. Fungal Divers. 2011, 47, 19–27. [Google Scholar] [CrossRef]
- Singer, E.; Bonnette, J.; Woyke, T.; Juenger, T.E. Conservation of endophyte bacterial community structure across two panicum grass species. Front. Microbiol. 2019, 10, 2181. [Google Scholar] [CrossRef]
- Grady, K.L.; Sorensen, J.W.; Stopnisek, N.; Guittar, J.; Shade, A. Assembly and seasonality of core phyllosphere microbiota on perennial biofuel crops. Nat. Commun. 2019, 10, 4135. [Google Scholar] [CrossRef]
- Wemheuer, B.; Thomas, T.; Wemheuer, F. Fungal endophyte communities of three agricultural important grass species differ in their response towards management regimes. Microorganisms 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.; Uphoff, N. Symbiotic root-endophytic soil microbes improve crop productivity and provide environmental benefits. Scientifica 2019, 2019, 9106395. [Google Scholar] [CrossRef]
- Young, C.A.; Hume, D.E.; McCulley, R.L. Forages and pastures symposium: Fungal endophytes of tall fescue and perennial ryegrass: Pasture friend or foe? J. Anim. Sci. 2013, 91, 2379–2394. [Google Scholar] [CrossRef]
- Song, H.; Nan, Z.; Song, Q.; Xia, C.; Li, X.; Yao, X.; Xu, W.; Kuang, Y.; Tian, P.; Zhang, Q. Advances in research on Epichloë endophytes in Chinese native grasses. Front. Microbiol. 2016, 7, 1399. [Google Scholar] [CrossRef] [PubMed]
- Skladanka, J.; Nedelnik, J.; Adam, V.; Dolezal, P.; Moravcova, H.; Dohnal, V. Forage as a primary source of mycotoxins in animal diets. Int. J. Environ. Res. Public Health 2011, 8, 37–50. [Google Scholar] [CrossRef]
- Pirelli, G.J.; Anderson, N.P.; Craig, A.M.; Young, C.A. Endophyte Toxins in Grass and Other Feed Sources: Risks to Livestock. Oregon State University Endophyte Service Laboratory. 2016. Available online: https://emt.oregonstate.edu/sites/agscid7/files/emt/endophyte-lab/ext-pub-nov-2016.pdf (accessed on 25 June 2022).
- Tian, P.; Le, T.N.; Ludlow, E.J.; Smith, K.F.; Forster, J.W.; Guthridge, K.M.; Spangenberg, G.C. Characterisation of novel perennial ryegrass host–Neotyphodium endophyte associations. Crop Pasture Sci. 2013, 64, 716–725. [Google Scholar] [CrossRef]
- Ruemmele, B.A.; Brilman, L.A.; Huff, D.R. Fine fescue germplasm diversity and vulnerability. Crop Sci. 1995, 35, 313–316. [Google Scholar] [CrossRef]
- Guerre, P. Ergot alkaloids produced by endophytic fungi of the genus Epichloë. Toxins 2015, 7, 773–790. [Google Scholar] [CrossRef] [PubMed]
- Low, S.G. Signal grass (Brachiaria decumbens) toxicity in grazing ruminants. Agriculture 2015, 5, 971–990. [Google Scholar] [CrossRef]
- Cheeke, P.R. Endogenous toxins and mycotoxins in forage grasses and their effects on livestock. J. Anim. Sci. 1995, 73, 909–918. [Google Scholar] [CrossRef]
- Lin, W.; Kuang, Y.; Wang, J.; Duan, D.; Xu, W.; Tian, P.; Nzabanita, C.; Wang, M.; Li, M.; Ma, B. Effects of seasonal variation on the alkaloids of different ecotypes of Epichloë endophyte-Festuca sinensis associations. Front. Microbiol. 2019, 10, 1695. [Google Scholar] [CrossRef]
- Saikkonen, K.; Young, C.A.; Helander, M.; Schardl, C.L. Endophytic Epichloë species and their grass hosts: From evolution to applications. Plant Mol. Biol. 2016, 90, 665–675. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Sullivan, J.J. The impact of herbivory on plants in different resource conditions: A meta-analysis. Ecology 2001, 82, 2045–2058. [Google Scholar] [CrossRef]
- Saikkonen, K.; Gundel, P.E.; Helander, M. Chemical ecology mediated by fungal endophytes in grasses. J. Chem. Ecol. 2013, 39, 962–968. [Google Scholar] [CrossRef]
- Ryan, G.D.; Rasmussen, S.; Parsons, A.J.; Newman, J.A. The effects of carbohydrate supply and host genetic background on Epichloë endophyte and alkaloid concentrations in perennial ryegrass. Fungal Ecol. 2015, 18, 115–125. [Google Scholar] [CrossRef]
- Helander, M.; Phillips, T.; Faeth, S.H.; Bush, L.P.; McCulley, R.; Saloniemi, I.; Saikkonen, K. Alkaloid quantities in endophyte-infected tall fescue are affected by the plant-fungus combination and environment. J. Chem. Ecol. 2016, 42, 118–126. [Google Scholar] [CrossRef]
- McCulley, R.L.; Bush, L.P.; Carlisle, A.E.; Ji, H.; Nelson, J.A. Warming reduces tall fescue abundance but stimulates toxic alkaloid concentrations in transition zone pastures of the U.S. Front. Chem. 2014, 2, 88. [Google Scholar] [CrossRef]
- Bush, L.P.; Wilkinson, H.H.; Schardl, C.L. Bio-protective alkaloids of grass-fungal endophyte symbioses. Plant Physiol. 1997, 114, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Young, C.A.; Pan, J.; Florea, S.; Takach, J.E.; Panaccione, D.; Farman, M.L.; Webb, J.S.; Jaromczyk, J.; Charlton, N.D.; et al. Currencies of mutualisms: Sources of alkaloid genes in vertically transmitted Epichloë. Toxins 2013, 5, 1064–1088. [Google Scholar] [CrossRef] [PubMed]
- Montañez, A.; Blanco, A.R.; Barlocco, C.; Beracochea, M.; Sicardi, M. Characterization of cultivable putative endophytic plant growth promoting bacteria associated with maize cultivars (Zea mays L.) and their inoculation effects in-vitro. Appl. Soil Ecol. 2012, 58, 21–28. [Google Scholar] [CrossRef]
- Sessitsch, A.; Hardoim, P.; Döring, J.; Weilharter, A.; Krause, A.; Woyke, T.; Woyke, T.; Mitter, B.; Hauberg-Lotte, L.; Friedrich, F.; et al. Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol. Plant Microbe Interact. 2012, 25, 28–36. [Google Scholar] [CrossRef]
- Kauppinen, M.; Saikkonen, K.; Helander, M.; Pirttilä, A.M.; Wäli, P.R. Epichloë grass endophytes in sustainable agriculture. Nat. Plants 2016, 2, 15224. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.J.; De Bonth, A.C.M.; Briggs, L.R.; Caradus, J.R.; Finch, S.C.; Fleetwood, D.J.; Fletcher, L.R.; Hume, D.E.; Johnson, R.D.; Popay, A.J.; et al. The exploitation of Epichloë endophytes for agricultural benefit. Fungal Divers. 2013, 60, 17188. [Google Scholar] [CrossRef]
- Finch, S.C.; Fletcher, L.R.; Babu, J.V. The evaluation of endophyte toxin residues in sheep fat. N. Z. Vet. J. 2012, 60, 5660. [Google Scholar] [CrossRef]
- Tapper-Bor, A.; Lane, G.A. Janthitrems Found in a Neotyphodium Endophyte of Perennial Ryegrass, Proceedings of the 5th International Symposium on Neotyphodium/Grass Interactions Fayetteville, Fayetteville, AR, USA, 23–26 May 2004; Kallenbach, R., Rosenkrans, C.J., Lock, T.R., Eds.; AR University of Arkansas Press: Fayetteville, AR, USA, 2014; p. 301. [Google Scholar]
- Bacon, C.W. Abiotic stress tolerances (moisture, nutrients) and photosynthesis in endophyte-infected tall fescue. Agric. Ecosyst. Environ. 1993, 44, 123–141. [Google Scholar] [CrossRef]
- Bacon, C.W. Toxic endophyte-infected tall fescue and range grasses: Historic perspectives. J. Anim. Sci. 1995, 73, 86170. [Google Scholar] [CrossRef]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef]
- Netzker, T.; Fischer, J.; Weber, J.; Mattern, D.J.; König, C.C.; Valiante, V.; Schroeckh, V.; Brakhage, A.A. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front. Microbiol. 2015, 6, 299. [Google Scholar] [CrossRef]
- Schardl, C.L.; Panaccione, D.G. Biosynthesis of ergot and loline alkaloids. In Neotyphodium in Cool-Season Grasses; Roberts, C.A., West, C.P., Spiers, D.E., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2005; pp. 73–92. [Google Scholar]
- Ola, A.R.B.; Thomy, D.; Lai, D.; Brötz-Oesterhelt, H.; Proksch, P. Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through co-culture with Bacillus subtilis. J. Nat. Prod. 2013, 76, 2094–2099. [Google Scholar] [CrossRef]
- Schroeckh, V.; Scherlach, K.; Nützmann, H.W.; Shelest, E.; Schmidt-Heck, W.; Schuemann, J.; Martin, K.; Hertweck, C.; Brakhage, A.A. Intimate bacterial–fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 2009, 106, 14558–14563. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.; Krischke, M.; Mueller, M.J.; Krauss, J. Plant age and seasonal timing determine endophyte growth and alkaloid biosynthesis. Fungal Ecol. 2017, 29, 52–58. [Google Scholar] [CrossRef]
- Hovermale, J.T.; Craig, A.M. Correlation of ergovaline and lolitrem B levels in endophyte infected perennial ryegrass (Lolium perenne). J. Vet. Diagn. Investig. 2001, 13, 323–327. [Google Scholar] [CrossRef]
- Tor-Agbidye, J.; Blythe, L.L.; Craig, A.M. Correlation of endophyte toxins (ergovaline and lolitrem B) with clinical disease: Fescue foot and perennial ryegrass staggers. Vet. Hum. Toxicol. 2001, 43, 140–146. [Google Scholar] [PubMed]
- Siegel, M.R.; Bush, L.P. Defensive chemicals in grass-fungal endophyte associations. In Phytochemical Diversity and Redundancy in Ecological Interactions; Romeo, J.T., Ed.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 3081, p. 119. [Google Scholar]
- Faeth, S.H.; Fagan, W.F. Fungal endophytes: Common host plant symbionts but uncommon mutualists. Integr. Comp. Biol. 2002, 42, 360–368. [Google Scholar] [CrossRef]
- Hegazi, N.; Hartmann, A.; Ruppel, S. The plant microbiome: Exploration of plant-microbe interactions for improving agricultural productivity. J. Adv. Res. 2019, 19, 1–2. [Google Scholar] [CrossRef]
- Doekes, H.M.; de Boer, R.J.; Hermsen, R. Toxin production spontaneously becomes regulated by local cell density in evolving bacterial populations. PLoS Comput. Biol. 2019, 15, 1007333. [Google Scholar] [CrossRef]
- Albinsson, M.E.; Negri, A.P.; Blackburn, S.I.; Bolch, C.J.S. Bacterial community affects toxin production by Gymnodinium catenatum. PLoS ONE 2014, 9, 104623. [Google Scholar] [CrossRef]
- Wicklow, D.T. Metabolites in the co-evolution of fungal chemical defence systems. In Co-Evolution of Fungi with Plants and Animals; Pirozynski, K.A., Hawksworth, D., Eds.; Academic Press: New York, NY, USA, 1988; pp. 173–201. [Google Scholar]
- Palumbo, J.D.; Mahoney, N.E.; Light, D.M. Navel orange worm (Amyelois transitella) as a vector of Aspergillus flavus on almonds. Phytopathology 2008, 98, S119. [Google Scholar]
- Watts, D.; Palombo, E.A.; Jaimes Castillo, A.; Zaferanloo, B. Endophytes in agriculture: Potential to improve yields and tolerances of agricultural crops. Microorganisms 2023, 11, 1276. [Google Scholar] [CrossRef]
- Goldson, S.L. An examination of the relationship between Argentine stem weevil (Listronotus bonariensis (Kuschel) and several of its host grasses. N. Z. J. Agri Res. 1982, 25, 395–403. [Google Scholar] [CrossRef][Green Version]
- Vikuk, V.; Young, C.A.; Lee, S.T.; Nagabhyru, P.; Krischke, M.; Mueller, M.J.; Krauss, J. Infection rates and alkaloid patterns of different grass species with systemic Epichloë endophytes. Appl. Environ. Microbiol. 2019, 85, e00465-19. [Google Scholar] [CrossRef] [PubMed]
- Nichea, M.J.; Palacios, S.A.; Chiacchiera, S.M.; Sulyok, M.; Krska, R.; Chulze, S.N.; Torres, A.M.; Ramirez, M.L. Presence of multiple mycotoxins and other fungal metabolites in native grasses from a wetland ecosystem in Argentina intended for grazing cattle. Toxins 2015, 7, 3309–3329. [Google Scholar] [CrossRef] [PubMed]
- Gopal, M.; Gupta, A. Microbiome selection could spur next-generation plant breeding strategies. Front. Microbiol. 2016, 7, 1971. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Bloom, E.H.; Atallah, S.S.; Casteel, C.L. Motivating organic farmers to adopt practices that support the pest-suppressive microbiome relies on understanding their beliefs. Renew. Agric. Food Syst. 2024, 39, e8. [Google Scholar] [CrossRef]
- Moebius, N.; Üzüm, Z.; Dijksterhuis, J.; Lackner, G.; Hertweck, C. Active invasion of bacteria into living fungal cells. Elife 2014, 3, 03007. [Google Scholar] [CrossRef]
- Torres-Cortés, G.; Ghignone, S.; Bonfante, P.; Schüβler, A. Mosaic genome of endobacteria in arbuscular mycorrhizal fungi: Transkingdom gene transfer in an ancient mycoplasma-fungus association. Proc. Natl. Acad. Sci. USA 2015, 112, 7785–7790. [Google Scholar] [CrossRef]
- Giorni, P.; Bertuzzi, T.; Battilani, P. Impact of fungi co-occurrence on mycotoxin contamination in maize during the growing season. Front. Microbiol. 2019, 10, 1265. [Google Scholar] [CrossRef]
- Kovalsky, P.; Kos, G.; Nahrer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize—An extensive survey. Toxins 2016, 8, 363. [Google Scholar] [CrossRef]
- Culig, B.; Bevardi, M.; Bosnir, J.; Serdar, S.; Lasic, D.; Racz, A.; Galić, A.; Kuharić, Ž. Presence of citrinin in grains and its possible health effects. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 22–30. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Rocha, A.; Sulyok, M.; Krska, R.; Mallmann, C.A. Natural mycotoxin contamination of maize (Zea mays L.) in the South region of Brazil. Food Control 2017, 73, 127–132. [Google Scholar] [CrossRef]
- Obradovic, A.; Krnjaja, V.; Nikolic, M.; Delibasic, G.; Filipovic, M.; Stankovic, G.; Stankovic, S. Impacts of climate conditions on aflatoxin B1 and fumonisins contamination of maize kernels and their co-occurrence. Biotechnol. Anim. Husb. 2018, 34, 469–480. [Google Scholar] [CrossRef]
- DongHo, K.; SungYong, H.; JeaWoo, K.; SungMin, C.; KvuRi, L.; TaeKyung, A.; Lee, C.; Chung, S.H. Simultaneous determination of multi-mycotoxins in cereal grains collected from South Korea by LC/MS/MS. Toxins 2017, 9, 9030106. [Google Scholar] [CrossRef] [PubMed]
- Adekova, I.; Obadina, A.; Phoku, J.; Boevre, M.; de Saeger, S.; de Niobeh, P. Fungal and mycotoxin contamination of fermented foods from selected South African markets. Food Control 2018, 90, 295–303. [Google Scholar] [CrossRef]
- Gundel, P.E.; Perez, L.I.; Helander, M.; Saikkonen, K. Symbiotically modified organisms: Nontoxic fungal endophytes in grasses. Trends Plant Sci. 2013, 18, 420–427. [Google Scholar] [CrossRef]
- Saikkonen, K.; Saari, S.; Helander, M. Defensive mutualism between plants and endophytic fungi? Fungal Divers. 2010, 41, 101–113. [Google Scholar] [CrossRef]
- Saikkonen, K.; Wäli, P.; Helander, M.; Faeth, S.H. Genetic compatibility determines endophyte-grass combinations. PLoS ONE 2010, 395, e11395. [Google Scholar] [CrossRef]
- Clay, K.; Schardl, C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 2002, 160, 99–127. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, A.; Sicardi, M.; Frioni, L. Plant genotype and nitrogen fertilization effects on abundance and diversity of diazotrophic bacteria associated with maize (Zea mays L.). Biol. Fertil. Soils 2015, 51, 391–402. [Google Scholar] [CrossRef]
- Ding, T.; Melcher, U. Influences of plant species, season and location on leaf endophytic bacterial communities of non-cultivated plants. PLoS ONE 2016, 11, 0150895. [Google Scholar] [CrossRef] [PubMed]
- Partida-Martínez, L.P.; Heil, M. The microbe-free plant: Fact or artifact? Front. Plant Sci. 2011, 2, 100. [Google Scholar] [CrossRef]
- Bacon, C.W.; Glenn, A.E.; Yates, I.E. Fusarium verticillioides: Managing the endophytic association with maize for reduced fumonisins accumulation. Toxin Rev. 2008, 27, 411–446. [Google Scholar] [CrossRef]
- Gilbert, S.F.; Mcdonald, E.; Boyle, N.; Buttino, N.; Gyi, L.; Mai, M.; Prakash, N.; Robinson, J. Symbiosis as a source of selectable epigenetic variation: Taking the heat for the big guy. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 671–678. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Afkhami, M.E.; Rúa, M.A.; Davitt, A.J.; Hammer, S.; Huguet, V.M. A fungus among us: Broad patterns of endophyte distribution in the grasses. Ecology 2009, 80, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, N.; Keller, N.P. Mycotoxins in conversation with bacteria and fungi. Front. Microbiol. 2019, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Vannier, N.; Agler, M.; Hacquard, S. Microbiota-mediated disease resistance in plants. PLoS Pathog. 2019, 15, 1007740. [Google Scholar] [CrossRef]
- Hacquard, S.; Spaepen, S.; Garrido-Oter, R.; Schulze-Lefert, P. Interplay between innate immunity and the plant microbiota. Annu. Rev. Phytopathol. 2017, 55, 565–589. [Google Scholar] [CrossRef]
- Cagnano, G.; Roulund, N.; Jensen, C.S.; Forte, F.P.; Asp, T.; Leuchtmann, A. Large scale screening of Epichloë endophytes infecting Schedonorus pratensis and other forage grasses reveals a relation between microsatellite-based haplotypes and loline alkaloid levels. Front. Plant Sci. 2019, 10, 765. [Google Scholar] [CrossRef]
- Piano, E.; Bertoli, F.B.; Romani, M.; Tava, A.; Riccioni, L.; Valvassori, M.; Carroni, A.M.; Pecetti, L. Specificity of host–endophyte association in tall fescue populations from Sardinia, Italy. Crop Sci. 2005, 45, 1456–1463. [Google Scholar] [CrossRef]
- Faeth, S.H.; Saikkonen, K. Variability is the Nature of the Endophyte-Grass Interaction, Proceedings of the 6th International Symposium on Fungal Endophytes in Grasses, Christchurch, New Zealand, 25–28 March 2007; Popay, A.J., Thorn, E.R., Eds.; New Zealand Grassland Association: Christchurch, New Zealand, 2007; pp. 37–48. [Google Scholar]
- Faeth, S.H. Are endophytic fungi defensive plant mutualists? Oikos 2002, 98, 25–36. [Google Scholar] [CrossRef]
- Wang, W.; Liang, X.; Li, Y.; Wang, P.; Keller, N.P. Genetic regulation of mycotoxin biosynthesis. J. Fungi 2022, 22, 21. [Google Scholar] [CrossRef]
- Howitz, K.T.; Sinclair, D.A. Xenohormesis: Sensing the chemical cues of other species. Cell 2008, 133, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.; Pandey, S.S.; Pandey, A.; Srivastava, M.; Shanker, K.; Kalra, A. Endophytic consortium with diverse gene-regulating capabilities of benzylisoquinoline alkaloids biosynthetic pathway can enhance endogenous morphine biosynthesis in Papaver somniferum. Front. Microbiol. 2019, 10, 925. [Google Scholar] [CrossRef]
- Pandey, S.S.; Singh, S.; Babu, C.V.; Shanker, K.; Srivastava, N.K.; Kalra, A. Endophytes of opium poppy differentially modulate host plant productivity and genes for the biosynthetic pathway of benzylisoquinoline alkaloids. Planta 243, 1097–1114. [CrossRef]
- Pandey, S.S.; Singh, S.; Babu, C.V.; Shanker, K.; Srivastava, N.K.; Shukla, A.K.; Kalra, A. Fungal endophytes of Catharanthus roseus enhance vindoline content by modulating structural and regulatory genes related to terpenoid indole alkaloid biosynthesis. Sci. Rep. 6, 26583. [CrossRef]
- Sreekanth, D.; Kristin, I.M.; Brett, A.N. Endophytic fungi from Cathranthus roseus: A potential resource for the discovery of antimicrobial polyketides. Nat. Prod. Chem. Res. 2017, 5, 256. [Google Scholar]
- Irmer, S.; Podzun, N.; Langel, D.; Heidemann, F.; Kaltenegger, E.; Schemmerling, B.; Geilfus, C.; Zorb, C.; Ober, D. New aspect of plant–rhizobia interaction: Alkaloid biosynthesis in Crotalaria depends on nodulation. Proc. Natl. Acad. Sci. USA 2015, 112, 4164–4169. [Google Scholar] [CrossRef]
- Abdallah, M.F.; De Boevre, M.; Landschoot, S.; De Saeger, S.; Haesaert, G.; Audenaert, K. Fungal endophytes control Fusarium graminearum and reduce Trichothecenes and Zearalenone in Maize. Toxins 2018, 10, 493. [Google Scholar] [CrossRef]
- Hewitt, K.G.; Hofmann, R.W.; Ball, O.J.; Finch, S.C.; Bryant, R.H.; Popay, A.J. Phosphorus induced changes in food quality enhance porina fitness feeding on Epichloë endophyte free forage grasses. Sci Rep. 2025, 15, 6448. [Google Scholar] [CrossRef] [PubMed]
- Taheur, F.B.; Kouidhi, B.; Al Qurashi, Y.M.A.; Salah-Abbes, J.B.; Chaieb, K. Review: Biotechnology of mycotoxins detoxification using microorganisms and enzymes. Toxicon 2019, 160, 12–22. [Google Scholar] [CrossRef]
- Vanhoutte, I.; Audenaert, K.; De Gelder, L. Biodegradation of mycotoxins: Tales from known and unexplored worlds. Front. Microbiol. 2016, 7, 561. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P. Microbial detoxification of mycotoxins. J. Chem. Ecol. 2013, 39, 907–918. [Google Scholar] [CrossRef]
- Tétard-Jones, C.; Edwards, R. Potential roles for microbial endophytes in herbicide tolerance in plants. Pest. Manag. Sci. 2016, 72, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Kuklinsky-Sobral, J.; Araújo, W.L.; Mendes, R.; Pizzirani-Kleiner, A.A.; Azevedo, J.L. Isolation and characterization of endophytic bacteria from soybean (Glycine max) grown in soil treated with glyphosate herbicide. Plant Soil 2005, 273, 91–99. [Google Scholar] [CrossRef]
- Trognitz, F.; Hackl, E.; Widhalm, S.; Sessitsch, A. The role of plant–microbiome interactions in weed establishment and control. FEMS Microbiol Ecol. 2016, 92, fiw138. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Neve, P.; Roux, F. A unified approach to the estimation and interpretation of resistance costs in plants. Heredity 2011, 107, 386–394. [Google Scholar] [CrossRef]
- Cummins, T.R.; Dib-Hajj, S.D.; Black, J.A.; Akopian, A.N.; Wood, J.N.; Waxman, S.G. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J. Neurosci. 1999, 19, RC43. [Google Scholar] [CrossRef]
- Edwards, R.; Brazier-Hicks, M.; Dixon, D.P.; Cummins, I. Chemical manipulation of antioxidant defences in plants. Adv. Bot. Res. 2005, 42, 1–32. [Google Scholar]
- Young, C.A.; Felitti, S.; Shields, K.; Spangenberg, G.; Johnson, R.D.; Bryan, G.T.; Saikia, S.; Scott, B. A complex gene cluster for indole-diterpene biosynthesis in the grass endophyte Neotyphodium lolii. Fungal Genet. Biol. 2006, 43, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Choi, S.K.; Kloepper, J.W.; Ryu, C.M. Genome sequence of the plant endophyte Bacillus pumilus INR7, triggering induced systemic resistance in field crops. Genome Announc. 2014, 2, 01093-14. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.S.; Yang, J.W.; Ryu, C.M. ISR meets SAR outside: Additive action of the endophyte Bacillus pumilus INR7 and the chemical inducer, benzothiadiazole, on induced resistance against bacterial spot in field-grown pepper. Front. Plant Sci. 2013, 4, 122. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Ryu, C.M. Bacterial endophytes as elicitors of induced systemic resistance. In Microbial Root Endophytes; Schulz, B., Boyle, C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 33–52. [Google Scholar]
- Constantin, M.E.; de Lamo, F.J.; Vlieger, B.V.; Rep, M.; Takken, F.L.W. Endophyte-mediated resistance in tomato to Fusarium oxysporum is independent of ET, JA, and SA. Front. Plant Sci. 2019, 10, 979. [Google Scholar] [CrossRef]
- Xu, H.; Song, J.; Luo, H.; Zhang, Y.; Li, Q.; Zhu, Y.; Xu, J.; Li, Y.; Song, C.; Wang, B.; et al. Analysis of the genome sequence of the medicinal plant Salvia miltiorrhiza. Mol. Plant 2016, 9, 949–952. [Google Scholar] [CrossRef]
- Salwan, R.; Sharma, M.; Sharma, A.; Sharma, V. Insights into plant beneficial microorganism-triggered induced systemic resistance. Plant Stress. 2023, 7, 100140. [Google Scholar] [CrossRef]
- Compant, S.; Clement, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Kusajima, M.; Shima, S.; Fujita, M.; Minamisawa, K.; Che, F.S.; Yamakawa, H.; Nakashita, H. Involvement of ethylene signaling in Azospirillum sp. B510-induced disease resistance in rice. Biosci. Biotechnol. Biochem. 2018, 82, 1522–1526. [Google Scholar] [CrossRef]
- Plett, J.M.; Martin, F.M. Know your enemy, embrace your friend: Using omics to understand how plants respond differently to pathogenic and mutualistic microorganisms. Plant J. 2018, 93, 729–746. [Google Scholar] [CrossRef]
- Rashid, M.H.; Chung, Y.R. Induction of systemic resistance against insect herbivores in plants by beneficial soil microbes. Front. Plant Sci. 2017, 8, 1816. [Google Scholar] [CrossRef]
- Campos, M.L.; Kang, J.H.; Howe, G.A. Jasmonate-triggered plant immunity. J. Chem. Ecol. 2014, 40, 657–675. [Google Scholar] [CrossRef]
- Mejía, L.C.; Herre, E.A.; Sparks, J.P.; Winter, K.; García, M.N.; Van Bael, S.A.; Stitt, J.; Shi, Z.; Zhang, Y.; Guiltinan, M.J.; et al. Pervasive effects of a dominant foliar endophytic fungus on host genetic and phenotypic expression in a tropical tree. Front. Microbiol. 2014, 5, 479. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; de Vos, M.; Sun, J.Y.; Sønderby, I.E.; Halkier, B.A.; Wittstock, U.; Jander, G. Differential effects of indole and aliphatic glucosinolates on lepidopteran herbivores. J. Chem. Ecol. 2010, 36, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Sergaki, C.; Lagunas, B.; Lidbury, I.; Gifford, M.L.; Schäfer, P. Challenges and approaches in microbiome research: From fundamental to applied. Front. Plant Sci. 2018, 9, 1205. [Google Scholar] [CrossRef]
- van der Heijden, M.G.; Schlaeppi, K. Root surface as a frontier for plant microbiome research. Proc. Nat. Acad. Sci. USA 2015, 112, 2299. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.; Bork, P.; Fraser, C.; Knight, R.; Wang, J. The microbiome explored: Recent insights and future challenges. Nat. Rev. Microbiol. 2013, 11, 213–217. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Sturz, A.V.; Matheson, B.G.; Arsenault, W.; Kimpinski, J.; Christie, B.R. Weeds as a source of plant growth promoting rhizobacteria in agricultural soils. Can. J. Microbiol. 2001, 47, 1013–1024. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Wang, L. Fungal endophytes: Beyond herbivore management. Front. Microbiol. 2018, 9, 544. [Google Scholar] [CrossRef]
- Pérez-Jaramillo, J.E.; Mendes, R.; Raaijmakers, J.M. Impact of plant domestication on rhizosphere microbiome assembly and functions. Plant Mol. Biol. 2016, 90, 635–644. [Google Scholar] [CrossRef]
- Van Wees, S.; Van der Ent, S.; Pieterse, C. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar] [CrossRef]
- Marasco, R.; Rolli, E.; Ettoumi, B.; Vigani, G.; Mapelli, F.; Borin, S.; Abou-hadid, A.F.; El-Behairy, U.A.; Sorlini, C.; Cherif, A.; et al. A drought resistance-promoting microbiome is selected by root system under desert farming. PLoS ONE 2012, 7, 48479. [Google Scholar] [CrossRef]
- Jones, D.L.; Nguyen, C.; Finlay, R.D. Carbon flow in the rhizosphere: Carbon trading at the soil-root interface. Plant Soil 2009, 321, 5–33. [Google Scholar] [CrossRef]
- Panke-Buisse, K.; Poole, A.; Goodrich, J.; Lay, R.E.; Kniffin, J.K. Selection on soil microbiomes reveals reproducible impacts on plant function. ISME J. 2015, 9, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.E.; Kent, A.D.; Brisson, V.L.; Gaudin, A.C.M. Agricultural management and plant selection interactively affect rhizosphere microbial community structure and nitrogen cycling. Microbiome 2019, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Nelkner, J.; Henke, C.; Lin, T.W.; Pätzold, W.; Hassa, J.; Jaenicke, S.; Grosch, R.; Pühler, A.; Sczyrba, A.; Schlüter, A. Effect of long-term farming practices on agricultural soil microbiome members represented by metagenomically assembled genomes (MAGs) and their predicted plant-beneficial genes. Genes 2019, 10, 424. [Google Scholar] [CrossRef]
- Hendgen, M.; Hoppe, B.; Döring, J.; Friedel, M.; Kauer, R.; Frisch, M.; Dahl, A.; Kellner, H. Effects of different management regimes on microbial biodiversity in vineyard soils. Sci. Rep. 2018, 8, 9393. [Google Scholar] [CrossRef]
- Attwood, G.T.; Wakelin, S.A.; Leahy, S.C.; Rowe, S.; Clarke, S.; Chapman, D.F.; Muirhead, R.; Jacobs, J.M.E. Applications of the soil, plant and rumen microbiomes in pastoral agriculture. Front. Nutr. 2019, 6, 107. [Google Scholar] [CrossRef]
- Garrett, K.A.; Frank, E.E.; Dendy, S.P.; Leslie, J.F.; Saleh, A. Microbiome engineers: Grazers, browsers, and the phytobiome stampede. bioRxiv 2017, 087494. [Google Scholar] [CrossRef]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. inner plant values: Diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda Mdel, C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Quiza, L.; St-Arnaud, M.; Yergeau, E. Harnessing phytomicrobiome signaling for rhizosphere microbiome engineering. Front. Plant Sci. 2015, 6, 507. [Google Scholar] [CrossRef]
- Jochum, M.D.; McWilliams, K.L.; Pierson, E.A.; Jo, Y.K. Host-mediated microbiome engineering (HMME) of drought tolerance in the wheat rhizosphere. PLoS ONE 2019, 14, 0225933. [Google Scholar] [CrossRef]
- Jones, P.; Garcia, B.J.; Furches, A.; Tuskan, G.A.; Jacobson, D. Plant host-associated mechanisms for microbial selection. Front. Plant Sci. 2019, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Philippe, G. Lolitrem B and indole diterpene alkaloids produced by endophytic fungi of the genus Epichloë and their toxic effects in livestock. Toxins 2016, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Vázquez de Aldana, B.R.; San Emeterio, L.; Zabalgogeazcoa, I. A survey of culturable fungal endophytes from Festuca rubra subsp. pruinosa, a grass from marine cliffs, reveals a core microbiome. Front. Microbiol. 2019, 9, 3321. [Google Scholar]
- Compant, S.; Saikkonen, K.; Mitter, B.; Campisano, A.; Mercado-Blanco, J. Editorial special issue: Soil, plants and endophytes. Plant Soil 2016, 405, 1–11. [Google Scholar] [CrossRef]
- Rasmussen, S.; Parsons, A.J.; Newman, J.A. Metabolomics analysis of the Lolium perenne-Neotyphodium lolii symbiosis: More than just alkaloids? Phytochem. Rev. 2009, 8, 535–550. [Google Scholar] [CrossRef]
- Panaccione, D.G.; Johnson, R.D.; Wang, J.; Young, C.A.; Damrongkool, P.; Scott, B.; Schardl, C.L. Elimination of ergovaline from a grass-Neotyphodium endophyte symbiosis by genetic modification of the endophyte. Plant Biol. 2001, 98, 12820–12825. [Google Scholar] [CrossRef]
- Hettiarachchige, I.K.; Elkins, A.C.; Reddy, P.; Mann, R.C.; Guthridge, K.M.; Sawbridge, T.I.; Forster, J.W.; Spangenberg, G.C. Genetic modification of asexual Epichloë endophytes with the perA gene for peramine biosynthesis. Mol. Genet. Genom. 2019, 294, 315–328. [Google Scholar] [CrossRef]
- Oyserman, B.O.; Medema, M.H.; Raaijmakers, J.M. Road MAPs to engineer host microbiomes. Curr. Opin. Microbiol. 2018, 43, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Vorholt, J.A.; Vogel, C.; Carlström, C.I.; Müller, D.B. Establishing causality: Opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 2017, 22, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Uroz, S.; Courty, P.E.; Oger, P. Plant symbionts are engineers of the plant-associates microbiome. Trends Plant Sci. 2019, 24, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Hestrin, R.; Lee, M.R.; Whitaker, B.K.; Pett-Ridge, J. The switchgrass microbiome: A review of structure, function, and taxonomic distribution. Phytobiomes J. 2020, 5, 14–28. [Google Scholar] [CrossRef]
- Saikkonen, K.; Nissinen, R.; Helander, M. Toward Comprehensive Plant Microbiome Research. Front. Ecol. Evol. 2020, 8, 61. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).