Combination of Vrn Alleles Assists in Optimising the Vernalization Requirement in Barley (Hordeum vulgare L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Research Field Experiments and Field Trials
2.3. Meteorological Conditions of the Experimental Spot
2.4. Assessment of Developmental Stages in Barley Plants
2.5. Molecular Analyses
2.6. Statistical Treatments
3. Results
3.1. Field Trial Tests for Phenotypic Evaluation in Barley Plants
3.2. Genetic Polymorphism of the Vrn-H1 Gene
3.3. Genetic Diversity of the Vrn-H2 Gene
3.4. Genetic Variability of the Vrn-H3 Gene
3.5. Barley Germplasm Classification for Vernalization: Comparison of Genotyping and Phenotyping Results
3.6. Examples of Successful Application of Genotyping for Vrn Genes in Barley Breeding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Hashash, E.F.; El-Absy, K.M. Barley (Hordeum vulgare L.) breeding. In Advances in Plant Breeding Strategies: Cereals; Al-Khayri, J., Jain, S., Johnson, D., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–45. [Google Scholar] [CrossRef]
- Zhou, M.X. Barley production and consumption. In Genetics and Improvement of Barley Malt Quality. Advanced Topics in Science and Technology in China; Zhang, G., Li, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–17. [Google Scholar] [CrossRef]
- Elakhdar, A.; Solanki, S.; Kubo, T.; Abed, A.; Elakhdar, I.; Khedr, R.; Hamwieh, A.; Capo-chichi, L.J.A.; Abdelsattar, M.; Franckowiak, J.D.; et al. Barley with improved drought tolerance: Challenges and perspectives. Environ. Exp. Bot. 2022, 201, 104965. [Google Scholar] [CrossRef]
- FAOSTAS. Available online: http://www.fao.org/faostat (accessed on 24 March 2025).
- Rizza, F.; Karsai, I.; Morcia, C.; Badeck, F.W.; Terzi, V.; Pagani, D.; Kiss, T.; Stanca, A.M. Association between the allele compositions of major plant developmental genes and frost tolerance in barley (Hordeum vulgare L.) germplasm of different origin. Mol. Breed. 2016, 36, 156. [Google Scholar] [CrossRef]
- Tondelli, A.; Pagani, D.; Ghafoori, I.N.; Rahimi, M.; Ataei, R.; Rizza, F.; Flavell, A.; Cattivelli, L. Allelic variation at Fr-H1/Vrn-H1 and Fr-H2 loci is the main determinant of frost tolerance in spring barley. Environ. Exp. Bot. 2014, 106, 148–155. [Google Scholar] [CrossRef]
- Baidyussen, A.; Khassanova, G.; Utebayev, M.; Jatayev, S.; Kushanova, R.; Khalbayeva, S.; Amangeldiyeva, A.; Yerzhebayeva, R.; Bulatova, K.; Schramm, C.; et al. Assessment of molecular markers and marker-assisted selection for drought tolerance in barley (Hordeum vulgare L.). J. Integr. Agric. 2024, 23, 20–38. [Google Scholar] [CrossRef]
- Fernández-Calleja, M.; Casas, A.M.; Igartua, E. Major flowering time genes of barley: Allelic diversity, effects, and comparison with wheat. Theor. Appl. Genet. 2021, 134, 1867–1897. [Google Scholar] [CrossRef]
- Trevaskis, B.; Hemming, M.N.; Dennis, E.S.; Peacock, W.J. The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci. 2007, 12, 352–357. [Google Scholar] [CrossRef]
- Distelfeld, A.; Li, C.; Dubcovsky, J. Regulation of flowering in temperate cereals. Curr. Opin. Plant Biol. 2009, 12, 178–184. [Google Scholar] [CrossRef]
- Ga, Z.; Gao, L.; Quzong, X.; Mu, W.; Zhuoma, P.; Taba, X.; Jiao, G.; Dondup, D.; Namgyal, L.; Sang, Z. Metabolomics, phytohormone and transcriptomics strategies to reveal the mechanism of barley heading date regulation to responds different photoperiod. BMC Genom. 2024, 25, 879. [Google Scholar] [CrossRef]
- Zitzewitz, J.; Szucs, P.; Dubcovsky, J.; Yan, L.; Francia, E.; Pecchioni, N.; Casas, A.; Chen, T.; Hayes, P.; Skinner, J. Molecular and structural characterization of barley vernalization genes. Plant Mol. Biol. 2005, 59, 449–467. [Google Scholar] [CrossRef]
- Fu, D.; Szűcs, P.; Yan, L.; Helguera, M.; Skinner, J.S.; von Zitzewitz, J.; Hayes, P.; Dubcovsky, J. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol. Genet. Genom. 2005, 273, 54–65. [Google Scholar] [CrossRef]
- Fernández-Calleja, M.; Ciudad, F.J.; Casas, A.M.; Igartua, E. Hybrids provide more options for fine-tuning flowering time responses of winter barley. Front. Plant Sci. 2022, 13, 827701. [Google Scholar] [CrossRef] [PubMed]
- Guerra, D.; Morcia, C.; Badeck, F.; Rizza, F.; Delbono, S.; Francia, E.; Milc, J.A.; Monostori, I.; Galiba, G.; Cattivelli, L.; et al. Extensive allele mining discovers novel genetic diversity in the loci controlling frost tolerance in barley. Theor. Appl. Genet. 2022, 135, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Danyluk, J.; Kane, N.A.; Breton, G.; Limin, A.E.; Fowler, D.B.; Sarhan, F. TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiol. 2003, 132, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Loukoianov, A.; Tranquilli, G.; Helguera, M.; Fahima, T.; Dubcovsky, J. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA 2003, 100, 6263–6268. [Google Scholar] [CrossRef]
- Casao, M.C.; Igartua, E.; Karsai, I.; Bhat, P.R.; Cuadrado, N.; Gracia, M.P.; Lasa, J.M.; Casas, A.M. Introgression of an intermediate VRNH1 allele in barley (Hordeum vulgare L.) leads to reduced vernalization requirement without affecting freezing tolerance. Mol. Breed. 2011, 28, 475–484. [Google Scholar] [CrossRef]
- Abu-Elenein, J.; Al-Sayaydeh, R.; Akkeh, Z.; Al-Ajlouni, Z.; Al-Bawalize, A.A.; Hasan, S.; Alhindi, T.; Albdaiwi, R.N.; Ayad, J.Y.; Al-Abdallat, A.M. Agronomic performance and flowering behavior in response to photoperiod and vernalization in barley (Hordeum vulgare L.) genotypes with contrasting drought tolerance behaviour. Environ. Exp. Bot. 2021, 192, 104661. [Google Scholar] [CrossRef]
- Karsai, I.; Szűcs, P.; Mészáros, K.; Filichkina, T.; Hayes, P.M.; Skinner, J.S.; Láng, L.; Bedö, Z. The Vrn-H2 locus is a major determinant of flowering time in a facultative winter growth habit barley (Hordeum vulgare L.) mapping population. Theor. Appl. Genet. 2005, 110, 1458–1466. [Google Scholar] [CrossRef]
- Yan, L.; Fu, D.; Li, C.; Blechl, A.; Tranquilli, G.; Bonafede, M.; Sanchez, A.; Valarik, M.; Yasuda, S.; Dubcovsky, J. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl. Acad. Sci. USA 2006, 103, 19581–19586. [Google Scholar] [CrossRef]
- Kikuchi, R.; Kawahigashi, H.; Ando, T.; Tonooka, T.; Handa, H. Molecular and functional characterization of PEBP genes in barley reveal the diversification of their roles in flowering. Plant Physiol. 2009, 149, 1341–1353. [Google Scholar] [CrossRef]
- Turner, A.; Beales, J.; Faure, S.; Dunford, R.P.; Laurie, D.A. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 2005, 310, 1031–1034. [Google Scholar] [CrossRef]
- Takahashi, R.; Yasuda, S. Genetics of earliness and growth habit in barley. In Barley Genetics II; Nilan, R.A., Ed.; Washington State University Press: Pullman, WA, USA, 1971; pp. 388–408. [Google Scholar]
- Zlotina, M.M.; Kovaleva, O.N.; Loskutov, I.G.; Potokina, E.K. Use of allele-specific markers of the Ppd and Vrn genes for predicting growing season duration in barley cultivars. Vavilov J. Genet. Breed. 2014, 17, 50–62. (In Russian) [Google Scholar] [CrossRef]
- Loskutov, I.G.; Kovaleva, O.N.; Blinova, E.V. Guidelines for Study and Conservation of the World Collection of Barley and Oats. VIR: St. Petersburg, Russia, 2012. (In Russian) [Google Scholar]
- Köppen Climate Classification; Wikipedia. Available online: https://en.wikipedia.org/wiki/K%C3%B6ppen_climate_classification (accessed on 15 July 2024).
- Climate in Kazakhstan. Climate Data. Available online: https://ru.climate-data.org (accessed on 17 July 2024).
- UPOV. International union for the protection of new varieties of plants Hordeum vulgare L. 5 May 2017. Available online: https://www.upov.int/edocs/mdocs/upov/en/twa_46/tg_19_11_proj_2.pdf (accessed on 17 July 2024).
- Dellaporta, S.L.; Wood, J.; Hicks, J.B. A plant DNA minipreparation: Version II. Plant Mol. Biol. Rep. 1983, 1, 19–21. [Google Scholar] [CrossRef]
- Shavrukov, Y. Cleaved Amplified Polymorphic Sequences (CAPS) Markers in Plant Biology. Nova Science Publishers: New York, NY, USA, 2014. [Google Scholar]
- JASP Version 0.19.3. Available online: https://jasp-stats.org/ (accessed on 12 May 2025).
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014. [Google Scholar]
- Cohen’s Kappa. Available online: https://datatab.net/tutorial/cohens-kappa (accessed on 17 July 2024).
- Cockram, J.; Norris, C.; O’Sullivan, D.M. PCR-based markers diagnostic for spring and winter seasonal growth habit in barley. Crop. Sci. 2009, 49, 403–410. [Google Scholar] [CrossRef]
- Kóti, K.; Karsai, I.; Szűcs, P.; Horváth, C.; Mészáros, K.; Kiss, G.B.; Bedő, Z.; Hayes, P.M. Validation of the two-gene epistatic model for vernalization response in a winter × spring barley cross. Euphytica 2006, 152, 17–24. [Google Scholar] [CrossRef]
- Szűcs, P.; Skinner, J.S.; Karsai, I.; Cuesta-Marcos, A.; Haggard, K.G.; Corey, A.E.; Chen, T.H.H.; Hayes, P.M. Validation of the VRN-H2/VRN-H1 epistatic model in barley reveals that intron length variation in VRN-H1 may account for a continuum of vernalization sensitivity. Mol. Genet. Genom. 2007, 277, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Loukoianov, A.; Blechl, A.; Tranquilli, G.; Ramakrishna, W.; SanMiguel, P.; Bennetzen, J.L.; Echenique, V.; Dubcovsky, J. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 2004, 303, 1640–1644. [Google Scholar] [CrossRef]
- Mohammadi, M.; Torkamaneh, D.; Nikkhah, H.R. Correlation of vernalization loci VRN-H1 and VRN-H2 and growth habit in barley germplasm. Int. J. Plant Genom. 2013, 2013, 924043. [Google Scholar] [CrossRef][Green Version]
- Cockram, J.; Chiapparino, E.; Taylor, S.A.; Stamati, K.; Donini, P.; Laurie, D.A.; O’Sullivan, D.M. Haplotype analysis of vernalization loci in European barley germplasm reveals novel VRN-H1 alleles and a predominant winter VRN-H1/VRN-H2 multi-locus haplotype. Theor. Appl. Genet. 2007, 115, 993–1001. [Google Scholar] [CrossRef]
- Alabushev, A.V.; Dontsova, A.A.; Filippov, E.G.; Dontsov, D.P.; Pepchuk, I.N.; Teplyakova, S.B.; Potokina, E.K. Search for the correlation between allelic polymorphism of the Ppd and Vrn genes with the variability of the main economically valuable traits of winter barley. Zernovoe Hozyaistvo Ross. 2019, 3, 19–25. (In Russian) [Google Scholar] [CrossRef]
- Trevaskis, B.; Hemming, M.N.; Peacock, W.J.; Dennis, E.S. HvVRN2 responds to daylength, whereas HvVRN1 is regulated by vernalization and developmental status. Plant Physiol. 2006, 140, 1397–1405. [Google Scholar] [CrossRef]
- Dubcovsky, J.; Chen, C.; Yan, L. Molecular characterization of the allelic variation at the VRN-H2 vernalization locus in barley. Mol. Breed. 2005, 15, 395–407. [Google Scholar] [CrossRef]
- Dinh, H.X.; Pourkheirandish, M.; Park, R.F.; Singh, D. The genetic basis and interaction of genes conferring resistance to Puccinia hordei in an ICARDA barley breeding line GID 5779743. Front. Plant Sci. 2022, 13, 988322. [Google Scholar] [CrossRef] [PubMed]
- Venegas, J.; Guttieri, M.J.; Boehm, J.D.J.; Graybosch, R.; Bai, G.; Amand, P.C.S.; Palmer, N.; Hussain, W.; Blecha, S.; Baenziger, P.S. Genetic architecture of the high inorganic phosphate phenotype derived from a low phytate mutant in winter wheat. Crop. Sci. 2022, 62, 1228–1241. [Google Scholar] [CrossRef]
- Iqbal, I.; Desta, Z.A.; Tripathi, R.K.; Beattie, A.; Badea, A.; Singh, J. Interaction and association analysis of malting related traits in barley. PLoS ONE 2023, 18, e0283763. [Google Scholar] [CrossRef] [PubMed]
- Qamar, Z.U.; Uzair, M.; Hameed, A.; Zafar, S.A.; Li, X. Identification of a novel mutation in the OsMRP5 gene in low phytate Basmati rice mutant and development of CAPS marker for marker-assisted breeding. Front. Plant Sci. 2024, 15, 1455219. [Google Scholar] [CrossRef]
- Saygili, I. Barley yield and malt quality affected by fall and spring planting under rainfed conditions. PeerJ 2023, 11, e15802. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Amatriaín, M.; Hernandez, J.; Herb, D.; Baenziger, P.S.; Bochard, A.M.; Capettini, F.; Casas, A.; Cuesta-Marcos, A.; Einfeldt, C.; Fisk, S.; et al. Perspectives on low temperature tolerance and vernalization sensitivity in barley: Prospects for facultative growth habit. Front. Plant Sci. 2020, 11, 585927. [Google Scholar] [CrossRef]
- Wiegmann, M.; Maurer, A.; Pham, A.; March, T.J.; Al-Abdallat, A.; Thomas, W.T.; Bull, H.; Shahid, M.; Eglinton, J.; Baum, M.; et al. Barley yield formation under abiotic stress depends on the interplay between flowering time genes and environmental cues. Sci. Rep. 2019, 9, 6397. [Google Scholar] [CrossRef]

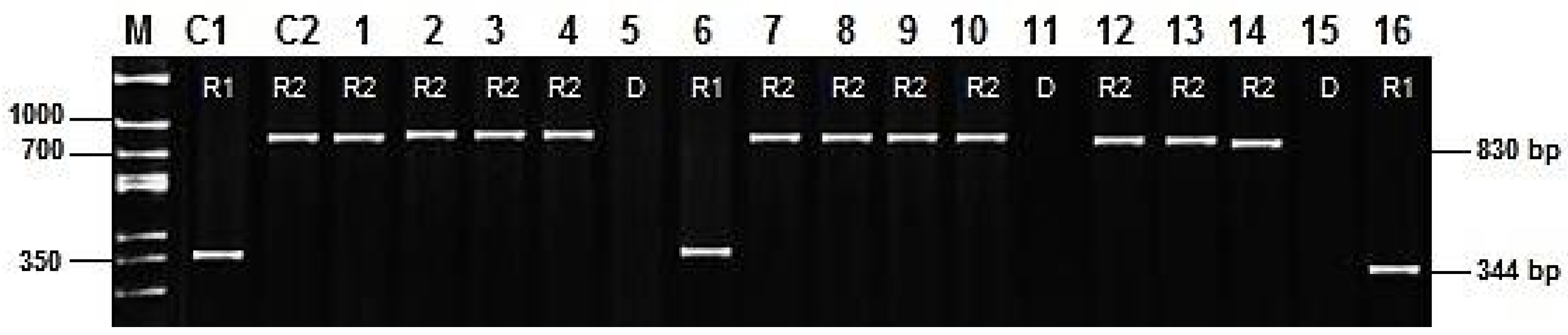
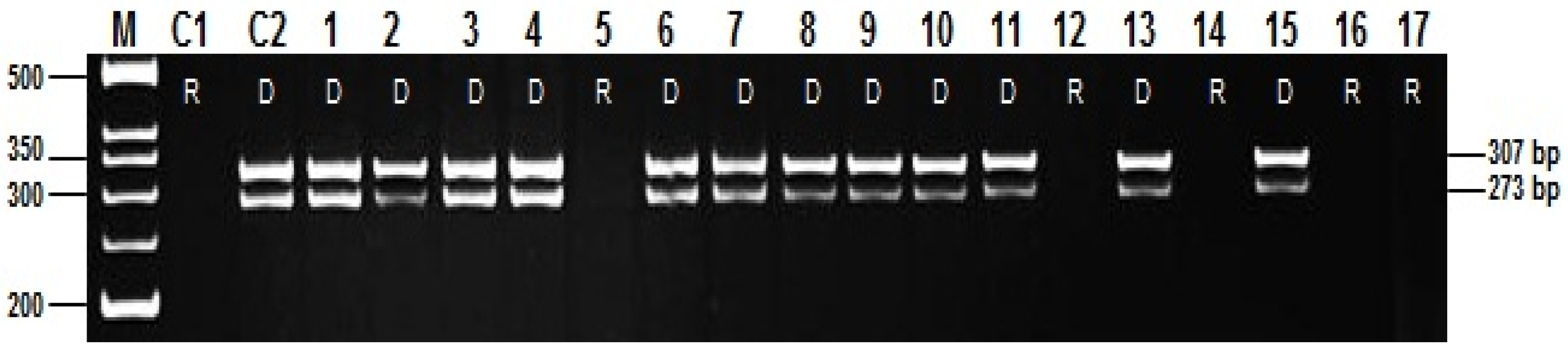
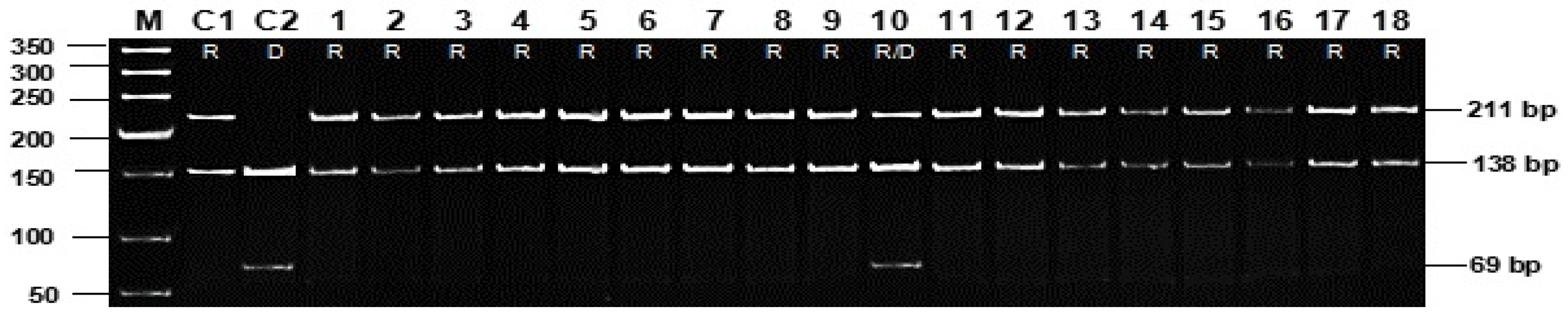
| Gene | Molecular Markers and Primers | Allele | Earing Genotypes | No Earing Genotypes | Total Genotypes | χ2 | df | p |
|---|---|---|---|---|---|---|---|---|
| Vrn-H1 | Marker 1 (HvBM5A intron1 F3b/R3b) | D | 207 | 7 | 214 | 171.5 | 1 | <0.001 |
| R | 39 | 87 | 126 | |||||
| Marker 2 (HvBM5.84/85 F/R) | D | 169 | 7 | 176 | 102.1 | 1 | <0.001 | |
| R | 77 | 87 | 164 | |||||
| Marker 3 (HvBM5A exon2F1/R1) | D | 243 | 83 | 326 | 18.9 | 1 | <0.001 | |
| R | 3 | 11 | 14 | |||||
| Vrn-H2 | Marker 4 (HvZCCT.06F/07R) | D | 30 | 51 | 81 | 66.3 | 1 | <0.001 |
| R | 216 | 43 | 259 | |||||
| Marker 5 (ZCCTH.14F/19R) | D | 46 | 34 | 80 | 11.5 | 1 | <0.004 | |
| R | 200 | 60 | 260 | |||||
| Marker 6 (ZCCTb.8F/11R) | D | 43 | 28 | 71 | 6.2 | 1 | <0.01 | |
| R | 203 | 66 | 269 | |||||
| Marker 7 (ZCCT.HcF/HcR) | D | 71 | 62 | 133 | 39.3 | 1 | <0.001 | |
| R | 175 | 32 | 207 | |||||
| Marker 8 (HvZCCT.001/002) | D | 30 | 22 | 52 | 6.5 | 1 | <0.01 | |
| R | 215 | 72 | 287 | |||||
| Marker 9 (HvSnf2.01F/2.03R) | D | 21 | 17 | 38 | 6.2 | 1 | <0.01 | |
| R | 225 | 77 | 302 | |||||
| Vrn-H3 | Marker 10 (HvFT1-F/R) | D | 2 | 6 | 8 | 9.2 | 2 | <0.002 |
| R | 244 | 88 | 332 |
| Genotyping Classification | Phenotyping Evaluation, Number of Accessions | ||
|---|---|---|---|
| Earing | Non-Earing | Total | |
| Spring | 211 | 7 | 218 |
| Winter | 7 | 56 | 63 |
| Facultative | 28 | 31 | 59 |
| Total | 246 | 94 | 340 |
| Chi-square result | χ2 = 197.6; df = 2; p =< 0.001 | ||
| Growth Habit | Accession Name | Pedigree | Formula Vrn-H | Winter Sowing | Spring Sowing | ||
|---|---|---|---|---|---|---|---|
| Yield t/ha | TSW, g | Yield t/ha | TSW, g | ||||
| Spring | [Standard] Bereke-54 | Krasnovodopad. Breeding Station | DDR | 4.1 a | 42.6 a | 2.3 a | 39.6 a |
| 70/08-3 | Bulbul × Yuzhno- Kazakhstansky-43 | DRR | 5.1 b | 47.2 ab | 2.7 ab | 44.4 b | |
| Winter | [Standard] Aydin | Vavilon//Zazjan /80-5151 | RDR | 4.0 a | 38.0 a | - | - |
| 67/08-6 | АI-19 × Yuzhno- Kazakhstansky-43 | RDR | 5.6 b | 50.4 b | - | - | |
| Facultative | 76/13-4 | 946 × 579 NBat-2 | RRR | 5.3 b | 49.4 b | 3.4 b | 44.6 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yerzhebayeva, R.; Bazylova, T.; Zhumaliyeva, G.; Bastaubayeva, S.; Baimuratov, A.; Sariev, B.; Shegebayev, G.; Ergün, N.; Shavrukov, Y. Combination of Vrn Alleles Assists in Optimising the Vernalization Requirement in Barley (Hordeum vulgare L.). Agriculture 2025, 15, 1389. https://doi.org/10.3390/agriculture15131389
Yerzhebayeva R, Bazylova T, Zhumaliyeva G, Bastaubayeva S, Baimuratov A, Sariev B, Shegebayev G, Ergün N, Shavrukov Y. Combination of Vrn Alleles Assists in Optimising the Vernalization Requirement in Barley (Hordeum vulgare L.). Agriculture. 2025; 15(13):1389. https://doi.org/10.3390/agriculture15131389
Chicago/Turabian StyleYerzhebayeva, Raushan, Tamara Bazylova, Gaziza Zhumaliyeva, Sholpan Bastaubayeva, Askar Baimuratov, Burabai Sariev, Galym Shegebayev, Namuk Ergün, and Yuri Shavrukov. 2025. "Combination of Vrn Alleles Assists in Optimising the Vernalization Requirement in Barley (Hordeum vulgare L.)" Agriculture 15, no. 13: 1389. https://doi.org/10.3390/agriculture15131389
APA StyleYerzhebayeva, R., Bazylova, T., Zhumaliyeva, G., Bastaubayeva, S., Baimuratov, A., Sariev, B., Shegebayev, G., Ergün, N., & Shavrukov, Y. (2025). Combination of Vrn Alleles Assists in Optimising the Vernalization Requirement in Barley (Hordeum vulgare L.). Agriculture, 15(13), 1389. https://doi.org/10.3390/agriculture15131389







