The Acaricidal Activity of Essential Oil Vapors and Its Effect on the Varroa Mite Varroa destructor
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Isolation of EOs
2.3. EO Analysis
2.4. Varroa Mite Collection
2.5. Acaridical Activity Bioassays
2.6. Statistical Analysis
3. Results
3.1. EO Yields and Chemical Composition Analysis
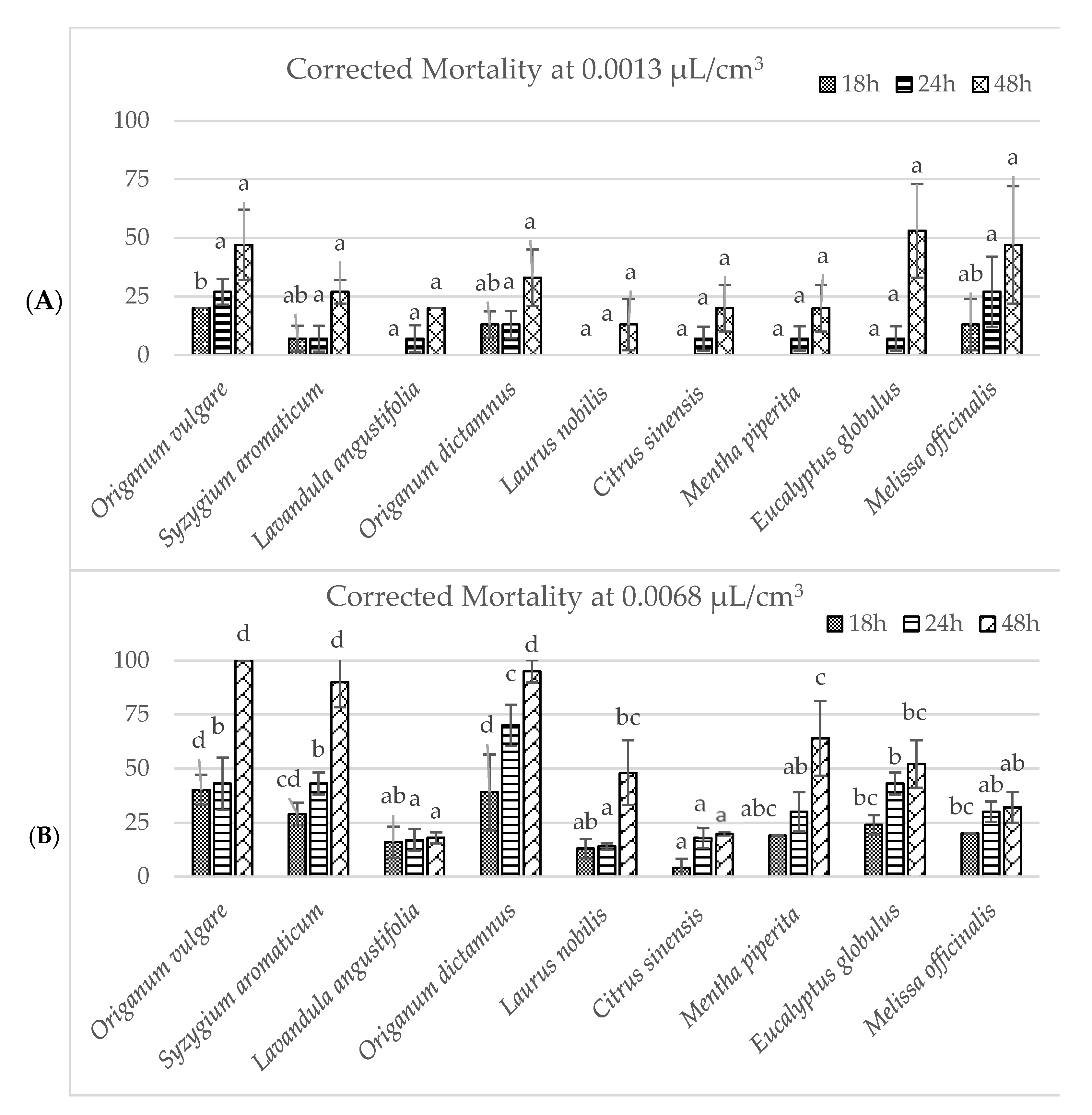
3.2. Miticide Screening Test and Activity Against V. destructor
3.3. Miticide Concentration Response Test and LC50 Values of O. vulgare, S. aromaticum, and O. dictamus Against V. destructor
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bradbear, N. Bees and Their Role in Forest Livelihoods: A Guide to the Services Provided by Bees and the Sustainable Harvesting, Processing and Marketing of Their Products. Non-Wood Forest Products. 2009. Available online: http://www.fao.org/3/i0842e/i0842e00.pdf (accessed on 10 October 2024).
- García, N.L. The current situation on the international honey market. Bee World 2018, 95, 89–94. [Google Scholar] [CrossRef]
- Brunet, J.; Fragoso, F.P. What Are the Main Reasons for the Worldwide Decline in Pollinator Populations? CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources Volume 19, Issue 1, January 2024. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabireviews.2024.0016 (accessed on 15 November 2024).
- Dequenne, I.; de Foy, J.M.P.; Cani, P.D. Developing Strategies to Help Bee Colony Resilience in Changing Environments. Animals 2022, 12, 3396. [Google Scholar] [CrossRef]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Regulation-(EC)-No-470/2009. Regulation (EC) No 470/2009 of 6 May 2009 Laying Down Community Procedures for the Establishment of Residue Limits of Pharmacologically Active Substances in Foodstufs of Animal Origin, Repealing Council Regulation (EEC) No 2377/90 and Amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. Official Journal of the European Union. 16.6.2009. L 152/11. Available online: https://eur-lex.europa.eu/eli/reg/2009/470/oj (accessed on 5 September 2024).
- Rinkevich, F.D. Detection of amitraz resistance and reduced treatment efficacy in the Varroa Mite, Varroa destructor, within commercial beekeeping operations. PLoS ONE 2020, 15, e0227264. [Google Scholar] [CrossRef]
- Farina, P.; Giunti, G.; Campolo, O.; Maggi, F.; Ricciardi, R.; Lucchi, A.; Canale, A.; Pavela, R.; Guedes, R.N.C.; Desneux, N.; et al. Nano- and microformulated botanicals for managing ticks and mites of medical and veterinary importance: Past, present, and future. Ind. Crops Prod. 2024, 222, 119809. [Google Scholar] [CrossRef]
- Ntalli, N.; Kopiczko, A.; Radtke, K.; Marciniak, P.; Rosinski, G.; Adamski, Z. Biological activity of Melia azedarach extracts against Spodoptera exigua. Biologia 2014, 69, 1606–1614. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. A Review of Resistance Mechanisms of Synthetic Insecticides and Botanicals, Phytochemicals, and Essential Oils as Alternative Larvicidal Agents Against Mosquitoes. Front. Physiol. 2020, 10, 1591. [Google Scholar] [CrossRef]
- Stuhl, C.J. The development of an attract-and-kill bait for controlling the small hive beetle (Coleoptera: Nitidulidae). Apidologie 2020, 51, 428–435. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Medici, S.K.; Maggi, M.D.; Sarlo, E.G.; Ruffinengo, S.; Marioli, J.M.; Eguaras, M.J. The presence of synthetic acaricides in beeswax and its influence on the development of resistance in Varroa destructor. J. Apicult Res. 2015, 54, 267–274. [Google Scholar] [CrossRef]
- Bogdanov, S. Contaminants of bee products. Apidologie 2006, 37, 1–18. [Google Scholar] [CrossRef]
- Lambert, O.; Piroux, M.; Puyo, S.; Thorin, C.; L’Hostis, M.; Wiest, L.; Buleté, A.; Delbac, F.; Pouliquen, H. Widespread Occurrence of Chemical Residues in Beehive Matrices from Apiaries Located in Different Landscapes of Western France. PLoS ONE 2013, 8, e67007. [Google Scholar] [CrossRef] [PubMed]
- Bava, R.; Castagna, F.; Lupia, C.; Ruga, S.; Musella, V.; Conforti, F.; Marrelli, M.; Argentieri, M.P.; Britti, D.; Statti, G.; et al. Chemical Profile of Essential Oils of Selected Lamiaceae Plants and In Vitro Activity for Varroosis Control in Honeybees (Apis mellifera). Vet. Sci. 2023, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Bava, R.; Castagna, F.; Palma, E.; Marrelli, M.; Conforti, F.; Musolino, V.; Carresi, C.; Lupia, C.; Ceniti, C.; Tilocca, B.; et al. Essential Oils for a Sustainable Control of Honeybee Varroosis. Vet. Sci. 2023, 10, 308. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Lupia, C.; Ruga, S.; Conforti, F.; Marrelli, M.; Argentieri, M.P.; Musella, V.; Britti, D.; Statti, G.; et al. Phytochemical Composition and Pharmacological Efficacy Evaluation of Calamintha nepeta, Calamintha sylvatica, Lavandula austroapennina and Mentha piperita Essential Oils for the Control of Honeybee (Apis mellifera) Varroosis. Animals 2024, 14, 69. [Google Scholar] [CrossRef]
- Kraus, B.; Koeniger, N.; Fuchs, S. Screening of Substances for Their Effect on Varroa-Jacobsoni—Attractiveness, Repellency, Toxicity and Masking Effects of Ethereal Oils. J. Apic. Res. 1994, 33, 34–43. [Google Scholar] [CrossRef]
- Puntener, W. Manual for field trials. In Plant Protection, 2nd ed.; Ciba-Geigy: Basel, Switzerland, 1981; p. 205. [Google Scholar]
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-Logistic Analysis of Herbicide Dose-Response Relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Daferera, D.J.; Ziogas, B.N.; Polissiou, M.G. GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J. Agric. Food Chem. 2000, 48, 2576–2581. [Google Scholar] [CrossRef]
- Tsoumani, E.S.; Kosma, I.S.; Badeka, A. Chemometric Screening of Oregano Essential Oil Composition and Properties for the Identification of Specific Markers for Geographical Differentiation of Cultivated Greek Oregano. Sustainability 2022, 14, 14762. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Arnold, N.A.; Menichini, F.; Senatore, F. Composition, antibacterial, antioxidant and antiproliferative activities of essential oils from three Origanum species growing wild in Lebanon and Greece. Nat. Prod. Res. 2016, 30, 735–739. [Google Scholar] [CrossRef]
- Gong, H.; Liu, W.; Lv, G.; Zhou, X.Y. Analysis of essential oils of Origanum vulgare from six production areas of China and Pakistan. Rev. Bras. Farmacogn. 2014, 24, 25–32. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and Antibacterial Capacities of Origanum vulgare L. Essential Oil from the Arid Andean Region of Chile and its Chemical Characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Teles, A.M.; Silva-Silva, J.V.; Fernandes, J.M.P.; Abreu-Silva, A.L.; Calabrese, K.D.S.; Mendes Filho, N.E.; Mouchrek, A.N.; Almeida-Souza, F. GC-MS Characterization of Antibacterial, Antioxidant, and Antitrypanosomal Activity of Syzygium aromaticum Essential Oil and Eugenol. Evid. Based Complement. Altern. Med. 2021, 2021, 6663255. [Google Scholar] [CrossRef]
- Dong, G.; Bai, X.; Aimila, A.; Aisa, H.A.; Maiwulanjiang, M. Study on Lavender Essential Oil Chemical Compositions by GC-MS and Improved pGC. Molecules 2020, 25, 3166. [Google Scholar] [CrossRef] [PubMed]
- Azadmanesh, R.; Tatari, M.; Asgharzade, A.; Taghizadeh, S.F.; Shakeri, A. GC/MS Profiling and Biological Traits of Eucalyptus globulus L. Essential Oil Exposed to Solid Lipid Nanoparticle (SLN). J. Essent. Oil Bear. Plants 2021, 24, 863–878. [Google Scholar] [CrossRef]
- Bourakna, Z.; Righi, K.; Righi, F.A. GC/MS Analysis of Eucalyptus globulus L. (Myrtaceae) Leaves Essential Oil from Algeria and their Insecticidal Activity Against Adults of Bactrocera oleae (Rossi) (Diptera; Tephritidae). J. Essent. Oil Bear. Plants 2022, 25, 876–887. [Google Scholar] [CrossRef]
- Taherpour, A.A.; Khaef, S.; Yari, A.; Nikeafshar, S.; Fathi, M.; Ghambari, S. Chemical composition analysis of the essential oil of Mentha piperita L. from Kermanshah, Iran by hydrodistillation and HS/SPME methods. J. Anal. Sci. Technol. 2017, 8, 11. [Google Scholar] [CrossRef]
- Mohammed, I.O.; Alrasheid, A.A.; Ayoub, S.M.H. GC-MS Analysis and Study of the Antimicrobial Activity of Citrus paradisi, Citrus aurantifolia, and Citrus sinensis Peel Essential Oils as Hand Sanitizer. Int. J. Microbiol. 2024, 2024, 4957712. [Google Scholar] [CrossRef]
- Peris, I.; Blázquez, M.A. Comparative GC-MS Analysis of Bay Leaf (Laurus nobilis L.) Essential Oils in Commercial Samples. Int. J. Food Prop. 2015, 18, 757–762. [Google Scholar] [CrossRef]
- Stojanovic, N.M.; Mladenovic, M.Z.; Maslovaric, A.; Stojiljkovic, N.; Randjelovic, P.J.; Radulovic, N.S. Lemon balm (Melissa officinalis L.) essential oil and citronellal modulate anxiety-related symptoms—In vitro and in vivo studies. J. Ethnopharmacol. 2022, 284, 114788. [Google Scholar] [CrossRef]
- Saeb, K.; Gholamrezaee, S. Variation of essential oil composition of Melissa officinalis L. leaves during different stages of plant growth. Asian Pac. J. Trop. Biomed. 2012, 2, S547–S549. [Google Scholar] [CrossRef]
- Gashout, H.A.; Guzman-Novoa, E. Acute toxicity of essential oils and other natural compounds to the parasitic mite, Varroa destructor, and to larval and adult worker honey bees (Apis mellifera L.). J. Apicult Res. 2009, 48, 263–269. [Google Scholar] [CrossRef]
- Özüiçli, M.; Baykalir, Y. Evaluation of efficiency of thyme oil, Cinnamomum verum, Melaleuca viridiflora, Syzygium aromaticum essential oils, and amitraz for Varroa mite (Acari: Varroidae) control in honeybee (hymenoptera: Apidae) colonies under field conditions. Kafkas Univ. Vet. Fak. 2024, 30, 541–548. [Google Scholar] [CrossRef]
- Li, L.; Lin, Z.G.; Wang, S.; Su, X.L.; Gong, H.R.; Li, H.L.; Hu, F.L.; Zheng, H.Q. The effects of clove oil on the enzyme activity of Varroa destructor Anderson and Trueman (Arachnida: Acari: Varroidae). Saudi J. Biol. Sci. 2017, 24, 996–1000. [Google Scholar] [CrossRef]
- Maggi, M.; Peralta, L.; Ruffinengo, S.; Fuselli, S.; Eguaras, M. Body size variability of Varroa destructor and its role in acaricide tolerance. Parasitol. Res. 2012, 110, 2333–2340. [Google Scholar] [CrossRef]
- Maggi, M.D.; Ruffinengo, S.R.; Gende, L.B.; Sarlo, E.G.; Eguaras, M.J.; Bailac, P.N.; Ponzi, M.I. Laboratory evaluations of Syzygium aromaticum (L.) Merr. et perry essential oil against Varroa destructor. J. Essent. Oil Res. 2010, 22, 119–122. [Google Scholar] [CrossRef]
- Bisrat, D.; Begna, T.; Ulziibayar, D.; Jung, C. Acaricidal activity of essential oil derived components from Thymus schimperi Ronninger against Varroa destructor Anderson and Trueman. J. Apic. Res. 2024, 63, 664–670. [Google Scholar] [CrossRef]
- Ariana, A.; Ebadi, R.; Tahmasebi, G. Laboratory evaluation of some plant essences to control Varroa destructor (Acari: Varroidae). Exp. Appl. Acarol. 2002, 27, 319–327. [Google Scholar] [CrossRef]
- Chaimanee, V.; Thongtue, U.; Sornmai, N.; Songsri, S.; Pettis, J.S. Antimicrobial activity of plant extracts against the honeybee pathogens, Paenibacillus larvae and Ascosphaera apis and their topical toxicity to Apis mellifera adults. J. Appl. Microbiol. 2017, 123, 1160–1167. [Google Scholar] [CrossRef]
- Aglagane, A.; Laghzaoui, E.; Soulaimani, B.; Er-Rguibi, O.; Abbad, A.; El Mouden, E.; Aourir, M. Acaricidal activity of Mentha suaveolens subsp. timija, Chenopodium ambrosioides, and Laurus nobilis essential oils, and their synergistic combinations against the ectoparasitic bee mite, Varroa destructor (Acari: Varroidae). J. Apic. Res. 2022, 61, 9–18. [Google Scholar] [CrossRef]
- Kütükoglu, F.; Girisgin, A.O.; Aydin, L. Varroacidal efficacies of essential oils extracted from Lavandula officinalis, Foeniculum vulgare, and Laurus nobilis in naturally infested honeybee (Apis mellifera L.) Colonies. Turk. J. Vet. Anim. Sci. 2012, 36, 554–559. [Google Scholar] [CrossRef]
- Ahumada, M.F.; Marcos, J.L.; Cadavid, A.; Baiiares, G.V.; Silva, C.M.; Olivares, Y.A.; Müller, H.Y. Evaluation of the efficacy of essential oils of Lavandula angustifolia and Eucalyptus globulus for the control of Varroa destructor in Apis mellifera: A randomised field study. Austral J. Vet. Sci. 2022, 54, 83–87. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Piras, C.; Palma, E.; Cringoli, G.; Musolino, V.; Lupia, C.; Perri, M.R.; Statti, G.; Britti, D.; et al. In Vitro Evaluation of Acute Toxicity of Five Citrus spp. Essential Oils towards the Parasitic Mite Varroa destructor. Pathogens 2021, 10, 1182. [Google Scholar] [CrossRef]
- Karimi, P.; Malekifard, F.; Tavassoli, M. Medicinal plant essential oils as promising Anti-Varroa agents: Oxidative/nitrosative screens. S. Afr. J. Bot. 2022, 148, 344–351. [Google Scholar] [CrossRef]
- Scalerandi, E.; Flores, G.A.; Palacio, M.; Defagó, M.T.; Carpinella, M.C.; Valladares, G.; Bertoni, A.; Palacios, S.M. Understanding Synergistic Toxicity of Terpenes as Insecticides: Contribution of Metabolic Detoxification in Musca domestica. Front. Plant Sci. 2018, 9, 1579. [Google Scholar] [CrossRef]
- Guillermo, A.M.F.; Nahuel, F.; Maria, T.D.; Andres, M.V.; Sara, M.P. Adulticidal effect of seven terpenes and a binary combination against Aedes aegypti. J. Vector Dis. 2020, 57, 356–358. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Zunino, M.P.; Herrera, J.M.; Pizzolitto, R.P.; Areco, V.A.; Zygadlo, J.A. Terpenes: Natural Products for Controlling Insects of Importance to Human Health—A Structure-Activity Relationship Study. Psyche 2016, 2016, 4595823. [Google Scholar] [CrossRef]
| Plant Species | Plant Part Used for Water Distillation | Yield a (mL/100 g of dw) | |
|---|---|---|---|
| 1 | Origanum vulgare (oregano) | stalks, flowers, leaves | 3.1 ± 0.01 |
| 2 | Syzygium aromaticum (clove) | flower buds | 13 ± 0.5 |
| 3 | Lavandula angustifolia (lavender) | stalks, flowers, leaves | 1.1 ± 0.1 |
| 4 | Origanum dictamnus (dittany) | stalks, flowers, leaves | 0.96 ± 0.01 |
| 5 | Laurus nobilis (bay laurel) | leaves | 1.97 ± 0.01 |
| 6 | Citrus sinensis (sweet orange) | peel | 0.3 ± 0.2 |
| 7 | Mentha piperita (peppermint) | stalks, flowers, leaves | 1.65 ± 0.02 |
| 8 | Eucalyptus globulus (blue gum) | leaves | 0.71 ± 0.03 |
| 9 | Melissa officinalis (lemon balm) | stalks, flowers, leaves | 0.10 ± 0.03 |
| Origanum vulgare | LC50 (μL/cm3 Petri) | R2 | St. Error | Cl95% |
|---|---|---|---|---|
| 18 h | >0.0068 | n.a. | n.a. | n.a. |
| 24 h | 0.003 | 0.992 | 0.0003 | 0.002–0.005 |
| 48 h | 0.001 | 0.940 | 0.0013 | 0.000–0.002 |
| Syzygium aromaticum | lC50 (μL/cm3 Petri) | R2 | n.a. | n.a. |
| 18 h | >0.0068 | n.a. | n.a. | n.a. |
| 24 h | >0.0068 | n.a. | n.a. | n.a. |
| 48 h | 0.002 | 0.946 | 0.0004 | 0.001–0.002 |
| Origanum dictamnus | LC50 (μL/cm3 Petri) | R2 | n.a. | n.a. |
| 18 h | >0.0068 | n.a. | n.a. | n.a. |
| 24 h | >0.0068 | n.a. | n.a. | n.a. |
| 48 h | 0.002 | 0.931 | 0.0007 | 0.001–0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntalli, N.G.; Samara, M.; Stathakis, T.; Barda, M.; Kapaxidi, E.; Manea-Karga, E.; Gounari, S.; Goras, G.; Kasiotis, K.M.; Karamaouna, F. The Acaricidal Activity of Essential Oil Vapors and Its Effect on the Varroa Mite Varroa destructor. Agriculture 2025, 15, 1379. https://doi.org/10.3390/agriculture15131379
Ntalli NG, Samara M, Stathakis T, Barda M, Kapaxidi E, Manea-Karga E, Gounari S, Goras G, Kasiotis KM, Karamaouna F. The Acaricidal Activity of Essential Oil Vapors and Its Effect on the Varroa Mite Varroa destructor. Agriculture. 2025; 15(13):1379. https://doi.org/10.3390/agriculture15131379
Chicago/Turabian StyleNtalli, Nikoletta G., Maria Samara, Theodoros Stathakis, Myrto Barda, Eleftheria Kapaxidi, Elektra Manea-Karga, Sofia Gounari, Georgios Goras, Konstantinos M. Kasiotis, and Filitsa Karamaouna. 2025. "The Acaricidal Activity of Essential Oil Vapors and Its Effect on the Varroa Mite Varroa destructor" Agriculture 15, no. 13: 1379. https://doi.org/10.3390/agriculture15131379
APA StyleNtalli, N. G., Samara, M., Stathakis, T., Barda, M., Kapaxidi, E., Manea-Karga, E., Gounari, S., Goras, G., Kasiotis, K. M., & Karamaouna, F. (2025). The Acaricidal Activity of Essential Oil Vapors and Its Effect on the Varroa Mite Varroa destructor. Agriculture, 15(13), 1379. https://doi.org/10.3390/agriculture15131379










