Organic Acids Metabolic Response and Transcription Factor Expression Changes of Highland Barley Seedlings Under Na2SeO3 Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Processing
2.2. Determination of Physiological Indicators
2.3. Determination of Selenium Content
2.4. Transcriptome Sequencing and Analysis
2.5. LC-MS/MS Analysis
2.6. qRT-PCR for Gene Expression Validation
2.7. Data Statistics
3. Results
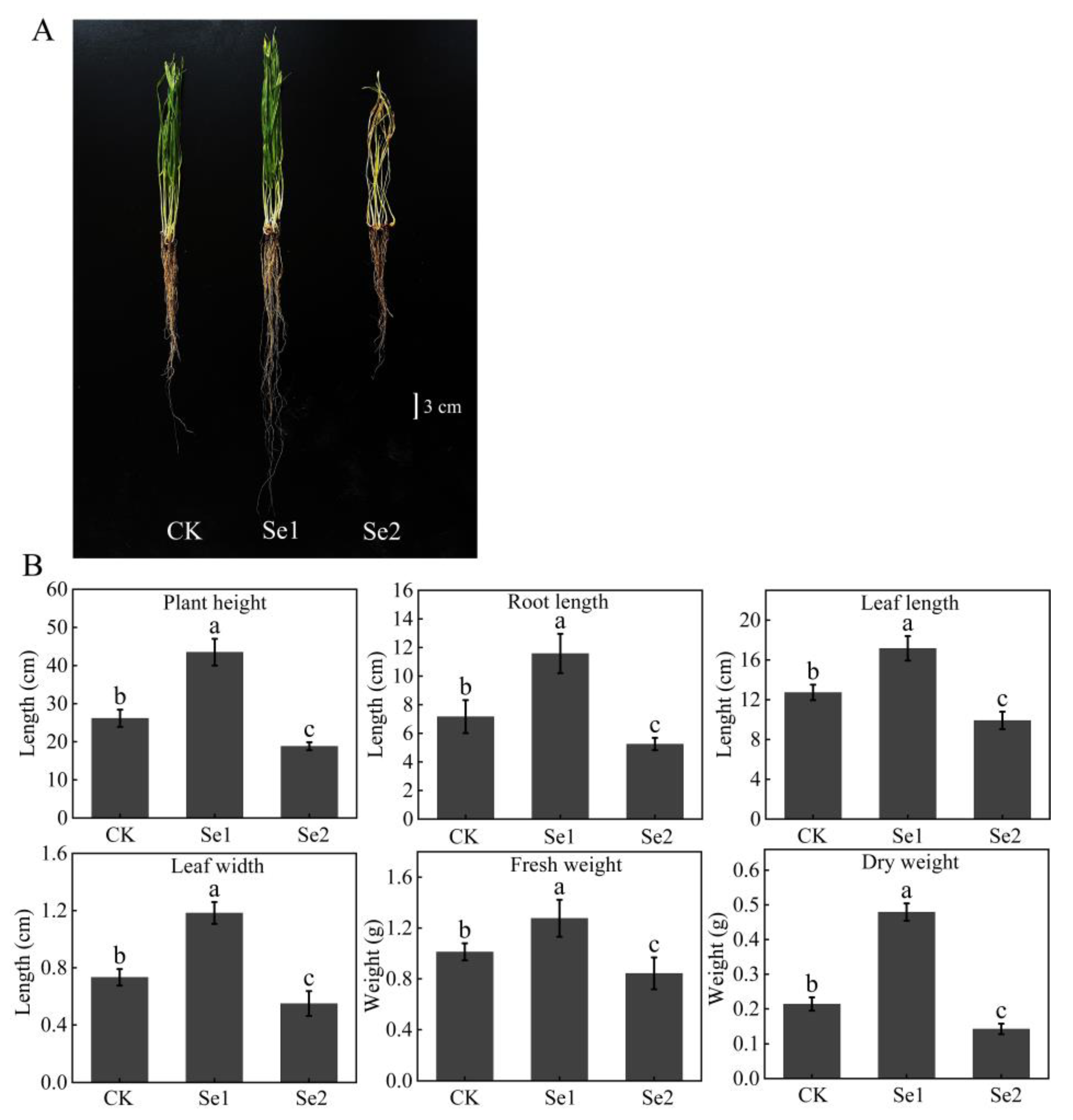
3.1. Growth Effects of Highland Barley Seedlings Under Na2SeO3 Treatment
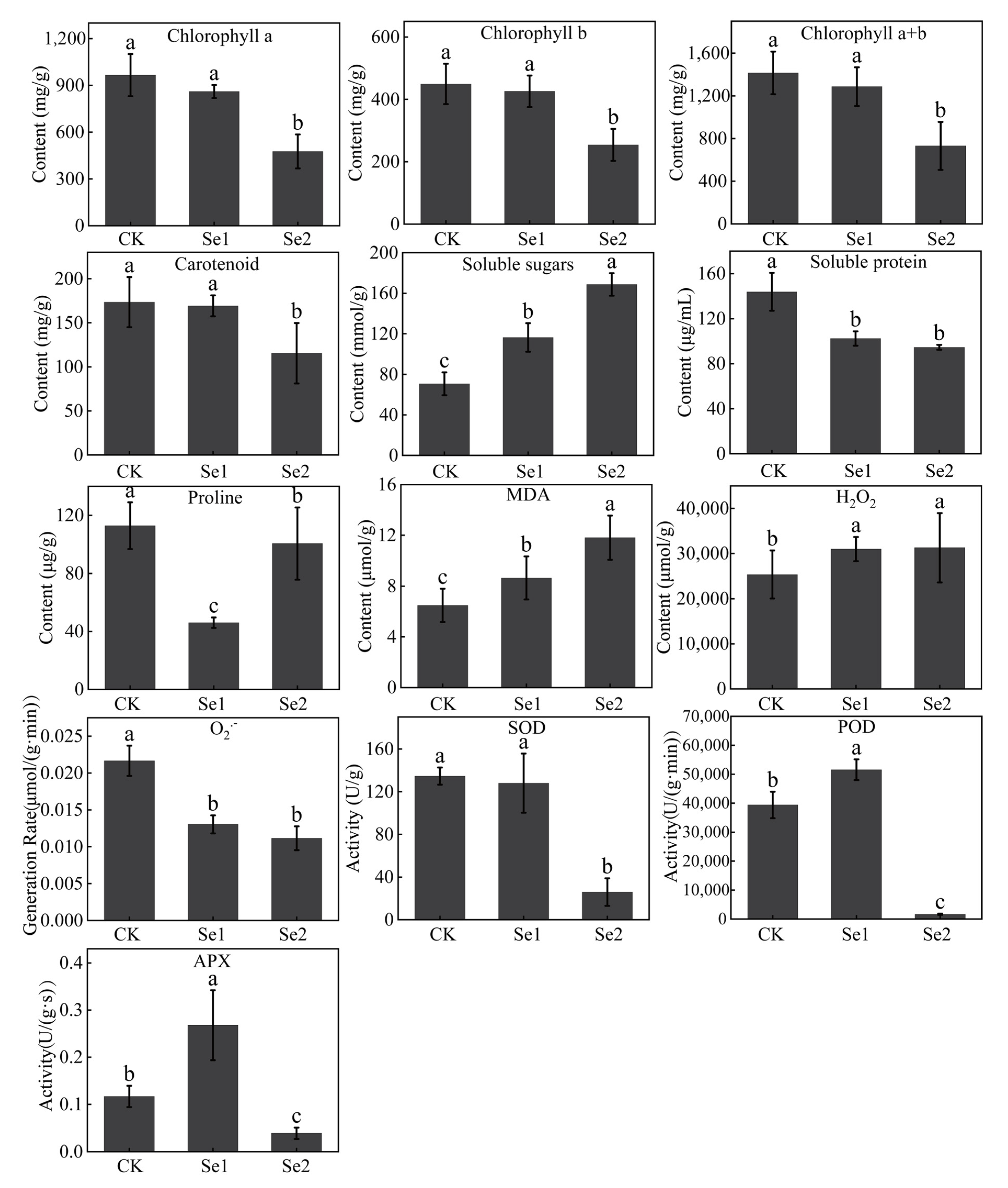
3.2. Physiological Effects of Highland Barley Seedlings Under Na2SeO3 Treatment
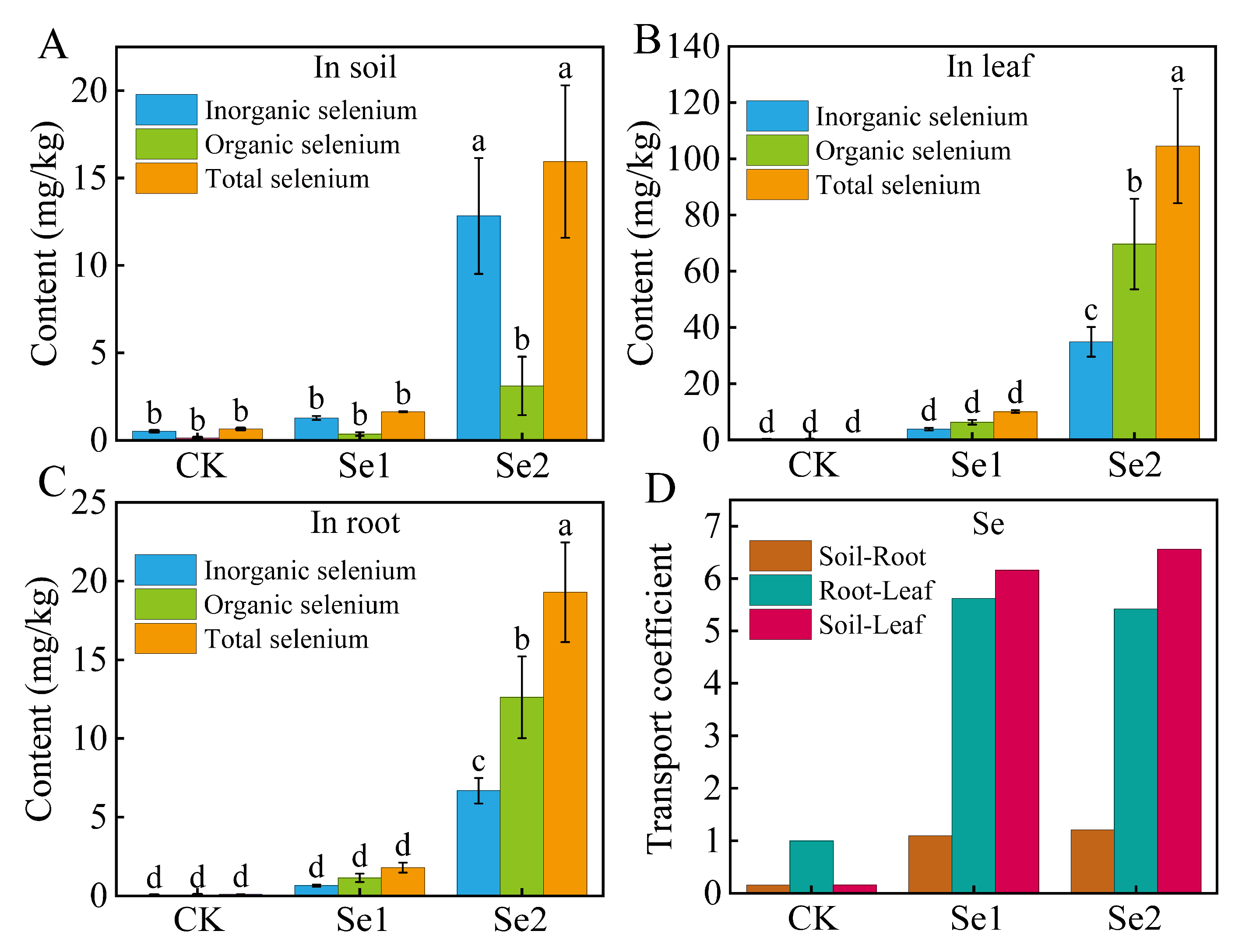
3.3. Selenium Content and Transport Coefficient of Soil and Highland Barley Seedlings Under Na2SeO3 Treatment
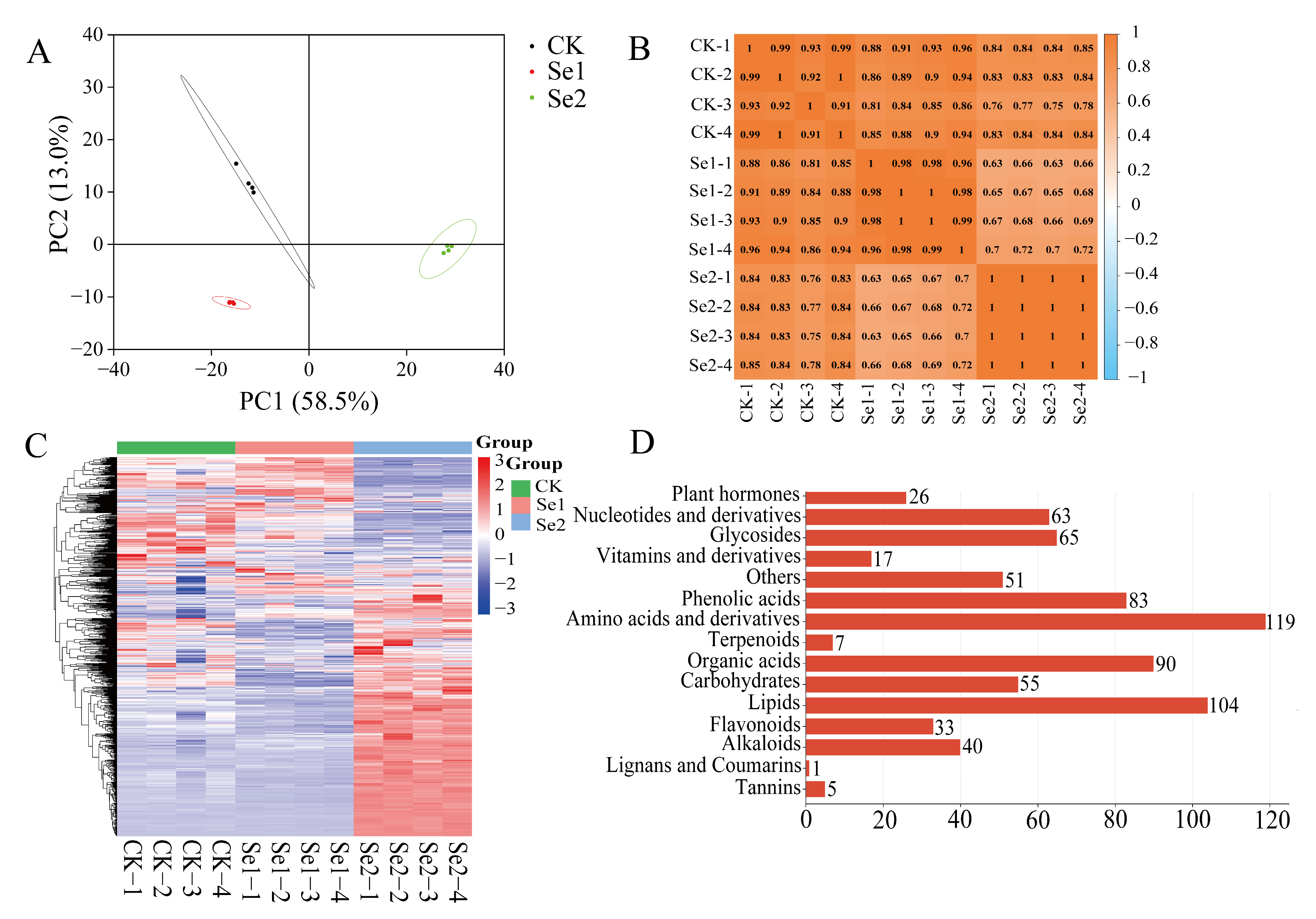
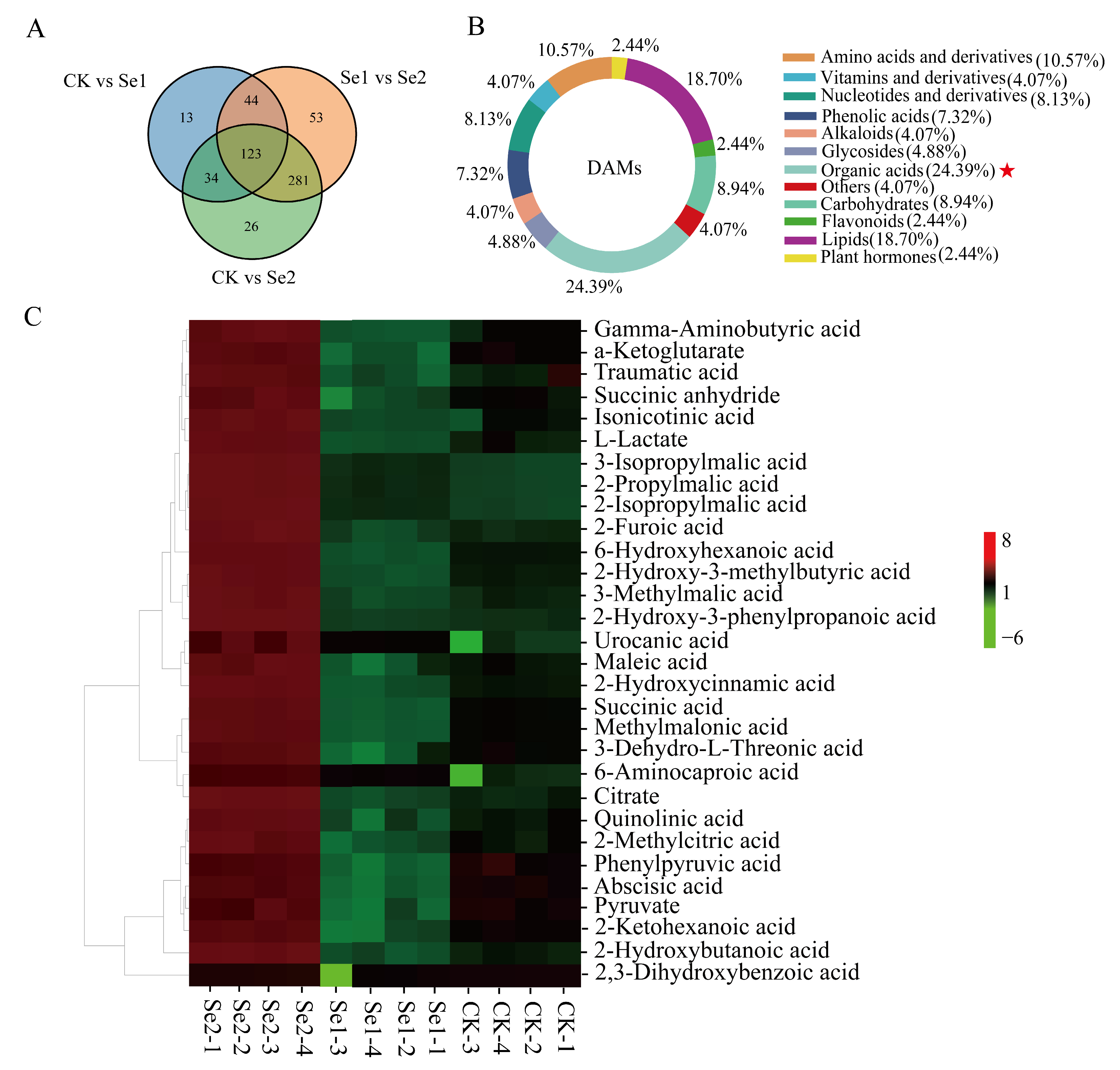
3.4. Metabonomic Analysis of Highland Barley Seedlings Under Na2SeO3 Treatment
3.5. Thirty DAMs of Organic Acids in Highland Barley Seedlings Under Na2SeO3 Treatment
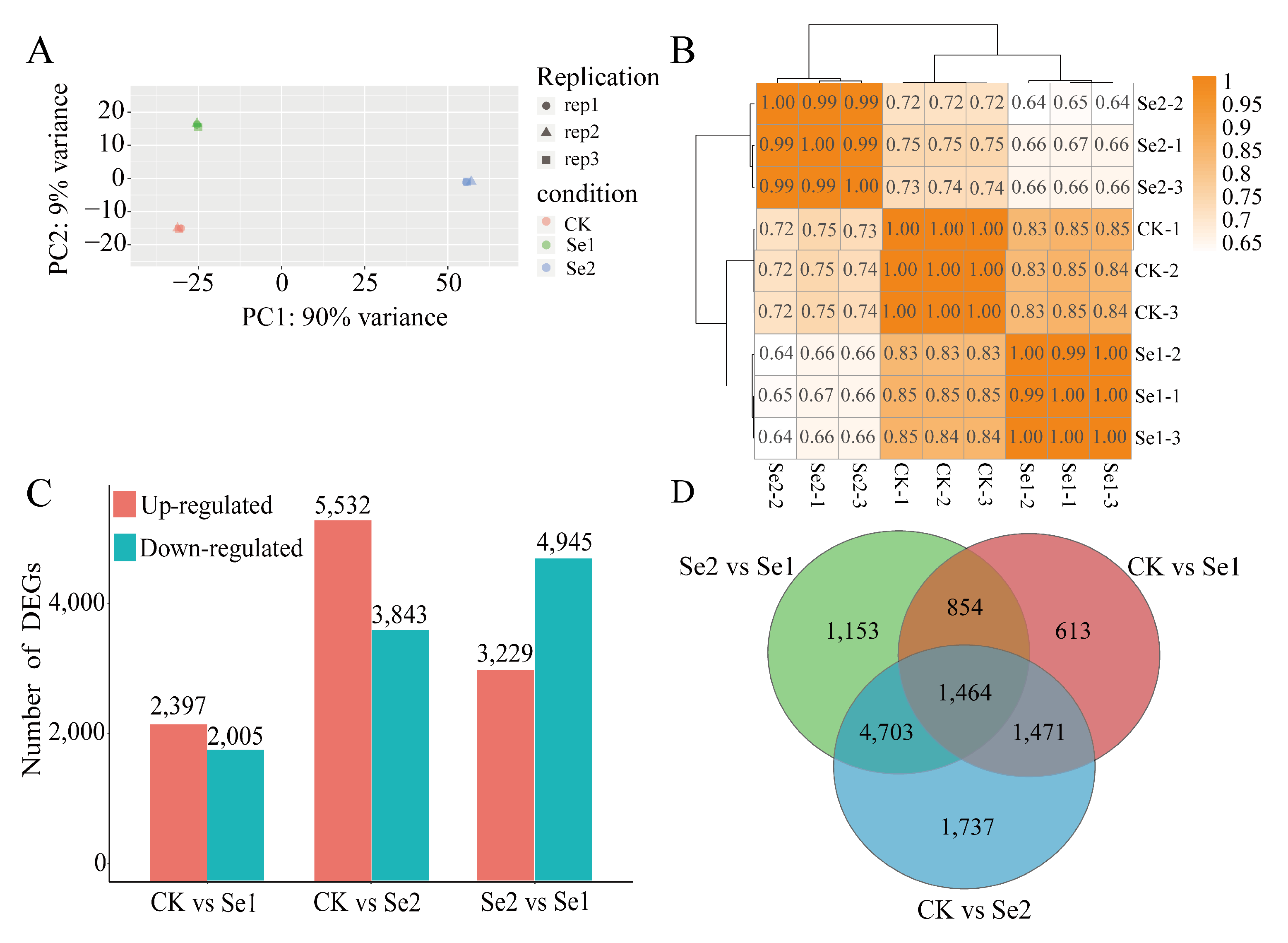
3.6. Transcriptome Analysis of Highland Barley Seedlings Under Na2SeO3 Treatment
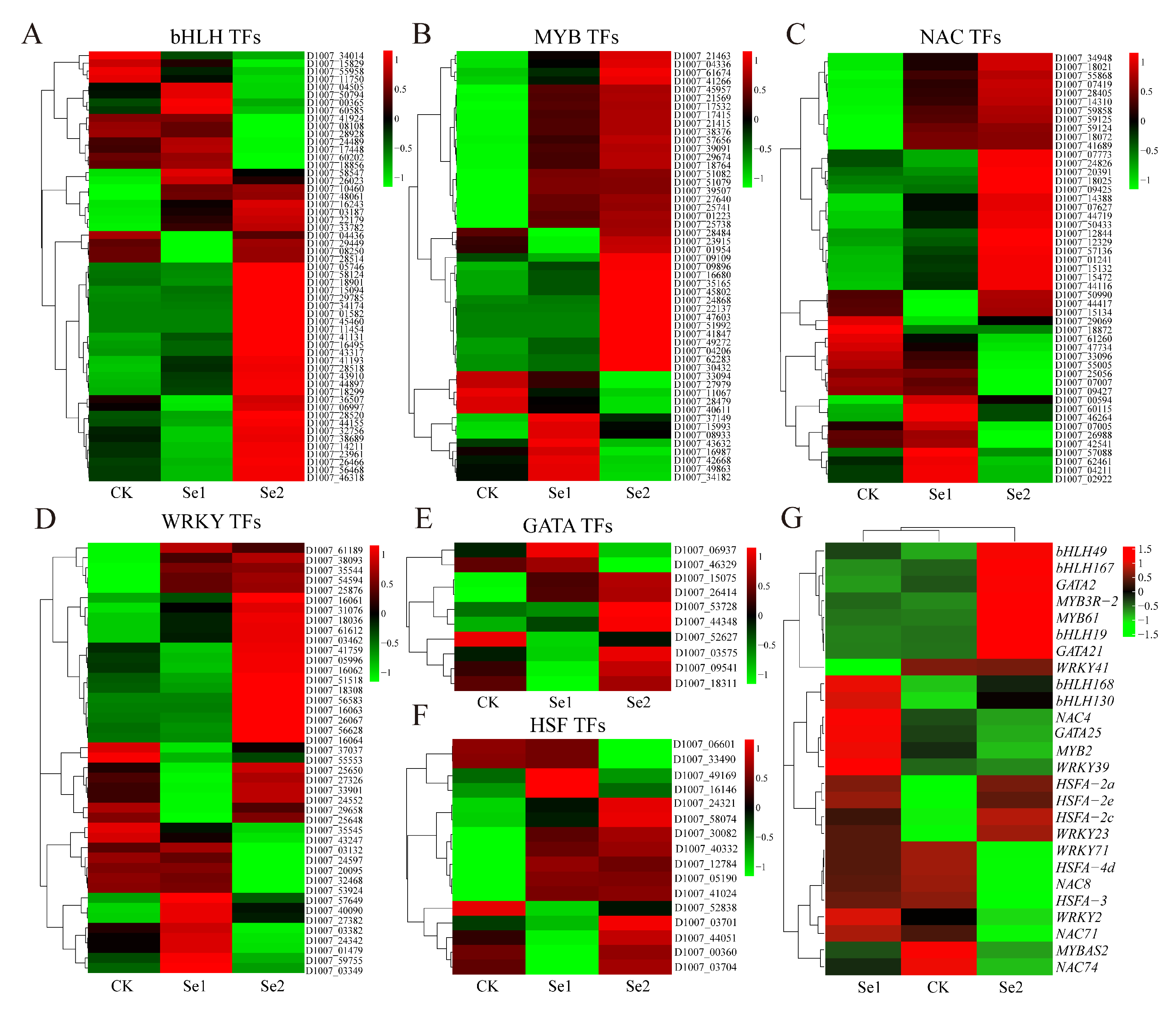
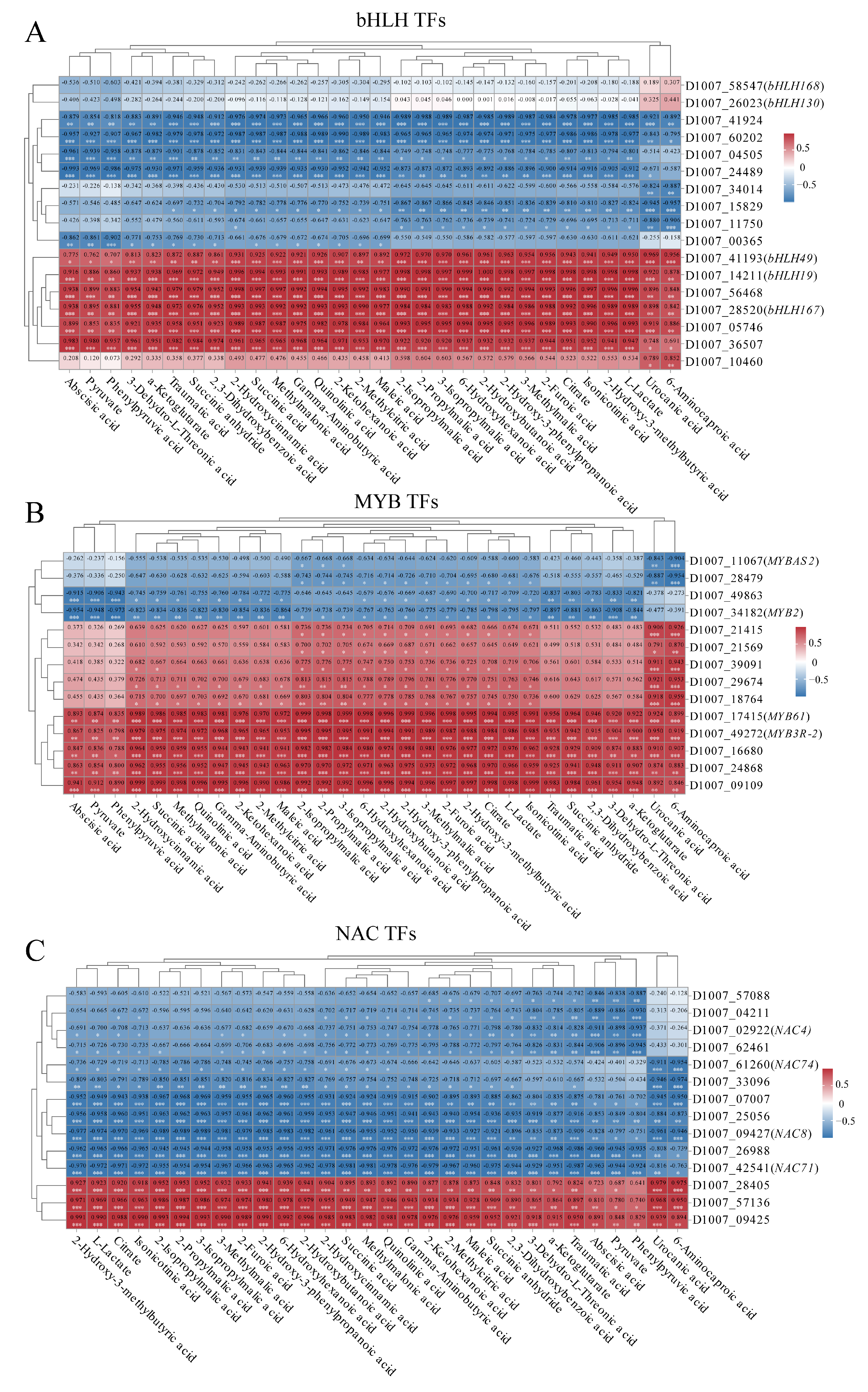
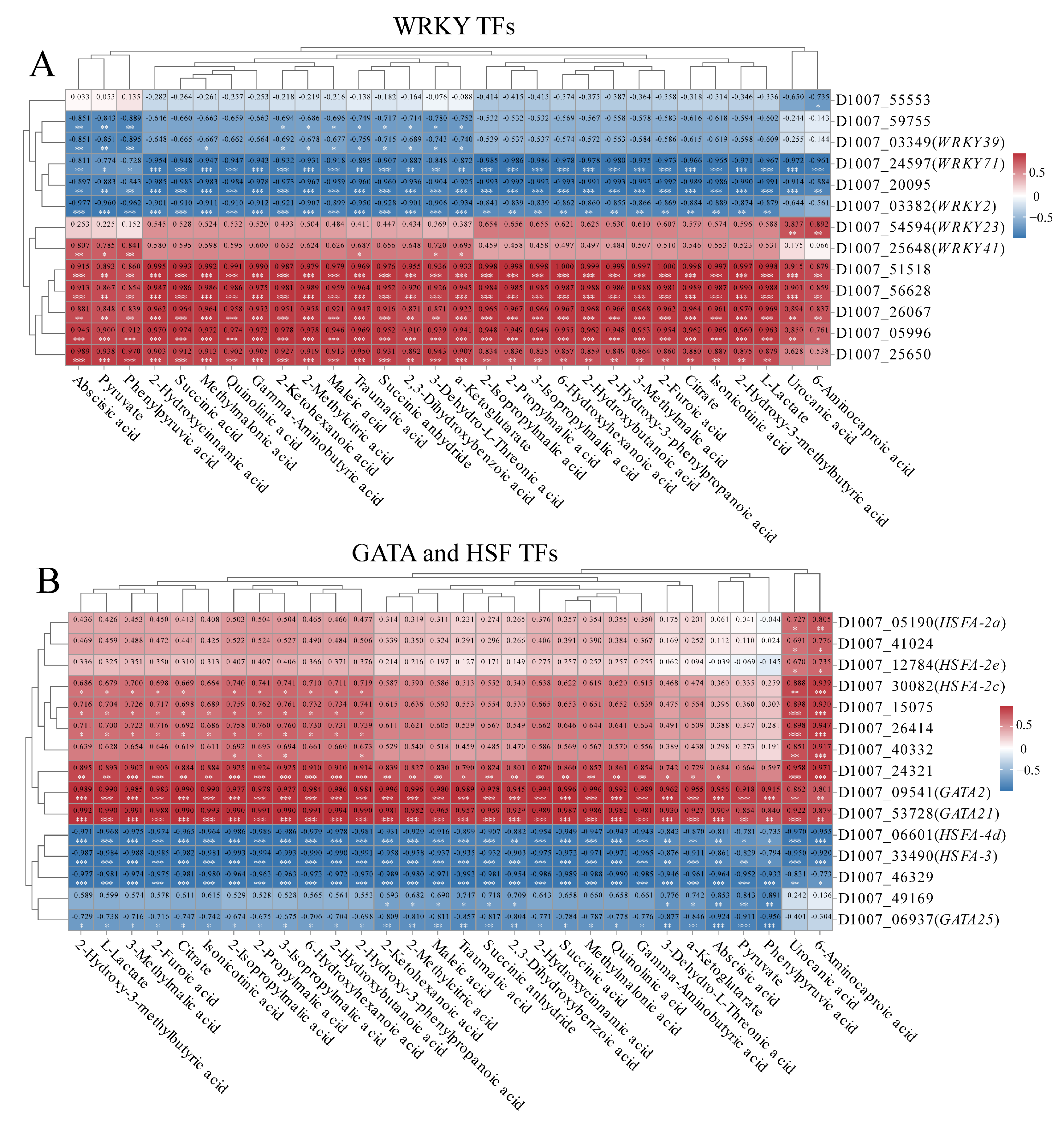
3.7. Differential Expression of Transcription Factors of Highland Barley Seedlings Under Na2SeO3 Treatment
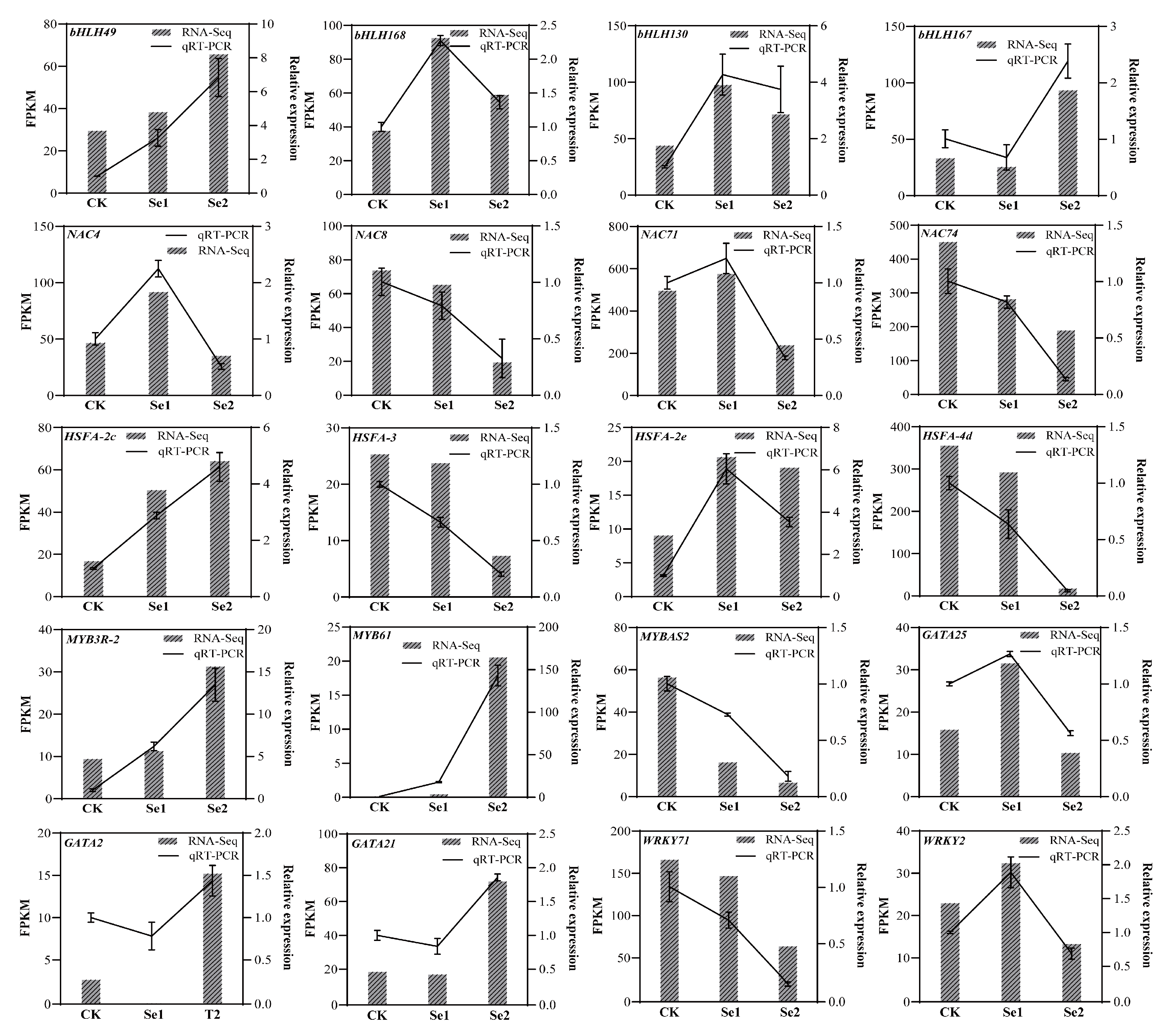
3.8. qRT—PCR Validation of Transcription Factors of Highland Barley Seedlings Under Na2SeO3 Treatment
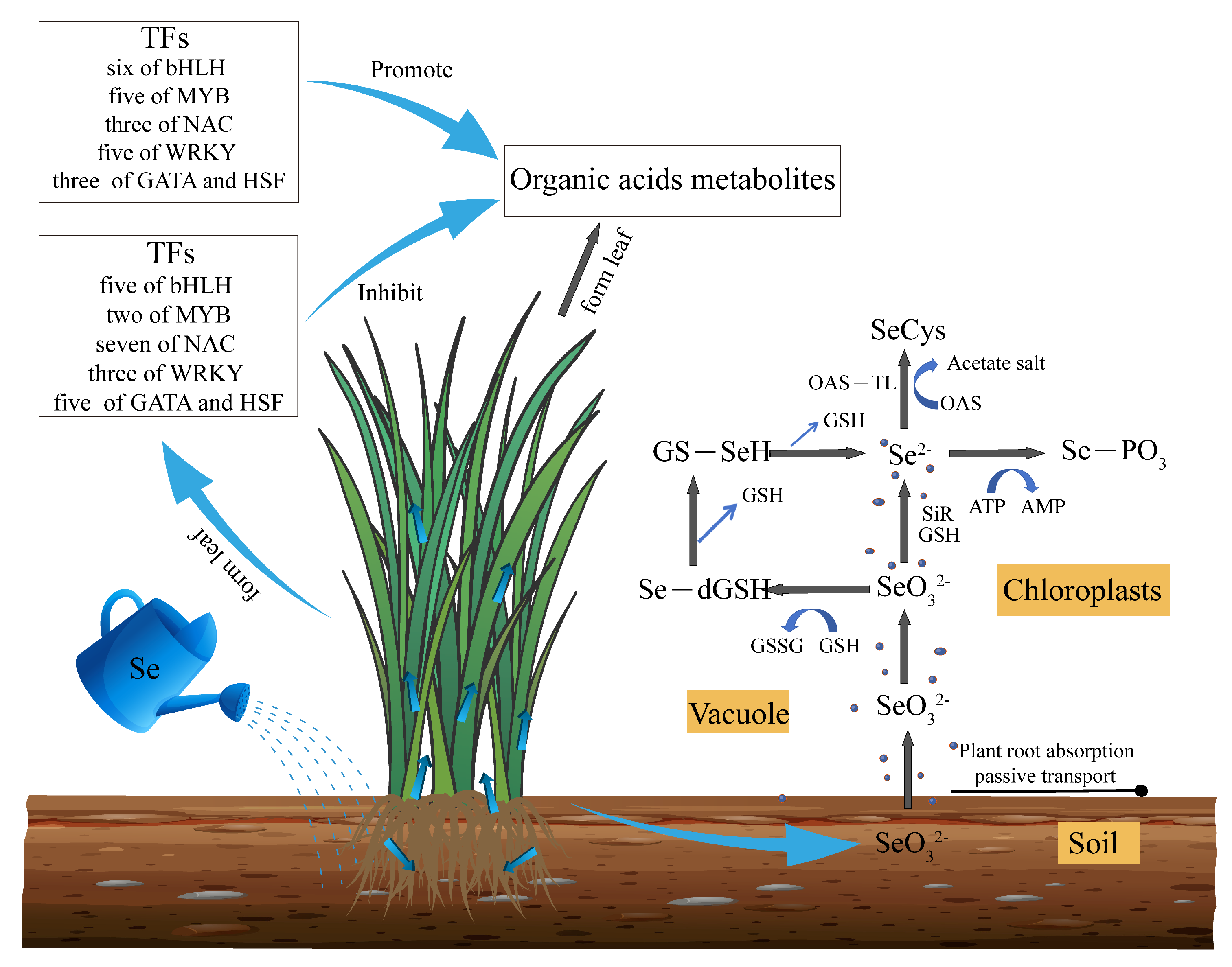
3.9. Correlation Analysis of Key Transcription Factors, Physiological Indexes and Metabolites Under Na2SeO3 Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDA | Malondialdehyde |
| O2.− | Superoxide anion radical |
| H2O2 | Hydrogen peroxide |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| APX | Ascorbate peroxidase |
| TFs | Transcription factors |
| bHLH | Basic helix-loop-helix |
| MYB | v-myb avian myeloblastosis viral oncogene homolog |
| NAC | NAM, ATAF1/2 and CUC2 |
| HSF | Heat stress transcription factor |
| GATA | GATA-binding protein |
| WRKY | WRKY DNA-binding protein |
| I(Se) | Inorganic selenium |
| O(Se) | Organic selenium |
| T(Se) | Total selenium |
References
- Kieliszek, M. Selenium-Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef]
- Zhou, B.; Cao, H.; Wu, Q.; Mao, K.; Yang, X.; Su, J.; Zhang, H. Agronomic and Genetic Strategies to Enhance Selenium Accumulation in Crops and Their Influence on Quality. Foods 2023, 12, 4442. [Google Scholar] [CrossRef] [PubMed]
- Mangiapane, E.; Pessione, A.; Pessione, E. Selenium and Selenoproteins: An Overview on Different Biological Systems. Curr. Protein Pept. Sci. 2014, 15, 598–607. [Google Scholar] [CrossRef]
- Winkel, L.H.E.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium Cycling across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Anjum, S.; Skalicky, M.; Waraich, E.A.; Muhammad Sabir Tariq, R.; Ayub, M.A.; Hossain, A.; Hassan, M.M.; Brestic, M.; Sohidul Islam, M.; et al. Selenium Alleviates the Adverse Effect of Drought in Oilseed Crops Camelina (Camelina sativa L.) and Canola (Brassica napus L.). Molecules 2021, 26, 1699. [Google Scholar] [CrossRef] [PubMed]
- Cappa, J.J.; Cappa, P.J.; El Mehdawi, A.F.; McAleer, J.M.; Simmons, M.P.; Pilon-Smits, E.A.H. Characterization of Selenium and Sulfur Accumulation across the Genus Stanleya (Brassicaceae): A Field Survey and Common-Garden Experiment. Am. J. Bot. 2014, 101, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Schneberg, K.A.; Pilon-Smits, E.A.H. Sulfur-Selenium-Molybdenum Interactions Distinguish Selenium Hyperaccumulator Stanleya Pinnata from Non-Hyperaccumulator Brassica Juncea (Brassicaceae). Planta 2014, 239, 479–491. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, W.; Pang, F. Selenium in Soil-Plant-Microbe: A Review. Bull. Environ. Contam. Toxicol. 2022, 108, 167–181. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Pan, Y.; Ma, L.; Fang, Y.; Pan, C.; Qiang, Y.; Cao, X.; Xu, H. Nano-Selenium Promotes the Product Quality and Plant Defense of Salvia Miltiorrhiza by Inducing Tanshinones and Salvianolic Acids Accumulation. Ind. Crops Prod. 2023, 195, 116436. [Google Scholar] [CrossRef]
- Xiang, J.; Rao, S.; Chen, Q.; Zhang, W.; Cheng, S.; Cong, X.; Zhang, Y.; Yang, X.; Xu, F. Research Progress on the Effects of Selenium on the Growth and Quality of Tea Plants. Plants 2022, 11, 2491. [Google Scholar] [CrossRef]
- Yi, Q.; Sun, X.; Tang, S.; Xu, P.; Pang, Y.; Huang, X.; Huang, Q.; Huang, J.; Zhang, M. Comparation of Se Accumulation and Distribution of Two Rice (Oryza sativa L.) Cultivars with High- and Low- Se Efficiency as Affected by Exogenous Application of Selenite. J. Cereal Sci. 2022, 105, 103475. [Google Scholar] [CrossRef]
- Zou, Y.; Han, C.; Wang, F.; Tan, Y.; Yang, S.; Huang, C.; Xie, S.; Xiao, X. Integrated Metabolome and Transcriptome Analysis Reveal Complex Molecular Mechanisms Underlying Selenium Response of Aloe vera L. J. Plant Biol. 2021, 64, 135–143. [Google Scholar] [CrossRef]
- Li, L.; Yu, J.; Li, L.; Rao, S.; Wu, S.; Wang, S.; Cheng, S.; Cheng, H. Treatment of Ginkgo biloba with Exogenous Sodium Selenite Affects Its Physiological Growth, Changes Its Phytohormones, and Synthesizes Its Terpene Lactones. Molecules 2022, 27, 7548. [Google Scholar] [CrossRef]
- Zhou, W.; Duan, Y.; Zhang, Y.; Wang, H.; Huang, D.; Zhang, M. Effects of Foliar Selenium Application on Growth and Rhizospheric Soil Micro-Ecological Environment of Atractylodes macrocephala Koidz. S. Afr. J. Bot. 2021, 137, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Xu, J.; Dai, Z.; Elyamine, A.M.; Huang, W.; Han, D.; Dang, B.; Xu, Z.; Jia, W. Selenium in Soil-Plant System: Transport, Detoxification and Bioremediation. J. Hazard. Mater. 2023, 452, 131272. [Google Scholar] [CrossRef]
- Tao, J.; Leng, J.; Lei, X.; Wan, C.; Li, D.; Wu, Y.; Yang, Q.; Wang, P.; Feng, B.; Gao, J. Effects of Selenium (Se) Uptake on Plant Growth and Yield in Common Buckwheat (Fagopyrum esculentum Moench). Field Crops Res. 2023, 302, 109070. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The Dual Effects of Two Inorganic Selenium Forms on the Growth, Selected Physiological Parameters and Macronutrients Accumulation in Cucumber Plants. Acta Physiol. Plant 2015, 37, 41. [Google Scholar] [CrossRef]
- Silva, A.R.; Fernandes, Â.; García, P.A.; Barros, L.; Ferreira, I.C.F.R. Cytinus hypocistis (L.) L. Subsp. macranthus Wettst.: Nutritional Characterization. Molecules 2019, 24, 1111. [Google Scholar] [CrossRef]
- Song, F.; Han, X.; Zhu, X.; Herbert, S.J. Response to Water Stress of Soil Enzymes and Root Exudates from Drought and Non-Drought Tolerant Corn Hybrids at Different Growth Stages. Can. J. Soil. Sci. 2012, 92, 501–507. [Google Scholar] [CrossRef]
- Jiao, L.; Cao, X.; Wang, C.; Chen, F.; Zou, H.; Yue, L.; Wang, Z. Crosstalk between in Situ Root Exudates and Rhizobacteria to Promote Rice Growth by Selenium Nanomaterials. Sci. Total Environ. 2023, 878, 163175. [Google Scholar] [CrossRef]
- Xu, C.; Gui, Z.; Huang, Y.; Yang, H.; Luo, J.; Zeng, X. Integrated Transcriptomics and Metabolomics Analyses Provide Insights into Qingke in Response to Cold Stress. J. Agric. Food Chem. 2023, 71, 18345–18358. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xi, M.; Liu, T.; Wu, X.; Ju, L.; Wang, D. The Central Role of Transcription Factors in Bridging Biotic and Abiotic Stress Responses for Plants’ Resilience. New Crops 2024, 1, 100005. [Google Scholar] [CrossRef]
- Wei, L.; Liu, H.; Ni, Y.; Xiong, R.; Li, S.; Sun, R.; Wei, Y.; Zhang, H.; Dong, J.; Chang, L.; et al. Integrative Analysis of Metabolome and Transcriptome Reveals New Insights into Major Quality Formation and Regulation in Two Strawberry Cultivars with Different Fruit Flavor. Veg. Res. 2024, 4, e012. [Google Scholar] [CrossRef]
- Li, S.-J.; Yin, X.-R.; Wang, W.-L.; Liu, X.-F.; Zhang, B.; Chen, K.-S. Citrus CitNAC62 Cooperates with CitWRKY1 to Participate in Citric Acid Degradation via Up-Regulation of CitAco3. J. Exp. Bot. 2017, 68, 3419–3426. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, X.; Gou, B.; Wang, D.; Liu, C.; Sun, J.; Yin, X.; Grierson, D.; Li, S.; Chen, K. The Interaction Between CitMYB52 and CitbHLH2 Negatively Regulates Citrate Accumulation by Activating CitALMT in Citrus Fruit. Front. Plant Sci. 2022, 13, 848869. [Google Scholar] [CrossRef]
- Li, S.; Yin, X.; Xie, X.; Allan, A.C.; Ge, H.; Shen, S.; Chen, K. The Citrus Transcription Factor, CitERF13, Regulates Citric Acid Accumulation via a Protein-Protein Interaction with the Vacuolar Proton Pump, CitVHA-C4. Sci. Rep. 2016, 6, 20151. [Google Scholar] [CrossRef]
- Hu, D.-G.; Sun, C.-H.; Ma, Q.-J.; You, C.-X.; Cheng, L.; Hao, Y.-J. MdMYB1 Regulates Anthocyanin and Malate Accumulation by Directly Facilitating Their Transport into Vacuoles in Apples. Plant Physiol. 2016, 170, 1315–1330. [Google Scholar] [CrossRef]
- Hu, D.-G.; Li, Y.-Y.; Zhang, Q.-Y.; Li, M.; Sun, C.-H.; Yu, J.-Q.; Hao, Y.-J. The R2R3-MYB Transcription Factor MdMYB73 Is Involved in Malate Accumulation and Vacuolar Acidification in Apple. Plant J. 2017, 91, 443–454. [Google Scholar] [CrossRef]
- Jia, D.; Wu, P.; Shen, F.; Li, W.; Zheng, X.; Wang, Y.; Yuan, Y.; Zhang, X.; Han, Z. Genetic Variation in the Promoter of an R2R3-MYB Transcription Factor Determines Fruit Malate Content in Apple (Malus domestica Borkh.). Plant Physiol. 2021, 186, 549–568. [Google Scholar] [CrossRef]
- Peng, Y.; Yuan, Y.; Chang, W.; Zheng, L.; Ma, W.; Ren, H.; Liu, P.; Zhu, L.; Su, J.; Ma, F.; et al. Transcriptional Repression of MdMa1 by MdMYB21 in Ma Locus Decreases Malic Acid Content in Apple Fruit. Plant J. 2023, 115, 1231–1242. [Google Scholar] [CrossRef]
- Pankin, A.; von Korff, M. Co-Evolution of Methods and Thoughts in Cereal Domestication Studies: A Tale of Barley (Hordeum Vulgare). Curr. Opin. Plant Biol. 2017, 36, 15–21. [Google Scholar] [CrossRef]
- Zeng, X.; Guo, Y.; Xu, Q.; Mascher, M.; Guo, G.; Li, S.; Mao, L.; Liu, Q.; Xia, Z.; Zhou, J.; et al. Origin and Evolution of Qingke Barley in Tibet. Nat. Commun. 2018, 9, 5433. [Google Scholar] [CrossRef]
- Liang, J.; Chen, X.; Deng, G.; Pan, Z.; Zhang, H.; Li, Q.; Yang, K.; Long, H.; Yu, M. Dehydration Induced Transcriptomic Responses in Two Tibetan Hulless Barley (Hordeum Vulgare Var. Nudum) Accessions Distinguished by Drought Tolerance. BMC Genom. 2017, 18, 775. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Hong, Y.; Gu, Z.; Cheng, L.; Li, Z.; Li, C.; Ban, X. Highland Barley Starch: Structures, Properties, and Applications. Foods 2023, 12, 387. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A Critical Review on Production and Industrial Applications of Beta-Glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press (US): Washington, DC, USA, 2000; ISBN 978-0-309-06949-6. [Google Scholar]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium Speciation from Food Source to Metabolites: A Critical Review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef]
- Cheng, C.; Zhao, X.; Yang, H.; Coldea, T.E.; Zhao, H. Mechanism of Selenite Tolerance during Barley Germination: A Combination of Tissue Selenium Metabolism Alterations and Ascorbate-Glutathione Cycle Modulation. Plant Physiol. Biochem. 2023, 205, 108189. [Google Scholar] [CrossRef] [PubMed]
- Nagdalian, A.A.; Blinov, A.V.; Siddiqui, S.A.; Gvozdenko, A.A.; Golik, A.B.; Maglakelidze, D.G.; Rzhepakovsky, I.V.; Kukharuk, M.Y.; Piskov, S.I.; Rebezov, M.B.; et al. Effect of Selenium Nanoparticles on Biological and Morphofunctional Parameters of Barley Seeds (Hordéum vulgáre L.). Sci. Rep. 2023, 13, 6453. [Google Scholar] [CrossRef]
- Yao, X.; Yao, Y.; An, L.; Li, X.; Bai, Y.; Cui, Y.; Wu, K. Accumulation and Regulation of Anthocyanins in White and Purple Tibetan Hulless Barley (Hordeum vulgare L. var. Nudum Hook. F.) Revealed by Combined de Novo Transcriptomics and Metabolomics. BMC Plant Biol. 2022, 22, 391. [Google Scholar] [CrossRef]
- Yu, M.; Wang, M.; Gyalpo, T.; Basang, Y. Stem Lodging Resistance in Hulless Barley: Transcriptome and Metabolome Analysis of Lignin Biosynthesis Pathways in Contrasting Genotypes. Genomics 2021, 113, 935–943. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Zhou, J.; Shang, R.; Wang, Y.; Jing, P. Comparative Study on Several Determination Methods of Chlorophyll Content in Plants. IOP Conf. Ser. Mater. Sci. Eng. 2020, 730, 012066. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Shi, J.; Pan, Y.; Yang, S.; Xue, Y. Combined Metabolome and Transcriptome Analysis Reveals the Key Pathways Involved in the Responses of Soybean Plants to High Se Stress. Ecotoxicol. Environ. Saf. 2024, 287, 117262. [Google Scholar] [CrossRef]
- He, Y.; Zhang, M.; Zhou, W.; Ai, L.; You, J.; Liu, H.; You, J.; Wang, H.; Wassie, M.; Wang, M.; et al. Transcriptome Analysis Reveals Novel Insights into the Continuous Cropping Induced Response in Codonopsis Tangshen, a Medicinal Herb. Plant Physiol. Biochem. 2019, 141, 279–290. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tadayon, M.R.; Nadeem, M.; Cheema, M.; Razmjoo, J. Proline-Mediated Changes in Antioxidant Enzymatic Activities and the Physiology of Sugar Beet under Drought Stress. Acta Physiol. Plant 2019, 41, 23. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid Peroxidation as Measured by Chromatographic Determination of Malondialdehyde. Human Plasma Reference Values in Health and Disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Li, X.; Shen, T.-L.; Qian, W.-J.; Xian, M. A Sweet H2S/H2O2 Dual Release System and Specific Protein S-Persulfidation Mediated by Thioglucose/Glucose Oxidase. J. Am. Chem. Soc. 2021, 143, 13325–13332. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, P.; Zhang, T.; Li, Y.; Wang, Y.; Gao, C. Comprehensive Analysis of BpHSP Genes and Their Expression under Heat Stresses in Betula Platyphylla. Environ. Exp. Bot. 2018, 152, 167–176. [Google Scholar] [CrossRef]
- Hadwan, M.H.; Hussein, M.J.; Mohammed, R.M.; Hadwan, A.M.; Saad Al-Kawaz, H.; Al-Obaidy, S.S.M.; Al Talebi, Z.A. An Improved Method for Measuring Catalase Activity in Biological Samples. Biol. Methods Protoc. 2024, 9, bpae015. [Google Scholar] [CrossRef]
- Sadak, M.S.; Abd El-Hameid, A.R.; Zaki, F.S.A.; Dawood, M.G.; El-Awadi, M.E. Physiological and Biochemical Responses of Soybean (Glycine Max L.) to Cysteine Application under Sea Salt Stress. Bull. Natl. Res. Cent. 2019, 44, 1. [Google Scholar] [CrossRef]
- Hosseini, F.; Mosaddeghi, M.R.; Dexter, A.R.; Sepehri, M. Maize Water Status and Physiological Traits as Affected by Root Endophytic Fungus Piriformospora Indica under Combined Drought and Mechanical Stresses. Planta 2018, 247, 1229–1245. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q. Novel Mechanistic Insights of Selenium Induced Microscopic, Histochemical and Physio-Biochemical Changes in Tomato (Solanum lycopersicum L.) Plant. An Account of Beneficiality or Toxicity. J. Hazard. Mater. 2022, 434, 128830. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liao, X.; Yu, L.; Rao, S.; Chen, Q.; Zhu, Z.; Cong, X.; Zhang, W.; Ye, J.; Cheng, S.; et al. Combined Metabolome and Transcriptome Analysis Reveal the Mechanism of Selenate Influence on the Growth and Quality of Cabbage (Brassica oleracea var. capitata L.). Food Res. Int. 2022, 156, 111135. [Google Scholar] [CrossRef]
- Sun, B.; Liu, G.-L.; Phan, T.T.; Yang, L.-T.; Li, Y.-R.; Xing, Y.-X. Effects of Cold Stress on Root Growth and Physiological Metabolisms in Seedlings of Different Sugarcane Varieties. Sugar Tech. 2017, 19, 165–175. [Google Scholar] [CrossRef]
- Banerjee, M.; Kalwani, P.; Chakravarty, D.; Singh, B.; Ballal, A. Functional and Mechanistic Insights into the Differential Effect of the Toxicant “Se(IV)” in the Cyanobacterium Anabaena PCC 7120. Aquat. Toxicol. 2021, 236, 105839. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Gao, M.; Shi, R.; Song, S.; Zhang, Y.; Su, W.; Liu, H. The Combination of Selenium and LED Light Quality Affects Growth and Nutritional Properties of Broccoli Sprouts. Molecules 2020, 25, 4788. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Biswas, D.K.; Li, W.-D.; Chen, S.-B.; Zhang, L.; Jiang, G.-M.; Li, Y.-G. Photosynthesis and Yield Responses of Ozone-Polluted Winter Wheat to Drought. Photosynthetica 2007, 45, 582–588. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments: A Review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Devi, D.D.; Shanker, A.K.; Sheeba, J.A.; Bangarusamy, U. Selenium – an Antioxidative Protectant in Soybean during Senescence. Plant Soil. 2005, 272, 77–86. [Google Scholar] [CrossRef]
- Pandey, P.; Srivastava, R.K.; Rajpoot, R.; Rani, A.; Pandey, A.K.; Dubey, R.S. Water Deficit and Aluminum Interactive Effects on Generation of Reactive Oxygen Species and Responses of Antioxidative Enzymes in the Seedlings of Two Rice Cultivars Differing in Stress Tolerance. Environ. Sci. Pollut. Res. Int. 2016, 23, 1516–1528. [Google Scholar] [CrossRef]
- Chu, J.; Yao, X.; Zhang, Z. Responses of Wheat Seedlings to Exogenous Selenium Supply under Cold Stress. Biol. Trace Elem. Res. 2010, 136, 355–363. [Google Scholar] [CrossRef]
- Huang, C.; Qin, N.; Sun, L.; Yu, M.; Hu, W.; Qi, Z. Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Strawberry Seedlings under Low-Temperature Stress. Int. J. Mol. Sci. 2018, 19, 1913. [Google Scholar] [CrossRef]
- Liu, K.; Li, S.; Han, J.; Zeng, X.; Ling, M.; Mao, J.; Li, Y.; Jiang, J. Effect of Selenium on Tea (Camellia sinensis) under Low Temperature: Changes in Physiological and Biochemical Responses and Quality. Environ. Exp. Bot. 2021, 188, 104475. [Google Scholar] [CrossRef]
- Hartikainen, H.; Xue, T.; Piironen, V. Selenium as an Anti-Oxidant and pro-Oxidant in Ryegrass. Plant Soil 2000, 225, 193–200. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, J.; Zhang, K.; Wen, Q.; Ming, K.; Xiong, H.; Ning, F. Peanut Selenium Distribution, Concentration, Speciation, and Effects on Proteins after Exogenous Selenium Biofortification. Food Chem. 2021, 354, 129515. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Feng, T.; Wang, B.; He, R.; Xu, Y.; Gao, P.; Zhang, Z.-H.; Zhang, L.; Fu, J.; Liu, Z.; et al. Enhancing Organic Selenium Content and Antioxidant Activities of Soy Sauce Using Nano-Selenium during Soybean Soaking. Front. Nutr. 2022, 9, 970206. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhou, W.; Li, D.; Wang, H.; Yang, Y.; You, J.; Liu, H.; Ai, L.; Zhang, M. Combined Transcriptome and Metabolome Analyses Reveal the Effects of Selenium on the Growth and Quality of Lilium Lancifolium. Front. Plant Sci. 2024, 15, 1399152. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Wang, C.-K.; Zhao, Y.-W.; Sun, C.-H.; Hu, D.-G. Mechanisms and Regulation of Organic Acid Accumulation in Plant Vacuoles. Hortic. Res. 2021, 8, 227. [Google Scholar] [CrossRef]
- Tang, M.; Bie, Z.; Wu, M.; Yi, H.; Feng, J. Changes in Organic Acids and Acid Metabolism Enzymes in Melon Fruit during Development. Sci. Hortic. 2010, 123, 360–365. [Google Scholar] [CrossRef]
- D’Amato, R.; Fontanella, M.C.; Falcinelli, B.; Beone, G.M.; Bravi, E.; Marconi, O.; Benincasa, P.; Businelli, D. Selenium Biofortification in Rice (Oryza sativa L.) Sprouting: Effects on Se Yield and Nutritional Traits with Focus on Phenolic Acid Profile. J. Agric. Food Chem. 2018, 66, 4082–4090. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, G.; Qin, H.; Guan, Y.; Wang, T.; Ni, W.; Xie, H.; Xing, Y.; Tian, G.; Lyu, M.; et al. Metabolomics Combined with Physiology and Transcriptomics Reveal Key Metabolic Pathway Responses in Apple Plants Exposure to Different Selenium Concentrations. J. Hazard. Mater. 2024, 464, 132953. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liao, Y.; Deng, S.; Li, J.; Bu, X.; Zhu, C.; Zhang, W.; Cong, X.; Cheng, S.; Chen, Q.; et al. Genome-Wide Analysis of NAC Transcription Factors and Exploration of Candidate Genes Regulating Selenium Metabolism in Broussonetia Papyrifera. Planta 2024, 260, 1. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Xie, H.; Ma, J.; Geng, G.; Yang, X.; Qiao, F. Organic Acids Metabolic Response and Transcription Factor Expression Changes of Highland Barley Seedlings Under Na2SeO3 Treatment. Agriculture 2025, 15, 1364. https://doi.org/10.3390/agriculture15131364
Wu X, Xie H, Ma J, Geng G, Yang X, Qiao F. Organic Acids Metabolic Response and Transcription Factor Expression Changes of Highland Barley Seedlings Under Na2SeO3 Treatment. Agriculture. 2025; 15(13):1364. https://doi.org/10.3390/agriculture15131364
Chicago/Turabian StyleWu, Xiaozhuo, Huichun Xie, Jianxia Ma, Guigong Geng, Xiaoli Yang, and Feng Qiao. 2025. "Organic Acids Metabolic Response and Transcription Factor Expression Changes of Highland Barley Seedlings Under Na2SeO3 Treatment" Agriculture 15, no. 13: 1364. https://doi.org/10.3390/agriculture15131364
APA StyleWu, X., Xie, H., Ma, J., Geng, G., Yang, X., & Qiao, F. (2025). Organic Acids Metabolic Response and Transcription Factor Expression Changes of Highland Barley Seedlings Under Na2SeO3 Treatment. Agriculture, 15(13), 1364. https://doi.org/10.3390/agriculture15131364




