Monitoring Moringa oleifera Lam. in the Mediterranean Area Using Unmanned Aerial Vehicles (UAVs) and Leaf Powder Production for Food Fortification
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
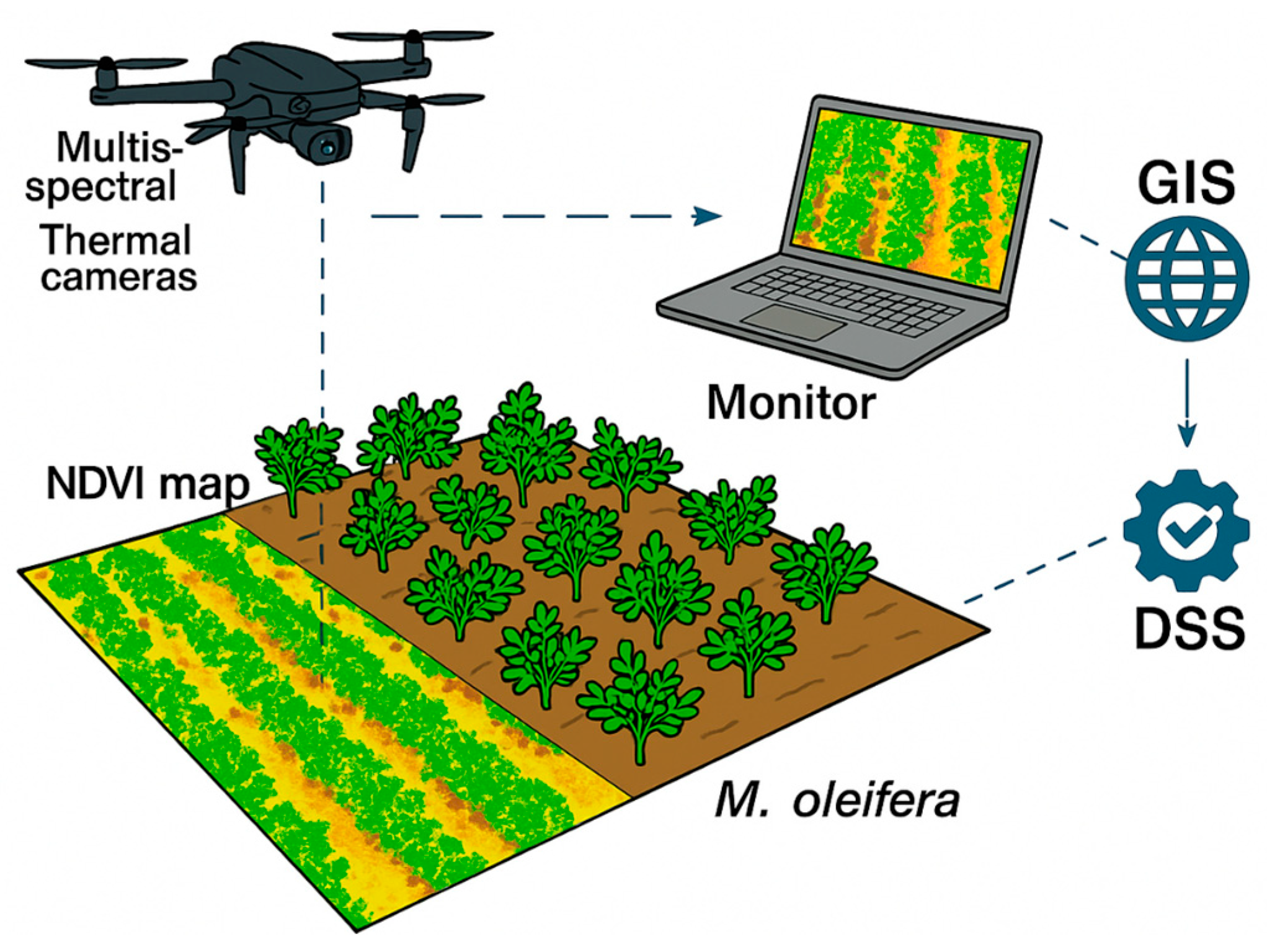
2.2. Moringa oleifera Growth Monitoring Through Precision Agriculture Technologies
2.3. Thermal and Multispectral Images Acquisition and Processing
2.4. Moringa oleifera Leaves Collection, Drying and Powder Production
2.5. Microbiological Analysis of Moringa oleifera Leaf Powders
2.6. Statistical Analysis
3. Results and Discussion
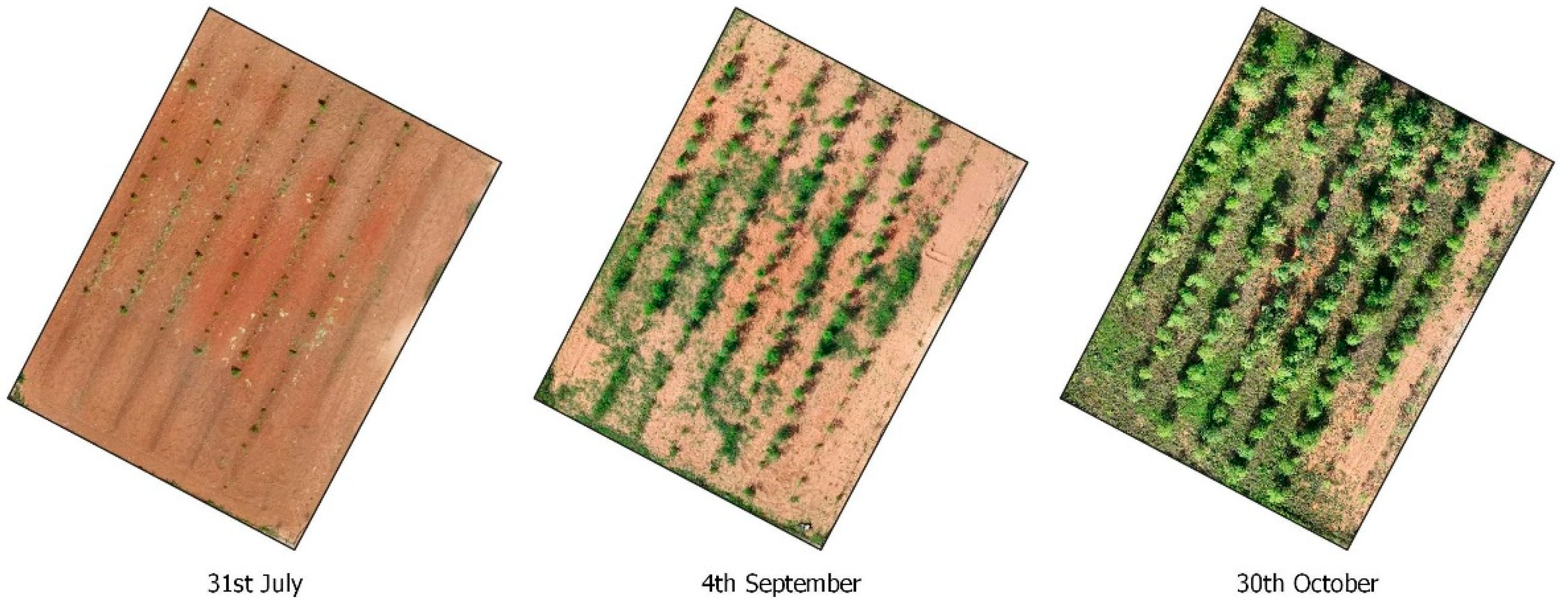
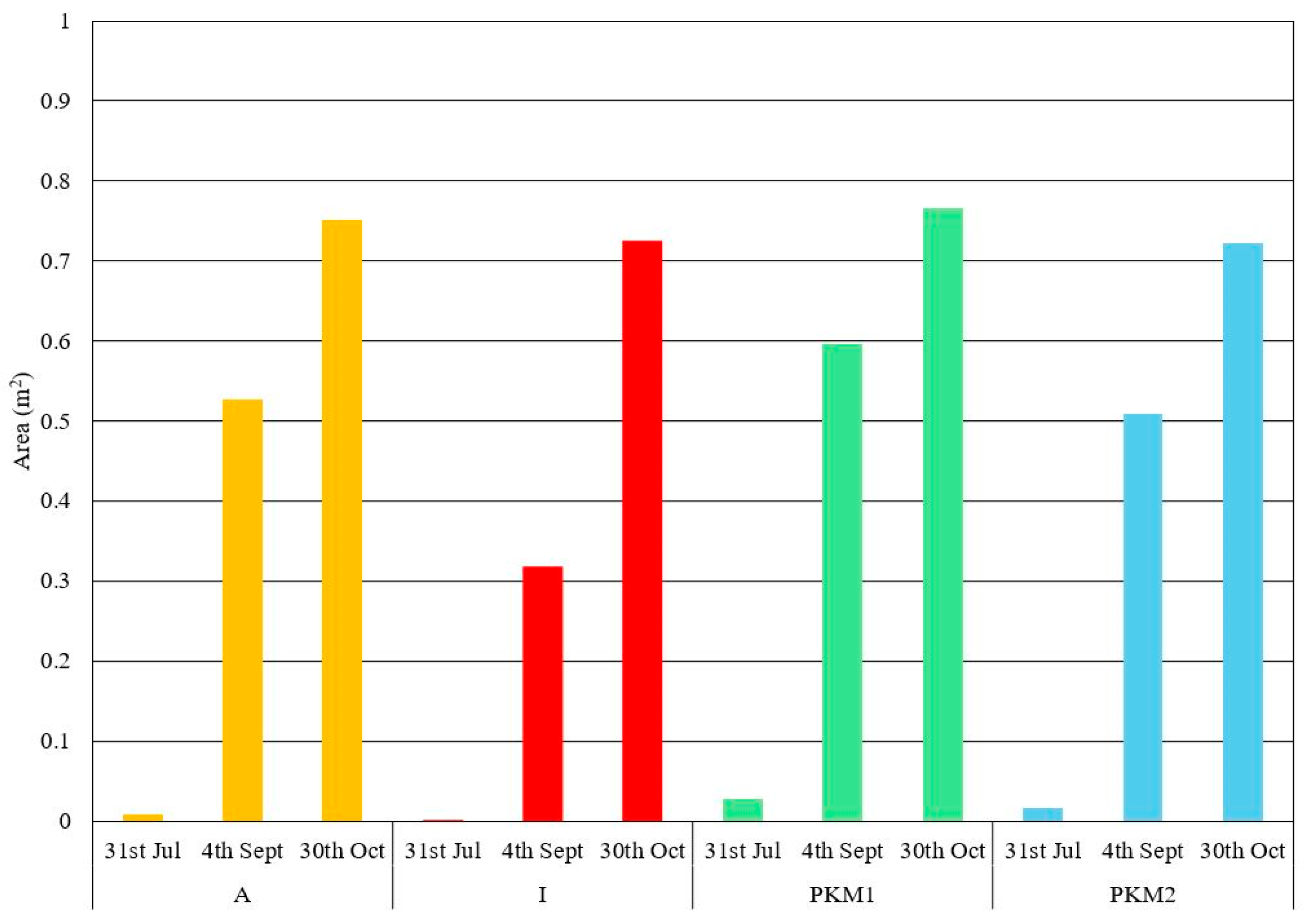
3.1. NDVI-Based Determination of Optimal Harvest Time for Moringa oleifera
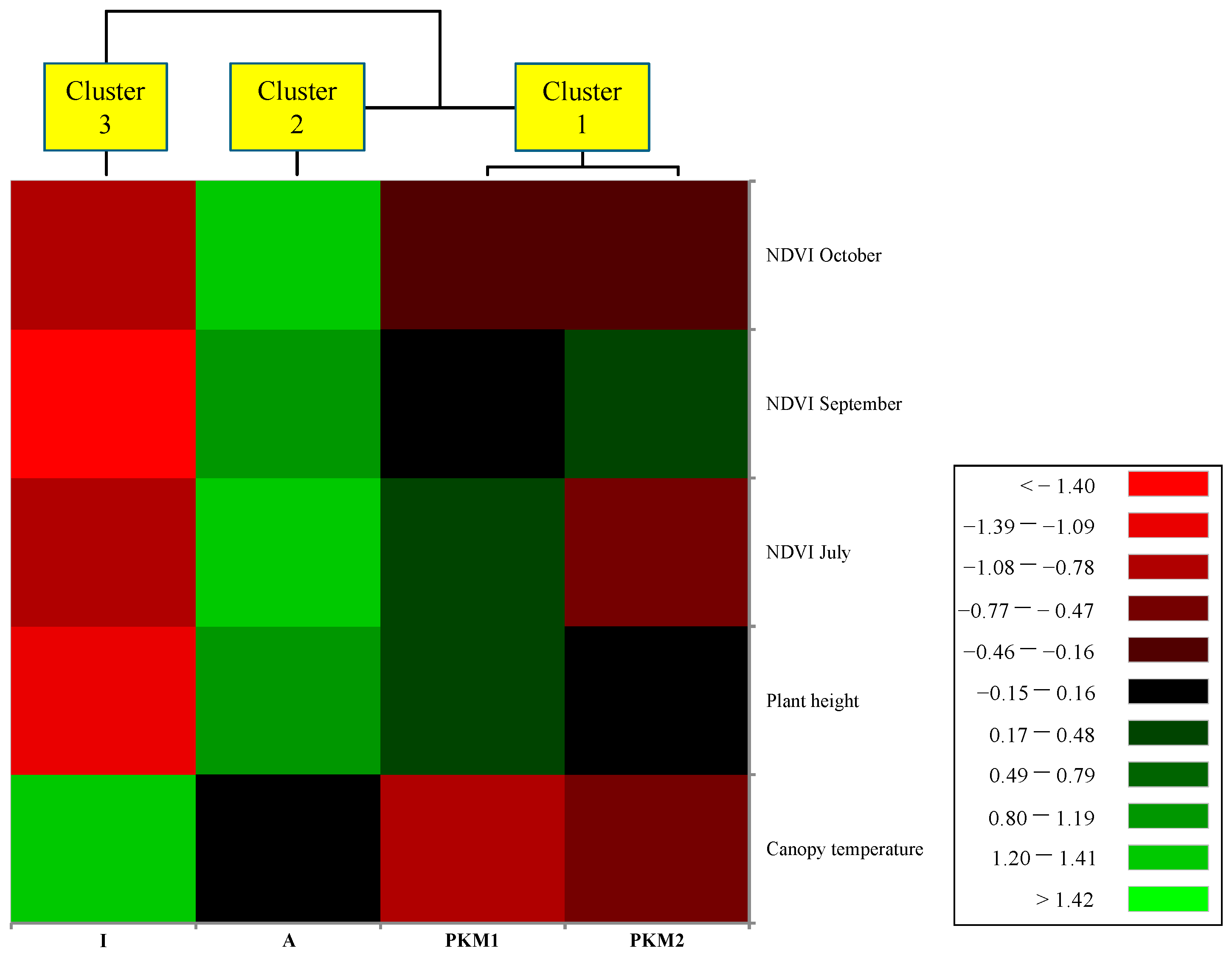
3.2. NDVI, Thermal and H Canopy Images
3.3. Hygiene and Safety Assessment of Moringa oleifera Leaf Powders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taha, M.F.; Mao, H.; Zhang, Z.; Elmasry, G.; Awad, M.A.; Abdalla, A.; Mousa, S.; Elwakeel, A.E.; Elsherbiny, O. Emerging Technologies for Precision Crop Management Towards Agriculture 5.0: A Comprehensive Overview. Agriculture 2025, 15, 582. [Google Scholar] [CrossRef]
- Fountas, S.; Malounas, I.; Athanasakos, L.; Avgoustakis, I.; Espejo-Garcia, B. AI-assisted vision for agricultural robots. AgriEngineering 2022, 4, 674–694. [Google Scholar] [CrossRef]
- Fountas, S.; Espejo-García, B.; Kasimati, A.; Gemtou, M.; Panoutsopoulos, H.; Anastasiou, E. Agriculture 5.0: Cutting-Edge Technologies, Trends, and Challenges. IT Prof. 2024, 26, 40–47. [Google Scholar] [CrossRef]
- Karagiannis, P.; Kotsaris, P.; Xanthakis, V.; Vasilaros, P.; Michalos, G.; Makris, S.; van Evert, F.K.; Nieuwenhuizen, A.T.; Fountas, S.; Chryssolouris, G. On an intelligent system to plan agricultural operations. Smart Agric. Technol. 2025, 10, 100707. [Google Scholar] [CrossRef]
- Mourtzis, D.; Angelopoulos, J.; Panopoulos, N. A Literature Review of the Challenges and Opportunities of the Transition from Industry 4.0 to Society 5.0. Energies 2022, 15, 6276. [Google Scholar] [CrossRef]
- Behmann, J.; Mahlein, A.-K.; Rumpf, T.; Römer, C.; Plümer, L. A Review of Advanced Machine Learning Methods for the Detection of Biotic Stress in Precision Crop Protection. Precis. Agric. 2015, 16, 239–260. [Google Scholar] [CrossRef]
- Shafi, U.; Mumtaz, R.; García-Nieto, J.; Hassan, S.A.; Zaidi, S.A.R.; Iqbal, N. Precision Agriculture Techniques and Practices: From Considerations to Applications. Sensors 2019, 19, 3796. [Google Scholar] [CrossRef]
- Gemtou, M.; Isakhanyan, G.; Fountas, S. Advancing climate-smart agriculture: integrating technology, behavioural insights and policy for a sustainable future. Smart Agric. Technol. 2025, 11, 100861. [Google Scholar] [CrossRef]
- Polymeni, S.; Skoutas, D.N.; Sarigiannidis, P.; Kormentzas, G.; Skianis, C. Smart agriculture and greenhouse gas emission mitigation: A 6G-IoT perspective. Electronics 2024, 13, 1480. [Google Scholar] [CrossRef]
- Ndubuaku, U.M.; Uchenna, N.V.; Baiyeri, K.P.; Ukonze, J. Anti nutrient, vitamin and other phytochemical compositions of old and succulent moringa (Moringa oleifera Lam) leaves as influenced by poultry manure application. Afr. J. Biotech. 2015, 14, 2502–2509. [Google Scholar] [CrossRef]
- Daba, M. Miracle tree: a review on multi-purposes of Moringa oleifera and its implication for climate change mitigation. J. Earth. Sci. Clim. Change 2016, 7, 366. [Google Scholar] [CrossRef]
- Yang, K.H.; Lee, M.H.; Yoe, H. Designing an Open Field Precision Agriculture System Using Drones. In Proceedings of the 2023 Congress in Computer Science, Computer Engineering & Applied Computing (CSCE), Las Vegas, NV, USA, 24–27 July 2023; pp. 1691–1694. [Google Scholar] [CrossRef]
- Garofalo, G.; Buzzanca, C.; Ponte, M.; Barbera, M.; D’Amico, A.; Greco, C.; Mammano, M.M.; Franciosi, E.; Piazzese, D.; Guarrasi, V.; et al. Comprehensive analysis of Moringa oleifera leaves’ antioxidant properties in ovine cheese. Food Biosci. 2024, 61, 104974. [Google Scholar] [CrossRef]
- Conte, L.; Prakofjewa, J.; Floridia, T.; Stocco, A.; Comar, V.; Gonella, F.; Lo Cascio, M. Learning from farmers on potentials and limits for an agroecological transition: a participatory action research in Western Sicily. Front. Environ. Sci. 2024, 12, 1347915. [Google Scholar] [CrossRef]
- Jiang, R.; Sánchez-Azofeifa, G.A.; Laakso, K.; Wang, P.; Xu, Y.; Zhou, Z.; Luo, X.; Lan, Y.; Zhao, G.; Chen, X. UAV-based partially sampling system for rapid NDVI mapping in the evaluation of rice nitrogen use efficiency. J. Clean. Prod. 2021, 289, 125705. [Google Scholar] [CrossRef]
- Solano-Alvarez, N.; Valencia-Hernández, J.A.; Vergara-Pineda, S.; Millán-Almaraz, J.R.; Torres-Pacheco, I.; Guevara-González, R.G. Comparative Analysis of the NDVI and NGBVI as Indicators of the Protective Effect of Beneficial Bacteria in Conditions of Biotic Stress. Plants 2022, 11, 932. [Google Scholar] [CrossRef]
- Malik, M.S.; Shukla, J.P.; Mishra, S. Relationship of LST, NDBI and NDVI using Landsat-8 data in Kandaihimmat Watershed, Hoshangabad, India. Indian J. Geo-Mar. Sci. 2019, 48, 25–31. [Google Scholar]
- Govil, H.; Guha, S.; Diwan, P.; Gill, N.; Dey, A. Analyzing linear relationships of LST with NDVI and MNDISI using various resolution levels of Landsat 8 OLI and TIRS data. In Data Management, Analytics and Innovation: Proceedings of ICDMAI 2019; Springer: Singapore, 2020; Volume 1, pp. 171–184. [Google Scholar]
- Budiman, I.; Asri, E.D.; Aidha, Z.R. The Smart Agriculture based on Reconstructed Thermal Image. In Proceedings of the 2nd International Conference on Intelligent Technologies (CONIT), Hubli, India, 24–26 June 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.; Catania, P.; Orlando, S.; Vallone, M.; Mammano, M.M. Assessment of vegetation indices as tool to decision support system for aromatic crops. In Proceedings of the Conference SHWA: Safety, Health and Welfare in Agriculture and Agro-food Systems 2024, Ragusa, Italy, 6–9 September 2023; pp. 322–331. [Google Scholar]
- Mammano, M.M.; Comparetti, A.; Ciulla, S.; Greco, C.; Orlando, S. A Prototype of Photovoltaic Dryer for Nutraceutical and Aromatic Plants. In AIIA 2022: Biosystems Engineering Towards the Green Deal. AIIA 2022; Ferro, V., Giordano, G., Orlando, S., Vallone, M., Cascone, G., Porto, S.M.C., Eds.; Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2023; Volume 337. [Google Scholar] [CrossRef]
- Greco, C.; Gaglio, R.; Settanni, L.; Sciurba, L.; Ciulla, S.; Orlando, S.; Mammano, M.M. Smart Farming Technologies for Sustainable Agriculture: A Case Study of a Mediterranean Aromatic Farm. Agriculture 2025, 15, 810. [Google Scholar] [CrossRef]
- Greco, C.; Catania, P.; Orlando, S.; Vallone, M.; Mammano, M.M. A Sustainable Smart Dryer for Aromatic Herbs. In Biosystems Engineering Promoting Resilience to Climate Change—AIIA 2024—Mid-Term Conference. MID-TERM AIIA 2024; Sartori, L., Tarolli, P., Guerrini, L., Zuecco, G., Pezzuolo, A., Eds.; Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2025; Volume 586. [Google Scholar] [CrossRef]
- Costa, J.M.; Grant, O.; Chaves, M. Use of Thermal Imaging in Viticulture: Current Application and Future Prospects. In Methodologies and Results in Grapevine Research; Delrot, S., Medrano, H., Or, E., Bavaresco, L., Grando, S., Eds.; Springer: Dordrecht, the Netherlands, 2010. [Google Scholar] [CrossRef]
- Wen, T.; Li, J.H.; Wang, Q.; Gao, Y.Y.; Hao, G.F.; Song, B.A. Thermal imaging: The digital eye facilitates high-throughput phenotyping traits of plant growth and stress responses. Sci. Total Environ. 2023, 899, 165626. [Google Scholar] [CrossRef] [PubMed]
- Pou, A.; Diago, M.P.; Medrano, H.; Baluja, J.; Tardaguila, J. Validation of thermal indices for water status identification in grapevine. Agric. Water Manag. 2014, 134, 60–72. [Google Scholar] [CrossRef]
- Boesch, R. THERMAL REMOTE SENSING WITH UAV-BASED WORKFLOWS. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2017, XLII-2/W6, 41–46. [Google Scholar] [CrossRef]
- Ipate, G.; Cotici, C.D.; Ionescu, A.; Fătu, V.; Găgeanu, I.; Cujbescu, D.; Nicolau, A.M. Evaluation of the thermal behavior of plants in the microgreenhouse with microbolometric image sensors. INMATEH Agric. Eng. 2023, 71, 558–565. [Google Scholar] [CrossRef]
- Almeida, T.A.B.; Montenegro, A.A.A.; da Silva, R.A.B.; de Lima, J.L.M.P.; Carvalho, A.A.d.; da Silva, J.R.L. Evaluating Daily Water Stress Index (DWSI) Using Thermal Imaging of Neem Tree Canopies under Bare Soil and Mulching Conditions. Remote Sens. 2024, 16, 2782. [Google Scholar] [CrossRef]
- Gerhards, M.; Rock, G.; Schlerf, M.; Udelhoven, T. Water stress detection in potato plants using leaf temperature, emissivity, and reflectance. Int. J. Appl. Earth Obs. Geoinf. 2016, 53, 27–39. [Google Scholar] [CrossRef]
- Kusnierek, K.; Korsaeth, A. Challenges in using an analog uncooled microbolometer thermal camera to measure crop temperature. Int. J. Agric. Biol. Eng. 2014, 7, 60–74. [Google Scholar]
- Möller, M.; Alchanatis, V.; Cohen, Y.; Meron, M.; Tsipris, J.; Naor, A.; Ostrovsky, V.; Sprintsin, M.; Cohen, S. Use of thermal and visible imagery for estimating crop water status of irrigated grapevine. J. Exp. Bot. 2007, 58, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989. [Google Scholar] [CrossRef]
- Shafiekhani, A.; Fritschi, F.B.; DeSouza, G.N. A New 4D-RGB Mapping Technique for Field-Based High-Throughput Phenotyping. In Proceedings of the BMVC 2018, Newcastle, UK, 3–6 September 2018; p. 322. [Google Scholar]
- Raza, S.E.A.; Prince, G.; Clarkson, J.P.; Rajpoot, N.M. Automatic detection of diseased tomato plants using thermal and stereo visible light images. PLoS ONE 2015, 10, e0123262. [Google Scholar] [CrossRef] [PubMed]
- Aversano, R. Integration of thermal and RGB sensors in precision agriculture: A review. Agric. Syst. 2022, 195, 103303. [Google Scholar]
- Poblete, T.; Gonzalez-Dugo, V.; Hornero, A.; Zarco-Tejada, P.J. Water stress detection in olive at the farm level using high-resolution multispectral airborne imagery: assessment against canopy temperature. In Proceedings of the II International Symposium on Precision Management of Orchards and Vineyards 2023, Tatura, VIC, Australia, 3–8 December 2023; Volume 1395, pp. 23–30. [Google Scholar]
- Zubler, D.; Yoon, S. Smartphone-enabled thermal monitoring for field applications. Agric. Sci. 2020, 11, 873–884. [Google Scholar]
- Younsuk, D.; Sloan, G.; Chappuies, J. Open-Source Time-Lapse Thermal Imaging Camera for CanopyTemperature Monitoring. Smart Agric. Technol. 2024, 7, 100430. [Google Scholar] [CrossRef]
- Chappuies, J.; Dong, Y. Creating Thermographic Profiles of Blueberry Plants using Open-Source Thermal Imaging and RGB Cameras. In Proceedings of the 2024 ASABE Annual International Meeting, Anaheim, CA, USA, 28–31 July 2024; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2024; p. 1. [Google Scholar]
- Messina, G.; Modica, G. Applications of UAV Thermal Imagery in Precision Agriculture: State of the Art and Future Research Outlook. Remote Sens. 2020, 12, 1491. [Google Scholar] [CrossRef]
- Miceli, A.; Gaglio, R.; Francesca, N.; Ciminata, A.; Moschetti, G.; Settanni, L. Evolution of shelf life parameters of ready-to-eat escarole (Cichorium endivia var. Latifolium) subjected to different cutting operations. Sci. Hortic. 2019, 247, 175–183. [Google Scholar]
- Alfonzo, A.; Gaglio, R.; Miceli, A.; Francesca, N.; Di Gerlando, R.; Moschetti, G.; Settanni, L. Shelf life evaluation of fresh-cut red chicory subjected to different minimal processes. Food Microbiol. 2018, 73, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Viola, E.; Buzzanca, C.; Tinebra, I.; Settanni, L.; Farina, V.; Gaglio, R.; Di Stefano, V. A functional end-use of avocado (cv. Hass) waste through traditional semolina sourdough bread production. Foods 2023, 12, 3743. [Google Scholar] [CrossRef]
- ISO 4833; Microbiology of Food and Animal Feeding Stuffs: Horizontal Method for the Enumeration of Microorganisms: Colony-Count Technique at 30 °C. International Organization for Standardization: Geneva, Switzerland, 2003.
- ISO 6888-2; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase Positive Staphylococci (Staphylococcus aureus and Other Species): Part 2: Technique Using Rabbit Plasma Fibrinogen Agar Medium. International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 7251: 2005; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Presumptive Escherichia coli—Most Probable Number Technique. International Standardization Organization: Geneva, Switzerland, 2005.
- ISO 11290-1; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 6579-1; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella–Part 1: Detection of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2017.
- Greco, C.; Catania, P.; Orlando, S.; Calderone, G.; Mammano, M.M. Rosemary Biomass Estimation from UAV Multispectral Camera. In Biosystems Engineering Promoting Resilience to Climate Change—AIIA 2024—Mid-Term Conference. MID-TERM AIIA 2024; Sartori, L., Tarolli, P., Guerrini, L., Zuecco, G., Pezzuolo, A., Eds.; Lecture Notes in Civil Engineering; Springer: Cham, Switzerland, 2025; Volume 586. [Google Scholar] [CrossRef]
- Commission Regulation (EC). No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 1–26. [Google Scholar]
- Banach, J.L.; Stratakou, I.; van der Fels-Klerx, H.J.; Besten, H.M.W.; de Zwietering, M.H. European alerting and monitoring data as inputs for the risk assessment of microbiological and chemical hazards in spices and herbs. Food Control. 2016, 69, 237–249. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). FDA Draft Risk Profile: Pathogens and Filth in Spices; Center for Food Safety and Applied Nutrition, US Department of Health and Human Services: College Park, MD, USA, 2013.
- Gerba, C.P.; Pepper, I.L. Microbial contaminants. Environ. Pollut. Sci. 2019, 191–217. [Google Scholar]
- ICMSF. International Commission on Microbiological Specifications for Foods. Spices, herbs, and vegetable seasonings. In Microorganisms in Foods, Microbial Ecology of Food Commodities; ICMSF: Montreal, QC, Canada, 2005; pp. 360–372. [Google Scholar]


| Genotype | NDVI (30 October) | Canopy Temp (°C) | CWSI | Biomass Yield (kg/Plant) | Leaf Dry Weight (%) | Chlorophyll Index (SPAD) |
|---|---|---|---|---|---|---|
| A | 0.88 | 30.1 | 0.42 | 2.4 | 22.5 | 45.2 |
| I | 0.82 | 31.4 | 0.55 | 2.1 | 20.1 | 41.6 |
| PKM1 | 0.79 | 32.0 | 0.61 | 1.8 | 19.8 | 40.3 |
| PKM2 | 0.91 | 29.3 | 0.36 | 2.7 | 23.3 | 47.5 |
| Parameter | UAV-Derived Measurement | Ground Measurement | Correlation Coefficient (R2) | RMSE |
|---|---|---|---|---|
| Plant height (m) | Based on DSM/DTM | Manual measurement (ruler/tape) | 0.92 | 0.08 m |
| NDVI (unitless) | Multispectral sensor (Red and NIR) | SPAD chlorophyll meter | 0.85 | 0.04 |
| Canopy temperature (°C) | Thermal infrared imaging | Handheld IR thermometer | 0.81 | 1.2 °C |
| Sample | TMM | CPS | E. coli | L. monocytogenes | Salmonella spp. |
|---|---|---|---|---|---|
| A | 2.95 ± 0.29 a | n.d. | n.d. | n.d. | n.d. |
| I | 3.07 ± 0.18 a | n.d. | n.d. | n.d. | n.d. |
| PKM1 | 3.14 ± 0.15 a | n.d. | n.d. | n.d. | n.d. |
| PKM2 | 2.84 ± 0.23 a | n.d. | n.d. | n.d. | n.d. |
| p value | 0.405 | n.e. | n.e. | n.e. | n.e. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, C.; Gaglio, R.; Settanni, L.; Alfonzo, A.; Orlando, S.; Ciulla, S.; Mammano, M.M. Monitoring Moringa oleifera Lam. in the Mediterranean Area Using Unmanned Aerial Vehicles (UAVs) and Leaf Powder Production for Food Fortification. Agriculture 2025, 15, 1359. https://doi.org/10.3390/agriculture15131359
Greco C, Gaglio R, Settanni L, Alfonzo A, Orlando S, Ciulla S, Mammano MM. Monitoring Moringa oleifera Lam. in the Mediterranean Area Using Unmanned Aerial Vehicles (UAVs) and Leaf Powder Production for Food Fortification. Agriculture. 2025; 15(13):1359. https://doi.org/10.3390/agriculture15131359
Chicago/Turabian StyleGreco, Carlo, Raimondo Gaglio, Luca Settanni, Antonio Alfonzo, Santo Orlando, Salvatore Ciulla, and Michele Massimo Mammano. 2025. "Monitoring Moringa oleifera Lam. in the Mediterranean Area Using Unmanned Aerial Vehicles (UAVs) and Leaf Powder Production for Food Fortification" Agriculture 15, no. 13: 1359. https://doi.org/10.3390/agriculture15131359
APA StyleGreco, C., Gaglio, R., Settanni, L., Alfonzo, A., Orlando, S., Ciulla, S., & Mammano, M. M. (2025). Monitoring Moringa oleifera Lam. in the Mediterranean Area Using Unmanned Aerial Vehicles (UAVs) and Leaf Powder Production for Food Fortification. Agriculture, 15(13), 1359. https://doi.org/10.3390/agriculture15131359










