A Systematic Review of the Seven Most Cultivated Mushrooms: Production Processes, Nutritional Value, Bioactive Properties and Impact on Non-Communicable Diseases
Abstract
1. Introduction
2. Method
2.1. Systematic Review Aim and Strategy
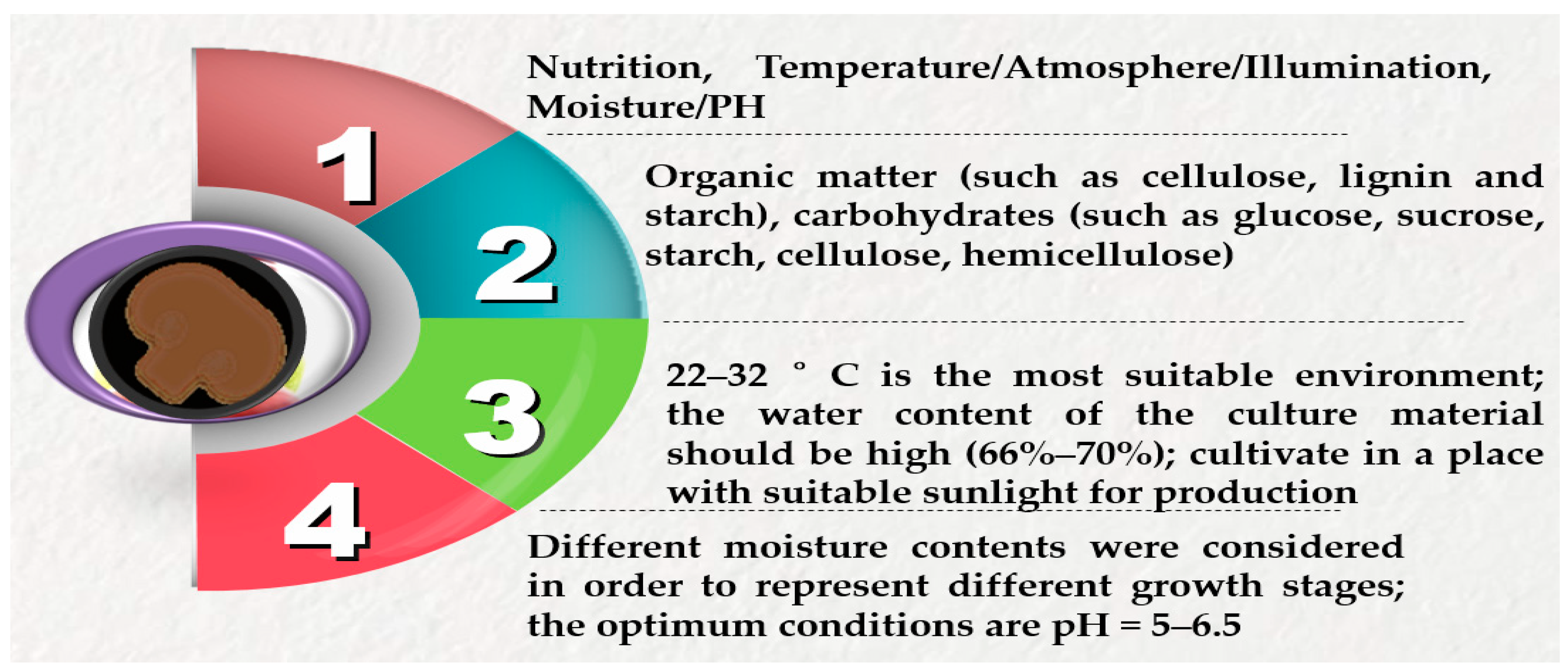
2.2. Literature Search, Study Selection, Eligibility Criteria and Quality Assessment
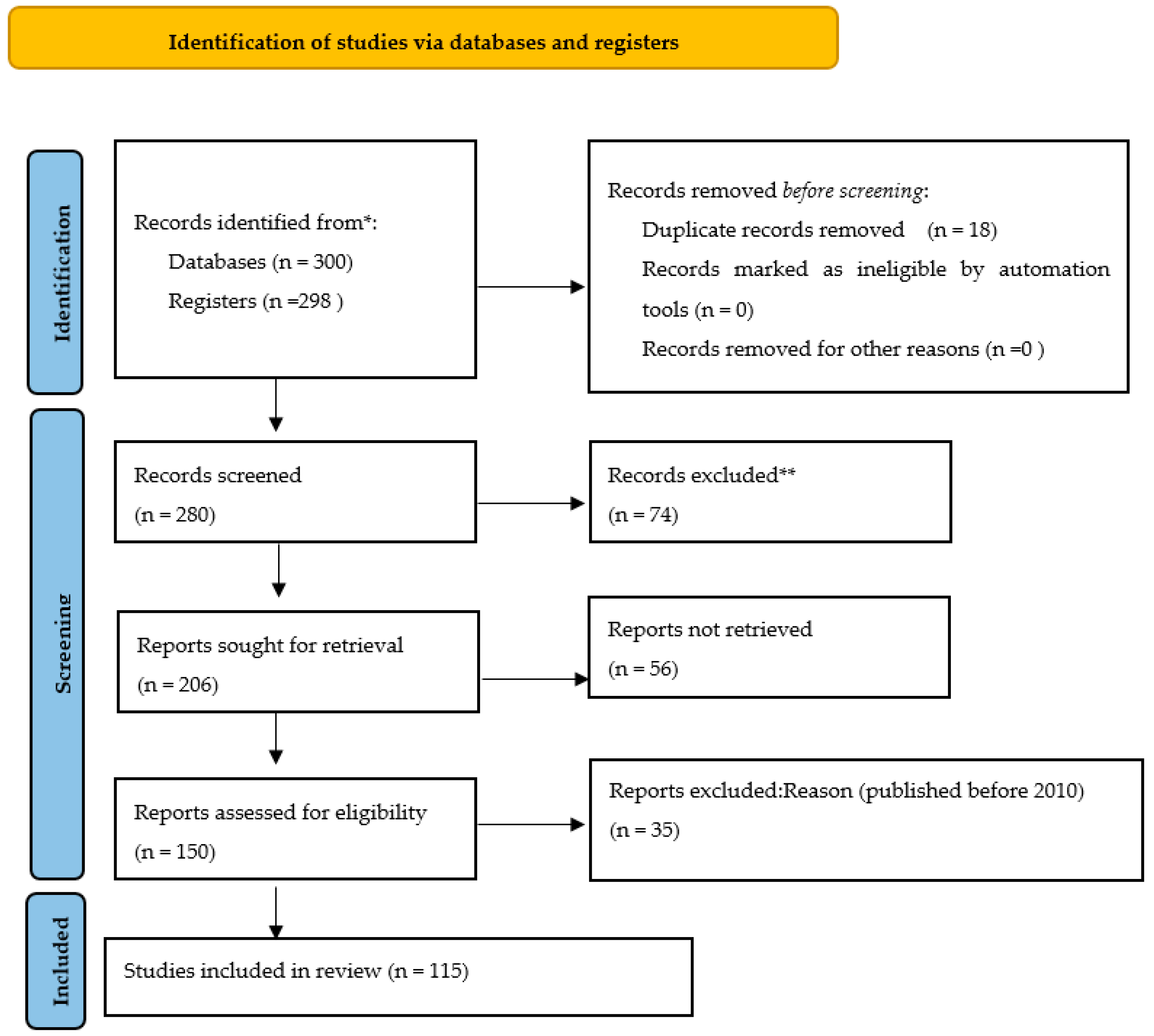
3. Results
3.1. Most Cultivated Mushrooms Worldwide: Production, Alternative Source of Protein, Post-Harvest Techniques
3.1.1. The Most Commonly Cultivated Species
- Agaricus bisporus;
- Lentinula edodes or Shiitake;
- Pleurotus spp.;
- Auricularia auricula-judae;
- Volvariella volvacea;
- Flammulina velutipes;
- Tuber spp.
3.1.2. Agaricus bisporus
3.1.3. Lentinula edodes
3.1.4. Pleurotus spp.
3.1.5. Auricularia spp.
3.1.6. Volvariella spp.
3.1.7. Flammulina velutipes
3.1.8. Tuber spp.
3.2. Most-Cultivated Mushrooms Worldwide: Alternative Source of Protein and Comparison with a Plant-Based Protein Source
Alternative Source of Protein
3.3. Most-Cultivated Mushrooms Worldwide: Post-Harvest Techniques with Important Benefits and Low Cost
Post-Harvest Techniques to Improve Their Nutritional Profile
3.4. Therapeutic Properties of the Most-Cultivated Mushrooms Worldwide and Comparison with Wild Mushrooms as Concerns Their Pharmaceutical Value
3.4.1. Non-Communicable Diseases
3.4.2. Cardiovascular Disease
3.4.3. Cancer
3.4.4. Diabetes Mellitus
3.4.5. Dyslipidemia
3.4.6. Hypertension
3.4.7. Neurological Disorders
- (a)
- Scientific studies have shown that the human brain and the cells of the nervous system are particularly sensitive to oxidative stress because of the following [97]:
- (b)
- The brain accounts for 2% of total body weight and consumes 20% of the oxygen supply.
- (c)
- It is rich in polyunsaturated fatty acids, which are easily oxidized.
- (d)
- It does not have a strong antioxidant defense in its cells.
- (e)
- It contains large amounts of iron and ascorbate, which catalyze lipid peroxidation.
- (f)
- Many neurotransmitters auto-oxidize and produce free radicals.
- (g)
- In some areas, nerve cells are damaged by mitosis and oxidative damage.
3.4.8. Osteoporosis
3.4.9. Other Effects
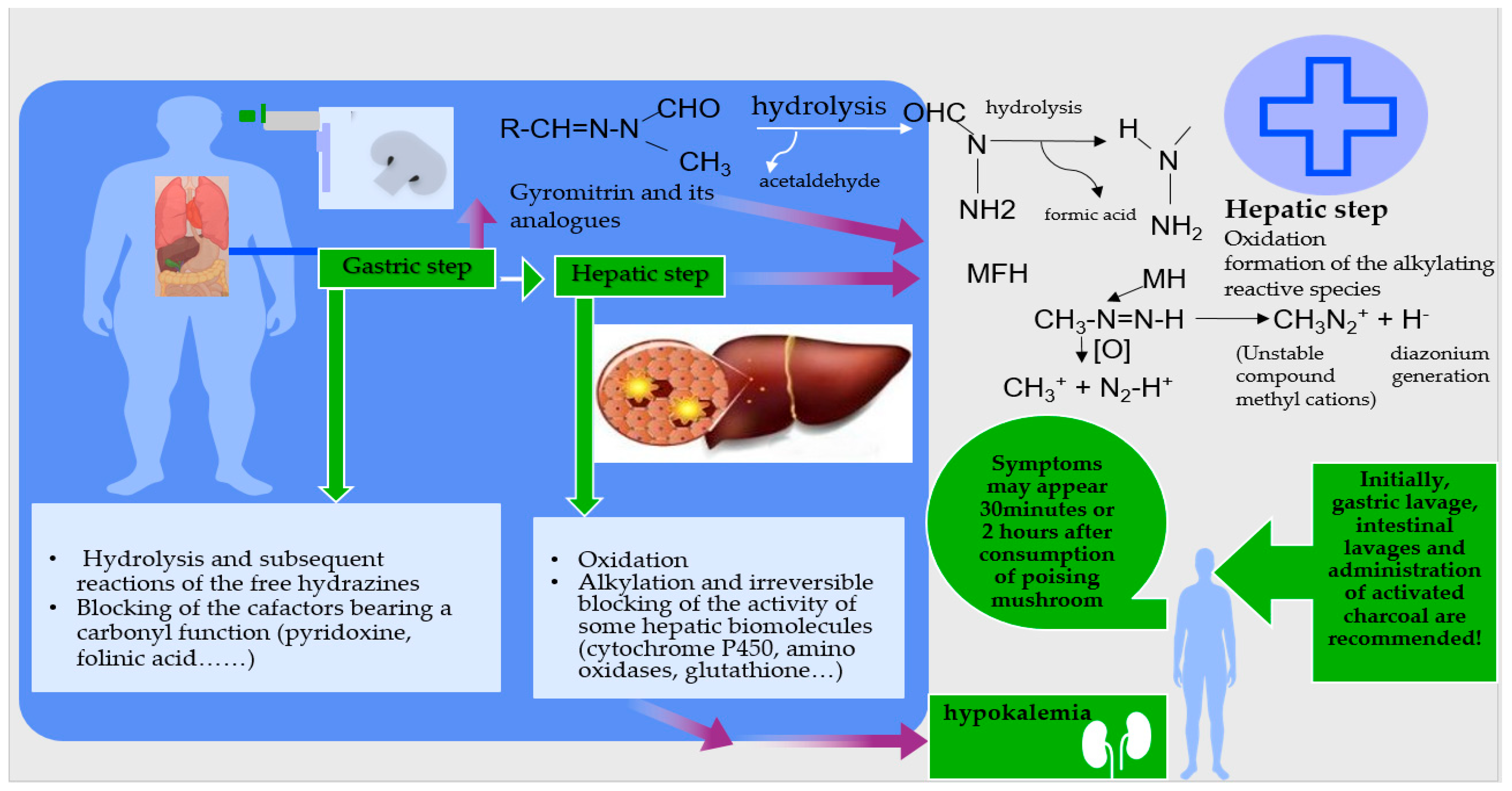
3.4.10. Side Effects
- Gastroenteritis
- Phalloid poisoning
- Muscarinic poisoning
- Mycoatropin poisoning
- Orleans poisoning
- Gyrometry poisoning
- Psilocybin poisoning
4. Discussion
- Regulation of cholesterol and sugar levels;
- Strengthening of the immune system;
- Support for anemia;
- Prevention of various forms of cancer;
- Control of body weight;
- Protection against inflammation;
- Cartilage formation and bone calcification;
- Prevention of cardiovascular diseases;
- Slowing down the natural aging process of cells;
- Regulating the metabolism of carbohydrates, lipids and proteins;
- Good functioning of the gastrointestinal tract.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Section/Topic | # | Checklist Item | Reported on Page |
|---|---|---|---|
| INFORMATION SOURCES AND METHODS | |||
| Database name | 1 | Name each individual database searched, stating the platform for each. | 2–5 |
| Multi-database searching | 2 | If databases were searched simultaneously on a single platform, state the name of the platform, listing all of the databases searched. | 2–5 |
| Study registries | 3 | List any study registries searched. | 3 |
| Online resources and browsing | 4 | Describe any online or print source purposefully searched or browsed (e.g., tables of contents, print conference proceedings, web sites), and how this was done. | - |
| Citation searching | 5 | Indicate whether cited references or citing references were examined, and describe any methods used for locating cited/citing references (e.g., browsing reference lists, using a citation index, setting up email alerts for references citing included studies). | 3–5 |
| Contacts | 6 | Indicate whether additional studies or data were sought by contacting authors, experts, manufacturers, or others. | - |
| Other methods | 7 | Describe any additional information sources or search methods used. | 3 |
| SEARCH STRATEGIES | |||
| Full search strategies | 8 | Include the search strategies for each database and information source, copied and pasted exactly as run. | 2–5 |
| Limits and restrictions | 9 | Specify that no limits were used, or describe any limits or restrictions applied to a search (e.g., date or time period, language, study design) and provide justification for their use. | 2–5 |
| Search filters | 10 | Indicate whether published search filters were used (as originally designed or modified), and if so, cite the filter(s) used. | 3 |
| Prior work | 11 | Indicate when search strategies from other literature reviews were adapted or reused for a substantive part or all of the search, citing the previous review(s). | 3 |
| Updates | 12 | Report the methods used to update the search(es) (e.g., rerunning searches, email alerts). | 3 |
| Dates of searches | 13 | For each search strategy, provide the date when the last search occurred. | 2,3 |
| PEER REVIEW | |||
| Peer review | 14 | Describe any search peer review process. | - |
| MANAGING RECORDS | |||
| Total Records | 15 | Document the total number of records identified from each database and other information sources. | 5 |
| Deduplication | 16 | Describe the processes and any software used to deduplicate records from multiple database searches and other information sources. | - |
References
- Dimopoulou, M.; Vareltzis, P.; Gortzi, O. A Systematic Review of the Twelve Most Popular Bean Varieties, Highlighting Their Potential as Functional Foods Based on the Health Benefits Derived from Their Nutritional Profiles, Focused on Non-Communicable Diseases. Appl. Sci. 2024, 14, 10215. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Bargiota, A.; Barmpa, E.; Outskouni, Z.; Stagos, D.; Trachana, V.; Androutsos, O.; Gortzi, O. Postprandial Glucose Response in Type 2 Diabetes Mellitus Patients and Possible Antioxidant Properties of a Plant-Based Snack Bar. Foods 2024, 13, 4123. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Vareltzis, P.; Floros, S.; Androutsos, O.; Bargiota, A.; Gortzi, O. Development of a Functional Acceptable Diabetic and Plant-Based Snack Bar Using Mushroom (Coprinus comatus) Powder. Foods 2023, 12, 2702. [Google Scholar] [CrossRef]
- Grimm, D.; Wösten, H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [PubMed]
- Valverde, M.; Hernández-Pérez, T.; Paredes-Lopez, O. Review Article Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 14. [Google Scholar] [CrossRef]
- Garcia-Barreda, S.; Camarero, J.J.; Vicente-Serrano, S.M.; Serrano-Notivoli, R. Variability and trends of black truffle production in Spain (1970–2017): Linkages to climate, host growth, and human factors. Agric. For. Meteorol. 2020, 287, 107951. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms: Technology and Applications; Diego, C.Z., Pardo-Gimenez, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar]
- Dimopoulou, M.; Kolonas, A.; Mourtakos, S.; Androutsos, O.; Gortzi, O. Nutritional composition and biological properties of sixteen edible mushroom species. Appl. Sci. 2022, 12, 8074. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Regis, J.; Geösel, A.; Geösel, R.M. Cultivation of Auricularia species: A review of the history, health benefits, principles, practices, environmental conditions, research methods, and recent trends. Sydowia 2024, 76, 21. [Google Scholar]
- Sun, X.; Yang, C.; Ma, Y.; Zhang, J.; Wang, L. Research progress of Auricularia heimuer on cultivation physiology and molecular biology. Front. Microbiol. 2022, 13, 1048249. [Google Scholar] [CrossRef] [PubMed]
- Priya, R.; Geetha, D.; Darshan, S. Biology and cultivation of black ear mushroom—Auricularia spp. Adv. Life Sci. 2016, 5, 10252–10254. [Google Scholar]
- Walker, A.; Wannasawang, N.; Taliam, W.; Keokanngeun, L.; Luangharn, T.; Thongklang, N. Optimal conditions for mycelial growth and nutritional values of the Auricularia cornea. Stud. Fungi 2023, 8, 19. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, Y.; Malysheva, V.F.; Du, P.; Dai, Y.C. Species clarification of the most important and cultivated Auricularia mushroom “Heimuer”: Evidence from morphological and molecular data. Phytotaxa 2014, 186, 241–253. [Google Scholar] [CrossRef]
- Mingyou, W.; Weidong, S.; Shuaiyang, W.; Dehuan, Z.; Jiaoling, W.; Tianhang, D. Research on production technology of Auricularia auricula in China. J. Chin. Agric. Mech. 2022, 43, 99. [Google Scholar]
- Rugolo, M.; Levin, L.; Lechner, B.E. Flammulina velutipes: An option for “alperujo” use. Rev. Iberoam. Micol. 2016, 33, 242–247. [Google Scholar] [CrossRef]
- Zambonelli, A.; Iotti, M.; Hall, I. Current status of truffle cultivation: Recent results and future perspectives. Ital. J. Mycol. 2015, 44, 31–40. [Google Scholar]
- Coleman, M.D.; Berch, S.; Bonito, G.; Allen, B.; Andrews, E.; Arechiga Carvajal, E.T.; Cook, S.P.; D’Amours, C.; Garibay-Orijel, R.; Guevara, G. Status of truffle science and cultivation in North America. Plant Soil 2024, 508, 625–661. [Google Scholar] [CrossRef]
- Büntgen, U.; Oliach, D.; Martínez-Peña, F.; Latorre, J.; Egli, S.; Krusic, P.J. Black truffle winter production depends on Mediterranean summer precipitation. Environ. Res. Lett. 2019, 14, 074004. [Google Scholar] [CrossRef]
- Sakinah, N.M.; Misran, A.; Mahmud, T.; Abdullah, S. A review: Production and postharvest management of Volvariella volvacea. Int. Food Res. J. 2019, 26, 367–376. [Google Scholar]
- Amir, N.F.; Mohd-Aris, A. Nutritional composition of Volvariella volvacea grow using different cultivation techniques and substrate utilization. J. Acad. 2024, 12, 132–138. [Google Scholar]
- Yuen, S.K.; Kalianon, K.; Atong, M. Effect of different drying temperatures on the nutritional quality of edible wild mushroom, Volvariella volvacea obtained nearby forest areas. Int. J. Adv. Res. 2014, 2, 859–864. [Google Scholar]
- Carrasco, J.; Zied, D.C.; Navarro, M.J.; Gea, F.J.; Pardo-Giménez, A. Commercial cultivation techniques of mushrooms. In Advances in Macrofungi; Sridhar, K.R., Deshmukn, S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 11–40. [Google Scholar]
- Atila, F.; Owaid, M.N.; Shariati, M.A. The nutritional and medical benefits of Agaricus bisporus: A review. J. Microbiol. Biotechnol. Food Sci. 2017, 7, 281–286. [Google Scholar] [CrossRef]
- Straatsma, G.; Sonnenberg, A.S.; Van Griensven, L.J. Development and growth of fruit bodies and crops of the button mushroom, Agaricus bisporus. Fungal Biol. 2013, 117, 697–707. [Google Scholar] [CrossRef]
- Zhang, K.; Pu, Y.-Y.; Sun, D.-W. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Rzymski, P.; Mleczek, M.; Niedzielski, P.; Siwulski, M.; Gąsecka, M. Cultivation of Agaricus bisporus enriched with selenium, zinc and copper. J. Sci. Food Agric. 2017, 97, 923–928. [Google Scholar] [CrossRef]
- Sonnenberg, A.S.; Baars, J.J.; Gao, W.; Visser, R.G. Developments in breeding of Agaricus bisporus var. bisporus: Progress made and technical and legal hurdles to take. Appl. Microbiol. Biotechnol. 2017, 101, 1819–1829. [Google Scholar]
- Pardo-Giménez, A.; Pardo González, J.E.; Zied, D.C. Casing materials and techniques in Agaricus bisporus cultivation. In Edible and Medicinal Mushrooms: Technology and Applications; Diego, C.Z., Pardo-Gimenez, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 149–174. [Google Scholar]
- Leiva, F.; Saenz-Díez, J.; Martínez, E.; Jiménez, E.; Blanco, J. Environmental impact of Agaricus bisporus cultivation process. Eur. J. Agron. 2015, 71, 141–148. [Google Scholar] [CrossRef]
- Park, Y.; Bak, W.-C.; Koo, C.-D.; Lee, B.-H. Cultural characteristics of new cultivar of Lentinula edodes, Poongnyunko. Korean J. Mycol. 2015, 43, 26–32. [Google Scholar] [CrossRef]
- Bach, F.; Helm, C.V.; De Lima, E.A.; Bellettini, M.B.; Haminiuk, C.W. Influence of cultivation methods on the chemical and nutritional characteristics of Lentinula edodes. Emir. J. Food Agric. 2018, 30, 1006–1014. [Google Scholar]
- Bisko, N.; Mustafin, K.; Al-Maali, G.; Suleimenova, Z.; Lomberg, M.; Narmuratova, Z.; Mykchaylova, O.; Mytropolska, N.; Zhakipbekova, A. Effects of cultivation parameters on intracellular polysaccharide production in submerged culture of the edible medicinal mushroom Lentinula edodes. Czech Mycol. 2020, 72, 1–17. [Google Scholar] [CrossRef]
- Jang, Y.; Jeong, Y.S.; Ryoo, R.; Ka, K.-H. Comparison of cultivation, mushroom yield, and fruiting body characteristics of Lentinula edodes strains according to the inoculation method. Korean J. Mycol. 2021, 49, 525–530. [Google Scholar]
- Trang, N.T.H.; Thuy, N.T.B.; Mo, N.T.; Luyen, N.T.; Nghien, N.X. Optimal Culture Conditions for the Enhanced Mycelial Growth and Cultivation of Shiitake Mushroom (Lentinula edodes). Vietnam. J. Agric. Sci. 2023, 6, 1958–1968. [Google Scholar] [CrossRef]
- Shanmugaraj, C.; Saranraj, K.; Biswas, M. Assessment of Various Substrates for Shiitake Mushroom (Lentinula edodes) Cultivation in the Agro-Climatic Conditions of West Bengal. J. Adv. Biol. Biotechnol. 2024, 27, 918–928. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, D.; Zhang, L.; Li, Q.; Song, C.; Shang, X.; Bao, D.; Tan, Q.; Chen, H.; Lv, B. Corncob as a substrate for the cultivation of Lentinula edodes. Waste Biomass Valorization 2022, 13, 929–939. [Google Scholar] [CrossRef]
- Shukla, S.; Jaitly, A. Morphological and biochemical characterization of different oyster mushroom (Pleurotus spp.). J. Phytol. 2011, 3, 18–20. [Google Scholar]
- Sekan, A.S.; Myronycheva, O.S.; Karlsson, O.; Gryganskyi, A.P.; Blume, Y. Green potential of Pleurotus spp. in biotechnology. PeerJ 2019, 7, e6664. [Google Scholar] [CrossRef]
- Viruthambigai, S.; Kannan, R.; Ramamoorthy, V.; Reihana, R.; Parthiban, V. Studies on morphological and growth characters of new Pleurotus isolates. J. Pharmacogn. Phytochem. 2019, 8, 3328–3330. [Google Scholar]
- Raman, J.; Jang, K.-Y.; Oh, Y.-L.; Oh, M.; Im, J.-H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and nutritional value of prominent Pleurotus spp.: An overview. Mycobiology 2021, 49, 1–14. [Google Scholar] [CrossRef]
- Ritota, M.; Manzi, P. Pleurotus spp. cultivation on different agri-food by-products: Example of biotechnological application. Sustainability 2019, 11, 5049. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar]
- Thiribhuvanamala, G.; Krishnamoorthy, S.; Manoranjitham, K.; Praksasm, V.; Krishnan, S. Improved techniques to enhance the yield of paddy straw mushroom (Volvariella volvacea) for commercial cultivation. Afr. J. Biotechnol. 2012, 11, 12740–12748. [Google Scholar]
- Amir, N.; Mohd-Aris, A.; Mohamad, A.; Abdullah, S.; Yusof, F.; Umor, N. Spawn production and cultivation technology for Volvariella volvacea: A perspective. Food Res. 2023, 7, 93–101. [Google Scholar]
- Li, Z.; Chen, M.; Yu, C.; Li, Q.; Zhou, F.; Li, Y. Five Steps to Cultivate Volvariella volvacea. Agric. Sci. Technol. 2017, 18, 1593–1594. [Google Scholar]
- Luu, T.-T.-H.; Bui, D.-K.; Huynh, N.; Le, T.-L.; Green, I. Effect of the Cultivation Technology on the Yield of Paddy Straw Mushroom (Volvariella volvacea). Korean J. Mycol. 2022, 50, 161–171. [Google Scholar]
- Garcia-Barreda, S.; Camarero, J.J. Tree ring and water deficit indices as indicators of drought impact on black truffle production in Spain. For. Ecol. Manag. 2020, 475, 118438. [Google Scholar] [CrossRef]
- Feeney, M.J.; Dwyer, J.; Hasler-Lewis, C.M.; Milner, J.A.; Noakes, M.; Rowe, S.; Wu, D. Mushrooms and health summit proceedings. J. Nutr. 2014, 144, 1128S–1136S. [Google Scholar] [CrossRef]
- Ketnawa, S.; Rawdkuen, S. Properties of Texturized Vegetable Proteins from Edible Mushrooms by Using Single-Screw Extruder. Foods 2023, 12, 1269. [Google Scholar] [CrossRef]
- Kadnikova, I.A.; Costa, R.; Kalenik, T.K.; Guruleva, O.N.; Yanguo, S. Chemical composition and nutritional value of the mushroom Auricularia auricula-judae. J. Food Nutr. Res. 2015, 3, 478–482. [Google Scholar]
- Islam, T.; Yao, F.; Kang, W.; Lu, L.; Xu, B. A systematic study on mycochemical profiles, antioxidant, and anti-inflammatory activities of 30 varieties of Jew’s ear (Auricularia auricula-judae). Food Sci. Hum. Wellness 2022, 11, 781–794. [Google Scholar] [CrossRef]
- Rahman, M.A.; Masud, A.; Lira, N.Y.; Shakil, S. Proximate analysis, phytochemical screening and antioxidant activity of different strains of Auricularia auricula-judae (ear mushroom). Int. J. Tradit. Complement. Med. 2020, 5, 29. [Google Scholar]
- Kim, T.-H.; Jo, S.-H.; Kim, M.-J.; Yu, Y.-B.; Jang, M.-H.; Park, K.-M. Comparative study on nutritional contents of Auricularia spp. J. Mushroom 2012, 10, 29–36. [Google Scholar]
- Liu, E.; Ji, Y.; Zhang, F.; Liu, B.; Meng, X. Review on Auricularia auricula-judae as a functional food: Growth, chemical composition, and biological activities. J. Agric. Food Chem. 2021, 69, 1739–1750. [Google Scholar] [CrossRef]
- Bohrer, B.M. Nutrient density and nutritional value of meat products and non-meat foods high in protein. Trends Food Sci. Technol. 2017, 65, 103–112. [Google Scholar] [CrossRef]
- Muszyńska, B.; Pazdur, P.; Lazur, J.; Sułkowska-Ziaja, K. Lentinula edodes (Shiitake)—Biological activity. Med. Int. Rev. 2017, 27, 189–195. [Google Scholar]
- Muszyńska, B.; Kała, K.; Rojowski, J.; Grzywacz, A.; Opoka, W. Composition and Biological Properties of Agaricus bisporus Fruiting Bodies—A Review. Pol. J. Food Nutr. Sci. 2017, 67, 173–181. [Google Scholar] [CrossRef]
- Bandara, A.R.; Rapior, S.; Mortimer, P.E.; Kakumyan, P.; Hyde, K.D.; Xu, J. A review of the polysaccharide, protein and selected nutrient content of Auricularia, and their potential pharmacological value. Mycosphere J. 2019, 10, 579–607. [Google Scholar] [CrossRef]
- Ahlawat, O.; Manikandan, K.; Singh, M. Proximate composition of different mushroom varieties and effect of UV light exposure on vitamin D content in Agaricus bisporus and Volvariella volvacea. Mushroom Res. 2016, 25, 1–8. [Google Scholar]
- Eguchi, F.; Kalaw, S.P.; Dulay, R.M.R.; Miyasawa, N.; Yoshimoto, H.; Seyama, T.; Reyes, R.G. Nutrient composition and functional activity of different stages in the fruiting body development of Philippine paddy straw mushroom, Volvariella volvacea (Bull.: Fr.) Sing. Adv. Environ. Biol. 2015, 9, 54–66. [Google Scholar]
- Ali, S.; Yousaf, N.; Usman, M.; Javed, M.A.; Nawaz, M.; Ali, B.; Azam, M.; Ercisli, S.; Tirasci, S.; Ahmed, A.E. Volvariella volvacea (paddy straw mushroom): A mushroom with exceptional medicinal and nutritional properties. Heliyon 2024, 10, e39747. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Meenu, M.; Xu, B. Nutritional value and antioxidant activity of Chinese black truffle (Tuber indicum) grown in different geographical regions in China. LWT 2021, 135, 110226. [Google Scholar] [CrossRef]
- Alirezalu, K.; Azadmard-Damirchi, S.; Achachlouei, B.F.; Hesari, J.; Emaratpardaz, J.; Tavakolian, R. Physicochemical properties and nutritional composition of black truffles grown in Iran. Chem. Nat. Compd. 2016, 52, 290–293. [Google Scholar] [CrossRef]
- Γεδεών, Ι. The Types of Truffle in Epirus; University of Ioannina: Ioannina, Greece, 2022. [Google Scholar]
- Vahdani, M.; Rastegar, S.; Rahimizadeh, M.; Ahmadi, M.; Karmostaji, A. Physicochemical characteristics, phenolic profile, mineral and carbohydrate contents of two truffle species. J. Agr. Sci. Tech. 2017, 19, 1091–1101. [Google Scholar]
- Wahiba, B.; Wafaà, T.; Asmaà, K.; Bouziane, A.; Mohammed, B. Nutritional and antioxidant profile of red truffles (Terfezia claveryi) and white truffle (Tirmania nivea) from southwestern of Algeria. Pharm. Lett. 2016, 8, 134–141. [Google Scholar]
- Alrawi, A.; AL-Azzami, A.; Hasan, A. Amino Acids In Three Iraqi Truffles Type. Eur. J. Mol. Clin. Med. 2020, 7, 401–405. Available online: www.researchgate.net/publication/346631236 (accessed on 1 January 2020).
- Morgunova, M.; Shashkina, S.; Malygina, E.; Dmitrieva, M.; Tiguntseva, N.; Belyshenko, A.Y.; Vlasova, A.; Evstaf′ev, S.; Aksenov-Gribanov, D. Preliminary Assessment of Fatty-Acid Composition and Low-Molecular-Mass Natural Compounds from Russian Truffle Tuber macrosporum. Chem. Nat. Compd. 2023, 59, 759–761. [Google Scholar] [CrossRef]
- Wang, S.; Marcone, M.F. The biochemistry and biological properties of the world’s most expensive underground edible mushroom: Truffles. Food Res. Int. 2011, 44, 2567–2581. [Google Scholar] [CrossRef]
- Kim, K.-J.; Jin, S.-W.; Choi, B.-S.; Kim, J.-K.; Koh, Y.-W.; Ban, S.-E.; Seo, K.-S. Evaluation of the nutrition properties of Flammulina velutipes. J. Mushroom 2016, 14, 44–50. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Liu, H.; Zhang, G.; Zhao, J.; Liu, L.; Piao, X.; Song, H.; Zhang, S.; Li, Y. Determination of the available energy values and amino acid digestibility of Flammulina velutipes stem waste and its effects on carcass trait and meat quality fed to growing-finishing pigs. J. Anim. Sci. Biotechnol. 2020, 11, 41. [Google Scholar] [CrossRef]
- Hong, H.-S.; Kang, N.-K.; Lee, J.-H.; Choi, Y.; Nam, J.-S. Nutritional Components and Antioxidant Activities of Solvent Extracts from White and Brown Flammulina velutipes. Korean J. Food Nutr. 2022, 35, 378–388. [Google Scholar]
- Vetter, J. Biological values of cultivated mushrooms—A review. Acta Aliment. 2019, 48, 229–240. [Google Scholar] [CrossRef]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.M.F.; Freitas, A.C.; Rocha-Santos, T.A.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Duarte, A.C. Chemical composition and nutritive value of Pleurotus citrinopileatus var cornucopiae, P. eryngii, P. salmoneo stramineus, Pholiota nameko and Hericium erinaceus. J. Food Sci. Technol. 2015, 52, 6927–6939. [Google Scholar] [CrossRef]
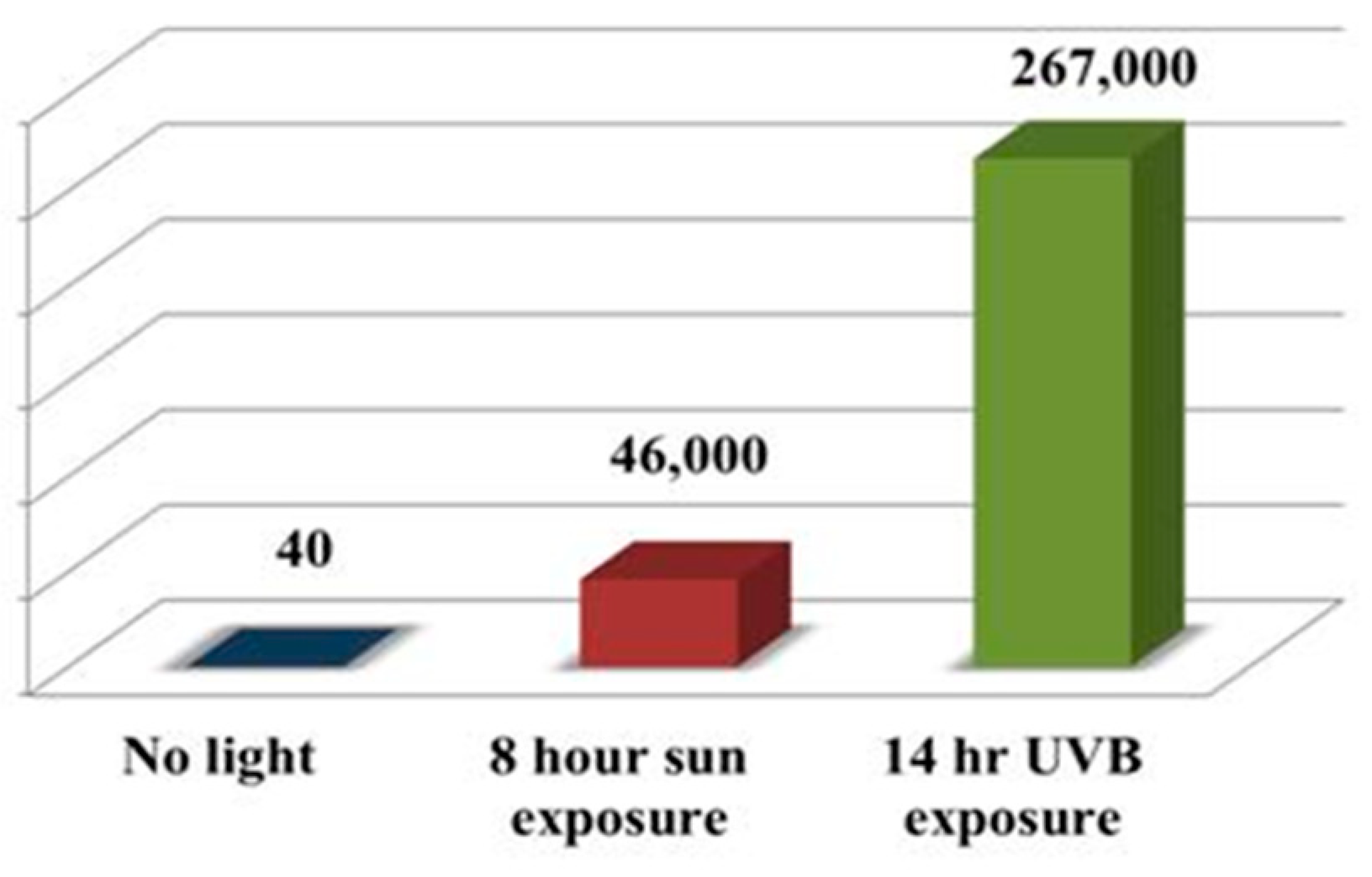
- Hidalgo-Sanz, R.; Del-Castillo-Alonso, M.Á.; Monforte, L.; Tomás-Las-Heras, R.; Sanz, S.; Olarte, C.; Núñez-Olivera, E. Ultraviolet-B radiation, mushrooms, and vitamin D: From technology to bioavailability. LWT 2023, 186, 115210. [Google Scholar] [CrossRef]
- Phillips, K.M.; Ruggio, D.M.; Horst, R.L.; Minor, B.; Simon, R.R.; Feeney, M.J.; Byrdwell, W.C.; Haytowitz, D.B. Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States. J. Agric. Food Chem. 2011, 59, 7841–7853. [Google Scholar] [CrossRef]
- Edward, T. Effect of ultraviolet-A and ultraviolet-C light on the concentration of vitamin D2 and mechanical properties of Oyster mushrooms during growth. J. Biophys. 2014, 2014, 687028. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Phillips, K.M.; Horst, R.L.; Munro, I.C. Vitamin D mushrooms: Comparison of the composition of button mushrooms (Agaricus bisporus) treated postharvest with UVB light or sunlight. J. Agric. Food Chem. 2011, 59, 8724–8732. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef]
- Phillips, K.M.; Rasor, A.S. A nutritionally meaningful increase in vitamin D in retail mushrooms is attainable by exposure to sunlight prior to consumption. J. Nutr. Food Sci. 2013, 3, 1. [Google Scholar]
- Phillips, K.M.; Horst, R.L.; Koszewski, N.J.; Simon, R.R. Vitamin D4 in mushrooms. PLoS ONE 2012, 7, e407702. [Google Scholar] [CrossRef] [PubMed]
- Uffelman, C.N.; Chan, N.I.; Davis, E.M.; Wang, Y.; McGowan, B.S.; Campbell, W.W. An assessment of mushroom consumption on cardiometabolic disease risk factors and morbidities in humans: A systematic review. Nutrients 2023, 15, 1079. [Google Scholar] [CrossRef]
- Ahmad, I.; Arif, M.; Mimi, X.; Zhang, J.; Ding, Y.; Lyu, F. Therapeutic values and nutraceutical properties of shiitake mushroom (Lentinula edodes): A review. Trends Food Sci. Technol. 2023, 134, 123–135. [Google Scholar] [CrossRef]
- Garcia, J.; Afonso, A.; Fernandes, C.; Nunes, F.M.; Marques, G.; Saavedra, M.J. Comparative antioxidant and antimicrobial properties of Lentinula edodes Donko and Koshin varieties against priority multidrug-resistant pathogens. S. Afr. J. Chem. Eng. 2021, 35, 98–106. [Google Scholar] [CrossRef]
- Zeng, X.; Suwandi, J.; Fuller, J.; Doronila, A.; Ng, K. Antioxidant capacity and mineral contents of edible wild Australian mushrooms. Food Sci. Technol. Int. 2012, 18, 367–379. [Google Scholar] [CrossRef]
- Ziaja-Sołtys, M.; Radzki, W.; Nowak, J.; Topolska, J.; Jabłońska-Ryś, E.; Sławińska, A.; Skrzypczak, K.; Kuczumow, A.; Bogucka-Kocka, A. Processed Fruiting Bodies of Lentinus edodes as a Source of Biologically Active Polysaccharides. Appl. Sci. 2020, 10, 470. [Google Scholar] [CrossRef]
- Łysakowska, P.; Sobota, A.; Wirkijowska, A. Medicinal Mushrooms: Their Bioactive Components, Nutritional Value and Application in Functional Food Production—A Review. Molecules 2023, 28, 5393. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Chang, C.-J.; Lin, C.-S.; Lu, C.-C.; Ko, Y.-F.; Tseng, S.-F.; Lai, H.-C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2017, 13, 149–160. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic potential of mushrooms in diabetes mellitus: Role of polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef]
- Elkhateeb, W.; Galappaththi, M.C.A.; El-Ghwas, D.E.; Daba, G. The anti-diabetic potential of mushrooms: A review. Curr. Trends Biotechnol. Pharm. 2023, 17, 1415–1424. [Google Scholar] [CrossRef]
- Khan, A.A.; Lu, L.-X.; Yao, F.-J.; Fang, M.; Wang, P.; Zhang, Y.-M.; Meng, J.-J.; Ma, X.-X.; He, Q.; Shao, K.-S. Characterization, antioxidant activity, and mineral profiling of Auricularia cornea mushroom strains. Front. Nutr. 2023, 10, 1167805. [Google Scholar] [CrossRef] [PubMed]
- Anwar, H.; Suchodolski, J.S.; Ullah, M.I.; Hussain, G.; Shabbir, M.Z.; Mustafa, I.; Sohail, M.U. Shiitake culinary-medicinal mushroom, Lentinus edodes (Agaricomycetes), supplementation alters gut microbiome and corrects dyslipidemia in rats. Int. J. Med. Mushrooms 2019, 21, 79–88. [Google Scholar] [CrossRef]
- Yadav, S.K.; Ir, R.; Jeewon, R.; Doble, M.; Hyde, K.D.; Kaliappan, I.; Jeyaraman, R.; Reddi, R.N.; Krishnan, J.; Li, M. A mechanistic review on medicinal mushrooms-derived bioactive compounds: Potential mycotherapy candidates for alleviating neurological disorders. Planta Med. 2020, 86, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.K.; Khan, M.A.H.N.A.; Das, S.K. Beneficial Role of Mushroom in Recovering Complications of Hypercholesterolemia. IDJPCR 2021, 4, 1–14. [Google Scholar] [CrossRef]
- Lee, W.; Fujihashi, A.; Govindarajulu, M.; Ramesh, S.; Deruiter, J.; Majrashi, M.; Almaghrabi, M.; Nadar, R.M.; Moore, T.; Agrawal, D.C. Role of mushrooms in neurodegenerative diseases. In Medicinal Mushrooms: Recent Progress in Research and Development; Agrawal, D.C., Dhanasekaran, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 223–249. [Google Scholar]
- Ye, S.; Gao, Y.; Hu, X.; Cai, J.; Sun, S.; Jiang, J. Research progress and future development potential of Flammulina velutipes polysaccharides in the preparation process, structure analysis, biology, and pharmacology: A review. Int. J. Biol. Macromol. 2024, 267, 131467. [Google Scholar] [CrossRef]
- Yamac, M.; Kanbak, G.; Zeytinoglu, M.; Senturk, H.; Bayramoglu, G.; Dokumacioglu, A.; Van Griensven, L.J.L.D. Pancreas protective effect of button mushroom Agaricus bisporus (JE Lange) imbach (Agaricomycetidae) extract on rats with streptozotocin-induced dia betes. Int. J. Med. Mushrooms 2010, 12, 379–389. [Google Scholar] [CrossRef]
- Chugh, R.M.; Mittal, P.; Mp, N.; Arora, T.; Bhattacharya, T.; Chopra, H.; Cavalu, S.; Gautam, R.K. Fungal mushrooms: A natural compound with therapeutic applications. Front. Pharmacol. 2022, 13, 925387. [Google Scholar] [CrossRef]
- Won, D.J.; Seong, K.S.; Jang, C.H.; Lee, J.S.; Ko, J.A.; Bae, H.; Park, H.J. Effects of vitamin D2-fortified shiitake mushroom on bioavailability and bone structure. Biosci. Biotechnol. Biochem. 2019, 83, 942–951. [Google Scholar] [CrossRef]
- Lindequist, U.; Haertel, B. Medicinal mushrooms for prevention and therapy of osteoporosis. Int. J. Med. Mushrooms 2021, 23, 13–22. [Google Scholar] [CrossRef]
- Qu, H.; Yi, J.; Gao, X.; Zhao, H.; Wang, Z. Anti-disuse osteoporosis activity of a complex of calcium-binding peptide from Auricularia auricula protein hydrolysates. J. Food Sci. 2019, 84, 1909–1919. [Google Scholar] [CrossRef]
- González-Ibáñez, L.; Meneses, M.E.; Sánchez-Tapia, M.; Pérez-Luna, D.; Torres, N.; Torre-Villalvazo, I.; Bonilla, M.; Petlacalco, B.; Castillo, I.; López-Barradas, A. Edible and medicinal mushrooms (Pleurotus ostreatus, Ustilago maydis, Ganoderma lucidum) reduce endoplasmic reticulum stress and inflammation in adipose tissue of obese Wistar rats fed with a high fat plus saccharose diet. Food Funct. 2023, 14, 5048–5061. [Google Scholar] [CrossRef] [PubMed]
- Merdivan, S.; Lindequist, U. Medicinal mushrooms with antiallergic activities. In Medicinal Plants and Fungi: Recent Advances in Research and Development; Agrawal, D.C., Tsay, H.-S., Shyur, L.-F., Wu, Y.-C., Wang, S.-Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 93–110. [Google Scholar]
- Seo, D.J.; Choi, C. Antiviral bioactive compounds of mushrooms and their antiviral mechanisms: A review. Viruses 2021, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Senti, G.; Leser, C.; Lundberg, M.; Wuthrich, B. Allergic asthma to shiitake and oyster mushroom. Allergy 2000, 55, 975–976. [Google Scholar] [CrossRef]
- Yin, X.; Yang, A.-A.; Gao, J.-M. Mushroom toxins: Chemistry and toxicology. J. Agric. Food Chem. 2019, 67, 5053–5071. [Google Scholar] [CrossRef] [PubMed]
- Blumfield, M.; Abbott, K.; Duve, E.; Cassettari, T.; Marshall, S.; Fayet-Moore, F. Examining the health effects and bioactive components in Agaricus bisporus mushrooms: A scoping review. J Nutr. Biochem. 2020, 84, 108453. [Google Scholar] [CrossRef]
- Stojković, D.; Barros, L. (Eds.) Edible Fungi: Chemical Composition, Nutrition and Health Effects; The Royal Society of Chemistry: Cambridge, UK, 2022; Volume 36, pp. 38–48. [Google Scholar]
- Wong, J.H.; Ng, T.B.; Chan, H.H.L.; Liu, Q.; Man, G.C.W.; Zhang, C.Z.; Guan, S.; Ng, C.C.W.; Fang, E.F.; Wang, H.; et al. Mushroom extracts and compounds with suppressive action on breast cancer: Evidence from studies using cultured cancer cells, tumor-bearing animals, and clinical trials. Appl. Microbiol. Biotechnol. 2020, 104, 4675–4703. [Google Scholar] [CrossRef]
- Rauf, A.; Joshi, P.B.; Ahmad, Z.; Hemeg, H.A.; Olatunde, A.; Naz, S.; Hafeez, N.; Simal-Gandara, J. Edible mushrooms as potential functional foods in amelioration of hypertension. Phytother. Res. 2023, 37, 2644–2660. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Y.; Sang, X.; Fan, L. Nutritional value, chemical composition and antioxidant activity of three Tuber species from China. AMB Express 2017, 7, 136. [Google Scholar] [CrossRef]
- Anusiya, G.; Gowthama Prabu, U.; Yamini, N.V.; Sivarajasekar, N.; Rambabu, K.; Bharath, G.; Banat, F. A review of the therapeutic and biological effects of edible and wild mushrooms. Bioengineered 2021, 12, 11239–11268. [Google Scholar] [CrossRef]
- Lee, H.; Nam, K.; Zahra, Z.; Farooqi, M.Q.U. Potentials of truffles in nutritional and medicinal applications: A review. Fungal Biol. Biotechnol. 2020, 7, 9. [Google Scholar] [CrossRef]
- Leser, S. The 2013 FAO report on dietary protein quality evaluation in human nutrition: Recommendations and implications. Nutr. Bull. 2013, 38, 421–428. [Google Scholar] [CrossRef]
- Oyedepo, T.A.; Morakinyo, A.E. Medicinal Mushrooms. In Herbal Product Development: Formulation and Applications; Sharma, A.K., Keservani, R.K., Gautam, S.P., Eds.; Apple Academic Press: New York, NY, USA, 2020; pp. 167–203. [Google Scholar]
- Huecker, M.; Sarav, M.; Pearlman, M.; Laster, J. Protein Supplementation in Sport: Source, Timing, and Intended Benefits. Curr. Nutr. Rep. 2019, 8, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, S.; Mishra, S.; Rajotiya, S.; Debnath, S.; Raj, P.; Bareth, H.; Singh, M.; Nathiya, D.; Tomar, B.S. The effects of vitamin D levels on physical, mental health, and sleep quality in adults: A comprehensive investigation. Front. Nutr. 2024, 11, 1451037. [Google Scholar] [CrossRef]
- Dawadi, E.; Magar, P.B.; Bhandari, S.; Subedi, S.; Shrestha, S.; Shrestha, J. Nutritional and post-harvest quality preservation of mushrooms: A review. Heliyon 2022, 8, e12093. [Google Scholar] [CrossRef]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef]
- Kubicka-Figiel, M.; Martyka, A.; Taborska, N. Vitamin D–the sunshine vitamin–could be correlated with both depression and anxiety. Environ. Med. Med. Srodowiskowa 2023, 26, 103. [Google Scholar] [CrossRef]
- Friedman, M. Mushroom polysaccharides: Chemistry and antiobesity, antidiabetes, anticancer, and antibiotic properties in cells, rodents, and humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef]
- Manjit, S.; Kamal, S.; Sharma, V.P. Status and trends in world mushroom production-III-World Production of Different Mushroom Species in 21st Century. Mushroom Res. 2021, 29, 75. [Google Scholar]
- Reid, T.; Munyanyi, M.; Mduluza, T. Effect of cooking and preservation on nutritional and phytochemical composition of the mushroom Amanita zambiana. Food Sci. Nutr. 2016, 5, 538–544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vlasenko, K.; Kuznetsova, O.; Heisun, A.; Matrosov, O. Comparative characteristics of aroma profile of wild and cultured edible mushrooms. Food Sci. Technol. 2022, 16, 2073–8684. [Google Scholar] [CrossRef]
- Çağlarirmak, N. Edible mushrooms: An alternative food item. In Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products (ICMBMP7),World Society for Mushroom Biology and Mushroom Products, Arcachon, France, 4–7 October 2011. [Google Scholar]
- Cai, Q.; Li, Y.; Pei, G. Polysaccharides from Ganoderma lucidum attenuate microglia-mediated neuroinflammation and modulate microglial phagocytosis and behavioural response. J. Neuroinflammation 2017, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Stilinović, N.; Čapo, I.; Vukmirović, S.; Rašković, A.; Tomas, A.; Popović, M.; Sabo, A. Chemical composition, nutritional profile and in vivo antioxidant properties of the cultivated mushroom Coprinus comatus. R. Soc. Open Sci. 2020, 7, 200900. [Google Scholar] [CrossRef]
- Teng, F.; Bito, T.; Takenaka, S.; Yabuta, Y.; Watanabe, F. Vitamin B12[c-lactone], a biologically inactive corrinoid compound, occurs in cultured and dried lion’s mane mushroom (Hericium erinaceus) fruiting bodies. J. Agric. Food Chem. 2014, 62, 1726–1732. [Google Scholar] [CrossRef]
- McKenna, D.J.; Jones, K.; Hughes, K.; Tyler, V.M. Botanical Medicines: The Desk Reference for Major Herbal Supplements, 1st ed.; Routledge: Oxfordshire, UK, 2012. [Google Scholar]
- Jaworska, G.; Pogoń, K.; Skrzypczak, A.; Bernaś, E. Composition and antioxidant properties of wild mushrooms Boletus edulis and Xerocomus badius prepared for consumption. J. Food Sci. Technol. 2015, 52, 7944–7953. [Google Scholar] [CrossRef] [PubMed]
- Farhan, E.M.; Chechan, R.A. Effect of Some Agricultural Substrates on Production Efficiency of Lentinula edodes (OM432157) and Evaluation of its Vitamins Content. Egypt. J. Bot. 2023, 63, 715–726. [Google Scholar] [CrossRef]
- Zohmangaiha, C. Morphological characterization and nutritional value of Lentinula edodes. J. Agric. Food Environ. (JAFE) 2023, 4, 9–12. [Google Scholar]
- Rahman, M.A.; Abdullah, N.; Aminudin, N. Inhibitory Effect on In Vitro LDL Oxidation and HMG Co-A Reductase Activity of the Liquid-Liquid Partitioned Fractions of Hericium erinaceus (Bull.) Persoon (Lion’s Mane Mushroom). BioMed Res. Int. 2014, 2014, 828149. [Google Scholar] [CrossRef]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a nutraceutical source: Promising bioactive compounds and market value. J. Fungi 2021, 7, 397. [Google Scholar] [CrossRef]

| Database | Keywords | MeSH Terms (PubMed) | Initial Articles | Duplicates Removed | Final Articles for Analysis | Contribution to Study | Reason for Inclusion |
|---|---|---|---|---|---|---|---|
| PubMed | #Mushrooms, #Cultivation, #Post-harvest techniques, #Protein, #Vitamin D, #Amino acids, #Diet, #Nutritional profile, #Nutrients, #Bioactive compounds, #Non-communicable diseases, #Health benefits, #Cardiovascular disease, #Cancer, #Diabetes mellitus, #Dyslipidemia, #Hypertension, #Neurological disorders, #Nutrition, and #Diet. | #Mushrooms, #Cultivation, #Post-harvest techniques, #Non-communicable diseases, #Nutritional profile, #Nutrients, #Bioactive compounds and #Interventions, #Diet, #Health. | 6 | 6 | 6 | Provided a broad understanding of the interplay between mushroom cultivation and post-harvest techniques to improve their nutritional profile but also consumption, interventions and health benefits; MeSH terms ensured precision in the search. | Widely recognized as a premier biomedical database, often used for reviews in healthcare research. |
| Web of Science | #Mushrooms, #Cultivation, #Post-harvest techniques, #Diet, #Nutritional profile, #Nutrients, #Bioactive compounds, #Health benefits, #Cardiovascular disease, #Diabetes mellitus, #Hypertension, #Cancer, #Dyslipidemia, #Neurological disorders, #Nutrition, and #Diet. | N/A (Web of Science doesn’t use MeSH terms) | 298 | 18 | 280 | Enhanced the overall coverage of literature related to mushroom nutrients, interventions and their impact on overall health. | Covering a wide range of scientific disciplines. |
| Scopus | #Mushrooms, #Cultivation, #Post-harvest techniques, #Nutrition, #Health, #Side Effects and #Evidence-Based Interventions. | #Mushrooms, #Health | 5 | 5 | 5 | Strengthened the evidence base by focusing on bean bioactive compounds related to evidence-based interventions; MeSH terms ensured specificity. | Renowned for reviews and emphasis on evidence-based interventions in healthcare research. |
| Cochrane Library | #Mushrooms, #Cultivation, #Post-harvest techniques, #Nutrition, #Health, #Side Effects and #Evidence-Based Interventions. | #Mushrooms, #Nutrition, #Health, #Side effects, #Evidence-Based Interventions | 6 | 6 | 6 | Strengthened the evidence base by focusing on bean bioactive compounds related to evidence-based interventions; MeSH terms ensured specificity. | Renowned for reviews and emphasis on evidence-based interventions in healthcare research. |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Reported in English | Does not report primary and/or secondary outcomes |
| Treated with techniques that have been described in detail | No Comparator Group (i.e., control or alternative dietary intervention) |
| In vitro and in vivo studies | Non-English language publication |
| Randomized Controlled trial or cohort study or reviews | Sample parameters (small sample) |
| Mushroom Species | Ash (g/100 g) | Energy (Kcal) | Protein (g/100 g) | Sugar (%) | Carbohydrates (g/100 g) | Fat (g/100 g) | Salt (g/100 g) | References |
|---|---|---|---|---|---|---|---|---|
| Agaricus bisporus edible | 9.3 | 336 Kcal/100 g— 1406.76 KJ/100 g | 25.1 | <0.1 | 52.7 | 1.7 | <0.1 | [8] |
| Agaricus bisporus cultivated | 8.7 | 303–324 Kcal/100 1268.6 g— 1356.52 KJ/100 g | 14.0–36.3 | <0.1 | 50.9–74 | 0.8–2.5 | <0.1 | [24,53,60,62,76] |
| Lentinula edodes edible | 6.1 | 340 Kcal/100 g— 1443 KJ/100 g | 20.7 | <0.1 | 59.5 | 1.3 | <0.1 | [8] |
| Lentinula edodes cultivated | 6.7 | 386 Kcal/100 g— 1616.1 KJ/100 g | 4.4–20.5 | <0.1 | 67.9–87 | 1.7–6.3 | <0.1 | [76,77] |
| Pleurotus ostreatus edible | 8 | 421 Kcal/100 g— 1762.64 KJ/100 g | 13.2 | <0.1 | 75.11 | 3.58 | <0.1 | [77] |
| Pleurotus ostreatus cultivated | 6 | 416 Kcal/100 g— 1762.64 KJ/100 g | 7–23.8 | <0.1 | 51.9–85 | 0.5–5.4 | <0.1 | [76,77] |
| Pleurotus eryngii edible | 6 | 421 Kcal/100 g— 1762.64 KJ/100 g | 16.2–26.6 | <0.1 | −64.9 | −3.5 | <0.1 | [78] |
| Pleurotus eryngii cultivated | 6 | 421 Kcal/100 g— 1762.64 KJ/100 g | 11–22 | <0.1 | 70.5–81.4 | 1.45–1.57 | <0.1 | [76,77] |
| Pleurotus citrinopileatus edible | 7.9 | 330 Kcal/100 g— 1395 KJ/100 g | 37.6 | <0.1 | 36.3 | 2.2 | <0.1 | [8,24] |
| Pleurotus citrinopileatus cultivated | 7 | 421 Kcal/100 g— 1762.64 KJ/100 g | 11–22.1 | <0.1 | 52.7 | 2.3 | <0.1 | [77,78] |
| Pleurotus ferulae edible | 5 | 330 Kcal/100 g— 1395 KJ/100 g | 30.3 | <0.1 | 47.8 | 5.7 | <0.1 | [53,77] |
| Auricularia auricula edible | 3.6 | 140 Kcal/100 g— 586.15 KJ/100 g | 12.5 | <0.1 | 66.1 | 1.7 | <0.1 | [57] |
| Auricularia auricula cultivated | 3.6 | 97–140 Kcal/100 g— 406.12–586.15 KJ/100 g | 5.7–15.5 | <0.1 | 77–91 | 0.4–4.5 | <0.1 | [53,54,55,56,57,61,76] |
| Volvariella volvacea edible | 8–10 | 346.04 Kcal/100 g— 1448.8 KJ/100 g | 28–32 | <0.1 | 50–52 | 2–4 | <0.1 | [64] |
| Volvariella volvacea cultivated | 8 | 346.04 Kcal/100 g— 1448.8 KJ/100 g | 32 | <0.1 | 56.8 | 5.7 | <0.1 | [22,23,24,62,63,64,76] |
| Flammulina velutipes edible | 9.4 | 346 Kcal/100 g— 1448.63 KJ/100 g | 17.89 | <0.1 | 70.85 | 1.84 | <0.1 | [77] |
| Flammulina velutipes White cultivated | 0.8 | 467 Kcal/100 g— 1372 KJ/100 g | 23.8 | <0.1 | 73.8 | 1.9 | <0.1 | [73,75,76] |
| Flammulina velutipes Brown cultivated | 0.68 | 467 Kcal/100 g— 1372 KJ/100 g | 24.8 | <0.1 | 76.2 | 1.7 | <0.1 | [73,75,76] |
| Black Truffle edible | 5 | 345.20 kcal/100— 1443.48 KJ/100 g | 20.9–23.2 | 7.32–6.35 | 51.7–49.6 | 5.58–6.59 | <0.1 | [65,66,68,72] |
| Black Truffle cultivated (T.pseudohima layense) | 8.77 | 339.6 Kcal/100— 1421.84 KJ/100 g | 14.28 | 23.89 | 74.4 | 2.55 | <0.1 | [115] |
| White truffle edible (Tirmania nivea) | 5.5 | 368 kcal/100— 1540.74 KJ/100 g | 11.97 | 5 | 68 | 2.15 | <0.1 | [69] |
| White truffle cultivated (T.latisporum) | 8.33 | 378.64 kcal/100— 1585.29 KJ/100 g | 14.64 | 50 | 74.63 | 2.4 | <0.1 | [67,69,71,115] |
| White truffle cultivated (T. subglobosum) | 8.13 | 378.6 kcal/100— 1585.12 KJ/100 g | 10.96 | 29.98 | 78.68 | 2.23 | <0.1 | [115] |
| Mushroom Species | Val | Leu | Ile | Thr | Met | Lys | Phe | Try | His | Total | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Agaricus bisporus | 3.9 | 6 | 3.1 | 3.4 | 1.4 | 6.1 | 3.1 | - | 4.23 | 27 | [52,77] |
| white | 5 | 6.3 | 3.8 | 4.6 | 1.4 | 5.9 | 4.4 | - | 4.12 | 31.4 | [52,77] |
| brown | 5 | 6.1 | 3.6 | 4.3 | 1.3 | 5.3 | 4.9 | - | 4.11 | 30.5 | [52,77] |
| Lentinula edodes | 3.8 | 6.4 | 3.3 | 5.6 | 2.2 | 5 | 3.8 | 1.9 | 4.22 | 32 | [52,77] |
| Pleurotus ostreatus | 4.7 | 6.8 | 4.3 | 5 | 1.9 | 6 | 4.3 | 1.4 | 4.44 | 34.4 | [52,77] |
| Pleurotus ferulae | 11.8 | 22.3 | 11.38 | 8.06 | 3.62 | 12.9 | 8.68 | - | 4.47 | 37.1 | [53,77] |
| Auricularia auricula | 3.53 | 4.89 | 1.89 | 4.89 | 0.29 | 4.04 | 2.76 | - | 2.16 | 34.7 | [53,77] |
| Volvariella volvacea | 3.5 | 7.55 | 3.3 | 4.88 | 1 | 5.2 | 6.22 | 1.7 | 2.16 | 35.8 | [64,77] |
| Flammulina velutipes | 4.6 | 6.1 | 4.4 | 4.7 | 1.4 | 5.7 | 4.7 | 1.5 | 2.3 | 34.8 | [74,75,77] |
| Truffle black | 4.9 | 6.1 | 3.6 | 4.6 | 1.5 | 5.5 | 4.9 | - | 2.17 | 31.1 | [65,66,67,70,117] |
| Truffle white | 4.9 | 6.1 | 3.6 | 4.6 | 1.5 | 5.5 | 4.9 | - | 2.17 | 31.1 | [70] |
| Standard protein (FAO/WHO) | 5 | 7 | 4 | 4 | 3.5 | 5.4 | 6.1 | 1 | 1 | 36 | [118] |
| Steak [58] | Dried Shiitake Mushrooms [59] |
|---|---|
| 20% Calories from proteins | 20% Calories from proteins |
| Saturated Fats | Unsaturated Fats |
| Higher lipidemic content | Lower lipidemic content |
| No Fiber | High in fiber |
| Iron 0.79 mg | Iron 18 mg |
| Magnesium 17 mg | Magnesium 17 mg |
| Calcium 35 mg | Calcium 22 mg |
| Potassium 288 mg | Potassium 464 mg |
| Vitamin C 0 mg | Vitamin C 25 mg |
| Depletes the earth | Adds needed nitrogen to the soil |
| Mushrooms Species | Vitamin D2 | Ergo Sterol | Ergo Sterol Ergosta7,22-Dienol | Ergosta-5,7dienol | Ergosta-7enol |
|---|---|---|---|---|---|
| Agaricus bisporus | 0.11 | 56.3 | 1.78 | 6.03 | 1.34 |
| Agaricus bisporus irradiated with UVB | 11.2 | 11.2 | 1.73 | 4.7 | 1.28 |
| Lentinula edodes | 0.44 | 84.9 | 2.26 | 6.51 | 5.03 |
| Pleurotus ostreatus | 0.72 | 68.0 | <1.66 | 8.89 | <1.7 |
| Volvariella volvacea | 0.50 | 84.9 | 2.26 | 6.51 | 5.13 |
| Volvariella volvacea irradiated with UVB | 19.2 | 19.2 | 1.73 | 4.7 | 1.28 |
| Flammulina veluptipes | 0.14 | 35.5 | <1.49 | 16.5 | 2.32 |
| Food Sources of Vitamin D | Vitamin D (μg/100 g) |
|---|---|
| Fish | |
| Cod liver oil | 210–250 |
| Salmon | 13.1–24.7 |
| Farm salmon | 6.0 |
| Herring | 5.7–15.4 |
| Smoked salmon | 5–27 |
| Dairy products | |
| Full-fat milk | 0.1 |
| Butter | 3.4–8.4 |
| Egg yolk | 0.5–5.4 |
| Beef liver | 1.2 |
| Mushrooms | |
| Agaricus bisporus fresh | 0.7–2.3 |
| Lentinula edodes sundried | 40 |
| Authors (Year) | Mushroom Species | Medical Value | References |
|---|---|---|---|
| Khursheed et al. (2020) | Agaricus bisporus, Pleurotus ostreatus, Lentinula edodes | Improves serum/plasma triglycerides and hs-CRP. | [93] |
| Ahmad et al. (2023) | Lentinula edodes | Acts as an antimicrobial, antiviral, anticancer/antitumor, antidiabetic, antihyperlipidemic, anticholesterol | [87] |
| Garcia et al. (2021) | Lentinula edodes | May be potential source of natural antioxidants, antibacterial agents and anti-aging agents. | [88] |
| Zeng et al. (2012) | Pleurotus eryngii, and Flammulina velutipes | The methanolic extracts of the dried caps of the mushrooms were determined using a number of different chemical reactions in evaluating multi-mechanistic antioxidant activities | [89] |
| Ziaja-Sołtys et al. (2020) | Lentinula edodes | Anticancer properties from Lentinus edodes fruiting bodies on human breast cancer | [90] |
| Łysakowska et al. (2023) | Shiitake (Lentinula edodes) | Acts as anti-obesogenic, antiviral, anticancer/antitumor, antidiabetic, antihyperlipidemic, anticholesterol | [91] |
| Martel et al. (2017) | Agaricus bisporus, Pleurotus ostreatus, Lentinula edodes | Anti-obesogenic and antidiabetic effects | [92] |
| Khursheed et al. (2020) | Pleurotus spp. | The polysaccharides from mushrooms are effective against type ΙΙ diabetes mellitus by reducing oxidative stress and also act as prebiotics and reduce gut dysbiosis, thereby helping in managing insulin resistance and type ΙΙ diabetes mellitus | [93] |
| Elkhateeb et al. (2023) | A. auricula-judae, L. edodes | Antidiabetic properties of mushroom species were promising | [94] |
| Khan et al. (2023) | Auricularia cornea | The crude polysaccharides from A. cornea mushroom strains act as natural antioxidants | [95] |
| Anwar et al. (2019) | Lentinula edodes | Total cholesterol and LDL cholesterol concentrations were reduced | [96] |
| Yadav et al. (2020) | Pleurotus giganteus | Protective effect against neuronal dysfunction | [97] |
| Kundu et al. (2021) | Agaricus bisporus, Pleurotus spp. | Source of important nutrients having hepatoprotective and antihyperlipidemic actions | [98] |
| Lee et al. (2019) | Pleurotus giganteus | Decrease neurotoxicity through various neuroprotective molecular mechanisms | [99] |
| Ye et al. (2024) | Flammulina velutipes | Immunomodulatory, anti-inflammatory and antibacterial properties | [100] |
| Yamac et al. (2010) | Agaricus bisporus | Pancreas protective effect of button mushroom Agaricus bisporus | [101] |
| Chugh et al. (2022) | Agaricus arvensis and Agaricus silvaticus | 130 medicinal activities like antitumor, immunomodulation, antioxidant, radical scavenging, cardioprotective and antiviral actions | [102] |
| Won et al. (2029) | Shiitake | Protection against osteoporosis | [103] |
| Lindequist et al. (2021) | Lentinula and Pleurotus | May be able to improve bone stability by influencing different steps of bone formation, mineralization or resorption. | [104] |
| Qu et al. (2019) | Auricularia auricula | May be protection against osteoporosis | [105] |
| González-Ibáñez et al. (2023) | Pleurotus ostreatus | Pleurotus ostreatus reduces endoplasmic reticulum stress and inflammation in adipose tissue of obese subjects | [106] |
| Merdivan et al. (2017) | Agaricus subrufescens, Flammulina velutipes, Pleurotus ostreatus and P. pulmonarius | Antiallergic activities | [107] |
| Seo et al. (2021) | Lentinus (Lentinula), Auricularia, Flammulina, Pleurotus, Agaricus | Some mushroom compounds that act against HIV, influenza A virus and hepatitis C virus showed antiviral effects comparable to those of antiviral drugs | [108] |
| Yan et al. (2017) | Tuber latisporum, T. subglobosum and T. pseudohimalayense | Antioxidant properties | [115] |
| Rauf et al. (2023) | Agaricus bisporus, Pleurotus ostreatus, Lentinula edodes, Flammulina velutipes | Helpful in alleviating hypertension and other cardiovascular malfunctions | [114] |
| Blumfield et al. (2017) | Agaricus bisporus | Beneficial impact on biomarkers correlated with metabolic syndrome and gastrointestinal health | [111] |
| Atila et al. (2017) | Agaricus bisporus | Has been reported to have antimicrobial, anticancer, antidiabetic, antihypercholesterolemic, antihypertensive, hepatoprotective and antioxidant activities | [112] |
| Wong et al. (2020) | Agaricus bisporus, Pleurotus abalonus, Pleurotus eryngii, P. ostreatus | Antineoplastic effectiveness in human clinical trials | [113] |
| Dimopoulou et al. (2022) | Agaricus bisporus, Pleurotus citrinopileatus, Lentinula edodes | Act as an antimicrobial, antiviral, anticancer/antitumor, antidiabetic, antihyperlipidemic, anticholesterol | [8] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimopoulou, M.; Chinou, I.; Gortzi, O. A Systematic Review of the Seven Most Cultivated Mushrooms: Production Processes, Nutritional Value, Bioactive Properties and Impact on Non-Communicable Diseases. Agriculture 2025, 15, 1329. https://doi.org/10.3390/agriculture15131329
Dimopoulou M, Chinou I, Gortzi O. A Systematic Review of the Seven Most Cultivated Mushrooms: Production Processes, Nutritional Value, Bioactive Properties and Impact on Non-Communicable Diseases. Agriculture. 2025; 15(13):1329. https://doi.org/10.3390/agriculture15131329
Chicago/Turabian StyleDimopoulou, Maria, Ioanna Chinou, and Olga Gortzi. 2025. "A Systematic Review of the Seven Most Cultivated Mushrooms: Production Processes, Nutritional Value, Bioactive Properties and Impact on Non-Communicable Diseases" Agriculture 15, no. 13: 1329. https://doi.org/10.3390/agriculture15131329
APA StyleDimopoulou, M., Chinou, I., & Gortzi, O. (2025). A Systematic Review of the Seven Most Cultivated Mushrooms: Production Processes, Nutritional Value, Bioactive Properties and Impact on Non-Communicable Diseases. Agriculture, 15(13), 1329. https://doi.org/10.3390/agriculture15131329








