Molecular Characterization and Ex Situ Conservation of Wild Grapevines Grown in the Area Around the Neolithic Settlement of Dikili Tash, Greece
Abstract
1. Introduction
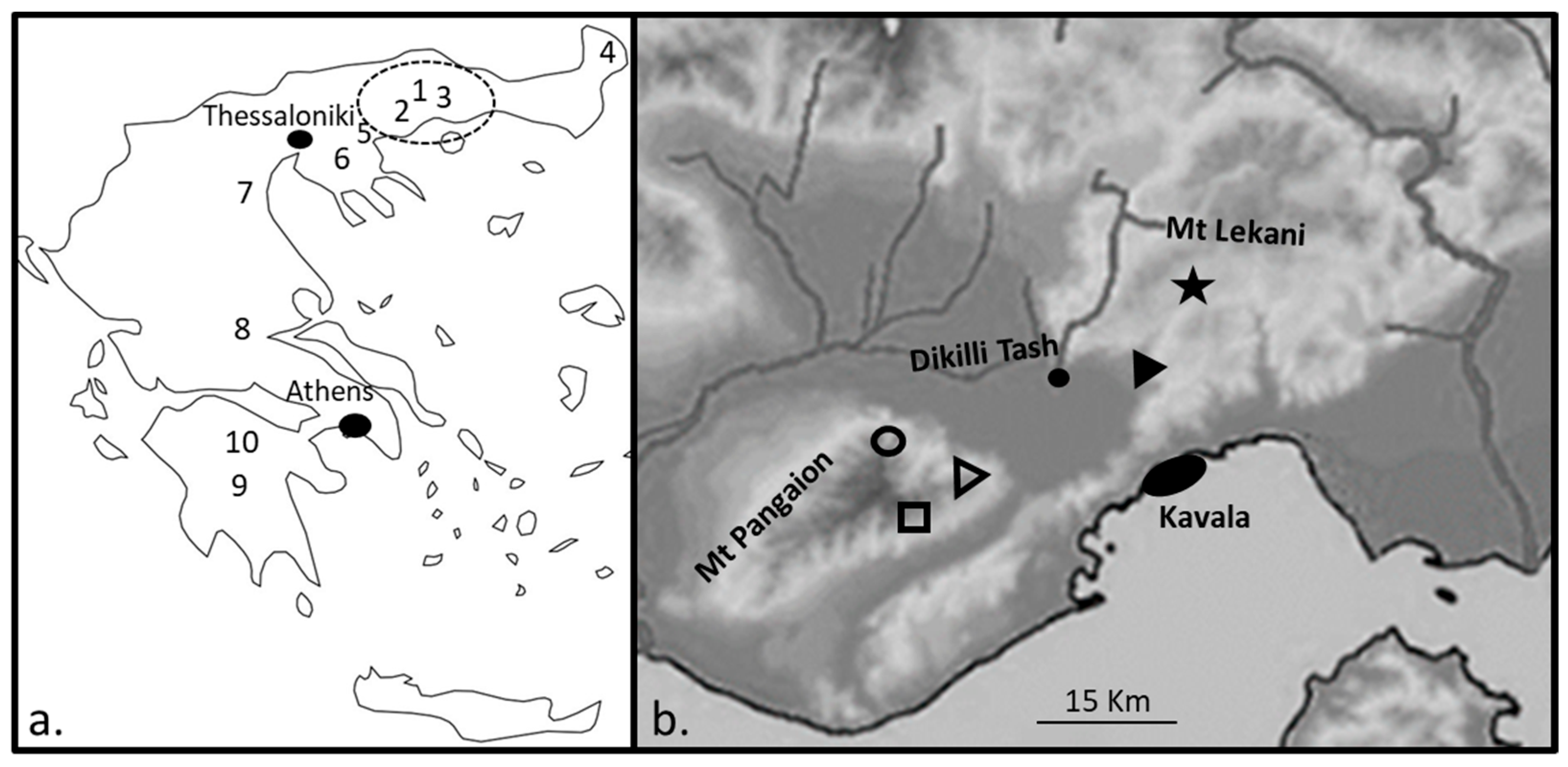
2. Materials and Methods
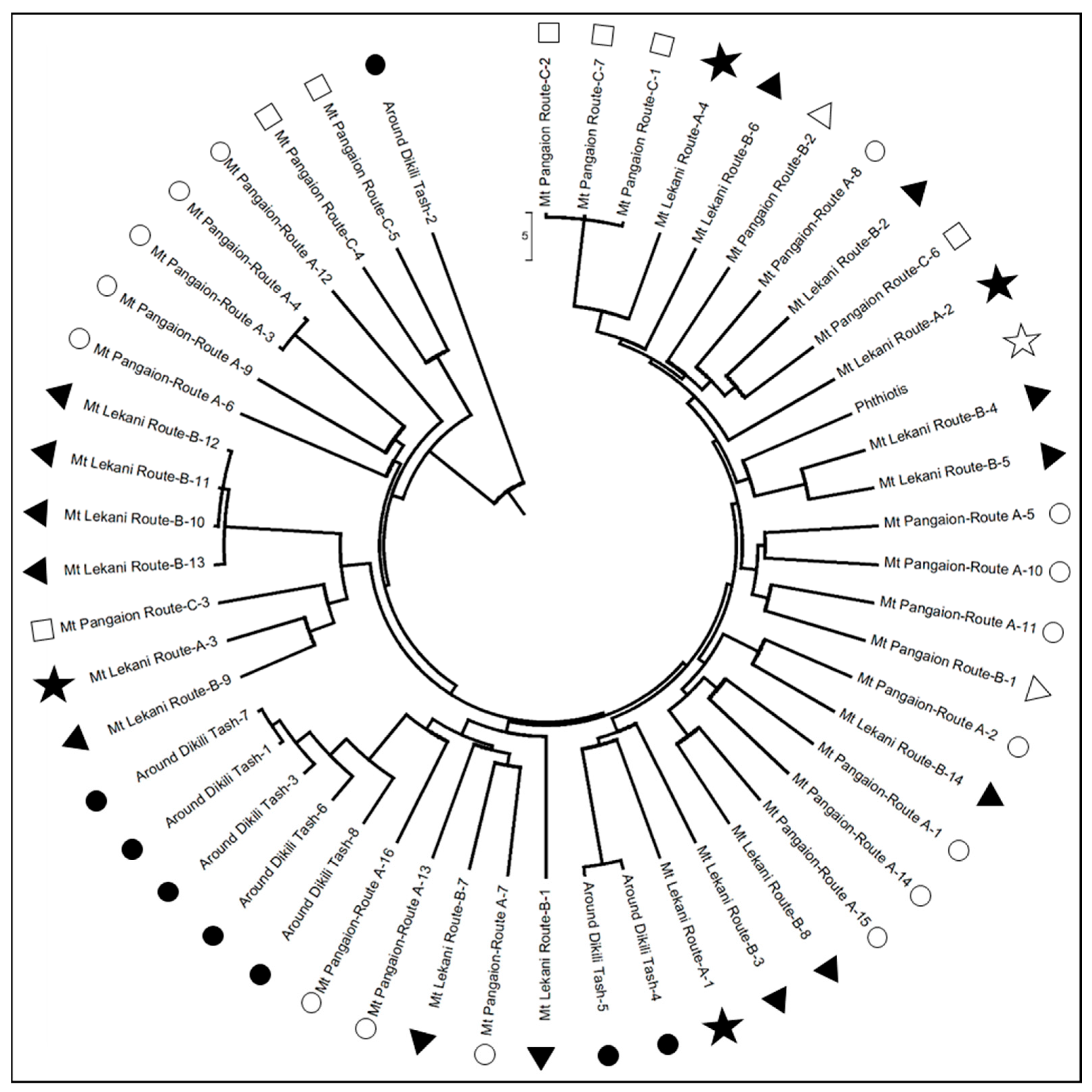
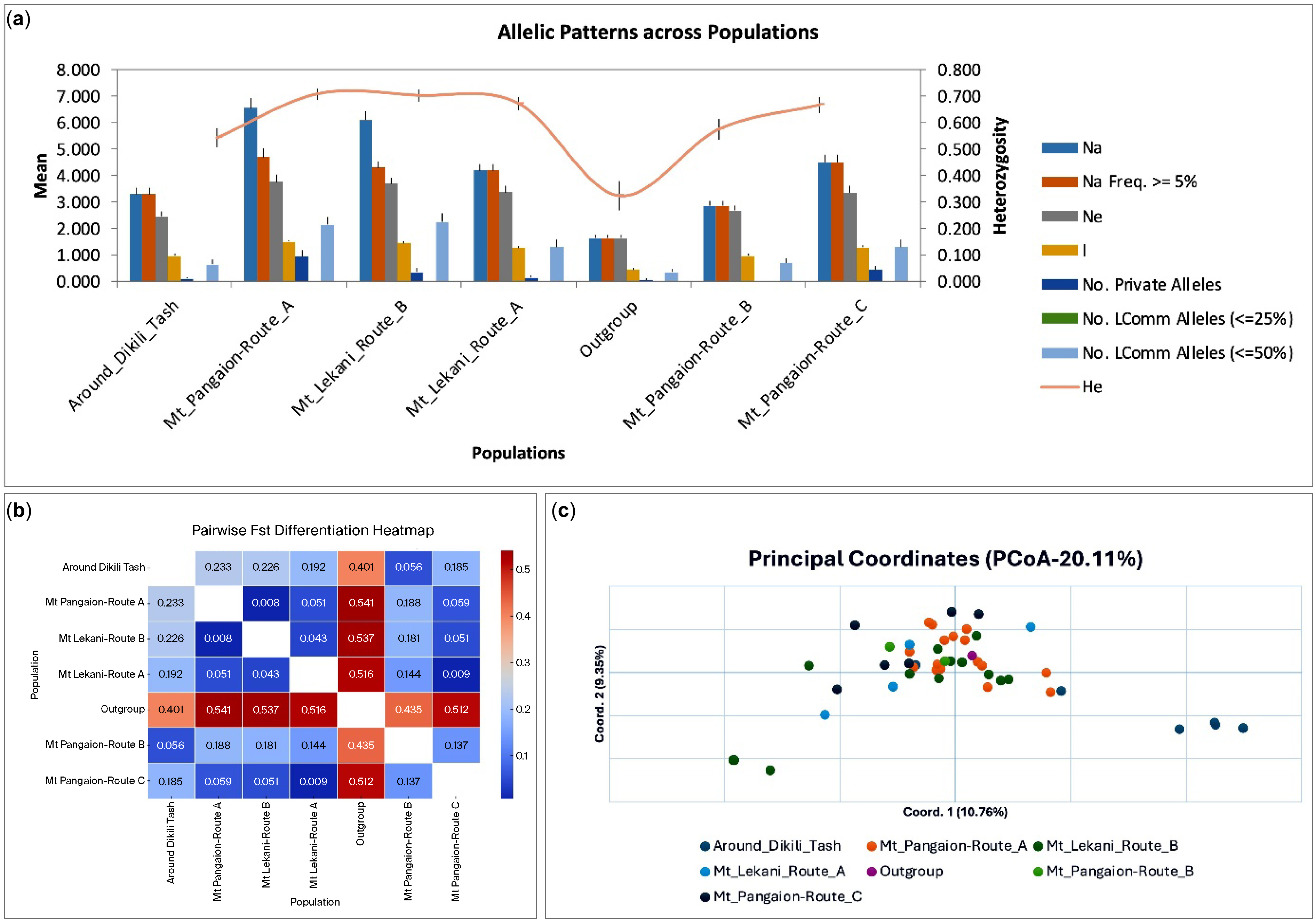
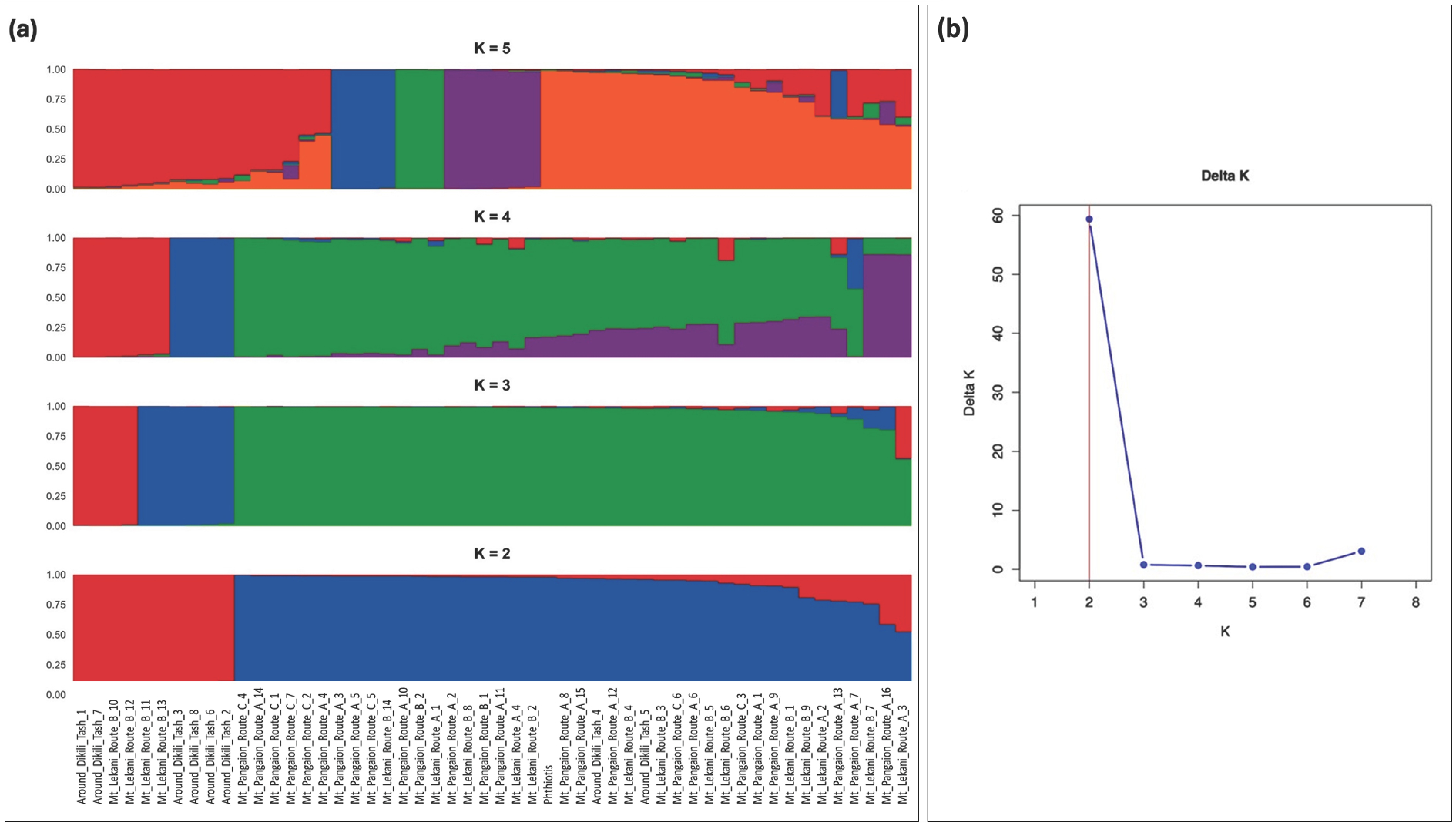
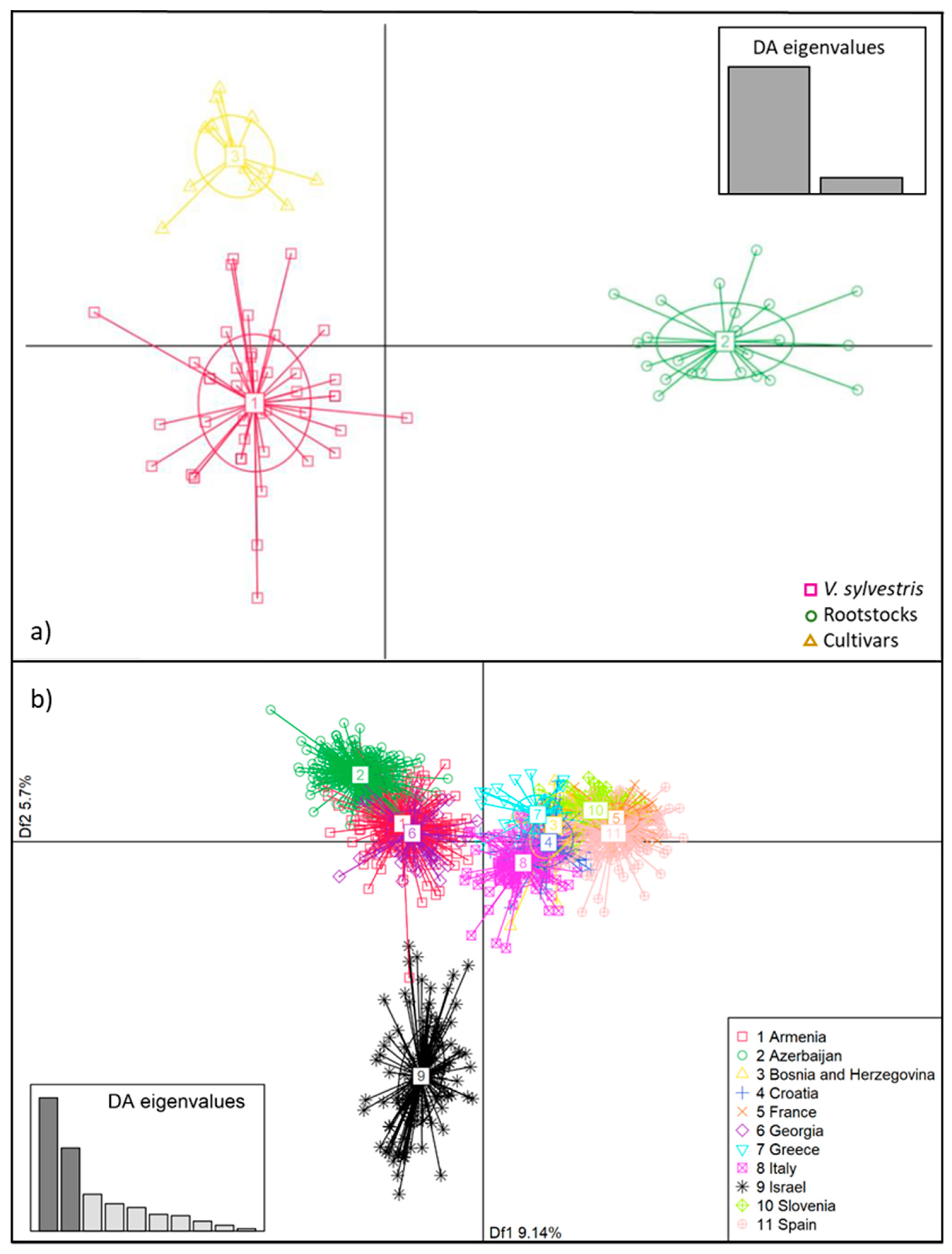
3. Results and Discussion
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ELGO-DIMITRA | Hellenic Agricultural Organisation DIMITRA |
| JKI | Julius Kühn Institute |
| PCR | Polymerase Chain Reaction |
| ECPGR | European Cooperative Programme for Plant Genetic Resources |
References
- Cola, G.; De Lorenzis, G.; Failla, O.; Kvaliashvili, N.; Kikilashvili, S.; Kikvadze, M.; Mamasakhlisashvili, L.; Mdinaradze, I.; Chipashvili, R.; Maghradze, D. The Status of Wild Grapevine (Vitis vinifera L. subsp. sylvestris (C.C. Gmel.) Hegi) Populations in Georgia (South Caucasus). Plants 2025, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Valamoti, S.M.; Pagnoux, C.; Ntinou, M.; Bouby, L.; Bonhomme, V.; Terral, J.-F. More than meets the eye: New archaeobotanical evidence on Bronze Age viticulture and wine making in the Peloponnese, Greece. Veg. Hist. Archaeobot. 2020, 29, 35–50. [Google Scholar] [CrossRef]
- Garnier, N.; Valamoti, S.M. Prehistoric wine-making at Dikili Tash (Northern Greece): Integrating residue analysis and archaeobotany. J. Archaeol. Sci. 2016, 74, 195–206. [Google Scholar] [CrossRef]
- Vaporidis, A.Z. Lykourgos, the King of Mt Pangaion, and Other Myths of the Area; Kapa Ekdotiki: Peristeri, Greece, 2015. (In Greek) [Google Scholar]
- Valamoti, S.M. Harvesting the ‘wild’? Exploring the context of fruit and nut exploitation at Neolithic Dikili Tash, with special reference to wine. Veg. Hist. Archaeobot. 2015, 24, 35–46. [Google Scholar] [CrossRef]
- Pagnoux, C.; Bouby, L.; Valamoti, S.M.; Bonhomme, V.; Ivorra, S.; Gkatzogia, E.; Karathanou, A.; Kotsachristou, D.; Kroll, H.; Terral, J.F. Local domestication or diffusion? Insights into viticulture in Greece from Neolithic to Archaic times, using geometric morphometric analyses of archaeological grape seeds. J. Archaeol. Sci. 2021, 125, 105263. [Google Scholar] [CrossRef]
- Nistelberger, H.M.; Smith, O.; Wales, N.; Star, B.; Boessenkool, S. The efficacy of high-throughput sequencing and target enrichment on charred archaeobotanical remains. Sci. Rep. 2016, 6, 37347. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, B.C. Les Vignes Sauvages (Vitis vinifera L. ssp. silvestris Gmel.) en tant que Matériel Primitif Viticole en Grèce; Scientific Annals of the Aristotle University, Department of Agriculture and Forest: Thessaloniki, Greece, 1962; pp. 1–43. [Google Scholar]
- Boursiquot, J.M.; Lacombe, T.; Laucou, V.; Bakasietas, K. La “Vigne de Pausanias”: Mythe et ADN. Food Hist. 2013, 11, 27–34. [Google Scholar] [CrossRef]
- Biniari, K.; Stavrakakis, M.N. The “vine of Pafsanias” and the group of grape cultivars ‘Mavroudia’ of the vineyard of Peloponnese. In 3rd International Symposium: Trends in World Vitiviniculture Development; Ampelos: Santorini, Greece, 2013. [Google Scholar]
- Srivastava, R.; Bazakos, C.; Tsachaki, M.; Žanko, D.; Kalantidis, K.; Tsiantis, M.; Laurent, S. Genealogical Analyses of 3 Cultivated and 1 Wild Specimen of Vitis vinifera from Greece. Genome Biol. Evol. 2023, 15, evad226. [Google Scholar] [CrossRef]
- Lukšić, K.; Zdunić, G.; Hančević, K.; Mihaljević, M.Ž.; Mucalo, A.; Maul, E.; Riaz, S.; Pejić, I. Identifcation of powdery mildew resistance in wild grapevine (Vitis vinifera subsp. sylvestris Gmel Hegi) from Croatia and Bosnia and Herzegovina. Sci. Rep. 2022, 12, 2128. [Google Scholar] [CrossRef]
- Margaryan, K.; Töpfer, R.; Gasparyan, B.; Arakelyan, A.; Trapp, O.; Röckel, F.; Maul, E. Wild grapes of Armenia: Unexplored source of genetic diversity and disease resistance. Front. Plant Sci. 2023, 14, 1276764. [Google Scholar] [CrossRef]
- Cordero-Bueso, G.; Vigentini, I.; Foschino, R.; Maghradze, D.; Cantoral, J.M. Diversidad genética de levaduras aisladas a partir de uvas de Vitis vinifera ssp. sylvestris (Gmelin) Hegi en el área Euroasiática [Genetic diversity of yeasts isolated from Eurasian populations of Vitis vinifera ssp. sylvestris (Gmelin) Hegi]. BIO Web Conf. 2017, 9, 02019. [Google Scholar] [CrossRef]
- Cantu, D.; Massonnet, M.; Cochetel, N. The wild side of grape genomics. Trends Genet. 2024, 40, 601–612. [Google Scholar] [CrossRef]
- Röckel, F.; Margaryan, K.; Merkouropoulos, G.; Laucou, V.; de Lorenzis, G.; Tello, J.; Zdunić, G.; de Andrés, M.; Baeta, F.; Cunha, J.; et al. A population genetic study of Vitis vinifera L. subsp. sylvestris Gmelin based on 3.000 individuals from 20 countries. In Proceedings of the 45th World Congress of Vine and Wine, Dijon, France, 14–18 October 2024. [Google Scholar]
- Pizzi, S.; Conti, A.; Di Canito, A.; Casagrande Pierantoni, D.; Foschino, R.; Setati, M.E.; Vigentini, I. Endophytic diversity in Vitis vinifera with different vineyard managements and Vitis sylvestris populations from northern Italy: A comparative study of culture-dependent and amplicon sequencing methods. Biology 2025, 14, 293. [Google Scholar] [CrossRef] [PubMed]
- Belkina, D.; Stepanov, I.; Makarkina, M.; Porotikova, E.; Lifanov, I.; Kozhevnikov, E.; Gorislavets, S.; Vinogradova, S. In-depth population genetic study of Vitis vinifera ssp. sylvestris from the Black Sea region and its virome. Front. Plant Sci. 2025, 16, 1536862. [Google Scholar] [CrossRef]
- Maghradze, D.; Melyan, G.; Salimov, V.; Chipashvili, R.; Miñiguez, M.; Puras, P.; Melendez, E.; Vaca, R.; Ocete, C.; Rivera, D.; et al. Wild grapevine (Vitis sylvestris C.C.Gmel.) wines from the Southern Caucasus region. OENO One 2020, 54, 849–862. [Google Scholar] [CrossRef]
- Maghradze, D.; Kikilashvili, S.; Gotsiridze, O.; Maghradze, T.; Fracassetti, D.; Failla, O.; Rustioni, L. Comparison between the grape technological characteristics of Vitis vinifera subsp. sylvestris and subsp. sativa. Agronomy 2021, 11, 472. [Google Scholar] [CrossRef]
- Hajnalová, M.; Látková, M.; Kajanová, M.; Eliáš, j.P.; Ďurišová, L. Wild or cultivated? A study of Vitis sylvestris in natura in Slovakia and implications for archaeology and archaeobotany (morphometric approach). Veg. Hist. Archaeobot. 2023, 32, 321–337. [Google Scholar] [CrossRef]
- Beukers, M.; Hondelink, M. Grape (Vitis vinifera) use in the early modern Low Countries: A tentative combination of aDNA-analysis and historical sources. Veg. Hist. Archaeobot. 2025. [Google Scholar] [CrossRef]
- Thomas, M.R.; Scott, N.S. Microsatellite repeats in grapevine reveal DNA polymorphisms when analyzed as Sequence-Tagged Sites (STSs). Theor. Appl. Genet. 1993, 86, 985–990. [Google Scholar] [CrossRef]
- Bowers, J.E.; Dangl, G.S.; Vignani, R.; Meredith, C.P. Isolation and characterization of new polymorphic simple sequence repeat loci in grape (Vitis vinifera L). Genome 1996, 39, 628–633. [Google Scholar] [CrossRef]
- Bowers, J.E.; Dangl, G.S.; Meredith, C.P. Development and characterization of additional microsatellite DNA markers for grape. Am. J. Enol. Vitic. 1999, 50, 243–246. [Google Scholar] [CrossRef]
- Sefc, K.M.; Regner, F.; Turetschek, E.; Glossl, J.; Steinkellner, H. Identification of microsatellite sequences in Vitis riparia and their applicability for genotyping of different Vitis species. Genome 1999, 42, 367–373. [Google Scholar] [CrossRef]
- Maul, E.; Topfer, R. Vitis International Variety Catalogue (VIVC): A cultivar database referenced by genetic profiles and morphology. BIO Web Conf. 2015, 5, 01009. [Google Scholar] [CrossRef]
- Merkouropoulos, G.; Michailidou, S.; Alifragkis, A.; Zioziou, E.; Koundouras, S.; Argiriou, A.; Nicolaou, N. A combined approach involving ampelographic description, berry oenological traits and molecular analysis to study native grapevine varieties of Greece. Vitis 2015, 54, 99–103. [Google Scholar]
- Margaryan, K.; Melyan, G.; Röckel, F.; Topfer, R.; Maul, E. Genetic diversity of Armenian grapevine (Vitis vinifera L.) germplasm: Molecular characterization and parentage analysis. Biology 2021, 10, 1279. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research–an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 583–590. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Res. 2010, 10, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Earl, D.A.; von Holdt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. CLUMPAK: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Perko, A.; Trapp, O.; Maul, E.; Rockel, F.; Piltaver, A.; Vršic, S. Monitoring and Genotyping of Wild Grapevine (Vitis vinifera L. subsp. sylvestris) in Slovenia. Plants 2024, 13, 1234. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merkouropoulos, G.; Ganopoulos, I.; Doupis, G.; Maul, E.; Röckel, F. Molecular Characterization and Ex Situ Conservation of Wild Grapevines Grown in the Area Around the Neolithic Settlement of Dikili Tash, Greece. Agriculture 2025, 15, 1301. https://doi.org/10.3390/agriculture15121301
Merkouropoulos G, Ganopoulos I, Doupis G, Maul E, Röckel F. Molecular Characterization and Ex Situ Conservation of Wild Grapevines Grown in the Area Around the Neolithic Settlement of Dikili Tash, Greece. Agriculture. 2025; 15(12):1301. https://doi.org/10.3390/agriculture15121301
Chicago/Turabian StyleMerkouropoulos, Georgios, Ioannis Ganopoulos, Georgios Doupis, Erika Maul, and Franco Röckel. 2025. "Molecular Characterization and Ex Situ Conservation of Wild Grapevines Grown in the Area Around the Neolithic Settlement of Dikili Tash, Greece" Agriculture 15, no. 12: 1301. https://doi.org/10.3390/agriculture15121301
APA StyleMerkouropoulos, G., Ganopoulos, I., Doupis, G., Maul, E., & Röckel, F. (2025). Molecular Characterization and Ex Situ Conservation of Wild Grapevines Grown in the Area Around the Neolithic Settlement of Dikili Tash, Greece. Agriculture, 15(12), 1301. https://doi.org/10.3390/agriculture15121301






