Effects of Tillage Systems and Bacterial Inoculation on Enzyme Activities and Selected Soil Chemical Properties
Abstract
1. Introduction
2. Materials and Methods
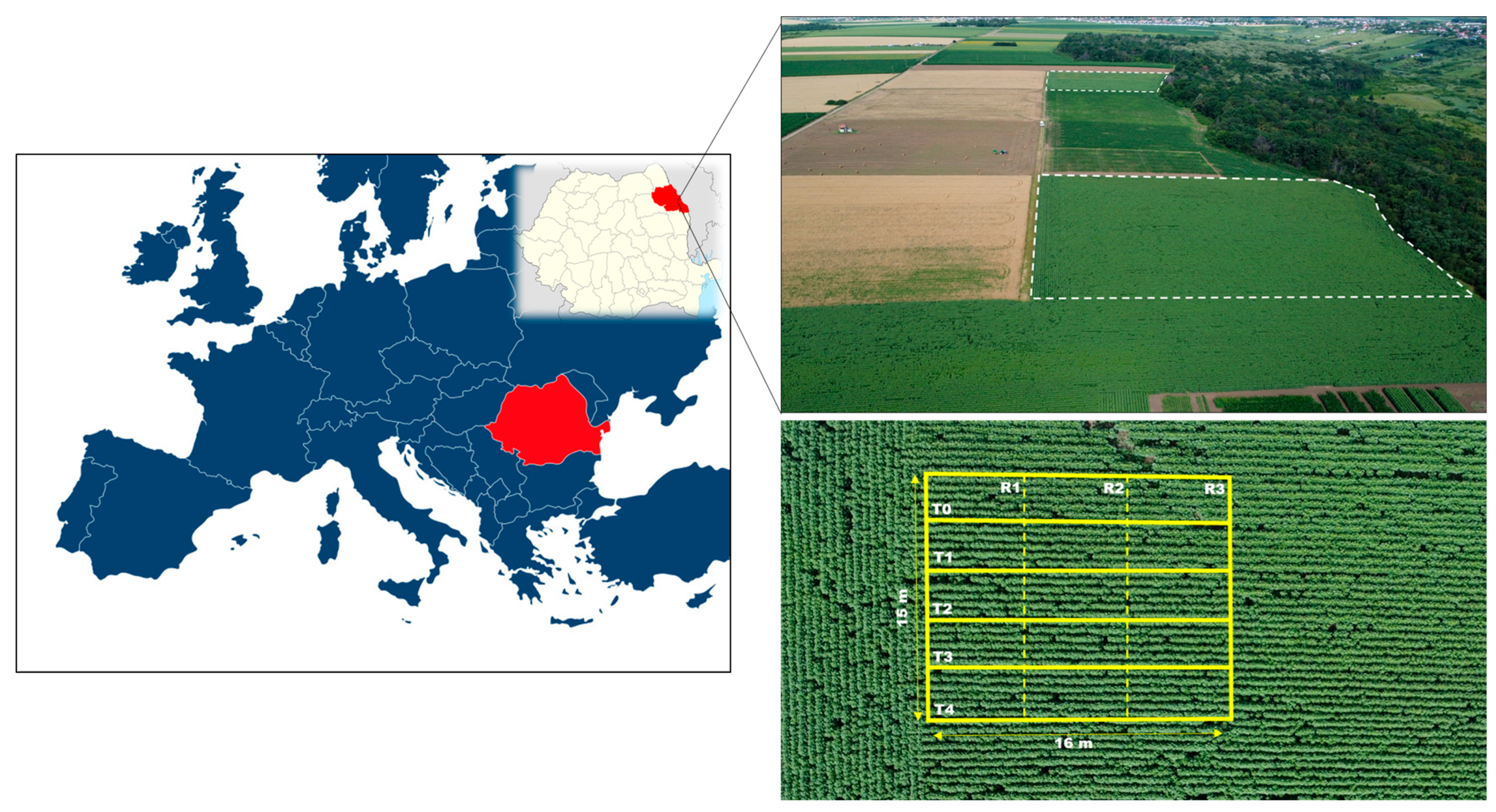
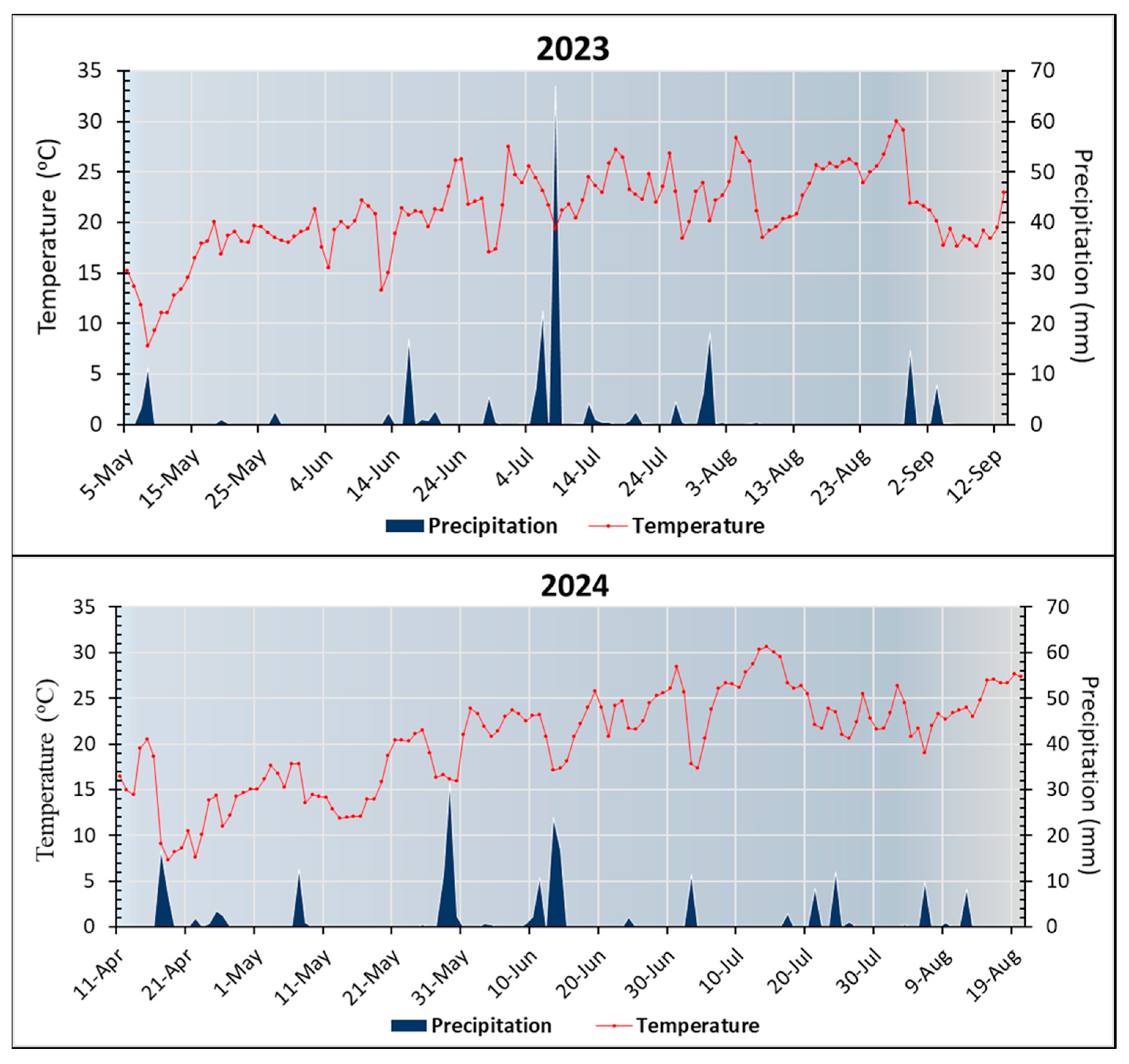
2.1. Experimental Site and Field Experiment Design
2.2. Soil Sampling
2.3. Soil Analysis
2.4. Statistical Data Processing
3. Results
3.1. Soil Enzymatic Activity
3.1.1. Dehydrogenase Activity
3.1.2. Catalase Activity
3.1.3. Acid Phosphatase Activity
3.1.4. Alkaline Phosphatase Activity
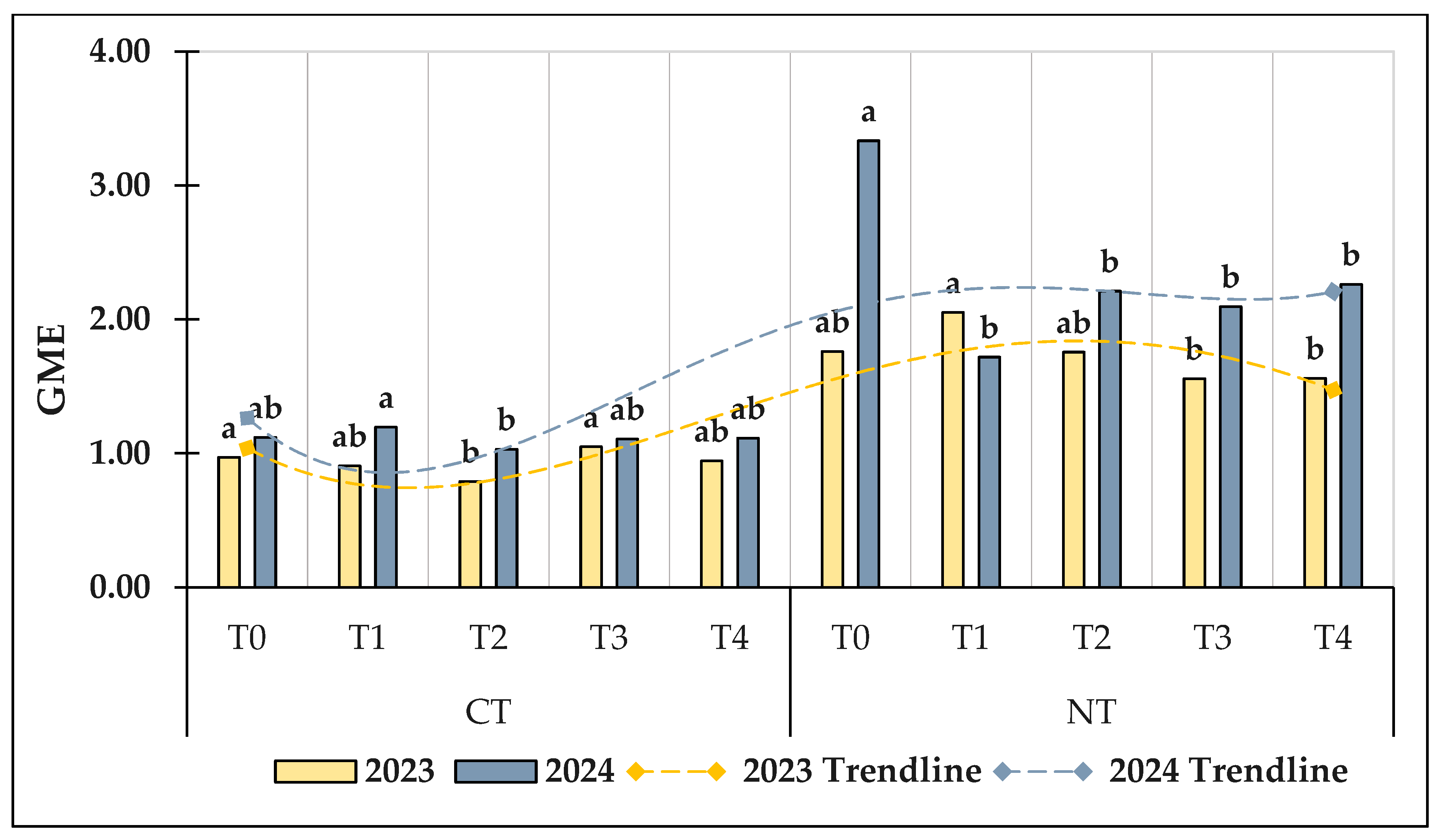
3.1.5. Geometric Mean of Studied Enzymes (GME)
3.1.6. Effects of Tillage, Fertilization, and Their Interaction on Soil Enzymatic Activity
3.1.7. Soil Enzyme Activity Correlation
3.2. Soil Chemical Properties
3.2.1. Soil pH, Organic Carbon (SOC), and Total Nitrogen (TN)
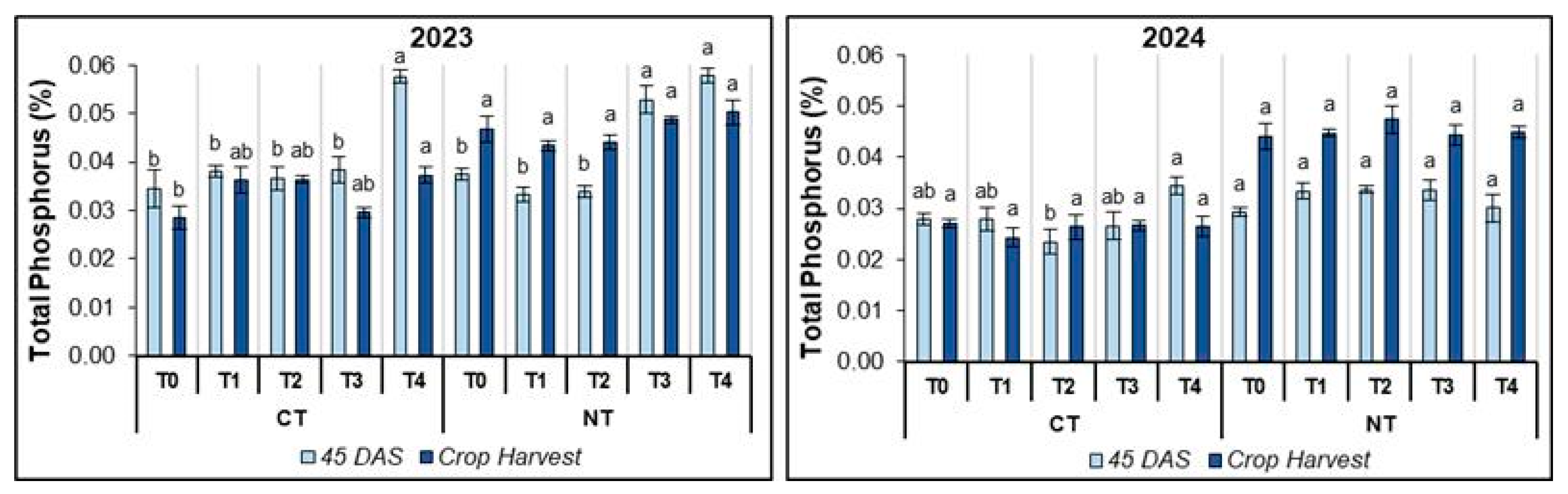
3.2.2. Soil Total Phosphorus (TP)
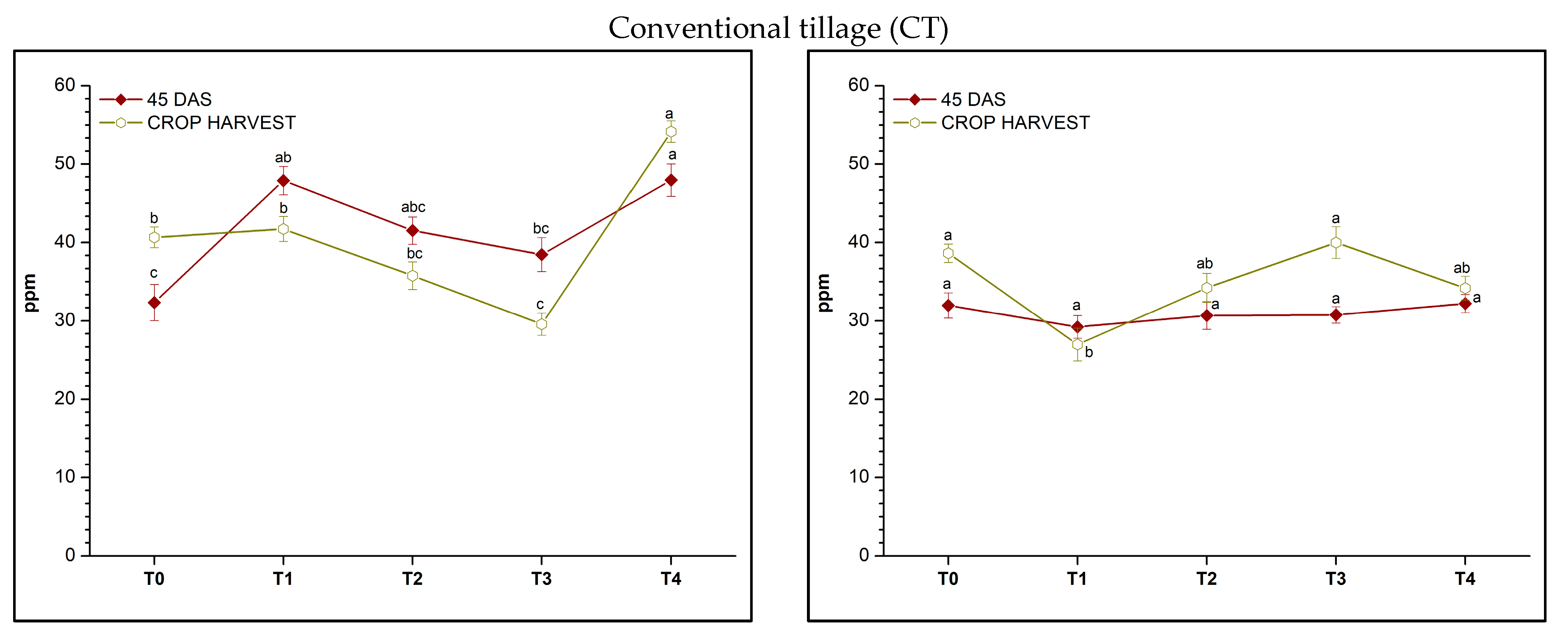
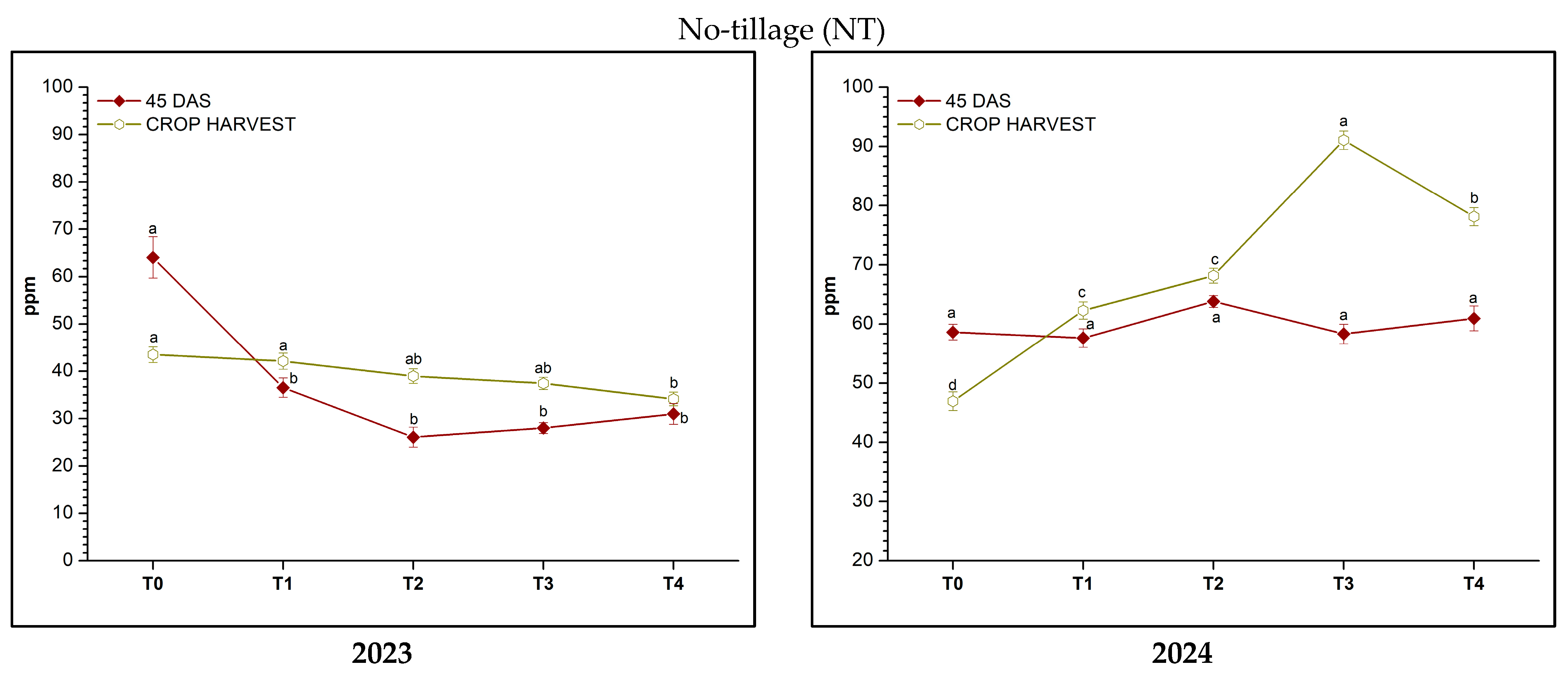
3.2.3. Soil Available Phosphorus (AP)
3.2.4. Effects of Tillage, Fertilization, and Their Interaction on Soil Chemical Properties
3.2.5. Soil Chemical Properties Correlation
4. Discussion
4.1. Soil Enzyme Responses to Fertilization and Tillage Systems
4.2. Soil Chemical Properties in Response to Fertilization and Tillage Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Cline, R.G.; Harris, R.F.; Schuman, G.E. Soil quality: A concept, definition, and framework for evaluation. Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef]
- Garrigues, E.; Corson, M.S.; Angers, D.A.; van der Werf, H.M.; Walter, C. Soil quality in Life Cycle Assessment: Towards development of an indicator. Ecol. Indic. 2012, 18, 434–442. [Google Scholar] [CrossRef]
- Stockdale, E.A.; Shepherd, M.A.; Fortune, S.; Cuttle, S.P. Soil fertility in organic farming systems—Fundamentally different? Soil Use Manag. 2002, 18, 301–308. [Google Scholar] [CrossRef]
- Szostek, M.; Szpunar-Krok, E.; Pawlak, R.; Stanek-Tarkowska, J.; Ilek, A. Effect of Different Tillage Systems on Soil Organic Carbon and Enzymatic Activity. Agronomy 2022, 12, 208. [Google Scholar] [CrossRef]
- Lv, L.; Gao, Z.; Liao, K.; Zhu, Q.; Zhu, J. Impact of conservation tillage on the distribution of soil nutrients with depth. Soil Tillage Res. 2023, 225, 105527. [Google Scholar] [CrossRef]
- Topa, D.; Cara, I.G.; Jităreanu, G. Long term impact of different tillage systems on carbon pools and stocks, soil bulk density, aggregation and nutrients: A field meta-analysis. Catena 2021, 199, 105102. [Google Scholar] [CrossRef]
- Mihu, G.-D.; Aostăcioaei, T.G.; Ghelbere, C.; Calistru, A.-E.; Țopa, D.C.; Jităreanu, G. Exploring Soil Hydro-Physical Improvements Under No-Tillage: A Sustainable Approach for Soil Health. Agriculture 2025, 15, 981. [Google Scholar] [CrossRef]
- Cakpo, S.S.; Aostăcioaei, T.G.; Mihu, G.-D.; Molocea, C.-C.; Ghelbere, C.; Ursu, A.; Țopa, D.C. Long-Term Effect of Tillage Practices on Soil Physical Properties and Winter Wheat Yield in North-East Romania. Agriculture 2025, 15, 989. [Google Scholar] [CrossRef]
- da Silva, M.F.; Fernandes, M.M.H.; Fernandes, C.; da Silva, A.M.R.; Ferraudo, A.S.; Coelho, A.P. Contribution of tillage systems and crop succession to soil structuring. Soil Tillage Res. 2021, 209, 104924. [Google Scholar] [CrossRef]
- Zhang, Y.; Dalal, R.C.; Bhattacharyya, R.; Meyer, G.; Wang, P.; Menzies, N.W.; Kopittke, P.M. Effect of long-term no-tillage and nitrogen fertilization on phosphorus distribution in bulk soil and aggregates of a Vertisol. Soil Tillage Res. 2021, 205, 104760, ISSN 0167-1987. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Lü, Y.; Zhang, X.; Liang, W. Contributions of soil biota to C sequestration varied with aggregate fractions under different tillage systems. Soil Biol. Biochem. 2013, 62, 147–156. [Google Scholar] [CrossRef]
- Zikeli, S.; Gruber, S. Reduced Tillage and No-Till in Organic Farming Systems, Germany—Status Quo, Potentials and Challenges. Agriculture 2017, 7, 35. [Google Scholar] [CrossRef]
- Sadiq, M.; Rahim, N.; Tahir, M.M.; Alasmari, A.; Alqahtani, M.M.; Albogami, A.; Ghanem, K.Z.; Abdein, M.A.; Ali, M.; Mehmood, N.; et al. Conservation tillage: A way to improve yield and soil properties and decrease global warming potential in spring wheat agroecosystems. Front. Microbiol. 2024, 15, 1356426. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Iqbal, A.; He, L.; Zhao, Q.; Wei, S.; Ali, I.; Ullah, S.; Yan, B.; Jiang, L. Long-term no-tillage and straw retention management enhances soil bacterial community diversity and soil properties in southern China. Agronomy 2020, 10, 1233. [Google Scholar] [CrossRef]
- Qiu, Z.; Egidi, E.; Liu, H.; Kaur, S.; Singh, B.K. New frontiers in agriculture productivity: Optimised microbial inoculants and in situ microbiome engineering. Biotechnol. Adv. 2019, 37, 107371. [Google Scholar] [CrossRef]
- Kavadia, A.; Omirou, M.; Fasoula, D.; Ioannides, I.M. The importance of microbial inoculants in a climate-changing agriculture in eastern Mediterranean region. Atmosphere 2020, 11, 1136. [Google Scholar] [CrossRef]
- Muniswami, D.M.; Chinnadurai, S.; Sachin, M.; Jithin, H.; Ajithkumar, K.; Narayanan, G.S.; Gopinath, B.S.; Rasheeq, A.A.; Preethi, B.A.; Dineshkumar, R. Comparative study of biofertilizer/biostimulant from seaweeds and seagrass in Abelmoschus esculentus crop. Biomass Convers. Biorefin. 2023, 13, 11005–11022. [Google Scholar] [CrossRef]
- Chauhan, A.; Siani, R.; Sharma, J.C. Plant growth promoting rhizobacteria and their biological properties for soil enrichment and growth promotion. J. Plant Nutr. 2021, 45, 273–299. [Google Scholar] [CrossRef]
- Bechtaoui, N.; Rabiu, M.K.; Raklami, A.; Oufdou, K.; Hafidi, M.; Jemo, M. Phosphate-Dependent Regulation of Growth and Stresses Management in Plants. Front. Plant Sci. 2021, 12, 679916. [Google Scholar] [CrossRef]
- Pang, F.; Li, Q.; Solanki, M.K.; Wang, Z.; Xing, Y.-X.; Dong, D.-F. Soil phosphorus transformation and plant uptake driven by phosphate-solubilizing microorganisms. Front. Microbiol. 2024, 15, 1383813. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, J.; Puga, M.I.; Rojas-Triana, M.; Martinez-Hevia, I.; Diaz, S.; Poza-Carrión, C.; Miñambres, M.; Leyva, A. Plant Adaptation to Low Phosphorus Availability: Core Signaling, Crosstalks, and Applied Implications. Mol. Plant 2022, 15, 104–124. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.N. Plant microbiomes for sustainable agriculture: Current research and future challenges. In Plant Microbiomes for Sustainable Agriculture; Yadav, A., Singh, J., Rastegari, A., Yadav, N., Eds.; Springer: Cham, Switzerland, 2020; pp. 475–482. [Google Scholar]
- Yadav, A.N.; Kumar, R.; Kumar, S.; Kumar, V.; Sugitha, T.; Singh, B.; Chauhan, V.; Dhaliwal, H.S.; Saxena, A.K. Beneficial microbiomes: Biodiversity and potential biotechnological applications for sustainable agriculture and human health. J. Appl. Biol. Biotechnol. 2017, 5, 45–57. [Google Scholar]
- Biedendieck, R.; Knuuti, T.; Moore, S.J.; Jahn, D. The “beauty in the beast”—The multiple uses of Priestia megaterium in biotechnology. Appl. Microbiol. Biotechnol. 2021, 105, 5719–5737. [Google Scholar] [CrossRef]
- Biswas, J.K.; Banerjee, A.; Rai, M.; Naidu, R.; Biswas, B.; Vithanage, M.; Dash, M.C.; Sarkar, S.K.; Meers, E. Potential application of selected metal resistant phosphate solubilizing bacteria isolated from the gut of earthworm (Metaphire posthuma) in plant growth promotion. Geoderma 2018, 330, 117–124. [Google Scholar] [CrossRef]
- Aliyat, F.Z.; Maldani, M.; El Guilli, M.; Nassiri, L.; Ibijbijen, J. Phosphate-solubilizing bacteria isolated from phosphate solid sludge and their ability to solubilize three inorganic phosphate forms: Calcium, iron, and aluminum phosphates. Microorganisms 2022, 10, 980. [Google Scholar] [CrossRef]
- Singh, P.; Banik, R.M. Effect of purified alkaline phosphatase from Bacillus licheniformis on growth of Zea mays L. Plant Sci. Today 2019, 6, 583–589. [Google Scholar]
- Shrivastava, M.; Srivastava, P.C.; D’souza, S.F. Phosphate-solubilizing microbes: Diversity and phosphates solubilization mechanism. In Role of Rhizospheric Microbes in Soil: Volume 2: Nutrient Management and Crop Improvement; Springer: Singapore, 2018; pp. 137–165. [Google Scholar]
- Maurya, S.; Abraham, J.S.; Somasundaram, S.; Toteja, R.; Gupta, R.; Makhija, S. Indicators for assessment of soil quality: A mini-review. Environ. Monit. Assess. 2020, 192, 604. [Google Scholar] [CrossRef]
- Kobierski, M.; Lemanowicz, J.; Wojewódzki, P.; Kondratowicz-Maciejewska, K. The Effect of Organic and Conventional Farming Systems with Different Tillage on Soil Properties and Enzymatic Activity. Agronomy 2020, 10, 1809. [Google Scholar] [CrossRef]
- Gajda, A.M.; Przewloka, B.; Gawryjolek, K. Changes in soil quality associated with tillage system applied. Int. Agrophys. 2013, 27, 133–141. [Google Scholar] [CrossRef]
- Erdel, E.; Şimşek, U.; Kesimci, T.G. Effects of Fungi on Soil Organic Carbon and Soil Enzyme Activity under Agricultural and Pasture Land of Eastern Türkiye. Sustainability 2023, 15, 1765. [Google Scholar] [CrossRef]
- Bielińska, E.J.; Mocek-Płóciniak, A. Impact of the tillage system on the soil enzymatic activity. Arch. Environ. Prot. 2012, 38, 75–82. [Google Scholar] [CrossRef]
- Zhu, B.; Gu, H.; He, J.; Li, F.; Yu, J.; Liu, W.; Chen, Q.; Lai, Y.; Yu, S. The impact of smash-ridge tillage on agronomic traits of tobacco plants, soil enzymatic activity, microbial community structure, and functional diversity. Plant Signal. Behav. 2023, 18, 2260640. [Google Scholar] [CrossRef] [PubMed]
- Mijangos, I.; Pérez, R.; Albizu, I.; Garbisu, C. Effects of fertilization and tillage on soil biological parameters. Enzyme Microb. Technol. 2006, 40, 100–106. [Google Scholar] [CrossRef]
- Seifert, S.; Shaw, D.R.; Zablatowicz, R.M.; Wesley, R.A.; Kingery, W.L. Effect of tillage on microbial characteristics and herbicide degradation in a Sharkey clay soil. Weed Sci. 2001, 49, 685–693. [Google Scholar] [CrossRef]
- Available online: www.fieldclimate.com (accessed on 12 March 2025).
- Lorenz, N.; Dick, R.P. Sampling and pretreatment of soil before enzyme analysis. Methods Soil Enzymol. 2011, 9, 85–101. [Google Scholar]
- Casida Jr, L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Furtak, K.; Gajda, A.M. Activity of dehydrogenases as an indicator of soil environment quality. Pol. J. Soil Sci. 2017, 50, 33–40. [Google Scholar] [CrossRef]
- Johnson, J.L.; Temple, K.L. Some variables affecting the measurement of “catalase activity” in soil. Soil Sci. Soc. Am. J. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Aruna Kumari, J.; Rao, P.C.; Padmaja, G.; Madhavi, M. Effect of Temperature on Soil Enzyme Acid Phosphatase. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1234–1240. [Google Scholar]
- Hinojosa, M.; Carreira, J.A.; Garcła-Rułz, R. Soil moisture pre-treatment effects on enzyme activities as indicators of heavy metal-contaminated and reclaimed soils. Soil Biol. Biochem. 2004, 36, 1559–1568. [Google Scholar] [CrossRef]
- ISO 7184/14-79; Soils. Determination of total phosphorus. Romanian Association for Standardization (ASRO): Bucharest, Romania, 1979.
- Rusu, M.; Cara, I.-G.; Stoica, F.; Țopa, D.; Jităreanu, G. Quality Parameters of Plum Orchard Subjected to Conventional and Ecological Management Systems in Temperate Production Area. Horticulturae 2024, 10, 907. [Google Scholar] [CrossRef]
- Calistru, A.E.; Filipov, F.; Cara, I.G.; Cioboată, M.; Topa, D.; Jităreanu, G. Tillage and Straw Management Practices Influences Soil Nutrient Distribution: A Case Study from North-Eastern Romania. Land 2024, 13, 625. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, M.-S.; Kim, J.-G.; Kim, S.-O. Use of Soil Enzymes as Indicators for Contaminated Soil Monitoring and Sustainable Management. Sustainability 2020, 12, 8209. [Google Scholar] [CrossRef]
- Niewiadomska, A.; Płaza, A.; Wolna-Maruwka, A.; Budka, A.; Głuchowska, K.; Rudziński, R.; Kaczmarek, T. Consortia of plant growth-promoting rhizobacteria and selected catch crops for increasing microbial activity in soil under spring barley grown as an organic farming system. Appl. Sci. 2023, 13, 5120. [Google Scholar] [CrossRef]
- Utobo, E.B.; Tewari, L. Soil enzymes as bioindicators of soil ecosystem status. Appl. Ecol. Environ. Res. 2015, 13, 147–169. [Google Scholar]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Samuel, A.D.; Mondici, S.; Brejea, R. Evaluation of soil enzyme activities under different croplands. Rom. Agric. Res. 2021, 38, 271–279. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Zwydak, M. The Relationship Between Soil Properties, Enzyme Activity and Land Use; Poland Forest Research Institute: Sekocin Stary, Poland, 2017. [Google Scholar]
- Wolińska, A.; Stępniewska, Z. Dehydrogenase activity in the soil environment. Dehydrogenases 2012, 10, 183–210. [Google Scholar]
- Bayraklı, B. Effect of Bacillus megaterium var. phosphaticum applied together with rock phosphate on wheat yield and some soil properties in a calcareous soil. Eurasian J. Soil Sci. 2022, 11, 198–205. [Google Scholar]
- Bai, B.; Yang, X.; Zhao, Q.; Liu, R.; Ren, J. Inoculations with Pseudomonas fluorescens and Bacillus cereus affect the soil enzyme activity, growth and rhizosphere microbial diversity of Taxus chinensis var. mairei. Plant Soil 2020, 455, 41–52. [Google Scholar] [CrossRef]
- Mohammadi, K.; Heidari, G.; Javaheri, M.; Karimi Nezhad, M.T. Soil microbial response to tillage systems and fertilization in a bac rhizosphere. Arch. Agron. Soil Sci. 2012, 59, 899–910. [Google Scholar] [CrossRef]
- Furtak, K.; Gałązka, A. Enzymatic activity as a popular parameter used to determine the quality of the soil environment. Pol. J. Agron. 2019, 37, 22–30. [Google Scholar]
- Burns, R.G.; Dick, R.P. Enzymes in the Environment: Activity, Ecology, and Applications; Marcel Dekker Inc.: New York, NY, USA, 2002; p. 614. [Google Scholar]
- Dunca, S.; Ștefan, M.; Nimițan, E.; Ailiesei, O. Microbiological Study of a Bacillus megaterium Strain with Soil Phosphorus Solubilization Potential. Rom. J. Biol.—Plant Biol. 2005, 49–50, 31–39. [Google Scholar]
- Saeed, S.W.Z.; Naseer, I.; Zahir, Z.A.; Hilger, T.; Shahid, S.; Iqbal, Z.; Ahmad, M. Bacillus Strains with Catalase Enzyme Improve the Physiology and Growth of Rice (Oryza sativa L.). Stresses 2023, 3, 736–748. [Google Scholar] [CrossRef]
- Su-mei, Z.; Man, Z.; Ke-ke, Z.; Xi-wen, Y.; De-xian, H.; Jun, Y.; Chen-yang, W. Effects of reduced nitrogen and suitable soil moisture on wheat (Triticum aestivum L.) rhizosphere soil microbiological, biochemical properties and yield in the Huanghuai Plain, China. J. Integr. Agric. 2020, 19, 234–250. [Google Scholar]
- Muhammad, I.; Yang, L.; Ahmad, S.; Zeeshan, M.; Farooq, S.; Ali, I.; Khan, A.; Zhou, X.B. Irrigation and Nitrogen Fertilization Alter Soil Bacterial Communities, Soil Enzyme Activities, and Nutrient Availability in Maize Crop. Front. Microbiol. 2022, 13, 833758. [Google Scholar] [CrossRef]
- Szczepanek, M.; Siwik-Ziomek, A.; Lemańczyk, G.; Lamparski, R.; Graczyk, R. Effect of Reduced Tillage on Soil Enzyme Activity, Pests Pressure and Productivity of Organically Grown Spring Wheat Species. Agronomy 2023, 13, 287. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, T.; Sha, G.; Zhu, Q.; Liu, Z.; Ren, K.; Yang, C. Soil enzyme activities of typical plant communities after vegetation restoration on the Loess Plateau, China. Appl. Soil Ecol. 2022, 170, 104292. [Google Scholar] [CrossRef]
- Ren, C.; Kang, D.; ping Wu, J.; Zhao, F.; Yang, G.; Han, X.; Ren, G. Temporal variation in soil enzyme activities after afforestation in the Loess Plateau, China. Geoderma 2016, 282, 103–111. [Google Scholar] [CrossRef]
- Ge, G.F.; Li, Z.J.; Zhang, J.; Wang, L.G.; Xu, M.G.; Zhang, J.B.; Wang, J.K.; Xie, X.L.; Liang, Y.C. Geographical and climatic differences in long-term effect of organic and inorganic amendments on soil enzymatic activities and respiration in field experimental stations of China. Ecol. Complex. 2009, 6, 421–431. [Google Scholar] [CrossRef]
- Khatun, M.R.; Mukta, R.H.; Islam, M.A.; Hud, A.N. Insight into Citric Acid-Induced Chromium Detoxification in Rice (Oryza sativa L). Int. J. Phytoremediat. 2019, 21, 1234–1240. [Google Scholar] [CrossRef]
- Habbib, H.; Verzeaux, J.; Nivelle, E.; Roger, D.; Lacoux, J.; Catterou, M.; Hirel, B.; Dubois, F.; Tétu, T. Conversion to No-Till Improves Maize Nitrogen Use Efficiency in a Continuous Cover Cropping System. PLoS ONE 2016, 11, e0164234. [Google Scholar] [CrossRef]
- Tan, X.; Xie, B.; Wang, J.; He, W.; Wang, X.; Wei, G. County-Scale spatial distribution of soil enzyme activities and enzyme activity indices in agricultural land: Implications for soil quality assessment. Sci. World J. 2014, 2014, 535768. [Google Scholar] [CrossRef]
- Suh, J.S.; Kim, S.J.; Noh, H.J.; Kwon, J.S.; Jung, W.K. Long-term Composting and Fertilization Impact on Dehydrogenase-producing Bacteria and Dehydrogenase Activity in Rice Paddy Soil. Korean J. Soil Sci. Fertil. 2007, 40, 229–233. [Google Scholar]
- Micuți, M.M.; Bădulescu, L.; Burlacu, A.; Israel-Roming, F. Activity of peroxidase and catalase in soils as influenced by some insecticides and fungicides. AgroLife Sci. J. 2018, 7. Available online: https://agrolifejournal.usamv.ro/index.php/agrolife/article/view/232 (accessed on 12 March 2025).
- Piotrowska-Długosz, A.; Kobierski, M.; Długosz, J. Enzymatic Activity and Physicochemical Properties of Soil Profiles of Luvisols. Materials 2021, 14, 6364. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Aparna, K.; Dotaniya, C.K.; Singh, M.; Regar, K.L. Role of soil enzymes in sustainable crop production. In Enzymes in Food Biotechnology: Production, Applications, and Future Prospects; Elsevier: Amsterdam, The Netherlands, 2018; pp. 569–589. [Google Scholar]
- Piotrowska Długosz, A.; Charzyński, P. The impact of the soil sealing degree on microbial biomass, enzymatic activity, and physicochemical properties in the Ekranic Technosols of Toruń (Poland). J. Soils Sediments 2015, 15, 47–59. [Google Scholar] [CrossRef]
- Criquet, S.; Ferre, E.; Farner, E.M.; Le Petit, J. Annual dynamics of phosphatase activities in an evergreen oak litter—Influence of biotic and abiotic factors. Soil Biol. Biochem. 2004, 36, 1111–1118. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Yadav, R.S.; Meena, S.C. Comparative efficiency of acid phosphatase originated from plant and fungal sources. J Plant Nutr. Soil Sci. 2001, 164, 279–282. [Google Scholar] [CrossRef]
- Długosz, J. Different Response of Carbon and P-Related Soil Properties toward Fertilization in Red Soil. Agronomy 2023, 13, 2751. [Google Scholar] [CrossRef]
- Janes-Bassett, V.; Blackwell, M.S.; Blair, G.; Davies, J.; Haygarth, P.M.; Mezeli, M.M.; Stewart, G. A meta-analysis of phosphatase activity in agricultural settings in response to phosphorus deficiency. Soil Biol. Biochem. 2022, 165, 108537. [Google Scholar] [CrossRef]
- Ibarra-Galeana, J.A.; Castro-Martínez, C.; Fierro-Coronado, R.A.; Armenta-Bojórquez, A.D.; Maldonado-Mendoza, I.E. Characterization of phosphate-solubilizing bacteria exhibiting the potential for growth promotion and phosphorus nutrition improvement in maize (Zea mays L.) in calcareous soils of Sinaloa, Mexico. Ann. Microbiol. 2017, 67, 801–811. [Google Scholar] [CrossRef]
- Widdig, M.; Schleuss, P.M.; Weig, A.R.; Guhr, A.; Biederman, L.A.; Borer, E.T.; Crawley, M.J.; Kirkman, K.P.; Seabloom, E.W.; Wragg, P.D.; et al. Nitrogen and Phosphorus Additions Alter the Abundance of Phosphorus-Solubilizing Bacteria and Phosphatase Activity in Grassland Soils. Front. Environ. Sci. 2019, 7, 185. [Google Scholar] [CrossRef]
- Kalembasa, S.; Symanowicz, B. Enzymatic activity of soil after applying various waste organic materials, ash, and mineral fertilizers. Pol. J. Environ. Stud. 2012, 21, 1635–1641. [Google Scholar]
- Mohammadi, K.; Heidari, G.; Karimi Nezhad, M.T.; Ghamari, S.; Sohrabi, Y. Contrasting soil microbial responses to fertilization and tillage systems in canola rhizosphere. Saudi J. Biol. Sci. 2012, 19, 377–383. [Google Scholar] [CrossRef]
- Avasilcai, L.; Bireescu, G.; Vieriu, M.; Bibire, N.; Crivoi, F.; Cioanca, O.; Morariu, I.D. Study on the Enzymatic Activity of the Soil in Racos Protected Area from Brasov. Rev. Chim. 2020, 71, 358–366. [Google Scholar] [CrossRef]
- Silva, A.; Babujia, L.; Franchini, J.; Ralisch, R.; Hungria, M.; Guimar, E. Soil structure and its influence on microbial biomass in different soil and crop management systems. Soil Till. Res. 2014, 142, 42–53. [Google Scholar] [CrossRef]
- Heidari, G.; Mohammadi, K.; Sohrabi, Y. Responses of soil microbial biomass and enzyme activities to tillage and fertilization systems in soybean (Glycine max L.) production. Front. Plant Sci. 2016, 7, 1730. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Zhao, X.; Guo, X.; Sui, B.; Liu, J.; Wang, H.; Li, J. Tillage Methods Change Nitrogen Distribution and Enzyme Activities in Maize Rhizosphere and Non-Rhizosphere Chernozem in Jilin Province of China. Processes 2023, 11, 3253. [Google Scholar] [CrossRef]
- Nugroho, P.A. Indicative Soil Biological Parameters in Long-Term Conventional and Conservation Tillage Experiment. Ph.D. Thesis, Magyar Agrár-és Élettudományi Egyetem, Gödöllő, Hungary, 2024. [Google Scholar]
- Carter, M.R. Microbial biomass as an index for tillage-induced changes in soil biological properties. Soil Tillage Res. 1986, 7, 29–40. [Google Scholar] [CrossRef]
- Lemanowicz, J. Activity of selected enzymes as markers of ecotoxicity in technogenic salinization soils. Environ. Sci. Pollut. Res. 2019, 26, 13014–13024. [Google Scholar] [CrossRef]
- Teslya, A.V.; Iashnikov, A.V.; Poshvina, D.V.; Stepanov, A.A.; Vasilchenko, A.S. Extracellular Enzymes of Soils Under Organic and Conventional Cropping Systems: Predicted Functional Potential and Actual Activity. Agronomy 2024, 14, 2634. [Google Scholar] [CrossRef]
- Maphuhla, N.G.; Lewu, F.B.; Oyedeji, O.O. The effects of physicochemical parameters on analysed soil enzyme activity from Alice landfill site. Int. J. Environ. Res. Public Health 2021, 18, 221. [Google Scholar] [CrossRef]
- López-Valdez, F.; Fernández-Luqueño, F.; Ceballos-Ramírez, J.M.; Marsch, R.; Olalde-Portugal, V.; Dendooven, L. A strain of Bacillus subtilis stimulates sunflower growth (Helianthus annuus L.) temporarily. Sci. Hortic. 2011, 128, 499–505. [Google Scholar] [CrossRef]
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Shapiro, C.A.; Wortmann, C.S.; Drijber, R.A.; Mamo, M.; Shaver, T.M.; Ferguson, R.B. Soil organic carbon: The value to soil properties. J. Soil Water Conserv. 2013, 68, 129A–134A. [Google Scholar] [CrossRef]
- Raimi, A.; Adeleke, R.; Roopnarain, A. Soil fertility challenges and Biofertiliser as a viable alternative for increasing smallholder farmer crop productivity in sub-Saharan Africa. Cogent Food Agric. 2017, 3, 1400933. [Google Scholar] [CrossRef]
- Javeed, H.M.R.; Qamar, R.; Rehman, A.U.; Ali, M.; Rehman, A.; Farooq, M.; Zamir, S.I.; Nadeem, M.; Cheema, M.A.; Shehzad, M.; et al. Improvement in Soil Characteristics of Sandy Loam Soil and Grain Quality of Spring Maize by Using Phosphorus Solublizing Bacteria. Sustainability 2019, 11, 7049. [Google Scholar] [CrossRef]
- Yilmaz, E.; Sönmez, M. The role of organic/bio–fertilizer amendment on aggregate stability and organic carbon content in different aggregate scales. Soil Tillage Res. 2017, 168, 118–124. [Google Scholar] [CrossRef]
- Just, B.S.; Marks, E.A.N.; Roquer-Beni, L.; Llenas, L.; Ponsà, S.; Vilaplana, R. Biofertilization increases soil organic carbon concentrations: Results of a meta-analysis. Int. J. Agric. Sustain. 2024, 22, 2361578. [Google Scholar] [CrossRef]
- Goswami, L.; Gorai, P.S.; Mandal, N.C. Microbial fortification during vermicomposting: A brief review. In Recent Advancement in Microbial Biotechnology; Academic Press: Cambridge, MA, USA, 2021; pp. 99–122. [Google Scholar]
- Oorts, K.; Garnier, P.; Findeling, A.; Mary, B.; Richard, G.; Nicolardot, B. Modeling soil carbon and nitrogen dynamics in no-till and conventional tillage using PASTIS model. Soil Sci. Soc. Am. J. 2007, 71, 336–346. [Google Scholar] [CrossRef]
- Andruschkewitsch, R.; Geisseler, D.; Koch, H.J.; Ludwig, B. Effects of tillage on contents of organic carbon, nitrogen, water-stable aggregates and light fraction for four different long-term trials. Geoderma 2013, 192, 368–377. [Google Scholar] [CrossRef]
- Masrahi, A.S.; Alasmari, A.; Shahin, M.G.; Qumsani, A.T.; Oraby, H.F.; Awad-Allah, M.M.A. Role of Arbuscular Mycorrhizal Fungi and Phosphate Solubilizing Bacteria in Improving Yield, Yield Components, and Nutrients Uptake of Barley under Salinity Soil. Agriculture 2023, 13, 537. [Google Scholar] [CrossRef]
- Amalraj, E.L.D.; Maiyappan, S.; Peter, A.J. In vivo and in vitro studies of Bacillus megaterium var. phosphaticum on nutrient mobilization, antagonism and plant growth promoting traits. J. Ecobiotechnol. 2012, 4. Available online: https://updatepublishing.com/journal/index.php/jebt/article/view/165 (accessed on 12 March 2025).
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]
- Liang, J.L.; Liu, J.; Jia, P.; Yang, T.T.; Zeng, Q.W.; Zhang, S.C.; Liao, B.; Shu, W.S.; Li, J.T. Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef]
- Cheng, J.; Zhuang, W.; Li, N.N.; Tang, C.L.; Ying, H.J. Efficient biosynthesis of d-ribose using a novel co-feeding strategy in Bacillus subtilis without acid formation. Lett. Appl. Microbiol. 2017, 64, 73–78. [Google Scholar] [CrossRef]
- Itelima, J.; Bang, W.; Onyimba, I.; Oj, E. A review: Biofertilizer; a key player in enhancing soil fertility and crop productivity. J. Microbiol. Biotechnol. Rep. 2018, 2, 22–28. [Google Scholar]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Goswami, D. Phosphorus solubilization and mobilization: Mechanisms, current developments, and future challenge. In Advances in Plant Microbiome and Sustainable Agriculture: Functional Annotation and Future Challenges; Springer: Singapore, 2020; pp. 1–20. [Google Scholar]
- Saeid, A.; Prochownik, E.; Dobrowolska-Iwanek, J. Phosphorus Solubilization by Bacillus Species. Molecules 2018, 23, 2897. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A.; Mishra, A.K.; Shah, S.N.; Bhat, M.A.; Jan, S.; Rahman, S.; Baek, K.-H.; Jan, A.T. Soil and Mineral Nutrients in Plant Health: A Prospective Study of Iron and Phosphorus in the Growth and Development of Plants. Curr. Issues Mol. Biol. 2024, 46, 5194–5222. [Google Scholar] [CrossRef]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef]
| Tillage System | CT | NT | ||
|---|---|---|---|---|
| Experimental Year | 2023 | 2024 | 2023 | 2024 |
| Bulk density (g/cm3) | 1.29 | 1.23 | 1.44 | 1.35 |
| pH | 5.79 | 5.80 | 5.95 | 5.87 |
| Organic C | 1.69 | 1.63 | 2.63 | 2.38 |
| Total N (%) | 0.148 | 0.145 | 0.229 | 0.207 |
| Total P (%) | 0.0312 | 0.0358 | 0.0389 | 0.0339 |
| Available P (ppm) | 58.37 | 29.06 | 69.83 | 58.56 |
| Soil Type | Cambic Chernozem | |||
| Texture | Clay–Loam | |||
| Fertilizer Treatments | Initial Fertilization | Second Fertilization | ||
|---|---|---|---|---|
| 05 May 2023 and 11 April 2024 | 20 June 2023 and 26 May 2024 | |||
| NPK 20:10:5 (240 kg ha−1) | Ecofertil (1 × 1010 CFU) (15 L ha−1 year−1) | Corona N (3 kg ha−1) | ||
| T0-control | + | – | – | – |
| T1 | + | 100% | 100% | – |
| T2 | + | 75% | 75% | + |
| T3 | + | 100% | 100% | + |
| T4 | + | 125% | 125% | + |
| Enzyme | Dehydrogenase (µg TPF g −1 h−1) | ||||||
|---|---|---|---|---|---|---|---|
| Tillage System | CT | NT | |||||
| Year | Treatment | Mean | Min. | Max. | Mean | Min. | Max. |
| 2023 | T0 | 0.94 ± 0.03 a B | 0.74 | 1.15 | 1.05 ± 0.07 c A | 0.75 | 1.56 |
| T1 | 0.78 ± 0.03 b B | 0.58 | 1.03 | 1.29 ± 0.21 b A | 0.40 | 2.79 | |
| T2 | 0.67 ± 0.27 c B | 0.51 | 0.84 | 1.46 ± 0.05 a A | 1.06 | 1.70 | |
| T3 | 0.98 ± 0.03 a ns | 0.80 | 1.19 | 0.92 ± 0.05 d ns | 0.56 | 1.13 | |
| T4 | 0.82 ± 0.03 b B | 0.64 | 1.05 | 1.00 ± 0.04 cd A | 0.75 | 1.29 | |
| 2024 | T0 | 0.82 ± 0.07 ab B | 0.51 | 1.46 | 2.03 ± 0.16 a A | 1.13 | 3.18 |
| T1 | 0.84 ± 0.04 a ns | 0.67 | 1.40 | 0.87 ± 0.04 d ns | 0.64 | 1.22 | |
| T2 | 0.71 ± 0.05 cd B | 0.41 | 1.09 | 1.13 ± 0.09 c A | 0.78 | 1.63 | |
| T3 | 0.70 ± 0.02 d B | 0.59 | 0.84 | 1.06 ± 0.07 c A | 0.68 | 1.50 | |
| T4 | 0.73 ± 0.01 bcd B | 0.65 | 0.81 | 1.34 ± 0.08 b A | 0.98 | 1.96 | |
| Enzyme | Catalase (µmol H2O2 g−1 h−1) | ||||||
|---|---|---|---|---|---|---|---|
| Tillage System | CT | NT | |||||
| Year | Treatment | Mean | Min. | Max. | Mean | Min. | Max. |
| 2023 | T0 | 1.43 ± 0.05 d ns | 1.21 | 1.79 | 1.38 ± 0.07 b ns | 1.00 | 1.75 |
| T1 | 1.50 ± 0.09 c A | 1.17 | 2.17 | 1.19 ± 0.08 e B | 0.83 | 1.67 | |
| T2 | 1.56 ± 0.15 b A | 0.46 | 2.12 | 1.34 ± 0.04 c B | 1.13 | 1.63 | |
| T3 | 1.91 ± 0.10 a A | 1.33 | 2.54 | 1.23 ± 0.05 d B | 0.88 | 1.42 | |
| T4 | 1.89 ± 0.08 a A | 1.63 | 2.50 | 1.53 ± 0.03 a B | 1.33 | 1.75 | |
| 2024 | T0 | 2.06 ± 0.05 c A | 1.77 | 2.40 | 1.21 ± 0.06 d B | 0.94 | 1.62 |
| T1 | 1.94 ± 0.06 d A | 1.68 | 2.31 | 1.89 ± 0.05 a B | 1.66 | 2.13 | |
| T2 | 2.17 ± 0.07 b A | 1.81 | 2.48 | 1.56 ± 0.08 c B | 1.15 | 1.96 | |
| T3 | 2.32 ± 0.04 a A | 2.06 | 2.53 | 1.58 ± 0.10 c B | 0.88 | 2.15 | |
| T4 | 2.16 ± 0.04 b A | 1.94 | 2.40 | 1.76 ± 0.09 b B | 1.18 | 2.10 | |
| Enzyme | Acid Phosphatase (µg p-Nitrophenol g−1 h−1) | ||||||
|---|---|---|---|---|---|---|---|
| Tillage System | CT | NT | |||||
| Year | Treatment | Mean | Min. | Max. | Mean | Min. | Max. |
| 2023 | T0 | 1.12 ± 0.02 a B | 0.97 | 1.24 | 1.21 ± 0.03 b A | 0.98 | 1.38 |
| T1 | 1.01 ± 0.02 c B | 0.84 | 1.12 | 1.52 ± 0.05 a A | 1.15 | 1.78 | |
| T2 | 0.93 ± 0.03 d B | 0.69 | 1.06 | 1.22 ± 0.04 b A | 0.99 | 1.49 | |
| T3 | 1.07 ± 0.02 b B | 0.95 | 1.20 | 1.22 ± 0.04 b A | 0.99 | 1.52 | |
| T4 | 0.90 ± 0.01 d B | 0.78 | 0.98 | 1.20 ± 0.05 b A | 0.95 | 1.47 | |
| 2024 | T0 | 1.10 ± 0.01 c B | 1.04 | 1.16 | 1.96 ± 0.05 a A | 1.69 | 2.40 |
| T1 | 1.17 ± 0.02 b B | 1.03 | 1.34 | 1.49 ± 0.05 d A | 1.28 | 1.81 | |
| T2 | 1.13 ± 0.01 bc B | 1.03 | 1.22 | 1.64 ± 0.04 c A | 1.31 | 1.83 | |
| T3 | 1.06 ± 0.03 d B | 0.91 | 1.19 | 1.84 ± 0.08 b A | 1.27 | 2.28 | |
| T4 | 1.27 ± 0.01 a B | 1.19 | 1.36 | 1.61 ± 0.04 c A | 1.37 | 1.88 | |
| Enzyme | Alkaline Phosphatase (µg p-Nitrophenol g−1 h−1) | ||||||
|---|---|---|---|---|---|---|---|
| Tillage System | CT | NT | |||||
| Year | Treatment | Mean | Min. | Max. | Mean | Min. | Max. |
| 2023 | T0 | 0.16 ± 0.01 bc B | 0.11 | 0.21 | 0.46 ± 0.03 b A | 0.31 | 0.62 |
| T1 | 0.18 ± 0.01 a B | 0.13 | 0.24 | 0.52 ± 0.04 a A | 0.32 | 0.77 | |
| T2 | 0.16 ± 0.01 bc B | 0.09 | 0.23 | 0.33 ± 0.02 c A | 0.23 | 0.43 | |
| T3 | 0.14 ± 0.01 c B | 0.09 | 0.22 | 0.45 ± 0.02 b A | 0.36 | 0.59 | |
| T4 | 0.17 ± 0.01 ab B | 0.10 | 0.24 | 0.34 ± 0.01 c A | 0.28 | 0.43 | |
| 2024 | T0 | 0.18 ± 0.004 a B | 0.14 | 0.20 | 0.60 ± 0.04 a A | 0.42 | 0.84 |
| T1 | 0.19 ± 0.01 a B | 0.14 | 0.24 | 0.30 ± 0.01 e A | 0.23 | 0.38 | |
| T2 | 0.15 ± 0.004 b B | 0.13 | 0.18 | 0.45 ± 0.04 b A | 0.31 | 0.68 | |
| T3 | 0.18 ± 0.005 a B | 0.16 | 0.22 | 0.38 ± 0.03 c A | 0.23 | 0.65 | |
| T4 | 0.16 ± 0.002 b B | 0.14 | 0.17 | 0.36 ± 0.03 d A | 0.26 | 0.60 | |
| Source | df | MS | F | Significance |
|---|---|---|---|---|
| Dependent variable: Catalase | ||||
| Tillage system | 1 | 13.631 | 105.259 | 0.000 *** |
| Fertilizer treatment | 4 | 0.896 | 6.922 | 0.000 *** |
| Tillage system × Fertilizer treatment | 4 | 0.537 | 4.150 | 0.003 ** |
| Error | 290 | 0.129 | ||
| Dependent variable: Dehydrogenase | ||||
| Tillage system | 1 | 12.996 | 105.682 | 0.000 *** |
| Fertilizer treatment | 4 | 0.819 | 6.656 | 0.000 *** |
| Tillage system × Fertilizer treatment | 4 | 0.710 | 5.776 | 0.000 *** |
| Error | 290 | 0.123 | ||
| Dependent variable: Acid phosphatase | ||||
| Tillage system | 1 | 12.888 | 216.116 | 0.000 *** |
| Fertilizer treatment | 4 | 0.134 | 2.254 | 0.063 |
| Tillage system × Fertilizer treatment | 4 | 0.059 | 0.984 | 0.416 |
| Error | 290 | 0.060 | ||
| Dependent variable: Alkaline phosphatase | ||||
| Tillage system | 1 | 4.795 | 507.697 | 0.000 *** |
| Fertilizer treatment | 4 | 0.079 | 8.373 | 0.000 *** |
| Tillage system × Fertilizer treatment | 4 | 0.068 | 7.245 | 0.000 *** |
| Error | 290 | 0.009 | ||
| Parameter | GME | Catalase | Dehydrogenase | Acid Phosphatase | Alkaline Phosphatase |
|---|---|---|---|---|---|
| GME | 1 | ||||
| Catalase | −0.460 ** | 1 | |||
| Dehydrogenase | 0.860 ** | −0.572 ** | 1 | ||
| Acid phosphatase | 0.858 ** | −0.330 ** | 0.604 ** | 1 | |
| Alkaline phosphatase | 0.892 ** | −0.638 ** | 0.731 ** | 0.703 ** | 1 |
| Tillage System | 45 DAS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CT | NT | |||||||||
| Fertilization Treatment | T0 | T1 | T2 | T3 | T4 | T0 | T1 | T2 | T3 | T4 |
| Indicator | ||||||||||
| pH | 6.0 a | 5.8 a | 5.8 a | 5.7 a | 5.8 a | 5.7 a | 5.7 a | 5.7 a | 5.7 a | 5.7 a |
| SE | 0.095 | 0.084 | 0.118 | 0.091 | 0.069 | 0.101 | 0.160 | 0.104 | 0.124 | 0.111 |
| SOC (%) | 1.91 a | 1.76 d | 1.86 bc | 1.91 ab | 1.85 c | 2.76 ab | 2.54 d | 2.67 c | 2.75 b | 2.82 a |
| SE | 0.007 | 0.012 | 0.017 | 0.003 | 0.005 | 0.007 | 0.008 | 0.017 | 0.017 | 0.010 |
| TN (%) | 0.168 a | 0.155 a | 0.162 a | 0.165 a | 0.162 a | 0.239 ab | 0.221 c | 0.234 b | 0.240 ab | 0.244 a |
| SE | 0.002 | 0.003 | 0.002 | 0.003 | 0.004 | 0.000 | 0.003 | 0.001 | 0.001 | 0.001 |
| Crop Harvest | ||||||||||
| pH | 5.5 a | 5.3 a | 5.5 a | 5.6 a | 5.3 a | 5.7 a | 5.9 a | 5.8 a | 5.8 a | 5.7 a |
| SE | 0.074 | 0.092 | 0.105 | 0.115 | 0.127 | 0.100 | 0.073 | 0.076 | 0.085 | 0.065 |
| SOC (%) | 2.13 a | 1.98 b | 1.73 c | 1.89 b | 2.07 a | 2.56 a | 2.29 c | 2.31 bc | 2.34 b | 2.24 d |
| SE | 0.023 | 0.034 | 0.010 | 0.007 | 0.010 | 0.003 | 0.010 | 0.003 | 0.008 | 0.010 |
| TN (%) | 0.185 a | 0.174 ab | 0.151 c | 0.168 b | 0.177 ab | 0.224 a | 0.198 b | 0.201 b | 0.205 b | 0.196 b |
| SE | 0.003 | 0.002 | 0.003 | 0.001 | 0.002 | 0.002 | 0.000 | 0.002 | 0.000 | 0.003 |
| Tillage System | 45 DAS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CT | NT | |||||||||
| Fertilization Treatment | T0 | T1 | T2 | T3 | T4 | T0 | T1 | T2 | T3 | T4 |
| Indicator | ||||||||||
| pH | 5.9 a | 5.9 a | 5.8 a | 5.8 a | 5.7 a | 5.7 a | 5.7 a | 5.7 a | 5.6 a | 5.6 a |
| SE | 0.100 | 0.091 | 0.115 | 0.093 | 0.134 | 0.121 | 0.104 | 0.074 | 0.075 | 0.058 |
| SOC (%) | 1.84 b | 1.74 cd | 2.00 a | 1.78 bc | 1.70 d | 2.08 c | 2.26 a | 2.23 ab | 2.21 ab | 2.20 b |
| SE | 0.007 | 0.027 | 0.013 | 0.012 | 0.003 | 0.010 | 0.017 | 0.010 | 0.010 | 0.017 |
| TN (%) | 0.157 ab | 0.153 b | 0.175 a | 0.156 ab | 0.148 b | 0.182 b | 0.196 a | 0.190 ab | 0.188 ab | 0.185 ab |
| SE | 0.004 | 0.005 | 0.003 | 0.005 | 0.005 | 0.004 | 0.003 | 0.001 | 0.001 | 0.002 |
| Crop Harvest | ||||||||||
| pH | 5.8 a | 5.8 a | 5.7 a | 5.8 a | 5.7 a | 5.6 a | 5.8 a | 5.8 a | 5.6 a | 5.8 a |
| SE | 0.045 | 0.050 | 0.072 | 0.113 | 0.062 | 0.069 | 0.087 | 0.134 | 0.083 | 0.066 |
| SOC (%) | 1.84 a | 1.84 a | 1.65 b | 1.61 b | 1.91 a | 2.26 c | 2.35 bc | 1.42 d | 2.45 ab | 2.52 a |
| SE | 0.017 | 0.023 | 0.010 | 0.010 | 0.010 | 0.020 | 0.015 | 0.015 | 0.034 | 0.035 |
| TN (%) | 0.157 a | 0.156 a | 0.144 b | 0.137 b | 0.161 a | 0.190 b | 0.200 ab | 0.126 c | 0.207 a | 0.212 a |
| SE | 0.002 | 0.002 | 0.003 | 0.001 | 0.002 | 0.003 | 0.001 | 0.001 | 0.003 | 0.004 |
| Source | df | MS | F | Significance |
|---|---|---|---|---|
| Dependent variable: pH | ||||
| Tillage system | 1 | 0.001 | 0.025 | 0.876 |
| Fertilizer treatment | 4 | 0.023 | 0.595 | 0.667 |
| Tillage system × Fertilizer treatment | 4 | 0.048 | 1.223 | 0.305 |
| Error | 110 | 0.039 | ||
| Dependent variable: SOC | ||||
| Tillage system | 1 | 7.874 | 153.421 | 0.000 *** |
| Fertilizer treatment | 4 | 0.137 | 2.666 | 0.036 * |
| Tillage system × Fertilizer treatment | 4 | 0.070 | 1.371 | 0.249 |
| Error | 110 | 0.051 | ||
| Dependent variable: TN | ||||
| Tillage system | 1 | 0.056 | 136.909 | 0.000 *** |
| Fertilizer treatment | 4 | 0.001 | 1.911 | 0.114 |
| Tillage system × Fertilizer treatment | 4 | 0.000 | 1.107 | 0.357 |
| Error | 110 | 0.000 | ||
| Dependent variable: TP | ||||
| Tillage system | 1 | 0.003 | 42.590 | 0.000 *** |
| Fertilizer treatment | 4 | 0.000 | 4.097 | 0.004 ** |
| Tillage system × Fertilizer treatment | 4 | 5.909 × 10−5 | 0.948 | 0.439 |
| Error | 110 | 6.233 × 10−5 | ||
| Dependent variable: AP | ||||
| Tillage system | 1 | 8247.058 | 32.988 | 0.000 *** |
| Fertilizer treatment | 4 | 226.480 | 0.906 | 0.463 |
| Tillage system × Fertilizer treatment | 4 | 323.585 | 1.294 | 0.277 |
| Error | 110 | 249.998 | ||
| Parameter | pH | SOC | TN | TP | AP |
|---|---|---|---|---|---|
| pH | 1 | ||||
| SOC | −0.080 | 1 | |||
| TN | −0.064 | 0.992 ** | 1 | ||
| TP | 0.091 | 0.461 ** | 0.482 ** | 1 | |
| AP | −0.080 | 0.292 ** | 0.262 ** | 0.223 * | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ursu, A.; Cara, I.G.; Bireescu, G.; Rusu, M.; Mihu, G.D.; Cakpo, S.S.; Țopa, D.; Jităreanu, G. Effects of Tillage Systems and Bacterial Inoculation on Enzyme Activities and Selected Soil Chemical Properties. Agriculture 2025, 15, 1285. https://doi.org/10.3390/agriculture15121285
Ursu A, Cara IG, Bireescu G, Rusu M, Mihu GD, Cakpo SS, Țopa D, Jităreanu G. Effects of Tillage Systems and Bacterial Inoculation on Enzyme Activities and Selected Soil Chemical Properties. Agriculture. 2025; 15(12):1285. https://doi.org/10.3390/agriculture15121285
Chicago/Turabian StyleUrsu, Ana, Irina Gabriela Cara, Geanina Bireescu, Mariana Rusu, Gabriel Dumitru Mihu, Segla Serginho Cakpo, Denis Țopa, and Gerard Jităreanu. 2025. "Effects of Tillage Systems and Bacterial Inoculation on Enzyme Activities and Selected Soil Chemical Properties" Agriculture 15, no. 12: 1285. https://doi.org/10.3390/agriculture15121285
APA StyleUrsu, A., Cara, I. G., Bireescu, G., Rusu, M., Mihu, G. D., Cakpo, S. S., Țopa, D., & Jităreanu, G. (2025). Effects of Tillage Systems and Bacterial Inoculation on Enzyme Activities and Selected Soil Chemical Properties. Agriculture, 15(12), 1285. https://doi.org/10.3390/agriculture15121285







