Effect of Ns Gene Dosage and Temperature on the Level of Potato Resistance to PVS
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. PVS Isolates
2.3. Preparation of the Virus Source
2.4. Potato Plants
2.5. Mechanical and Graft Inoculation of Plants
- 1.
- At the end of the vegetative period, tubers were collected separately from each inoculated (mechanical and grafted) and control plant;
- 2.
- The following year, the pre-sprouted tubers were planted in pots in a greenhouse and six weeks after planting, the presence of the virus was again verified by DAS-ELISA. Clones were classified in the same way as for primary infected plants.
2.6. PVS Detection—Serological Test
2.7. Gene
3. Results
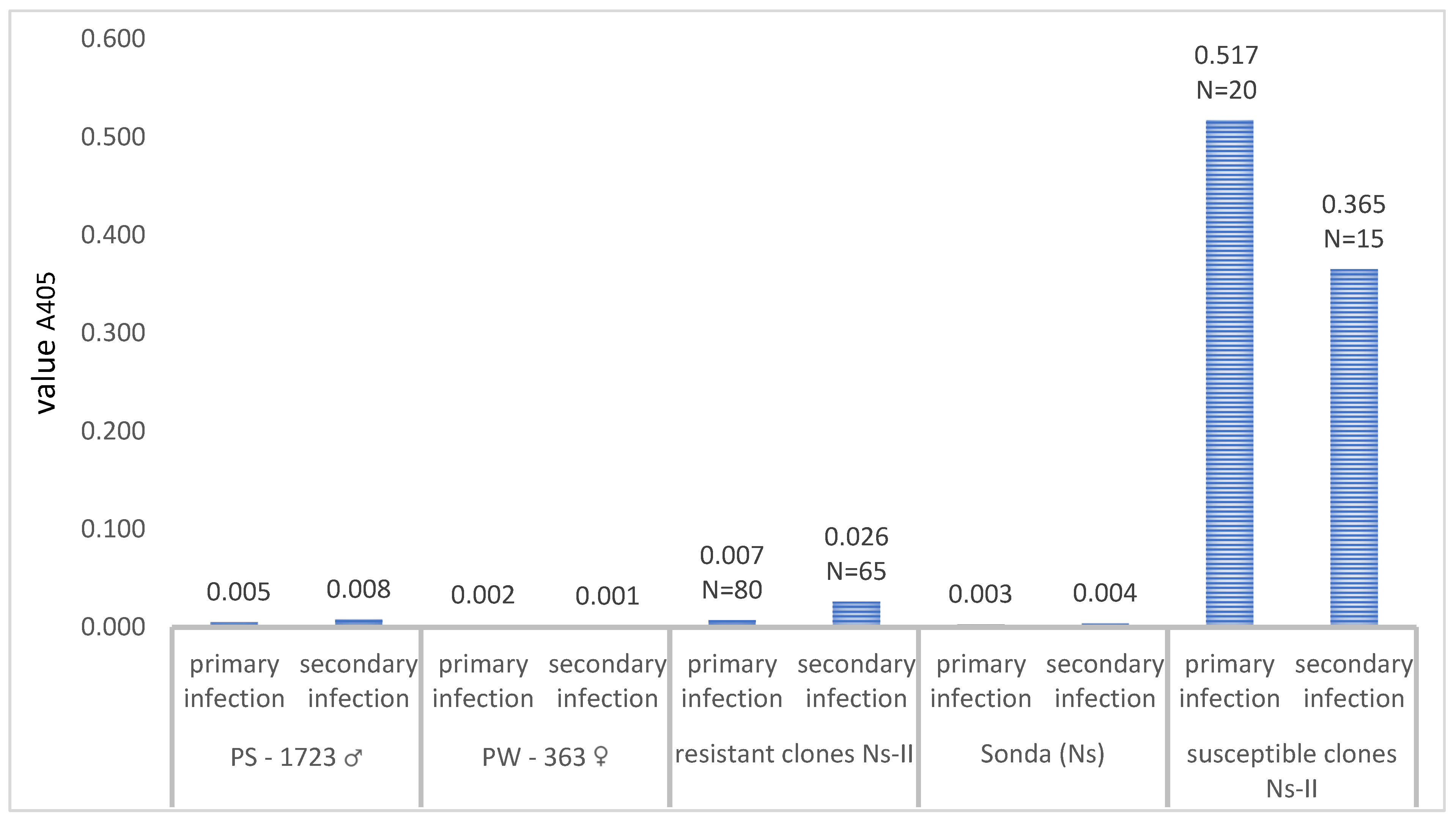
3.1. Segregation of Resistance to PVS
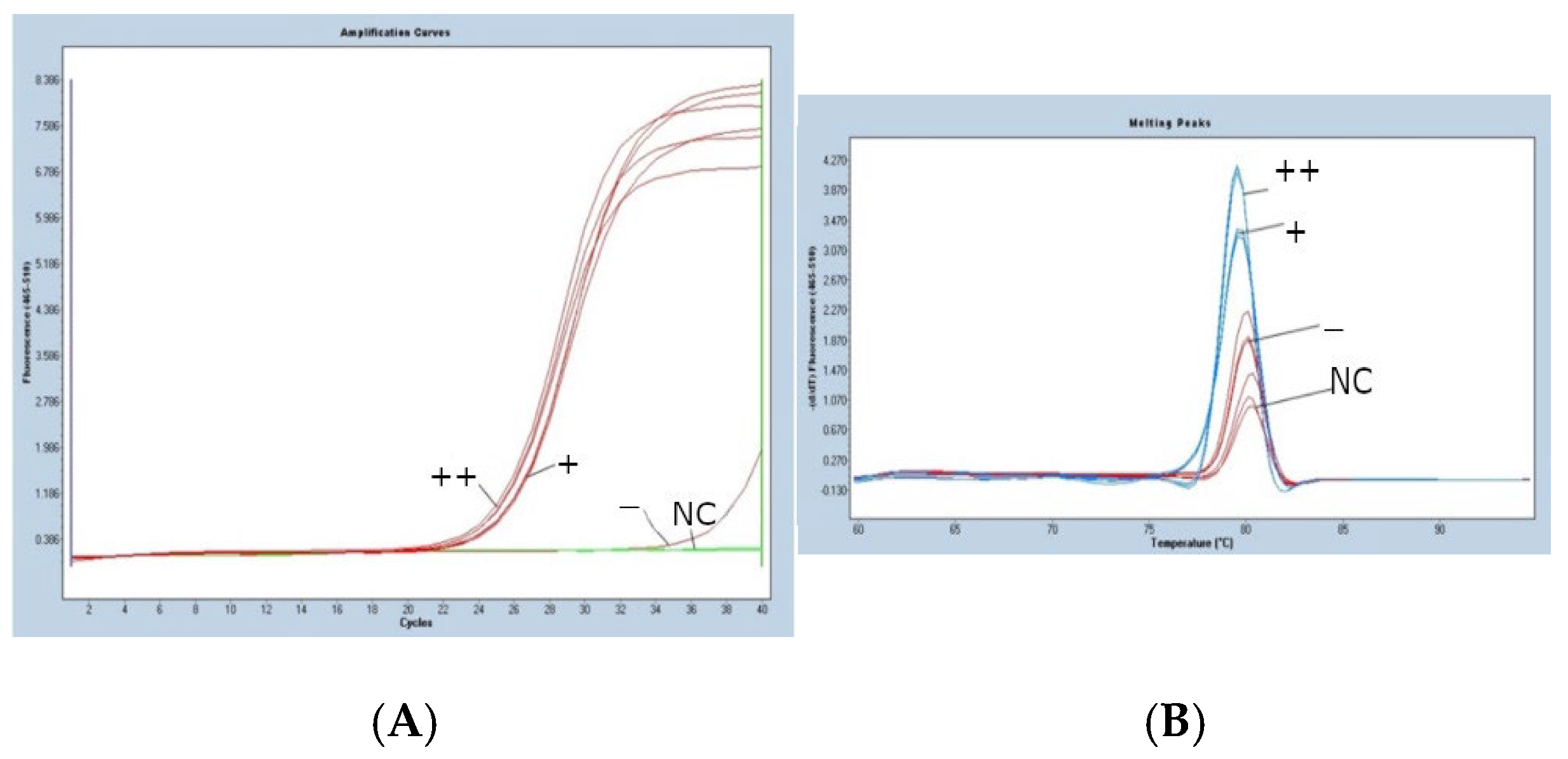
3.2. Ns Gene Dosage in Potato Clones from Population Ns-II
- (A)
- The real-time PCR amplification curves produced, revealed using EvaGreen® dye: (+) simplex form; (++) duplex form; (sample -) susceptible clone and NC (negative control).
- (B)
- The melting curves of the products of the real-time PCR shown by the use of EvaGreen dye:
- -
- The blue lines correspond to the melting point of the tested PCR product for the sample.
- -
- The red lines show the melting curve of the non-specific product for susceptible clone (sample -) and water (NC, negative control).
3.3. Effect of the Cultivation Temperatures on the Resistance Reaction to Two PVS Isolates—Mechanical Inoculation (Primary and Secondary Infection)
3.4. Effect of Cultivation Temperatures on the Resistance Reaction to Two PVS Isolates—Graft Inoculation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- King, A.M.; Lefkowitz, E.; Adams, M.J.; Carstens, E.B. (Eds.) Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2011; Volume 9, ISBN 978-0-12-384684-6. [Google Scholar]
- Duan, G.; Zhan, F.; Du, Z.; Ho, S.Y.; Gao, F. Europe was a hub for the global spread of potato virus S in the 19th century. Virology 2018, 525, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Kreuze, J.F.; Souza-Dias, J.A.C.; Jeevalatha, A.; Figueira, A.R.; Valkonen, J.P.T.; Jones, R.A.C. Viral Diseases in Potato. In The Potato Crop; Campos, H., Ortiz, O., Eds.; Springer: NewYork, NY, USA, 2020; pp. 389–430. [Google Scholar]
- Stevenson, W.R.; Loria, R.; Franc, G.D.; Weingartner, D.P. Compendium of Potato Diseases, 2nd ed.; American Phytopathological Society, Press: St. Paul, MN, USA, 2001. [Google Scholar]
- Cox, B.A.; Jones, R.A.C. Genetic variability of the coat protein gene of Potato virus S and distinguishing its biologically distinct strains. Arch. Virol. 2010, 155, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Zimnoch-Guzowska, E.; Yin, Z.; Chrzanowska, M.; Flis, B. Sources and effectiveness of potato PVY resistance in IHAR’s breeding research. Am. J. Potato Res. 2013, 90, 21–27. [Google Scholar] [CrossRef]
- Chung, B.N.; Canto, T.; Tenllado, F.; Choi, K.S.; Joa, J.H.; Ahn, J.J.; Kim, C.H.; Do, K.S. The effects of high temperature on infection by Potato virus Y, Potato virus A, and Potato leafroll virus. Plant Pathol. J. 2016, 32, 321. [Google Scholar] [CrossRef]
- Obrępalska-Stęplowska, A.; Renaut, J.; Planchon, S.; Przybylska, A.; Wieczorek, P.; Barylski, J.; Palukaitis, P. Effect of temperature on the pathogenesis, accumulation of viral and satellite RNAs and on plant proteome in peanut stunt virus and satellite RNA-infected plants. Front. Plant Sci. 2015, 6, 903. [Google Scholar] [CrossRef]
- Del Toro, F.J.; Aguilar, E.; Hernandez-Walias, F.J.; Tenllado, F.; Chung, B.N.; Canto, T. High temperature, high ambient CO2 affect the interactions between three positive-sense RNA viruses and a compatible host differentially, but not their silencing suppression efficiencies. PLoS ONE 2015, 10, e0136062. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Chen, A.; Peterson, T.; Ma, K.; Bertzky, M.; Poulter, B. Global priority conservation areas in the face of 21st century climate change. PLoS ONE 2013, 8, e54839. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Ashoub, A.; Baeumlisberger, M.; Neupaertl, M.; Karas, M.; Brüggemann, W. Characterization of common and distinctive adjustments of wild barley leaf proteome under drought acclimation, heat stress and their combination. Plant Mol. Biol. 2015, 87, 459–471. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; 184p, Available online: https://www.ipcc.ch/report/ar6/syr/downloads/report/IPCC_AR6_SYR_FullVolume.pdf (accessed on 3 June 2025).
- Jones, R.A. Plant virus emergence and evolution: Origins, new encounter scenarios, factors driving emergence, effects of changing world conditions, and prospects for control. Virus Res. 2009, 141, 113–130. [Google Scholar] [CrossRef]
- Szajko, K.; Chrzanowska, M.; Witek, K.; Strzelczyk-Żyta, D.; Zagórska, H.; Gebhardt, C.; Hennig, J.; Marczewski, W. The novel gene Ny-1 on potato chromosome IX confers hypersensitive resistance to Potato virus Y and is an alternative to Ry genes in potato breeding for PVY resistance. Theor. Appl. Genet. 2008, 116, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Liang, Z.; Nie, B.; Murphy, A.; Singh, M. Studies on varietal response to different strains of Potato virus Y (PVY) reveal hypersensitive resistance in Exploits to PVYO and extreme resistance in F87084 to all tested strains. Am. J. Potato Res. 2015, 92, 23–31. [Google Scholar] [CrossRef]
- Ohki, T.; Sano, M.; Asano, K.; Nakayama, T.; Maoka, T. Effect of temperature on resistance to Potato virus Y in potato cultivars carrying the resistance gene. Rychc. Plant Pathol. 2018, 67, 1629–1635. [Google Scholar] [CrossRef]
- Pájtli, É. Whole Genome Molecular Analysis of Potato Virus S(PVS) Isolates. Ph.D. Thesis, Corvinus University of Budapest, Budapest, Hungary, 2015. [Google Scholar]
- Santillan, F.W.; Fribourg, C.E.; Adams, I.P.; Gibbs, A.J.; Boonham, N.; Kehoe, M.A.; Maina, S.; Jones, R.A.C. The biology and phylogenetics of Potato virus S isolates from the Andean region of South America. Plant Dis. 2018, 102, 869–885. [Google Scholar] [CrossRef]
- Tatarowska, B.; Plich, J.; Milczarek, D.; Flis, B. Temperature-dependent resistance to potato virus M in potato (Solanum tuberosum). Plant Pathol. 2020, 69, 1445–1452. [Google Scholar] [CrossRef]
- Witek, K.; Strzelczyk-Żyta, D.; Hennig, J.; Marczewski, W. A multiplex PCR approach to simultaneously genotype potato towards the resistance alleles Ry-fsto and Ns. Mol. Breed. 2006, 18, 273–275. [Google Scholar] [CrossRef]
- Szajko, K.; Chrzanowska, M.; Strzelczyk-Żyta, D.; Zagórska, H.; Marczewski, W. Endonuclease restriction of SCAR amplicons SC811 is required to identify Ns-false-positive markers in PVS-susceptible potato cultivars. J. Appl. Genet. 2008, 49, 45–48. [Google Scholar] [CrossRef]
- Szajko, K.; Sołtys-Kalina, D.; Szarzyńska, B.; Strzelczyk-Żyta, D.; Szweykowska-Kulinska, Z.; Marczewski, W. A comparative proteomic analysis of the PVY-induced hypersensitive response in leaves of potato (Solanum tuberosum L.) plants that differ in Ny-1 gene dosage. Eur. J. Plant Pathol. 2019, 153, 385–396. [Google Scholar] [CrossRef]
- Torrance, L.; Talianksy, M.E. Potato Virus Y Emergence and Evolution from the Andes of South America to Become a Major Destructive Pathogen of Potato and Other Solanaceous Crops Worldwide. Viruses 2020, 12, 1430. [Google Scholar] [CrossRef]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive responses of potato (Solanum tuberosum L.) plants to heat stress and infection with potato virus Y. Front. Microbiol. 2018, 9, 2582. [Google Scholar] [CrossRef]
- Prasch, C.M.; Sonnewald, U. Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol. 2013, 162, 1849–1866. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Sarmiento, C.; Kiisma, M.; Koivumäki, S.; Lemmetty, A.; Truve, E.; Lehto, K. Effects of viral silencing suppressors on tobacco ringspot virus infection in two Nicotiana species. J. Gen. Virol. 2008, 89, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, K.; Renovell, A.; Comellas, M.; Serra, P.; García, M.L.; Pina, J.A.; Navarro, L.; Moreno, P.; Guerri, J. Effect of temperature on RNA silencing of a negative-stranded RNA plant virus: Citrus psorosis virus. Plant Pathol. 2010, 59, 982–990. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Singh, J.; Li, D.; Qu, F. Temperature-dependent survival of Turnip crinkle virus-infected arabidopsis plants relies on an RNA silencing-based defense that requires DCL2, AGO2, and HEN1. J. Virol. 2012, 86, 6847–6854. [Google Scholar] [CrossRef]
- Ghoshal, B.; Sanfaçon, H. Temperature-dependent symptom recovery in Nicotiana benthamiana plants infected with tomato ringspot virus is associated with reduced translation of viral RNA2 and requires ARGONAUTE 1. Virology 2014, 456, 188–197. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Y.; Chen, L.; Zhu, L.; Ren, H.; Lin, H.; Xi, D. Temperature dependent defense of Nicotiana tabacum against cucumber mosaic virus and recovery occurs with the formation of dark green islands. J. Plant Biol. 2016, 59, 293–301. [Google Scholar] [CrossRef]
- Király, L.; Hafez, Y.M.; Fodor, J.; Király, Z. Suppression of tobacco mosaic virus-induced hypersensitive-type necrotization in tobacco at high temperature is associated with downregulation of NADPH oxidase and superoxide and stimulation of dehydroascorbate reductase. J. Gen. Virol. 2008, 89, 799–808. [Google Scholar] [CrossRef]
- Anfoka, G.; Moshe, A.; Fridman, L.; Amrani, L.; Rotem, O.; Kolot, M.; Zeidan, M.; Czosnek, H.; Gorovits, R. Tomato yellow leaf curl virus infection mitigates the heat stress response of plants grown at high temperatures. Sci. Rep. 2016, 6, 19715. [Google Scholar] [CrossRef]
- Honjo, M.N.; Emura, N.; Kawagoe, T.; Sugisaka, J.; Kamitani, M.; Nagano, A.J.; Kudoh, H. Seasonality of interactions between a plant virus and its host during persistent infection in a natural environment. ISME J. 2020, 14, 506–518. [Google Scholar] [CrossRef]
- Marczewski, W.; Ostrowska, K.; Zimnoch-Guzowska, E. Identification of RAPD markers linked to the Ns locus in potato. Plant Breed. 1998, 117, 88–90. [Google Scholar] [CrossRef]
- Valkonen, J.P.T. Novel resistances to four potyviruses in tuber-bearing potato species, and temperature-sensitive expression of hypersensitive resistance to potato virus Y. Ann. Appl. Biol. 1997, 130, 91–104. [Google Scholar] [CrossRef]
- Szajko, K.; Strzelczyk-Żyta, D.; Marczewski, W. Ny-1 and Ny-2 genes conferring hypersensitive response to potato virus Y (PVY) in cultivated potatoes: Mapping and marker-assisted selection validation for PVY resistance in potato breeding. Mol. Breed. 2014, 34, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Erickson, F.L.; Dinesh-Kumar, S.P.; Holzberg, S.; Ustach, C.V.; Dutton, M.; Handley, V.; Corr, C.; Baker, B.J. Interactions between tobacco mosaic virus and the tobacco N gene. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1999, 354, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Toxopeus, H.J. On the significance of multiplex parental material in breeding for resistance to some disease in the potato. Euphytica 1953, 2, 139–146. [Google Scholar] [CrossRef]
- Kaeppler, S. Heterosis: Many genes, many mechanisms—End the search for an undiscovered unifying theory. Int. Sch. Res. Not. 2012, 2012, 682824. [Google Scholar] [CrossRef]
- Slater, A.T.; Cogan, N.O.I.; Hayes, B.J.; Schultz, L.; Dale, M.F.B.; Bryan, G.J.; Forster, J.W. Improving breeding efficiency in potato using molecular and quantitative genetics. Theor. Appl. Genet. 2014, 127, 2279–2292. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Pinto, C.A.B.P.; Andrade, C.M.; dos Santos, J.B.; dos Reis Figueira, A. SCAR marker for the selection of Ry-duplex potato clones immune to potato virus Y. Crop Breed. Appl. Biotechnol. 2006, 6, 1–8. [Google Scholar] [CrossRef]
- Andrade, C.M.; Pinto, C.A.B.P.; de Paula Ribeiro, S.R.R.; Peixouto, L.S.; Santos, L. Potato clones with multiple copies of the Ryadg allele conferring resistance to PVY. Crop Breed. Appl. Biotechnol. 2009, 9, 286–292. [Google Scholar] [CrossRef]




| Plant Material | |
|---|---|
| Cross ♂ x ♀ | PS-1723 × PW-363 |
| Number of clones evaluated in mechanical inoculation | 100 |
| Number of clones resistant in primary infection (R) | 80 |
| Number of clones susceptible in primary infection (S) | 20 |
| Number of clones resistant in secondary infection (R) | 65 |
| Number of clones susceptible in secondary infection (S) | 15 |
| Clones selected for detailed experiments | Ns-II-3, Ns-II-4, Ns-II-6, Ns-II-10, Ns-II-11, Ns-II-40, Ns-II-43, Ns-II-81 |
| Standard cultivars | Etola cv., susceptible to PVS (nulliplex)Sonda cv., resistant to PVS (simplex) |
| Parental clones | PS-1723 clone, resistant to PVS (simplex)PW-363 clone, resistant to PVS (simplex) |
| Control of DNA duplex gene Ns | Colchicinated DW 83-3121 (duplex) |
| Type of Inoculation | Name of Population | Cross ♂ x ♀ | Number Evaluated Clones | The Mean Values and Range of A405 for Clones Resistant to PVS | The Mean Values and Range of A405 for Clones Susceptible to PVS |
|---|---|---|---|---|---|
| Mechanical inoculation— primary infection | Ns-II | PS-1723 × PW-363 | N = 100 | A405 = 0.007 (0.010−0.042) N = 80 | A405 = 0.517 (0.058–1.651) N = 20 |
| Mechanical inoculation—secondary infection | N = 80 | N = 65 A405 = 0.026 (0.003–0.080) | N = 15 A405 = 0.365 (0.075–1.635) |
| Clone/Cultivar | Level of Ns Dosage | Mean Ct | 2−ΔCt |
|---|---|---|---|
| Colchicinated DW 83-3127 | duplex | 20.03 | 2.91 |
| PW-363 | simplex | 21.55 | 1.00 |
| PS-1723 | simplex | 21.11 | 1.00 |
| Standard susceptible cv. Etola | nulliplex | 37.47 | 0.00 |
| Mean values for 8 clones | simplex | 22.55 | 0.87 |
| Mean value for 42 clones | duplex | 20.14 | 3.83 |
| Mean value for 15 clones not defined | undetermined | 1.96 |
| Clone/ cultivar | Mechanical Inoculation—Primary Infection | Level of Resistance to PVS | Gene Dosage | |||
|---|---|---|---|---|---|---|
| S-966 | S-923 | |||||
| A405 20 °C | A405 28 °C | A405 20 °C | A405 28 °C | |||
| Ns-II-3 | 0.008 | 0.158 | 0.013 | 0.189 | TD (a) | undetermined |
| Ns-II-4 | 0.020 | 0.333 | 0.039 | 0.453 | TD | duplex |
| Ns-II-6 | 0.019 | 0.015 | 0.006 | 0.011 | R (b) | duplex |
| Ns-II-10 | 0.008 | 0.006 | 0.008 | 0.022 | R | duplex |
| Ns-II-11 | 0.625 | 1.002 | 0.213 | 0.361 | S (c) | nulliplex |
| Ns-II-40 | 0.007 | 0.007 | 0.007 | 0.009 | R | duplex |
| Ns-II-43 | 0.011 | 0.011 | 0.017 | 0.021 | R | duplex |
| Ns-II-81 | 0.018 | 0.173 | 0.016 | 0.548 | TD | duplex |
| PS-1723♂ | 0.009 | 0.005 | 0.004 | 0.010 | R | simplex |
| PW-363♀ | 0.012 | 0.009 | 0.013 | 0.012 | R | simplex |
| Sonda (R) | 0.010 | 0.013 | 0.007 | 0.006 | R | simplex |
| Etola (S) | 1.161 | 1.293 | 0.829 | 0.605 | S | nulliplex |
| Clone/ Cultivar | Mechanical Inoculation—Secondary Infection | Level of Resistance to PVS | Gene Dosage | |||
|---|---|---|---|---|---|---|
| S-966 | S-923 | |||||
| A405 20 °C | A405 28 °C | A405 20 °C | A405 28 °C | |||
| Ns-II-3 | 0.017 | 0.514 | 0.011 | 0.154 | TD (a) | undetermined |
| Ns-II-4 | 0.012 | 0.449 | 0.014 | 0.225 | TD | duplex |
| Ns-II-6 | 0.005 | 0.011 | 0.000 | 0.004 | R (b) | duplex |
| Ns-II-10 | 0.010 | 0.010 | 0.006 | 0.015 | R | duplex |
| Ns-II-11 | 0.713 | 0.651 | 0.182 | 0.321 | S (c) | nulliplex |
| Ns-II-40 | 0.006 | 0.009 | 0.009 | 0.021 | R | duplex |
| Ns-II-43 | 0.006 | 0.009 | 0.022 | 0.036 | R | duplex |
| Ns-II-81 | 0.013 | 0.655 | 0.009 | 0.501 | TD | duplex |
| PS-1723♂ | 0.009 | 0.003 | 0.004 | 0.021 | R | simplex |
| PW-363♀ | 0.011 | 0.010 | 0.012 | 0.008 | R | simplex |
| Sonda (R) | 0.015 | 0.010 | 0.011 | 0.011 | R | simplex |
| Etola (S) | 1.551 | 1.720 | 0.947 | 0.432 | S | nulliplex |
| Clone/ Cultivar | Graft Inoculation—Primary Infection | Level of Resistance to PVS | Gene Dosage | |||
|---|---|---|---|---|---|---|
| S-966 | S-923 | |||||
| A405 20 °C | A405 28 °C | A405 20 °C | A405 28 °C | |||
| Ns-II-3 | 0.021 | 1.390 | 0.006 | 0.449 | TD (a) | undetermined |
| Ns-II-4 | 0.013 | 0.877 | 0.015 | 0.211 | TD | duplex |
| Ns-II-6 | 0.017 | 0.018 | 0.011 | 0.020 | R (b) | duplex |
| Ns-II-10 | 0.029 | 0.015 | 0.031 | 0.011 | R | duplex |
| Ns-II-11 | 0.655 | 2.397 | 0.520 | 0.668 | S (c) | nulliplex |
| Ns-II-40 | 0.029 | 0.016 | 0.003 | 0.008 | R | duplex |
| Ns-II-43 | 0.026 | 0.015 | 0.058 | 0.018 | R | duplex |
| Ns-II-81 | 0.021 | 1.569 | 0.007 | 0.745 | TD | duplex |
| PS-1723♂ | 0.022 | 0.012 | 0.014 | 0.008 | R | simplex |
| PW-363♀ | 0.008 | 0.013 | 0.005 | 0.005 | R | simplex |
| Sonda (R) | 0.011 | 0.010 | 0.006 | 0.010 | R | simplex |
| Etola (S) | 1.871 | 1.468 | 0.575 | 0.540 | S | nulliplex |
| Clone/ Cultivar | Graft Inoculation—Secondary Infection | Level of Resistance to PVS | Gene Dosage | |||
|---|---|---|---|---|---|---|
| S-966 | S-923 | |||||
| A405 20 °C | A405 28 °C | A405 20 °C | A405 28 °C | |||
| Ns-II-3 | 0.012 | 1.121 | 0.017 | 0.617 | TD (a) | undetermined |
| Ns-II-4 | 0.023 | 0.499 | 0.020 | 0.224 | TD | duplex |
| Ns-II-6 | 0.023 | 0.020 | 0.013 | 0.012 | R (b) | duplex |
| Ns-II-10 | 0.017 | 0.007 | 0.014 | 0.014 | R | duplex |
| Ns-II-11 | 0.591 | 1.758 | 0.592 | 0.332 | S (c) | nulliplex |
| Ns-II-40 | 0.014 | 0.015 | 0.011 | 0.008 | R | duplex |
| Ns-II-43 | 0.020 | 0.007 | 0.096 | 0.020 | R | duplex |
| Ns-II-81 | 0.012 | 0.878 | 0.006 | 0.487 | TD | duplex |
| PS-1723♂ | 0.012 | 0.010 | 0.008 | 0.014 | R | simplex |
| PW-363♀ | 0.014 | 0.015 | 0.009 | 0.010 | R | simplex |
| Sonda (R) | 0.009 | 0.010 | 0.008 | 0.011 | R | simplex |
| Etola (S) | 2.979 | 1.157 | 0.806 | 0.657 | S | nulliplex |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatarowska, B.; Milczarek, D.; Szajko, K.; Plich, J.; Boguszewska-Mańkowska, D. Effect of Ns Gene Dosage and Temperature on the Level of Potato Resistance to PVS. Agriculture 2025, 15, 1239. https://doi.org/10.3390/agriculture15121239
Tatarowska B, Milczarek D, Szajko K, Plich J, Boguszewska-Mańkowska D. Effect of Ns Gene Dosage and Temperature on the Level of Potato Resistance to PVS. Agriculture. 2025; 15(12):1239. https://doi.org/10.3390/agriculture15121239
Chicago/Turabian StyleTatarowska, Beata, Dorota Milczarek, Katarzyna Szajko, Jarosław Plich, and Dominika Boguszewska-Mańkowska. 2025. "Effect of Ns Gene Dosage and Temperature on the Level of Potato Resistance to PVS" Agriculture 15, no. 12: 1239. https://doi.org/10.3390/agriculture15121239
APA StyleTatarowska, B., Milczarek, D., Szajko, K., Plich, J., & Boguszewska-Mańkowska, D. (2025). Effect of Ns Gene Dosage and Temperature on the Level of Potato Resistance to PVS. Agriculture, 15(12), 1239. https://doi.org/10.3390/agriculture15121239





