Response of Four Shrubs to Drought Stress and Comprehensive Evaluation of Their Drought Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Research Methods
2.2.1. Experimental Design
2.2.2. Measurement Indicators and Methods
2.2.3. Data Processing and Evaluation Methods
3. Results
3.1. Characteristics of Changes in Physiological and Biochemical Indicators
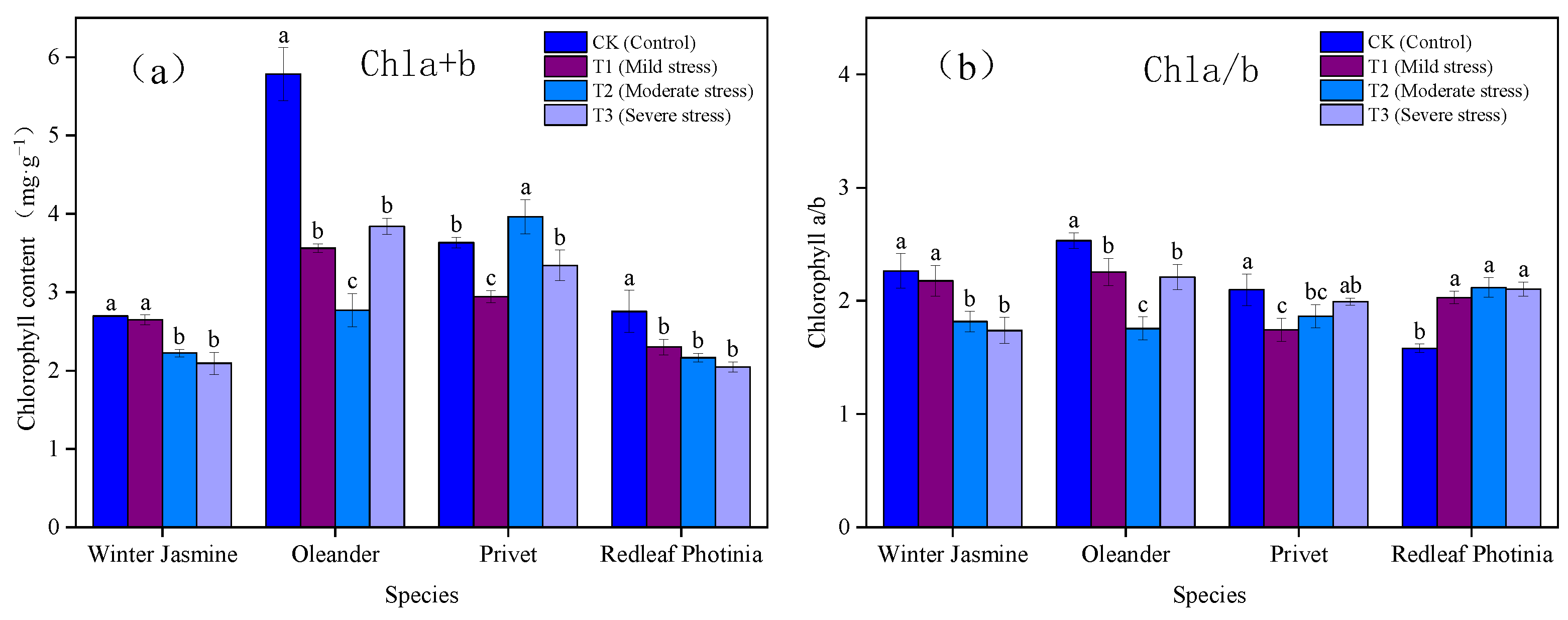
3.1.1. Effects of Drought Stress on Chlorophyll Content and Chlorophyll a/b Value
3.1.2. Effects of Drought Stress on Relative Water Content and Relative Electrical Conductivity of Leaves
3.1.3. Effects of Drought Stress on Proline Content and Soluble Sugar Content of Plants
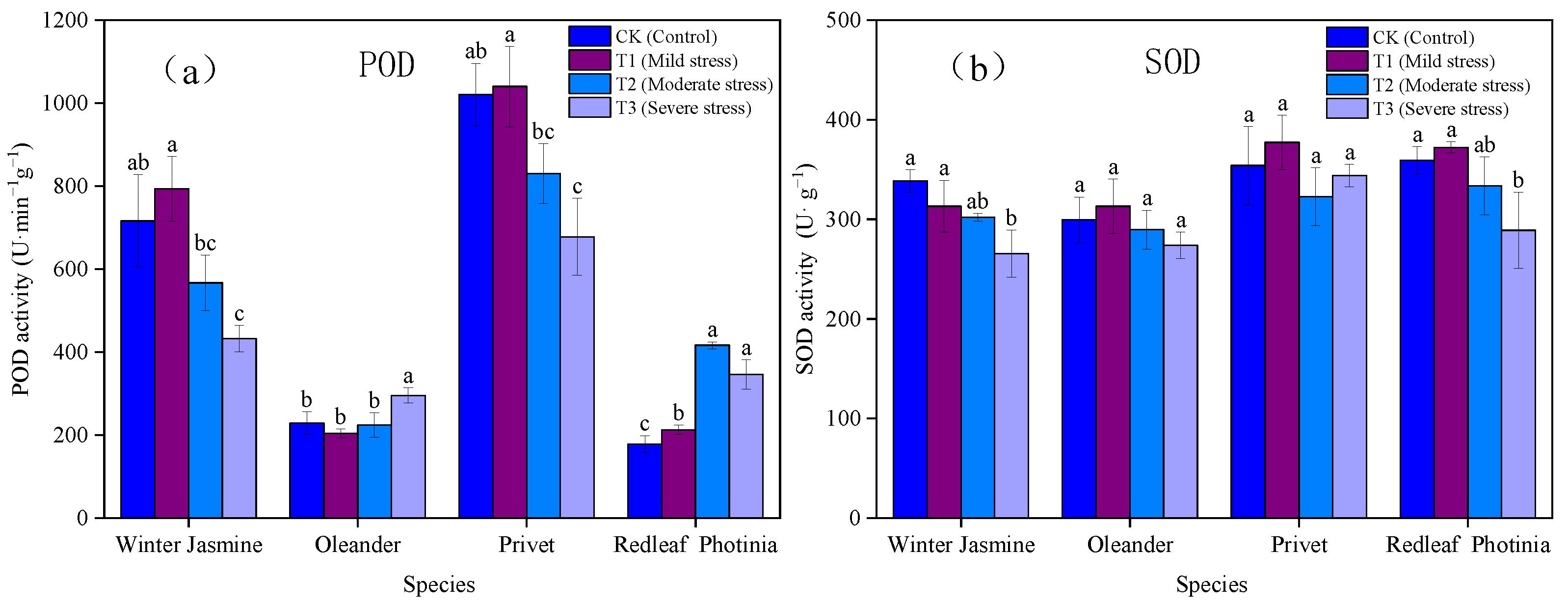
3.1.4. Effects of Drought Stress on the Activities of Peroxidase (POD) and Superoxide Dismutase (SOD) in Plants
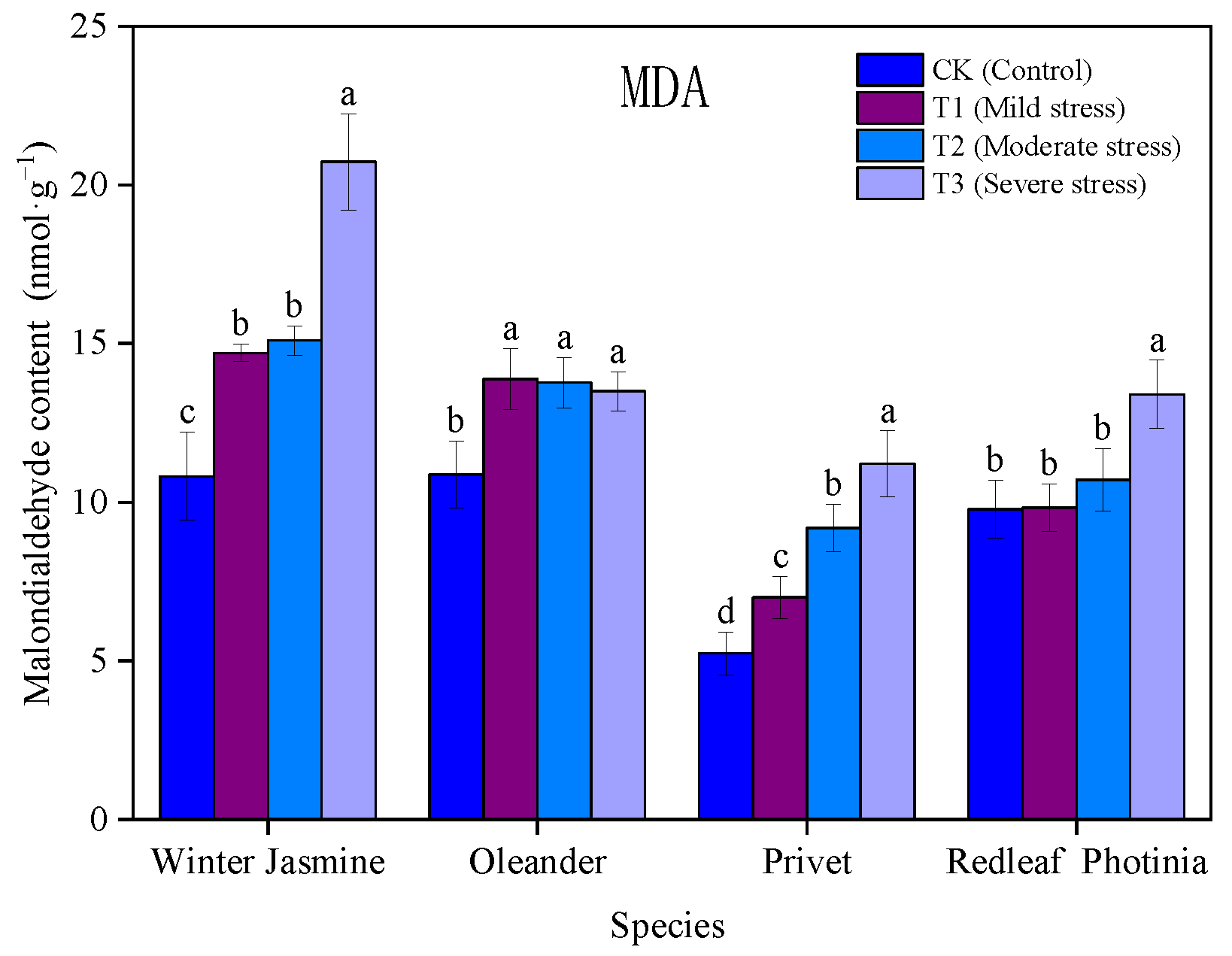
3.1.5. Effects of Drought Stress on the Content of Malondialdehyde in Plants
3.2. Correlation Analysis and Principal Component Analysis of Each Index
3.3. Fuzzy Comprehensive Evaluation of Drought Resistance Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Chla+b | Chlorophyll content |
| Pro | Proline |
| SS | Soluble sugar |
| MDA | Malondialdehyde |
| POD | Peroxidase |
| SOD | Superoxide dismutase |
| CK | Control |
| T1 | Mild stress |
| T2 | Moderate stress |
| T3 | Severe stress |
| Chla | Chlorophyll a content |
| Chlb | Chlorophyll b content |
| Chla/b | Chlorophyll a/b ratio |
| RWC | Relative water content |
| REC | Relative electrical conductivity |
| NBT | Nitroblue tetrazolium |
| PCA | Principal component analysis |
| ANOVA | One-way analysis of variance |
| HSD | Honestly Significant Difference |
| ROS | Reactive oxygen species |
References
- Wang, M.; Ding, Z.; Wu, C.; Song, L.; Ma, M.; Yu, P.; Lu, B.; Tang, X. Divergent responses of ecosystem water-use efficiency to extreme seasonal droughts in Southwest China. Sci. Total Environ. 2021, 760, 143427. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Chen, X.Y. Main processes affecting global average surface temperature. Chin. Sci. Bull. 2021, 66, 4017–4027. (In Chinese) [Google Scholar] [CrossRef]
- Ding, Y.Q. A Review of Research on Drought Resistance of Trees and Drought Resistant Silvicultural Techniques. J. Agric. Catastrophol. 2024, 14, 70–72. [Google Scholar]
- La, B.; Hu, J.; Zhang, X.P. Research Progress on the Physiological Effects of Drought Stress on Plants and the Response of Molecular Mechanism. Qinghai Pratacult. 2022, 31, 31–35. (In Chinese) [Google Scholar]
- Deng, P.Y.; Zhou, J.L.; Tao, D.H.; Liu, K.; Wu, Y.X. Effects of Drought Stress on Photosynthesis and Chlorophyll Fluorescence Parameters of Six Woody Vine Species. J. Hunan Agric. Univ. (Nat. Sci.) 2015, 41, 263–270. (In Chinese) [Google Scholar]
- Cui, T.; Yu, H.; Li, H.; Bian, X.; Wang, L. Effect of drought stress and rewatering on physiological characteristics of Pennisetum alopecuroides seedlings. Pratacult. Sci. 2017, 34, 788–793. [Google Scholar]
- Zhao, X.; Zhang, X.Z.; Mou, H.X.; Sun, X.; He, H.Y.; Yang, K.W.; Shan, Y.Y.; Li, C.Y. Effect of drought stress on the water physiological characteristics and the osmoticregulation substances of Xanthoceras sorbifollia seedings from different provenances. J. Northeast For. Univ. 2017, 45, 17–21. (In Chinese) [Google Scholar]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.-K.; Takahashi, Y.; Munemasa, S.; Schroeder, J.I. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.E.; Schulze, E.-D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef]
- Chandregowda, M.H.; Murthy, K.; Bagchi, S. Woody shrubs increase soil microbial functions and multifunctionality in a tropical semi-arid grazing ecosystem. J. Arid Environ. 2018, 155, 65–72. [Google Scholar] [CrossRef]
- Liu, M.; Xu, X.; Wang, D.; Sun, A.Y.; Wang, K. Karst catchments exhibited higher degradation stress from climate change than the non-karst catchments in southwest China: An ecohydrological perspective. J. Hydrol. 2016, 535, 173–180. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhao, W.Z.; Zhang, C.; Wang, X.P.; Liao, H.M.; Liu, D.M. Comprehensive evaluation of drought tolerance of three species of Miscanthaceae. Jiangsu Agric. Sci. 2021, 49, 92–96. [Google Scholar]
- Zhang, F.; Chen, H.Y.; Chen, S.; Sun, Y.M.; Li, H.Y.; Ma, Y.; Ding, W.L.; Tang, K.X. Effects of drought stress on photosynthetic characteristics of Nerium indicum. Forestry Science & Technology. 2022, 47, 18–22. [Google Scholar]
- Wang, C.; Zhou, C.N.; Lu, J.; Jiang, L.L. Study on Leaf Functional Traits of Photinia fraseri under Different Habitat Conditions. J. Plateau Agric. 2021, 5, 217–225. [Google Scholar]
- Zhang, Q.W.; Zhu, S.D.; Jansen, S.; Cao, K.F. Topography strongly affects drought stress and xylem embolism resistance in woody plants from a karst forest in Southwest China. Funct. Ecol. 2021, 35, 566–577. [Google Scholar] [CrossRef]
- Xu, W.; Ji, S.F. Effects of different extraction methods on chlorophyll content. J. Langfang Norm. Univ. (Nat. Sci. Ed.) 2022, 22, 56–59. [Google Scholar]
- Yang, Y.; Zhou, Y.; Ban, X.W.; Zhou, M.Q.; Wang, J.; Yang, X.Y.; Lei, J.; Yang, C.L. Effects of morphological and physiological characteristics of coixlacryma-jobi L. seedlings under drought stress. Molecular Plant Breeding. 2023, 1–19. [Google Scholar]
- Wang, Y.; Yu, S.M.; Wang, J.Y.; Song, H.L.; Lei, Y.D.; Zhang, H.Y.; Liu, B. Effects of drought stress and rehydration on physiological characteristics of Hemerocallis fulva. Chin. Agric. Sci. Bull. 2023, 39, 58–64. [Google Scholar]
- Liu, R.; Wang, C.Z.; Ma, Y.H. Analysis of proline content and HrP5CS gene expression in Hippophae rhamnoides under drought stress. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2023, 52, 846–852. [Google Scholar]
- Gao, Y.N.; Xuan, Z.Y.; Zhang, K.H.; Li, Y.; Chang, J.; Ayimaimu, S.; Ma, G.C. Effects of drought stress on physiological traits of different drought-resistant turnip seedlings. Agric. Res. Arid Areas 2023, 41, 127–133. [Google Scholar]
- Li, Y.H.; Cui, Y.F.; Zhang, Y.C.; Su, Z.W.; Mou, N.N.; Guo, J.H.; Cui, Y.X. Evaluation of drought-resistance of nine landscape ground cover plants. J. Anhui Agric. Sci. 2021, 49, 112–115. [Google Scholar]
- Li, G.Q.; Zhang, H.Y.; Ji, L.; Zhao, E.K.; Liu, J.B.; Li, L.; Zhang, J.R. Comprehensive evaluation on drought resistance of different soybean varieties. Chin. J. Appl. Ecol. 2006, 17, 2408–2412. [Google Scholar]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Liu, Q.F.; Zhou, Z.Y.; Du, N.N. Effects of drought stress and re-watering on photosynthetic characteristics of maize at seedling sage. Agric. Eng. 2021, 11, 112–120. (In Chinese) [Google Scholar]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant. 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef]
- Matysik, J.; Alia; Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Che, C.; Xiao, S.; Peng, X.; Ding, A.; Su, J. Radial growth of Korshinsk peashrub and its response to drought in different sub-arid climate regions of northwest China. J. Environ. Manag. 2023, 326, 116708. [Google Scholar] [CrossRef]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]


| Plant Name | Family | Morphological Characteristics, Habits, and Ecological Functions |
|---|---|---|
| Winter Jasmine (Jasminum nudiflorum) | Oleaceae | Deciduous shrub, light-loving and slightly shade-tolerant; likes a warm, humid climate but also cold conditions; exhibits drought tolerance but not tolerance of water and humidity; the soil requirements are not strict; shallow roots, strong growth, and strong germination |
| Oleander (Nerium oleander) | Apocynaceae | Evergreen upright shrub; light-loving; prefers warm, humid conditions; hardy; strong drought resistance; the soil requirements are not strict; and it can grow on alkaline soil |
| Privet (Ligustrum lucidum) | Oleaceae | Evergreen shrubs or small trees with dense foliage; good cold resistance and water and wet resistance; prefers a warm and humid climate; light and shade tolerance; deep-rooted species with developed fibrous roots, fast growth, strong germination, and resistance to pruning |
| Redleaf Photinia (Photinia × fraseri) | Rosaceae | Evergreen shrub, light-tolerant, slightly shade-tolerant, warm and humid climate-tolerant; prefers dry and barren conditions and does not like wet conditions |
| Processing Number | Stress Gradient | Soil Water Content (%) | Ratio of Soil Water Content to Field Water Capacity (%) |
|---|---|---|---|
| CK | Control | 22~23 | 75 |
| T1 | Mild stress | 18~19 | 60 |
| T2 | Moderate stress | 13~14 | 45 |
| T3 | Severe stress | 10~11 | 35 |
| Index | Chla | Chlb | Chla+b | Chla/b | RWC | REC | Pro | MDA | POD | SOD |
|---|---|---|---|---|---|---|---|---|---|---|
| Chlb | 0.919 | |||||||||
| Chla+b | 0.994 | 0.956 | ||||||||
| Chla/b | 0.608 | 0.258 | 0.522 | |||||||
| RWC | −0.229 | −0.294 | −0.251 | −0.005 | ||||||
| REC | −0.417 | −0.472 | −0.439 | −0.075 | −0.329 | |||||
| Pro | −0.200 | −0.145 | −0.190 | −0.267 | −0.608 | 0.464 | ||||
| MDA | −0.299 | −0.378 | −0.328 | −0.071 | −0.470 | 0.533 | 0.526 | |||
| POD | −0.031 | 0.057 | −0.006 | −0.105 | −0.005 | 0.360 | 0.013 | −0.426 | ||
| SOD | −0.093 | 0.009 | −0.065 | −0.179 | 0.548 | −0.287 | −0.331 | −0.798 | 0.387 | |
| SS | −0.088 | −0.035 | −0.075 | −0.121 | −0.632 | 0.369 | 0.713 | 0.253 | 0.306 | −0.318 |
| Principal Component | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| Eigenvalue | 3.957 | 3.032 | 1.763 | 1.014 |
| Contribution rate/% | 35.977 | 27.563 | 16.03 | 9.22 |
| Cumulative contribution rate/% | 35.977 | 63.54 | 79.571 | 88.791 |
| Index | Chla | Chlb | Chla+b | Chla/b | RWC | REC | Pro | MDA | POD | SOD | SS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.799 | 0.763 | 0.803 | 0.463 | 0.288 | −0.721 | −0.639 | −0.708 | −0.002 | 0.368 | −0.478 |
| 2 | 0.585 | 0.533 | 0.580 | 0.340 | −0.844 | 0.157 | 0.506 | 0.437 | −0.122 | −0.684 | 0.536 |
| 3 | 0.018 | 0.191 | 0.067 | −0.254 | −0.178 | 0.227 | 0.254 | −0.491 | 0.883 | 0.481 | 0.508 |
| 4 | 0.080 | −0.219 | 0.000 | 0.675 | 0.135 | 0.524 | −0.256 | 0.057 | 0.350 | −0.103 | −0.096 |
| Species of Trees | Drought Stress Gradient | Composite Indicator Values by Means of the Value of the Affiliation Function Method | Comprehensive Valuation | Rank | ||||
|---|---|---|---|---|---|---|---|---|
| Principal Component 1 | Principal Component 2 | Principal Component 3 | Principal Component 4 | D | Average D | |||
| Winter Jasmine | CK | 0.486 | 0.729 | 0.607 | 0.000 | 0.533 | 0.407 | 4 |
| T1 | 0.356 | 0.536 | 0.591 | 0.016 | 0.419 | |||
| T2 | 0.196 | 0.511 | 0.610 | 0.499 | 0.400 | |||
| T3 | 0.000 | 0.235 | 0.765 | 0.615 | 0.275 | |||
| Oleander | CK | 0.483 | 0.519 | 0.625 | 0.535 | 0.525 | 0.575 | 2 |
| T1 | 1.000 | 0.000 | 0.936 | 0.423 | 0.618 | |||
| T2 | 0.612 | 0.382 | 1.000 | 0.553 | 0.605 | |||
| T3 | 0.453 | 0.500 | 0.970 | 0.964 | 0.614 | |||
| Privet | CK | 0.543 | 0.110 | 0.868 | 0.509 | 0.464 | 0.465 | 3 |
| T1 | 0.483 | 0.519 | 0.625 | 0.535 | 0.525 | |||
| T2 | 0.763 | 0.768 | 0.207 | 0.407 | 0.627 | |||
| T3 | 0.588 | 0.873 | 0.045 | 0.662 | 0.586 | |||
| Redleaf Photinia | CK | 0.583 | 0.222 | 0.128 | 0.654 | 0.396 | 0.654 | 1 |
| T1 | 0.343 | 0.100 | 0.000 | 0.783 | 0.251 | |||
| T2 | 0.483 | 0.519 | 0.625 | 0.535 | 0.525 | |||
| T3 | 0.580 | 0.960 | 0.825 | 1.000 | 0.786 | |||
| Weight | 40.52% | 31.04% | 18.05% | 10.38% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Hu, H.; Liu, X.; Wang, Q.; Zhou, H.; Chen, S.; Liu, J.; Li, Y. Response of Four Shrubs to Drought Stress and Comprehensive Evaluation of Their Drought Resistance. Agriculture 2025, 15, 1211. https://doi.org/10.3390/agriculture15111211
Ma B, Hu H, Liu X, Wang Q, Zhou H, Chen S, Liu J, Li Y. Response of Four Shrubs to Drought Stress and Comprehensive Evaluation of Their Drought Resistance. Agriculture. 2025; 15(11):1211. https://doi.org/10.3390/agriculture15111211
Chicago/Turabian StyleMa, Bing, Haibo Hu, Xingyu Liu, Qi Wang, Hongwei Zhou, Sheng Chen, Jiacai Liu, and Yuyan Li. 2025. "Response of Four Shrubs to Drought Stress and Comprehensive Evaluation of Their Drought Resistance" Agriculture 15, no. 11: 1211. https://doi.org/10.3390/agriculture15111211
APA StyleMa, B., Hu, H., Liu, X., Wang, Q., Zhou, H., Chen, S., Liu, J., & Li, Y. (2025). Response of Four Shrubs to Drought Stress and Comprehensive Evaluation of Their Drought Resistance. Agriculture, 15(11), 1211. https://doi.org/10.3390/agriculture15111211





