Utilization of Gluconacetobacter diazotrophicus in Tomato Crop: Interaction with Nitrogen and Phosphorus Fertilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Location
2.2. Plant Material
2.3. Microbial Cultures
2.4. Preparation of Greenhouses
2.5. Experimental Design
2.6. Transplant and Crop Management
2.7. Evaluation of Yield and Quality
2.8. Statistical Analysis
3. Results
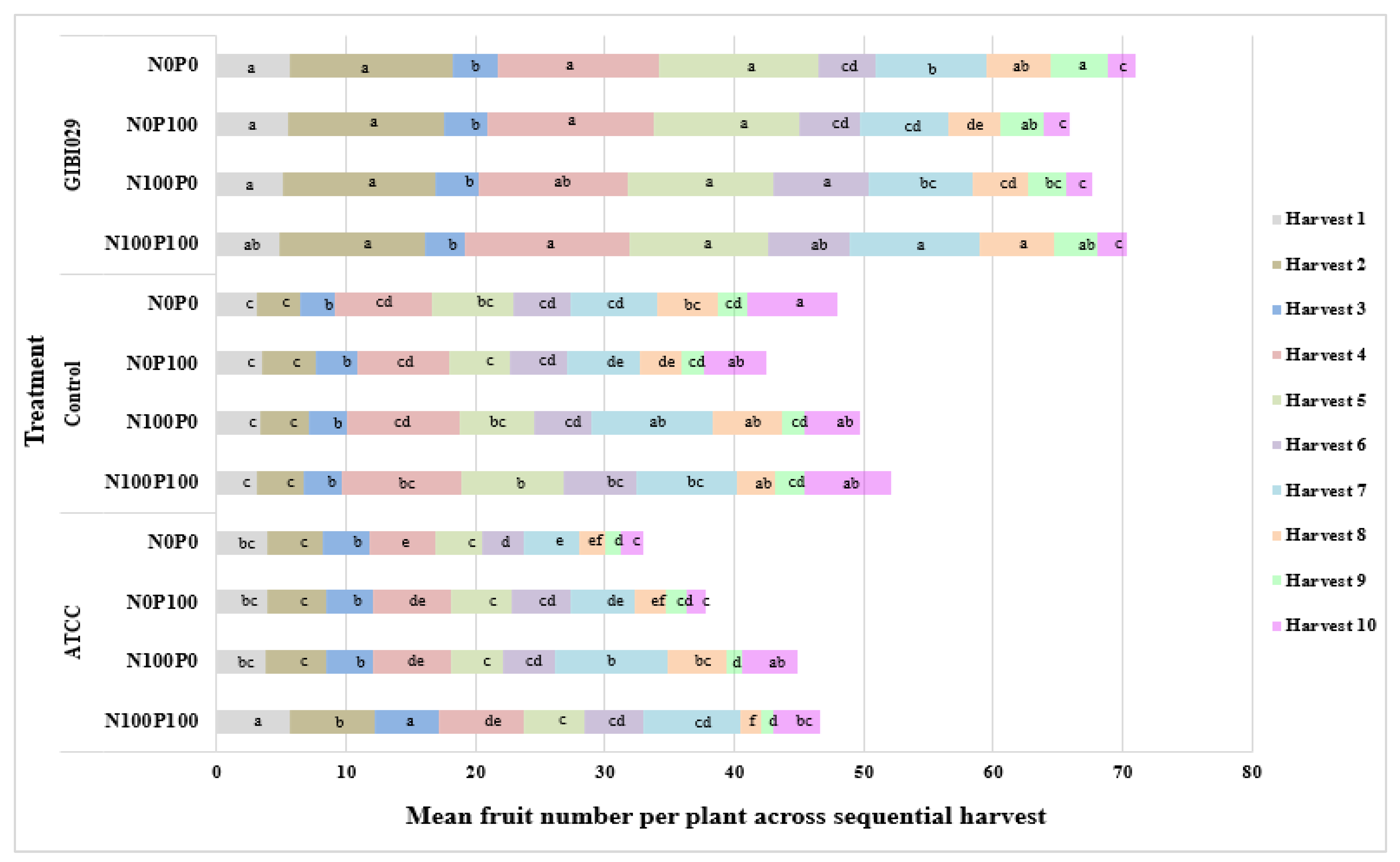
3.1. Number of Fruits per Plant
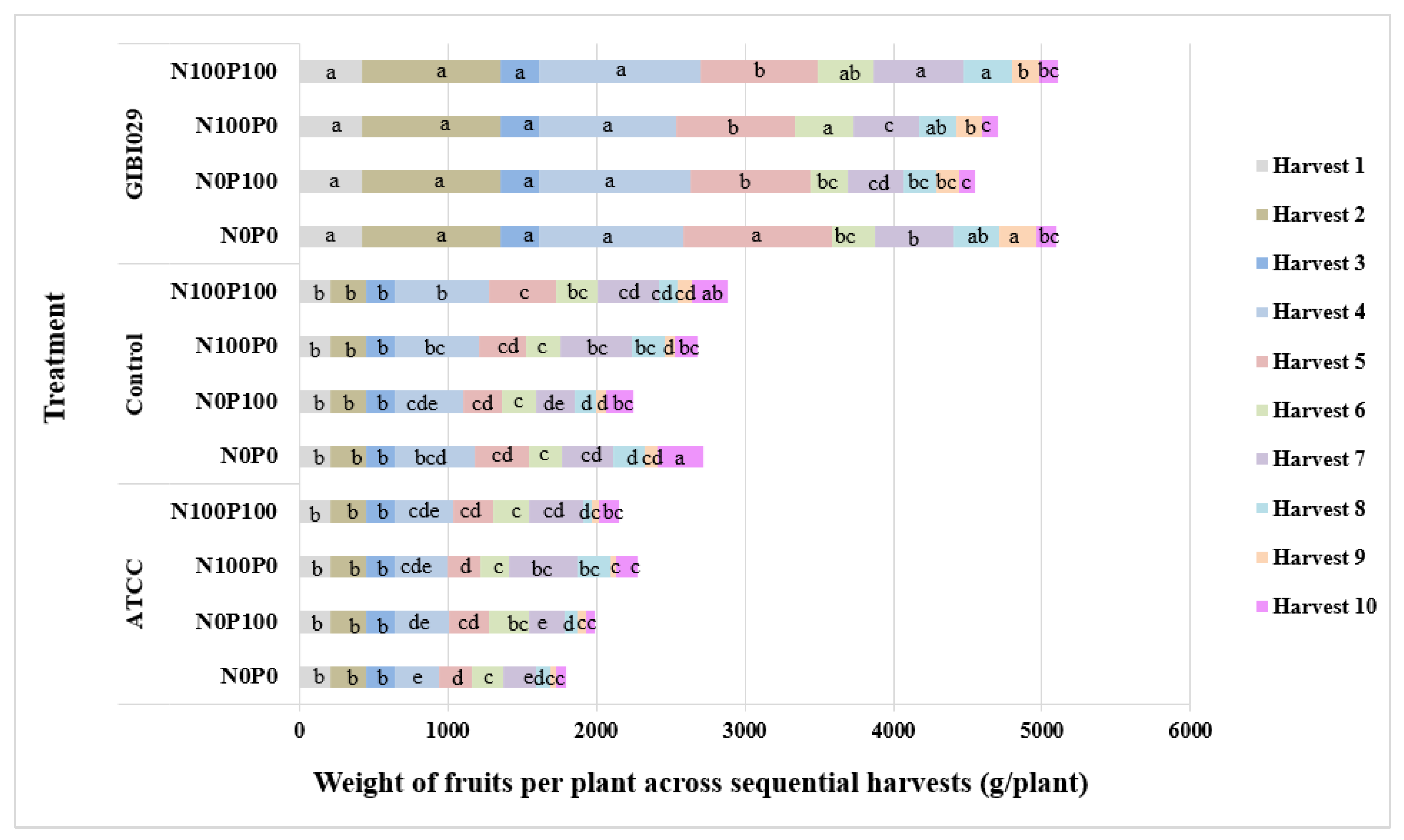
3.2. Weight of Fruits per Plant
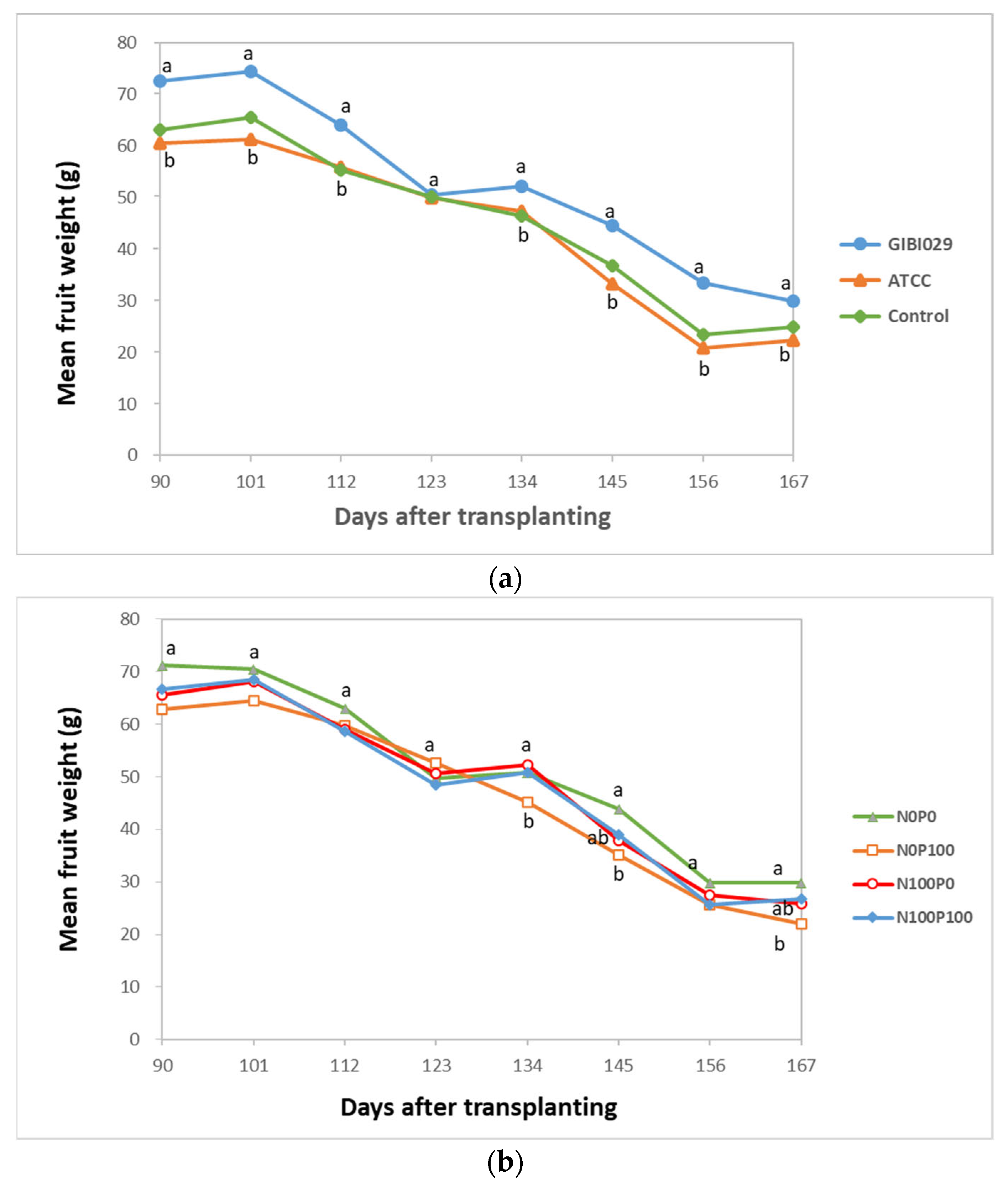
3.3. Mean Fruit Weight
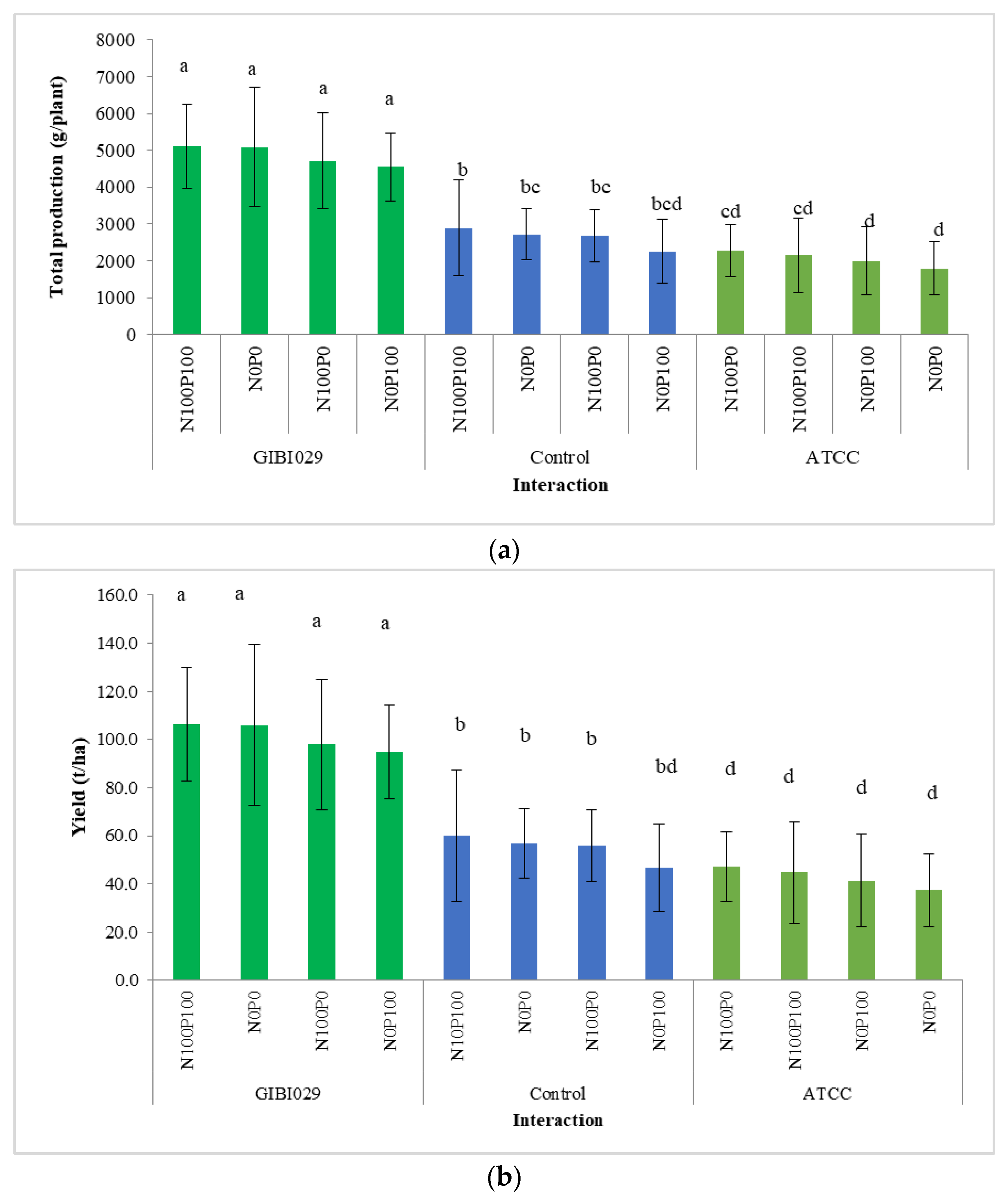
3.4. Total Number of Fruits per Plant and Total Production per Plant
3.5. Crop Yield
4. Discussion
4.1. Soil Analysis
4.2. Fruit Number and Weight
4.3. Per Plant Production and Mean Fruit Weight
4.4. Crop Yield
4.5. Harvest-Stage Analysis
4.6. Overall Comparison of Results on Yield Components
4.7. Comparison of Native Isolate and Reference Strain
4.8. Advantages of Native Colombian GIBI029 Isolate
4.9. Limitations of This Study
4.10. Comparison with Findings from Previous Studies
4.11. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Units | Value |
|---|---|---|
| N | % | 0.53 |
| pH | 4.8 | |
| Na | cmol/kg | 0.43 |
| Ca | cmol/kg | 2.38 |
| Mg | cmol/kg | 0.65 |
| K | cmol/kg | 0.19 |
| P | ppm | 299 |
| Fe | ppm | 669 |
| Cu | ppm | 7.97 |
| Zn | ppm | 28.65 |
| Mn | ppm | 38.35 |
| B | ppm | 1.9 |
| S | ppm | 25.54 |
| OM | % | 14.08 |
| Texture | Sand—76%; Silt—13%; Clay—11% | |
| Type of Treatment | Nitrogen (%) | Phosphorus (mg/kg) |
|---|---|---|
| N0P0 | 0.61 a | 220.33 b |
| N0P100 | 0.59 a | 223.67 b |
| N100P0 | 0.58 a | 273.67 a |
| N100P100 | 0.60 ab | 259.22 ab |
| ATCC 49037 | 0.60 b | 227.33 b |
| GIBI029 | 0.64 a | 284.75 a |
| No bacteria | 0.56 c | 220.58 b |
References
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.-L.; Li, Q.; Zeng, X.-P.; Liu, Y.; Li, Y.-R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Xu, M.; Li, R.; Zheng, L.; Liu, S.; Reis, S.; Wang, H.; Lu, C.; Zhang, W.; Gao, H. Optimizing nitrogen fertilizer use for more grain and less pollution. J. Clean. Prod. 2022, 360, 132180. [Google Scholar] [CrossRef]
- Pöyry, J.; Carvalheiro, L.G.; Heikkinen, R.K.; Kühn, I.; Kuussaari, M.; Schweiger, O.; Valtonen, A.; van Bodegom, P.M.; Franzén, M. The effects of soil eutrophication propagate to higher trophic levels. Glob. Ecol. Biogeogr. 2017, 26, 18–30. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Pooniya, V.; Bana, R.; Kumar, A.; Singh, U. Mitigating pulse productivity constraints through phosphorus fertilization—A review. Agric. Rev. 2014, 35, 314–319. [Google Scholar] [CrossRef]
- Rojas, M.M.; Rodríguez, A.J.; Trujillo, I.D.; Heydrich, M. Relación de la fijación de nitrógeno y la producción de auxinas en cepas de Gluconacetobacter diazotrophicus procedentes de diferentes cultivos (Relationships between nitrogen fixation and auxin production in Gluconacetobacter diazotrophicus strains from different crops). Rev. Colomb. Biotecnol. 2009, 11, 84–93. (In Spanish) [Google Scholar]
- Cavalcante, V.A.; Döbereiner, J. A new acid-tolerant nitrogen-fixing bacterium associated with sugar cane. Plant Soil 1988, 108, 23–31. [Google Scholar] [CrossRef]
- Cocking, E.C. Endophytic colonization of plant roots by nitrogen-fixing bacteria. Plant Soil 2003, 252, 169–175. [Google Scholar] [CrossRef]
- Leandro, M.; Andrade, L.; De Souza Vespoli, L.; Soares, F.; Moreira, J.; Pimentel, V.; Barbosa, R.; De Oliveira, M.; Silveira, V.; De Souza Filho, G. Combination of osmotic stress and sugar stress response mechanisms is essential for Gluconacetobacter diazotrophicus tolerance to high-sucrose environments. Appl. Microbiol. Biotechnol. 2021, 105, 7463–7473. [Google Scholar] [CrossRef]
- Ceballos-Aguirre, N.; Cuellar, J.A.; Restrepo, G.M.; Sánchez, Ó.J. Effect of the application of Gluconacetobacter diazotrophicus and its interaction with nitrogen and phosphorus fertilization on carrot yield in the field. Int. J. Agron. 2023, 2023, 6899532. [Google Scholar] [CrossRef]
- Ríos Rocafull, Y.; Sánchez López, M.; Dibut Álvarez, B.; Ortega García, M.; Tejeda González, G.; Rodríguez Sánchez, J.; Rojas Badía, M. The culture medium effect in plant growth promotion activity of Gluconacetobacter diazotrophicus in carrot and sugar beet. Rev. Bio Cienc. 2019, 6, e470. [Google Scholar] [CrossRef]
- Jiménez, T.; Fuentes, L.E.; Tapia, A.; Macarua, M.; Martínez, E.; Caballero, J. Coffea arabica L., a new host plant for Acetobacter diazotrophicus, and isolation of other nitrogen-fixing acetobacteria. Appl. Environ. Microbiol. 1997, 63, 3676–3683. [Google Scholar] [CrossRef] [PubMed]
- Tapia, A.; Bustillos, M.R.; Jiménez, T.; Caballero, J.; Fuentes, L.E. Natural endophytic occurrence of Acetobacter diazotrophicus in pineapple plants. Microb. Ecol. 2000, 39, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Dibut, B.; Martínez, R.; Ríos, Y.; Plana, L.; Rodríguez, J.; Ortega, M.; Tejada, G. Estudio de la asociación Gluconacetobacter diazotrophicus-viandas tropicales en suelo ferralítico rojo. I. selección de cepas efectivas para la biofertilización de boniato, yuca y malanga (Study of the association Gluconacetobacter diazotrophicus-tropical foods in red ferralite Soil I. Selection of effective strains for the biofertilization of sweet potato, cassava and taro). Cultiv. Tropic. 2010, 31, 51–57. (In Spanish) [Google Scholar]
- Madhaiyan, M.; Saravanan, V.; Jovi, D.B.S.S.; Lee, H.; Thenmozhi, R.; Hari, K.; Sa, T. Occurrence of Gluconacetobacter diazotrophicus in tropical and subtropical plants of Western Ghats, India. Microbiol. Res. 2004, 159, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, V.; Madhaiyan, M.; Osborne, J.; Thangaraju, M.; Sa, T. Ecological occurrence of Gluconacetobacter diazotrophicus and nitrogen-fixing Acetobacteraceae members: Their possible role in plant growth promotion. Microb. Ecol. 2008, 55, 130–140. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 5 March 2025).
- Gil, R.; Bojacá, C.R.; Schrevens, E. Understanding the heterogeneity of smallholder production systems in the Andean tropics—The case of Colombian tomato growers. NJAS Wagening J. Life Sci. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Herrera, H.J.; Hurtado-Salazar, A.; Ceballos-Aguirre, N. Estudio técnico y económico del tomate tipo cereza élite (Solanum lycopersicum L. var. cerasiforme) bajo condiciones semicontroladas (Economic study of the elite cherry tomato type (Solanum lycopersicum L. var. cerasiforme) under semicontrolled conditions). Rev. Colomb. Cienc. Hortic. 2015, 9, 290–300. (In Spanish) [Google Scholar] [CrossRef]
- Rahman, K.A.; Zhang, D. Effects of fertilizer broadcasting on the excessive use of inorganic fertilizers and environmental sustainability. Sustainability 2018, 10, 759. [Google Scholar] [CrossRef]
- Baez-Rogelio, A.; Morales-García, Y.E.; Quintero-Hernández, V.; Muñoz-Rojas, J. Next generation of microbial inoculants for agriculture and bioremediation. Microb. Biotechnol. 2017, 10, 19–21. [Google Scholar] [CrossRef]
- De Andrade, L.A.; Santos, C.; Frezarin, E.T.; Sales, L.; Rigobelo, E. Plant growth-promoting rhizobacteria for sustainable agricultural production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef]
- Ladha, J.K.; Peoples, M.B.; Reddy, P.M.; Biswas, J.C.; Bennett, A.; Jat, M.L.; Krupnik, T.J. Biological nitrogen fixation and prospects for ecological intensification in cereal-based cropping systems. Field Crops Res. 2022, 283, 108541. [Google Scholar] [CrossRef] [PubMed]
- Cocking, E.C.; Stone, P.J.; Davey, M.R. Intracellular colonization of roots of Arabidopsis and crop plants by Gluconacetobacter diazotrophicus. Vitr. Cell. Dev. Biol.-Plant 2006, 42, 74–82. [Google Scholar] [CrossRef]
- Luna, M.F.; Aprea, J.; Crespo, J.M.; Boiardi, J.L. Colonization and yield promotion of tomato by Gluconacetobacter diazotrophicus. Appl. Soil Ecol. 2012, 61, 225–229. [Google Scholar] [CrossRef]
- Botta, A.L.; Santacecilia, A.; Ercole, C.; Cacchio, P.; Del Gallo, M. In vitro and in vivo inoculation of four endophytic bacteria on Lycopersicon esculentum. N. Biotechnol. 2013, 30, 666–674. [Google Scholar] [CrossRef]
- Pallucchini, M.; Franchini, M.; El-Ballat, E.M.; Narraidoo, N.; Pointer-Gleadhill, B.; Palframan, M.J.; Hayes, C.J.; Dent, D.; Cocking, E.C.; Perazzolli, M. Gluconacetobacter diazotrophicus AZ0019 requires functional nifD gene for optimal plant growth promotion in tomato plants. Front. Plant Sci. 2024, 15, 1469676. [Google Scholar] [CrossRef]
- Caballero-Mellado, J.; Fuentes-Ramirez, L.E.; Reis, V.M.; Martinez-Romero, E. Genetic structure of Acetobacter diazotrophicus populations and identification of a new genetically distant group. Appl. Environ. Microbiol. 1995, 61, 3008–3013. [Google Scholar] [CrossRef]
- Kirchhof, G.; Reis, V.M.; Baldani, J.I.; Eckert, B.; Döbereiner, J.; Hartmann, A. Occurrence, physiological and molecular analysis of endophytic diazotrophic bacteria in gramineous energy plants. Plant Soil 1997, 194, 45–55. [Google Scholar] [CrossRef]
- Muthukumarasamy, R.; Revathi, G.; Lakshminarasimhan, C. Influence of N fertilisation on the isolation of Acetobacter diazotrophicus and Herbaspirillum spp. from Indian sugarcane varieties. Biol. Fertil. Soils 1999, 29, 157–164. [Google Scholar] [CrossRef]
- Bueno dos Reis Junior, F.; Massena Reis, V.; Urquiaga, S.; Döbereiner, J. Influence of nitrogen fertilisation on the population of diazotrophic bacteria Herbaspirillum spp. and Acetobacter diazotrophicus in sugar cane (Saccharum spp.). Plant Soil 2000, 219, 153–159. [Google Scholar] [CrossRef]
- Hari, K.; Srinivasan, T. Response of sugarcane varieties to application of nitrogen fixing bacteria under different nitrogen levels. Sugar Tech. 2005, 7, 28–31. [Google Scholar] [CrossRef]
- Medeiros, A.; Polidoro, J.; Reis, V. Nitrogen source effect on Gluconacetobacter diazotrophicus colonization of sugarcane (Saccharum spp.). Plant Soil 2006, 279, 141–152. [Google Scholar] [CrossRef]
- Tufail, M.A.; Touceda-González, M.; Pertot, I.; Ehlers, R.-U. Gluconacetobacter diazotrophicus Pal5 enhances plant robustness status under the combination of moderate drought and low nitrogen stress in Zea mays L. Microorganisms 2021, 9, 870. [Google Scholar] [CrossRef] [PubMed]
- Van Long, V.; Duong, V.T.; Van Dung, T.; Nghia, N.K.; Nam, T.S.; Viet, N.Q.; Van Cuong, L.; Van Tien, H. Gluconacetobacter diazotrophicus bacteria combined nitrogen fertilization promotes rice yield and soil quality in the paddy field in the Vietnamese Mekong Delta Region. Malays. J. Soil Sci. 2024, 28, 359–368. [Google Scholar]
- Delaporte-Quintana, P.; Grillo-Puertas, M.; Lovaisa, N.C.; Teixeira, K.R.; Rapisarda, V.A.; Pedraza, R.O. Contribution of Gluconacetobacter diazotrophicus to phosphorus nutrition in strawberry plants. Plant Soil 2017, 419, 335–347. [Google Scholar] [CrossRef]
- Delaporte-Quintana, P.; Grillo-Puertas, M.; Lovaisa, N.C.; Rapisarda, V.A.; Teixeira, K.R.; Pedraza, R.O. Solubilization of different sources of insoluble phosphate of Gluconacetobacter diazotrophicus. Rev. Agron. Noreste Argent. 2016, 36, 33–35. [Google Scholar]
- Idogawa, N.; Ryuta, A.; Kousaku, M.; Kawai, S. Phosphate enhances levan production in the endophytic bacterium Gluconacetobacter diazotrophicus Pal5. Bioengineered 2014, 5, 173–179. [Google Scholar] [CrossRef]
- Restrepo, G.M.; Sánchez, Ó.J.; Marulanda, S.M.; Galeano, N.F.; Taborda, G. Evaluation of plant-growth promoting properties of Gluconacetobacter diazotrophicus and Gluconacetobacter sacchari isolated from sugarcane and tomato in West Central region of Colombia. Afr. J. Biotechnol. 2017, 16, 1619–1629. [Google Scholar] [CrossRef]
- Universidad de Caldas. Sistema de Granjas (System of Farms). Available online: http://www.ucaldas.edu.co/portal/sistema-de-granjas/ (accessed on 12 October 2020). (In Spanish).
- ImpulSemillas. Tomate Híbrido Roble 956 F1 (Roble 956 F1 Hybrid Tomato). Available online: https://www.impulsemillas.com/documentos/fichas/Toamate_Roble_compressed.pdf (accessed on 4 June 2024). (In Spanish).
- ImpulSemillas. Tomate Híbrido Roble 956 F1 (Roble 956 F1 Hybrid Tomato). Available online: https://www.impulsemillas.com/producto/tomate-roble-956-f1/ (accessed on 1 February 2022). (In Spanish).
- Restrepo, G.M.; Ceballos, N.; Valencia, L.F.; Sánchez, Ó.J. Plant growth promotion by Gluconacetobacter diazotrophicus and its interaction with genotype and phosphorus availability in tomato seedlings. Org. Agr. 2021, 11, 601–614. [Google Scholar] [CrossRef]
- Jaramillo, J.; Rodríguez, V.P.; Guzmán, M.; Zapata, M.; Rengifo, T. Buenas Prácticas Agrícolas (BPA) en la Producción de Tomate bajo Condiciones Protegidas (Good Agricultural Practices (GAP) in Tomato Production Under Protected Conditions); Organización de las Naciones Unidas para la Agricultura y la Alimentación (FAO): Rome, Italy, 2007. (In Spanish) [Google Scholar]
- Jaramillo, J.E.; Rodríguez, V.P.; Gil, L.F.; García, M.C.; Clímaco, J.; Quevedo, D.; Sánchez, G.D.; Aguilar, P.A.; Pinzón, L.M.; Zapata, M.Á.; et al. Tecnología para el Cultivo de Tomate bajo Condiciones Protegidas (Technology for Growing Tomatoes Under Protected Conditions); Corpoica: Bogotá, Colombia, 2012. (In Spanish) [Google Scholar]
- United States Standards for Grades of Fresh Tomatoes. Available online: https://www.ams.usda.gov/sites/default/files/media/Tomato_Standard%5B1%5D.pdf (accessed on 1 October 2022).
- Análisis de Suelos y su Interpretación (Soil Analysis and Its Interpretation). Available online: www.infoagro.go.cr/Inforegiones/RegionCentralOriental/Documents/Suelos/SUELOS-AMINOGROWanalisiseinterpretacion.pdf (accessed on 1 February 2024). (In Spanish).
- IGAC; CORPOCALDAS. Estudio Semidetallado de Suelos de los Municipios de Manizales, Chinchiná, Palestina, Neira y Villamaría (Semi-Detailed Soil Study of the Municipalities of Manizales, Chinchiná, Palestina, Neira, and Villamaría); IGAC & CORPOCALDAS: Manizales, Colombia, 2013. (In Spanish) [Google Scholar]
- Hernández, M.I.; Chailloux, M. Las micorrizas arbusculares y las bacterias rizosfericas como alternativa a la nutricion mineral del tomate (Arbuscular mycorrhizae and rhizosphere bacteria as an alternative to mineral nutrition of tomato). Cultiv. Tropic. 2004, 25, 5–12. (In Spanish) [Google Scholar]
- Aguilar, J.E.; Sánchez, M. Efecto de una rizobacteria nitrofijadora y niveles de fertilizante en el comportamiento agronómico del tomate Lycopersicon esculentum var (Effect of nitrogen fixing rhizobacteria and chemical fertilization on yield of Lycopersicon esculentum Mill var Santa Clara). Acta Agron. 1998, 48, 60–70. (In Spanish) [Google Scholar]
- Reis, V.; Lee, S.; Kennedy, C. Biological nitrogen fixation in sugarcane. In Associative and Endophytic Nitrogen-Fixing Bacteria and Cyanobacterial Associations; Elmerich, C., Newton, W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 213–232. [Google Scholar]
- Dibut, B.; Martínez-Viera, R.; Ortega, M.; Ríos, Y.; Planas, L.; Rodríguez, J.; Tejeda, G. Situación actual y perspectiva de las relaciones endófitas planta-bacteria. Estudio de caso Gluconacetobacter diazotrophicus-cultivos de importancia económica (Current situation and perspective of plant-bacteria endophytic relationships. Gluconacetobacter diazotrophicus-cultures of economic importance case study). Cultiv. Tropic. 2009, 30, 16–23. (In Spanish) [Google Scholar]
- Aujla, M.; Thind, H.; Buttar, G. Fruit yield and water use efficiency of eggplant (Solanum melongena L.) as influenced by different quantities of nitrogen and water applied through drip and furrow irrigation. Sci. Hortic. 2007, 112, 142–148. [Google Scholar] [CrossRef]
- Direkvandi, S.N.; Ansari, N.A.; Dehcordie, F.S. Effect of different levels of nitrogen fertilizer with two types of bio-fertilizers on growth and yield of two cultivars of tomato (Lycopersicon esculentum Mill). Asian J. Plant Sci. 2008, 7, 757–761. [Google Scholar] [CrossRef]
- Cifras Agropecuarias (Agricultural Figures). Available online: http://www.agronet.gov.co/estadistica/Paginas/Precios.aspx (accessed on 1 March 2016). (In Spanish)
- Alfonso, E.T.; Galán, A.L. Evaluación agrobiológica de la coinoculación micorrizas-rizobacterias en tomate (Agrobiological evaluation of coinoculation micorrhyza-rhyzobacteria in tomato). Agron. Costarric. 2006, 30, 65–73. (In Spanish) [Google Scholar]
- Bashan, Y.; Holguin, G. Azospirillum—Plant relationships: Environmental and physiological advances (1990–1996). Can. J. Microbiol. 1997, 43, 103–121. [Google Scholar] [CrossRef]
- Marcelis, L. Sink strength as a determinant of dry matter partitioning in the whole plant. J. Exp. Bot. 1996, 47, 1281–1291. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Zhou, W.; Shen, W.; Li, Y.; Hui, D. Interactive effects of temperature and moisture on composition of the soil microbial community. Eur. J. Soil Sci. 2017, 68, 909–918. [Google Scholar] [CrossRef]
- Ceballos-Aguirre, N.; Hurtado-Salazar, A.; Restrepo, G.M.; Sánchez, Ó.J.; Hernández, M.C.; Montoya, M. Technical and economic assessment of tomato cultivation through a macro-tunnel production system with the application of Gluconacetobacter diazotrophicus. Horticulturae 2024, 10, 1110. [Google Scholar] [CrossRef]
- Restrepo, G.M.; Rincón, A.; Sánchez, Ó.J. Kinetic analysis of Gluconacetobacter diazotrophicus cultivated on a bench scale: Modeling the effect of pH and design of a sucrose-based medium. Fermentation 2023, 9, 705. [Google Scholar] [CrossRef]
- Ceballos-Aguirre, N.; Restrepo, G.M.; Hurtado-Salazar, A.; Cuellar, J.A.; Sánchez, Ó.J. Economic feasibility of Gluconacetobacter diazotrophicus in carrot cultivation. Rev. Ceres 2022, 69, 40–47. [Google Scholar] [CrossRef]

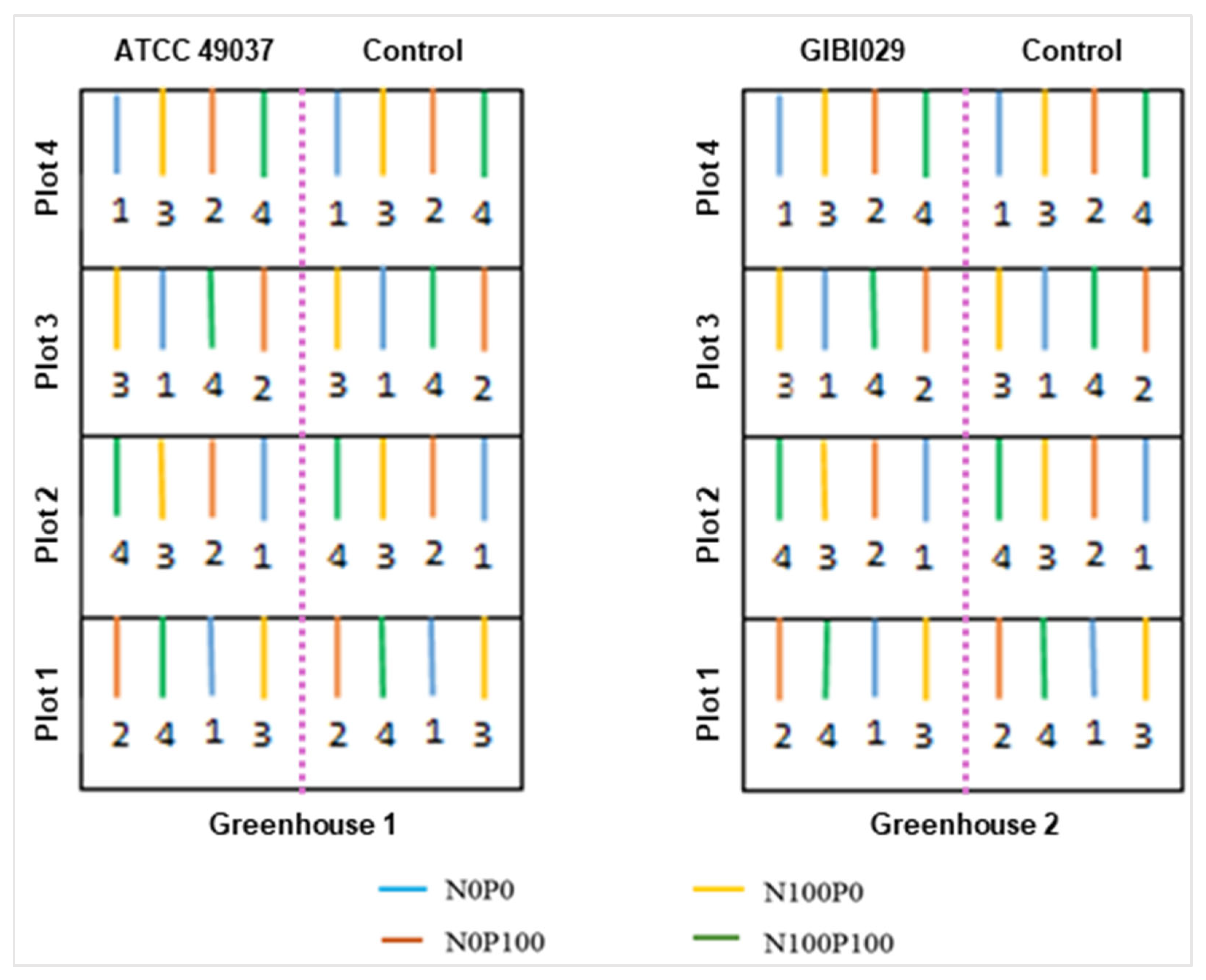

| Treatments | Degree of Fertilization | Code | Fertilization Source | Days After Transplanting [g per Plant] | Dosage [kg ha−1] | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 150 | |||||
| 1 | 0% N + 0% P | N0P0 | - | - | - | - | - | - | 0 |
| 2 | 0% N + 100% P | N0P100 | TSP | 8.0 | 4.0 | 4.2 | - | - | 338 |
| 3 | 100% N + 0% P | N100P0 | Urea | 8.0 | 4.0 | 4.0 | - | - | 335 |
| 4 | 100% N + 100% P | N100P100 | TSP | 8.0 | 4.0 | 4.2 | - | - | 338 |
| Urea | 8.0 | 4.0 | 4.0 | - | - | 335 | |||
| 1–4 | Micronutrients | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 205 | ||
| 1–4 | KCl | 6.0 | 6.0 | 12.0 | 12.0 | 9.0 | 937 | ||
| 1–4 | MgSO4 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 520 | ||
| Variable | Factor | Harvest | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |||
| Number of fruits per plant | Type of bacteria | GIBI029 | 5.25 a | 11.94 a | 3.32 b | 12.38a | 11.38 a | 5.72 a | 8.35 a | 4.78 a | 3.49 a | 2.18 b |
| ATCC | 4.31 b | 4.98 b | 3.97 a | 5.95c | 4.23 c | 4.13 a | 6.33 b | 2.65 b | 1.24 c | 2.77 b | ||
| Control | 3.28 c | 3.65 c | 2.97 b | 8.17b | 6.19 b | 4.73 b | 7.33 ab | 4.10 a | 1.99 b | 5.65 a | ||
| Type of fertilization | N0P0 | 3.93 a | 5.85 a | 3.11 a | 8.13a | 7.27 ab | 5.62 a | 6.41 b | 4.20 ab | 2.57 a | 4.37 a | |
| N0P100 | 4.11 a | 6.19 a | 3.35 a | 8.29a | 6.30 b | 4.54 ab | 5.79 b | 3.22 b | 2.05 a | 3.32 a | ||
| N100P0 | 3.93 a | 5.95 a | 3.23 a | 8.75a | 6.69 ab | 5.03 ab | 8.92 a | 4.87 a | 1.930 a | 3.73 a | ||
| N100P100 | 4.15 a | 6.23 a | 3.52 a | 9.49a | 7.73 a | 5.62 a | 8.22 a | 3.35 b | 2.17 a | 4.84 a | ||
| Weight of fruits [g per plant] | Type of bacteria | GIBI029 | 418.0 a | 940.0 a | 260.0 a | 996.8a | 846.2 a | 331.0 a | 487.2 a | 275.4 a | 189.9 a | 117.9 b |
| ATCC | 207.0 b | 240.0 b | 191.0 b | 356.1c | 244.9 c | 230.9 b | 321.8 b | 114.0 c | 43.6 c | 101.7 b | ||
| Control | 207.0 b | 240.0 b | 191.0 b | 554.8b | 354.0 b | 238.8 b | 372.5 b | 177.4 b | 81.3 b | 218.8 a | ||
| Type of fertilization | N0P0 | 259.8 a | 415.0 a | 208.3 a | 591.8a | 492.1 a | 238.6 a | 353.6 b | 209.8 ab | 116.8 b | 195.4 a | |
| N0P100 | 259.8 a | 415.0 a | 208.3 a | 580.4a | 396.9 a | 246.9 a | 286.4 b | 146.2 c | 87.1 a | 131.1 a | ||
| N100P0 | 259.8 a | 415.0 a | 208.3 a | 602.2a | 419.3 a | 260.3 a | 462.9 a | 229.3 a | 87.4 a | 142.6 a | ||
| N100P100 | 259.8 a | 415.0 a | 208.3 a | 688.0a | 490.8 a | 293.6 a | 451.0 a | 158.8 bc | 104.4 a | 187.9 a | ||
| Variable | Factor | Treatment | Total Value |
|---|---|---|---|
| Total number of fruits per plant | Type of bacteria | GIBI029 | 69.0 a ± 16.5 |
| ATCC | 40.5 c ± 15.5 | ||
| Control | 48.0 b ± 16.1 | ||
| Type of fertilization | N0P0 | 49.9 bc ± 19.0 | |
| N0P100 | 47.1 c ± 18.0 | ||
| N100P0 | 53.1 ab ± 17.2 | ||
| N100P100 | 55.3 a ± 21.6 | ||
| Total production [g per plant] | Type of bacteria | GIBI029 | 4862.4 a ± 1268.8 |
| ATCC | 2051.1 c ± 854.6 | ||
| Control | 2635.6 b ± 937.5 | ||
| Type of fertilization | N0P0 | 3081.3 a ± 1579.4 | |
| N0P100 | 2758.2 b ± 1365.7 | ||
| N100P0 | 3087.2 a ± 1294.4 | ||
| N100P100 | 3258.1 a ± 1620.5 | ||
| Yield [t ha−1] | Type of bacteria | GIBI029 | 101.3 a ± 26.4 |
| ATCC | 42.7 c ± 17.8 | ||
| Control | 54.9 b ± 19.5 | ||
| Type of fertilization | N0P0 | 64.2 a ± 32.9 | |
| N0P100 | 57.5 b ± 28.4 | ||
| N100P0 | 64.3 a ± 26.9 | ||
| N100P100 | 67.9 a ± 33.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceballos-Aguirre, N.; Restrepo, G.M.; Patiño, S.; Cuéllar, J.A.; Sánchez, Ó.J. Utilization of Gluconacetobacter diazotrophicus in Tomato Crop: Interaction with Nitrogen and Phosphorus Fertilization. Agriculture 2025, 15, 1191. https://doi.org/10.3390/agriculture15111191
Ceballos-Aguirre N, Restrepo GM, Patiño S, Cuéllar JA, Sánchez ÓJ. Utilization of Gluconacetobacter diazotrophicus in Tomato Crop: Interaction with Nitrogen and Phosphorus Fertilization. Agriculture. 2025; 15(11):1191. https://doi.org/10.3390/agriculture15111191
Chicago/Turabian StyleCeballos-Aguirre, Nelson, Gloria M. Restrepo, Sergio Patiño, Jorge A. Cuéllar, and Óscar J. Sánchez. 2025. "Utilization of Gluconacetobacter diazotrophicus in Tomato Crop: Interaction with Nitrogen and Phosphorus Fertilization" Agriculture 15, no. 11: 1191. https://doi.org/10.3390/agriculture15111191
APA StyleCeballos-Aguirre, N., Restrepo, G. M., Patiño, S., Cuéllar, J. A., & Sánchez, Ó. J. (2025). Utilization of Gluconacetobacter diazotrophicus in Tomato Crop: Interaction with Nitrogen and Phosphorus Fertilization. Agriculture, 15(11), 1191. https://doi.org/10.3390/agriculture15111191








