Comprehensive Assessment of Alfalfa Aluminum Stress Resistance Using Growth and Physiological Trait Analysis
Abstract
1. Introduction
2. Results
2.1. Effects of Al Stress on Alfalfa Root Length
2.2. Analysis of Alfalfa Root Surface Area and Root Volume Under Al Stress
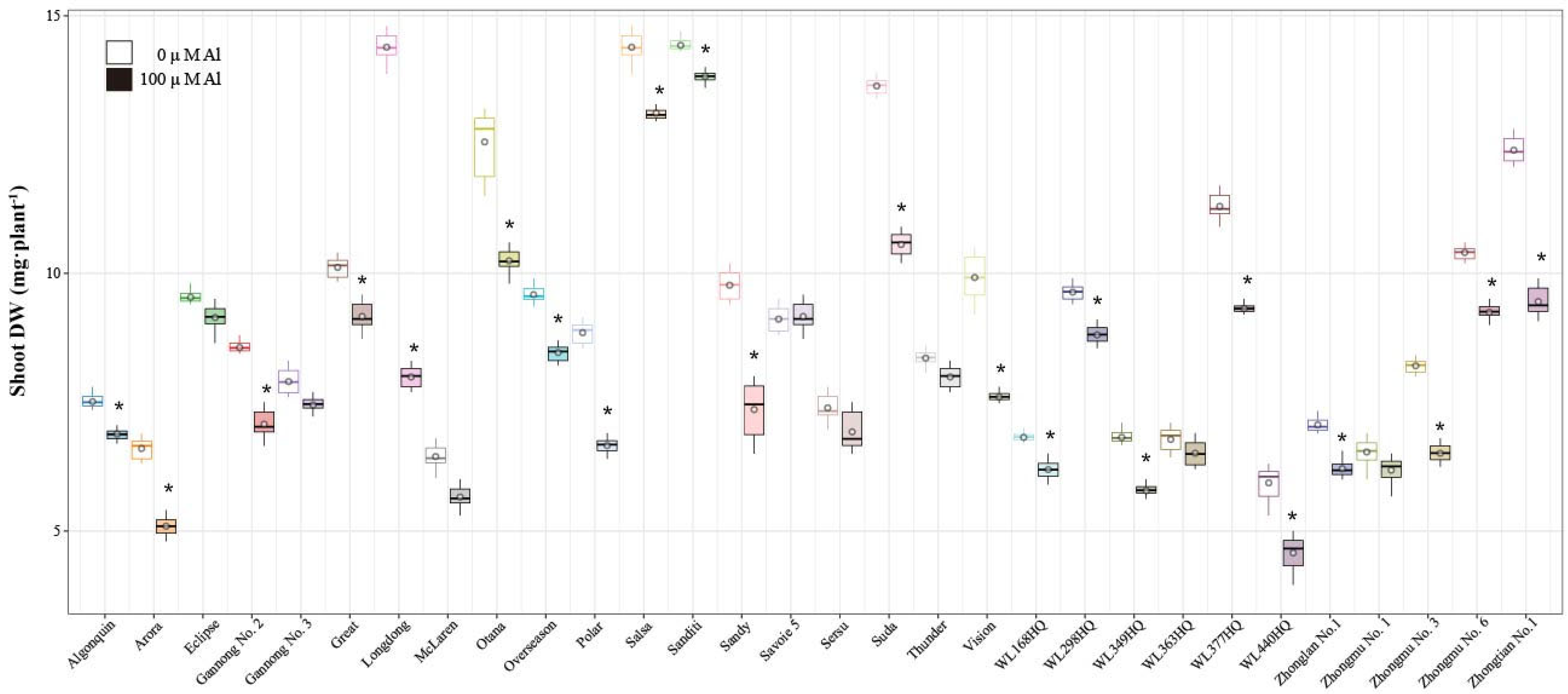
2.3. Analysis of Alfalfa Root Dry Weight (RDW), Shoot Dry Weight, and Root-to-Shoot Ratio Under Al Stress
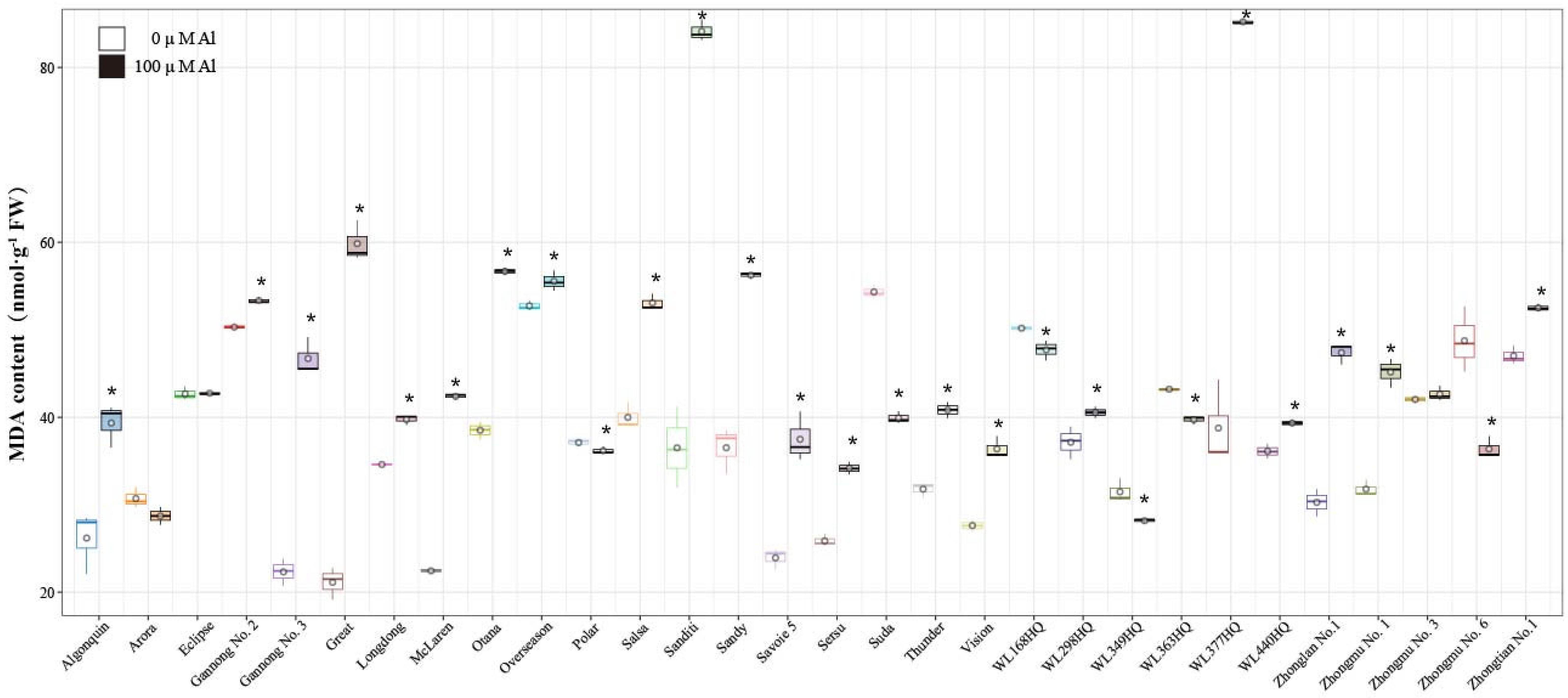
2.4. Effects of Al Stress on Malondialdehyde (MDA) Content in Alfalfa Roots
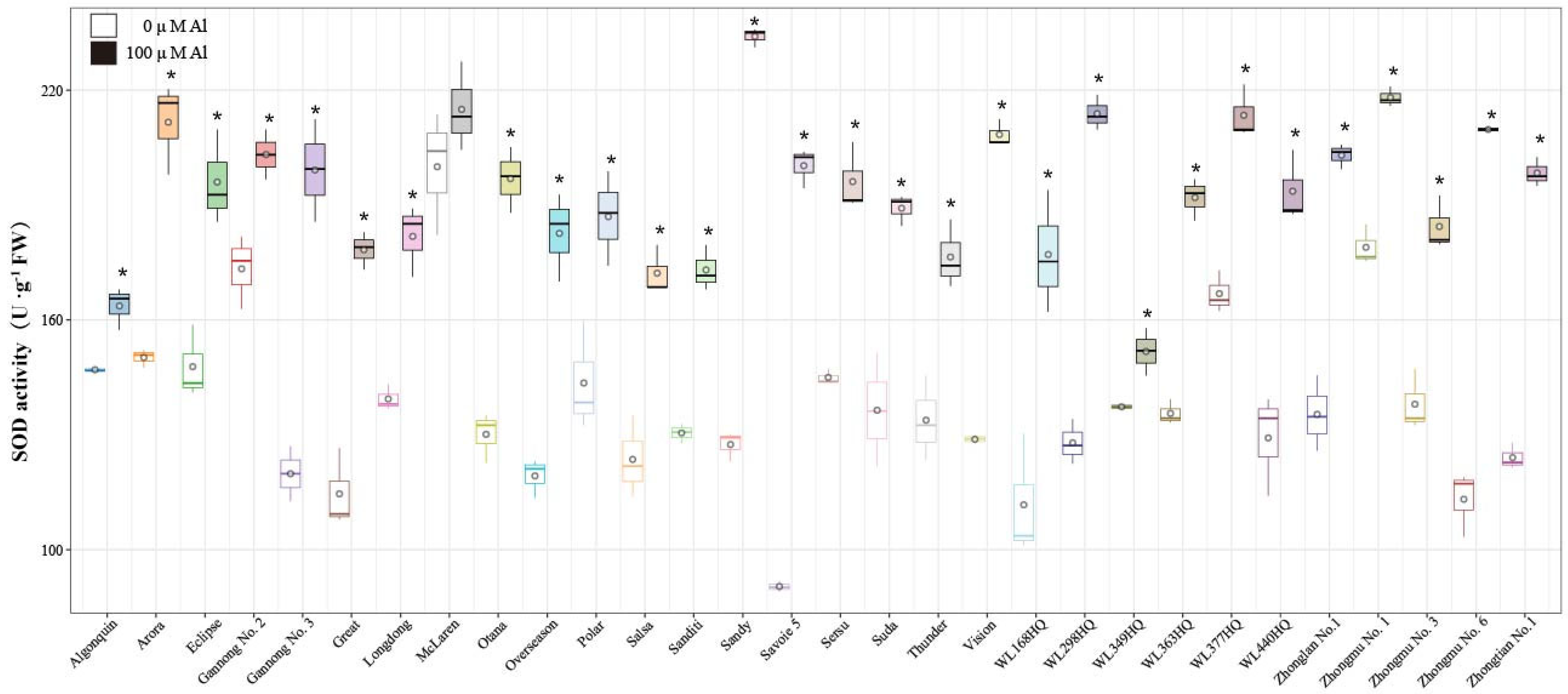
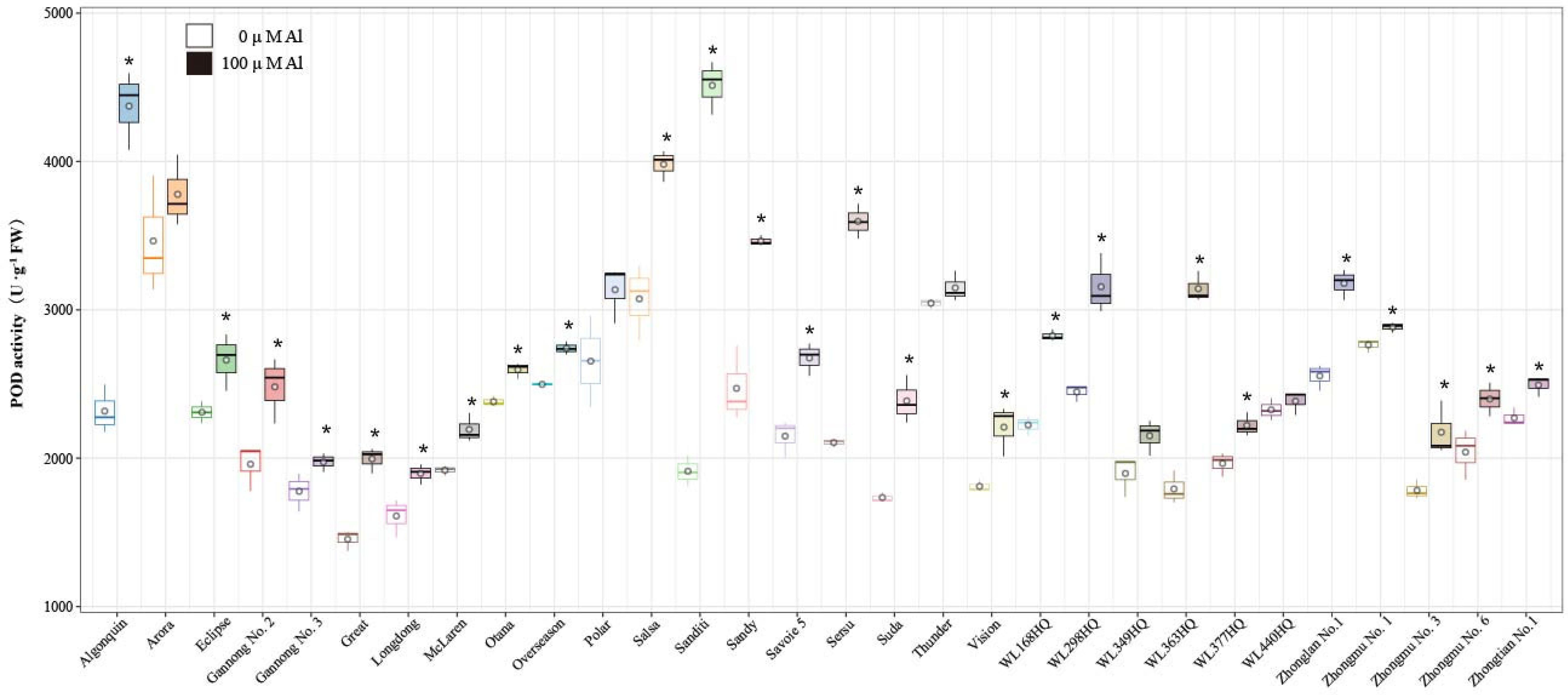
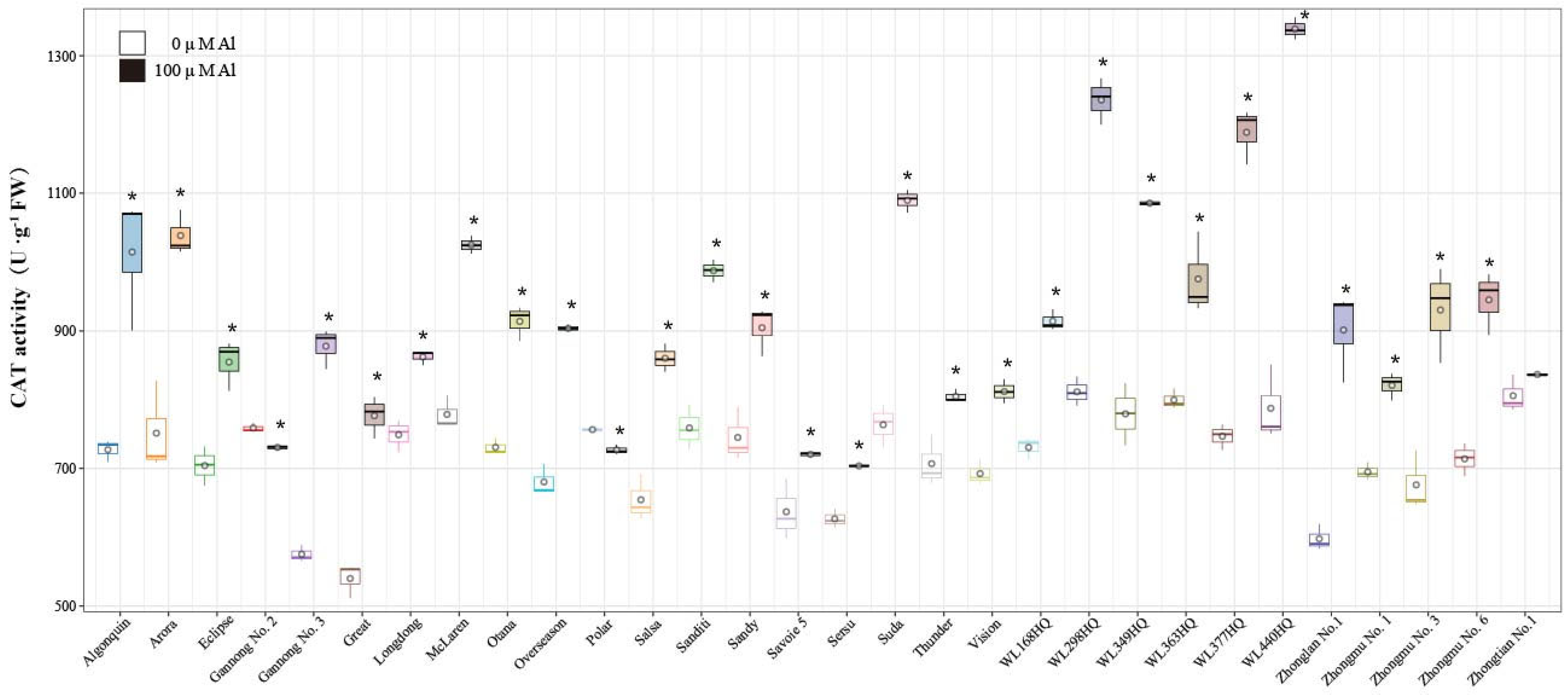
2.5. Effects of Al Stress on Antioxidant Enzyme Activities in Alfalfa Roots
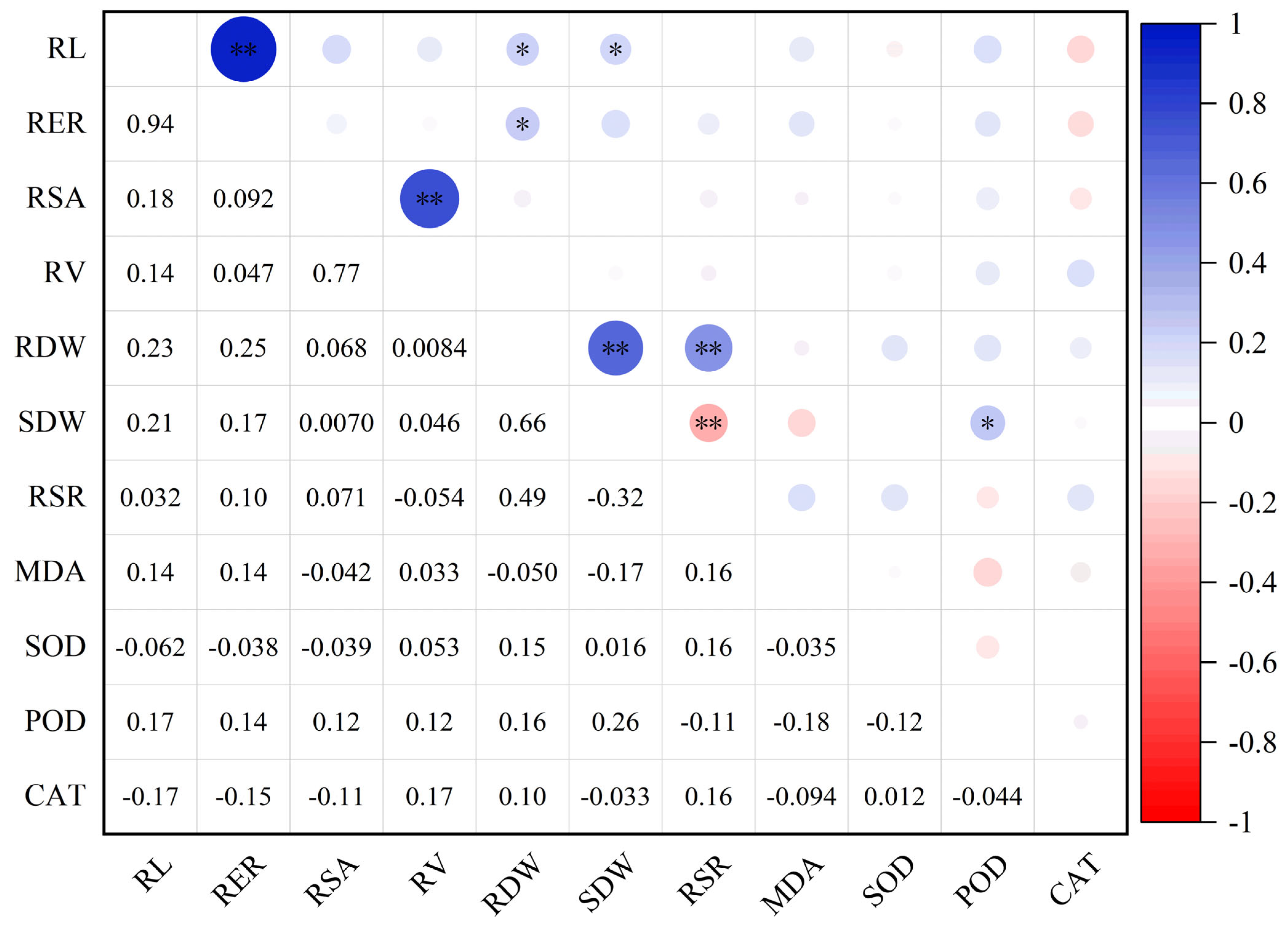
2.6. Al Tolerance Coefficient and Correlation Analysis of Alfalfa
2.7. PCA of Alfalfa Growth Physiological Indices
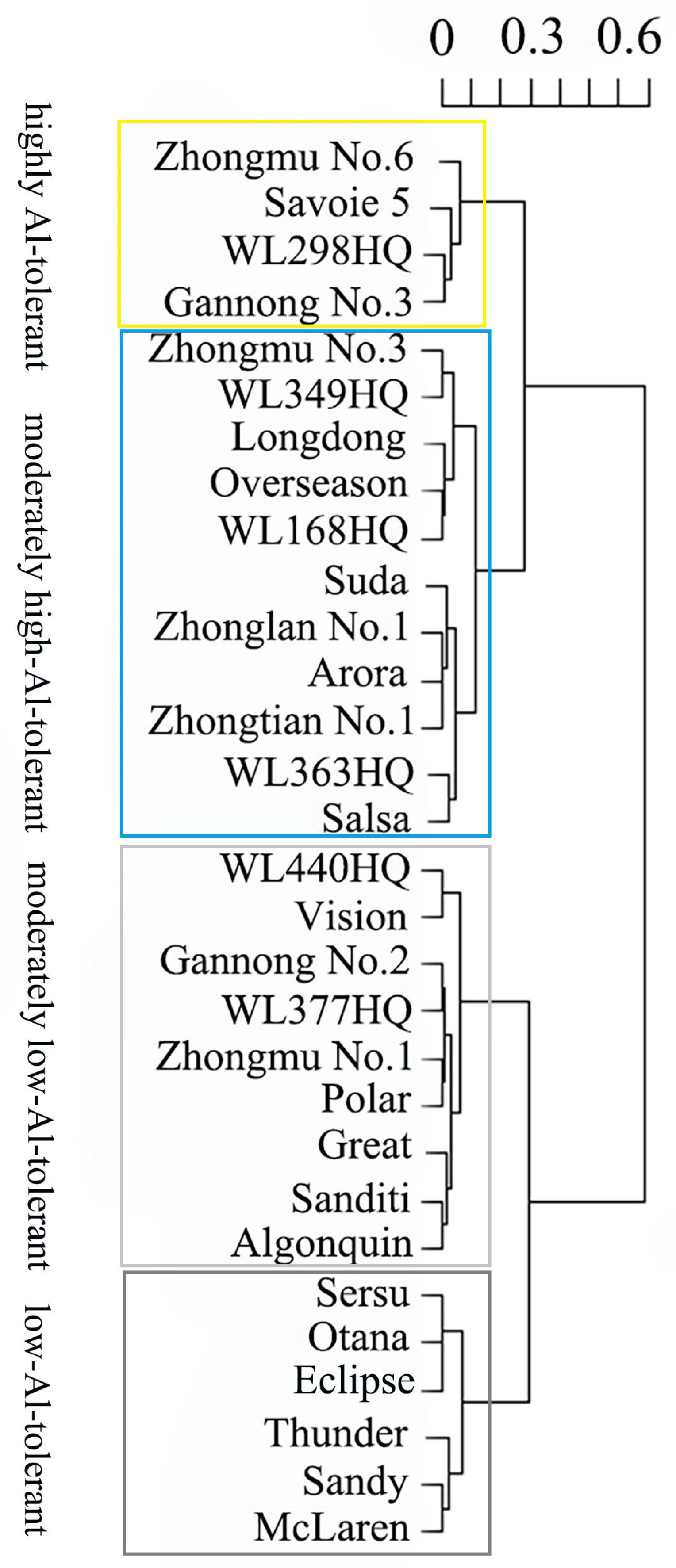
2.8. Comprehensive Evaluation of Al Tolerance of Alfalfa Cultivars
X1 (root elongation rate)
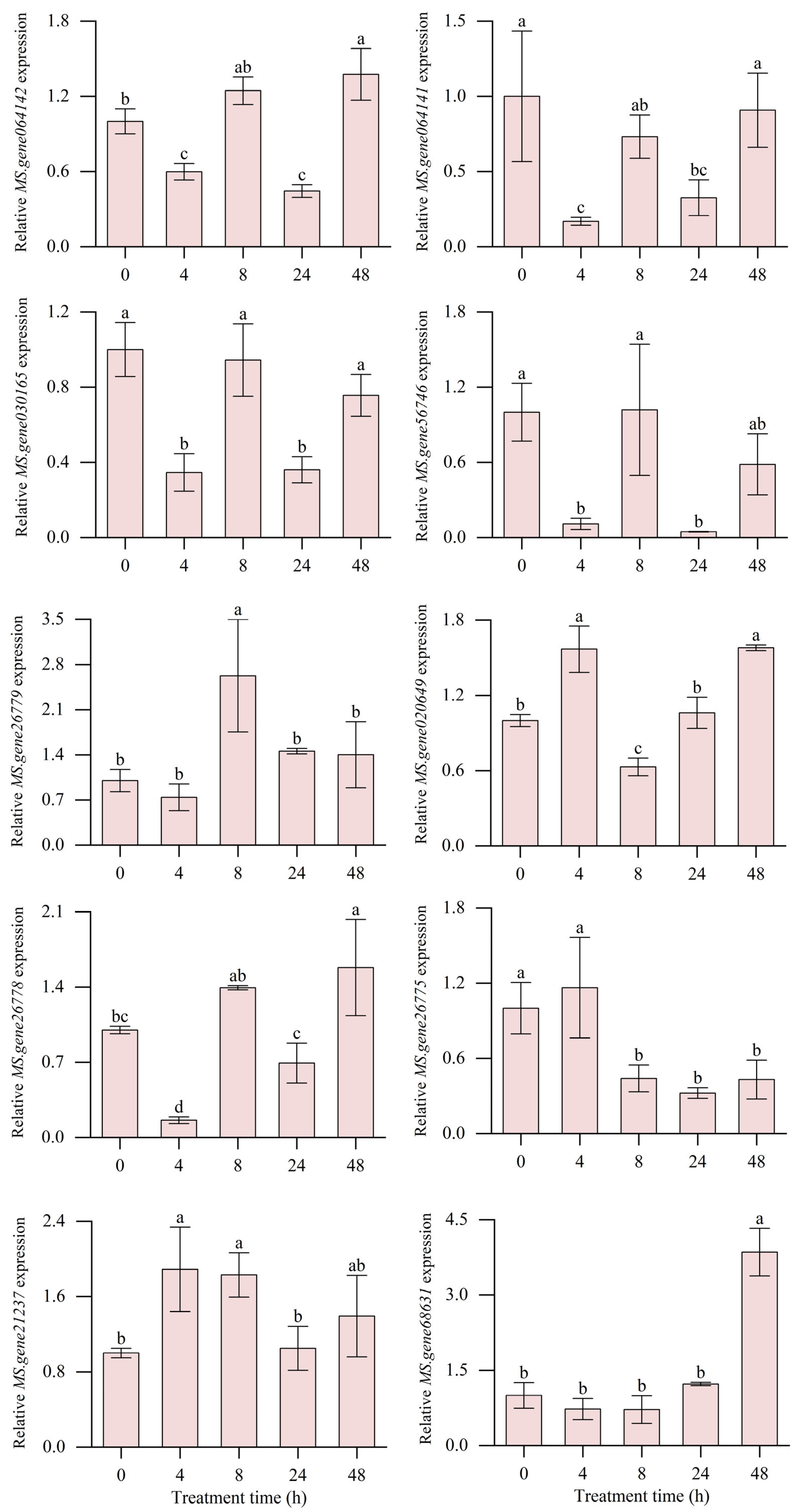
2.9. Expression Analysis of MsABC Genes in Response to Al Toxicity
3. Discussion
3.1. Effects of Al Stress on Alfalfa Growth
3.2. Effects of Al Stress on Alfalfa Antioxidant Mechanism
3.3. Gene Expression Pattern Analysis
4. Materials and Methods
4.1. Plant Materials
4.2. Experimental Design
4.3. Phenotypic Trait Assessment
4.3.1. Root Trait Indicators
Treatment) × 100%
Treatment) × 100%
4.3.2. Dry Weight
4.4. Malondialdehyde (MDA) Content Determination
4.5. Antioxidant Enzyme Activity Analysis
4.6. Analysis of Al Tolerance in Alfalfa
4.7. Quantitative Real-Time PCR Analysis
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Al | aluminum |
| SOD | superoxide dismutase |
| CAT | catalase |
| POD | peroxidase |
| ROS | reactive oxygen species |
| APX | ascorbate peroxidase |
| O2− | superoxide anions |
| H2O2 | hydrogen peroxide |
| PCA | principal component analysis |
| CV | coefficient of variation |
| RDW | root dry weight |
| FW | fresh weight |
| PC | principal component |
| RRE | relative root elongation |
References
- Seguel, A.; Cumming, J.R.; Klugh-Stewart, K.; Cornejo, P.; Borie, F. The role of arbuscular mycorrhizas in decreasing aluminium phytotoxicity in acidic soils: A review. Mycorrhiza 2013, 23, 167–183. [Google Scholar] [CrossRef]
- Kochian, L.V.; Pi Eros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.K.; Yadav, V.; Vaculík, M.; Gassmann, W.; Pike, S.; Arif, N.; Singh, V.P.; Deshmukh, R.; Sahi, S.; And Tripathi, D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Basit, F.; Liu, J.; An, J.; Chen, M.; He, C.; Zhu, X.; Li, Z.; Hu, J.; Guan, Y. Seed priming with brassinosteroids alleviates aluminum toxicity in rice via improving antioxidant defense system and suppressing aluminum uptake. Environ. Sci. Pollut. Res. 2022, 29, 10183–10197. [Google Scholar] [CrossRef]
- Phukunkamkaew, S.; Tisarum, R.; Pipatsitee, P.; Samphumphuang, T.; Maksup, S.; Cha-Um, S. Morpho-physiological responses of indica rice (Oryza sativa sub. indica) to aluminum toxicity at seedling stage. Environ. Sci. Pollut. Res. 2021, 28, 29321–29331. [Google Scholar] [CrossRef] [PubMed]
- Zelinová, V.; Halušková, L.; Huttová, J.; Illéš, P.; Mistrík, I.; Valentovičová, K.; Tamás, L. Short-term aluminium-induced changes in barley root tips. Protoplasma 2011, 248, 523–530. [Google Scholar] [CrossRef]
- Yan, J.F.; Zhu, W.B.; Wu, D.S.; Chen, X.Y.; Yang, S.X.; Xue, Y.B.; Liu, Y.; Liu, Y. Mechanisms of Aluminum Toxicity Impacting Root Growth in Shatian Pomelo. Int. J. Mol. Sci. 2024, 25, 13454. [Google Scholar] [CrossRef]
- Toth, B.; Moloi, M.J.; Szoke, L.; Danter, M.; Grusak, M.A. Cultivar Differences in the Biochemical and Physiological Responses of Common Beans to Aluminum Stress. Plants 2021, 10, 2097. [Google Scholar] [CrossRef]
- Liang, Y.; Bai, T.; Liu, B.; Yu, W.; Teng, W. Different antioxidant regulation mechanisms in response to aluminum-induced oxidative stress in Eucalyptus species. Ecotoxicol. Environ. Saf. 2022, 241, 113748. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 2022. [Google Scholar] [CrossRef]
- Furlan, F.; Borgo, L.; Rabêlo, F.H.S.; Rossi, M.L.; Martinelli, A.P.; Azevedo, R.A.; Lavres, J. Aluminum-induced stress differently modifies Urochloa genotypes responses on growth and regrowth: Root-to-shoot Al-translocation and oxidative stress. Theor. Exp. Plant Physiol. 2018, 30, 141–152. [Google Scholar] [CrossRef]
- Awasthi, J.P.; Saha, B.; Panigrahi, J.; Yanase, E.; Koyama, H.; Panda, S.K. Redox balance, metabolic fingerprint and physiological characterization in contrasting North East Indian rice for Aluminum stress tolerance. Sci. Rep. 2019, 9, 8681. [Google Scholar] [CrossRef] [PubMed]
- You-Sheng, W.; Zhi-Min, Y. Nitric Oxide Reduces Aluminum Toxicity by Preventing Oxidative Stress in the Roots of Cassia tora L. Plant Cell Physiol. 2005, 46, 1915–1923. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Ouyang, Z.; Liu, B.; Li, T.; Bai, T.; Teng, W. Effects of aluminum on metabolism of reactive oxygen species and reactive nitrogen species in root tips of different Eucalyptus species. BMC Plant Biol. 2025, 25, 55. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, Y.; Lin, X.; Liu, X.; Sun, C. Novel application of cyclo(-Phe-Pro) in mitigating aluminum toxicity through oxidative stress alleviation in wheat roots. Environ. Pollut. 2024, 363, 125241. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.Y.; Zhou, C.N.; Lin, H.; Luo, D.; Jain, D.; Chai, M.F.; Lu, Z.C.; Liu, Z.P.; Roy, S.; Dong, J.L.; et al. Medicago2035: Genomes, functional genomics and molecular breeding. Mol. Plant 2025, 18, 219–244. [Google Scholar] [CrossRef]
- Peng, W.X.; Cai, W.Q.; Pan, J.Y.; Su, X.R.; Dou, L.R. Molecular Mechanisms of Alfalfa Response to Abiotic Stresses. Plants 2025, 14, 487. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, S.; Su, L.; Lv, A.; Zhou, P.; An, Y. Aluminum toxicity in alfalfa (Medicago sativa) is alleviated by exogenous foliar IAA inducing reduction of Al accumulation in cell wall. Environ. Exp. Bot. 2017, 139, 1–13. [Google Scholar] [CrossRef]
- Singh, D.; Tripathi, A.; Bhati, J.; Taunk, J.; Singh, D.; Siddiqui, M.H.; Singh, M.P. Genome wide identification and expression profiling of ATP binding cassette (ABC) transporters gene family in lentil (Lens culinaris Medikus) under aluminium stress condition. Plant Physiol. Biochem. 2024, 211, 108710. [Google Scholar] [CrossRef]
- Huang, C.F.; Yamaji, N.; Chen, Z.C.; Ma, J.F. A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 2012, 69, 857–867. [Google Scholar] [CrossRef]
- Cao, J.; Wang, T.; Yu, D.; He, J.; Qian, W.; Tang, B.; Bi, X.; Wang, H.; Zhang, Y. MsDUF3700 overexpression enhances aluminum tolerance in alfalfa shoots. Plant Cell Rep. 2024, 43, 301. [Google Scholar] [CrossRef]
- Ryan, P.R.; Tyerman, S.D.; Sasaki, T.; Furuichi, T.; Yamamoto, Y.; Zhang, W.H.; Delhaize, E. The identification of aluminium-resistance genes provides opportunities for enhancing crop production on acid soils. J. Exp. Bot. 2011, 62, 9–20. [Google Scholar] [CrossRef]
- Takayuki, S.; Bunichi, E.; Hideaki, M. A gene encoding multidrug resistance (MDR)-like protein is induced by aluminum and inhibitors of calcium flux in wheat. Plant Cell Physiol. 2002, 48, 177–185. [Google Scholar] [CrossRef]
- Lei, G.J.; Yokosho, K.; Yamaji, N.; Fujii-Kashino, M.; Ma, J.F. Functional characterization of two half-size ABC transporter genes in aluminium-accumulating buckwheat. New Phytol. 2017, 215, 1080–1089. [Google Scholar] [CrossRef]
- Kocjan, A.; Kwasniewska, J.; Szurman-Zubrzycka, M. Understanding plant tolerance to aluminum: Exploring mechanisms and perspectives. Plant Soil 2025, 507, 195–219. [Google Scholar] [CrossRef]
- Panda, S.K.; Matsumoto, H. Molecular physiology of aluminum toxicity and tolerance in plants. Bot. Rev. 2007, 73, 326–347. [Google Scholar] [CrossRef]
- Passos, L.P.; Köpp, M.M.; Lédo, F.J.S. Performance of tetraploid alfalfa genotypes as exposed to aluminum toxicity. Agric. Sci. 2012, 3, 230–240. [Google Scholar] [CrossRef]
- Hijbeek, R.; van Loon, M.P.; Ouaret, W.; Boekelo, B.; van Ittersum, M.K. Liming agricultural soils in Western Kenya: Can long-term economic and environmental benefits pay off short term investments? Agric. Syst. 2021, 190, 103095. [Google Scholar] [CrossRef]
- Foy, C.D. Plant adaptation to acid, aluminum-toxic soils. Commun. Soil Sci. Plant Anal. 1988, 19, 959–987. [Google Scholar] [CrossRef]
- Shi, J.N.; Zhao, M.; Zhang, F.; Feng, D.D.; Yang, S.X.; Xue, Y.B.; Liu, Y. Physiological Mechanism through Which Al Toxicity Inhibits Peanut Root Growth. Plants 2024, 13, 325. [Google Scholar] [CrossRef]
- Kouki, R.; Ayachi, R.; Ferreira, R.; Sleimi, N. Behavior of Cucumis sativus L. in presence of aluminum stress: Germination, plant growth, and antioxidant enzymes. Food Sci. Nutr. 2021, 9, 3280–3288. [Google Scholar] [CrossRef]
- You, J.F.; He, Y.F.; Yang, J.L.; Zheng, S.J. A Comparison of Aluminum Resistance among Polygonum Species Originating on Strongly Acidic and Neutral Soils. Plant Soil 2005, 276, 143–151. [Google Scholar] [CrossRef]
- Horst, W.J.; Wang, Y.X.; Eticha, D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: A review. Ann. Bot. 2010, 106, 185–197. [Google Scholar] [CrossRef]
- Yang, J.L.; Zhu, X.F.; Zheng, C.; Zhang, Y.J.; Zheng, S.J. Genotypic differences in Al resistance and the role of cell-wall pectin in Al exclusion from the root apex in Fagopyrum tataricum. Ann. Bot. 2011, 107, 371–378. [Google Scholar] [CrossRef]
- Dong, G.; Lu, H.; Pan, X.; He, X.; Jiang, J.; Li, J.; Xu, R. Application of measuring electrochemical characteristics on plant root surfaces in screening Al-tolerant wheat. Environ. Pollut. 2021, 281, 116993. [Google Scholar] [CrossRef]
- Zhang, P.; Zhong, K.Z.; Tong, H.H.; Shahid, M.Q.; Li, J.Q. Association Mapping for Aluminum Tolerance in a Core Collection of Rice Landraces. Front. Plant Sci. 2016, 7, 1415. [Google Scholar] [CrossRef]
- Chang, S.; Jing-Hao, W.; Gao-Ling, S.; Lai-Qing, L.; Jun-Xia, D.; Jian-Lin, W.; Qing-Sheng, C. Different Aluminum Tolerance among Indica, Japonica and Hybrid Rice Varieties. Rice Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Hufnagel, B.; Guimaraes, C.T.; Craft, E.J.; Shaff, J.E.; Schaffert, R.E.; Kochian, L.V.; Magalhaes, J.V. Exploiting sorghum genetic diversity for enhanced aluminum tolerance: Allele mining based on the AltSB locus. Sci. Rep. 2018, 8, 10094. [Google Scholar] [CrossRef]
- Matsumoto, H. Cell biology of aluminum toxicity and tolerance in higher plants. Int. Rev. Cytol. 2000, 200, 1–46. [Google Scholar] [CrossRef]
- Malik, B.; Pirzadah, T.B.; Tahir, I.; Ul Rehman, R. Growth and physiological responses in chicory towards mercury induced in vitro oxidative stress. Plant Physiol. Rep. 2019, 24, 236–248. [Google Scholar] [CrossRef]
- Zhang, J.; He, Z.; Tian, H.; Zhu, G.; Peng, X. Identification of aluminium-responsive genes in rice cultivars with different aluminium sensitivities. J. Exp. Bot. 2007, 58, 2269–2278. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, J.L.; He, L.S.; Li, Y.Y.; Zheng, S.J. Effect of aluminum on cell wall, plasma membrane, antioxidants and root elongation in triticale. Biol. Plant 2008, 52, 87–92. [Google Scholar] [CrossRef]
- Ma, J.F.; Hiradate, S.; Nomoto, K.; Iwashita, T.; Matsumoto, H. Internal Detoxification Mechanism of Al in Hydrangea (Identification of Al Form in the Leaves). Plant Physiol. 1997, 113, 1033–1039. [Google Scholar] [CrossRef]
- Zhang, X.; Humphries, A.; Auricht, G. Genetic variability and inheritance of aluminium tolerance as indicated by long root regrowth in lucerne (Medicago sativa L.). Euphytica 2007, 157, 177–184. [Google Scholar] [CrossRef]
- Shi, J.N.; Li, J.Y.; Pan, Y.H.; Zhao, M.; Zhang, R.; Xue, Y.B.; Liu, Y. The Physiological Response Mechanism of Peanut Leaves under Al Stress. Plants 2024, 13, 1606. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Ali, A.; Tuteja, N.; Gill, S.; Pattanayak, A. Identification and expression pattern of aluminium-responsive genes in roots of rice genotype with reference to Al-sensitivity. Sci. Rep. 2023, 13, 12184. [Google Scholar] [CrossRef]
- Thiruvengadam, R.; Venkidasamy, B.; Easwaran, M.; Chi, H.Y.; Thiruvengadam, M.; Kim, S. Dynamic interplay of reactive oxygen and nitrogen species (ROS and RNS) in plant resilience: Unveiling the signaling pathways and metabolic responses to biotic and abiotic stresses. Plant Cell Rep. 2024, 43, 198. [Google Scholar] [CrossRef]
- Zhang, M.; Smith, J.A.C.; Harberd, N.P.; Jiang, C. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef]
- Huang, W.; Yang, X.; Yao, S.; Lwinoo, T.; He, H.; Wang, A.; Li, C.; He, L. Reactive oxygen species burst induced by aluminum stress triggers mitochondria-dependent programmed cell death in peanut root tip cells. Plant Physiol. Biochem. 2014, 82, 76–84. [Google Scholar] [CrossRef]
- Yamamoto, Y. Aluminum toxicity in plant cells: Mechanisms of cell death and inhibition of cell elongation. Soil Sci. Plant Nutr. 2019, 65, 41–55. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, J.; Sun, L.; Huang, B.; Ding, C.; Gu, Y.; Liao, J.; Hu, C.; Zhang, Z.; Yuan, S.; et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Yamamoto, Y. Lipid Peroxidation Is an Early Symptom Triggered by Aluminum, But Not the Primary Cause of Elongation Inhibition in Pea Roots. Plant Physiol. 2001, 125, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.; Wang, B.; Xie, H.; Wang, J.; Nan, Z. Transcriptome analysis of two soybean cultivars identifies an aluminum respon-sive antioxidant enzyme GmCAT1. Biosci. Biotechnol. Biochem. 2020, 84, 1394–1400. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, K.; Zhang, Y.; Phan, X.; Li, K.; Yu, Y.; Chen, L. Physiological and molecular responses of broad bean (Vicia faba L.) to aluminum stress. Acta Physiol. Plant 2012, 34, 2251–2263. [Google Scholar] [CrossRef]
- Guo, T.; Yao, P.; Zi-Dong, Z.; Wang, J.; Wang, M. Involvement of Antioxidative Defense System in Rice Seedlings Exposed to Aluminum Toxicity and Phosphorus Deficiency. Rice Sci. 2012, 19, 207–212. [Google Scholar] [CrossRef]
- Ling, J.Y.; Tan, J.H.; Chen, H.; Yang, Z.Q.; Luo, Q.F.; Jia, J. Physiology, Transcriptome and Root Exudates Analysis of Response to Aluminum Stress in Pinus massoniana. Forests 2023, 14, 1410. [Google Scholar] [CrossRef]
- Abo, M.; Yonehara, H.; Yoshimura, E. Aluminum stress increases carbon-centered radicals in soybean roots. J. Plant Physiol. 2010, 167, 1316–1319. [Google Scholar] [CrossRef]
- Borgo, L.; Rabêlo, F.H.; Carvalho, G.; Ramires, T.; Righetto, A.J.; Piotto, F.Â.; Boaretto, L.F.; Azevedo, R. Antioxidant performance and aluminum accumulation in two genotypes of Solanum lycopersicum in response to low pH and aluminum availability and under their combined stress. Sci. Hortic. 2020, 259, 108813. [Google Scholar] [CrossRef]
- Birouste, M.; Zamora-Ledezma, E.; Bossard, C.; Pérez-Ramos, I.M.; Roumet, C. Measurement of fine root tissue density: A comparison of three methods reveals the potential of root dry matter content. Plant Soil 2014, 374, 299–313. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Azizi, F.; Amiri, H.; Ismaili, A. Melatonin improves salinity stress tolerance of Phaseolus vulgaris L. cv. Pak by changing antioxidant enzymes and photosynthetic parameters. Acta Physiol. Plant 2022, 44, 40. [Google Scholar] [CrossRef]
- Garratt, L.C.; Janagoudar, B.S.; Lowe, K.C.; Anthony, P.; Power, J.B.; Davey, M.R. Salinity tolerance and antioxidant status in cotton cultures. Free Radic. Biol. Med. 2002, 33, 502–511. [Google Scholar] [CrossRef]
- Lv, A.M.; Su, L.T.; Fan, N.A.; Wen, W.W.; Wang, Z.; Zhou, P.; An, Y. Chloroplast-targeted late embryogenesis abundant 1 increases alfalfa tolerance to drought and aluminum. Plant Physiol. 2023, 193, 2750–2767. [Google Scholar] [CrossRef]
- Su, L.; Lv, A.; Wen, W.; Fan, N.; Li, J.; Gao, L.; Zhou, P.; An, Y. MsMYB741 is involved in alfalfa resistance to aluminum stress by regulating flavonoid biosynthesis. Plant J. 2022, 112, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Zhao, H. Activity test and mechanism study of an environmentally friendly wheat seed coating agent. Agric. Sci. 2013, 4, 334–339. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.A.; Barros, J.A.S.; Dal-Bianco, M.; Martins, S.C.V.; Magalhães, P.C.; Ribeiro, D.M.; Damatta, F.M.; Araújo, W.L.; Ribeiro, C. Metabolic and physiological adjustments of maize leaves in response to aluminum stress. Theor. Exp. Plant Physiol. 2020, 32, 133–145. [Google Scholar] [CrossRef]
- Dar, F.A.; Tahir, I.; Ul Rehman, R.; Alharby, H.F.; Alzahrani, Y.; Alsamadany, H.; Hakeem, K.R. Exogenous silicon alleviates aluminum phytotoxicity in Fagopyrum esculentum Moench by modulating physiological and antioxidant responses. S. Afr. J. Bot. 2024, 167, 367–384. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, W.; Hao, J.; Fan, D.; Chen, L.; Xu, H.; Li, K. Measuring the expression and activity of the CAT enzyme to determine Al resistance in soybean. Plant Physiol. Biochem. 2019, 144, 254–263. [Google Scholar] [CrossRef]
- Ma, B.; Gao, L.; Zhang, H.; Cui, J.; Shen, Z. Aluminum-induced oxidative stress and changes in antioxidant defenses in the roots of rice varieties differing in Al tolerance. Plant Cell Rep. 2012, 31, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhang, S.S.; Meng, Q.M.; Zou, J.; Jiang, W.S.; Liu, D.H. Effects of aluminum on nucleoli in root tip cells, root growth and the antioxidant defense system in Vicia faba L. Acta Biol. Crac. Ser. Bot. 2009, 51, 99–106. [Google Scholar] [CrossRef]
- Hwang, J.U.; Song, W.Y.; Hong, D.; Ko, D.; Yamaoka, Y.; Jang, S.; Yim, S.; Lee, E.; Khare, D.; Kim, K.; et al. Plant ABC Transporters Enable Many Unique Aspects of a Terrestrial Plant’s Lifestyle. Mol. Plant 2016, 9, 338–355. [Google Scholar] [CrossRef] [PubMed]
- Theodoulou, F.L.; Kerr, I.D. ABC transporter research: Going strong 40 years on. Biochem. Soc. Trans. 2015, 43, 1033–1040. [Google Scholar] [CrossRef]
- Sun, N.; Xie, Y.F.; Wu, Y.; Guo, N.; Li, D.H.; Gao, J.S. Genome-wide identification of ABCC gene family and their expression analysis in pigment deposition of fiber in brown cotton (Gossypium hirsutum). PLoS ONE 2021, 16, e0246649. [Google Scholar] [CrossRef]
- Larsen, P.B.; Cancel, J.; Rounds, M.; Ochoa, V. ArabidopsisALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta 2007, 225, 1447–1458. [Google Scholar] [CrossRef]
- Huang, J.J.; Li, X.Y.; Chen, X.; Guo, Y.R.; Liang, W.H.; Wang, H.H. Genome-Wide Identification of Soybean ABC Transporters Relate to Aluminum Toxicity. Int. J. Mol. Sci. 2021, 22, 6556. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; And Mo, L. Screening of grape genotypes for aluminum tolerance and evaluation on selecting indices. Soil Sci. Plant Nutr. 2020, 66, 560–568. [Google Scholar] [CrossRef]
- Luo, D.; Zhou, Q.; Wu, Y.G.; Chai, X.T.; Liu, W.X.; Wang, Y.R.; Yang, Q.C.; Wang, Z.Y.; Liu, Z.P. Full-length transcript sequencing and comparative transcriptomic analysis to evaluate the contribution of osmotic and ionic stress components towards salinity tolerance in the roots of cultivated alfalfa (Medicago sativa L.). BMC Plant Biol. 2019, 19, 32. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Bednarek, P.T.; Orlowska, R.; Niedziela, A. A relative quantitative Methylation-Sensitive Amplified Polymorphism (MSAP) method for the analysis of abiotic stress. BMC Plant Biol. 2017, 17, 79. [Google Scholar] [CrossRef] [PubMed]
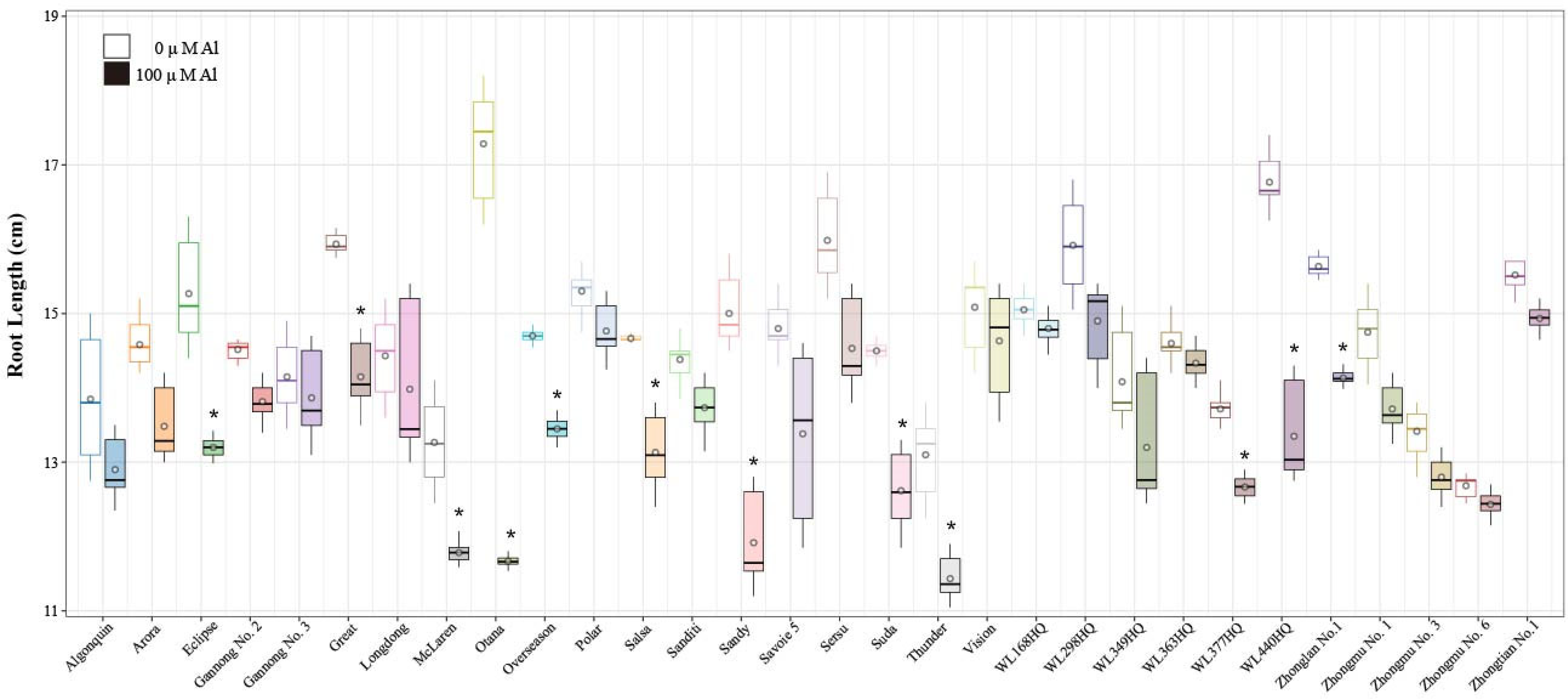
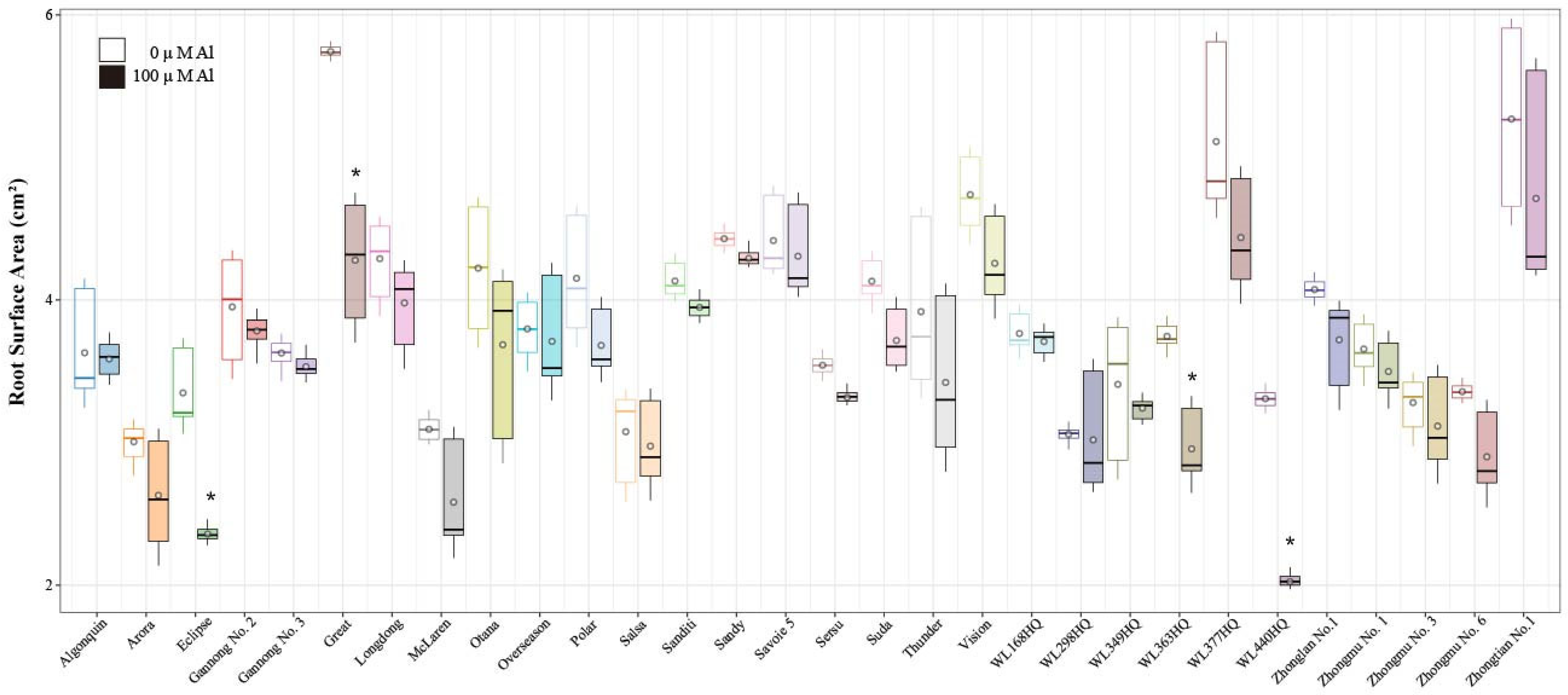
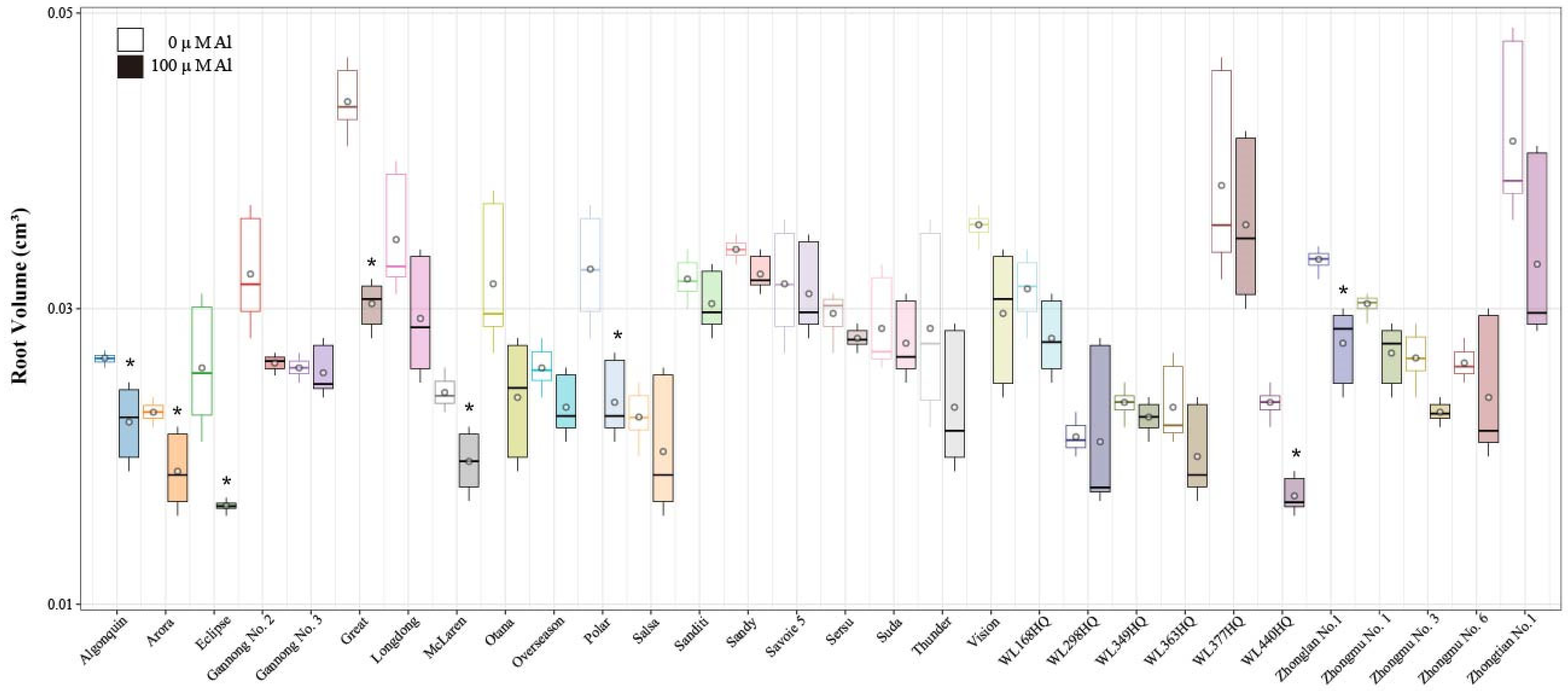
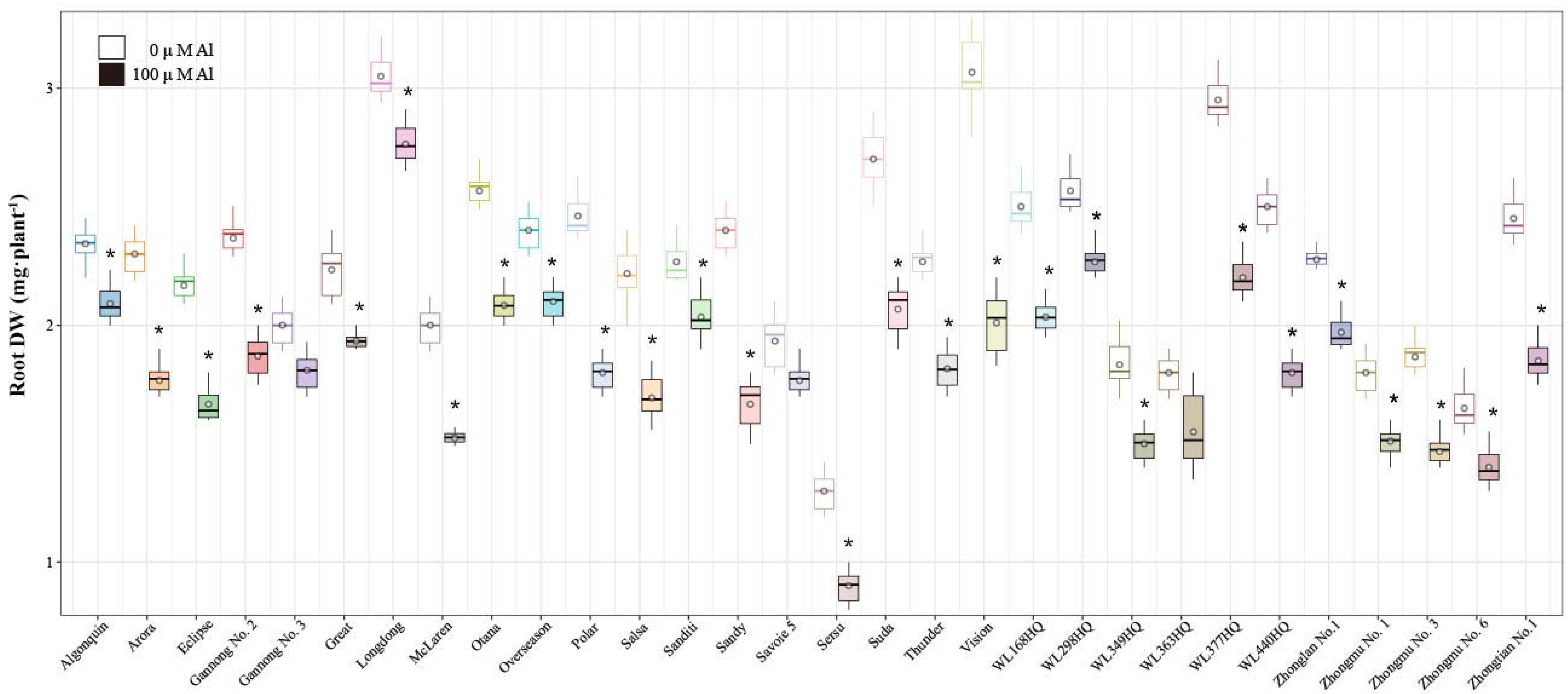
| Cultivars | Root Length | Root Elongation Rate | Root Surface Area | Root Volume | Root DW | Shoot DW | Root/Shoot Ratio | MDA Content | SOD Activity | POD Activity | CAT Activity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Algonquin | 0.93 ± 0.035 bcdef | 0.75 ± 0.152 defgh | 1.00 ± 0.161 a | 0.84 ± 0.118 ab | 0.89 ± 0.033 abc | 0.92 ± 0.028 abcd | 0.97 ± 0.018 ab | 0.67 ± 0.117 k | 1.11 ± 0.036 jkl | 1.89 ± 0.132 b | 1.39 ± 0.107 cdef |
| Arora | 0.92 ± 0.010 cdef | 0.72 ± 0.023 efghi | 0.87 ± 0.104 ab | 0.82 ± 0.095 ab | 0.77 ± 0.035 ghijkl | 0.77 ± 0.032 ijk | 0.99 ± 0.007 a | 1.07 ± 0.011 bcd | 1.41 ± 0.091 defghi | 1.10 ± 0.133 hij | 1.39 ± 0.076 cdef |
| Eclipse | 0.87 ± 0.0410 h | 0.54 ± 0.142 kl | 0.71 ± 0.073 bc | 0.65 ± 0.107 b | 0.77 ± 0.068 fghijkl | 0.96 ± 0.017 a | 0.81 ± 0.086 g | 1.00 ± 0.019 defg | 1.33 ± 0.124 efghijk | 1.15 ± 0.077 fghij | 1.22 ± 0.080 ghij |
| Gannong No. 2 | 0.95 ± 0.003 abcd | 0.86 ± 0.010 abcde | 0.96 ± 0.064 ab | 0.82 ± 0.100 ab | 0.79 ± 0.045 defghijk | 0.83 ± 0.036 fghi | 0.96 ± 0.033 abc | 0.94 ± 0.003 gh | 1.18 ± 0.097 ijkl | 1.28 ± 0.207 defgh | 0.96 ± 0.013 l |
| Gannong No. 3 | 0.98 ± 0.007 ab | 0.92 ± 0.034 ab | 0.98 ± 0.046 a | 0.99 ± 0.118 a | 0.91 ± 0.006 ab | 0.94 ± 0.026 abc | 0.96 ± 0.027 abc | 0.48 ± 0.02 lm | 1.66 ± 0.012 bc | 1.11 ± 0.060 ghij | 1.53 ± 0.081 bc |
| Great | 0.89 ± 0.016 fgh | 0.71 ± 0.033 fghi | 0.75 ± 0.099 abc | 0.69 ± 0.077 ab | 0.87 ± 0.073 abcdef | 0.91 ± 0.041 abcde | 0.96 ± 0.037 abc | 0.35 ± 0.034 n | 1.56 ± 0.102 cde | 1.37 ± 0.103 de | 1.44 ± 0.096 cde |
| Longdong | 0.97 ± 0.041 abc | 0.90 ± 0.130 abc | 0.93 ± 0.132 ab | 0.87 ± 0.236 ab | 0.91 ± 0.035 ab | 0.91 ± 0.035 abcde | 0.99 ± 0.003 a | 0.87 ± 0.013 h | 1.31 ± 0.097 fghijk | 1.18 ± 0.064 fghij | 1.15 ± 0.048 jk |
| McLaren | 0.89 ± 0.046 efgh | 0.68 ± 0.140 ghij | 0.84 ± 0.155 abc | 0.81 ± 0.058 ab | 0.76 ± 0.040 hijkl | 0.88 ± 0.043 defg | 0.87 ± 0.045 defg | 0.53 ± 0.009 l | 1.08 ± 0.041 l | 1.14 ± 0.068 fghij | 1.32 ± 0.027 efghi |
| Otana | 0.68 ± 0.030 j | 0.19 ± 0.083 m | 0.89 ± 0.244 ab | 0.78 ± 0.226 ab | 0.82 ± 0.078 bcdefghi | 0.82 ± 0.079 ghij | 0.99 ± 0.006 a | 0.68 ± 0.013 jk | 1.52 ± 0.141 cdefg | 1.09 ± 0.020 hij | 1.25 ± 0.036 fghij |
| Overseason | 0.92 ± 0.008 defg | 0.67 ± 0.050 ghij | 0.99 ± 0.200 a | 0.90 ± 0.155 ab | 0.88 ± 0.005 abcde | 0.88 ± 0.012 bcdef | 0.99 ± 0.007 a | 0.95 ± 0.021 fgh | 1.53 ± 0.149 cdef | 1.10 ± 0.016 hij | 1.33 ± 0.037 efg |
| Polar | 0.97 ± 0.010 abc | 0.91 ± 0.026 ab | 0.89 ± 0.079 ab | 0.73 ± 0.093 ab | 0.73 ± 0.046 jklm | 0.75 ± 0.022 k | 0.97 ± 0.036 ab | 1.03 ± 0.009 cdef | 1.32 ± 0.207 fghijk | 1.19 ± 0.080 fghij | 0.96 ± 0.011 l |
| Salsa | 0.90 ± 0.028 efgh | 0.71 ± 0.068 fghi | 0.97 ± 0.083 a | 0.90 ± 0.203 ab | 0.77 ± 0.050 ghijkl | 0.77 ± 0.047 ijk | 0.99 ± 0.007 a | 0.75 ± 0.036 ij | 1.40 ± 0.077 defghi | 1.30 ± 0.142 def | 1.32 ± 0.067 efghi |
| Sanditi | 0.95 ± 0.005 abcd | 0.82 ± 0.018 abcdef | 0.96 ± 0.028 ab | 0.95 ± 0.021 ab | 0.90 ± 0.049 abc | 0.96 ± 0.025 a | 0.94 ± 0.028 abcd | 0.43 ± 0.054 n | 1.33 ± 0.056 efghijk | 2.36 ± 0.099 a | 1.3 ± 0.067 efghi |
| Sandy | 0.79 ± 0.020 i | 0.23 ± 0.034 m | 0.97 ± 0.013 a | 0.95 ± 0.018 ab | 0.69 ± 0.027 klm | 0.75 ± 0.060 k | 0.92 ± 0.048 abcde | 0.65 ± 0.049 k | 1.84 ± 0.064 b | 1.41 ± 0.145 d | 1.22 ± 0.069 ghij |
| Savoie 5 | 0.90 ± 0.065 efgh | 0.74 ± 0.143 efgh | 0.97 ± 0.015 a | 0.98 ± 0.050 a | 0.92 ± 0.080 a | 0.96 ± 0.028 a | 0.96 ± 0.057 abc | 0.64 ± 0.039 k | 2.22 ± 0.088 a | 1.25 ± 0.122 defgh | 1.13 ± 0.074 jk |
| Sersu | 0.91 ± 0.002 defgh | 0.73 ± 0.009 efgh | 0.94 ± 0.038 ab | 0.95 ± 0.109 ab | 0.69 ± 0.024 lm | 0.94 ± 0.022 abcd | 0.74 ± 0.020 h | 0.76 ± 0.024 ij | 1.35 ± 0.043 efghi | 1.71 ± 0.077 c | 1.12 ± 0.026 jk |
| Suda | 0.87 ± 0.038 gh | 0.59 ± 0.088 ijkl | 0.90 ± 0.109 ab | 0.98 ± 0.217 a | 0.77 ± 0.030 ghijkl | 0.78 ± 0.028 ijk | 0.99 ± 0.004 a | 1.36 ± 0.006 a | 1.40 ± 0.179 defghi | 1.38 ± 0.113 de | 1.43 ± 0.072 cde |
| Thunder | 0.87 ± 0.025 gh | 0.50 ± 0.058 l | 0.90 ± 0.262 ab | 0.85 ± 0.283 ab | 0.80 ± 0.046 cdefghij | 0.96 ± 0.015 a | 0.84 ± 0.036 fg | 0.78 ± 0.008 i | 1.33 ± 0.174 efghijk | 1.03 ± 0.025 ij | 1.14 ± 0.068 jk |
| Vision | 0.97 ± 0.014 abc | 0.89 ± 0.053 abc | 0.9 ± 0.139 ab | 0.83 ± 0.144 ab | 0.66 ± 0.109 m | 0.77 ± 0.059 ijk | 0.86 ± 0.073 efg | 0.40 ± 0.021 mn | 1.62 ± 0.030 cd | 1.22 ± 0.078 efghi | 1.17 ± 0.008 ijk |
| WL168HQ | 0.98 ± 0.003 a | 0.94 ± 0.011 a | 0.99 ± 0.029 a | 0.90 ± 0.110 ab | 0.81 ± 0.030 bcdefghi | 0.91 ± 0.017 abcde | 0.89 ± 0.026 bcdef | 1.05 ± 0.027 bcde | 1.62 ± 0.342 cd | 1.27 ± 0.054 defgh | 1.25 ± 0.018 fghij |
| WL298HQ | 0.94 ± 0.023 abcde | 0.81 ± 0.068 abcdefg | 0.99 ± 0.194 a | 1.00 ± 0.353 a | 0.88 ± 0.040 abcd | 0.92 ± 0.021 abcd | 0.96 ± 0.026 abc | 0.92 ± 0.054 gh | 1.67 ± 0.073 bc | 1.29 ± 0.066 defg | 1.52 ± 0.008 bcd |
| WL349HQ | 0.94 ± 0.015 abcde | 0.79 ± 0.048 bcdefgh | 0.97 ± 0.168 a | 0.96 ± 0.040 ab | 0.82 ± 0.035 bcdefghi | 0.85 ± 0.020 efgh | 0.96 ± 0.024 abc | 1.12 ± 0.044 b | 1.11 ± 0.042 kl | 1.14 ± 0.026 fghij | 1.40 ± 0.085 cdef |
| WL363HQ | 0.98 ± 0.007 a | 0.92 ± 0.035 ab | 0.79 ± 0.077 abc | 0.85 ± 0.040 ab | 0.86 ± 0.100 abcdefg | 0.96 ± 0.016 a | 0.89 ± 0.091 bcdef | 1.09 ± 0.011 bc | 1.42 ± 0.072 defgh | 1.76 ± 0.126 bc | 1.22 ± 0.089 ghij |
| WL363HQ | 0.92 ± 0.008 cdef | 0.65 ± 0.081 hijk | 0.88 ± 0.175 ab | 0.96 ± 0.306 ab | 0.75 ± 0.015 ijklm | 0.83 ± 0.032 fghi | 0.90 ± 0.053 bcdef | 0.46 ± 0.057 lm | 1.28 ± 0.077 ghijkl | 1.13 ± 0.085 fghij | 1.59 ± 0.090 ab |
| WL440HQ | 0.80 ± 0.023 i | 0.57 ± 0.042 jkl | 0.61 ± 0.037 c | 0.73 ± 0.026 ab | 0.72 ± 0.036 jklm | 0.77 ± 0.031 ijk | 0.93 ± 0.007 abcd | 0.92 ± 0.019 gh | 1.51 ± 0.133 cdefg | 1.03 ± 0.065 j | 1.71 ± 0.120 a |
| Zhonglan No.1 | 0.90 ± 0.007 efgh | 0.68 ± 0.009 ghij | 0.92 ± 0.126 ab | 0.83 ± 0.110 ab | 0.87 ± 0.034 abcdef | 0.88 ± 0.025 cdefg | 0.98 ± 0.010 a | 0.64 ± 0.037 k | 1.51 ± 0.126 cdefg | 1.24 ± 0.058 defgh | 1.51 ± 0.087 bcd |
| Zhongmu No. 1 | 0.93 ± 0.011 cdef | 0.76 ± 0.030 cdefgh | 0.96 ± 0.142 ab | 0.89 ± 0.113 ab | 0.84 ± 0.061 abcdefgh | 0.95 ± 0.007 ab | 0.89 ± 0.069 cdef | 0.70 ± 0.046 ijk | 1.22 ± 0.040 hijkl | 1.04 ± 0.024 ij | 1.18 ± 0.050 hij |
| Zhongmu No. 3 | 0.95 ± 0.016 abcd | 0.84 ± 0.052 abcdef | 0.95 ± 0.129 ab | 0.87 ± 0.079 ab | 0.78 ± 0.018 efghijkl | 0.79 ± 0.015 hijk | 0.99 ± 0.008 a | 0.99 ± 0.016 efg | 1.34 ± 0.112 efghij | 1.22 ± 0.060 efghi | 1.38 ± 0.184 def |
| Zhongmu No. 6 | 0.98 ± 0.011 ab | 0.91 ± 0.027 ab | 0.87 ± 0.120 ab | 0.92 ± 0.251 ab | 0.85 ± 0.028 abcdefgh | 0.89 ± 0.023 bcde | 0.96 ± 0.047 abc | 1.34 ± 0.140 a | 1.86 ± 0.152 b | 1.18 ± 0.147 fghij | 1.33 ± 0.105 efgh |
| Zhongtian No.1 | 0.96 ± 0.009 abc | 0.89 ± 0.021 abcd | 0.89 ± 0.094 ab | 0.80 ± 0.047 ab | 0.76 ± 0.005 hijkl | 0.76 ± 0.011 jk | 0.99 ± 0.012 a | 0.90 ± 0.012 h | 1.60 ± 0.062 cd | 1.10 ± 0.034 hij | 1.04 ± 0.036 kl |
| DF | 29 | 29 | 29 | 29 | 29 | 29 | 29 | 29 | 29 | 29 | 29 |
| MS | 0.013 | 0.103 | 0.025 | 0.025 | 0.015 | 0.017 | 0.012 | 0.211 | 0.181 | 0.253 | 0.096 |
| p | ** | ** | ns | ns | ** | ** | ** | ** | ** | ** | ** |
| Trait | Component | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Root length | 0.271 | −0.207 | 0.249 | 0.086 | −0.001 | 0.217 |
| Root elongation rate | 0.253 | −0.196 | 0.272 | 0.178 | 0.022 | 0.171 |
| Root surface area | 0.231 | 0.17 | 0.02 | −0.451 | −0.145 | −0.208 |
| Root volume | 0.213 | 0.244 | −0.069 | −0.355 | −0.077 | 0.464 |
| Root dry weight | 0.233 | 0.186 | −0.171 | 0.386 | 0.108 | −0.364 |
| Shoot dry weight | 0.189 | −0.162 | −0.368 | 0.215 | 0.292 | −0.02 |
| Root/shoot ratio | 0.056 | 0.411 | 0.243 | 0.21 | −0.219 | −0.447 |
| MDA | 0.005 | 0.019 | 0.381 | 0.09 | 0.146 | 0.107 |
| SOD | 0.028 | 0.289 | −0.042 | −0.09 | 0.727 | 0.173 |
| POD | 0.15 | −0.133 | −0.244 | −0.098 | −0.39 | −0.132 |
| CAT | −0.038 | 0.271 | −0.138 | 0.383 | −0.369 | 0.661 |
| Eigenvalue | 2.827 | 1.739 | 1.694 | 1.281 | 0.989 | 0.86 |
| Contribution rate (%) | 25.702 | 15.813 | 15.396 | 11.647 | 8.99 | 7.817 |
| Cumulative contribution rate (%) | 25.702 | 41.515 | 56.911 | 68.558 | 77.548 | 85.365 |
| Cultivars | CI1 | CI2 | CI3 | CI4 | CI5 | CI6 | U(X1) | U(X2) | U(X3) | U(X4) | U(X5) | U(X6) | D | Rank |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Algonquin | 0.959 | −0.189 | −0.910 | 0.454 | −2.021 | −1.193 | 0.828 | 0.505 | 0.239 | 0.666 | 0.000 | 0.246 | 0.499 | 20 |
| Arora | −0.608 | 0.460 | 1.268 | 0.434 | −0.455 | 0.179 | 0.452 | 0.682 | 0.783 | 0.662 | 0.324 | 0.579 | 0.580 | 12 |
| Eclipse | −1.504 | −1.916 | −0.712 | 1.210 | 1.291 | −0.142 | 0.236 | 0.032 | 0.288 | 0.826 | 0.686 | 0.501 | 0.359 | 30 |
| Gannong No. 2 | 0.276 | −0.898 | 1.185 | −0.606 | −0.328 | −1.583 | 0.664 | 0.311 | 0.762 | 0.442 | 0.351 | 0.152 | 0.505 | 18 |
| Gannong No. 3 | 1.427 | 0.985 | −0.592 | 0.628 | 0.330 | 1.084 | 0.940 | 0.826 | 0.318 | 0.703 | 0.487 | 0.798 | 0.713 | 2 |
| Great | −0.580 | −0.168 | −1.145 | 2.035 | 0.222 | −0.762 | 0.458 | 0.510 | 0.180 | 1.000 | 0.464 | 0.351 | 0.482 | 22 |
| Longdong | 0.988 | −0.107 | 0.535 | 0.752 | 0.154 | −1.189 | 0.835 | 0.527 | 0.600 | 0.729 | 0.450 | 0.247 | 0.626 | 7 |
| McLaren | −0.767 | −1.086 | −0.578 | 0.172 | −0.760 | 0.214 | 0.413 | 0.259 | 0.322 | 0.606 | 0.261 | 0.587 | 0.394 | 27 |
| Otana | −2.044 | 1.622 | −1.203 | −0.393 | 0.167 | −2.208 | 0.107 | 1.000 | 0.165 | 0.487 | 0.453 | 0.000 | 0.361 | 29 |
| Overseason | 0.469 | 1.045 | 0.222 | 0.163 | 0.279 | −0.506 | 0.710 | 0.842 | 0.522 | 0.604 | 0.476 | 0.413 | 0.634 | 6 |
| Polar | −0.374 | −1.093 | 2.134 | −0.337 | 0.019 | −1.337 | 0.508 | 0.257 | 1.000 | 0.499 | 0.422 | 0.211 | 0.512 | 17 |
| Salsa | −0.152 | 0.704 | 0.569 | −0.734 | −1.014 | −0.206 | 0.561 | 0.749 | 0.608 | 0.415 | 0.209 | 0.486 | 0.540 | 15 |
| Sanditi | 1.677 | −0.474 | −1.864 | −0.126 | −1.694 | −0.745 | 1.000 | 0.427 | 0.000 | 0.543 | 0.068 | 0.355 | 0.494 | 21 |
| Sandy | −1.387 | 1.374 | −0.807 | −2.697 | 0.244 | 0.003 | 0.265 | 0.932 | 0.264 | 0.000 | 0.469 | 0.536 | 0.398 | 26 |
| Savoie 5 | 1.110 | 1.365 | −0.948 | −0.502 | 2.808 | −0.507 | 0.864 | 0.930 | 0.229 | 0.464 | 1.000 | 0.413 | 0.679 | 4 |
| Sersu | 0.141 | −2.034 | −1.182 | −2.079 | 0.121 | 1.361 | 0.631 | 0.000 | 0.170 | 0.131 | 0.444 | 0.866 | 0.365 | 28 |
| Suda | −0.376 | 1.158 | 0.879 | −0.406 | −0.944 | 0.764 | 0.507 | 0.873 | 0.686 | 0.484 | 0.223 | 0.721 | 0.593 | 10 |
| Thunder | −0.533 | −0.788 | −0.985 | −0.496 | 0.958 | −0.297 | 0.470 | 0.341 | 0.220 | 0.465 | 0.617 | 0.464 | 0.415 | 25 |
| Vision | −0.426 | −1.120 | 0.531 | −1.367 | 0.344 | 0.980 | 0.495 | 0.250 | 0.599 | 0.281 | 0.490 | 0.773 | 0.464 | 24 |
| WL168HQ | 0.957 | −0.417 | 0.523 | −0.354 | 0.929 | 0.734 | 0.827 | 0.442 | 0.597 | 0.495 | 0.611 | 0.713 | 0.635 | 5 |
| WL298HQ | 1.120 | 1.182 | −0.255 | 0.228 | 0.221 | 1.100 | 0.866 | 0.880 | 0.402 | 0.618 | 0.464 | 0.802 | 0.703 | 3 |
| WL349HQ | 0.469 | 0.295 | 0.822 | 0.011 | −1.175 | 0.558 | 0.710 | 0.637 | 0.672 | 0.572 | 0.175 | 0.671 | 0.610 | 8 |
| WL363HQ | 0.826 | −1.386 | −0.171 | 1.086 | 0.389 | 0.199 | 0.796 | 0.177 | 0.423 | 0.800 | 0.499 | 0.584 | 0.563 | 14 |
| WL377HQ | −0.402 | 0.248 | −0.563 | −0.209 | −1.254 | 1.915 | 0.501 | 0.624 | 0.325 | 0.526 | 0.159 | 1.000 | 0.505 | 19 |
| WL440HQ | −2.490 | 0.364 | 0.068 | 1.930 | −0.195 | 1.689 | 0.000 | 0.656 | 0.483 | 0.978 | 0.378 | 0.945 | 0.468 | 23 |
| Zhonglan No.1 | 0.043 | 0.849 | −0.515 | 0.919 | −0.316 | −0.175 | 0.608 | 0.789 | 0.338 | 0.764 | 0.353 | 0.493 | 0.576 | 13 |
| Zhongmu No. 1 | 0.446 | −0.656 | −0.381 | −0.174 | 0.239 | −0.318 | 0.705 | 0.377 | 0.371 | 0.533 | 0.468 | 0.458 | 0.512 | 16 |
| Zhongmu No. 3 | 0.136 | 0.349 | 1.221 | 0.041 | −0.912 | 0.229 | 0.630 | 0.652 | 0.772 | 0.579 | 0.230 | 0.591 | 0.606 | 9 |
| Zhongmu No. 6 | 0.770 | 0.514 | 1.101 | 0.745 | 1.665 | 1.009 | 0.782 | 0.697 | 0.741 | 0.727 | 0.763 | 0.780 | 0.749 | 1 |
| Zhongtian No.1 | −0.173 | −0.183 | 1.753 | −0.329 | 0.686 | −0.850 | 0.556 | 0.506 | 0.905 | 0.500 | 0.560 | 0.329 | 0.581 | 11 |
| W | 0.301 | 0.185 | 0.180 | 0.136 | 0.105 | 0.092 |
| Number | Cultivar | Source | Number | Cultivar | Source |
|---|---|---|---|---|---|
| 1 | Algonquin | Canada | 16 | Sersu | USA |
| 2 | Arora | USA | 17 | Suda | USA |
| 3 | Eclipse | China | 18 | Thunder | USA |
| 4 | Gannong No. 2 | China | 19 | Vision | USA |
| 5 | Gannong No. 3 | China | 20 | WL168HQ | USA |
| 6 | Great | USA | 21 | WL298HQ | USA |
| 7 | Longdong | China | 22 | WL349HQ | USA |
| 8 | McLaren | USA | 23 | WL363HQ | USA |
| 9 | Otana | USA | 24 | WL377HQ | USA |
| 10 | Overseason | USA | 25 | WL440HQ | USA |
| 11 | Polar | USA | 26 | Zhonglan No.1 | China |
| 12 | Salsa | USA | 27 | Zhongmu No. 1 | China |
| 13 | Sanditi | France | 28 | Zhongmu No. 3 | China |
| 14 | Sandy | USA | 29 | Zhongmu No. 6 | China |
| 15 | Savoie 5 | France | 30 | Zhongtian No.1 | China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, N.; Zeng, X.; Wei, J.; Li, Z.; Zhao, X.; Chen, J.; Gu, X.; Chen, C.; Dong, R. Comprehensive Assessment of Alfalfa Aluminum Stress Resistance Using Growth and Physiological Trait Analysis. Agriculture 2025, 15, 1168. https://doi.org/10.3390/agriculture15111168
Tang N, Zeng X, Wei J, Li Z, Zhao X, Chen J, Gu X, Chen C, Dong R. Comprehensive Assessment of Alfalfa Aluminum Stress Resistance Using Growth and Physiological Trait Analysis. Agriculture. 2025; 15(11):1168. https://doi.org/10.3390/agriculture15111168
Chicago/Turabian StyleTang, Nannan, Xiangming Zeng, Jizhi Wei, Zhou Li, Xuechun Zhao, Jihui Chen, Xinyao Gu, Chao Chen, and Rui Dong. 2025. "Comprehensive Assessment of Alfalfa Aluminum Stress Resistance Using Growth and Physiological Trait Analysis" Agriculture 15, no. 11: 1168. https://doi.org/10.3390/agriculture15111168
APA StyleTang, N., Zeng, X., Wei, J., Li, Z., Zhao, X., Chen, J., Gu, X., Chen, C., & Dong, R. (2025). Comprehensive Assessment of Alfalfa Aluminum Stress Resistance Using Growth and Physiological Trait Analysis. Agriculture, 15(11), 1168. https://doi.org/10.3390/agriculture15111168






