Tea Plant/Ophiopogon japonicus Intercropping Drives the Reshaping of Soil Microbial Communities in Terraced Tea Plantation’s Micro-Topographical Units
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Soil Physicochemical Properties and Enzyme Activity Measurements
2.3. Tea Plant Yield Measurement and Disease Survey
2.4. Total Soil DNA Extraction
2.5. 16S/ITS rDNA High-Throughput Sequencing Analysis
2.6. Data Analysis
3. Results
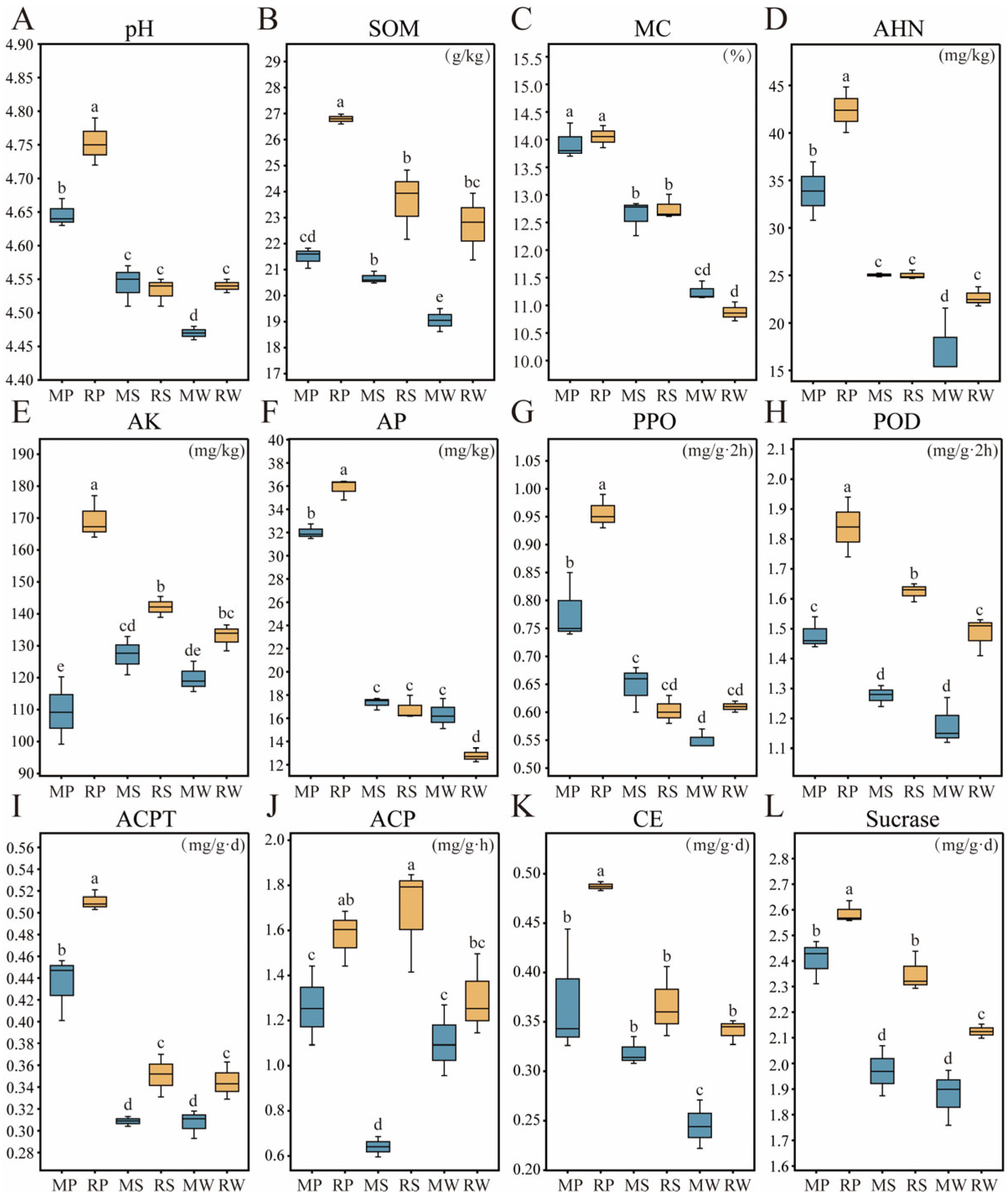
3.1. The Impact of O. japonicus Intercropping on Soil Physicochemical Properties in Different Micro-Topographical Units of Tea Plantations
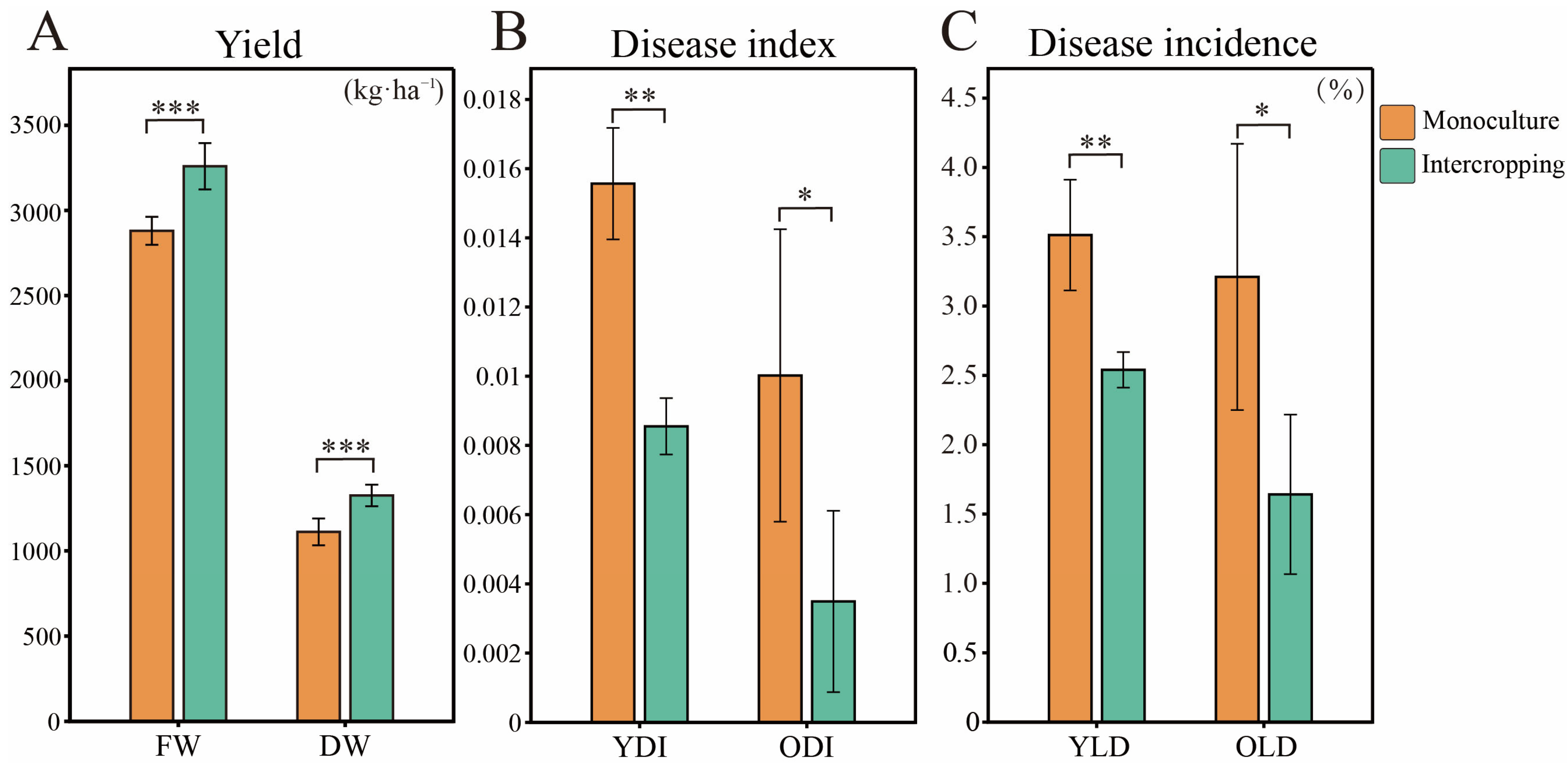
3.2. Tea Plant Yield Measurement and Disease Survey in Monoculture and O. japonicus Intercropping Systems
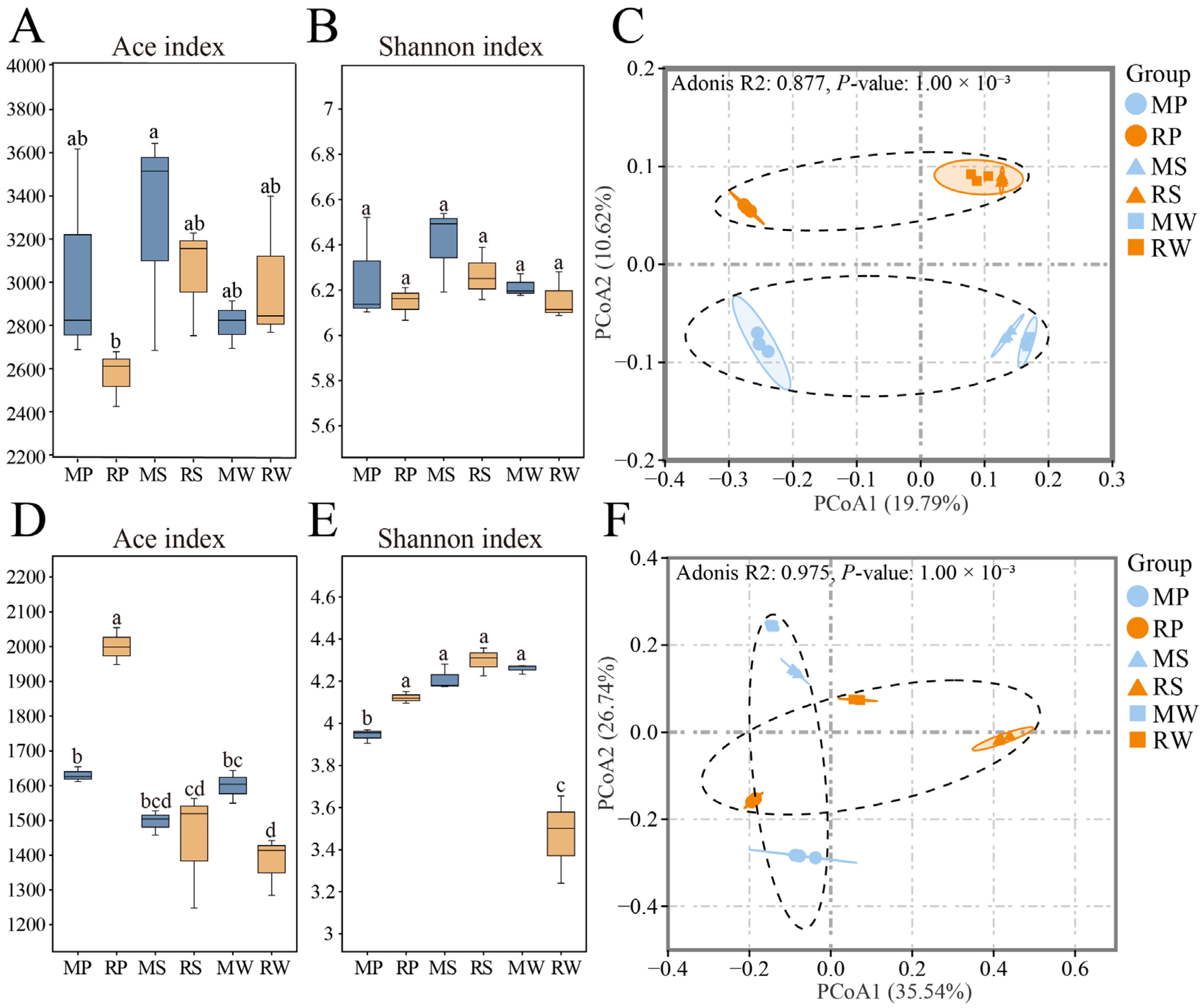
3.3. Analysis of Soil Microbial Diversity in Terraced Tea Plantations Under O. japonicus Intercropping
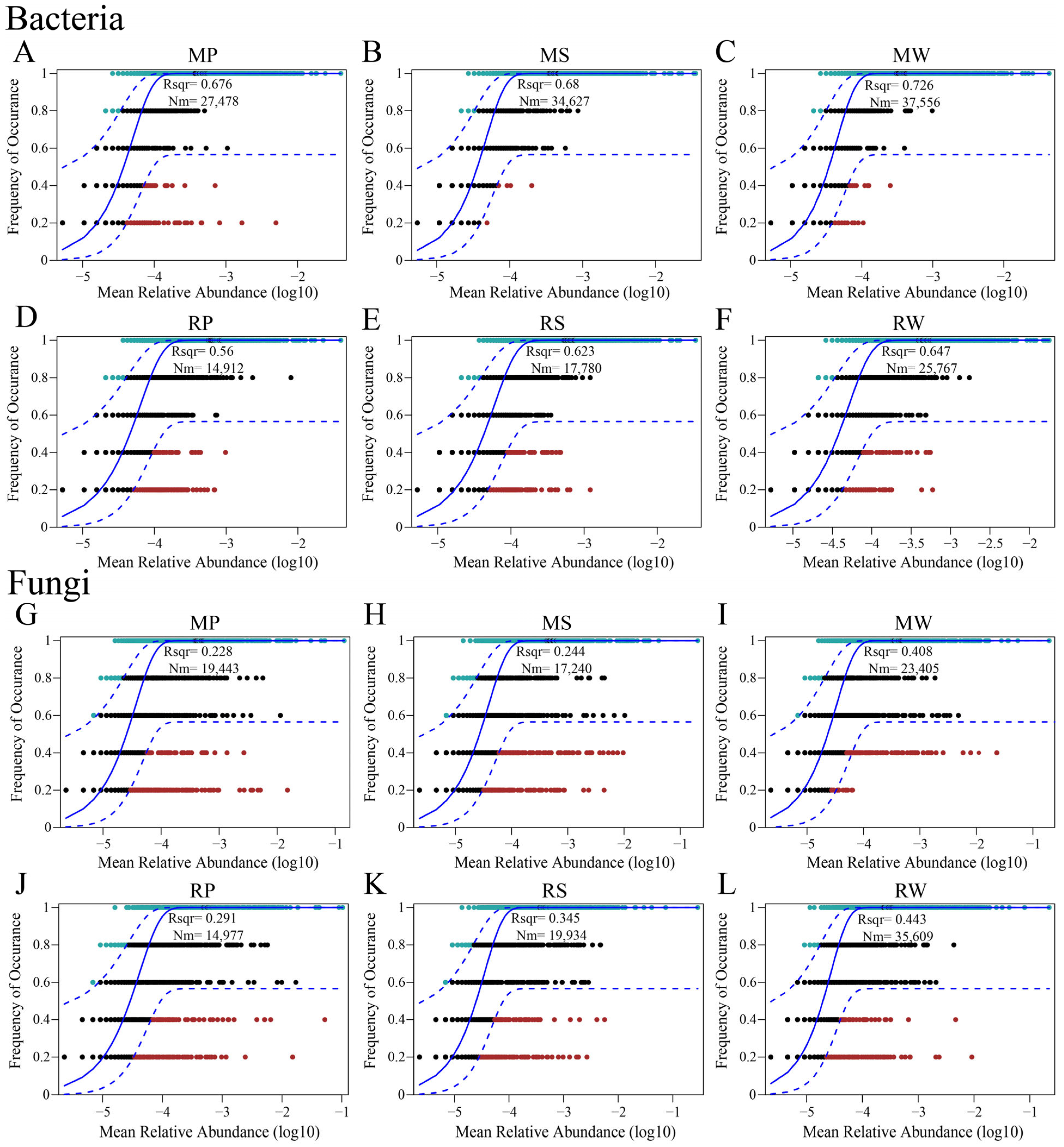
3.4. The Impact of O. japonicus Intercropping on Microbial Community Assembly and Diffusion in Terraced Tea Plantations
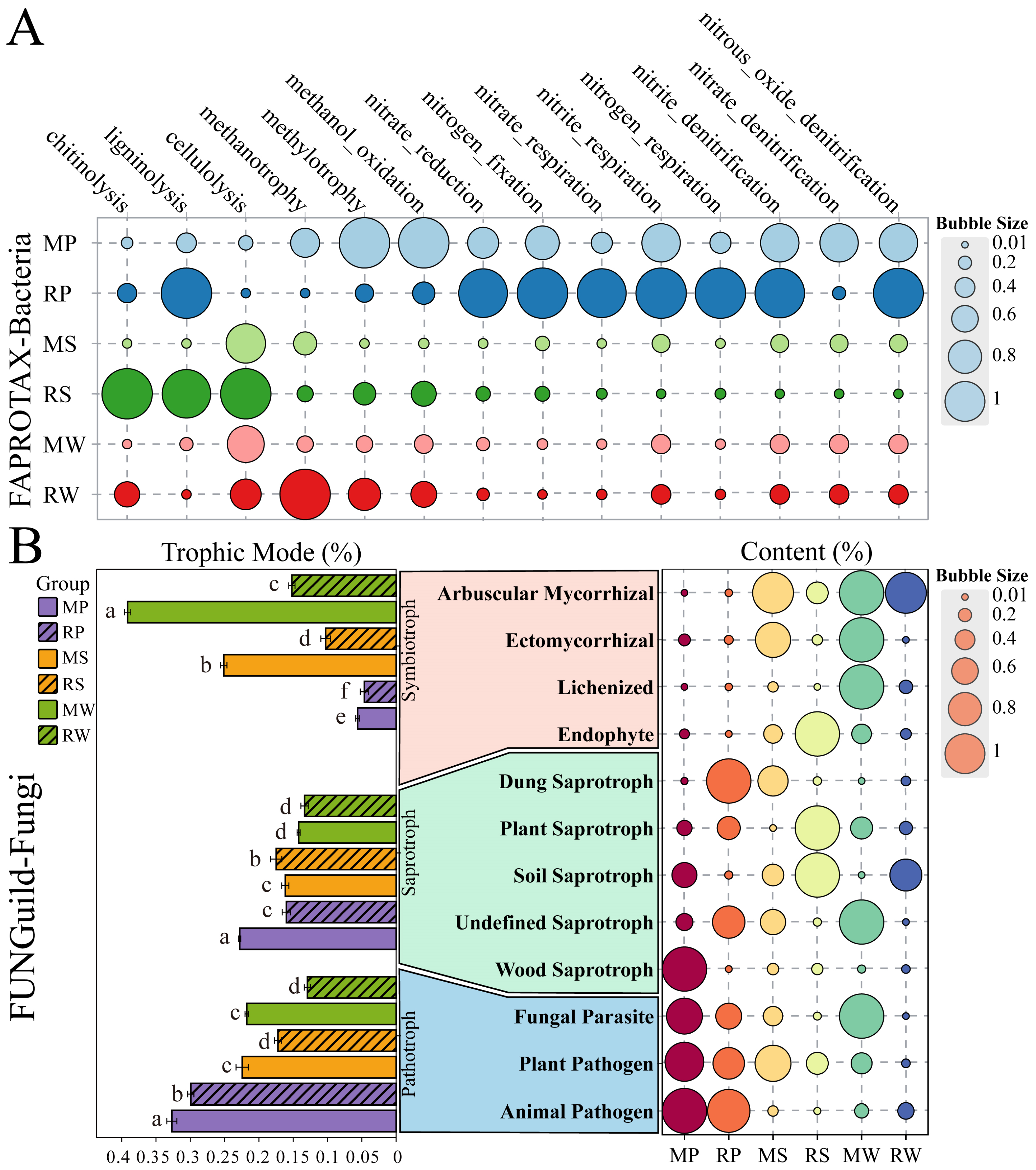
3.5. The Impact of O. japonicus Intercropping on Core Functional Microbial Groups in the Micro-Topographical Units of Terraced Tea Plantations
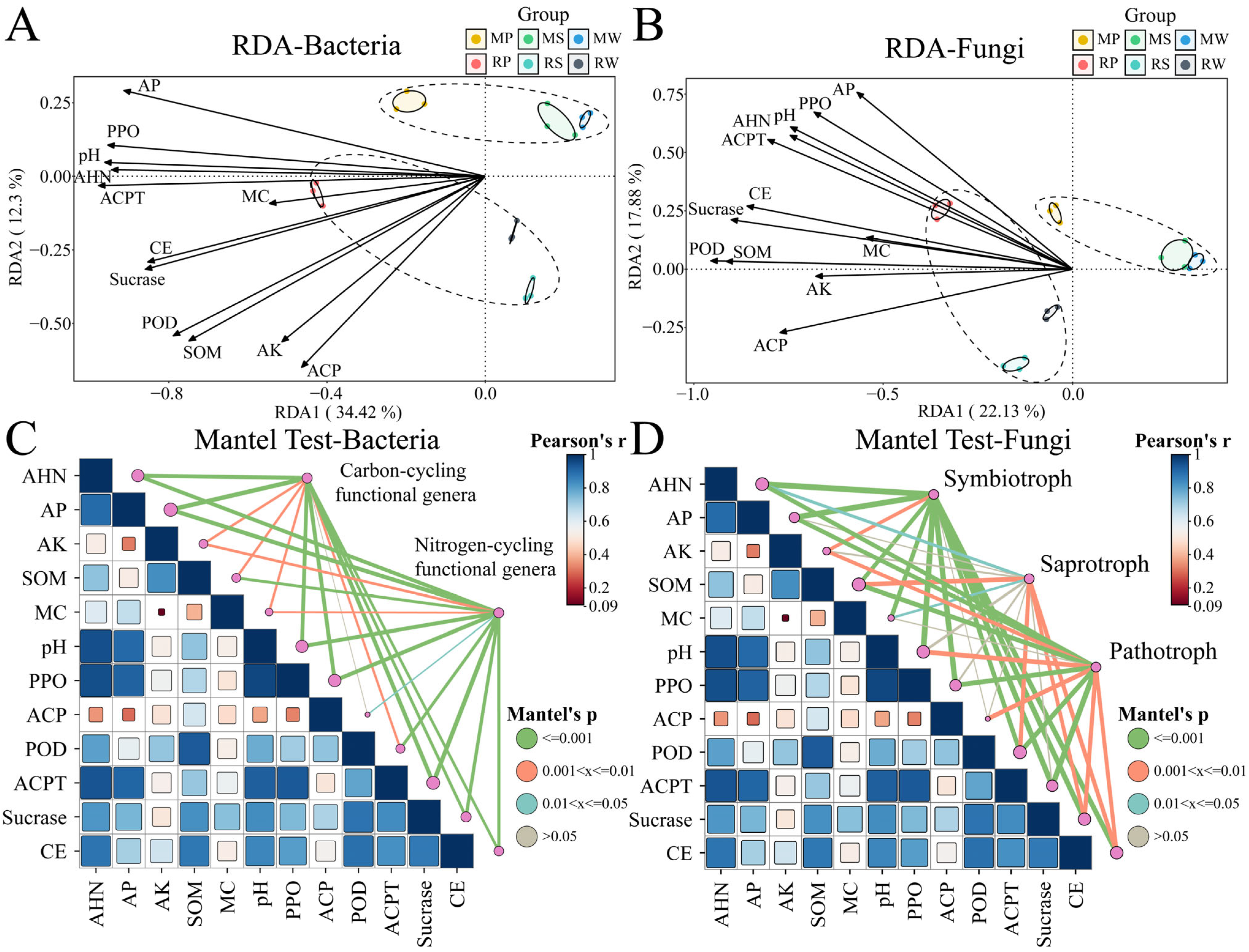
3.6. Correlation Between Soil Microbial Community Structure and Soil Physicochemical Properties
4. Discussion
4.1. Spatial Heterogeneity in Microbial Functional Community Assembly Driven by O. japonicus Intercropping in Terraced Tea Plantations
4.2. O. japonicus Intercropping Drives the Differentiation of Soil Microbial Carbon and Nitrogen Cycling Functions and Ecological Regulation in Tea Plantation Micro-Topographies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOM | Soil organic matter |
| MC | Moisture content |
| AHN | Alkaline hydrolyzable nitrogen |
| AK | Available potassium |
| AP | Available phosphorus |
| PPO | Polyphenol oxidase |
| POD | Peroxidase |
| ACPT | Protease |
| ACP | Acid phosphatase |
| CE | Cellulase |
| OTUs | Operational taxonomic units |
References
- Zhao, Z.; Song, Q.; Bai, D.; Niu, S.; He, Y.; Qiao, D.; Chen, Z.; Li, C.; Luo, J.; Li, F. Population structure analysis to explore genetic diversity and geographical distribution characteristics of cultivated-type tea plant in Guizhou Plateau. BMC Plant Biol. 2022, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fan, J.; Zhou, K. An Empirical Study on Spatial–Temporal Dynamics and Influencing Factors of Tea Production in China. Sustainability 2018, 10, 3037. [Google Scholar] [CrossRef]
- Wen, B.; Ren, S.; Zhang, Y.; Duan, Y.; Shen, J.; Zhu, X.; Wang, Y.; Ma, Y.; Zou, Z.; Fang, W. Effects of geographic locations and topographical factors on secondary metabolites distribution in green tea at a regional scale. Food Control 2020, 110, 106979. [Google Scholar] [CrossRef]
- Tian, X.; Chen, S.; Zhong, Q.; Wang, J.; Chen, J.; Chen, L.; Moon, D.; Ma, J. Widely Targeted Metabolomics Analysis Reveals the Effect of Cultivation Altitude on Tea Metabolites. Agronomy 2024, 14, 812. [Google Scholar] [CrossRef]
- Shao, Q.; Xie, X.; Pu, L.; Zhu, L.; Meadows, M.; Wu, T.; Jiang, G.; Xu, F. Response of soil physicochemical properties, enzyme activities, and bacterial community variation to tea plantation age in a subtropical hilly region of China. J. Soils Sediments 2025, 25, 1302–1313. [Google Scholar] [CrossRef]
- Camera, C.; Djuma, H.; Bruggeman, A.; Zoumides, C.; Eliades, M.; Charalambous, K.; Abate, D.; Faka, M. Quantifying the effectiveness of mountain terraces on soil erosion protection with sediment traps and dry-stone wall laser scans. CATENA 2018, 171, 251–264. [Google Scholar] [CrossRef]
- Li, L.; Tilman, D.; Lambers, H. Plant diversity and overyielding: Insights from belowground facilitation of intercropping in agriculture. New Phytol. 2014, 203, 63–69. [Google Scholar] [CrossRef]
- Li, C.; Lambers, H.; Jing, J.; Zhang, C.; Bezemer, T.M.; Klironomos, J.; Cong, W.-F.; Zhang, F. Belowground cascading biotic interactions trigger crop diversity benefits. Trends Plant Sci. 2024, 29, 1191–1202. [Google Scholar] [CrossRef]
- Laichao, S.; Zhanhai, N.; Shiliang, C.; Shilei, Z.; Ziyuan, Q.; Yu, W.; Xuewen, H.; Zhaotang, D.; Qingping, M. Effects of pea-tea intercropping on rhizosphere soil microbial communities. Plant Soil 2025, 506, 125–135. [Google Scholar] [CrossRef]
- Wang, T.; Duan, Y.; Liu, G.; Shang, X.; Liu, L.; Zhang, K.; Li, J.; Zou, Z.; Zhu, X.; Fang, W. Tea plantation intercropping green manure enhances soil functional microbial abundance and multifunctionality resistance to drying-rewetting cycles. Sci. Total Environ. 2022, 810, 151282. [Google Scholar] [CrossRef]
- Shah, T.; Lateef, S.; Noor, M.A. Carbon and Nitrogen Cycling in Agroecosystems: An Overview. In Carbon and Nitrogen Cycling in Soil; Datta, R., Meena, R.S., Pathan, S.I., Ceccherini, M.T., Eds.; Springer: Singapore, 2020; pp. 1–15. [Google Scholar] [CrossRef]
- Jiao, S.; Yang, Y.; Xu, Y.; Zhang, J.; Lu, Y. Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J. 2020, 14, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, Q.; Zhou, J.; Wang, M.; Liang, Y.; Sun, B.; Chu, H.; Yang, Y. The scale dependence of fungal community distribution in paddy soil driven by stochastic and deterministic processes. Fungal Ecol. 2019, 42, 100856. [Google Scholar] [CrossRef]
- Suriyavirun, N.; Krichels, A.H.; Kent, A.D.; Yang, W.H. Microtopographic differences in soil properties and microbial community composition at the field scale. Soil Biol. Biochem. 2019, 131, 71–80. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Shi, Y.; Zhao, S.; Liu, J.; Lin, Y.; Li, C.; Zhang, C. Effects of microtopography on soil microbial communities in alpine meadows on the Qinghai-Tibetan Plateau. CATENA 2024, 239, 107945. [Google Scholar] [CrossRef]
- Wen, B.; Zhang, X.; Ren, S.; Duan, Y.; Zhang, Y.; Zhu, X.; Wang, Y.; Ma, Y.; Fang, W. Characteristics of soil nutrients, heavy metals and tea quality in different intercropping patterns. Agrofor. Syst. 2020, 94, 963–974. [Google Scholar] [CrossRef]
- Lei, X.; Wang, T.; Yang, B.; Duan, Y.; Zhou, L.; Zou, Z.; Ma, Y.; Zhu, X.; Fang, W. Progress and perspective on intercropping patterns in tea plantations. Beverage Plant Res. 2022, 2, 18. [Google Scholar] [CrossRef]
- Shao, S.; Li, Z.; Ma, X.; Cui, J.; Zhu, Y.; Li, Y.; Wu, L.; Rensing, C.; Cai, P.; Zhang, J.; et al. Enhancing tea plant growth and soil microbial ecology through intercropping tea plants with Ophiopogon japonicus. Plant Soil 2025. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Li, Q.; Zhang, J.; Hou, R.; Wang, Z.; Zhu, Q.; Zhou, Y.; Chen, Y.; Huang, J. Integrated transcriptome and metabolome analysis provide insights into the mechanism of saponin biosynthesis and its role in alleviating cadmium-induced oxidative damage in Ophiopogon japonicum. Plant Physiol. Biochem. 2024, 210, 108634. [Google Scholar] [CrossRef]
- Li, Z.; Wang, M.; Yu, S.; Liu, J. Effects of the root’s distribution on the stability of slope. Geotech. Geol. Eng. 2024, 42, 1009–1019. [Google Scholar] [CrossRef]
- Hu, L.; Huang, R.; Zhou, L.; Qin, R.; He, X.; Deng, H.; Li, K. Effects of magnesium-modified biochar on soil organic carbon mineralization in citrus orchard. Front. Microbiol. 2023, 14, 1109272. [Google Scholar] [CrossRef]
- Guan, S. Soil Enzymes and Their Research Methods; Agricultural Press: Beijing, China, 1986. (In Chinese) [Google Scholar]
- Zhang, Q.; Zhang, Y.; Wang, Y.; Lin, S.; Chen, M.; Cheng, P.; Ye, J.; Miao, P.; Jia, X.; Wang, H. Effects of pruning on tea tree growth, tea quality, and rhizosphere soil microbial community. Microbiol. Spectr. 2023, 11, e01601–e01623. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Long, X.; Geng, Y. Companion Plants of Tea: From Ancient to Terrace to Forest. Plants 2023, 12, 3061. [Google Scholar] [CrossRef]
- Ge, S.; Wang, Y.; Shen, K.; Wang, Q.; Ahammed, G.J.; Han, W.; Jin, Z.; Li, X.; Shi, Y. Effects of differential shading on summer tea quality and tea garden microenvironment. Plants 2024, 13, 202. [Google Scholar] [CrossRef]
- Ulbrich, T.C.; Rivas-Ubach, A.; Tiemann, L.K.; Friesen, M.L.; Evans, S.E. Plant root exudates and rhizosphere bacterial communities shift with neighbor context. Soil Biol. Biochem. 2022, 172, 108753. [Google Scholar] [CrossRef]
- Zhang, X.; Dippold, M.A.; Kuzyakov, Y.; Razavi, B.S. Spatial pattern of enzyme activities depends on root exudate composition. Soil Biol. Biochem. 2019, 133, 83–93. [Google Scholar] [CrossRef]
- Štursová, M.; Bárta, J.; Šantrůčková, H.; Baldrian, P. Small-scale spatial heterogeneity of ecosystem properties, microbial community composition and microbial activities in a temperate mountain forest soil. FEMS Microbiol. Ecol. 2016, 92, fiw185. [Google Scholar] [CrossRef]
- Li, S.; Wu, F. Diversity and Co-occurrence Patterns of Soil Bacterial and Fungal Communities in Seven Intercropping Systems. Front. Microbiol. 2018, 9, 1521. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rabert, E.; van Amstel, C.; Smith, C.; Sloan, W.T.; Gonzalez-Cabaleiro, R. Environmental and ecological controls of the spatial distribution of microbial populations in aggregates. PLoS Comput. Biol. 2022, 18, e1010807. [Google Scholar] [CrossRef]
- Lv, W.; Liu, Y.; Du, J.; Tang, L.; Zhang, B.; Liu, Q.; Cui, X.; Xue, K.; Wang, Y. Microtopography mediates the community assembly of soil prokaryotes on the local-site scale. CATENA 2023, 222, 106815. [Google Scholar] [CrossRef]
- Huo, X.; Ren, C.; Wang, D.; Wu, R.; Wang, Y.; Li, Z.; Huang, D.; Qi, H. Microbial community assembly and its influencing factors of secondary forests in Qinling Mountains. Soil Biol. Biochem. 2023, 184, 109075. [Google Scholar] [CrossRef]
- Kang, P.; Pan, Y.; Yang, P.; Hu, J.; Zhao, T.; Zhang, Y.; Ding, X.; Yan, X. A comparison of microbial composition under three tree ecosystems using the stochastic process and network complexity approaches. Front. Microbiol. 2022, 13, 1018077. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Mei, R.; Liao, J.; Liu, W.-T. Nexus of Stochastic and Deterministic Processes on Microbial Community Assembly in Biological Systems. Front. Microbiol. 2019, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wang, Y.; Zhang, Y.; Fei, J.; Rong, X.; Peng, J.; Yin, L.; Zhou, X.; Luo, G. Enhanced productivity of maize through intercropping is associated with community composition, core species, and network complexity of abundant microbiota in rhizosphere soil. Geoderma 2024, 442, 116786. [Google Scholar] [CrossRef]
- Ma, H.-Y.; Surigaoge, S.; Xu, Y.; Li, Y.-C.; Christie, P.; Zhang, W.P.; Li, L. Responses of soil microbial community diversity and co-occurrence networks to interspecific interactions in soybean/maize and peanut/maize intercropping systems. Appl. Soil Ecol. 2024, 203, 105613. [Google Scholar] [CrossRef]
- Tao, S.; Veen, G.F.; Zhang, N.; Yu, T.; Qu, L. Tree and shrub richness modifies subtropical tree productivity by regulating the diversity and community composition of soil bacteria and archaea. Microbiome 2023, 11, 261. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, C.; Cao, Y.; Dai, J.; Cheng, X.; Hua, S.; Wang, W.; Duan, Y.; Petropoulos, E.; Wang, H. Tea plant–legume intercropping simultaneously improves soil fertility and tea quality by changing Bacillus species composition. Hortic. Res. 2022, 9, uhac046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, H.; He, X.; Thomas, B.W.; Lupwayi, N.Z.; Hao, X.; Thomas, M.C.; Shi, X. Fertilization Shapes Bacterial Community Structure by Alteration of Soil pH. Front. Microbiol. 2017, 8, 1325. [Google Scholar] [CrossRef]
- Zhang, C.; Jiao, S.; Shu, D.; Wei, G. Inter-phylum negative interactions affect soil bacterial community dynamics and functions during soybean development under long-term nitrogen fertilization. Stress Biol. 2021, 1, 15. [Google Scholar] [CrossRef]
- Hu, M.; Sardans, J.; Sun, D.; Yan, R.; Wu, H.; Ni, R.; Peñuelas, J. Microbial diversity and keystone species drive soil nutrient cycling and multifunctionality following mangrove restoration. Environ. Res. 2024, 251, 118715. [Google Scholar] [CrossRef]
- Balasundaram, A.; Sundaresan, P.; Bhavsar, A.; Mattu, M.; Kavitha, M.S.; Shaik, A. Tea leaf disease detection using segment anything model and deep convolutional neural networks. Results Eng. 2025, 25, 103784. [Google Scholar] [CrossRef]
- Dutta, J.; Gupta, S.; Thakur, D.; Handique, P.J. First report of Nigrospora leaf blight on tea caused by Nigrospora sphaerica in India. Plant Dis. 2015, 99, 417. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Dong, X.; Wang, Y.; Maker, G.; Agarwal, M.; Ding, Z. Tea-Soybean Intercropping Improves Tea Quality and Nutrition Uptake by Inducing Changes of Rhizosphere Bacterial Communities. Microorganisms 2022, 10, 2149. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Liang, L.; Xu, R.; Xu, H.; Sun, L.; Liao, H. Intercropping tea plantations with soybean and rapeseed enhances nitrogen fixation through shifts in soil microbial communities. Front. Agric. Sci. Eng. 2022, 9, 344–355. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Hu, Y.; Xi, X.; Tang, Y.; Chen, J.; Fu, X.; Sun, Y. Water organic pollution and eutrophication influence soil microbial processes, increasing soil respiration of estuarine wetlands: Site study in Jiuduansha wetland. PLoS ONE 2015, 10, e0126951. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, N.; Li, Y.; Zhu, C.; Qu, B.; Liu, H.; Li, R.; Bai, Y.; Shen, Q.; Falcao Salles, J. Bio-organic soil amendment promotes the suppression of Ralstonia solanacearum by inducing changes in the functionality and composition of rhizosphere bacterial communities. New Phytol. 2022, 235, 1558–1574. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic Amendments, Beneficial Microbes, and Soil Microbiota: Toward a Unified Framework for Disease Suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Sun, L.; Zhang, J.; Zhao, H.; Su, T.; Li, W.; Wu, L.; Cai, P.; Rensing, C.; Li, Y.; et al. Tea Plant/Ophiopogon japonicus Intercropping Drives the Reshaping of Soil Microbial Communities in Terraced Tea Plantation’s Micro-Topographical Units. Agriculture 2025, 15, 1150. https://doi.org/10.3390/agriculture15111150
Li Y, Sun L, Zhang J, Zhao H, Su T, Li W, Wu L, Cai P, Rensing C, Li Y, et al. Tea Plant/Ophiopogon japonicus Intercropping Drives the Reshaping of Soil Microbial Communities in Terraced Tea Plantation’s Micro-Topographical Units. Agriculture. 2025; 15(11):1150. https://doi.org/10.3390/agriculture15111150
Chicago/Turabian StyleLi, Yangxin, Le Sun, Jialin Zhang, Hongxue Zhao, Tejia Su, Wenhui Li, Linkun Wu, Pumo Cai, Christopher Rensing, Yuanping Li, and et al. 2025. "Tea Plant/Ophiopogon japonicus Intercropping Drives the Reshaping of Soil Microbial Communities in Terraced Tea Plantation’s Micro-Topographical Units" Agriculture 15, no. 11: 1150. https://doi.org/10.3390/agriculture15111150
APA StyleLi, Y., Sun, L., Zhang, J., Zhao, H., Su, T., Li, W., Wu, L., Cai, P., Rensing, C., Li, Y., Zhang, J., Wang, F., & Li, Q. (2025). Tea Plant/Ophiopogon japonicus Intercropping Drives the Reshaping of Soil Microbial Communities in Terraced Tea Plantation’s Micro-Topographical Units. Agriculture, 15(11), 1150. https://doi.org/10.3390/agriculture15111150





