Health Assessment of Natural Selenium-Rich Soil in Yuanzhou District Based on Selenium–Cadmium Principal Factors and the Accumulation of Selenium and Cadmium in the Area Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling Points
2.2. Sample Collection and Chemical Analysis
2.3. Risk Assessment and Selection of the Parameters for Risk Evaluation
2.4. Risk Assessment Model for Soil Heavy Metals in the Soil
2.5. Data Analysis
3. Results and Discussion
3.1. Total Se Levels in the Soil
3.2. Levels of Cd and Related Risk Assessment in Se-Rich Soil
3.3. Risk Assessment of Heavy Metals in Se-Rich Soil
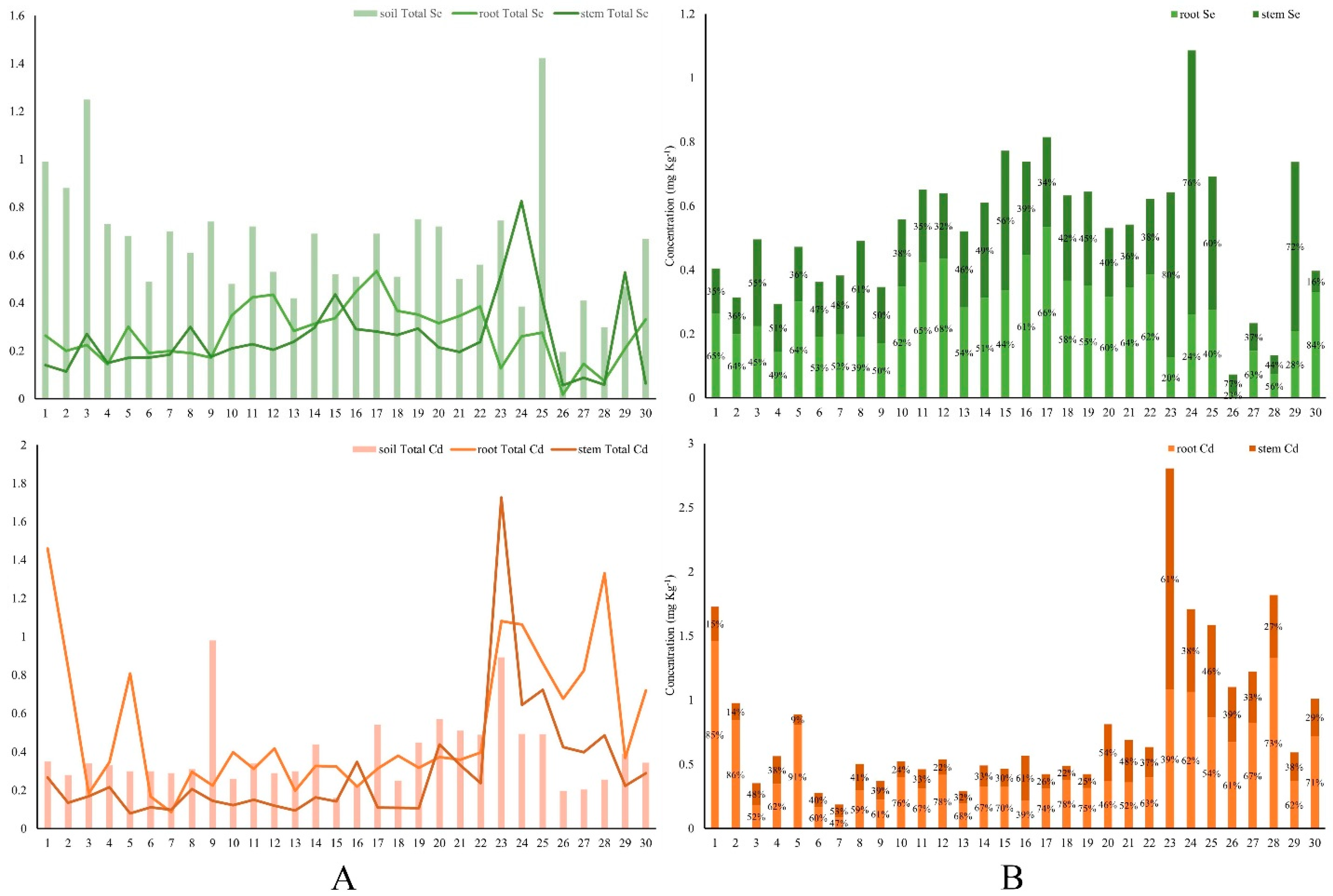
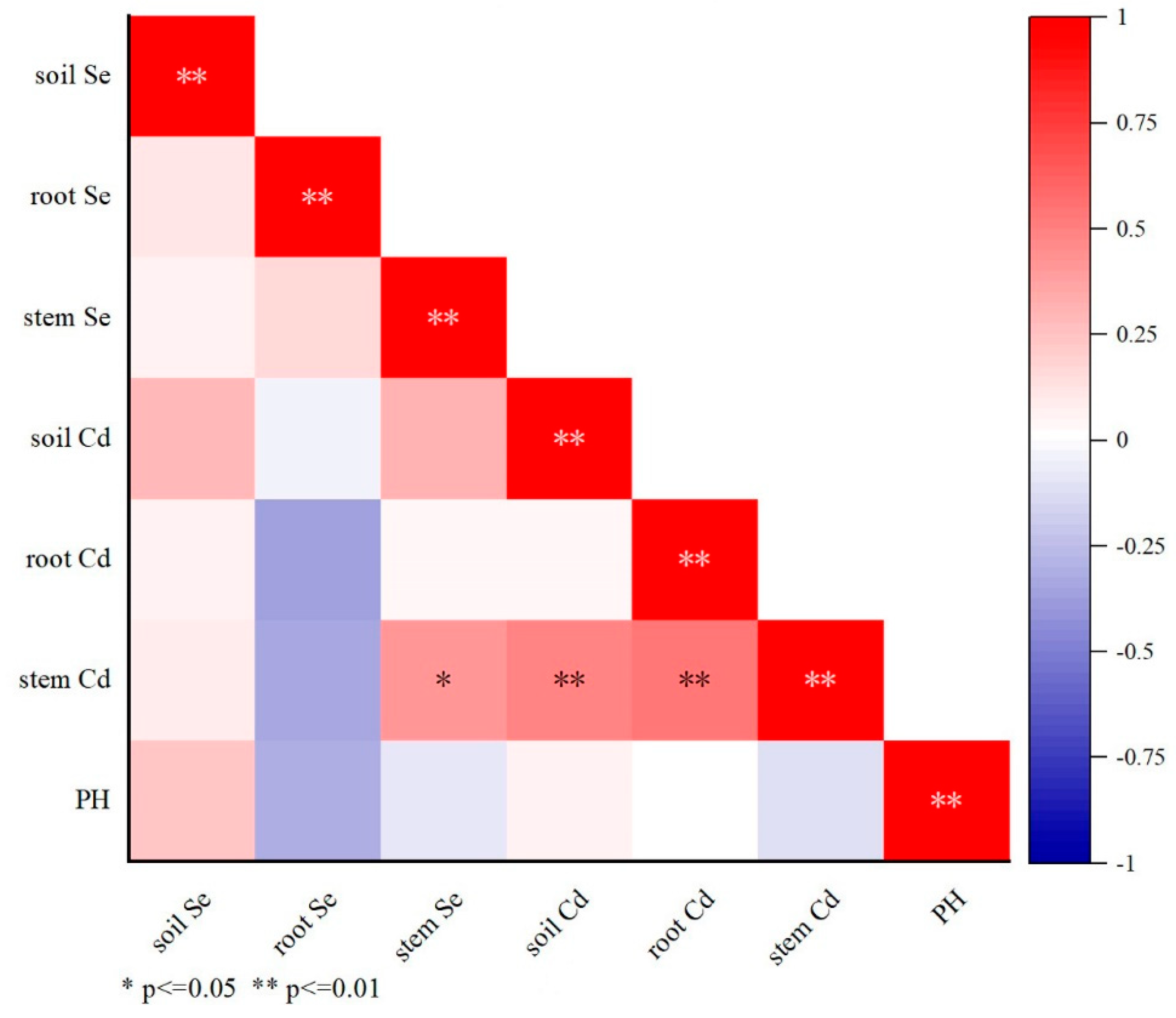
3.4. Accumulation of Se and Cd in Different Parts of Oilseed Rape Plants
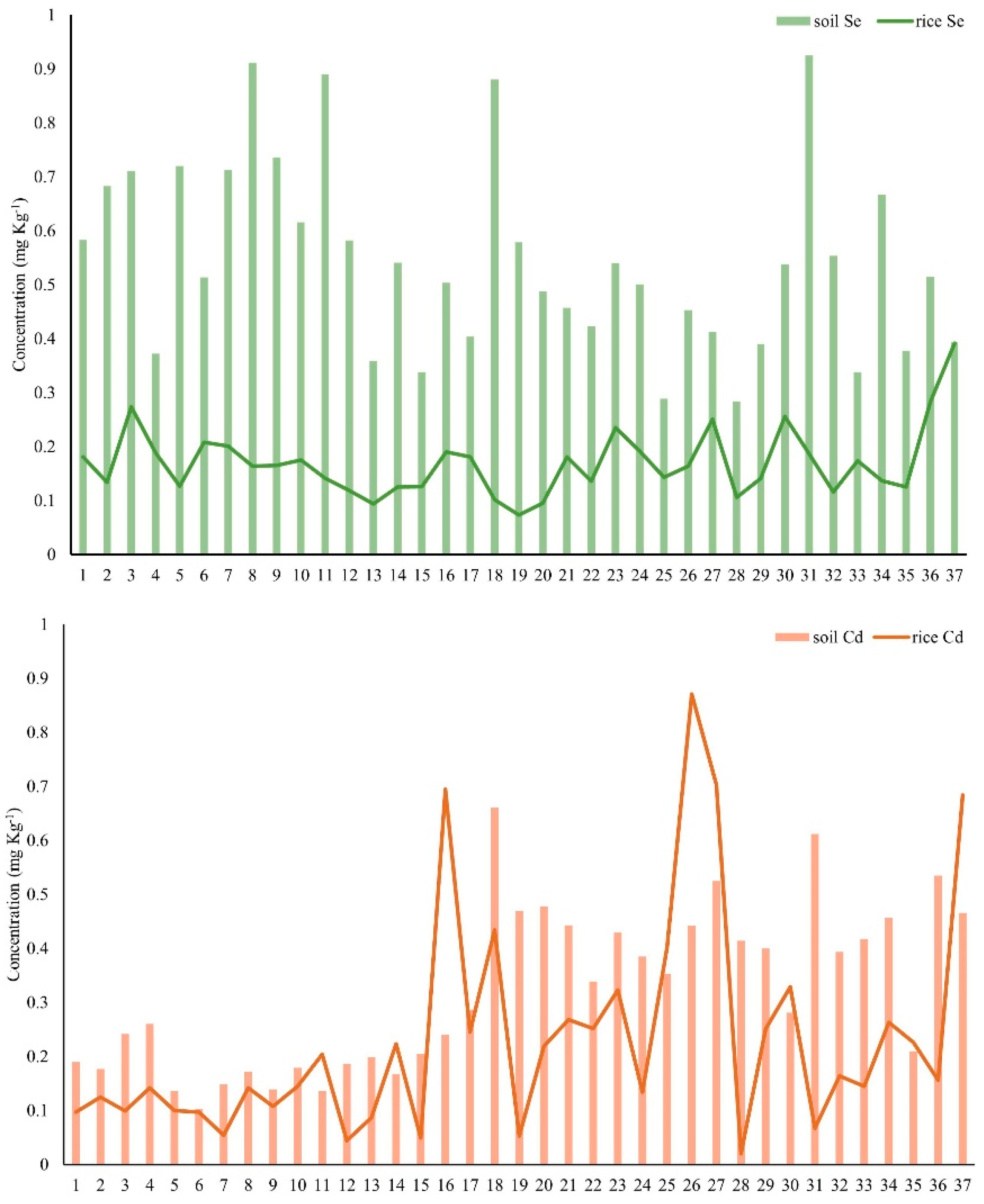
3.5. Accumulation of Se and Cd in the Soil–Rice System
3.6. Green Crop Rotation Patterns in Se-Rich Areas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, K.M.; Arthur, J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, F.M. Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology; Selinus, O., Ed.; Springer: New York, NY, USA, 2013; pp. 375–416. [Google Scholar] [CrossRef]
- Shi, Z.; Pan, P.; Feng, Y.; Kan, Z.; Li, Z.; Wei, F. Environmental water chemistry and possible correlation with Kaschin-Beck Disease (KBD) in northwestern Sichuan, China. Environ. Int. 2017, 99, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, Y. Biogeochemical cycling of selenium in Chinese environments. Appl. Geochem. 2001, 16, 1345–1351. [Google Scholar] [CrossRef]
- Sargsyan, E.; Witkowska, K.; Park, K.; Gladyshev, V.N. Molecular mechanisms of selenium-induced neurotoxicity through misincorporation into protein disulfide bonds. Redox Biol. 2020, 34, 101521. [Google Scholar] [CrossRef]
- Köhrle, J. Selenium and the thyroid: A close-knit connection in health and disease. Nat. Rev. Endocrinol. 2020, 16, 265–266. [Google Scholar] [CrossRef]
- Xu, Z.; Shao, H.; Li, S.; Zheng, C. Relationships between the selenium content in flue-cured tobacco leaves and the selenium content in soil in Enshi, China tobacco-growing area. Pak. J. Bot. 2012, 44, 1563–1568. [Google Scholar]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yanga, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Yang, L.; Li, Y.; Wei, B.; Yu, J.; Feng, F. Distribution and translocation of selenium from soil to highland barley in the Tibetan Plateau Kashin-Beck disease area. Environ. Geochem. Health 2017, 39, 221–229. [Google Scholar] [CrossRef]
- Chang, C.; Chen, C.; Yin, R.; Shen, Y.; Mao, K.; Yang, Z.; Feng, X.; Zhang, H. Bioaccumulation of Hg in rice leaf facilitates selenium bioaccumulation in rice (Oryza sativa L.) leaf in the Wanshan mercury mine. Environ. Sci. Technol. 2021, 54, 3228–3236. [Google Scholar] [CrossRef]
- Yang, B.B.; Yang, C.; Shao, Z.Y.; Wang, H.; Zan, S.T.; Zhu, M.; Zhou, S.B.; Yang, R.Y. Selenium (Se) does not reduce cadmium (Cd) uptake and translocation in rice (Oryza sativa L.) in naturally occurred Se-rich paddy fields with a high geological background of Cd. Bull. Environ. Contam. Toxicol. 2019, 103, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yuan, L.; Qi, S.; Yin, X. The threshold effect between the soil bioavailable molar Se: Cd ratio and the accumulation of Cd in corn (Zea mays L.) from natural Se-Cd rich soils. Sci. Total Environ. 2019, 688, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Gwóźdź, E.A. The message of nitric oxide in cadmium challenged plants. Plant Sci. 2011, 181, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.L.; Yang, X. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Marquès, M. The effects of some essential and toxic metals/metalloids in COVID-19: A review. Food Chem. Toxicol. 2021, 152, 112161. [Google Scholar] [CrossRef]
- Esplugas, R.; Mari, M.; Marquès, M.; Schuhmacher, M.; Domingo, J.L.; Nadal, M. Biomonitoring of trace elements in hair of schoolchildren living near a hazardous waste incinerator—A 20 years follow-up. Toxics 2019, 7, 52. [Google Scholar] [CrossRef]
- Lippert, J.; Montgomery, J.; DeMarco, C. Lead health fairs: A community-based approach to addressing lead exposure in Chicago. Health Educ. Behav. 2020, 48, 758–768. [Google Scholar] [CrossRef]
- Mo, A.; Huang, Y.; Gu, Z.; Liu, C.; Wang, J.; Yuan, Y. Health risk assessment and bioaccumulation of heavy metals in Procambarus clarkii from six provinces of China. Environ. Sci. Pollut. Res. 2022, 29, 2539–2546. [Google Scholar] [CrossRef]
- Rahman, M.S.; Saha, N.; Kumar, S.; Khan, M.D.H.; Islam, A.R.M.T.; Khan, M.N.I. Coupling of redundancy analysis with geochemistry and mineralogy to assess the behavior of dust arsenic as a base of risk estimation in Dhaka, Bangladesh. Chemosphere 2022, 287, 132048. [Google Scholar] [CrossRef]
- Subhani, M.; Mustafa, I.; Alamdar, A.; Katsoyiannis, I.A.; Ali, N.; Huang, Q.; Peng, S.; Shen, H.; Eqani, S.A.M.A.S. Arsenic levels from different land-use settings in Pakistan: Bio-accumulation and estimation of potential human health risk via dust exposure. Ecotoxicol. Environ. Saf. 2015, 115, 187–194. [Google Scholar] [CrossRef]
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Pirrone, N.; Thorne, R.J. Global sources and pathways of mercury in the context of human health. Int. J. Environ. Res. Public Health 2017, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zeng, X.; Wang, Q.; Liu, Y.; Liu, X.; Wu, Y.; Gong, Z.; Fang, M. Exposure and health risk assessment of heavy metal in crayfish from the middle and lower reaches of the Yangtze River. Biol. Trace Elem. Res. 2024, 202, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, R.; Guo, J.; Wu, F.; Xu, Y.; Feng, R. The effect of selenium on the subcellular distribution of antimony to regulate the toxicity of antimony in paddy rice. Environ. Sci. Pollut. Res. 2015, 22, 5111–5123. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.P.; Singh, P.K.; Awasthi, S.; Chakrabarty, D.; Trivedi, P.K.; Tripathi, R.D. Selenium ameliorates arsenic induced oxidative stress through modulation of antioxidant enzymes and thiols in rice (Oryza sativa L.). Ecotoxicology 2014, 23, 1153–1163. [Google Scholar] [CrossRef]
- Wan, Y.; Yu, Y.; Wang, Q.; Qiao, Y.; Li, H. Cadmium uptake dynamics and translocation in rice seedling: Influence of different forms of selenium. Ecotoxicol. Environ. Saf. 2016, 133, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dang, F.; Evans, R.D.; Zhong, H.; Zhao, J.; Zhou, D. Mechanistic understanding of MeHg-Se antagonism in soil-rice systems: The key role of antagonism in soil. Sci. Rep. 2016, 6, 19477. [Google Scholar] [CrossRef]
- Ding, Y.; Feng, R.W.; Wang, R.; Guo, J.; Zheng, X. A dual effect of Se on Cd toxicity: Evidence from plant growth, root morphology and responses of the antioxidative systems of paddy rice. Plant Soil 2013, 375, 289–301. [Google Scholar] [CrossRef]
- Feng, R.W.; Zhao, P.P.; Zhu, Y.M.; Yang, J.G.; Wei, X.Q.; Yang, L.; Liu, H.; Rensing, C.; Ding, Y.Z. Application of inorganic selenium to reduce accumulation and toxicity of heavy metals (metalloids) in plants: The main mechanisms, concerns and risks. Sci. Total Environ. 2021, 771, 144776. [Google Scholar] [CrossRef]
- Guo, Y.; Mao, K.; Cao, H.; Ali, W.; Lei, D.; Teng, D.; Chang, C.; Yang, X.; Yang, Q.; Niazi, N.K.; et al. Exogenous selenium (cadmium) inhibits the absorption and transportation of cadmium (selenium) in rice. Environ. Pollut. 2021, 268, 115829. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, X.; Tie, B.; Peng, L.; Li, H.; Wang, K.; Zeng, Q. Comparison of three types of oil crop rotation systems for effective use and remediation of heavy metal contaminated agricultural soil. Chemosphere 2017, 188, 148–156. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, C.; Wang, F.; Peng, L.; Zeng, Q.; Luo, S. Assessment of the potential for phytoremediation of cadmium polluted soils by various crop rotation patterns based on the annual input and output fluxes. J. Hazard. Mater. 2022, 423, 127183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, L.H.; Peng, L.; Luo, S.; Zeng, Q.R. Phytoremediation of heavy metals under an oil crop rotation and treatment of biochar from contaminated biomass for safe use. Chemosphere 2020, 247, 125856. [Google Scholar] [CrossRef] [PubMed]
- GB15618-2018; Soil Environmental Quality: Risk Control Standard for Soil Contamination of Agricultural Land (Trial). Environmental Standards. Ministry of Ecology and Environment of China, China Environmental Science Press: Beijing, China, 2018.
- Li, X.; Yang, H.; McGrath, S.P. Cadmium bioavailability in contrasting soils under rice-wheat rotation: The role of soil selenium. Chemosphere 2019, 235, 550–558. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, C.; Li, H.; Smith, S.E.; Smith, F.A. Geogenic cadmium pollution in multi-metal mining areas: A case study in Se-rich soils of Enshi, China. Environ. Sci. Technol. 2022, 56, 1623–1632. [Google Scholar]
- Yang, R.; He, Y.; Luo, L.; Zhu, M.; Zan, S.; Guo, F.; Wang, B.; Yang, B. The interaction between selenium and cadmium in the soil-rice-human continuum in an area with high geological background of selenium and cadmium. Ecotoxicol. Environ. Saf. 2021, 222, 112516. [Google Scholar] [CrossRef]
- NY/T 1104-2006; Agricultural Industry Standards of the People’s Republic of China: Determination of Selenium in Soils. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2006.
- GB2762-2017; National Standard for Food Safety: Limits for Contaminants in Food. Environmental Standards. Ministry of Ecology and Environment of China, China Environmental Science Press: Beijing, China, 2017.
- GB 5009.93–2017; National standards of the People’s Republic of China: National Food Safety Standard for the Determination of Se in Food. National Health and Family Planning Commission of the People’s Republic of China and National Medical Products Administration of the People’s Republic of China: Beijing, China, 2017.
- Tan, J.A.; Zhu, W.; Wang, W.; Li, R.; Hou, S.; Wang, D.; Yang, L. Selenium in soil and endemic diseases in China. Sci. Total Environ. 2002, 284, 227–235. [Google Scholar] [CrossRef]
- Liu, K.; Cai, H.S.; Zhang, X.L.; He, Q.G. Spatial correlation and influencing factors of soil selenium and heavy metal content in Yuanzhou district. Southwest China J. Agric. Sci. 2023, 36, 1492–1503. [Google Scholar]
- Natasha, M.S.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef]
- Zhao, Z.G. Selenium Health & Selenium Products; Science and Technology Literature Press: Beijing, China, 2022. [Google Scholar]
- Wang, J.; Li, H.R.; Li, Y.H.; Yu, J.P.; Yang, L.S.; Feng, F.J.; Chen, Z. Speciation, distribution and bioavailability of soil selenium in the Tibetan plateau KashinBeck disease area-a case study in Songpan County, Sichuan Province, China. Biol. Trace Elem. Res. 2013, 156, 367–375. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, N.; Zhang, Y.; Deng, H.; Yang, H. Spatial Distribution of Se Content and Its Influencing Factors in Cultivated Topsoil in Yunnan. Soils 2021, 53, 578–584. [Google Scholar] [CrossRef]
- Doane, T.A.; Horwáth, W.R. Soil organic matter decomposition and nitrogen mineralization in soil. Biol. Fertil. Soils 2003, 38, 219–227. [Google Scholar] [CrossRef]
- Lyu, C.; Chen, J.; Li, L.; Zhao, Z.; Liu, X. Characteristics of Se in water-soil-plant system and threshold of soil Se in seleniferous areas in Enshi, China. Sci. Total Environ. 2022, 827, 154372. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, J.; Wang, Y.; Smith, F.A. Arbuscular mycorrhizal fungi enhance selenium uptake and mitigate cadmium toxicity in oilseed rape (Brassica napus L.) through rhizosphere redox regulation. Soil Biol. Biochem. 2023, 176, 108891. [Google Scholar] [CrossRef]
- Rinklebe, H.; Shaheen, S.M.; Frohne, T. Redox-induced mobilization of cadmium, selenium, and other trace elements in flooded soils: Mechanisms and management strategies. J. Hazard. Mater. 2020, 384, 121432. [Google Scholar]
- Wu, X.; Song, H.; Guan, C.; Zhang, Z. Boron alleviates cadmium toxicity in brassica napus by promoting the chelation of cadmium onto the root cell wall components. Sci. Total Environ. 2020, 728, 138833. [Google Scholar] [CrossRef]
- Williams, P.N.; Lombi, E.; Sun, G.X.; Scheckel, K.; Zhu, Y.G.; Feng, X.; Zhu, J.; Carey, A.M.; Adomako, E.; Lawgali, Y.; et al. Selenium characterization in the global rice supply chain. Environ. Sci. Technol. 2009, 43, 6024–6030. [Google Scholar] [CrossRef]
- WHO. Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization and Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2004. [Google Scholar]
- Wen, B.Y.; Zhang, T.L.; Li, X.Z.; Xie, Z.D. A feasibility study of selenium-rich soil development in Longnan County of Jiangxi Province. Geol. China 2014, 41, 256–263. (In Chinese) [Google Scholar]
- Fujiwara, Y.; Lee, J.Y.; Tokumoto, M.; Satoh, M. Cadmium renal toxicity via apoptotic pathways. Biol. Pharm. Bull. 2012, 35, 1892–1897. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Zhao, W.; Xia, C.; Wu, M.; Wang, Q.; Wang, Z.; Jiang, Y.; Zuza, A.V.; Tian, X. Assessment of heavy metals should be performed before the development of the selenium-rich soil: A case study in China. Environ. Res. 2022, 210, 112990. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, H.; Huang, F.; Feng, X. Understanding the translocation and bioaccumulation of cadmium in the Enshi seleniferous area, China: Possible impact by the interaction of Se and Cd. Environ. Pollut. 2022, 300, 118927. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Bryson, R.J.; Meacham, M.C.; Bowen, H.C.; Johnson, S.E.; Zhao, F.J. Biofortification of UK food crops with selenium. Plant Physiol. 2010, 153, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Pilon-Smits, E.A.H.; Wirtz, M.; Hell, R.; Malagoli, M. Selenium fertilization alters sulfur and selenium metabolism in Brassica juncea. J. Exp. Bot. 2015, 66, 6011–6023. [Google Scholar] [CrossRef]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Kyozuka, J.; Fujiwara, T. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Plant Cell 2011, 23, 648–664. [Google Scholar] [CrossRef] [PubMed]

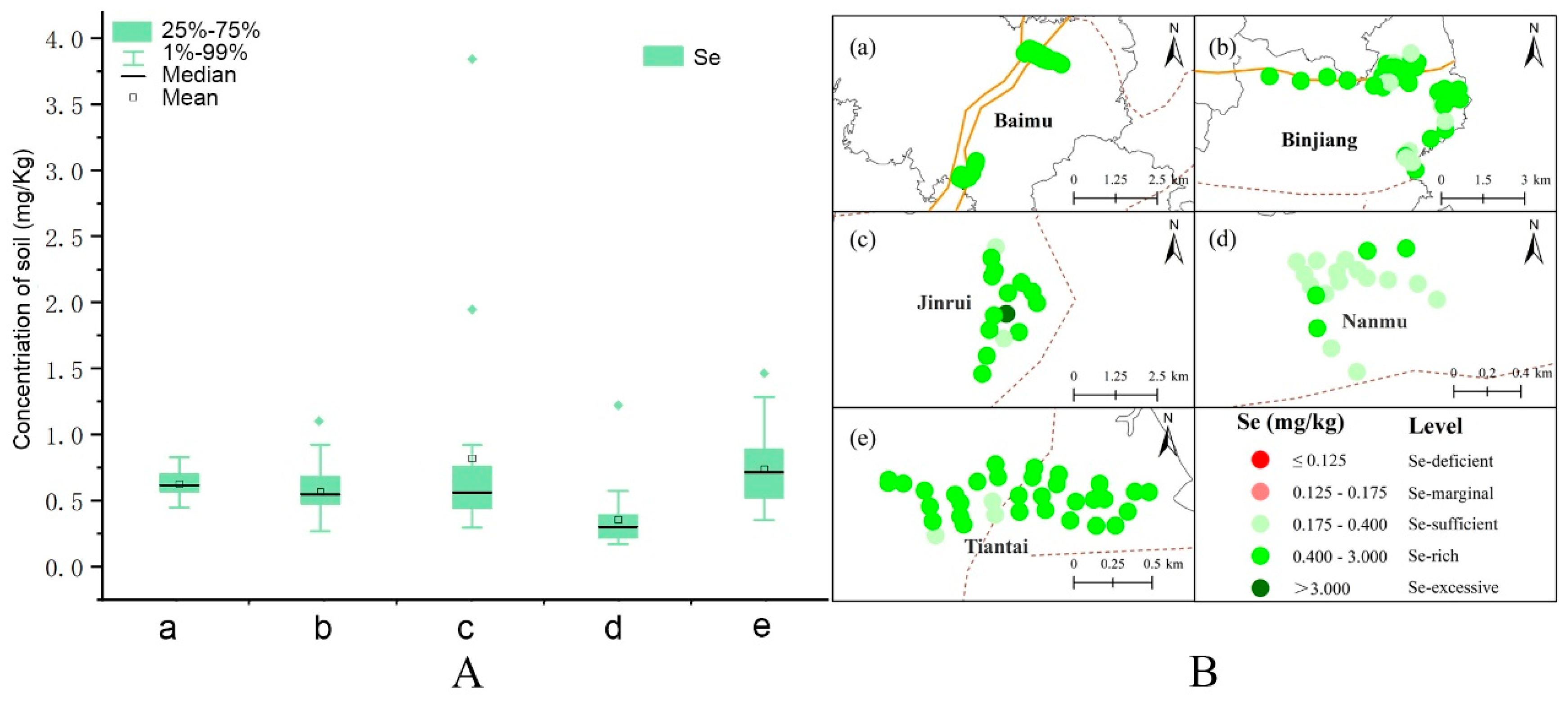
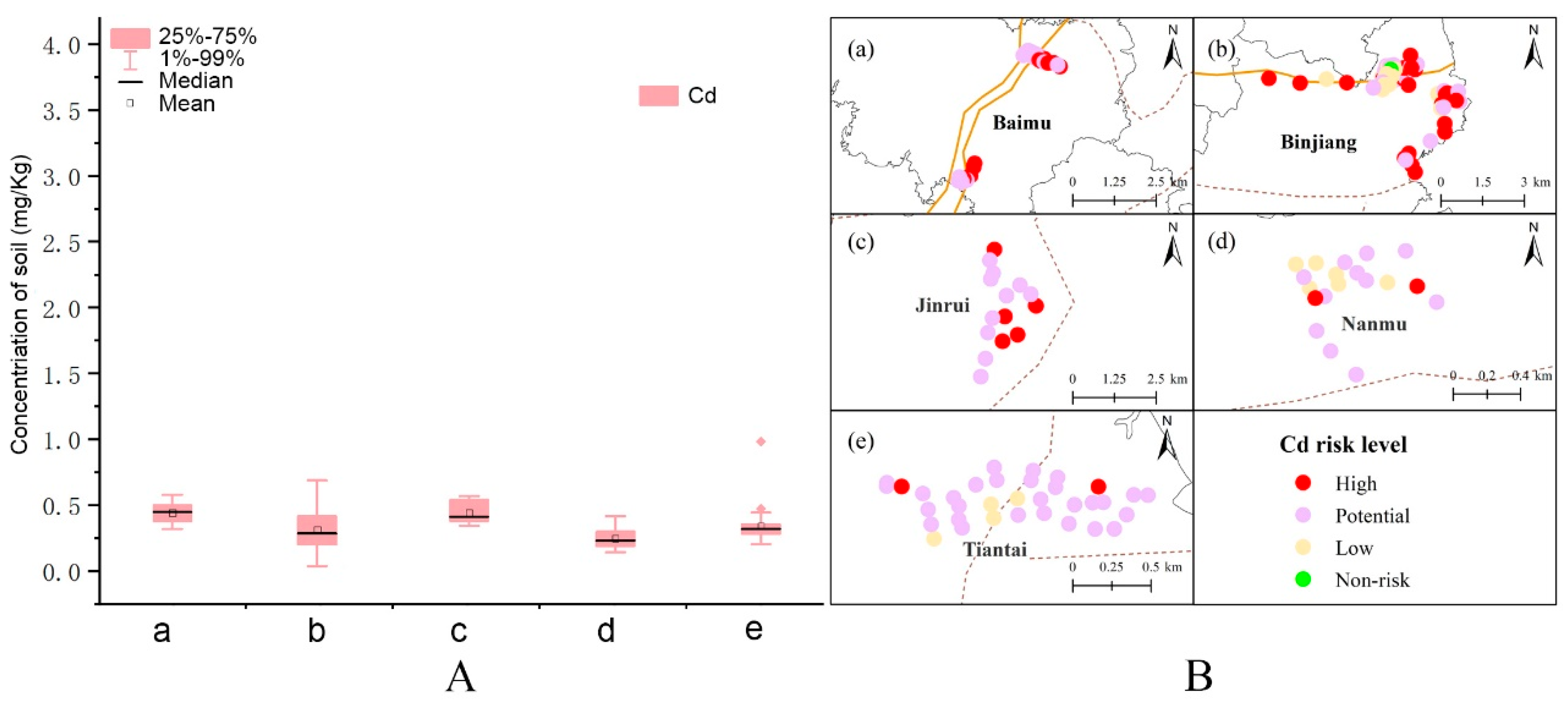



| Number | Site | Longitude | Latitude |
|---|---|---|---|
| a | Baimu Country | 114.50°–114.55° E | 27.75°–27.80° N |
| b | Binjiang Town | 114.65°–114.73° E | 27.85°–27.92° N |
| c | Jinrui Town | 114.35°–114.42° E | 27.68°–27.75° N |
| d | Nanmu Country | 114.58°–114.65° E | 27.60°–27.67° N |
| e | Tiantai Town | 114.78°–114.85° E | 27.95°–28.02° N |
| Heavy Metal | pH | High Risk | Potential Risk | Low Risk | Non-Risk |
|---|---|---|---|---|---|
| As | pH ≤ 5.5 | >30.00 | 18.0–30.00 | 9.00–18.00 | <9.00 |
| 5.5 < pH ≤ 6.5 | >30.00 | 18.0–30.00 | 9.00–18.00 | <9.00 | |
| 6.5 < pH ≤ 7.5 | >25.00 | 25.00–15.00 | 7.50–15.00 | <7.50 | |
| pH ≥ 7.5 | >20.00 | 12.00–20.00 | 6.00–12.00 | <6.00 | |
| Cd | pH ≤ 5.5 | >0.3 | 0.18–0.3 | 0.09–0.18 | <0.09 |
| 5.5 < pH ≤ 6.5 | >0.4 | 0.24–0.4 | 0.12–0.24 | <0.12 | |
| 6.5 < pH ≤ 7.5 | >0.6 | 0.36–0.6 | 0.18–0.36 | <0.18 | |
| pH ≥ 7.5 | >0.8 | 0.48–0.8 | 0.24–0.48 | <0.24 | |
| Cr | pH ≤ 5.5 | >250.00 | 150.00–250.00 | 75.00–150.00 | <75.00 |
| 5.5 < pH ≤ 6.5 | >250.00 | 150.00–250.00 | 75.00–150.00 | <75.00 | |
| 6.5 < pH ≤ 7.5 | >300.00 | 180.00–300.00 | 90.00–180.00 | <90.00 | |
| pH ≥ 7.5 | >350.00 | 210.00–350.00 | 105.00–210.00 | <105.00 | |
| Hg | pH ≤ 5.5 | >0.50 | 0.30–0.50 | 0.15–0.30 | <0.15 |
| 5.5 < pH ≤ 6.5 | >0.50 | 0.30–0.50 | 0.15–0.30 | <0.15 | |
| 6.5 < pH ≤ 7.5 | >0.60 | 0.36–0.60 | 0.18–0.36 | <0.18 | |
| pH ≥ 7.5 | >1.00 | 0.60–1.00 | 0.30–0.60 | <0.30 | |
| Pb | pH ≤ 5.5 | >80.00 | 48.00–80.00 | 24.00–48.00 | <24.00 |
| 5.5 < pH ≤ 6.5 | >100.00 | 60.00–10.00 | 30.00–60.00 | <30.00 | |
| 6.5 < pH ≤ 7.5 | >140.00 | 84.00–140.00 | 42.00–84.00 | <42.00 | |
| pH ≥ 7.5 | >240.00 | 144.00–240.00 | 72.00–144.00 | <72.00 |
| Dimension | Indicator | Indicator Weight | Indicator Properties |
|---|---|---|---|
| Heavy metal contamination risk elements in paddy fields | Cd | −0.4 (Non-risk: −0.1, Low risk: −0.2, Potential risk: −0.3, High risk: −0.4) | Negative direction |
| Hg | −0.15 (Non-risk: −0.01, Low risk: −0.05, Potential risk: −0.1, High risk: −0.15) | Negative direction | |
| As | −0.15 (Non-risk: −0.01, Low risk: −0.05, Potential risk: −0.1, High risk: −0.15) | Negative direction | |
| Pb | −0.15 (Non-risk: −0.01, Low risk; −0.05, Potential risk: −0.1, High risk: −0.15) | Negative direction | |
| Cr | −0.15 (Non-risk: −0.01, Low risk: −0.05, Potential risk: −0.1, High risk: −0.15) | Negative direction | |
| Healthy element in paddy fields | Se | 0.4 (Se-deficient: 0.1, Se-marginal: 0.2, Se-sufficient: 0.3, Se-rich: 0.4, Se-excessive: 0) | Positive direction |
| Fertility indicator | OM | 0.3 | Positive direction |
| Nutrients in paddy fields | TN | 0.3 | Positive direction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, N.; Su, Y.; Huang, F.; Yu, D.; Han, C.; Li, X.; Zhao, Z.; Sun, X. Health Assessment of Natural Selenium-Rich Soil in Yuanzhou District Based on Selenium–Cadmium Principal Factors and the Accumulation of Selenium and Cadmium in the Area Crops. Agriculture 2025, 15, 1149. https://doi.org/10.3390/agriculture15111149
He N, Su Y, Huang F, Yu D, Han C, Li X, Zhao Z, Sun X. Health Assessment of Natural Selenium-Rich Soil in Yuanzhou District Based on Selenium–Cadmium Principal Factors and the Accumulation of Selenium and Cadmium in the Area Crops. Agriculture. 2025; 15(11):1149. https://doi.org/10.3390/agriculture15111149
Chicago/Turabian StyleHe, Ning, Yuting Su, Fang Huang, De Yu, Chengyun Han, Xingjie Li, Zhigang Zhao, and Xian Sun. 2025. "Health Assessment of Natural Selenium-Rich Soil in Yuanzhou District Based on Selenium–Cadmium Principal Factors and the Accumulation of Selenium and Cadmium in the Area Crops" Agriculture 15, no. 11: 1149. https://doi.org/10.3390/agriculture15111149
APA StyleHe, N., Su, Y., Huang, F., Yu, D., Han, C., Li, X., Zhao, Z., & Sun, X. (2025). Health Assessment of Natural Selenium-Rich Soil in Yuanzhou District Based on Selenium–Cadmium Principal Factors and the Accumulation of Selenium and Cadmium in the Area Crops. Agriculture, 15(11), 1149. https://doi.org/10.3390/agriculture15111149







