Diversification of Cultivars and Production of Male Inflorescence Flours for More Sustainable Banana Cultivation
Abstract
1. Introduction
2. Materials and Methods
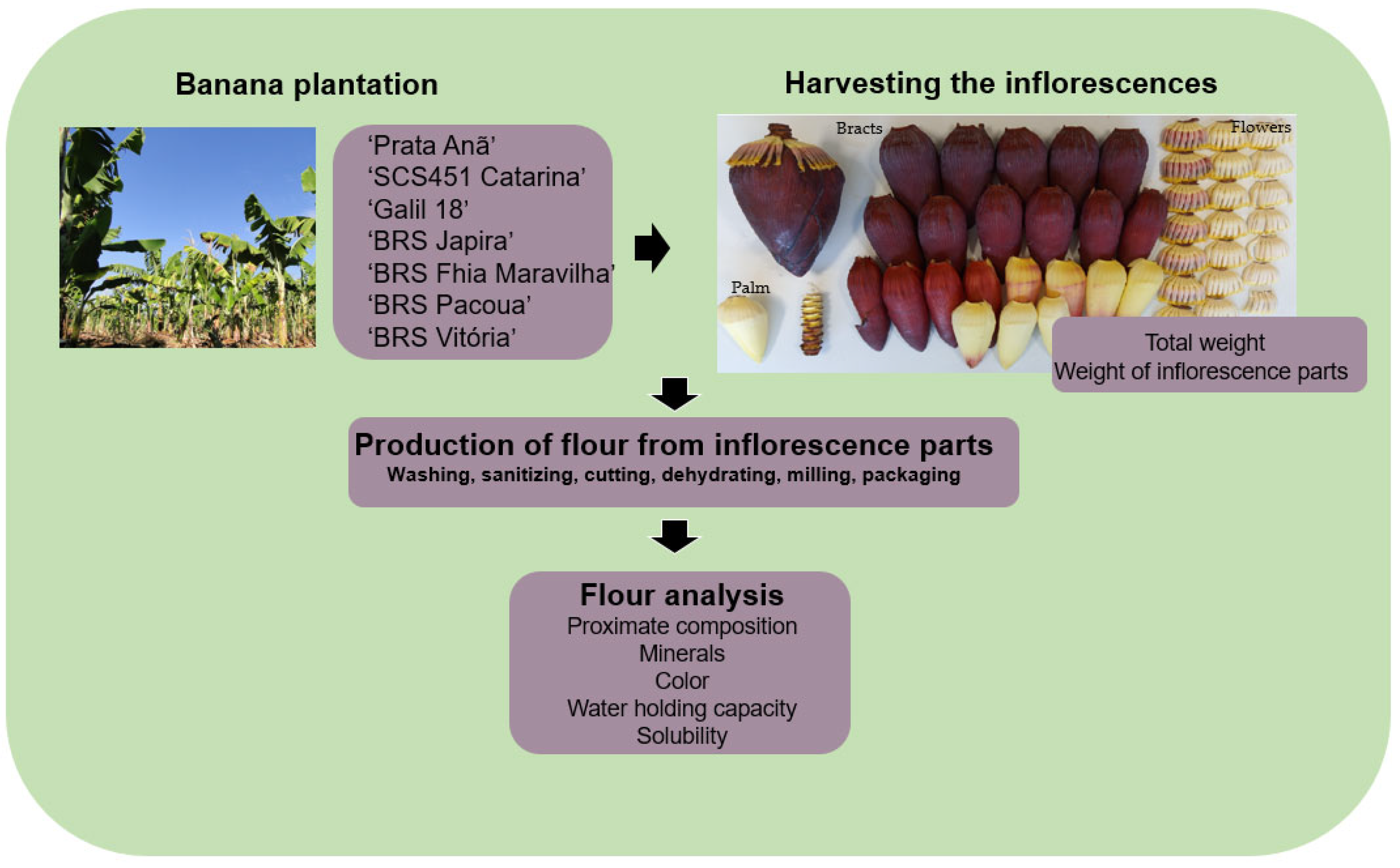
2.1. Banana Cultivation
2.2. Treatments and Experimental Design
2.3. Harvesting the Inflorescences
2.4. Production and Analysis of Flours
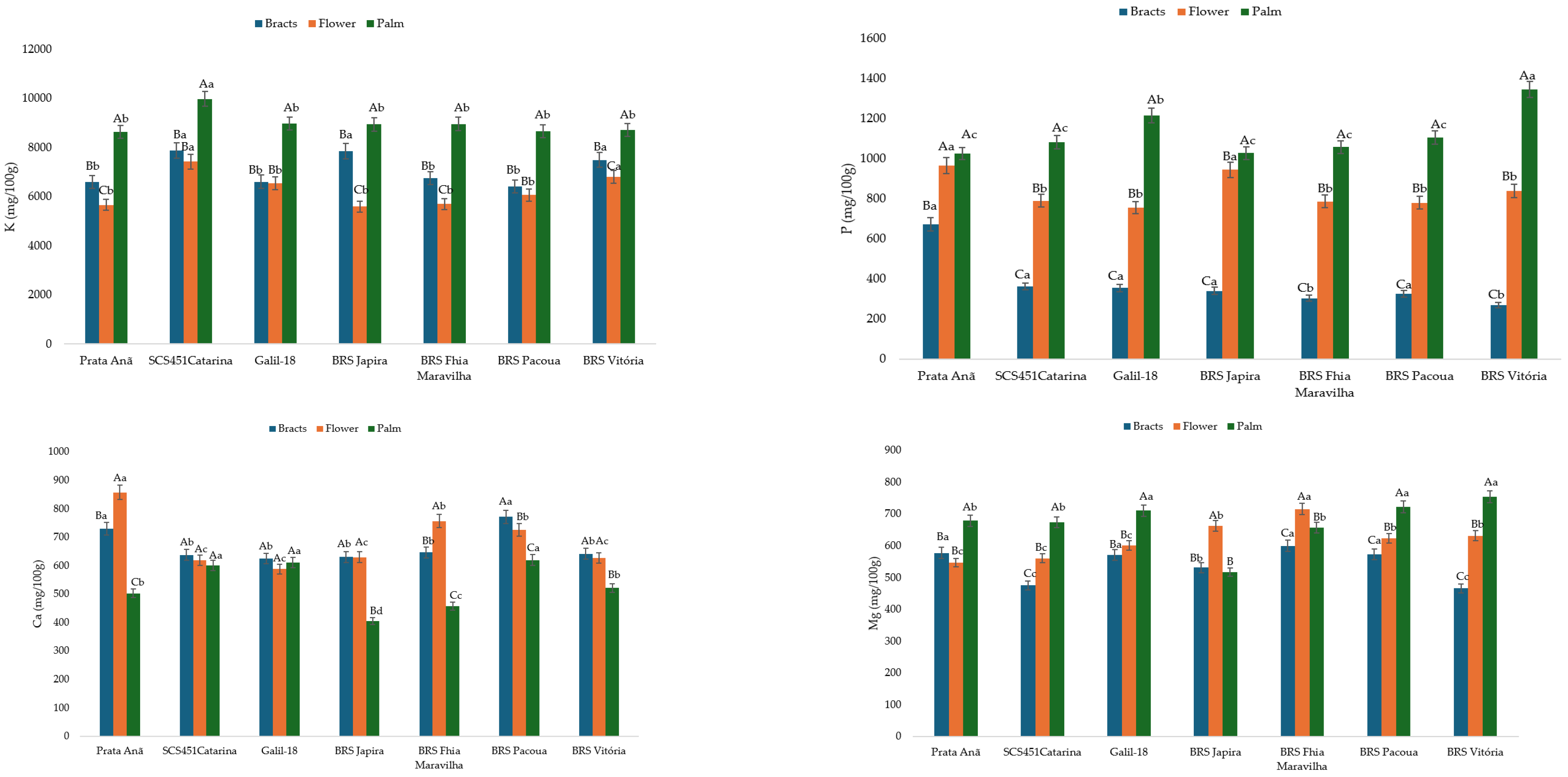
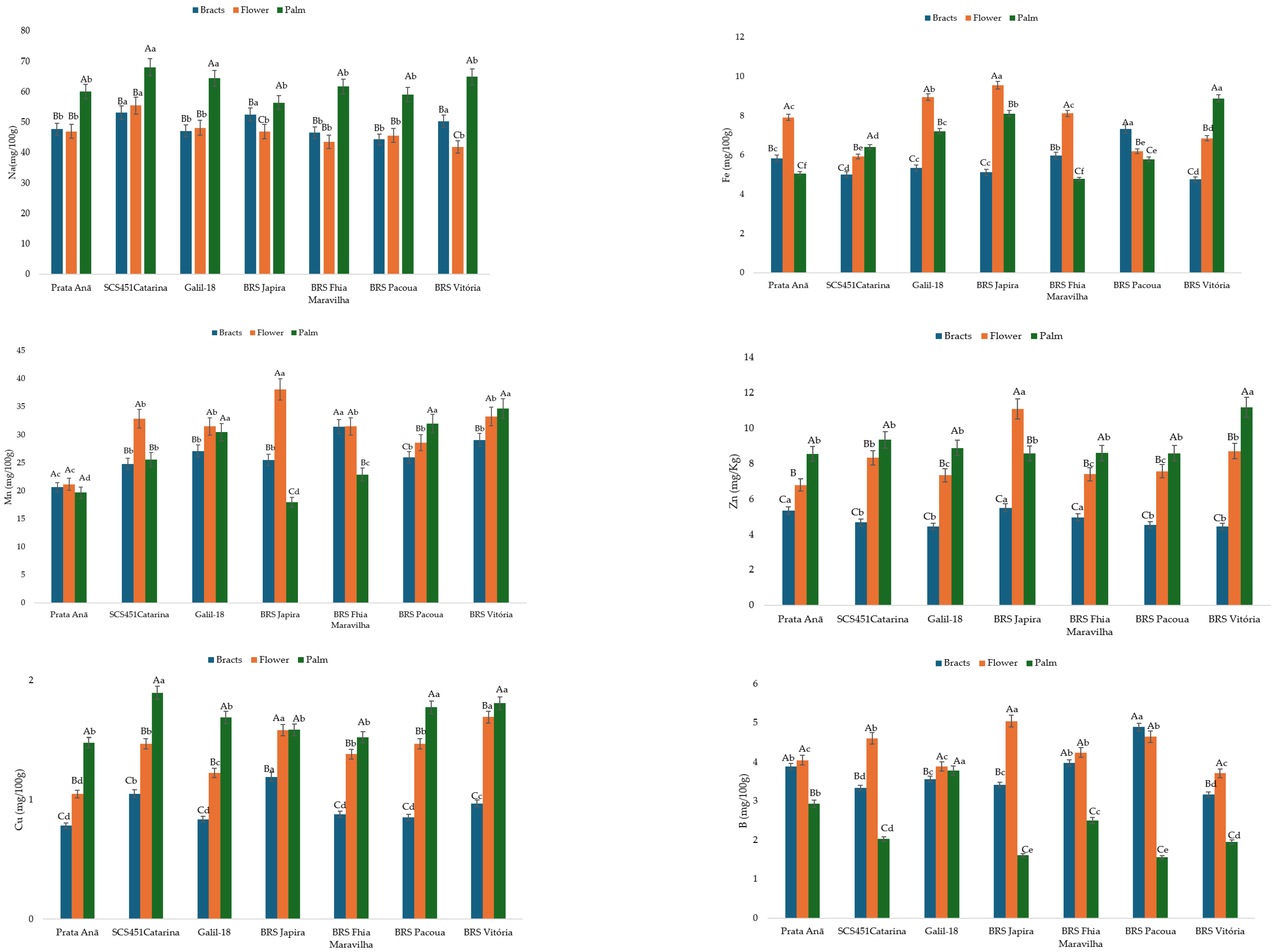
2.4.1. Proximate Composition and Mineral Elements Contents
2.4.2. Flour Color
2.4.3. Water Holding Capacity
2.4.4. Solubility
2.5. Statistical Analysis
3. Results
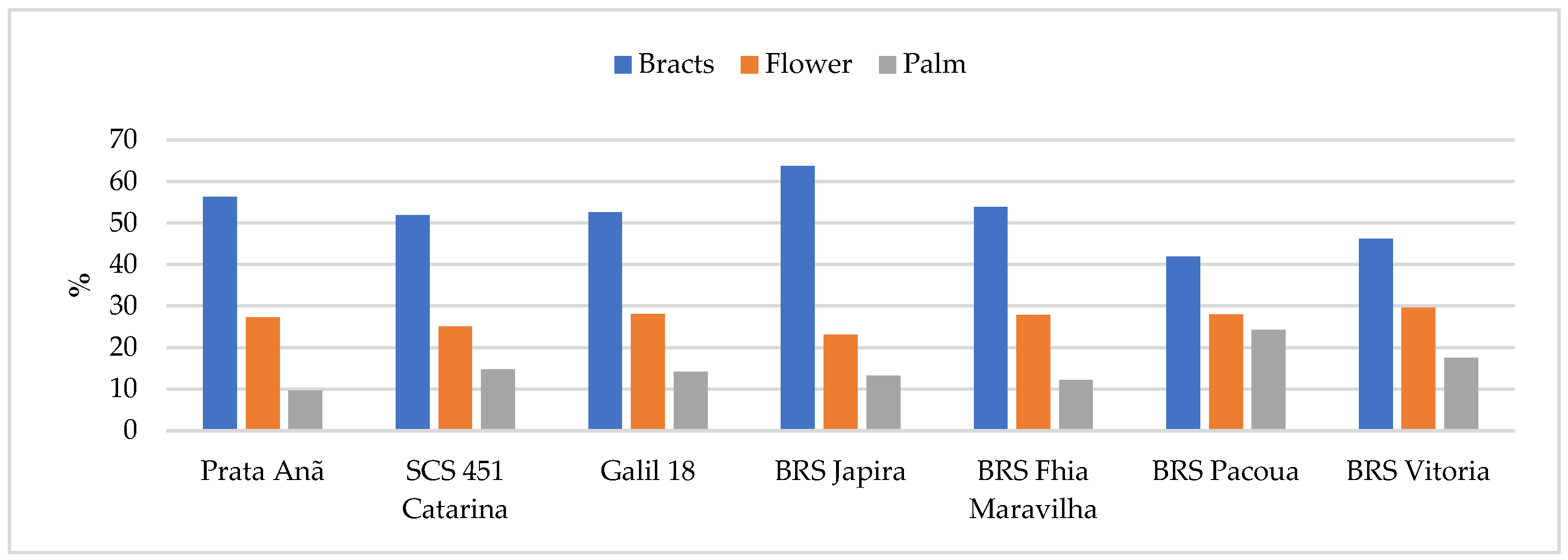
3.1. Fresh Weight of the Inflorescence Parts
3.2. Proximate Composition of Flours
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAOSTAT). Statistical Database 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 February 2025).
- Napoleão, G.M.; Jesus, P.R.R.; Leonel, S. Cultivar Diversification of Banana Production in Brazil. ASB 2021, 7, 1–14. [Google Scholar] [CrossRef]
- Senevirathna, N.; Karim, A. Banana Inflorescence as a New Source of Bioactive and Pharmacological Ingredients for the Food Industry. Food Chem. Adv. 2024, 5, 10814. [Google Scholar] [CrossRef]
- Borges, A.L.; Silva, A.L.; Batista, D.C. Sistema de Produção da Bananeira Irrigada; Embrapa: Brasília, Brazil, 2009; Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/110622/1/Sistema-de-Producao-da-Bananeira-Irrigada.pdf (accessed on 12 April 2025).
- Lau, B.F.; Kong, K.W.; Leong, K.H.; Sun, J.; He, X.; Wang, Z.; Mustafa, M.R.; Ling, T.C.; Ismail, A. Banana Inflorescence: Its Bio-prospects as an Ingredient for Functional Foods. Trends Food Sci. Technol. 2020, 97, 14–28. [Google Scholar] [CrossRef]
- Suprayitno, E.; Sulistiyati, T.D.; Sasmito, B.B.; Chamidah, A.; Panjaitan, M.A.P.; Tambunan, J.E.; Djamaludin, H. Banana Blossom Addition to Increase Food Fiber in Tuna (Thunnus sp.) Floss Product as Functional Food for Degenerative Disease’s Patient. IOP Conf. Ser. Earth Environ. Sci. 2022, 1036, 012095. [Google Scholar] [CrossRef]
- Fiallos-Cárdenas, M.; Pérez-Martínez, S.; Ramirez, A.D. Prospects for the Development of a Circular Bioeconomy around the Banana Value Chain. Sustain. Prod. Consum. 2022, 30, 541–555. [Google Scholar] [CrossRef]
- Begum, Y.A.; Deka, S.C. Chemical Profiling and Functional Properties of Dietary Fibre Rich Inner and Outer Bracts of Culinary Banana Flower. J. Food Sci. Technol. 2019, 56, 5298–5308. [Google Scholar] [CrossRef]
- Fingolo, C.E.; Braga, J.M.A.; Vieira, A.C.M.; Moura, M.R.L.; Kaplan, M.A.C. The Natural Impact of Banana Inflorescences (Musa acuminata) on Human Nutrition. Anais Acad. Bras. Cienc. 2012, 84, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Bolaños, S.; Díaz, S.; Ventura-Castellano, A.; Quirós-Pozo, R.; Rodríguez-Rodríguez, Á.; Castro, P.; Robaina, L. Assessing the Growth and Physiological Performance of Juvenile Tilapia (Oreochromis niloticus) with the Inclusion of New Banana By-products in Starter Diets. Aquac. Rep. 2023, 28, 101453. [Google Scholar] [CrossRef]
- Sumathy, V.; Lachumy, S.J.; Zakaria, Z.; Sasidharan, S. In Vitro Bioactivity and Phytochemical Screening of Musa acuminata Flower. Pharmacologyonline 2011, 2, 118–127. Available online: https://pharmacologyonline.silae.it/files/archives/2011/vol2/012.sasidharan.pdf (accessed on 31 March 2025).
- Vilhena, R.O.; Fachi, M.M.; Marson, B.M.; Dias, B.L.; Pontes, F.L.D.; Tonin, F.S.; Pontarolo, R. Antidiabetic Potential of Musa spp. Inflorescence: A Systematic Review. J. Pharm. Pharmacol. 2018, 70, 1583–1595. [Google Scholar] [CrossRef]
- Ramu, R.; Shirahatti, P.S.; Nanjunda Swamy, S.; Zameer, F.; Dhananjaya, B.L.; Nagendra Prasad, M.N. Correction: Assessment of In Vivo Antidiabetic Properties of Umbelliferone and Lupeol Constituents of Banana (Musa sp. var. Nanjangud rasa bale) Flower in Hyperglycaemic Rodent Model. PLoS ONE 2016, 11, e0160048. [Google Scholar] [CrossRef]
- Industry ARC™. Functional Flours Market—Industry Analysis, Market Size, Share, Trends, Application Analysis, Growth and Forecast 2022–2027. USA: Industry ARC™. 2022. Available online: https://www.industryarc.com/Research/Functional-Flours-Market-Research-504754 (accessed on 12 January 2025).
- Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018; p. 356. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Costa, F.S.; Coelho, E.F.; Borges, A.L.; Pamponet, A.J.M.; Silva, A.A.S.M.; Azevedo, N.F. Crescimento, Produção e Acúmulo de Potássio em Bananeira ‘Galil 18’ Sob Irrigação e Fertilização Potássica. Pesq. Agropecuária Bras. 2012, 47, 409–416. [Google Scholar] [CrossRef]
- Albuquerque, T.C.S.; Schurt, D.A.; Alves, A.B. BRS Japira: Cultivar Resistente à Sigatoka-Negra para Produção de Bananas de Qualidade em Roraima; Embrapa: Boa Vista, Brazil, 2022; p. 18. [Google Scholar]
- Silva, T.S.; Donato, S.L.R.; Rodrigues Filho, V.A.; Padilha Júnior, M.C.; Silva, Y.C.P. Características Agronômicas de Seis Cultivares de Bananeiras Tipo Prata. Rev. Verde Agroecol. Desenvolv. Sustent. 2015, 10, 1–4. [Google Scholar] [CrossRef][Green Version]
- EMBRAPA. BRS Pacoua: Nova Cultivar de Bananeira do Tipo Pacovan; Embrapa Mandioca e Fruticultura: Cruz das Almas, Brazil, 2016; Folder Técnico; 2p. [Google Scholar]
- Lichtemberg, L.A.; Hinz, R.H.; Malburg, J.L.; Sonego, M.; Peruch, L.A.M. SCS451 Catarina—Novo Cultivar de Bananeira do Subgrupo Prata. Agropecuária Catarin. 2011, 24, 70–75. [Google Scholar]
- Pereira, J.C.R.; Gasparotto, L.; Pereira, M.C.N. BRS Vitória: Nova Cultivar de Bananeira do Subgrupo Prata para o Agronegócio do Estado do Amazonas; Embrapa Amazônia Ocidental: Manaus, Brazil, 2005; Comunicado Técnico 34; p. 2. [Google Scholar]
- Jesus, P.R.R.; Leonel, S.; Leonel, M.; Cândido, H.T.; Molha, N.Z.; Domiciano, V.M.; Ouros, L.F.; Tecchio, M.A. Performance and Leaf Nutritional Content of Banana Cultivars Intercropped with Lemongrass. Rev. Caatinga 2024, 37, e12448. [Google Scholar] [CrossRef]
- Martins, A.N.; Narita, N.; Suguino, E.; Takata, W.H.S. Desempenho de Genótipos de Bananeiras em Cultivos Irrigado e Seco, na Região Centro Oeste Paulista. Colloq. Agrar. 2020, 16, 11–18. [Google Scholar] [CrossRef]
- Teixeira, L.A.J.; Nomura, E.S.; Damatto-Junior, E.R.; Fuzitani, E.J. Banana. In Instruções Agrícolas Para as Principais Culturas Econômicas, 1st ed.; Aguiar, A.T.E., Gonçalves, C., Paterniani, M.E.A.G., Tucci, M.G.S., Castro, C.E.F., Eds.; Instituto Agronômico de Campinas: Campinas, Brazil, 2014; pp. 46–51. [Google Scholar]
- Nomura, E.S.; Damatto-Junior, E.R.; Kobori, R.T.; Penteado, L.A.C. Tratos Culturais. In Cultivo da Bananeira, 1st ed.; Nomura, E.S., Damatto-Junior, E.R., Eds.; CDRS: Campinas, Brazil, 2020; Volume 1, pp. 49–71. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2007; p. 2. [Google Scholar]
- Altundag, H.; Tuzen, M. Comparison of Dry, Wet and Microwave Digestion Methods for the Multi-element Determination in Some Dried Fruit Samples by ICP-OES. Food Chem. Toxicol. 2011, 49, 2800–2807. [Google Scholar] [CrossRef]
- Borges, C.V.; Maraschin, M.; Coelho, D.S.; Leonel, M.; Gomez, H.A.G.; Belin, M.A.F.; Diamante, M.S.; Amorim, E.P.; Gianeti, T.; Castro, G.R.; et al. Nutritional Value and Antioxidant Compounds During the Ripening and After Domestic Cooking of Bananas and Plantains. Food Res. Int. 2020, 132, 109061. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioproc. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Smith, D.E. Color Analysis. In Food Analysis, 2nd ed.; Nielsen, S.S., Ed.; Springer: Cham, Switzerland, 2017; pp. 573–586. [Google Scholar]
- Rodríguez-Ambriz, S.L.; Islas-Hernández, J.J.; Agama-Acevedo, E.; Tovar, J.; Bello-Pérez, L.A. Characterization of a fibre-rich powder prepared by liquefaction of unripe banana flour. Food Chem. 2008, 107, 1515–1521. [Google Scholar] [CrossRef]
- de Sá Mendes, N.; Favre, L.C.; Rolandelli, G.; Ferreira, C.S.; Goncalves, E.C.B.A.; Buera, M.P. Flour from ’fruits and vegetables’ waste with addition of a SouthAmerican pepper (Capsicum baccatum) proposed as food ingredient. Int. J. Food Sci. Technol. 2020, 55, 1230–1237. [Google Scholar] [CrossRef]
- Ferreira, D.F. SISVAR: A Computer Statistical Analysis System. Ciência Agrotecnol. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Galvão, F.; Pinto, E.; Martins, Z.E.; Almeida, A.A.; Ferreira, I.M.P.L.V.O.; de Lima, V.A.; Felsner, M.L. Nutritional Composition and Minerals Bioaccessibility of Commercial Fruit Flours. J. Food Meas. Charact. 2023, 17, 2547–2554. [Google Scholar] [CrossRef]
- Pushpaveni, C.; Visagaperumal, D.; Chandy, V. A Review on Banana Blossom: The Heart of Banana. World J. Pharm. Res. 2019, 8, 440–450. [Google Scholar]
- Silva Neto, P.A.; Aires, M.L.P.; Cunha, F.E.T.; da Silva, L.M.R.; de Sousa, P.H.M.; Thomaz Gouveia, S.T. Processing of Banana Mangará: Microbiological Evaluation and Development of Preserves and Appetizers with a Focus on Sensory Qualities and Gastronomic Applications. Int. J. Gastron. Food Sci. 2024, 38, 101028. [Google Scholar] [CrossRef]
- ANVISA—Brazilian Health Regulatory Agency. Resolution No. 263/2005: Technical Regulation for Cereals, Starches, Flours and Flour Products; ANVISA: Brasília, Brazil, 2022. Available online: https://anvisalegis.datalegis.net/action/ActionDatalegis.php?acao=apresentacao&cod_menu=9434&cod_modulo=310 (accessed on 1 May 2025).
- Felisberto, M.H.F.; Beraldo, A.L.; Clerici, M.T.P. Young bamboo culm flour of Dendrocalamus asper: Technological properties for food applications. LWT 2017, 76, 230–235. [Google Scholar] [CrossRef]
- Nogueira, P.V.; Vilas Boas, A.C.; Suárez, N.F.; Abreu, R.A.A.; Carvalho, C.V.; Pio, L.A.S.; Pasqual, M. Composition and Functional Properties of Banana Tree Male Inflorescence Flour. J. Culin. Sci. Technol. 2024, 22, 242–262. [Google Scholar] [CrossRef]
- Moreira, A.; Fageria, N.K. Yield, Uptake, and Retranslocation of Nutrients in Banana Plants Cultivated in Upland Soil of Central Amazonian. J. Plant Nutr. 2009, 32, 443–457. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Prestes, R.C.; Kubota, E.H.; Scapin, G.; Mazutti, M.A. Evaluation of Antioxidant Activity of Extracts of Banana Inflorescences (Musa cavendishii). CyTA—J. Food 2015, 13, 498–505. [Google Scholar] [CrossRef]
- Tasnim, T.; Chandra Das, P.; Begum, A.A.; Nupur, A.H.; Mazumder, A.R. Nutritional, Textural and Sensory Quality of Plain Cake Enriched with Rice Rinsed Water Treated Banana Blossom Flour. J. Agric. Food Res. 2020, 2, 100071. [Google Scholar] [CrossRef]
- Sheng, Z.W.; Ma, W.H.; Jin, Z.Q.; Bi, Y.; Sun, Z.G.; Dou, H.T.; Li, J.Y.; Han, L.N. Investigation of Dietary Fiber, Protein, Vitamin E, and Other Nutritional Compounds of Banana Flower of Two Cultivars Grown in China. Afr. J. Biotechnol. 2010, 9, 3888–3895. [Google Scholar]
- ANVISA—Brazilian Health Regulatory Agency. Resolution No. 429/2020: Nutritional Labeling of Packaged Foods; ANVISA: Brasília, Brazil, 2012. Available online: https://anvisalegis.datalegis.net/action/ActionDatalegis.php?acao=apresentacao&cod_menu=9434&cod_modulo=310 (accessed on 1 May 2025).
- Lu, Z.-H.; Donner, E.; Yada, R.Y.; Liu, Q. Physicochemical properties and in vitro starch digestibility of potato starch/protein blends. Carbohydr. Polym. 2016, 154, 214–222. [Google Scholar] [CrossRef]
- Shewry, P.R.; Prins, A.; Kosik, O.; Lovegrove, A. Challenges to Increasing Dietary Fiber in White Flour and Bread. J. Agric. Food Chem. 2024, 72, 13513–13522. [Google Scholar] [CrossRef]
- Santos, D.; da Silva, J.A.L.; Pintado, M. Fruit and Vegetable By-products’ Flours as Ingredients: A Review on Production Process, Health Benefits, and Technological Functionalities. LWT—Food Sci. Technol. 2022, 154, 112707. [Google Scholar] [CrossRef]
- Coelho, E.M.; Gomes, R.G.; Machado, B.A.S.; Oliveira, R.S.; Lima, M.S.; Azêvedo, L.C.; Guez, M.A.U. Passion fruit peel flour: Technological properties and application in food products. Food Hydrocoll. 2017, 62, 158–164. [Google Scholar] [CrossRef]
- Kavya, M.H.; Manasa, R.; Deepika, M.; Shivananjappa, M.; Shekhara Naik, R. A Review on Banana Flower: Nutritional Composition, Processed Products, and Health Benefits. IP J. Nutr. Metabol. Health Sci. 2023, 6, 110–115. [Google Scholar] [CrossRef]
- Kraithong, S.; Issara, U. A Strategic Review on Plant By-product from Banana Harvesting: A Potentially Bio-based Ingredient for Approaching Novel Food and Agro-industry Sustainability. J. Saudi Soc. Agric. Sci. 2021, 20, 530–543. [Google Scholar] [CrossRef]
- Prasad, R.; Majumdar, K.; Shivay, Y.S.; Kapil, U. Minerals in Plant and Human Nutrition and Health; International Plant Nutrition Institute: Peachtree Corners, GA, USA, 2016. [Google Scholar]
- Basumatary, S.; Nath, N. Assessment of Chemical Compositions and In Vitro Antioxidant Properties of Musa balbisiana Colla Inflorescence. Int. J. Pharm. Res. 2018, 10, 80–85. [Google Scholar]
- Adeolu, A.T.; Enesi, D.O. Assessment of proximate, mineral, vitamin and phytochemical compositions of plantain (Musa paradisiaca) bract. Int. Res. J. Plant Sci. 2013, 4, 192–197. [Google Scholar]
- Rosa, R.H.; Fernandes, M.R.; Melo, E.S.P.; Arakaki, D.G.; Lima, N.V.; Leite, L.C.S.; Espindola, P.R.; Souza, I.D.; Nascimento, V.A.; Tschinkel, P.F.S.; et al. Determination of Macro- and Microelements in the Inflorescences of Banana Tree Using ICP OES: Evaluation of the Daily Recommendations of Intake for Humans. Sci. World J. 2020, 2020, 8383612. [Google Scholar] [CrossRef]
- Pohl, H.R.; Wheeler, J.S.; Murray, H.E. Sodium and Potassium in Health and Disease. In Metal Ions in Life Sciences; Springer: Berlin/Heidelberg, Germany, 2013; Volume 13, pp. 29–47. [Google Scholar] [CrossRef]
- Grober, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, X.; Yu, Y.; Wang, L.; Xie, D.; Zhang, Z.; Chen, L.; Lu, A.; Zhang, G.; Li, F. Disorders of Calcium and Phosphorus Metabolism and the Proteomics/Metabolomics-Based Research. Front. Cell Dev. Biol. 2020, 8, 576110. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Abib, B.; Qin, Z.; Ze, X.; Ali, S.E. Dietary Macrominerals: Updated Review of Their Role and Orchestration in Human Nutrition Throughout the Life Cycle with Sex Differences. Curr. Res. Food Sci. 2023, 6, 100450. [Google Scholar] [CrossRef] [PubMed]
- Vallinoto, P.; Moreira, E.G.; Maihara, V.A. Estimation of Daily Dietary Intake of Essential Minerals and Trace Elements in Commercial Complementary Foods Marketed in Brazil. Food Chem. Adv. 2022, 1, 100039. [Google Scholar] [CrossRef]
- Ferreira, M.P.; Tarley, C.R.T. Assessment of in vitro bioaccessibility of macrominerals and trace elements in green banana flour. J. Food Compos. Anal. 2020, 92, 103586. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Martin-Sánchez, A.; Sánchez-Zapata, E.; Fernández-López, J.; Sendra, E.; Sayas-Barberá, E.; Navarro, C.; Pérez-Álvarez, J.A. Chemical, Physico-Chemical, and Functional Properties of Pomegranate (Punica granatum L.) Bagasse Powder Coproduct. J. Food Eng. 2012, 110, 220–224. [Google Scholar] [CrossRef]
- Kunyanee, K.; Van Ngo, T.; Kusumawardani, S.; Luangsakul, N. Enhancing Banana Flour Quality through Physical Modifications and Its Application in Gluten-Free Chips Product. Foods 2024, 13, 593. [Google Scholar] [CrossRef]
- Larrosa, A.P.Q.; Otero, D.M. Flour Made from Fruit By-Products: Characteristics, Processing Conditions, and Applications. J. Food Process. Preserv. 2021, 45, e15398. [Google Scholar] [CrossRef]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple Pomace as a Source of Dietary Fibre and Polyphenols and Its Effect on the Rheological Characteristics and Cake Making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Hassan, F.A.; Ismail, A.; Hamid, A.A.; Azlan, A.; Al-sheraji, S.H. Characterisation of Fibre-Rich Powder and Antioxidant Capacity of Mangifera pajang K. Fruit Peels. Food Chem. 2010, 126, 283–288. [Google Scholar] [CrossRef]
- Lossolli, N.A.B.; Leonel, M.; Leonel, S.; Izidoro, M.; Cândido, H.T.; Assis, J.L.J.; Oliveira, L.A. Exploring Differences in the Physico-Chemical and Nutritional Properties of Mango Flours and Starches. Food Sci. Technol. Int. 2024. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.S.; Mahalakshimi, M.; Parimalavalli, R. Comparative study of banana flour, starch and autoclaved starch. Trends Carbohydr. Res. 2014, 6, 38–44. [Google Scholar]
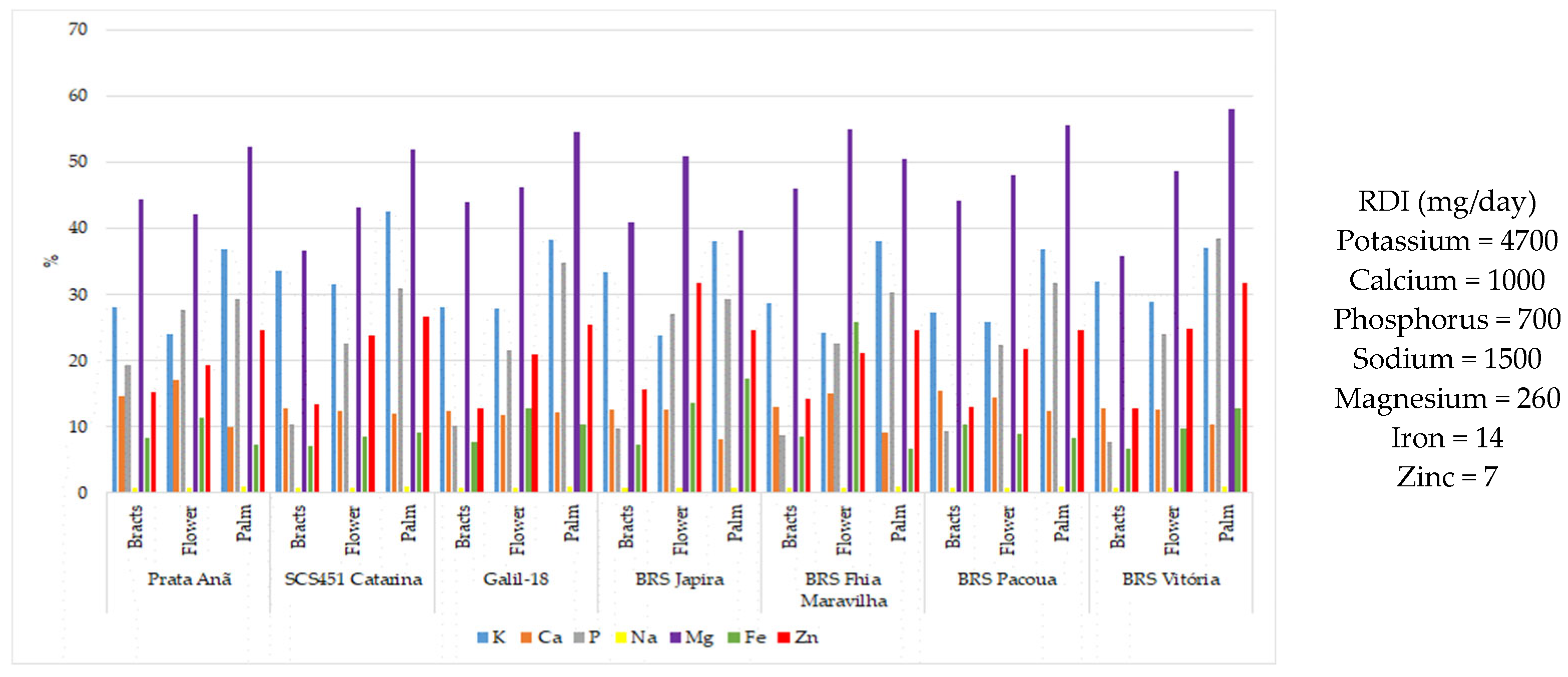
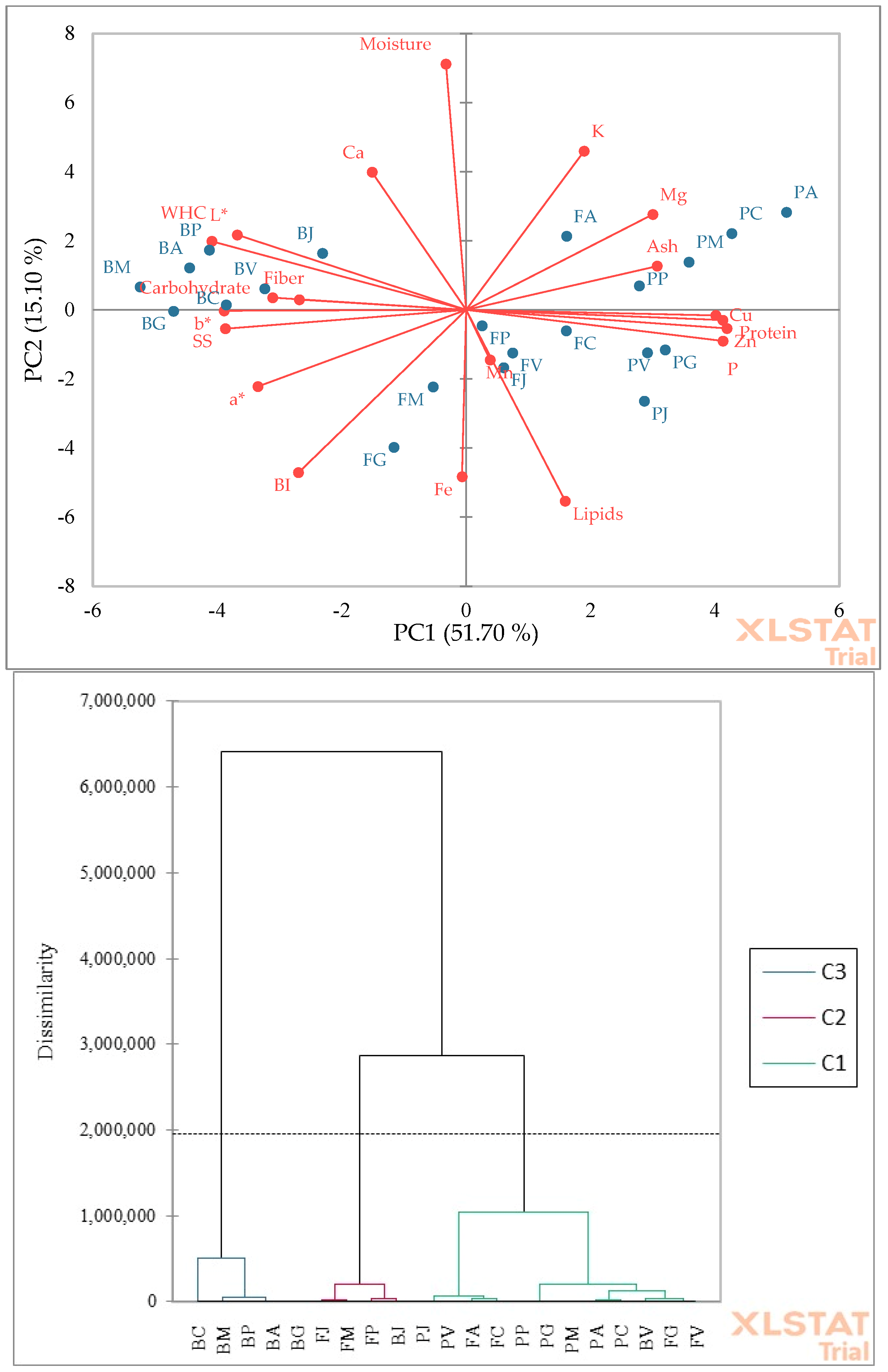
| Cultivars | Genotype | Plant Height (m) | Number Fruit/Bunch | Fruit Length (cm) | Fruit Mass (g) | Yield (t ha−1) |
|---|---|---|---|---|---|---|
| Prata Anã | AAB | 2.3 | 149–190.5 | 17.3–20 | 115–119 | 19.7 |
| SCS451 Catarina | AAB | 3.22–3.3 | 121.3 | 17 | 135–145 | 23.5–36.7 |
| Galil 18 | AAAB | 2.12–2.5 | 97.8–118 | 13.8–16.4 | 167.9 | 16.3–21 |
| BRS Japira | AAAB | 2.48 | 80.5 | 12.8–20.5 | 122.8–184.78 | 20 |
| BRS Fhia Maravilha | AAAB | 2.6–2.31 | 145.6–175 | 24.3–25 | 243–245 | 17.8 |
| BRS Pacoua | AAAB | 2.4–3.5 | 120–130 | 15 | 150 | 17.2–30 |
| BRS Vitória | AAAB | 3.10 | 98 | 18 | 145 | 15 |
| Susceptibility to pests and diseases | ||||||
| Prata Anã | Susceptible to Yellow Sigatoka (Mycosphaerella musicola, Leach) and Black Sigatoka (Mycosphaerella fijiensis, Morelet) and Moko (Ralstonia solanacearum), moderately susceptible to Panama Disease (Fusarium oxisporum. f. sp. cubense), moderately resistant to nematodes and the Rhizome Borer (Cosmolites sordidus). | |||||
| SCS451 Catarina | Susceptible to Yellow Sigatoka and Black Sigatoka, and moderately susceptible to Panama Disease. | |||||
| Galil 18 | Resistant to Black Sigatoka, moderately susceptible to Yellow Sigatoka, and tolerant to Panama Disease | |||||
| BRS Japira | Resistant to Black Sigatoka, Yellow Sigatoka, Panama Disease, and Anthracnose | |||||
| BRS Fhia Maravilha | Resistant to Black Sigatoka and Panama Disease, moderately resistant to Yellow Sigatoka, and moderately susceptible to Rhizome Borer | |||||
| BRS Pacoua | Resistant to Yellow Sigatoka and Panama disease and moderately resistant to Black Sigatoka. | |||||
| BRS Vitória | Resistant to Black Sigatoka, Yellow Sigatoka, and Panama Disease | |||||
| Cultivars | Inflorescence | Bracts | Flower | Palm |
|---|---|---|---|---|
| Prata Anã | 1245.42 ± 46.45 a | 786.74 ± 37.76 a | 339.02 ± 12.51 a | 119.66 ± 9.33 b |
| SCS 451 Catarina | 895.44 ± 39.54 c | 539.6 ± 29.9 c | 224.48 ± 8.13 c | 131.36 ± 9.09 b |
| Galil 18 | 874.14 ± 35.54 c | 505.6 ± 19.14 c | 245.3 ± 16.73 c | 123.26 ± 2.92 b |
| BRS Japira | 808.10 ± 29.93 d | 514.54 ± 17.03 c | 186.32 ± 10.62 d | 107.24 ± 4.56 c |
| BRS Fhia Maravilha | 1129.4 ± 27.09 b | 677.45 ± 20.84 b | 314.8 ± 14.40 b | 137.15 ± 11.17 b |
| BRS Pacoua | 777.42 ± 55.60 e | 371.06 ± 13.13 d | 217.8 ± 8.85 c | 188.56 ± 5.74 a |
| BRS Vitória | 681.26 ± 32.15 f | 360.82 ± 21.85 d | 201.16 ± 10.20 d | 119.28 ± 6.48 b |
| Moisture | Ash | Protein | |||||||
| Bracts | Flower | Palm | Bracts | Flower | Palm | Bracts | Flower | Palm | |
| Prata Anã | 9.9 ± 0.2 Ab | 9.6 ± 0.2 Ba | 10.0 ± 0.1 Aa | 8.8 ± 0.1 Bc | 8.1 ± 0.2 Ce | 13.0 ± 0.2 Aa | 7.8 ± 0.2 Cd | 13.6 ± 0.2 Bb | 19.4 ± 0.1 Aa |
| SCS 451 Catarina | 9.6 ± 0.2 Bc | 9.2 ± 0.1 Cb | 10.1 ± 0.1 Aa | 9.5 ± 0.2 Ba | 9.5 ± 0.2 Ba | 12.5 ± 0.3 Ab | 8.2 ± 0.1 Cc | 13.2 ± 0.1 Bc | 17.4 ± 0.2 Ad |
| Galil 18 | 9.5 ± 0.1 Ad | 8.4 ± 0.1 Cd | 9.0 ± 0.1 Bc | 8.3 ± 0.1 Cd | 8.6 ± 0.1 Bc | 11.6 ± 0.2 Ad | 7.2 ± 0.2 Ce | 11.8 ± 0.1 Be | 17.3 ± 0.3 Ad |
| BRS Japira | 9.6 ± 0.2 Ac | 9.2 ± 0.1 Bb | 8.8 ± 0.1 Cd | 9.5 ± 0.2 Ba | 8.0 ± 0.2 Ce | 11.0 ± 0.2 Af | 10.1 ± 0.2 Cb | 12.1 ± 0.1 Bd | 18.5 ± 0.2 Ab |
| BRS Fhia Maravilha | 9.4 ± 0.2 Bd | 8.8 ± 0.1 Cc | 9.8 ± 0.1 Ab | 8.4 ± 0.1 Bd | 8.1 ± 0.2 Ce | 12.3 ± 0.1 Ac | 7.5 ± 0.2 Cd | 11.1 ± 0.1 Bf | 17.8 ± 0.2 Ac |
| BRS Pacoua | 10.1 ± 0.1 Aa | 9.7 ± 0.1 Ba | 10.0 ± 0.1 Aa | 8.2 ± 0.1 Bd | 8.3 ± 0.1 Bd | 11.2 ± 0.1 Ae | 7.7 ± 0.2 Cd | 12.2 ± 0.2 Bd | 17.4 ± 0.4 Ad |
| BRS Vitória | 10.1 ± 0.1 Aa | 8.9 ± 0.1 Bc | 9.0 ± 0.1 Bc | 9.0 ± 0.1 Bb | 9.0 ± 0.1 Bb | 12.5 ± 0.1 Ab | 10.6 ± 0.1 Ca | 14.4 ± 0.1 Ba | 19.3 ± 0.3 Aa |
| Lipids | Fiber | Carbohydrate | |||||||
| Bracts | Flower | Palm | Bracts | Flower | Palm | Bracts | Flower | Palm | |
| Prata Anã | 5.0 ± 0.1 Ba | 5.9 ± 0.1 Ad | 4.3 ± 0.1 Cd | 34.8 ± 0.7 Bd | 37.2 ± 0.9 Ac | 27.7 ± 0.6 Ce | 34.0 ± 0.7 Aa | 25.6 ± 0.5 Bd | 25.7 ± 0.5 Bb |
| SCS 451 Catarina | 4.4 ± 0.1 Cb | 6.8 ± 0.1 Ab | 5.5 ± 0.1 Bc | 40.0 ± 0.6 Ab | 36.8 ± 0.9 Bc | 33.8 ± 0.8 Cb | 28.3 ± 0.9 Ad | 24.5 ± 0.9 Bd | 20.7 ± 0.8 Ce |
| Galil 18 | 5.0 ± 0.1 Ca | 8.0 ± 0.1 Aa | 5.8 ± 0.1 Bb | 37.6 ± 0.8 Ac | 35.3 ± 0.7 Bd | 32.9 ± 0.8 Cc | 32.4 ± 0.9 Ab | 27.9 ± 0.8 Bc | 23.4 ± 1.1 Cd |
| BRS Japira | 4.0 ± 0.1 Cc | 6.4 ± 0.1 Ac | 4.4 ± 0.2 Bd | 40.6 ± 0.7 Ab | 35.0 ± 0.8 Bd | 30.2 ± 0.43 Cd | 26.19 ± 0.6 Be | 29.4 ± 1.0 Ab | 27.1 ± 0.5 Ba |
| BRS Fhia Maravilha | 4.0 ± 0.1 Cc | 6.4 ± 0.1 Ac | 4.4 ± 0.10 Bd | 40.0 ± 0.9 Ab | 38.6 ± 0.6 Bb | 30.8 ± 0.4 Cd | 30.7 ± 1.3 Ac | 27.0 ± 0.6 Bc | 25.0 ± 0.4 Cc |
| BRS Pacoua | 3.9 ± 0.1 Bc | 6.7 ± 0.1 Ab | 6.6 ± 0.1 Aa | 41.9 ± 0.8 Aa | 31.1 ± 0.8 Ce | 35.4 ± 0.8 Ba | 28.3 ± 1.2 Bd | 32.1 ± 0.9 Aa | 19.3 ± 0.5 Cf |
| BRS Vitória | 4.3 ± 0.1 Cb | 6.0 ± 0.1 Ad | 5.6 ± 0.1 Bc | 39.9 ± 0.9 Ab | 40.7 ± 0.9 Aa | 32.4 ± 0.8 Bc | 26.1 ± 1.0 Ae | 21.0 ± 1.0 Be | 21.2 ± 1.3 Be |
| L* | a* | b* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bracts | Flower | Palm | Bracts | Flower | Palm | Bracts | Flower | Palm | |
| Prata Anã | 42.9 ± 0.3 Ab | 32.5 ± 0.3 Be | 32.8 ± 0.2 Bc | 5.1 ±0.1 Ac | 3.22 ± 0.1 Bg | 3.1 ± 0.0 Ce | 12.6 ± 0.2 Ab | 8.3 ± 0.2 Bf | 7.7 ± 0.1 Cf |
| SCS 451 Catarina | 39.1 ± 0.2 Ae | 33.5 ± 0.2 Cc | 33.9 ± 0.1 Bb | 5.0 ± 0.1 Ad | 3.94 ± 0.0 Bf | 3.6 ± 0.0 Cd | 11.7 ± 0.2 Ad | 9.7 ± 0.1 Bc | 8.9 ± 0.1 Cd |
| Galil 18 | 41.1 ± 0.2 Ac | 35.0 ± 0.1 Ba | 33.9 ± 0.2 Cb | 5.6 ± 0.0 Ab | 5.11 ± 0.0 Ba | 4.4 ± 0.1 Cc | 11.9 ± 0.0 Ac | 11.2 ± 0.2 Ba | 9.4 ± 0.1 Cc |
| BRS Japira | 38.8 ± 0.1 Ae | 32.9 ± 0.2 Cd | 34.1 ± 0.2 Bb | 4.8 ± 0.0 Ae | 4.05 ± 0.0 Ce | 4.4 ± 0.1 Bc | 10.8 ± 0.1 Ae | 8.9 ± 0.1 Be | 8.9 ± 0.1 Bd |
| BRS Fhia Maravilha | 44.2 ± 0.2 Aa | 33.0 ± 0.1 Cd | 34.0 ± 0.2 Bb | 5.8 ± 0.0 Aa | 4.5 ± 0.0 Bc | 4.4 ± 0.0 Cc | 12.9 ± 0.1 Aa | 9.0 ± 0.1 Be | 8.7 ± 0.1 Ce |
| BRS Pacoua | 42.9 ± 0.3 Ab | 34.3 ± 0.2 Cb | 35.8 ± 0.2 Ba | 5.0 ± 0.0 Ad | 4.43 ± 0.0 Cd | 4.7 ± 0.0 Bb | 11.8 ± 0.2 Ac | 9.5 ± 0.0 Bd | 9.7 ± 0.1 Bb |
| BRS Vitória | 39.8 ± 0.3 Ad | 34.9 ± 0.2 Ca | 35.5 ± 0.2 Ba | 5.1 ± 0.0 Ac | 4.6 ± 0.0 Cb | 4.8 ± 0.0 Ba | 11.9 ± 0.2 Ac | 10.0 ± 0.1 Bb | 9.9 ± 0.1 Ba |
| WI | BI | ||||||||
| Bracts | Flower | Palm | Bracts | Flower | Palm | ||||
| Prata Anã | 15.6 ± 0.2 Ab | 12.1 ± 0.2 Bf | 11.7 ± 0.1 Cf | 42.7 ± 0.3 Ac | 35.9 ± 0.4 Bf | 33.0 ± 0.3 Cf | |||
| SCS 451 Catarina | 14.9 ± 0.2 Ad | 13.3 ± 0.1 Bc | 12.6 ± 0.1 Ce | 43.9 ± 0.4 Aa | 42.0 ± 0.3 Bc | 37.5 ± 0.2 Ce | |||
| Galil 18 | 15.2 ± 0.1 Ac | 14.7 ± 0.1 Ba | 13.2 ± 0.1 Cc | 43.3 ± 0.2 Bb | 48.3 ± 0.6 Aa | 41.2 ± 0.2 Ca | |||
| BRS Japira | 14.2 ± 0.1 Ae | 12.7 ± 0.1 Be | 12.9 ± 0.1 Bd | 40.7 ± 0.4 Ad | 39.7 ± 0.3 Be | 39.1 ± 0.3 Cc | |||
| BRS Fhia Maravilha | 16.0 ± 0.1 Aa | 13.0 ± 0.1 Bd | 12.7 ± 0.1 Ce | 43.3 ± 0.3 Ab | 41.0 ± 0.4 Bd | 38.2 ± 0.3 Cd | |||
| BRS Pacoua | 14.9 ± 0.2 Ad | 13.3 ± 0.1 Bc | 13.4 ± 0.1 Bb | 40.0 ± 0.3 Be | 41.2 ± 0.3 Ad | 40.2 ± 0.3 Bb | |||
| BRS Vitória | 15.1 ± 0.2 Ac | 13.7 ± 0.1 Bb | 13.6 ± 0.1 Ba | 44.0 ± 0.6 Aa | 42.7 ± 0.2 Bb | 41.6 ± 0.3 Ca | |||
| WHC | SS | |||||
|---|---|---|---|---|---|---|
| Bracts | Flower | Palm | Bracts | Flower | Palm | |
| Prata Anã | 6.8 ± 0.1 Aa | 4.4 ± 0.1 Ba | 2.4 ± 0.1 Cc | 6.1 ± 0.2 Ba | 6.6 ± 0.1 Be | 7.5 ± 0.1 Ac |
| SCS 451 Catarina | 6.5 ± 0.1 Ab | 3.4 ± 0.1 Be | 2.6 ± 0.1 Cb | 5.5 ± 0.2 Cb | 8.2 ± 0.1 Bc | 8.6 ± 0.1 Aa |
| Galil 18 | 6.3 ± 0.1 Ac | 3.9 ± 0.1 Bc | 2.5 ± 0.1 Cc | 5.5 ± 0.1 Cb | 8.9 ± 0.1 Ab | 7.6 ± 0.1 Bc |
| BRS Japira | 5.9 ± 0.1 Ae | 3.6 ± 0.1 Bd | 2.2 ± 0.1 Ce | 5.3 ± 0.1 Cc | 9.2 ± 0.2 Aa | 8.0 ± 0.1 Bb |
| BRS Fhia Maravilha | 6.3 ± 0.1 Ac | 3.6 ± 0.1 Bd | 2.4 ± 0.1 Cc | 4.9 ± 0.1 Cd | 6.7 ± 0.2 Ae | 6.1 ± 0.1 Bd |
| BRS Pacoua | 6.2 ± 0.1 Ad | 3.5 ± 0.2 Bd | 2.8 ± 0.1 Ca | 5.1 ± 0.1 Cc | 8.1 ± 0.2 Ad | 7.6 ± 0.2 Bc |
| BRS Vitória | 5.6 ± 0.1 Af | 4.1 ± 0.1 Bb | 2.3 ± 0.1 Cd | 5.6 ± 0.1 Cb | 8.3 ± 0.2 Bc | 8.5 ± 0.4 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouros, L.F.d.; Leonel, M.; Leonel, S.; Molha, N.Z.; Jesus, P.R.R.d.; Cândido, H.T.; Tecchio, M.A.; Rechsteiner, M.S.; Ouros, C.C.d. Diversification of Cultivars and Production of Male Inflorescence Flours for More Sustainable Banana Cultivation. Agriculture 2025, 15, 1110. https://doi.org/10.3390/agriculture15101110
Ouros LFd, Leonel M, Leonel S, Molha NZ, Jesus PRRd, Cândido HT, Tecchio MA, Rechsteiner MS, Ouros CCd. Diversification of Cultivars and Production of Male Inflorescence Flours for More Sustainable Banana Cultivation. Agriculture. 2025; 15(10):1110. https://doi.org/10.3390/agriculture15101110
Chicago/Turabian StyleOuros, Lucas Felipe dos, Magali Leonel, Sarita Leonel, Nicholas Zanette Molha, Paulo Ricardo Rodrigues de Jesus, Hebert Teixeira Cândido, Marco Antonio Tecchio, Mayra Schmidt Rechsteiner, and Caio César dos Ouros. 2025. "Diversification of Cultivars and Production of Male Inflorescence Flours for More Sustainable Banana Cultivation" Agriculture 15, no. 10: 1110. https://doi.org/10.3390/agriculture15101110
APA StyleOuros, L. F. d., Leonel, M., Leonel, S., Molha, N. Z., Jesus, P. R. R. d., Cândido, H. T., Tecchio, M. A., Rechsteiner, M. S., & Ouros, C. C. d. (2025). Diversification of Cultivars and Production of Male Inflorescence Flours for More Sustainable Banana Cultivation. Agriculture, 15(10), 1110. https://doi.org/10.3390/agriculture15101110







