Comparative Phytochemical and Biological Profiling of Zea mays L. Varieties in Cotopaxi Region
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
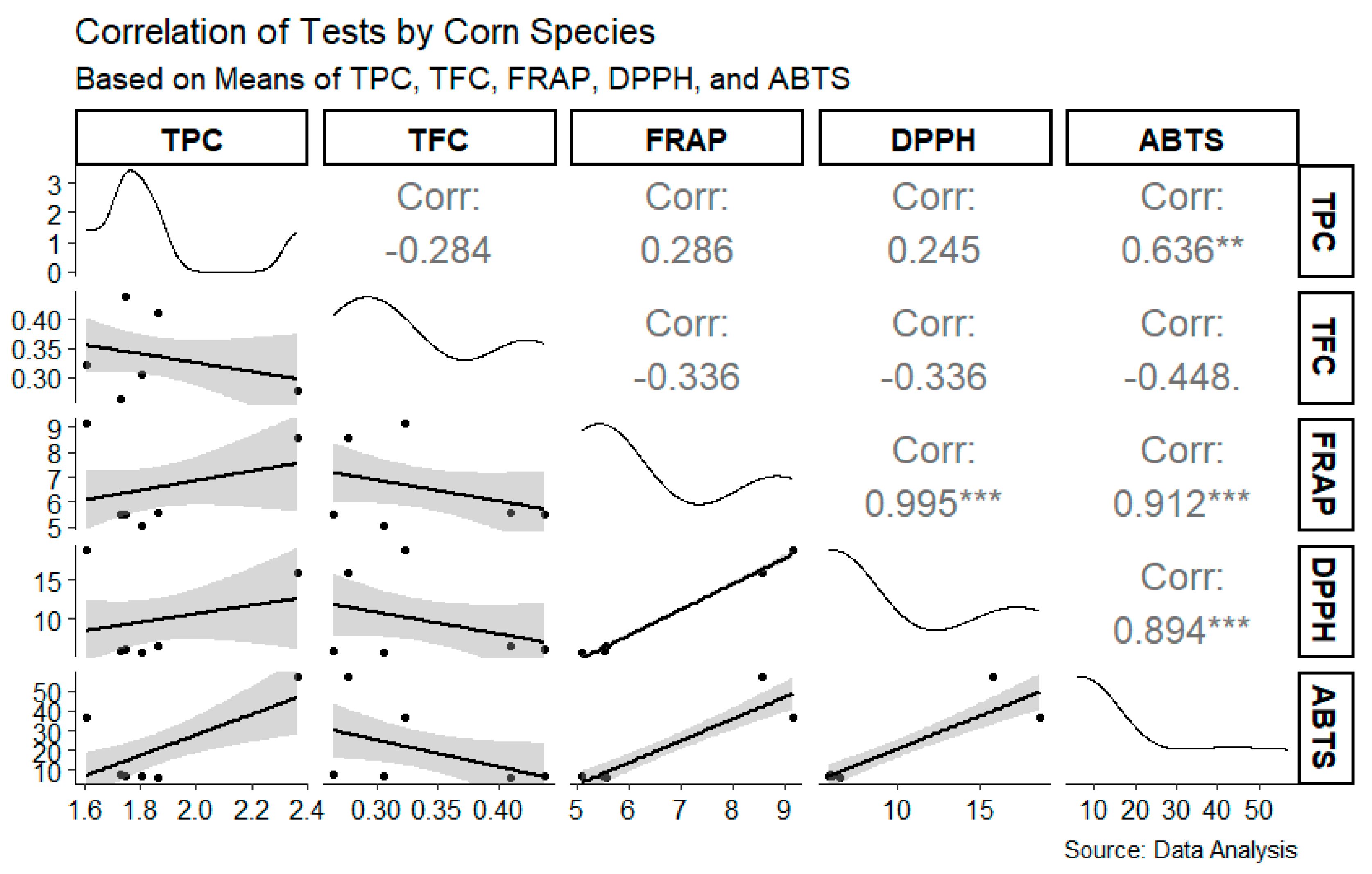
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, G.; Zhang, X.; Chen, W.; Zhang, R.; Li, Z.; Wen, W.; Warburton, M.L.; Li, J.; Li, H.; Yang, X. Population genomics of Zea species identifies selection signatures during maize domestication and adaptation. BMC Plant Biol. 2022, 22, 72. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.M.; Vidal, R.; de Almeida Silva, N.C.; Veasey, E.A.; de Oliveira Freitas, F.; Zucchi, M.I. Archaeological findings show the extent of primitive characteristics of maize in South America. Sci. Adv. 2024, 10, 9. [Google Scholar] [CrossRef]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Ranilla, L.G. The application of metabolomics for the study of cereal corn (Zea mays L.). Metabolites 2020, 10, 300. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2021. Making Agrifood Systems More Resilient to Shocks and Stresses; FAO: Rome, Italy, 2021. [Google Scholar]
- Zambrano, J.; Garcés, S.; Villacrés, E.; Velásquez, J.; Cartagena, Y.; Pintado, P.; Peñaherrera, D.; Sangoquiza, C.; León, J.; López, V.; et al. Guía Para la Producción Sustentable de Maíz en la Sierra Ecuatoriana; INIAP: Quito, Ecuador, 2021; Volume 122, p. 148. [Google Scholar]
- Tanumihardjo, S.A.; McCulley, L.; Roh, R.; Lopez-Ridaura, S.; Palacios-Rojas, N.; Gunaratna, N.S. Maize agro-food systems to ensure food and nutrition security in reference to the Sustainable Development Goals. Glob. Food Sec. 2020, 25, 100327. [Google Scholar] [CrossRef]
- Sión Saltos, G.J.; Velásquez Campozano, M.R.; Rodríguez Pincay, I. La cosmovisión en torno al consumo de la chicha de maíz en Ecuador. Rev. Cien. Multi. Sapientiae 2024, 7, 111–127. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Zolla, G.; Afaray-Carazas, A.; Vera-Vega, M.; Huanuqueño, H.; Begazo-Gutiérrez, H.; Chirinos, R.; Pedreschi, R.; Shetty, K. Integrated metabolite analysis and health-relevant in vitro functionality of white, red, and orange maize (Zea mays L.) from the Peruvian Andean race Cabanita at different maturity stages. Front. Nutr. 2023, 10, 1132228. [Google Scholar] [CrossRef]
- Jackson, D.; Tian, F.; Zhang, Z. Maize genetics, genomics, and sustainable improvement. Mol. Breed. 2022, 42, 2. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Sánchez-Nuño, Y.A.; Zermeño-Ruiz, M.; Vázquez-Paulino, O.D.; Nuño, K.; Villarruel-López, A. Bioactive Compounds from Pigmented Corn (Zea mays L.) and Their Effect on Health. Biomolecules 2024, 14, 338. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Rios-Gonzales, B.A.; Ramírez-Pinto, M.F.; Fuentealba, C.; Pedreschi, R.; Shetty, K. Primary and Phenolic Metabolites Analyses, In Vitro Health-Relevant Bioactivity and Physical Characteristics of Purple Corn (Zea mays L.) Grown at Two Andean Geographical Locations. Metabolites 2021, 11, 722. [Google Scholar] [CrossRef]
- Claros, P. Evaluacion de la Capacidad Antioxidante Total y Contenido de Polifenoles Totales del Phaseolus vulgaris “Frijol”. Bachelor’s Thesis, Universidad Nacional José Faustino Sánchez Carrión, Huacho, Peru, 2021. Available online: https://repositorio.unjfsc.edu.pe/handle/20.500.14067/5297 (accessed on 19 January 2025).
- López-Froilán, R.; Hernández-Ledesma, B.; Cámara, M.; Pérez-Rodríguez, M. Evaluation of the Antioxidant Potential of Mixed Fruit-Based Beverages: A New Insight on the Folin-Ciocalteu Method. Food Anal. Met. 2018, 11, 2897–2906. [Google Scholar] [CrossRef]
- Pekal, A.; Pyrzynska, K. Evaluation of aluminum complexation reaction for flavonoid content assay. Food Anal. Met. 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Rajurkar, N.S.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef]
- Sachett, A.; Gallas-Lopes, M.; Conterato, G.M.M.; Herrmann, A.; Piato, A. Antioxidant Activity by DPPH Assay: In Vitro Protocol. Protocols Io. 2021. Available online: https://www.protocols.io/view/antioxidant-activity-by-dpph-assay-in-vitro-protocbtbpnimn (accessed on 3 September 2023).
- Thaweesang, S. Antioxidant activity and total phenolic compounds of fresh and blanching banana blossom (Musa ABB CV. Kluai “Namwa”) in Thailand. IOP Conf. Ser. Mat. Sci. Eng. 2019, 639, 012047. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversosmétodos químicos para determinar actividad antioxidante en pulpa de frutos. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Tohma, H.; Koksal, E.; Kılıc, O.; Alan, Y.; Yılmaz, M.A.; Gulcin, I.; Bursal, E.; Alwasel, S.H. RP-HPLC/MS/MS analysis of the phenolic compounds, antioxidant and antimicrobial activities of Salvia L. species. Antioxidants 2016, 5, 38. [Google Scholar] [CrossRef]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C.G. LC-MS identification and quantification of phenolic compounds in solid residues from the essential oil industry. Antioxidants 2021, 10, 2016. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Cellier, G.; Moreau, A.; Chabirand, A.; Hostachy, B.; Ailloud, F.; Prior, P. A Duplex PCR Assay for the Detection of Ralstonia solanacearum Phylotype II Strains in Musa spp. PLoS ONE 2015, 10, e0122182. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Maize flavonoid biosynthesis, regulation, and human health relevance: A review. Molecules 2022, 27, 5166. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jensen, J.D.; Svensson, B.; Jørgensen, H.J.L.; Collinge, D.B.; Finnie, C. A maize gene regulatory network for phenolic metabolism. Mol. Plant-Microbe Interact. 2017, 30, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Yeo, J.D. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Górnaś, P.; Juhnevica-Radenkova, K.; Lacis, G. Phenolic compounds as potential natural antioxidants in cereal-based foods: Insights into their composition, stability, and bioavailability. Antioxidants 2020, 9, 469. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Hossain, F.; Muthusamy, V. Biofortification of maize: An Indian perspective. Indian J. Genet. 2015, 75, 1–22. [Google Scholar]
- Salinas-Moreno, Y.; Valle-Guadarrama, S.; García-Salinas, C.; Velázquez-Cardelas, G.A. Anthocyanin distribution and color variation in maize (Zea mays L.) landraces. J. Cereal Sci. 2005, 41, 259–266. [Google Scholar]
- Zhao, D.; Shahidi, F.; Xiao, C. Phenolic profiles and antioxidant activities of yellow and red maize kernels. J. Agric. Food Chem. 2019, 67, 697–707. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Saito, K. Integrated metabolomics for abiotic stress responses in plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef]


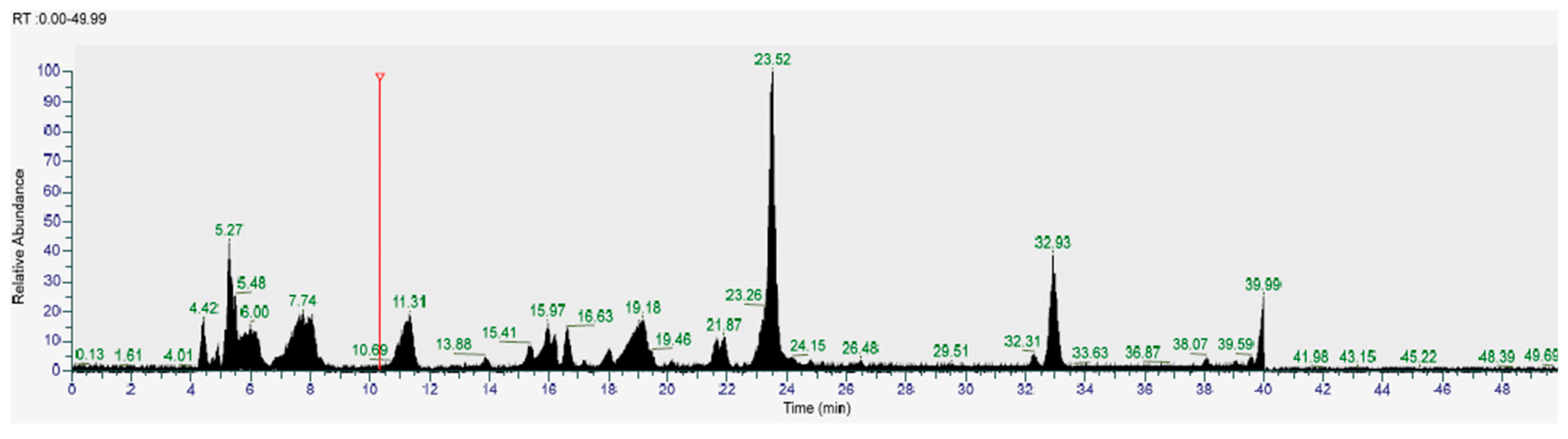

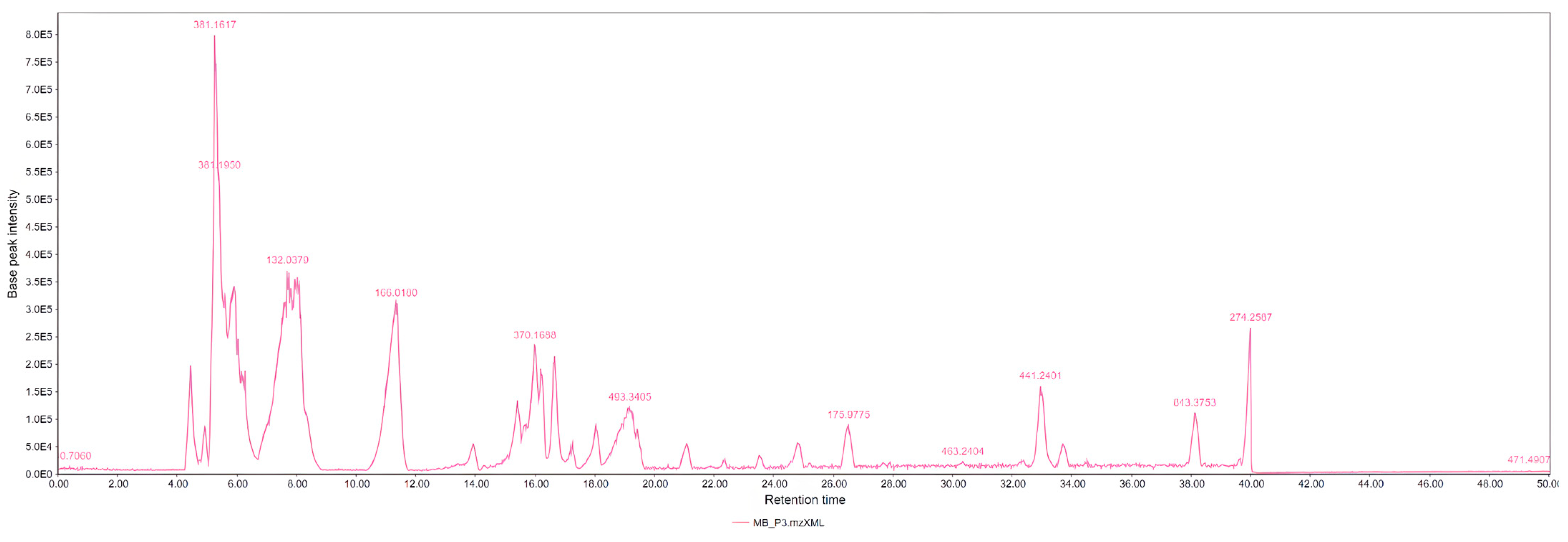
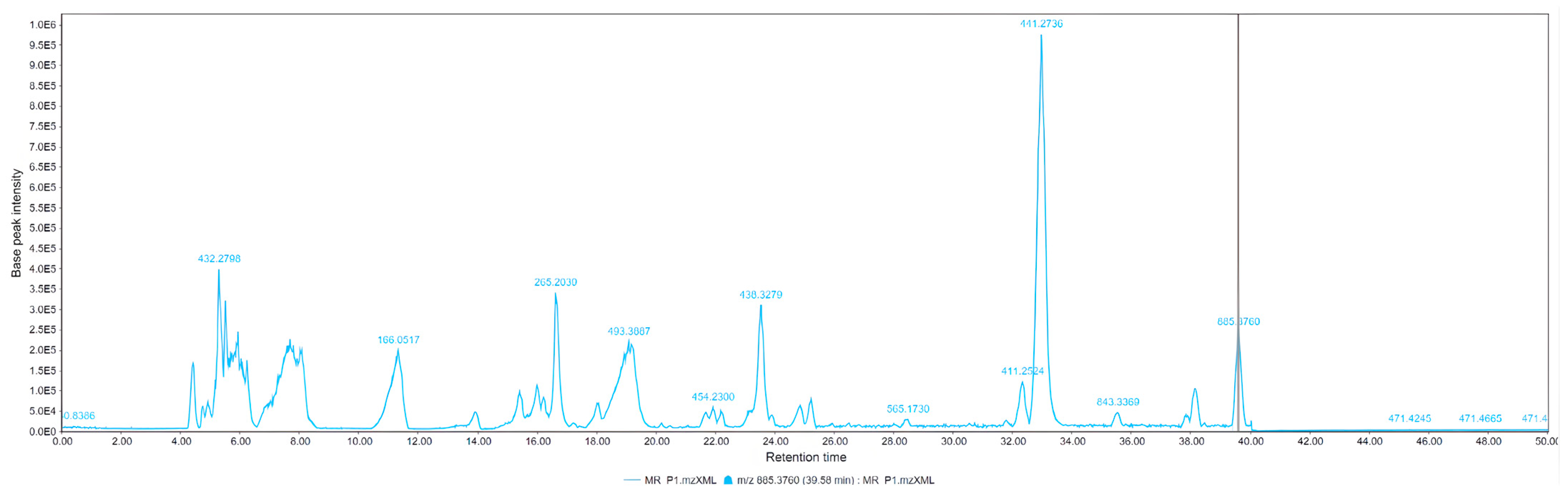
| LC-MS Positive Ions | |||||
|---|---|---|---|---|---|
| ID | Proposed Compound Identity | Molecular Formula | Retention Time | m/z | Molecular Ion |
| 19 | Daphnetin | C9H6O4 | 4.424 | 178.925 | M+H |
| 49 | Argininosuccinate | C10H18N4O6 | 4.898 | 290.915 | M+ |
| 59 | Arginine | C6H14N4O2 | 4.98 | 175.119 | M+H |
| 175 | Pumiloside | C21H24O11 | 5.447 | 513.229 | M+H |
| 218 | Glycerophosphorylcholine | C8H20NO6P | 5.861 | 258.145 | M+ |
| 284 | Biotin | C10H16N2O3S | 7.111 | 245.081 | M+H |
| 290 | L-Tyrosine | C9H11NO3 | 7.644 | 183.061 | M+H |
| 362 | 5′-Methylthioadenosine | C11H15N5O3S | 15.649 | 298.097 | M+H |
| 407 | gamma-Glutamylleucine | C11H20N2O4 | 16.912 | 261.161 | M+ |
| 416 | (2e)-3-(3,4-Dihydroxyphenyl)-N-[2-(4-Hydroxyphenyl)ethyl]acrylamide | C17H17NO4 | 19.959 | 338.186 | M+ |
| 548 | Glucose_6-phosphate | C6H13O9P | 23.502 | 261.127 | [M+Na]+ |
| 564 | Sinigrin hydrate | C10H16KNO9S2 | 23.68 | 438.317 | M+H |
| 580 | Isoshaftoside | C26H28O14 | 24.811 | 565.236 | [M+H]+ |
| 605 | Vicenin | C27H30O15 | 29.299 | 617.218 | [M+Na]+ |
| 693 | Abscisic acid | C15H20O4 | 31.05 | 265.138 | M+H |
| 859 | Ferulate | C10H10O4 | 38.501 | 195.016 | M+H |
| 886 | Pelargonidin-3-O-glucoside | C21H21O10 | 19.959 | 433.293 | M-H |
| HPLC-MS Negative Ions | |||||
| ID | Proposed compound identity | Molecular Formula | Retention Time | m/z | Molecular Ion |
| 62 | Glucose 1-phosphate | C6H13O9P | 5.175 | 259.147 | M-H |
| 70 | Sorbitol | C6H14O6 | 5.192 | 181.124 | M-H |
| 120 | Sucrose | C12H22O11 | 5.33 | 341.257 | M-H |
| 144 | Glucose, fructose, mannose | C6H12O6 | 5.3 | 179.133 | M-H |
| 459 | alpha, alpha-Trehalose | C12H22O11 | 19.878 | 179.063 | M-H |
| 503 | Caffeic acid | C9H8O4 | 21.053 | 190.073 | M+H |
| 563 | Trihydroxyflavone-C-hexoside-C-pentoside | C20H20O11 | 23.245 | 563.313 | M-H |
| 629 | Orientin | C21H20O11 | 24.594 | 447.141 | M-H |
| HPLC-MS Positive and Negative Ions | ||||
|---|---|---|---|---|
| ID | Proposed Compound Identity | Molecular Formula | m/z | Retention Time |
| 205 | L–Tryptophan | C11H12N2O2 | 205.098 | 15.4 |
| 265 | Feruloylputrescine | C14H20N2O3 | 265.155 | 5.26 |
| 381 | Sucrose | C12H22O11 | 341.108 | 16.6 |
| 438 | N1, N10-Bis(p-coumaroyl) spermidine | C26H32N2O4 | 437.244 | 23.5 |
| 441 | Bis-ferulamidobutane | C22H30N2O6 | 441.201 | 32.87 |
| HPLC-MS Positive and Negative Ions | ||||
|---|---|---|---|---|
| ID | Proposed Compound Identity | Molecular Formula | m/z | Retention Time |
| 265 | Feruloylputrescine | C14H20N2O3 | 265.155 | 15.4 |
| 411 | Diferuloyl putrescine | C12H22O11 | 343.124 | 5.26 |
| 438 | N1, N10-Bis(p-coumaroyl) spermidine | C26H32N2O4 | 437.244 | 16.6 |
| 441 | Bis-ferulamidobutane | C22H30N2O6 | 441.2001 | 23.5 |
| 454 | p-Coumaroyl caffeoyl spermidine | C21H28N2O5 | 389.208 | 32.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihai, R.A.; Vivanco Gonzaga, R.F.; Calero Rondal, D.O.; Teneda Jijón, D.A.; Cubi Insuaste, N.S.; Borja Tacuri, C.D.; Catana, R.D. Comparative Phytochemical and Biological Profiling of Zea mays L. Varieties in Cotopaxi Region. Agriculture 2025, 15, 1054. https://doi.org/10.3390/agriculture15101054
Mihai RA, Vivanco Gonzaga RF, Calero Rondal DO, Teneda Jijón DA, Cubi Insuaste NS, Borja Tacuri CD, Catana RD. Comparative Phytochemical and Biological Profiling of Zea mays L. Varieties in Cotopaxi Region. Agriculture. 2025; 15(10):1054. https://doi.org/10.3390/agriculture15101054
Chicago/Turabian StyleMihai, Raluca A., Ramiro Fernando Vivanco Gonzaga, Damián O. Calero Rondal, Dámaris A. Teneda Jijón, Nelson Santiago Cubi Insuaste, Christian D. Borja Tacuri, and Rodica D. Catana. 2025. "Comparative Phytochemical and Biological Profiling of Zea mays L. Varieties in Cotopaxi Region" Agriculture 15, no. 10: 1054. https://doi.org/10.3390/agriculture15101054
APA StyleMihai, R. A., Vivanco Gonzaga, R. F., Calero Rondal, D. O., Teneda Jijón, D. A., Cubi Insuaste, N. S., Borja Tacuri, C. D., & Catana, R. D. (2025). Comparative Phytochemical and Biological Profiling of Zea mays L. Varieties in Cotopaxi Region. Agriculture, 15(10), 1054. https://doi.org/10.3390/agriculture15101054








