Abstract
Ammonium (NH4+) content is one of the most important parameters in manure assessment. The accurate and rapid determination of this inorganic form of nitrogen is therefore important not only in agronomy, when calculating fertilizer application rates, but also in scientific studies, for example, in the study of greenhouse gas emissions from stored manure. There is not enough research to assess which analytical method is the most appropriate for the determination of (NH4+) in manure with additives such as perlite, vermiculite, or peat. We compared three analytical methods for NH4+ determination: distillation, ionometry, and spectrophotometry. The results showed that the distillation method had the lowest average coefficient of variation (Cv) between the two laboratory replicates, with a Cv = 0.77%, while the ionometry and spectrophotometry methods had average Cv values of 1.83% and 3.97%, respectively. A lower coefficient of variation indicates that the analytical method is less sensitive to various interferences, resulting in more reliable data. Experimental data also show that storing manure for 40 days reduces the NH4+ content from about 21,000 ppm to about 7000 ppm and that the use of additives such as perlite, vermiculite, or peat did not significantly affect NH4+ retention compared with control samples (without additives). Based on the results of our study, we recommend using the distillation method for the determination of NH4+ in the manure with additives in agrochemical and scientific laboratories.
1. Introduction
It is widely known that animal manure is an organic fertilizer used to fertilize plants. Manure contains valuable nutrients for plants. Nevertheless, manure can also be the cause of soil, water, and air pollution if handled incorrectly [1,2]. During the entire period of exposure to manure, plants take about 50–60% of the nitrogen contained in it from manure, up to 50% of phosphorus, and up to 80% of potassium, absorbing calcium, magnesium, iron, sulfur, etc. Manure improves the properties of the soil: fertilized heavy soils become more permeable to water and air, and lighter ones do not evaporate so quickly. Manure promotes plant growth, provides nutritious food to soil organisms, adds genetic and functional diversity to soils, and improves chemical and physical soil properties [3,4,5]. More than 1.4 billion t y−1 of manure are generated in the EU and UK and reapplied to soils [6]. Manure is also a highly important source of raw materials for biogas power plants. The yield of biogas also depends on the chemical composition of the manure and changes the chemical composition of the by-product of production (digestate) and its value as a biofertilizer [7].
Manure is not only an effective organic fertilizer, but it can also become a significant source of pollution if storage or fertilization recommendations are not followed. According to the European Union [8], during the year, one hectare of the farm must need so many livestock that the rate of nitrogen contained in their manure does not exceed 170 kg per hectare. If the nitrogen content is higher, then the herd should be reduced.
The theme of organic fertilizers, especially in the development of sustainable agricultural production, is very important and this importance is growing. According to one of the most used databases “Google Scholar”, which provides the broadest range of scientific literature, the number of scientific sources reporting topic of the manure storage and additives increased during half of a century increased since 88 mentions in 1973 to 9230 mentions in 2023 (Figure 1). Growth rates of this indicator have increased drastically in the last two decades and it demonstrates the general importance of the mentioned topic.
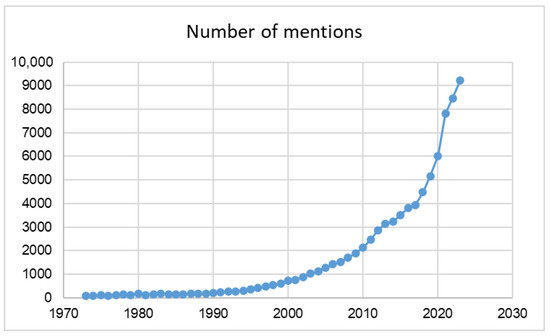
Figure 1.
Google Scholar search results on the topic of manure storage and additives (1973–2023). Note: each point marked on the graph shows the number of sources included by authors mentioned in the corresponding year.
Manure and slurry must be stored in such a way as to avoid contamination of surface water and ground waters as well as reduce air pollution. The method of manure storing affects its composition and quality. Degradation and transformation of organic matter by microbial results in the loss of organic carbon and nitrogen from composting, mainly as Greenhouse gas emissions. The complete oxidative decomposition of organic matter OM by the action of microorganisms produces carbon dioxide (CO2), as well as incomplete decomposition under partial anaerobic conditions produces methane (CH4). During the processes of composting, the organic nitrogen or non-dissolved nitrogen of the raw material, such as proteins and amino acids, is degraded by aerobic microorganisms to dissolved nitrogen, which is eventually degraded to NH4+; then, some of the NH4+ evaporates as NH3, and some of the NH4+ experienced incomplete nitrification and denitrification to produce N2O [9].
The use of animal manure as an organic fertilizer on agricultural soil not only enhances soil health and agricultural productivity, but also has a substantial influence on GHG emissions.
Agriculture represents the main source of anthropogenic ammonia (NH3) emissions [1,10,11]. In total, 81% of global NH3 emissions are a result of agriculture [12]. Applications of livestock manure to agricultural land are a significant source of NH3 emissions to the atmosphere, with potentially harmful impacts on the environment. Moreover, an excessive amount of NH4+ can be toxic to plants [13,14]. In the UK, NH3 losses following land spreading have been estimated at approximately 70 kt (ammonia nitrogen) NH3–N per year, accounting for 30% of the total emission from UK agriculture [15]. The volatilization of NH3 from manure management operations can vary depending on NH3 concentration, temperature, pH, storage time, etc. Determination of the chemical composition of manure in the laboratory must be carried out by reliable methods and urgently. So, the measurement of one of the most important components—NH4+ directly in the manure as well as in digests is one of the more important needs in laboratories conducting agricultural and environmental research [16].
Three main analytical methods are used successfully to measure NH4+ in manure samples (distillation, spectrophotometry, and ionometry). The choice of method depends on the available laboratory equipment and the regulatory documents and standards used in that country and in the institution. The distillation consists of heating an alkaline buffered solution and condensing the vapors into an acidic solution [17]. Peters et al. (2003) summarized three recommended methods for determining NH4+ and they were distillation/titration, colorimetric method, and electrode. Distillation and titration have fewer requirements for pretreatment. Colorimetric methods need centrifuged or filtrated samples. In manure samples, which can contain nitrate-N, the measurement should be included in the analysis. These variously evaluated methods do not demonstrate the suitability of these methods for analyzing manure samples which have in their composition—different sorbents.
The main objective of this study was to compare and statistically validate which of the NH4+ determination methods (distillation, ionometry, and spectrophotometry) was the most suitable for measuring NH4+ content in manure with perlite, vermiculite, and peat additives. Additionally, it aimed to assess the ability of manure additives to adsorb NH4+ and to determine its changes during storage.
2. Materials and Methods
2.1. Description of the Experimental Site
Our experiment consisted of two parallel studies: the determination of NH4+ content and its changes during manure storage using three different detection methods; and the measurement of greenhouse gas emissions. However, greenhouse gas emissions were not analyzed in this paper. These data were used to monitor the experiment’s progress.
The research was conducted under laboratory conditions using 90 L experimental manure tanks. The manure was stored in climate chambers, with microclimate parameters not being artificially regulated. The study was performed with fresh cattle manure. Manure (solid fraction and urine) was collected directly from dairy cattle in the barn and brought to the laboratory. In the laboratory, the solid fraction of the manure was mixed with urine in a ratio of 1.6:1. The manure was homogenized and placed in experimental manure tanks (7 kg per tank). The amount of sorbents was calculated as a percentage of the dry matter content in the manure. Each treatment had 4 replicates.
The experiment lasted for 40 days, with measurements taken until a significant reduction in gas emissions was observed. The manure was kept in climate chambers, where the ambient temperature fluctuated between 11.9 and 16.3 °C, and the relative humidity ranged from 44.9 to 67.5%.
During gas concentration measurements, the tanks with manure were sealed along with gas measuring devices. After each measurement, the tanks were left open to allow the manure to be naturally affected by environmental conditions, ensuring that gas emissions were not artificially suppressed [18]. For the gas concentration measurement period, the temperature and relative humidity of both the environment and the manure were recorded. The emissions of (CO2), (CH4), and (NH3) gasses were measured for evaluation of gas emissions.
The chemical composition of manure samples (n = 32) was determined at the Chemical Research Laboratory, Lithuanian Institute of Agriculture, Lithuanian Research Centre for Agriculture and Forestry. Located in Akademija, Kedainiai district, Lithuania. In total, 4 experiments in 2024 were studied. During the experiment, manure was placed in experimental tanks—control tanks without sorbents, and others with different sorbents and their mixtures. Treatments investigated were as follows: 1. K—manure without additives; 2. D—manure +15% carbon + 15% peat; 3. P—manure +15% carbon + 15% perlite; and 4. V—manure +15% carbon + 15% vermiculite. Four experimental replicates were performed. For our research tasks, manure samples were analyzed before the storage experiment and at the end of the 40-day experiment. The experiments were carried out at the Institute of Animal Science of the Lithuanian University of Health Sciences, located in Baisogala, Radviliskis district, Lithuania. Sampling and manure samples preparation for chemical analyses was performed as follows: manure samples (about 0.3 kg) were taken after homogenizing the manure. The sample is made by taking the homogenized manure from three places in the tank.
2.2. Chemical Analysis of Manure Ammonium
Chemical analyses of manure samples were performed at the Chemical Research Laboratory. Ammonium concentrations were determined using three analytical methods:
(a) by determination of NH4+ using steam distillation Kjeldahl system. Ammonium is predominant when the pH is below 8.75, and NH3 is predominant when the pH is above 9.75 [19]. About 6–7 g of manure sample was weighed in 250 mL FOSS digestion tube, and after that sample was wetted with about 20 mL (the exact amount of water is not important) deionized H2O and carefully mixed. A digestion tube was placed in Kjeltec 2200 steam distillation system as fast as possible, to prevent loss of volatile NH3. The following automated program setting was used for steam distillation: 10 mL of 8.8 N NaOH solution; no addition H2O; 30 mL of boron acid solution (H3BO3) as receiver; distillation time 6 min. NH3 collected after distillation was titrated with 0.1 N H2SO4 acid made from fixanal, using an automatic SI Analytics TitroLine 5000 titrator. The obtained nitrogen content value was expressed as ammonium concentration;
(b) for the potentiometric measurement’s electrochemical analyzer, Consort C6030 and selective NH4+ ion electrode Toshcon TISE-NH4 were used. In total, 4 g of sample was shaken with 100 mL of water for 1 h. According to the electrode manufacturer, 2 mL of 2 M lithium acetate (LiCH3COO) to 100 mL of standard and sample;
(c) for the spectrophotometric measurement UV/Vis Cary 50, Varian Inc spectrophotometer was used. A blue color compound formed by reaction with salicylate, ammonium, and hypochlorite ions in an alkaline solution in the presence of sodium nitroferricyanide was quantified at 655 nm; 1 M potassium chloride (KCl) solution was used for NH4+ extraction (1:2.5, w/v) [20]. In all three analytical methods, the final NH4+ concentration was expressed and used in this work as ppm in dry matter.
2.3. Statistical Analysis
The data were statistically analyzed using SAS Enterprise Guide 7.1. An analysis of variance (ANOVA) was carried out. Significant differences between means 95% (p < 0.05) were used.
3. Results and Discussion
3.1. Comparison of Analytical Methods for the Ammonium Determination in Manure Samples
To select the most appropriate method for the NH4+ determination in the manure with additives, the NH4+ content in the samples was determined by three analytical methods, as described in the methodological section. Usually, spectrophotometry is used as the main method to determine NH4+ content in various samples [21,22]. In our study, each analytical method was performed with two laboratory repetitions to determine repeatability. The NH4+ content results are shown in Table 1.

Table 1.
Comparison of average (n = 2) ammonium (NH4+, ppm, in DM) content in the cattle manure samples with additives determined by different methods.
Analyzing the data presented in Table 1, we can see that there are variations in the concentration of ammonium between two laboratory repetitions of the same sample depending on the method of determination used. The values of the coefficient of variation (Cv%) presented in the table show how strongly each method fluctuates during two laboratory repetitions. The lower these values, the smaller the discrepancies between laboratory repetitions are obtained and the more reliable the method is.
Minimal variation between two laboratory repetitions was observed for the direct distillation method. The coefficient of variation fluctuated from 0.02 to 3.93%, with an average value of 0.77%. Results obtained from ionometric and spectrophotometric measurements were more variable: average coefficient of variation was 1.83% and 3.97%, respectively. The calculated values of the determination coefficient (Figure 2) for each method of analysis showed that direct distillation was characterized by a very high determination coefficient, which reached 0.9995. A slightly lower value of the determination coefficient value: 0.9959 was shown by the ionometric method. On the other hand, the worst correlation of 0.9855 between laboratory repetitions was obtained by the spectrophotometric procedure for analysis of samples. In addition, in this case, the higher measurement differences between repetitions were obtained at NH4+ concentrations higher than 15,000 ppm. We can therefore conclude that the method of direct distillation was less sensitive to external factors that can negatively affect measurement accuracy. According to our data, the results can vary not only when two laboratory repetitions are analyzed, but also when comparing different analytical methods. This may indicate that not all the methods presented here are suitable for the accurate determination of NH4+ in manure with additives. The standard error values given in Table 1 are calculated by comparing all three methods, and the higher this value, the greater the measurement differences between the different methods of analysis. The standard error measured between the different analytical methods ranged from 98 to 4126 NH4+ ppm with an average of 1116 NH4+ ppm. The same is indicated by min and max values: the greater the difference between them, the greater the differences are obtained between different methods. For example, for the determination of the NH4+ content of sample 1-4V using the ionometric method, 16,904 ppm NH4+ was obtained by measuring the ammonium content by direct distillation and spectrophotometry, measured by 25,717 and 31,056 NH4+ ppm (with SE value 4126 NH4+ ppm), respectively. As we see from the results of analyses demonstrated, the measured concentration in the same sample can differ almost twice, depending on which analytical method is used. In addition, there was a tendency for the NH4+ content values measured by ionometry for all 32 samples to be lower compared to the other two methods. However, in the ANOVA test, to check whether there was a statistical difference between the data obtained by these methods of analysis, the calculated value of p was 0.2482, while the values of F and F crit were 1.4144 and 3.0943. These calculations confirm the zero hypothesis, which means that there was no statistically proven difference between the values of the NH4+ content measured by different methods. Like most methods of laboratory analysis, these methods used in our study have their own advantages and disadvantages, which directly affect the accuracy and reliability of the measurement results. Manure samples are often characterized by large quantities of fine water-insoluble organic and inorganic particles, intense color, as well as a large amount of soluble minerals, such as potassium, magnesium, calcium, and others. The mentioned characteristics of manure are important factors that can affect the NH4+ values in the sample. When analyzing manure samples by spectrophotometry, to obtain accurate quantification results, it is necessary to prepare an extract as transparent as possible, without intensive color and turbidity [23]. A solution of 1 M or 2 M KCl is often used for NH4+ extraction in various samples [24,25,26,27,28,29,30]. However, due to the remnants of the feed not being completely digested by the animal in the scandium, extracts of colloids, and dark color are obtained. These two factors mentioned increase the optical density of the solution, which leads to higher readings of the NH4+ concentration. This is precisely the trend that we observed in the data obtained (Figure 3)—the average NH4+ concentrations determined by spectrophotometry were higher compared to the concentrations obtained during direct distillation or ionometry. These two factors mentioned increase the optical density of the solution, which leads to higher readings of the NH4+ concentration. This is precisely the trend that we observed in the data obtained (Figure 3)—the average NH4+ concentrations determined by spectrophotometry were higher compared to the concentrations obtained during direct distillation or ionometry. These negative influences can be somewhat controlled using an ionic strength adjuster (ISA) [31,32,33], but in the presence of large amounts of ions capable of interfering in samples, even the use of ISA cannot always ensure the reliability of the analysis. In addition, ionometric measurements are greatly influenced by the temperature of the sample and standards; therefore, the maintenance of a constant temperature during measurement must be ensured. In contrast to the other two methods of analysis discussed above, the method of direct distillation is less sensitive to extraneous factors. All this follows from the very essence of the analysis. Strongly in an alkaline medium, mNH4+ are deprotonated [19], and the ammonia (NH3) formed is removed from the reaction medium by distillation with water vapor. Therefore, in this method of analysis, the turbidity, intense color, or anions/cations of the sample in the solution do not affect the results of the measurement. As another advantage of direct distillation, one can mention the fact that a larger amount of the laboratory sample under study can be used for this analysis, which gives an advantage over the remaining two analytical methods when is needed to analyze samples of low homogeneity.
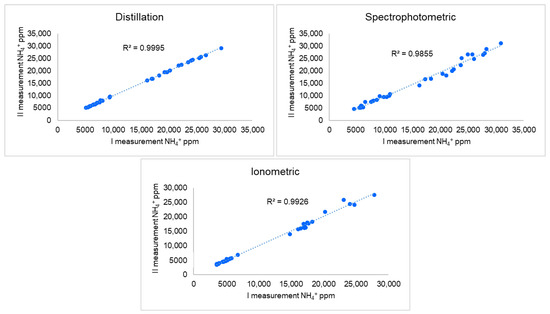
Figure 2.
Coefficient of determination of two laboratory repetitions.
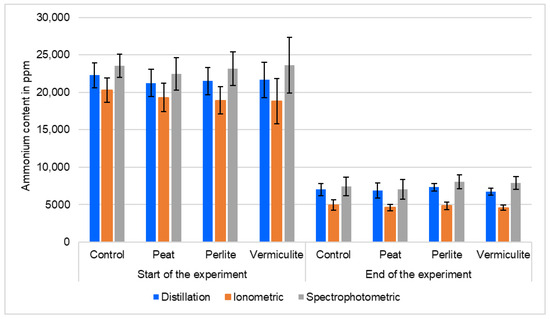
Figure 3.
Manure ammonium (NH4+) content in ppm at the start and end of the experiment. Average of four experimental replicates. Vertical bars represent standard error.
3.2. Effect of Manure Storage Time and Additives on the Change in the Ammonium Content
Figure 3 provides aggregated (Table 1) data. The data in Figure 3 demonstrate that the concentration of ammonium at the end of the experiment (after 40 days) was almost three times lower than at the beginning. Therefore, we can safely say that there was an emission of NH4+ (as ammonia or nitrogen oxides) or its transformation into another form of nitrogen, probably due to microbiological or chemical processes.
To give a more accurate estimate of the NH4+ reduction during the experiment, Figure 4 is presented. This figure shows the difference between the initial NH4+ concentration of the same sample and the NH4+ concentration of that sample at the end of the experiment. The more negative the number, the greater the NH4+ loss. Calculations were made for all three analytical methods used: distillation, ionometry, and spectrophotometry. As can be seen from the data presented, the greatest reduction in NH4+ content for all the additives analyzed was recorded using the spectrophotometric method. Meanwhile, the other analytical methods (distillation and ionometry) showed similar results between them.
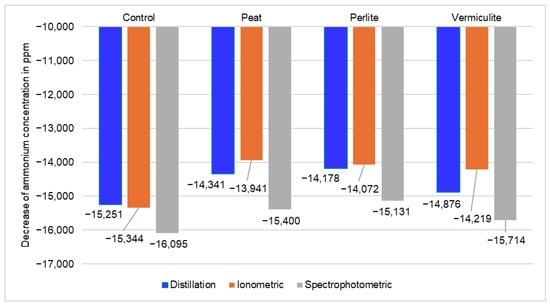
Figure 4.
Ammonium (NH4+) content decrease determined during the experiment from manure (control) and manure with sorbents. Average of four experimental replicates.
Looking at the average values of the four experimental replicates from the point of view of additives, the control and vermiculite showed very similar reductions in NH4+ content during storage. In contrast, peat and perlite showed slightly lower NH4+ losses, which may indicate better absorption properties of these additives (sorbents).
A two-way ANOVA analysis was used to assess whether this decrease in NH4+ concentrations could have been influenced by added substances (activated carbon, peat, perlite, or vermiculite), or due to random factors. One factor of investigation was time (the beginning of the experiment, the end of the experiment), and the other—the kind of additives to the manure. This mentioned method of statistical estimation has been applied to all analytical methods of determining the NH4+ content: direct distillation, ionometry, and spectrophotometry, to verify that all these methods of analysis have given the same trends in the change in NH4+ content in manure with additives.
Based on the results of the ANOVA analysis (Table 2), it can be seen that regardless of the method of analysis used to determine the NH4+ content in the samples, the time factor played a key role. The most obvious differences in the concentration of NH4+ were when comparing measurements at the beginning and end of the experiment; that is, a significant decrease in NH4+ content was observed. Using different methods of laboratory analysis, the p-value fluctuated between 5.05 × 10-11and 1.8 × 10-12, while F values were between 125.78 and 172.87, and the F crit value of 4.260 exceeded significantly.

Table 2.
Two-factor ANOVA test results for different ammonium (NH4+) determination methods.
However, the situation is somewhat different when assessing the influence of additives on the accumulated amount of NH4+. In this case, regardless of the analytical method used, F crit’s value of 3.009 was greater than the values of F, which ranged from 0.070 to 0.110. This indicates that no statistically significant differences were observed between the different manure additives used and the accumulated NH4+ content in the manure at the end of the experiment. The zero hypothesis is also confirmed by the high P-value, which averaged 0.961. Some studies show that slurry treatment technologies in the form of slurry additives represent an under-utilized means of reducing gaseous emissions and preserving the nutrient content of stored manures [34]. The results of our study demonstrate that the manure additives used (perlite and carbon, carbon and peat, as well as carbon and vermiculite) were not particularly effective at the time of the study and did not significantly reduce the loss of NH4+ content from the manure during storage. Nevertheless, during the course of the experiment, trends in the reduction of NH4+ loss during manure storage using sorbents were recorded.
4. Conclusions
To find the most effective method for the NH4+ content determination in manure with additives (sorbents), three analytical methods were compared: distillation, ionometry, and spectrophotometry. In the case of the spectrophotometry method, the turbidity and intense color of the sample have the greatest influence which increases the optical density of the solution. Also, this method of analysis requires higher time, reagent costs, and relatively expensive laboratory equipment. The cost of ionometry analysis equipment is relatively lower, but there are difficulties due to the very uneven ionic strength of the analyzed samples, as well as a large amount of interfering ions, such as potassium, magnesium, calcium, and others. In our research, laboratory repetitions of the same sample carried out using the distillation method differed the least, as shown by the lowest coefficient of variation. It can be concluded that the distillation method was the least sensitive to interfering factors, and this makes it possible to obtain more accurate results. The results of the ANOVA analysis did not show a significant statistical difference between the three methods of analysis: the value of P-value reached 0.2482, and the values of F and Fcrit were 1.4144 and 3.0943, respectively, which means that all three methods studied were valid and suitable for the determination of the NH4+ content, despite the fluctuations between the readings of different methods. Summarizing the results obtained, we recommend using the water vapor distillation method for the determination of NH4+ content in manure with additives, due to its quickness, simplicity, and a better match between laboratory repetitions.
During the experiment, when storing manure with different additives, after 40 days, a significant decrease (almost three times) in the content of NH4+ was observed. Results of our study demonstrate that the manure additives we used such as: peat, vermiculite, and perlite were not very effective in reducing NH4+ losses.
Further research is needed to examine the efficacy of more types of manure additives (sorbents), which can reduce the release of NH4+ from manure, as well as study the gas release process during different types of manure storage. Reducing the loss of NH4+ during manure storage would be an important factor in reducing air pollution and the overall quality of the environment.
Author Contributions
Conceptualization, I.P., V.J.; methodology, V.J. and I.P.; software, I.P.; validation, I.P., V.J., and A.Š.; formal analysis, I.P. and A.Š.; investigation, V.J.; data curation, I.P. and V.J.; writing—original draft preparation, I.P.; writing—review and editing, V.J., A.Š., and G.K.; visualization, I.P., A.Š.; supervision, A.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cattaneo, M.; Tayà, C.; Burgos, L.; Morey, L.; Noguerol, J.; Provolo, G.; Cerrillo, M.; Bonmatí, A. Assessing Ammonia and Greenhouse Gas Emissions from Livestock Manure Storage: Comparison of Measurements with Dynamic and Static Chambers. Sustainability 2023, 15, 15987. [Google Scholar] [CrossRef]
- Qi, J.; Yang, H.; Wang, X.; Zhu, H.; Wang, Z.; Zhao, C.; Li, B.; Liu, Z. State-of-the-Art on Animal Manure Pollution Control and Resource Utilization. J. Environ. Chem. Eng. 2023, 11, 110462. [Google Scholar] [CrossRef]
- Bilong, E.G.; Abossolo-Angue, M.; Nanganoa, L.T.; Anaba, B.D.; Ajebesone, F.N.; Madong, B.À.; Bilong, P. Organic Manures and Inorganic Fertilizers Effects on Soil Properties and Economic Analysis under Cassava Cultivation in the Southern Cameroon. Sci. Rep. 2022, 12, 20598. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Liptzin, D.; Maharjan, B. Long-Term Manure Application Improves Soil Health and Stabilizes Carbon in Continuous Maize Production System. Geoderma 2023, 430, 116338. [Google Scholar] [CrossRef]
- Fu, Y.; de Jonge, L.W.; Moldrup, P.; Paradelo, M.; Arthur, E. Improvements in Soil Physical Properties after Long-Term Manure Addition Depend on Soil and Crop Type. Geoderma 2022, 425, 116062. [Google Scholar] [CrossRef]
- Köninger, J.; Lugato, E.; Panagos, P.; Kochupillai, M.; Orgiazzi, A.; Briones, M.J.I. Manure Management and Soil Biodiversity: Towards More Sustainable Food Systems in the EU. Agric. Syst. 2021, 194, 103251. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Lončarić, Z.; Jović, J.; Samac, D.; Popović, B.; Tišma, M. Digestate Management and Processing Practices: A Review. Appl. Sci. 2022, 12, 9216. [Google Scholar] [CrossRef]
- European Union Council Directive 91/676/EEC of 12 December 1991. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31991L0676 (accessed on 10 November 2024).
- Huang, D.; Gao, L.; Cheng, M.; Yan, M.; Zhang, G.; Chen, S.; Du, L.; Wang, G.; Li, R.; Tao, J.; et al. Carbon and N Conservation during Composting: A Review. Sci. Total Environ. 2022, 840, 156355. [Google Scholar] [CrossRef]
- Kupper, T.; Häni, C.; Neftel, A.; Kincaid, C.; Bühler, M.; Amon, B.; VanderZaag, A. Ammonia and Greenhouse Gas Emissions from Slurry Storage—A Review. Agric. Ecosyst. Env. 2020, 300, 106963. [Google Scholar] [CrossRef]
- Sommer, S.G.; Hafner, S.D.; Laubach, J.; van der Weerden, T.J.; Leytem, A.B.; Pacholski, A. Model for Calculating Ammonia Emission from Stored Animal Liquid Manure. Biosyst. Eng. 2022, 223, 41–55. [Google Scholar] [CrossRef]
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia Emissions from Agriculture and Their Contribution to Fine Particulate Matter: A Review of Implications for Human Health. J. Environ. Manag. 2022, 323, 116285. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of Ammonium Toxicity and the Quest for Tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed]
- van der Eerden, L.J.M. Toxicity of Ammonia to Plants. Agric. Environ. 1982, 7, 223–235. [Google Scholar] [CrossRef]
- Misselbrook, T.H.; Nicholson, F.A.; Chambers, B.J. Predicting Ammonia Losses Following the Application of Livestock Manure to Land. Bioresour. Technol. 2005, 96, 159–168. [Google Scholar] [CrossRef]
- Baethgen, W.E.; Alley, M.M. A Manual Colorimetric Procedure for Measuring Ammonium Nitrogen in Soil and Plant Kjeldahl Digests. Commun. Soil. Sci. Plant Anal. 1989, 20, 961–969. [Google Scholar] [CrossRef]
- Peters, J.; Combs, S.; Hoskins, B.; Jarman, J.; Kovar, J.; Watson, M.; Wolf, A.; Wolf, N. Recommended Methods of Manure Analysis; University of Wisconsin Cooperative Extension Publishing: Madison, WI, USA, 2003; pp. 25–29. [Google Scholar]
- Wang, J.; Duan, C.; Ji, Y.; Sun, Y. Methane Emissions during Storage of Different Treatments from Cattle Manure in Tianjin. J. Environ. Sci. 2010, 22, 1564–1569. [Google Scholar] [CrossRef]
- Molins-Legua, C.; Meseguer-Lloret, S.; Moliner-Martinez, Y.; Campíns-Falcó, P. A Guide for Selecting the Most Appropriate Method for Ammonium Determination in Water Analysis. TrAC Trends Anal. Chem. 2006, 25, 282–290. [Google Scholar] [CrossRef]
- Jurgutis, L.; Šlepetienė, A.; Amalevičiūtė-Volungė, K.; Volungevičius, J.; Šlepetys, J. The Effect of Digestate Fertilisation on Grass Biogas Yield and Soil Properties in Field-Biomass-Biogas-Field Renewable Energy Production Approach in Lithuania. Biomass Bioenergy 2021, 153, 106211. [Google Scholar] [CrossRef]
- Utomo, W.P.; Wu, H.; Ng, Y.H. Quantification Methodology of Ammonia Produced from Electrocatalytic and Photocatalytic Nitrogen/Nitrate Reduction. Energies 2022, 16, 27. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, J.; Yuan, D.; Yang, Z.; Shi, X.; Li, H.; Jin, H.; Ran, L. Development of Analytical Methods for Ammonium Determination in Seawater over the Last Two Decades. TrAC Trends Anal. Chem. 2019, 119, 115627. [Google Scholar] [CrossRef]
- Penzel, S.; Mayer, T.; Goblirsch, T.; Borsdorf, H.; Rudolph, M.; Kanoun, O. A Novel Turbidity Compensation Method for Water Measurements by UV/Vis and Fluorescence Spectroscopy. Measurement 2025, 239, 115447. [Google Scholar] [CrossRef]
- Nelson, D.W. Determination of Ammonium in KCl Extracts of Soils by the Salicylate Method. Commun. Soil Sci. Plant Anal. 1983, 14, 1051–1062. [Google Scholar] [CrossRef]
- Kozloski, G.V.; Senger, C.C.D.; Perottoni, J.; Sanchez, L.M.B. Evaluation of Two Methods for Ammonia Extraction and Analysis in Silage Samples. Anim. Feed. Sci. Technol. 2006, 127, 336–342. [Google Scholar] [CrossRef]
- Holmboe, N.; Kristensen, E. Ammonium Adsorption in Sediments of a Tropical Mangrove Forest (Thailand) and a Temperate Wadden Sea Area (Denmark). Wetl. Ecol. Manag. 2002, 10, 453–460. [Google Scholar] [CrossRef]
- Dorich, R.A.; Nelson, D.W. Direct Colorimetric Measurement of Ammonium in Potassium Chloride Extracts of Soils. Soil Sci. Soc. Am. J. 1983, 47, 833–836. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, H.; Yuan, W.; Williams, D.; Walker, J.T.; Shi, W. Is Biochar-Manure Co-Compost a Better Solution for Soil Health Improvement and N2O Emissions Mitigation? Soil Biol. Biochem. 2017, 113, 14–25. [Google Scholar] [CrossRef]
- Abid, A.A.; Yu, S.; Zou, X.; Batool, I.; Castellano-Hinojosa, A.; Wang, J.; Li, D.; Zhang, Q. Unraveling Nitrogen Loss in Paddy Soils: A Study of Anaerobic Nitrogen Transformation in Response to Various Irrigation Practice. Environ. Res. 2024, 252, 118693. [Google Scholar] [CrossRef]
- Kachurina, O.M.; Zhang, H.; Raun, W.R.; Krenzer, E.G. Simultaneous Determination of Soil Aluminum, Ammonium- and Nitrate-nitrogen Using 1 M Potassium Chloride Extraction. Commun. Soil Sci. Plant Anal. 2000, 31, 893–903. [Google Scholar] [CrossRef]
- Available online: Https://Www.Hach.Com/p-Ammonium-Ionic-Strength-Adjustor-Isa-Pillows-Pk100/2980699?Srsltid=AfmBOopg3WgZlDUm59xXsmeJ8rugzJH2PU3RabQuk4EB-Vv3vlr1hJeo (accessed on 20 October 2023).
- Oh, S.Y.; Sung, H.K.; Shin, H.H.; Jeong, U.; Eom, I.; Kim, P.; Kim, Y. Effect of Ionic-Strength Adjusters on the Detection of Silver Ion Using Ion-Selective Electrode. Korean J. Chem. Eng. 2015, 32, 1924–1927. [Google Scholar] [CrossRef]
- Frant, M.; Ross, J.W. Use of a Total Ionic Strength Adjustment Buffer for Electrode Determination of Fluoride in Water Supplies. Anal. Chem. 1968, 40, 1169–1171. [Google Scholar] [CrossRef]
- Thorn, C.E.; Nolan, S.; Lee, C.S.; Friel, R.; O’Flaherty, V. Novel Slurry Additive Reduces Gaseous Emissions during Storage Thereby Improving Renewable Energy and Fertiliser Potential. J. Clean. Prod. 2022, 358, 132004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).