Abstract
The reclamation of post-mining land for agricultural purposes has continued to be a big challenge. Our study concerns the use of soil microfauna (nematodes) and mesofauna (mites and springtails) as indicators of soil quality after 6 years of agricultural reclamation of a post-mining area in west–central Poland. A new method, which involves rotation growing of industrial hemp (H) and alfalfa (A) and incorporating the resulting biomass into the soil, was used to reclaim two sites (5 and 15 years after mining) representing different types of post-mining deposits (clayey and sandy). On each site, two plots were established, where each crop was grown for three years, but in a different order during the rotation cycle (3H3A and 3A3H). The results showed significant differences in the abundance and structure of the fauna communities between 3H3A and 3A3H reclamation practices, as well as between the reclaimed plots and non-reclaimed (NR) plots, where spontaneous succession proceeded. The three animal groups were more abundant in the reclaimed soil compared to the NR soil. The highest densities for nematodes were observed in the 3H3A plots and for the mesofauna in the 3A3H plots. The reclamation practices had a positive effect on groups involved in the regulation of C and N mineralisation, particularly bacterial- and hyphal-feeding nematodes and oribatid mites, and a negative effect on plant-feeding nematodes and euedaphic collembolans. The finding that most of the parameters of the studied biota had values resembling those of agricultural soils after 6 years of reclamation clearly indicates the effectiveness of the applied practices for transforming degraded land into soils that mirror soils under agricultural use.
1. Introduction
Post-mining waste management plans and the reclamation of areas degraded by coal mining are mandatory steps and one of the most important mine activities after the cessation of mining. However, the transformation of degraded land into a fully functional and stable ecosystem seems to be a big challenge [1]. Agricultural reclamation is one of the most demanding methods of reclamation, as it requires restoration of the proper soil structure and improvement of its physicochemical and biological properties through agrotechnical treatments. The primary challenge in agricultural reclamation is restoration of the humus layer, which determines soil fertility and affects plant growth and development [2,3]. The main source of humus is soil organic matter, whose content, however, in most post-mining substrates is very low [2,4,5].
Selection of appropriate revegetation crops yielding high biomass, as well as appropriate crop rotation, can help rebuild soil organic matter, while agrotechnical procedures such as ploughing, fertilisation, etc. can accelerate the processes of its transformation (mineralisation and humification) and influence the biological activity of the reclaimed post-mine soil [6,7,8].
Currently, agricultural reclamation of degraded land is carried out by growing a limited number of crops, which are then used for energy, food, and fodder [9]. However, the agricultural reclamation practices used so far do not give satisfactory yield results. Soil devastated by excavation, with uneven composition and deficiencies in some important nutrients, is not suitable for the production of food or fodder crops. Due to frequent disease and pest infestation, such crops do not provide complete food for humans or animals [3]. In addition, some plants, such as miscanthus, energy willow, or kenaf, require high cultivation inputs and have been found to be dependent on climatic conditions [3].
A new method for the reclamation of this post-mining land by cultivating industrial hemp in rotation with alfalfa was implemented in the frame of the project LIFE11 ENV/PL/445 EKOHEMPKON, carried out in the period 2013–2018 [3]. Alfalfa in rotation with other plants, such as cereals, has often been used in the reclamation of post-mining or degraded areas [10,11,12], while its rotation with hemp was used for the first time in the EKOHEMPKON project.
Both plant species (industrial hemp (Cannabis sativa) and alfalfa (Medicago sativa)) were chosen because they provide a high biomass yield and have a well-developed root system. Hemp, an annual plant, can easily be included in crop rotation and its cultivation does not require the use of pesticides, as it is naturally disease- and pest-resistant, inhibits the growth of weeds due to its rapid growth yields, and is less dependent on weather conditions. Hemp is one of the fibre plants most frequently used in phytoremediation due to its high tolerance to soil contamination by heavy metals [13,14]. However, the possibility of using it in post-mining land reclamation or as an element of crop rotation is poorly documented in the literature [2,3,15].
The main assumption of the EKOHEMPKON project was that when ploughed into the soil, hemp fibre, producing a large amount of biomass, would enrich the soil in cellulose and contribute to faster restoration of the humus layer, while alfalfa, a plant living in symbiosis with nodule bacteria, would enrich the soil with nitrogen and accelerate the decomposition and mineralisation processes of organic matter. The results verified these assumptions [3,15]. During the six years of reclamation, a gradual increase in soil humus level and nutrients was observed. As the physical and chemical attributes of the soil on the reclaimed sites improved, they became progressively more productive over time [3,15].
A fundamental component of ecological restoration is the recovery of soil fauna, but despite this, few studies address this topic [16]. Soil fauna is crucial to soil formation, litter decomposition, nutrient cycling, biotic regulation, and the promotion of plant growth [17]. Meanwhile, plants influence soil communities through their input of litter and root exudates, mutualistic relationships, and habitat formation [18]. However, the EKOHEMPKON project and previous work [3,15] from the same sites did not explore how the soil faunal communities developed during the reclamation period. Therefore, our research carried out in the last (i.e., sixth) year of reclamation was focused primarily on microfauna (nematodes) and mesofauna (collembolans and mites), which are among the soil groups most often used as bioindicators. They can both influence and reflect changes taking place in the soil ecosystem. Nematodes in particular are a key group of soil invertebrates represented in all heterotrophic levels of the soil food web. The trophic structure of nematode communities is mainly determined by the availability of nutrients in the soil, and hence changes in the structure of the nematode community can be used to assess the ecological condition of the soil [19,20,21].
The aim of our study was to find out how the abundance and community structure of nematodes and microarthropods (mites and collembolans) reflected the state of the biological activity and processes in post-mining soil after six years of reclamation. The more specific objectives of the study were to evaluate the effect of (i) the plant species used in reclamation and (ii) the different reclamation schemes (a sequence of plant species in the six-year rotation cycle) on nematode, collembolan, and mite communities.
2. Materials and Methods
2.1. Study Area
The two research sites were located in the Kazimierz open pit (belonging to the Konin lignite mine) near the town of Kazimierz Biskupi (central–west Poland), where mining activity ended in 1997 (site I, 52°20′13.3″ N 18°06′19.0″ E) and in 2008 (site II, 52°20′47.5″ N 18°08′14.3″ E). On these sites, agricultural reclamation began in 2013 on a total area of 25 ha. Each site encompassed three plots: two plots where reclamation was carried out by growing industrial hemp (H) or alfalfa (A), and one non-reclaimed plot (NR) where spontaneous succession proceeded. In 2016, after three years of vegetation, at each reclaimed plot, crop rotation took place: the plot on which the alfalfa was earlier cultivated was subsequently sown for hemp growing (3A3H), and the alfalfa was sown in the plot previously under hemp (3H3A). The site characteristics and reclamation practices are presented in Table 1. Each year after the growing season, the plants were mown and the whole biomass was ploughed into the soil [3]. Every spring, fertilizers were applied in the following doses: phosphorus (150 kg/ha), potassium (215 kg/ha), and nitrogen (150 kg N/ha to hemp, while nitrogen fertilisation was not used for alfalfa). Hemp (Polish cultivar Białobrzeskie), an annual plant, was sown every year—40 kg/ha [3].

Table 1.
Characteristics of the sampling sites and reclamation practices.
2.2. Soil Sampling and Methods
Soil samples were collected at the end of June 2018. For physicochemical, microbiological, and nematode analyses, samples were taken with a steel soil corer (with an area of 2.5 cm2) and for mesofauna with a corer (with an area of 5 cm2) to a depth of 15 cm. For each type of analysis, five samples were taken from each plot.
The soil pH was determined with the potentiometric method using a soil-to-water ratio of 1:2.5 (w/v), and soil water content (WC%) was measured using the gravimetric method [22,23]. The soil organic matter (SOM) was calculated from the loss on ignition (LOI) after heating at 550 °C for 5 h, with SOMLOI% calculated as the difference between the oven-dry weight before and after ignition and related to the oven-dry soil [24]. The total nitrogen (TN%) and total organic carbon (TOC%) content were determined by the dry combustion technique with an automatic CHNS analyser (Thermo Scientific Flash Smart Elemental Analyzer, Thermo Fisher Scientific Inc., Bremen, Germany) according to the manufacturer’s method [25]. The C/N ratio was calculated as the ratio of the mass of organic carbon (TOC%) to the mass of total nitrogen (TN%).
Soil enzyme activities were measured using spectrophotometric methods. Dehydrogenase (EC 1.1.1.1) activity (DHA) was determined according to the Casida procedure [26]. The reaction product, triphenyl formazan (TPF), was expressed in μg TPF g−1 soil h−1. L-asparaginase (EC 3.5.1.1) activity (L-ASN) was measured with L-asparagine as the substrate in a phosphate buffer at pH 7.6. L-asparaginase activity was measured as the amount of ammonia released during the reaction with Nessler’s reagent and expressed in μg of NH4+ g−1 soil h−1 [27]. Invertase (EC 3.2.1.26) activity (INV) was assayed according to the Hoffman and Pallauf method [28]. The amount of glucose (reaction product) as a result of sucrose hydrolysis was expressed in mg glucose g−1 soil h−1.
Nematodes were extracted from the soil using a modified Baermann method [29] then heat-killed and preserved in 4% formaldehyde. The extracted nematodes were counted, and in each sample, all or when available, 100 randomly selected individuals were identified at the genus level under a light microscope (400×) according to the keys of Goodey [30], Andrássy [31], and Bongers [32]. All identified nematodes were assigned to a trophic group (plant feeders, hyphal feeders, bacterial feeders, omnivores, or predators) as per Yeates et al. [33]. The nematode analysis included total abundance, relative abundance of nematode trophic groups, and nematode generic richness and dominance.
Mites (Acari) and springtails (Collembola) were extracted from the soil for seven days using a Macfadyen high-gradient extractor [34]. The extracted animals were preserved in 70% ethyl alcohol and then submerged in Hoyer fluid [35] to prepare permanent microscopic slides. All the animals were counted and identified. Taxonomic identification of mites to order level was performed based on the studies of Baker and Wharton [36]. Springtails were assigned to ecological groups (epedaphic, hemiedaphic, and euedaphic) according to their vertical stratification following Chernova [37].
2.3. Statistical Analysis
One-way ANOVA was conducted to test the effect of reclamation practices on the abundance of the studied faunal groups and the soil enzyme activity. Due to the considerable differences between the sites (see Table 1), the analyses were conducted separately for each site. The differences between the means were tested by using Tukey’s honest significant difference (HSD) post hoc test at a significance level of p < 0.05. Owing to the lack of normal distribution for some data (density of micro- and mesofauna and enzymatic activity), Box–Cox or square-root transformation was applied. One-way ANOVA and data transformation were performed using STATISTICA ver. 13.3 (TIBCO Software Inc., Palo Alto, CA, USA, 1984–2017). Chi-squared tests (STATISTICA software) were used to compare the relative abundance of the nematode and springtail ecological groups.
In addition, principal component analysis (PCA), implemented using the CANOCO 5.0 software package [38], was selected as the most appropriate ordination method of animal abundance (total and ecological groups) and soil properties across the different sampling plots.
3. Results
3.1. Soil Physicochemical Properties
On site I, the soil pHH2O ranged from neutral in the 3A3H plot to slightly alkaline in the 3H3A and non-reclaimed (NR) plots (Table 2). The soil pH in the 3A3H plot was significantly lower than in the other two plots. On site II, the soil pH in all plots was moderately alkaline, and no significant differences between the plots were found.

Table 2.
Soil physicochemical properties in the reclaimed (3A3H, 3H3A) and NR plots. The different lower-case letters in the rows represent significant differences between the means on each site separately. For more information about the sites (I and II) and the reclamation practices, see Table 1. Abbreviations: WC—water content, SOM—soil organic matter, TOC—total organic carbon, TN—total nitrogen, C:N—carbon:nitrogen ratio.
The SOM content (SOMLOI%) ranged from 3.38% to 4.56% on site I and from 1.97% to 2.50% on site II. On site I, the SOMLOI% did not differ between the plots, while on site II, significantly more organic matter was found in the NR soil compared to the 3H3A plot (Table 2).
The content of the soil organic carbon (TOC%) was similar in all plots on both site I and site II. On site I, the highest total nitrogen content (TN%) was found in the 3H3A plot, and it was significantly higher (p < 0.05) than in the 3A3H and NR plots. On site II, the TN% was similar in all the plots (Table 2).
In both sites, the soil in the reclaimed plots (especially in the 3H3A plot) was characterised by a narrower C:N ratio compared to the NR plots (Table 2).
3.2. Soil Enzymatic Activity
The ANOVA results indicated a significant influence of the reclamation on the activity of the three analysed enzymes (dehydrogenase, L-asparaginase, and invertase) on site I and of the invertase on site II (Table 3).

Table 3.
Results of the one-way ANOVA for the overall effect of reclamation (non-reclaimed, 3A3H, 3H3A) on the abundance of the selected soil fauna groups and enzyme activity. The significant effects (p < 0.05) are in bold text. For more information about the sites (I and II) and the reclamation practices, see Table 1.
On site I, the highest dehydrogenase (DHA) and L-asparaginase (L-ASN) activity was found in the soil of plot I 3H3A, and it was significantly higher (p < 0.05) than the activity in the I 3A3H plot and the I NR plot (Figure 1A,B). No significant differences between the activity of these enzymes between the I 3A3H plot and the NR plot were noticed (Figure 1A,B).
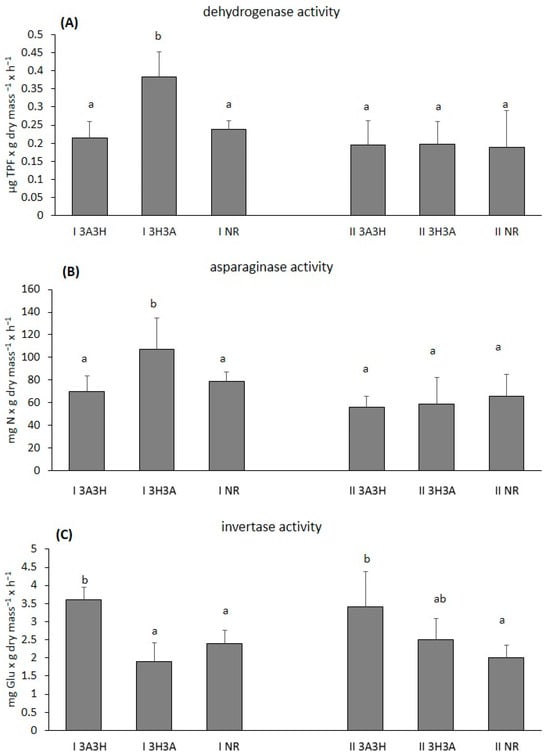
Figure 1.
Soil dehydrogenase (A), L-asparaginase (B) and invertase activity (C) at the reclaimed (3A3H, 3H3A) and non-reclaimed (NR) post-mine plots. The different lower-case letters represent significant differences between the means of the plots at each site separately according to Tukey’s honest significant difference test. The values are means + SD (n = 5). For more information about the sites (I and II) and the reclamation practices, see Table 1.
The highest activity of invertase (INV) was found in the I 3A3H plot, and it was significantly higher (p < 0.05) than the I NR and I 3H3A plots (Figure 1C).
3.3. Microfauna (Nematoda) Abundance and Community Structure
3.3.1. Nematode Density
The nematode densities in the study ranged from 4–6 × 105 ind. m−2 in the NR and 3A3H plots to 10–12 × 105 ind. m−2 in the 3H3A plots. The ANOVA results indicated a significant influence of the reclamation practices on the total nematode density and on some trophic groups (Table 3).
On both sites, the nematode communities were most abundant in the soil of the 3H3A plots (Figure 2A). On site I, the nematode communities were denser in the soil under reclamation than in the NR soil. However, the only significant difference (p < 0.05) was found between the I 3H3A and I NR plots. No differences in nematode density were observed between the reclaimed I 3H3A and I 3A3H plots.
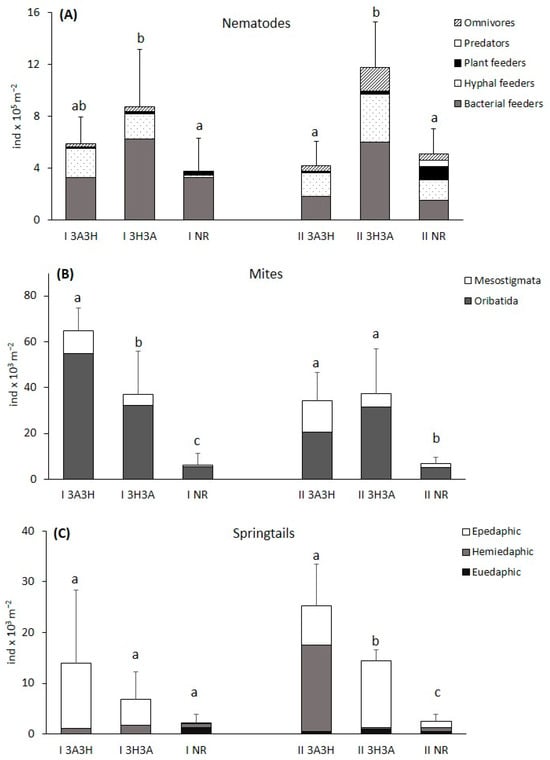
Figure 2.
Density of (A) micro- and (B,C) mesofauna (total and ecological groups) at the reclaimed (3A3H, 3H3A) and non-reclaimed (NR) post-mine plots. The different lower-case letters represent significant differences between the total means of the plots at each site separately according to Tukey’s honest significant difference test. The values are means + SD (n = 5). For more information about the sites (I and II) and the reclamation practices, see Table 1.
The density of bacterial feeders on site I did not differ significantly between the plots, but hyphal feeders were significantly more abundant (p < 0.05) in the reclaimed soil (the I 3H3A and I 3A3H plots) compared to the NR soil (Figure 2A).
On site II, the nematode densities (total, bacterial, fungal feeders, and omnivores) were significantly higher (p < 0.05) in the II 3H3A plot compared to the II 3A3H and II NR plots. In the II NR plot, we found significantly more (p < 0.05) plant feeders than in any of the reclaimed plots.
No predatory nematodes were found in the reclaimed plots on either site. A few predators were noticed only in the NR plots.
3.3.2. Nematode Trophic Structure
On site I, bacterial feeders constituted the largest percentage of the nematode community in all the plots, ranging from 56% in the I 3A3H plot to 87% in the I NR plot (Figure 3A). In both reclaimed plots, the relative abundance of bacterial feeders was lower, while that of hyphal feeders was significantly higher (p < 0.001) than in the I NR plot. Additionally, in the I 3A3H plot, the percentage of hyphal feeders was significantly higher (p < 0.05) than in the I 3H3A plot.
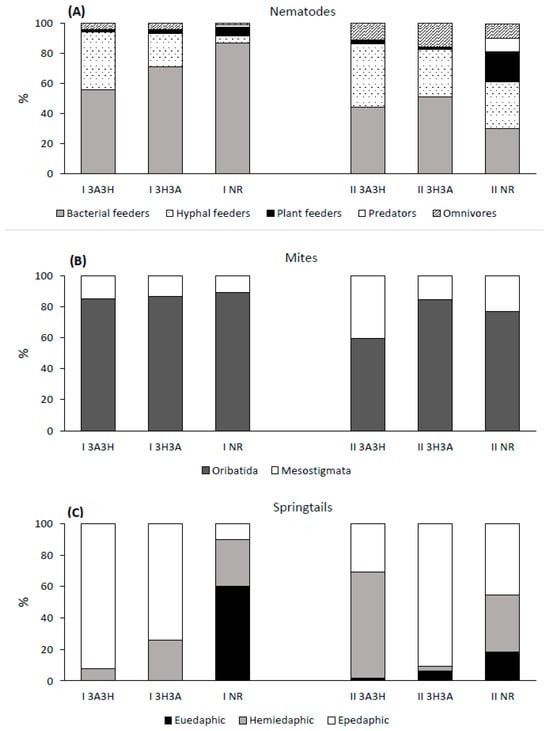
Figure 3.
Relative abundance of (A) micro- and (B,C) mesofauna ecological groups at the reclaimed (3A3H, 3H3A) and non-reclaimed (NR) post-mine plots. For more information about the sites (I and II) and the reclamation practices, see Table 1.
On site II, bacterial and hyphal feeders had very similar percentages in the II 3A3H and II NR plots, while in the II 3H3A plot, bacterial feeders prevailed over the hyphal feeders (Figure 3A). The relative abundance of the two above-mentioned trophic groups did not differ significantly between the plots. In the two reclaimed plots, the relative abundance of plant feeders was significantly lower (p < 0.001), and the relative abundance of omnivores was slightly higher than in the II NR plot.
3.3.3. Nematode Generic Richness and Dominance Structure
A total of 37 nematode genera were recorded in the study. At site I, more nematode genera (18 genera) were found in the reclaimed I 3H3A and I 3A3H plots than in the I NR plot (12 genera). At site II, the same number (20) of nematode genera was found in all the plots.
Of all nematodes, bacterial and hyphal feeders were the dominant taxa (Table 4). However, the dominant genera differed among the plots. At site I, a very high relative abundance (above 20%) was noted for the bacterial feeders (Bf) Acrobeloides and Prismatolaimus in the I NR plot and for Panagrolaimus and Acrobeloides in the I 3H3A plot. In the I 3A3H plot, two bacterial feeders (Bfs) (Panagrolaimus and Plectus) and two hyphal feeders (Hfs) (Aphelenchus and Aphelenchoides) showed high proportions (above 10%) in the communities (Table 4). At site II, Panagrolaimus (Bf) and Aphelenchoides (Hf) were the most dominant genera at the two reclaimed plots, while at the II NR plot, high proportions were indicated for Ditylenchus (Hf), Paratylenchus (plant feeder), and Prismatolaimus (Bf).

Table 4.
Nematode genera with ≥5% share of the total nematode communities at the reclaimed (3A3H, 3H3A) and non-reclaimed (NR) post-mine plots. Trophic groups abbreviations: Bf−bacterial feeder, Hf−hyphal feeder, Pf−plant feeder, Om−omnivorous, Pr−predator. For more information about the sites (I and II) and the reclamation practices, see Table 1.
3.4. Mesofauna (Acari and Collembola) Abundance and Community Structure
Both groups of microarthropods were influenced by the reclamation practices (Table 3). They reached significantly higher total densities in the reclaimed plots than in the NR ones (Figure 2B,C). Thus, while the mite densities of the NR plots slightly exceeded 6 × 103 ind. m−2, in the reclaimed plots, their density was 6 to even more than 10 times higher. Mite numbers ranged between 34 × 103 ind. m−2 in the II 3A3H plot to more than 64 × 103 ind. m−2 in the I 3A3H plot.
When the mite orders were examined, it was found that on site I, the average densities of Oribatida and Mesostigmata were significantly higher (p < 0.05) in the I 3A3H plot than in the I 3H3A and I NR plots, while on site II, the Oribatida densities in both reclaimed plots were significantly higher compared to the II NR plot (Figure 2B).
On both sites and plots, mites from the Oribatida order constituted the largest percentage of the communities, ranging from 60% in the II 3A3H plot to 90% in the I NR plot (Figure 3B). At site I, the relative abundance of Oribatida and Mesostigmata was similar in the three plots. At site II, the percentage of Oribatida in the II 3H3A plot was significantly higher (p < 0.05) than in the II 3A3H plot, while the percentage of Mesostigmata in the II 3A3H plot was significantly higher (p < 0.05) compared to the II 3H3A and II NR plots (Figure 3B).
The average density of springtails in the NR plots (I NR and II NR) was about 2 × 103 ind. m−2, while in the reclaimed plots it was higher, ranging between 8 × 103 ind. m−2 in the I 3H3A plot and 25 × 103 ind. m−2 in the II 3A3H plot (Figure 2C). However, the only significant differences in the density between reclaimed and NR plots were found on site II, where the density of springtails was also significantly higher (p < 0.05) at the 3A3H plot than at the 3H3A plot. As for the ecological groups, on both sites, significantly more epedaphic springtails were found in both reclaimed plots compared to the NR plots (Figure 2C).
Clear differences in the percentages of the three springtail ecological groups between the plots were also found (Figure 3C). At both sites, the share of euedaphic springtails was significantly higher in the NR plots (on the I NR plot, they constituted 60% of the total community) than in the reclaimed plots. Conversely, the relative abundance of epedaphic springtails in the reclaimed plots was significantly higher than in the NR plots. An extremely high percentage of epedaphic springtails (90%) was found in the I 3A3H and II 3H3A plots.
At site I, the percentage of hemiedaphic springtails in the 3A3H plots was significantly lower (p < 0.05) than in the 3H3A and NR plots, while at site II, hemiedaphic springtails in the 3A3H plots constituted almost 70% of the community, which was significantly higher in comparison to the NR and 3H3A plots.
3.5. Soil Properties and Soil Fauna Abundance across the Sampling Plots
The PCA results based on the animal abundance (total and ecological groups) and soil properties across the different sampling plots are represented in Figure 4.
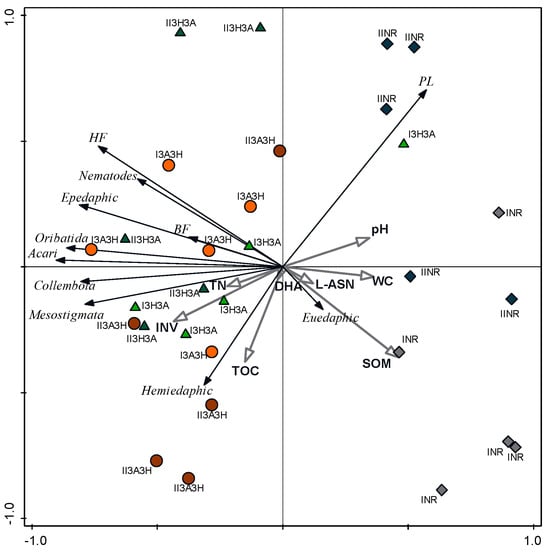
Figure 4.
Principal component (PCA) ordination of soil properties and abundance of the soil fauna groups in the reclaimed (3A3H, 3H3A) and non-reclaimed (NR) plots. For more information about the sites (I and II) and the reclamation practices, see Table 1. Abbreviations: SOM—soil organic matter, TN—total nitrogen, TOC—total organic carbon, WC—water content, Bf—bacterial feeders, Hf—hyphal feeders, Pf—plant feeders, DHA, L-ASN, INV—soil dehydrogenase, L-asparaginase, and invertase activity, respectively.
The first (horizontal) axis shows the most variation in response variables at approximately 48.3%. The second axis shows a further 15% variation in the response data. The coefficients 0.84 and 0.53 show that the correlation between the response-based and explanatory variable-based axes are highly significant.
The PCA ordination differentiated well between the reclaimed and the NR plots, and additionally between the two types of post-mine deposits. Considering the relationship between the animal groups and the sampling plots, most animal groups had a higher abundance in the reclaimed plots than in the NR plots. However, two groups—plant-feeding nematodes and euedaphic springtails—showed a clear positive relationship with the NR plots (Figure 4).
4. Discussion
4.1. Soil Physicochemical Properties in Reclaimed and Non-Reclaimed Post-Mine Soil
Soil organic matter is a key factor for structural development, aggregate formation, and increasing biological activity [10,39,40,41], and is a basic measure of post-mining reclamation success. Our results are similar to those obtained in the studies of Mańkowski et al. [3] and Pudełko et al. [15] from the same plots, showing unequivocally that six years of reclamation by planting industrial hemp in rotation with alfalfa contributed to a significant increase in the SOM and consequently the humus level. The humus content (about 2.4%) in the top layer of the studied post-mine soils reached the level observed in the agricultural soils of the region [3,15]. Our results for soil TN in 3H3A plots (on clay deposits) are similar to those found by Chen et al. [42] after 3 years of alfalfa reclamation of brown coal mining area in Germany. On the other hand, the doubled values for TOC% in our study compared to Chen et al. [42] can be explained by the high biomass introduced into the soil during the years of hemp cultivation.
The lack of differences in organic matter and TOC% content between the reclaimed and NR plots in our study indicates an accumulation of organic matter also during spontaneous succession (for 21 years on NR plots on site I and for 11 years on site II). However, the marked differences in the soil TN% and C:N values between the reclaimed and the NR plots show a different quality of organic matter, which implies different rates of decomposition and mineralisation processes. The higher TN% content (especially in the clay deposits) found in the soil under alfalfa than the soil under hemp and in the NR plots confirms that the use of legumes in reclamation practices supplies the soil with nitrogen, the deficiency of which, as shown in some previous studies, is the main factor limiting the decomposition of SOM in post-mining areas [43].
The introduction of alfalfa into the rotation cycle after hemp resulted in an increased pool of litter with a narrow (of about 15) C:N ratio [44]. Such substrates provide ample N, which likely stimulates microbial growth, mainly bacteria [15], as evidenced in our study by the higher dehydrogenase activity in the soil under alfalfa. Meanwhile, the incorporation of hemp biomass (yearly about 3.3 t/ha in the 3H3A plots and more than 5.3 t/ha in the 3A3H plots; see Mańkowski et al. [3]) into the soil led to a large contribution of cellulose to the soil, which is the basic substrate for cellulolytic enzymes, including invertase. According to Hayano [45] and Rhee et al. [46], fungi are considered the major source of cellulases in soils. An indirect indication of the predominantly fungal origin of the cellulases in the soil under hemp (3A3H plots) appeared to be the high relative abundance of hyphal-feeding nematodes.
4.2. Soil Fauna Responses to Reclamation of Post-Mine Soil
All three studied animal groups were found to respond clearly to the applied reclamation practices. In the sixth year of plant cultivation at the post-mining sites, the abundance and structure of micro- and mesofauna communities in the reclaimed plots differed significantly from those where natural succession took place. All animal groups had distinctly higher densities in the reclaimed plots compared to the NR ones. It is worth noting that after six years of reclamation, nematode densities reached the lowest values in the abundance range (0.3–8.0 × 106 ind. m−2) known from agroecosystems [47].
Additionally, we found that the sequence of plants (hemp or alfalfa) in the crop rotation had a strong effect on animal abundance. The highest densities for nematodes were observed in the 3H3A plots regardless of the deposit type. Unlike nematodes, the highest density of microarthropods was reached in the 3A3H plots and the clearest difference between 3A3H and 3H3A was particularly evident in the clay soil. Due to the variety of trophic adaptations, microarthropods can feed on organic matter at various stages of decomposition, as well as bacteria, fungi, protozoa, nematodes, and other groups of soil fauna [48]. An increase in organic matter in the litter and humus layers causes an increase in fungal biomass, which is the primary food source for oribatid mites [49]. In our study, the increase in the food base (large amounts of hemp biomass) may therefore be one of the main drivers of microarthropod density. In addition, the hemp and alfalfa roots, found to grow to a depth of 100 cm in reclaimed soil, as well as the agrotechnical practices, ploughing on heavy clay deposits [3], resulted in greater soil aeration and an increase in the number of pores, which provide microhabitats for microarthropods [50]. A marked positive response of Mesostigmata mites to reclamation practices could be related to increased nematode abundance. These mites are predators that mainly feed on different groups of soil animals, including nematodes.
Another finding of the study is that the particular trophic and ecological groups of the soil fauna were influenced differently by the reclamation practices. Bacterial (especially nematodes from the Panagrolaimus genus) and hyphal feeders (mainly Aphelenchus and Aphelenchoides), the two nematode trophic groups known to be involved in carbon and nitrogen mineralisation, responded most clearly. The high abundance of bacterial feeders (primarily of the so-called enrichment opportunists [51] with short life cycles and large food resource requirements, such as Panagrolaimus) in the 3H3A plot is most likely related to the alfalfa cultivation and easily decomposed alfalfa biomass used for soil enrichment (annually about 4.5 t/ha; see Mańkowski et al. [3]) in the last two years before sampling. As previously shown, such substrate stimulates bacterial growth and activity and has a positive effect on bacterium-feeding nematodes [15,44,52,53]. In soil restored for two years by alfalfa cultivation, Reichel et al. [53] found that bacterial feeders contributed to 98% of all free-living nematodes, which suggested that they had the potential to accelerate the remineralisation of N of the living microbial biomass [54,55], and this led to faster and almost complete decomposition and remineralisation of contained C and N [53]. Empirical evidence exists that N mineralisation exceeds N immobilisation if organic substrates such as alfalfa with C:N ratios lower than 20–25 are decomposed [56]. The weaker response of bacterial feeders in our study (with a contribution of 50% and 70% in the sandy and clay soils, respectively) than in the study by Reichel et al. [53] could be explained by the fact that prior to the alfalfa cultivation, the soil was reclaimed for three consecutive years by hemp (the 3H3A plot), and each year the hemp biomass (about 3.3 t/ha, with a high C:N ratio) was incorporated into the soil [3].
The high quantities of ploughed hemp biomass, containing about 70% cellulose, provided a good substrate for the growth of fungi, which are most likely to be a valuable food source supporting hyphal-feeding nematodes. This was confirmed by the fact that both reclamation practices (each with half based on hemp cultivation) have a positive effect on hyphal-feeding nematodes, but the proportion of these nematodes in the community structure was by far the highest in the 3A3H plots, where a large amount of hemp biomass (more than 5.3 t/ha) was ploughed into the soil in the last two years prior to the sampling [3].
The high proportion of hyphal feeders and the smaller proportion of bacterial nematodes (especially enrichment opportunists) in the reclaimed 3A3H plots indicate that decomposition and mineralisation processes are progressing slowly in comparison to the study by Reichel et al. [53]. This implies that the introduction of hemp in rotation with alfalfa, as in our study, is likely to avoid the negative consequences associated with very fast decomposition when only alfalfa is cultivated.
Our results clearly indicate that compared to the reclaimed plots, the high percentage of bacterial feeders, especially in I NR plots, was associated with the occurrence of nematodes from genera such as Acrobeloides, Plectus, and Prismatolaimus, known as habitat generalists with lower food requirements and longer life cycles compared to enrichment opportunists [51,57]. They are considered “basal condition” nematodes. The high share of these nematodes in the NP plots on clay deposits, even 20 years after mining activities ceased, indicates resource-poor conditions and suggests that enriching the soil with easily decomposable organic matter and nutrients is proceeding more slowly than in the reclaimed plots. An additional but very clear indicator of low levels of labile nutrients in the NR soil was the complete absence of enrichment opportunists among nematodes.
The lower percentage of plant-feeding nematodes in the reclaimed plots compared to the NR plots confirms the resistance of hemp to parasites, including plant-feeding nematodes [3]. Furthermore, as shown in earlier studies, the rapid growth rate of hemp increased weed suppression due to the high ground cover in early spring [3]. Thus, it is highly likely that the suppression of weed growth reduced the food base for plant-feeding nematodes in the reclaimed plots. On the other hand, more diverse vegetation on II NR plots, and thus more potential hosts, explains the higher share of plant feeders in the nematode community.
We expected that the heavy metals found in the post-mining soil [15] would have a negative impact on the abundance of predatory nematodes and euedaphic collembolan species. However, the lack of predatory nematodes and lower abundance of euedaphic collembolans in the reclaimed soil than in the NR soil indicates that they may be more sensitive to the physical disturbance during crop cultivation rather than to the heavy metals (Cu, Zn, Fe) in the post-mining soil, which, as shown in Pudełko’s et al. work [15], did not exceed acceptable levels for Polish soils. The lower tolerance of euedaphic species to abiotic stress in comparison to epedaphic species has often been previously observed [58,59,60].
5. Conclusions
Our study confirmed previous results [3,15] that six years of reclamation by planting industrial hemp in rotation with alfalfa contributed to a significant increase in the SOM. The significant differences we found in the soil organic quality (TN% and C:N) between NR and reclaimed plots imply a faster rate of decomposition and mineralisation processes as a result of reclamation.
We found that the cultivation of industrial hemp in rotation with alfalfa over six years contributed to significant transformation of post-mine deposits, which was readily reflected in the abundance and community structure of all three studied invertebrate groups.
The three animal groups were more abundant in the reclaimed soil compared to the NR soil. The highest densities for nematodes were observed in the 3H3A plots and for the mesofauna in the 3A3H plots. Most of the parameters of the studied biota were found to have values resembling those of agricultural soils. The reclamation practices had a positive effect on groups involved in the regulation of C and N mineralisation, particularly bacterial- and hyphal-feeding nematodes and oribatid mites, and a negative effect on plant-feeding nematodes and euedaphic collembolans.
Our results clearly indicate that the soils in the reclaimed plots became more productive and suitable for agricultural use, as their biological activity also increased along with their improved physical and chemical properties. This allows us to recommend this as an effective reclamation method that improves soil biological quality after mining.
Author Contributions
Conceptualisation, K.K. and K.I.-M.; methodology, K.I.-M., A.A.-K. and K.K.; formal analysis, K.I.-M., A.A.-K. and K.K.; investigation, K.I.-M., A.A.-K. and K.K.; resources, J.M. and J.K.; data curation, K.I.-M., A.A.-K. and K.K.; writing—original draft preparation, K.I.-M. and A.A.-K.; visualisation, K.K.; supervision of the experiments: J.M. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fagiewicz, K.; Łowicki, D. The dynamics of landscape pattern changes in mining areas: The case study of the Adamów-Koźmin Lignite Basin. Quaest. Geogr. 2019, 38, 151–162. [Google Scholar] [CrossRef]
- Mańkowski, J.; Kołodziej, J.; Kuback, A.; Baraniecki, P.; Pniewska, I. Cultivation of industrial hemp accelerating reclamation of land degraded by open mining of lignite. Chemik 2014, 68, 983–988. [Google Scholar]
- Mańkowski, J.; Kołodziej, J.; Pudełko, K.; Kozłowski, R.M. Bast fibres: The role of hemp (Cannabis sativa L.) in remediation of degraded lands. In Handbook of Natural Fibres, 2nd ed.; Kozłowski, R.M., Mackiewicz-Talarczyk, M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2020; pp. 393–417. ISBN 9780128187821. [Google Scholar] [CrossRef]
- Ganjegunte, G.K.; Wick, A.F.; Stahl, P.D.; Vance, G.F. Accumulation and composition of total organic carbon in reclaimed coal mine lands. Land Degrad. Dev. 2009, 20, 156–175. [Google Scholar] [CrossRef]
- Abakumov, E.V.; Cajthaml, T.; Brus, J.; Frouz, J. Humus accumulation, humification, and humic acid composition in soils of two post-mining chronosequences after coal mining. J. Soils Sediments 2013, 13, 491–500. [Google Scholar] [CrossRef]
- Pagliai, M.; Vignozzi, N.; Pellegrin, S. Soil structure and the effect of management practices. Soil Tillage Res. 2004, 79, 131–143. [Google Scholar] [CrossRef]
- Sen, S.; Kumar, V. Evaluating soil quality and bioefficacy study of Cajanus cajan L. in coal mine-degraded land. Turk. J. Agric. For. 2016, 40, 3. [Google Scholar] [CrossRef]
- Clayton, J.; Lemanski, K.; Bonkowski, M. Shifts in soil microbial stoichiometry and metabolic quotient provide evidence for a critical tipping point at 1% soil organic carbon in an agricultural post-mining chronosequence. Biol. Fertil. Soils 2021, 57, 435–446. [Google Scholar] [CrossRef]
- Mosier, S.; Córdova, S.C.; Robertson, G.P. Restoring soil fertility on degraded lands to meet food, fuel, and climate security needs via perennialization. Front. Sustain. Food Syst. 2021, 5, 706142. [Google Scholar] [CrossRef]
- Pihlap, E.; Vuko, M.; Lucas, M.; Steffens, M.; Schloter, M.; Vetterlein, D.; Endenich, M.; Kögel-Knabner, I. Initial soil formation in an agriculturally reclaimed open-cast mining area—The role of management and loess parent material. Soil Tillage Res. 2019, 191, 224–237. [Google Scholar] [CrossRef]
- Głowacki, A.; Mocek-Płóciniak, A.; Spychalski, W.; Kayzer, D. The influence of long-term land reclamation on the microbiological properties of post-mining soils. Soil Sci. Annu. 2020, 71, 359–370. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, N.; Wei, Q.; Cao, Y.; Li, D.; Cui, G. Alfalfa modified the effects of degraded black soil cultivated land on the soil microbial community. Front. Plant Sci. 2022, 13, 938187. [Google Scholar] [CrossRef] [PubMed]
- Linger, P.; Müssig, J.; Fischer, H.; Kobert, J. Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: Fibre quality and phytoremediation potential. Ind. Crops Prod. 2002, 16, 33–42. [Google Scholar] [CrossRef]
- Husain, R.; Weeden, H.; Bogush, D.; Deguchi, M.; Solimanm, M.; Potlakayala, S.; Katam, R.; Goldman, S.; Rudrabhatla, S. Enhanced tolerance of industrial hemp (Cannabis sativa L.) plants on abandoned mine land soil leads to overexpression of cannabinoids. PLoS ONE 2019, 14, e0221570. [Google Scholar] [CrossRef] [PubMed]
- Pudełko, K.; Kołodziej, J.; Mańkowski, J. Restoration of minesoil organic matter by cultivation of fiber hemp (Cannabis sativa L.) on lignite post-mining areas. Ind. Crops Prod. 2021, 171, 113921. [Google Scholar] [CrossRef]
- Borges, F.L.G.; Oliveira, M.R.; de Almeida, T.C.; Majer, J.D.; Garcia, L.C. Terrestrial invertebrates as bioindicators in restoration ecology: A global bibliometric survey. Ecol. Indic. 2021, 25, 107458. [Google Scholar] [CrossRef]
- Briones, M.J.I. The serendipitous value of soil fauna in ecosystem functioning: The unexplained explained. Front. Environ. Sci. 2018, 6, 149. [Google Scholar] [CrossRef]
- Kardol, P.; De Long, J. How anthropogenic shifts in plant community composition alter soil food webs. F1000Research 2018, 7, 4. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Ilieva-Makulec, K.; Tyburski, J.; Makulec, G. Soil nematodes in organic and conventional farming system: A comparison of the taxonomic and functional diversity. Pol. J. Ecol. 2016, 64, 303–320. [Google Scholar] [CrossRef]
- Machado, J.S.; Oliveira Filho, L.C.I.; Santos, J.C.P.; Paulino, A.T.; Baretta, D. Morphological diversity of springtails (Hexapoda: Collembola) as soil quality bioindicators in land use systems. Biota Neotrop. 2019, 19, e20180618. [Google Scholar] [CrossRef]
- Forster, J.C. Determination of soil pH. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; p. 55. ISBN 0125138407. [Google Scholar]
- Forster, J.C. Soil Physical Analysis. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; p. 105. ISBN 0125138407. [Google Scholar]
- Salehi, M.H.; Beni, O.H.; Harchegani, H.B.; Esfandiarpour-Borujeni, I.; Motaghian, H.R. Refining soil organic matter determination by loss-on-ignition. Pedosphere 2011, 21, 473–482. [Google Scholar] [CrossRef]
- Krotz, L.; Leone, F.; Giazzi, G. High Accuracy of Nitrogen, Carbon and Sulfur Analysis for Agronomy Applications Using the Thermo Scientific FlashSmart Elemental Analyzer; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2016; Application Note. 2016, AN42264 0716G. [Google Scholar]
- Casida, L.; Klein, D.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Kanazawa, S.; Kiyota, H. Estimation of L-glutaminase and L-asparaginase activities in soils by the indophenol method. Soil Sci. Plant Nutr. 1995, 41, 305–311. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Saccharase activity. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; pp. 354–355. ISBN 0125138407. [Google Scholar]
- Flegg, J.J.M.; Hooper, D.J. Extraction of free-living stages from soil. In Laboratory Methods for Work with Plant and Soil Nematodes; Southey, J.P., Ed.; Technical Bulletin of Ministry of Agriculture, Fisheries and Food: London, UK, 1970; pp. 5–23. ISBN 0112409024. [Google Scholar]
- Goodey, T. Soil and Freshwater Nematodes; John Wiley and Sons: New York, NY, USA, 1963. [Google Scholar]
- Andrássy, I. A Taxonomic Review of the Suborder Rhabditina (Nematoda: Secernentia); Office de la Recherche Scientifique et Technique Outre-Mer (ORSTOM): Paris, France, 1983; ISBN 2709906996. [Google Scholar]
- Bongers, T. The Nematodes of the Netherlands; Stichting Uitgeverij Koninklijke Nederlandse Natuurhistorische Vereniging: Utrecht, The Netherlands, 1998. (In Dutch) [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera. An outline for soil ecologists. J. Nematol. 1993, 25, 315–331. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2619405/ (accessed on 10 May 2022).
- Macfadyen, A. Improved funnel type extractors for soil arthropods. J. Anim. Ecol. 1961, 30, 171–184. [Google Scholar] [CrossRef]
- Cielecka, D.; Salamatin, R.; Garbacewicz, A. Usage of the Hoyer’s medium for diagnostics and morphological studies of some parasites. Wiad. Parazytol. 2009, 55, 265–270. (In Polish) [Google Scholar]
- Baker, E.W.; Wharton, G.W. An Introduction to Acarology; Macmillan: New York, NY, USA, 1952. [Google Scholar]
- Chernova, N.M. Collembolan populations. In Key Book of Collembola of the USSR Fauna; Chernova, N.M., Ed.; Nauka: Moscow, Russia, 1988; pp. 45–51. (In Russian) [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012; p. 496. [Google Scholar]
- Delschen, T. Impacts of long-term application of organic fertilizers on soil quality parameters in reclaimed loess soils of the Rhineland lignite mining area. Plant Soil 1999, 213, 43–54. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Till. Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Qiang, M.A.; Yu, W.-T.; Zhao, S.-H.; Zhang, L. Relationship between water-stable aggregates and nutrients in black soils after reclamation. Pedosphere 2007, 17, 538–544. [Google Scholar] [CrossRef]
- Chen, Y.F.; Mekete, T.; Dababat, A.A.; Daub, M.; Cao, Z.P.; Sikora, R.A. Response of nematode communities to reclamation of agricultural soils following degradation through brown coal strip-mining processes. Helminthologia 2014, 51, 53–62. [Google Scholar] [CrossRef]
- Kumari, S.; Maiti, S.K. Nitrogen recovery in reclaimed mine soil under different amendment practices in tandem with legume and non-legume revegetation: A review. Soil Use Manag. 2022, 38, 1113–1145. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E. Fungal and bacterial growth in soil with plant materials of different C/N ratios. FEMS Microbiol. Ecol. 2007, 62, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Hayano, K. Cellulase complex in a tomato field soil: Induction, localization and some properties. Soil Biol. Biochem. 1986, 18, 215–219. [Google Scholar] [CrossRef]
- Rhee, Y.H.; Hah, Y.C.; Hong, S.W. Relative contributions of fungi and bacteria to soil carboxymethylcellulase activity. Soil Biol. Biochem. 1987, 19, 479–481. [Google Scholar] [CrossRef]
- Wasilewska, L. The structure and function of soil nematode communities in natural ecosystems and agrocenoses. Pol. Ecol. Stud. 1979, 5, 97–145. [Google Scholar]
- Coleman, D.C.; Crossley, D.A., Jr.; Hendrix, P.F. Fundamentals of Soil Ecology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2004; ISBN 9780121797263. [Google Scholar]
- Princz, J.I.; Behan-Pelletier, V.M.; Scroggins, R.P.; Siciliano, S.D. Oribatid mites in soil toxicity testing—The use of Oppia nitens (C.L. Koch) as a new test species. Environ. Toxicol. Chem. 2010, 29, 971–979. [Google Scholar] [CrossRef]
- Minor, M.A.; Norton, R.A. Effects of soil amendments on assemblages of soil mites (Acari: Oribatida, Mesostigmata) in short-rotation willow plantings in Central New York. Can. J. For. Res. 2004, 34, 1417–1425. [Google Scholar] [CrossRef]
- Bongers, T. The Maturity Index, the evolution of nematode life history traits, adaptive radiation and c–p-scaling. Plant Soil 1999, 212, 13–22. [Google Scholar] [CrossRef]
- Insam, H.; Domsch, K.H. Relationship between soil organic carbon and microbial biomass on chronosequences of reclamation sites. Microb. Ecol. 1988, 15, 177–188. [Google Scholar] [CrossRef]
- Reichel, R.; Hänsch, M.; Brüggemann, N. Indication of rapid soil food web recovery by nematode-derived indices in restored agricultural soil after open-cast lignite mining. Soil Biol. Biochem. 2017, 115, 261–264. [Google Scholar] [CrossRef]
- Ferris, H.; Venette, R.C.; van der Meulen, H.R.; Lau, S.S. Nitrogen mineralization by bacterial-feeding nematodes: Verification and measurement. Plant Soil 1998, 203, 159–171. [Google Scholar] [CrossRef]
- Ferris, H.; Venette, R.C.; Scow, K.M. Soil management to enhance bacterivore and fungivore nematode populations and their nitrogen mineralization function. Appl. Soil Ecol. 2004, 24, 19–35. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of microbial nitrogen use efficiency to carbon: Nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 2014, 5, 3694. [Google Scholar] [CrossRef] [PubMed]
- Ilieva-Makulec, K. A comparative study of the life strategies of two bacterial-feeding nematodes under laboratory conditions. II. Influence of the initial food level on the population dynamics of Acrobeloides nanus (de Man 1880) Anderson 1968 and Dolichorhabditis dolichura (Schneider 1866) Andrassy 1983. Pol. J. Ecol. 2001, 49, 123–135. [Google Scholar]
- Bokhorst, S.; Phoenix, G.K.; Bjerke, J.W.; Callaghan, T.V.; Huyer-Brugman, F.; Berg, M.P. Extreme winter warming events more negatively impact small rather than large soil fauna: Shift in community composition explained by traits not taxa. Glob. Chang. Biol. 2012, 18, 1152–1162. [Google Scholar] [CrossRef]
- Da Silva, M.P.; Carvalho, F.; Dirilgen, T.; Stone, D.; Creamer, R.; Bolger, T.; Sousa, J.P. Traits of collembolan life-form indicate land use types and soil properties across an European transect. Appl. Soil Ecol. 2016, 97, 69–77. [Google Scholar] [CrossRef]
- Holmstrup, M.; Ehlers, B.K.; Slotsbo, S.; Ilieva-Makulec, K.; Sigurdsson, D.B.; Leblans, N.I.W.; Ellers, J.; Berg, M.P. Functional diversity of Collembola is reduced in soils subjected to short-term, but not long-term, geothermal warming. Funct. Ecol. 2018, 32, 1304–1316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).