1. Introduction
Rice is a fundamental and essential food for global food security [
1], especially in developing countries [
2]. It is one of the three most relevant crops in the world [
3] and serves as one of the main sources of energy in the diets of more than half of the population [
4]. Since the 12th century, rice has been grown in L’Albufera de Valencia, making it the first place in Spain where rice was cultivated and from which it expanded to other areas in the country [
5]. Among all the cultivated varieties,
Bomba is a well-recognized landrace that has been cultivated since the end of the 19th century [
6]. The
Bomba plants show an old morphological aspect; they are tall plants with few and weak stems and low yields. Today,
Bomba rice is highly valued in the Spanish market for its grain, cooking, and eating qualities.
Fungal infections are one of the most worrying diseases of the crop, because in addition to causing reductions in yield [
7] and losses in the quality of rice [
8], they can generate compounds that become toxic for consumption [
9]. The disease caused by
Magnaporthe oryzae is considered the most important fungal disease in rice cultivation worldwide due to its distribution and high destructive power [
10]. Furthermore, the pathogen is highly contagious and can spread rapidly, affecting nearby plants. It can be found throughout the field and causes significant yield losses [
11], with estimates of up to a 30% yield loss worldwide [
12]. Additionally, the fungus is responsible for a reduction in the surface area of the plant, which in turn results in a significant reduction in yield. This is due to the fungus affecting the weight of the grain as well as the percentage of mature spikelets and mature grains [
13]. The fungus affects the entire aerial part of the plant, including the leaves, stems, panicle neck, and the panicle itself [
14].
M. oryzae, along with other varieties of this fungus, has been and is currently a serious threat to rice production around the world [
15], with reports indicating yield losses up to 35% [
16]. Even under favorable crop growth conditions, the fungus tends to destroy the entire rice plant within 20 days, which can lead to a 100% yield loss [
17].
A 2005 study concluded that
M. oryzae destroyed enough rice each year to feed 60 million people and that 50% of the production could be lost in infested plots [
18]. The impact that the fungus has on the crop highlights the need to look for effective and environmentally friendly solutions. The traditional method is chemical control, i.e., the use of pesticides, especially fungicides [
19]. Their effectiveness depends on several factors, such as the substance itself, the application method, the degree of development of the infestation, the forecasting system and, above all, the time at which the treatment is applied [
20]. It should be considered that, in spite of its effectiveness, chemical control must be used correctly, since improper use, such as human exposure, can cause intoxication and harmful side effects in people [
21]. Good work practices are essential when applying products such as fungicides in order to guarantee the safety of exposed workers during the application of these types of chemical products [
22]. The extent of the disease, as well as the level of infestation, is traditionally assessed visually on symptomatic plants, which is time-consuming and labor-intensive [
23]. Integrated pest management (IPM), i.e., knowing the pathogen and monitoring its appearance, is a tool to apply control measures before yield losses exceed the cost of control [
12].
Remote sensing data provide the possibility of detecting changes in plant biophysical properties caused by pathogens [
24,
25], since reflectance is determined by the chemical and morphological characteristics of the plant surface [
26]. The study of the unique spectral signature of the crop makes it possible to recognize its coverage and condition, facilitating assessments and analysis using remote sensing techniques [
27]. Plant responses to initial crop infestation are difficult to quantify visually, however, these infestations usually affect the quantity and quality of electromagnetic radiation reflected in the spectral signature of the crop [
28,
29]. Previous studies have reported on the relationship between spectral reflectance and satellite sensors regarding the biophysical parameters of the crop [
30]. The application of remote sensing technology for early detection of infested plants is being extensively investigated [
31]. However, the effectiveness of this method may vary depending on the type of pest and crop. Therefore, it is essential to evaluate the sensitivity of remote sensing characteristics to ensure successful monitoring.
Early detection of
M. oryzae is crucial in rice farming to prevent significant damage and production losses. Traditional field monitoring of crop damage caused by diseases and pests can be time-consuming and labor-intensive [
32]. Furthermore, the reliability of the assessments carried out by individual field advisors, who carry out visual surveys of symptomatic plants, is questionable, leading to considerable discrepancies in the outcomes obtained [
29]. Remote sensing can be used as a tool to objectively characterize infected crops [
28], providing more accurate and consistent measurements than those obtained by field assessors [
32,
33,
34,
35,
36,
37]. This monitoring and classification are possible because diseases cause chemical and biological changes in plants, which can be detected using remote sensing technology. The interaction of leaf tissue with light is determined by its structural and chemical properties. During pathogenesis, leaf pathogens alter these properties, which in turn affects the leaf optics. As a result, the spectral reflectance of vegetation is determined by the morphological and chemical characteristics of the leaf surface or organ. This was previously reported by Mahlein [
28] and also discussed by Zhang et al. [
26]. Reflectance data can detect changes in the pathogen-related biophysical properties of plants and canopy [
24,
25,
38,
39,
40].
Therefore, the use of remote sensing techniques can provide a record of the severity of the disease and allows the level of infestation to be assessed more objectively [
37,
38] before it becomes visibly evident. The results obtained in the study by San Bautista et al. [
32] demonstrated the importance and need to evaluate all reflectance regions to study the sensitivity of any change in any of the bands as a new index.
Based on the critical moment for initiating an Integrated Pest Management program in rice and with the beginning of the appearance of differences between affected and unaffected canopies, the spectral signature is unique to each crop and is fundamental for the recognition of plant canopies. Therefore, in order to carry out crop studies with remote sensing techniques, it is essential to start by obtaining spectral signatures [
27]. The dynamics between reflectance in the visible and NIR bands follow the crop dynamics proposed by San Bautista et al. [
32]. Other previous studies in other species, such as wheat stripe rust [
41], wheat powdery mildew [
42], kiwi gray mold [
43], tomato late blight [
44], and leafroll of vine [
45] demonstrated that the analysis and representation of the spectral signature could anticipate the detection of the disease due to changes in the reflectance spectrum of some of the bands studied.
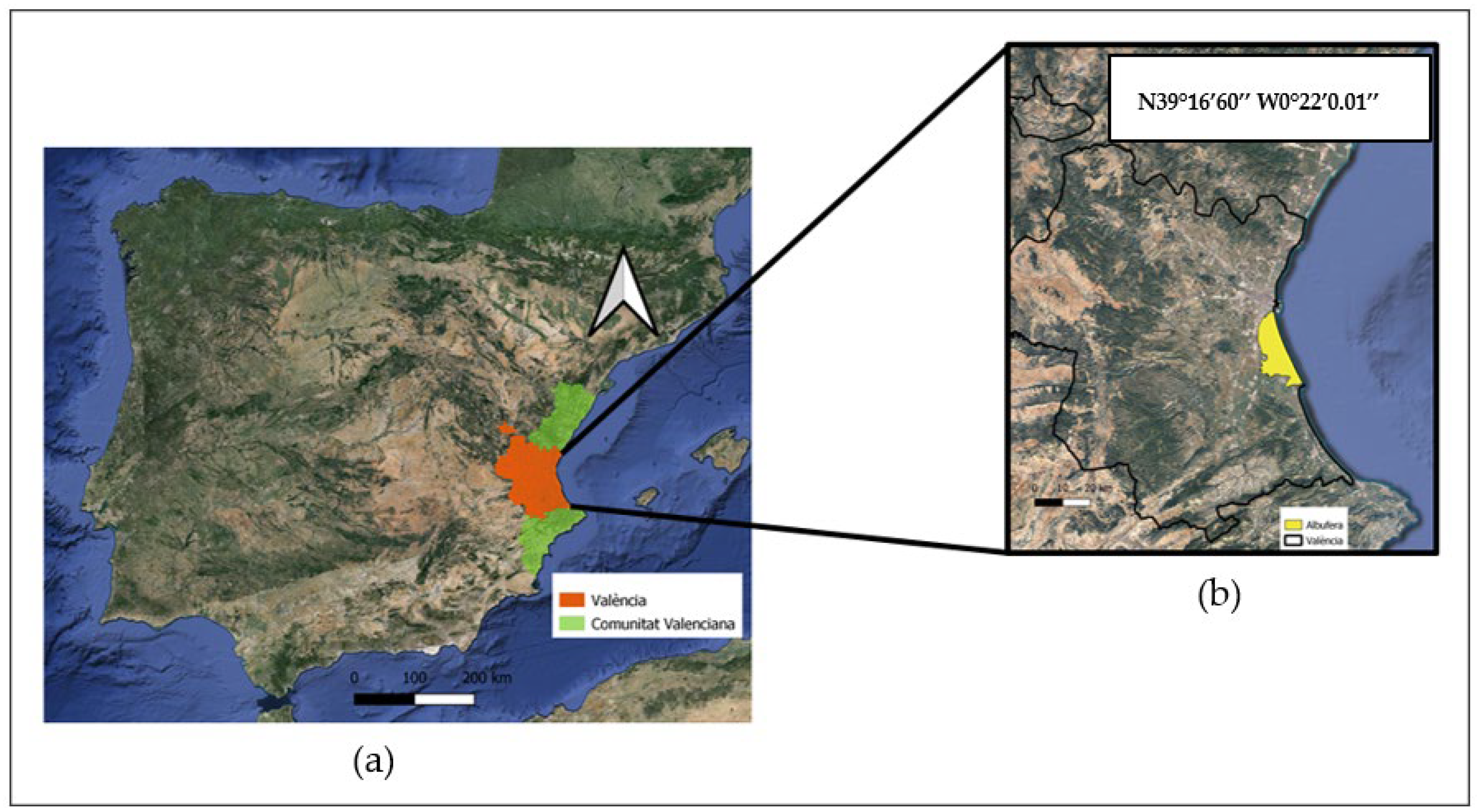
The objective of this work is to study the monitoring of M. oryzae infection in the Bomba variety at L’Albufera Natural Park (Valencia, Spain) in the years 2022 and 2023. For this, the reflectance values of different bands obtained from the Sentinel-2 satellite were used and, from these values, different vegetative indices used in rice cultivation were obtained for each year. From the results obtained, it is possible to quantify the degree of infestation before it is visually evident, which is of utmost importance in pest control. The crop response to M. oryzae infestation is also characterized by relating the evolution of reflectance and the different vegetative indices with field sampling. The results obtained in the early detection of the incidence of blast in rice crops could be the basis for advancements in the detection of the disease at the intra-field level. In this way, the proposed monitoring during the process of rice cultivation could be considered a digital tool capable of generating objective information to achieve Precision Agriculture.
4. Discussion
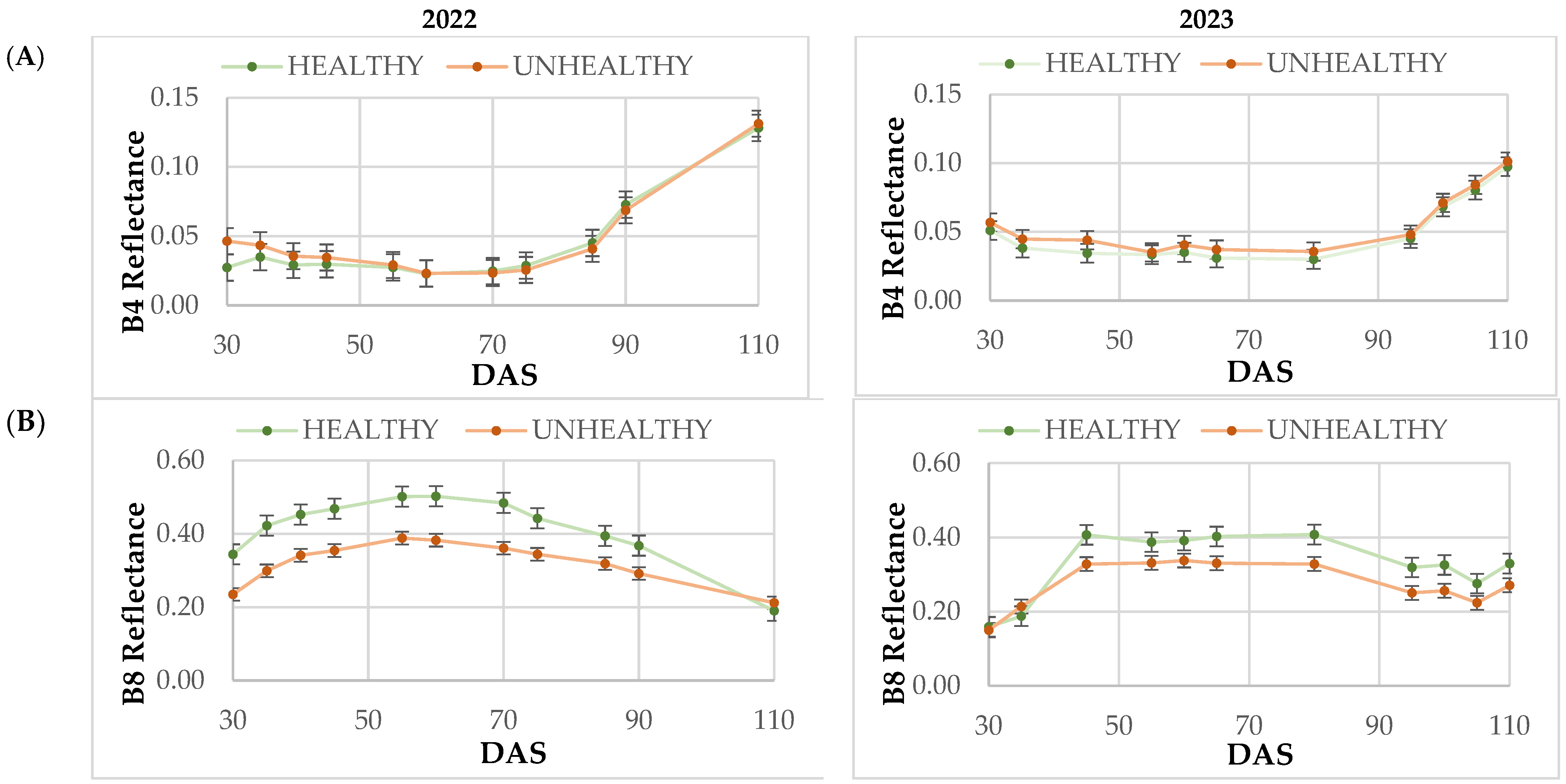
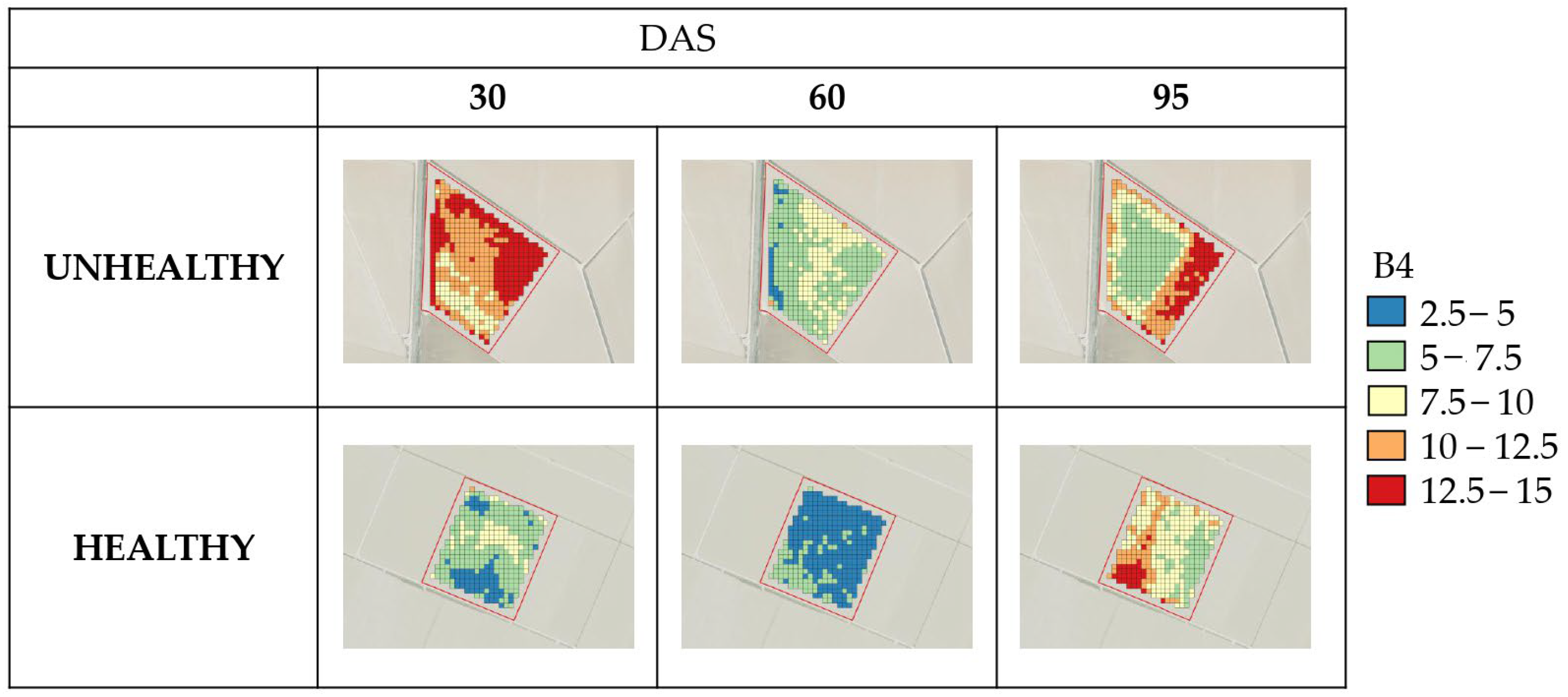
The analysis of the results presented in this work on how the frames have evolved across the different Sentinel-2 bands has proven to be helpful in categorizing them based on their health status, especially in the B8-NIR and Red Edge (B6 and B7) bands. This categorization was carried out throughout the growing season in the two years analyzed (2022 and 2023). These findings are in agreement with those reported by Zhang et al. [
31], who stated that VIS-NIR sensors are the most commonly used remote sensing systems for tracking diseases and pests in plants.
Several studies have found that the green, red, and near-infrared (NIR) spectral regions are sensitive to various plant diseases and pests [
62,
63]. In our study, there were statistical differences in reflectance in the B8-NIR band throughout the entire cycle for both years (2022 and 2023). The plants affected by blast showed a loss of biomass and plant cover, as was shown by the lowest values in the reflectance of B8-NIR band. The relationship between radiation in the red band and green biomass has an inverse nonlinear relationship, whereas in the near-infrared, the nonlinear relationship is directly due to the strong absorption of incident radiation by chlorophyll [
64]. The near-infrared (NIR) spectral region does not absorb much solar radiation and does not have a significant correlation with vegetation pigments [
32]. However, it is highly correlated with crop biomass and leaf area index (LAI) [
65]. This means that a higher biomass results in greater reflectance in B8-NIR and therefore in plants free of
M. oryzae.
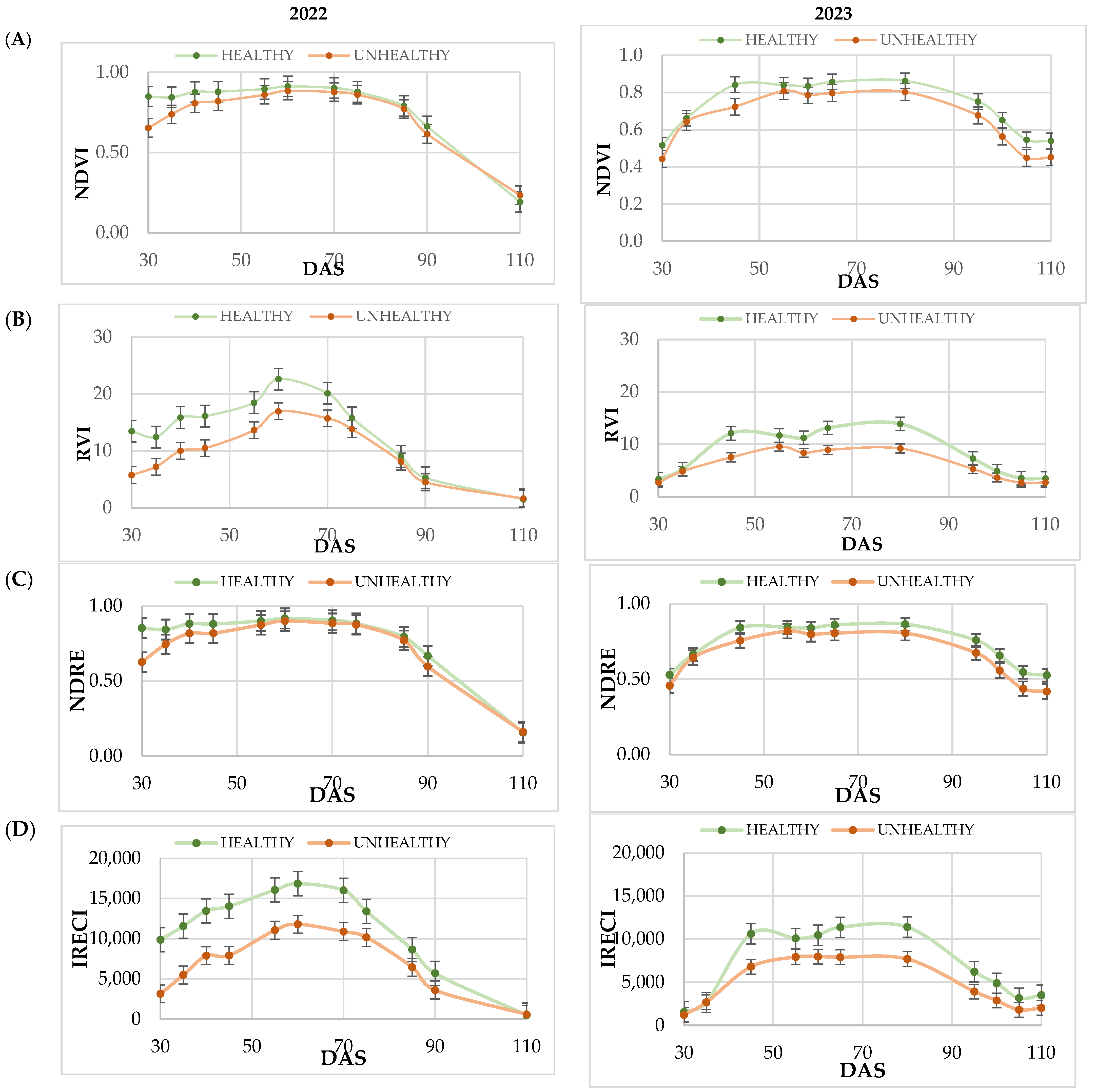
Vegetation indices were used to track the evolution according to the classification of plots according to disease incidence. VIs combining visible and NIR bands improve the sensitivity of green vegetation detection [
66]. Therefore, several indices have been studied: NDVI, RVI, NDRE, and IRECI. NDVI, despite being widely used due to its positive correlation with LAI (Leaf Area Index), has a saturation issue. NDVI is not very sensitive to high vegetation densities. However, NDRE improved the discretization of healthy vegetation, coinciding with the results reported by Frampton et al. [
41]. The RVI, calculated with the NIR and Red region as NDVI, allows for the study of disease stress [
32]. The results obtained in this work show that it could be considered as a VI amplifier of the differences between fields affected and not affected by blast. This ratio, used for monitoring crop growth and development, is highly sensitive to any changes in vegetation that may occur during the peak growth period [
67]. Finally, IRECI, compared to the rest of the indices, showed more significant differences between healthy and unhealthy fields, as reported in other works [
68]. Since its calculation consists of the three red edge bands, it decreases the saturation with respect to NDVI on high-density crops, such as rice. Moreover, it should be remembered that the red edge reflectance was already an excellent indicator to separate healthy and unhealthy plots. Based on the study of the VIs, the results indicate that NDVI is not a reliable indicator for distinguishing plots affected by blast, even though it is the most commonly used VI for studying agronomic conditions, developmental stages, and crop biomass [
69].
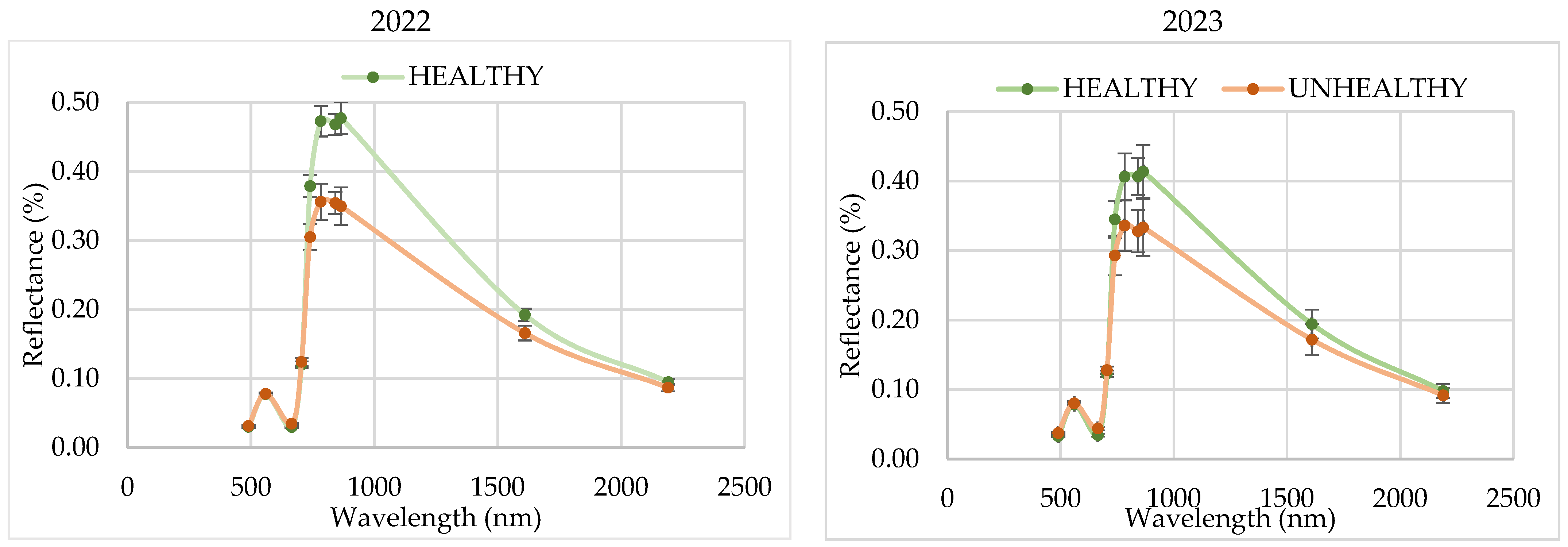
The spectral signature analysis at 45 DAS showed significant differences in reflectance in the visible (B4), infrared (B8), Red Edge (B6 and B7), and SWIR (B11) bands. The highlight of the results is the separation of the reflectance between blast-affected and unaffected fields, regardless of the year. Likewise, this separation can be evidenced with the RVI and IRECI vegetation indices, reaching a total variability close to 70% between the two factors considered in the statistical analysis (year and disease). These results in band reflectance coincide with those obtained by Zhang et al. [
31] in the study of powdery mildew in wheat. In this study, higher values were recorded in the visible region and lower values in the NIR region in infested areas at the canopy level.
5. Conclusions
The results obtained in this study demonstrate the great usefulness of remote sensing and the data it provides for monitoring disease in rice crops. M. oryzae is a fungus that affects the crop worldwide and it is considered one of the major concerns affecting yield, however, other factors such as climatic factors greatly condition the crop.
From the study of VIs, the results obtained indicate that the NDVI, despite being the most commonly used VI for the study of development stages and crop biomass, is not a reliable indicator to distinguish affected plots by blast. The highlight of the observed results is that the spectral signature at 45 DAS showed significant differences in reflectance in the visible (B4), infrared (B8), Red Edge (B6 and B7), and SWIR (B11) bands between fields affected and non-affected by blast. Likewise, these differences are observed with the RVI and IRECI vegetation indices, reaching a total variability close to 70% between the two factors considered in the statistical analysis (year and disease). This is why the RVI and the IRECI are more reliable than the NDVI or the NDRE for the purpose of distinguishing plots affected by blast.
The early difference in the values of bands, indices, and spectral signatures between infected plots with respect to healthy ones constitutes a promising result in the agronomic management of rice crops to anticipate and solve the problem. Consequently, the proposed monitoring during rice cultivation could be regarded as a tool in IPM, with the objective of enhancing the effectiveness of fungicide treatments. This is because it is not dependent only on the active substances, but also on the timing of application, which conditions the development and progression of the infestation in rice cultivation. The analysis of reflectance bands and VIs should be studied in other areas and in additional plots, to carry out a more exhaustive study. Furthermore, it would be interesting to study the evolution of the fungus and the band values in smaller areas within the same plot in order to be able to compare smaller plots.