1. Introduction
By 2050, the world population is estimated to grow to about 9.6 billion and the demand for animal protein to increase by 70% [
1]. With this rapid population growth, economic development, and urbanization, meeting the demand for feed and quality food and organic waste management require substantial resources, often with great environmental impact [
2].
The European Commission highlights organic waste as a key item of the circular and green economy, emphasizing its valorization into useful products. Several studies investigated their potential added value, mainly using anaerobic digestion and composting [
3]. Among the organic biomass, fruit and vegetable waste (FVW), a mixture of edible and inedible leftovers, plays an important role. The Food and Agriculture Organization (FAO) reports about 45% of fruits and vegetables (FV) are discarded (as waste) across the food supply chain [
2]. Furthermore, the United Nations predicts that 13% of all agricultural product is lost during harvest as well as retail, while 17% is wasted in houses, retail, and eating places [
4]. In the context of FVW valorization, one promising strategy is its exploitation as a substrate for mass rearing of edible insects to be used as a protein source for aquaculture, chicken farming, or as a lipid source for biodiesel production [
5,
6].
Saprophagous insects represent a potentially valid solution to two problems: the recovery of the growing amount of organic waste and the increasing global demand for feed and food. In a circular economy perspective, the application of Conversion of Organic Refuse by Saprophages (CORS) technology mediated by the black soldier fly (BSF),
Hermetia illucens L. (Diptera, Stratiomyidae, Hermetiinae), represents an important innovation that can significantly contribute to the development of supply chains that meet the principles of green economy and green chemistry. The species is distributed globally throughout temperate and tropical regions [
7]. BSF larvae (BSFL) can grow on various organic substrates, i.e., vegetable matter, manure, and catering waste, transforming them into high added-value biomass. It was observed that mature larvae, fed on different organic waste, contain up to 44% of dry matter and reach 42% of protein and 35% of fat [
8,
9,
10].
Larval performances, in terms of growth, size, biochemical composition, and life span, as well as the efficiency by which substrates are converted into larval biomass, vary considerably and are affected by the quality of the substrate [
11,
12,
13], substrate supply [
14], as well as temperature, oxygen availability, and moisture content [
15,
16].
Larvae can grow on substrate with a moisture content within the range of 60–90% [
17], with the optimal at 70–75% [
15]. For these reasons, the use of FVW as BSFL growth substrate may not be sufficient to create suitable conditions for the optimal larval development and the bioconversion of the waste into high added-value molecules, due to high moisture content of the substrate. Furthermore, in case of high moisture content of such a waste stream, the separation of BSFL from the frass might be challenging and time consuming [
18].
Several works have investigated the feasibility of mixing FVW with other types of waste that would optimize the bioconversion process, like fermented “spent coffee grounds” or bread waste from catering [
19,
20]. Besides abiotic factors, such as substrate quality, temperature, and moisture content, the feeding regime intended as substrate amount per larva (sometimes stated as larval density, i.e., number of larvae per area unit) is an important factor that needs to be optimized for its ability to improve or hinder the BSFL growth [
14,
21]. At low larval densities, which means a great amount of substrate per larva, BSFL may not achieve sufficient substrate conditioning and co-operative digestion, while too high densities lead to providing a low quantity of feed per larva, causing competition over the nutrients, and may negatively influence the growth [
22]. The density also influences other physical phenomena such as heat storage and evaporation, which change the feed properties and consequently affect the growth of the larvae [
23].
The bioconversion process reduces the volume by 50–70% of waste, a result that could alleviate the environmental and economic cost of their disposal [
24]. At the end of the bioconversion cycle, the larval biomass can be separated and used directly as feed or as raw material in a biorefinery conversion to produce protein meal, oil, biodiesel, and chitin [
25]. The rearing residue (frass) is a mixture of uneaten substrate, feces, and exuviae, which has interesting characteristics for improving soil and crop productivity [
26]. Several works have tested the frass as soil conditioner as such or after composting, demonstrating its potential as alternative organic fertilizer on different crops [
26,
27,
28,
29].
Historically, most of the studies concerning the knowledge on BSF feeding performance on different waste streams have been conducted at a laboratory scale (i.e., several hundred larvae per replicate on hundred grams of substrate) showing how BSFL growth and performance can vary on different waste or with different bioconversion conditions (feeding rate, moisture of the substrate, larval density, and feeding regime). The shifting from laboratory to industrial scale is not necessarily linear; many authors indicated that the findings of small-scale trials could not be necessarily moved to large production systems. For example, small-scale compared to large-scale studies, both carried out in the same conditions on swine manure, gave different results for larval development time until the mature stage (less in the small scale) or survivorship (higher in the small scale) or for the prepupal weight (less in the small scale) [
30]. The size of the pilot-level studies (also named large-scale or mass production or industrial-level studies) ranges from 10,000 to 20,000 larvae on 6–10 kg of substrate in a crate of about 30–40 L of volume, considering each crate as a bioconversion unit [
31,
32,
33,
34]. Gligorescu et al. [
32] found the best feeding treatment testing the bioconversion efficiency in crates with 20,000 BSFL on 8–10 kg of food waste. Yang-Jie et al. [
35] planned a 4-year full-scale study with containers filled with 6 kg of urban waste inoculated with 8000 BSFL. The containers were racked in rows and layers to treat 15 ton/day of waste. Over the 4 years, they could improve the production of larvae and the protein content modifying the frequency of pile turning or changing the fermentation agent of the initial substrate. Hence, the industrial bioconversion process must be developed by continuous technical optimization, according to the waste resource (type and quantity daily provided).
The HERMES project, funded within POR FESR Lazio 2014–2020 (Det. Reg. n. G09493 140721, 22/07/2021), aimed at making available a sustainable and environmentally friendly technology for the recovery and valorization of FVW from the large-scale retail trade by means of bioconversion mediated by BSFL. With a view of the likely seasonal variability of FVW, it was planned to carry out the bioconversion trials using waste provided by one supermarket and, since the large-scale tests required a longer time for the preparation and more space than the medium-scale laboratory tests, trials were diluted over time, within the time allowed for the completion of the Hermes project. Hence, a supermarket provided the mixture of FVW all over the year. To lower the moisture content of the FVW substrate, bakery waste bran, brewer’s spent grain, and dry stuff (such as cellulose ramekins and straw) were added one at a time in different tests. All the by-products were chosen since they were locally available.
The experiments were planned in order to (i) compare two feeding regimes and two rearing scales on bioconversion efficiency parameters (larval biomass, added-value products, and BSFL waste reduction); (ii) verify the scale-up of the system from experimental proof-of-concept on a small-scale lab environment to the large scale; and (iii) evaluate the field behavior of the composted residue.
4. Discussion
This aimed to analyze the influence of rearing conditions (feeding regimes and rearing scales) on bioconversion efficiency parameters of BSFL in view of system scale-up, comprising the whole supply chain till the field evaluation of the composted frass. Fruit- and vegetable-based diets represent the largest amount of useable organic waste from large-scale retail trade and from local markets, together with a wide range of by-products from the agrifood industry.
FVW supply from the supermarket was heterogeneous. The heterogeneity was not related to the seasonality because of the continuous presence of greenhouse-grown products provided off-season. A wide range of fruits and vegetables (domestic and exotic) were provided by the supermarket and used in the various tests in the different compositions and proportions as they arrived. Similarly, the supply of BW was very variable in quantity (sometimes huge, sometimes few, since it was as a priority targeted to ethical food banking) and quality (pizza and sweets contain fats and carbohydrates). For this reason and to respect a real scenario of a continuous supply by several supermarkets to a CORS-based bioconversion plant mediated by BSFL, the qualitative and quantitative compositions of each supply were not considered as a variable.
The addition of bakery waste (BW) or bran (BR) was effective in lowering the initial FVW moisture content, absorbing a part of the excess leachate, and facilitating the bioconversion process. In contrast, the addition of brewer’s spent grain (BSG) was not sufficient to lower the moisture content to the needs of BSFL. A combination of the three types of waste (FVW, BW, and BSG) could represent an improvement in the composition of the substrate and thus the efficiency of the bioconversion process. In addition to the moisture content, the feeding rate (amount of substrate/larva/day) is an important factor that influences the bioconversion process. Diener et al. [
14] evaluated the effects of different feeding rates on waste reduction and final biomass production, demonstrating that the optimal value was 0.1 g/larva/day if a balanced feed from the nutritional point of view was ensured. Parra Paz et al. [
21] estimated as the ideal condition of BSFL feeding rate a range between 0.095 and 0.163 mg/larva/day. The present study demonstrated that, with the combination FVW:BW, valuable yields of larval biomass and added-value products can be obtained at a low feeding rate (0.066–0.089 g/larva/day), no matter the production scale. Higher yields are attainable (as observed also in our work) with high feeding rate but, when applied to a firm scale, it means higher operating cost. Thus, a trade-off between valuable production and wise economic management must be considered.
BSFL can be reared on a wide range of organic substrates [
34] but the bioconversion process is influenced by feedstock characteristics [
10]. It was shown that dietary protein and nonstructural carbohydrate contents are primary determinants of bioconversion efficiency [
17,
22]. Nevertheless, high dietary protein levels do not necessarily lead to higher larval performance [
12] since the amount, quality, and ratio of protein, carbohydrates, and lipid should be considered as well [
52]. Compared to other studies [
53] and considering BSFL nutritional needs [
22], the substrates used in this work were poor in protein and lipid content, with a high percentage of carbohydrates. This led to a P:C ratio higher than the optimal rates of 1:2–1:3 [
11,
54]. Nevertheless, mature larval weights did not differ from those observed by other authors using FVW as the main substrate for BSFL rearing [
20,
55,
56]. At a low feeding rate, the maximum larval weight (MLW) was around 250 mg/BSFL for FVW:BW and remained around 200 mg/BSFL with FVW:BSG and FVW:BR mixtures. The best results in terms of MLW were achieved by using a substrate with a P:C ratio of 1:5.5–1:6.5, which means an unbalanced diet, with a high percentage of carbohydrates. Such data suggest that BSFL are able to exploit poor nutritional diets to reach a body mass comparable to supplying a richer diet, such as kitchen waste with a high level of protein and fat [
57].
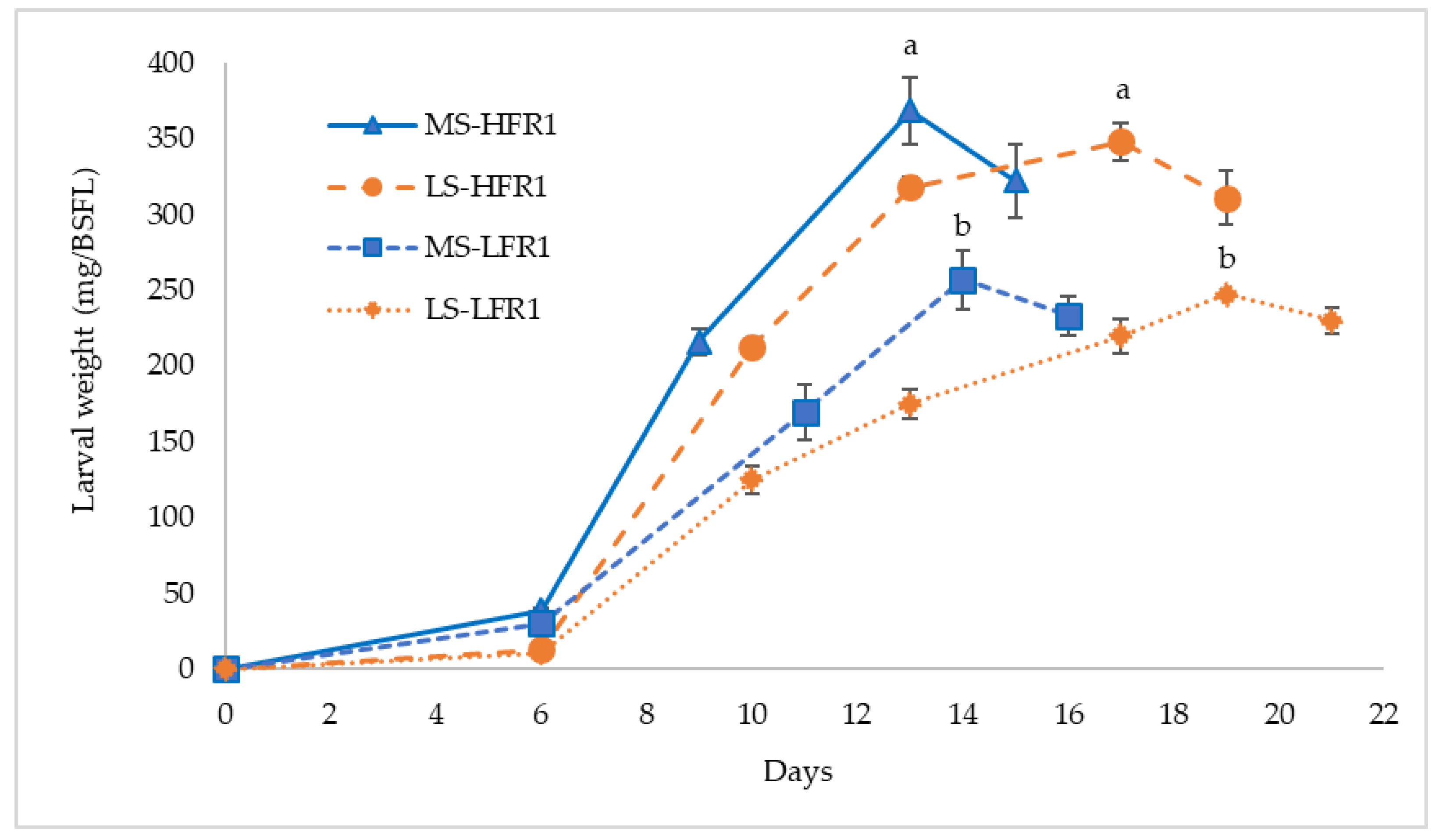
Shorter development time is especially important, as it leads to less production of greenhouse gases such as CO
2 and NH
3 during the waste bioconversion process by BSFL [
58]. In this work, the larval lifetime until the mature stage (i.e., at highest larval weight) was influenced by the scale of the trials, confirming how the transition from laboratory to mass rearing of insects could affect this parameter [
59,
60]. In the MS trial, BSFL had a development time not exceeding 14 days, while, in LS condition, the lifetime lasted up to 19 days. The weight decrease following the MLW is typical of the prepupae, the last larval stage before pupation. In this phase, the larvae empty their digestive tube, do not feed anymore, and move away from the food source towards a place to pupate [
10]. Similarly, Caruso et al. [
61] obtained mature larvae under mass production conditions in a time (14 days) longer than under laboratory conditions (9 days). Arabzadeh et al. [
62] evaluated that the required time to attain 40% prepupae was 15 days. In their study, they suggested that the faster development time could be due to higher levels of proteins in the diet rather than the presence of BSG and bread in addition to FVW. Other studies reported that BSFL fed with vegetable-based diets completed their larval cycle in 36–52 days [
34,
55]. Meneguz et al. [
63] found a significant difference in the development time comparing BSG diet (8 days) with FV diets (20–22 days), where BSG has a considerably higher protein content. However, protein level was not the key factor in our study, because, despite having a low protein content compared to the carbohydrates, the percentage of larval crude protein was in agreement with those of Arabzadeh et al. [
62].
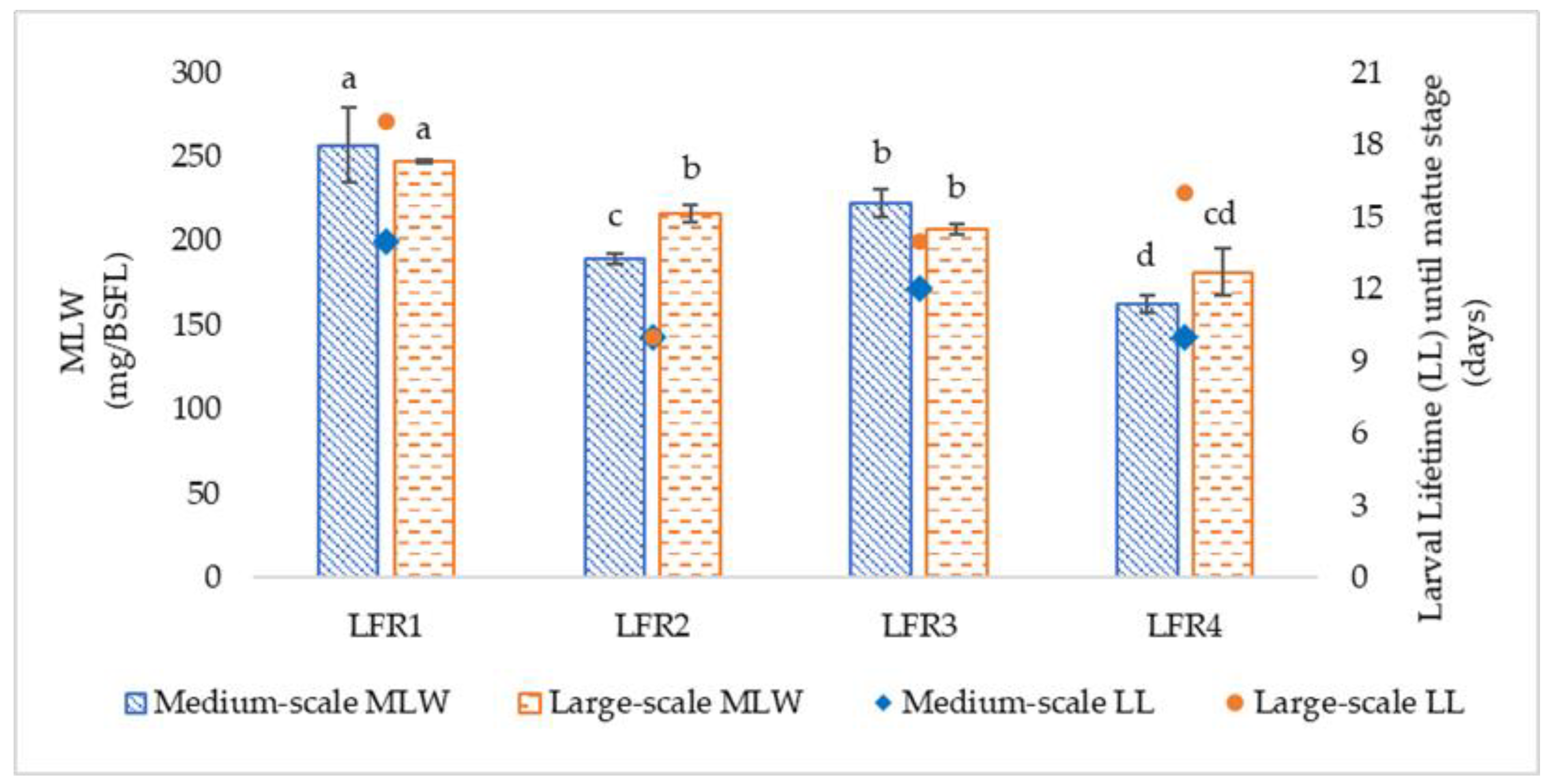
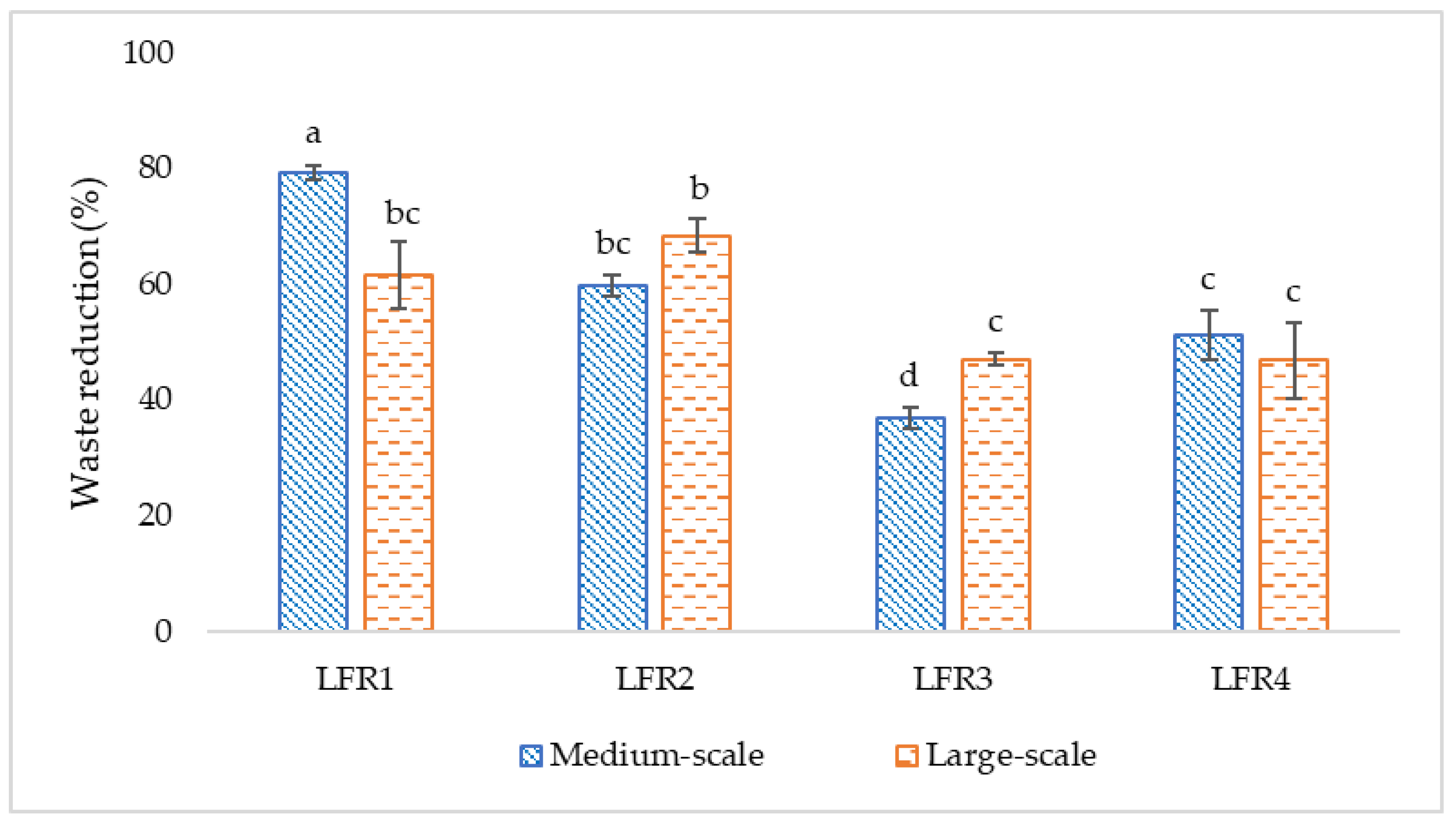
Although the addition of BW and BR did not show linearity between MS and LS trials, probably due to the better conditions in terms of moisture content, the percentage of waste reduction was the highest, ranging from 59.8 to 79.3%. These results are in line with Arabzadeh et al. [
62] and Candian et al. [
20], who showed that, with the addition of bread to FVW, the substrate reduction reached values of 66.8 and 76.9%, respectively.
In the current study, BSFL reared on FVW with added BW, in both the scales, had the highest crude lipid content. The role of carbohydrates-to-lipids conversion has been well documented [
53,
64]. Again, the results are nonlinear between MS and LS trials; only in LS does the lipid content of BSFL appear to be affected by the carbohydrate content of the substrates, with the lowest values in BSFL fed with FVW:BSG or FVW:DS. These results, ranging from 24.8 ± 0.3 to 45.7 ± 1.0%, are similar to values found in other studies for FVW-fed larvae [
62,
65]. Larval crude protein content, ranging from 23.7 ± 0.5 to 37.1 ± 1.3, with the lowest values in FVW:BW, appeared to mirror the protein content of the substrate. This result is in contrast with the findings of Spranghers et al. [
53] and Tschirner & Simon [
66], who reported that crude protein was higher in larvae fed on a diet with the lowest crude protein content. Nevertheless, the percentages of BSFL crude protein are in agreement with other authors [
9,
63].
A significantly lower percentage of crude protein content of substrate than BSFL confirmed the efficient capacity of larvae to convert dietary proteins into larval protein biomass [
22,
34]. The trend of PrCR values was not perfectly comparable between the medium and large scale. In MS trials, the highest values were obtained for FVW:BW and FVW:BSG, while, in LS trials, for FVW:BSG and FVW:DS. In addition, in LS condition, the PrCR showed higher values than in MS. In any case, the PrCR values were in line with the results of Lalander et al. [
34] and Arabzadeh et al. [
62], who tested FVW diets for BSFL feeding. Lower efficiency in PrCR values in the MS trial and, in general, the disagreement between the scales could be explained by the influence of the scale. At the laboratory scale, with small amounts of larvae, BSFL performance could be more affected by rearing conditions (substrate, temperature, and relative humidity), with a reduction in bioconversion efficiency [
65]. These results show how relevant the scale factor is for BSFL rearing and, as for key performance variables (e.g., waste reduction, lipid content, and PrCR), the transition from a benchtop to a large/industrial scale may not be linear [
30,
67]. In the setting up of the experiments, it was not possible to maintain the same proportion between the quantity of feed provided to BSFL and the size of the container. In LS trials, the fresh food column (about 12–13 cm) was reduced to 4–5 cm during the bioconversion process because of larval activity and water evaporation. Considering the number of starting larvae and the surface area of the container, the final density was about 3.5 BSFL per cm
2. In the MS trials, the larvae were more affected by the weight of the food column weighing on them. In a preliminary test we observed that the same rearing conditions produced much higher amounts of leachate than in LS. Under these conditions, the larvae were unable to co-operate in bioconversion easily because of the lack of available oxygen. Therefore, the food column was halved to about 6–7 cm, halving the number of larvae and the total amount of food. This way, we had about 1.7 BSFL per cm
2, which is still within the optimal range of larval density obtained by Parra Paz et al. [
21] between 1 and 5 BSFL/cm
2. Therefore, the conditions for the best performance of the bioconversion process and the parameters to be set (whether they are expressed as feeding regime, larval density, or feeding rate) cannot necessarily be the same on a small, medium, and large scale, thus causing the nonlinearity of the scaling up. Results on BSFL rearing may provide the basis to compare findings from previous small-scale studies. Moreover, they are a paradigm to help in optimizing the mass-rearing conditions of BSFL fed with FVW and agro-industrial by-products.
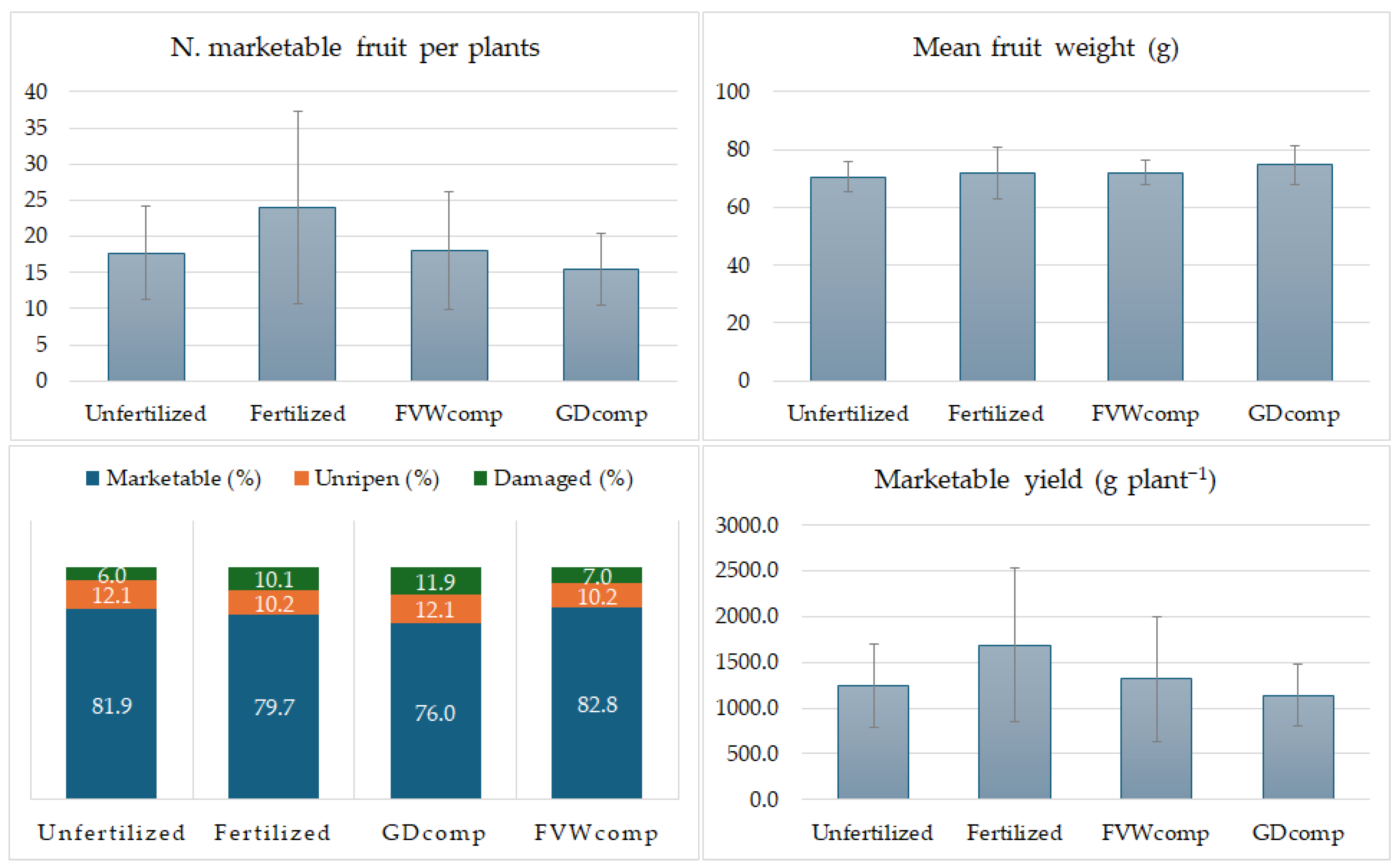
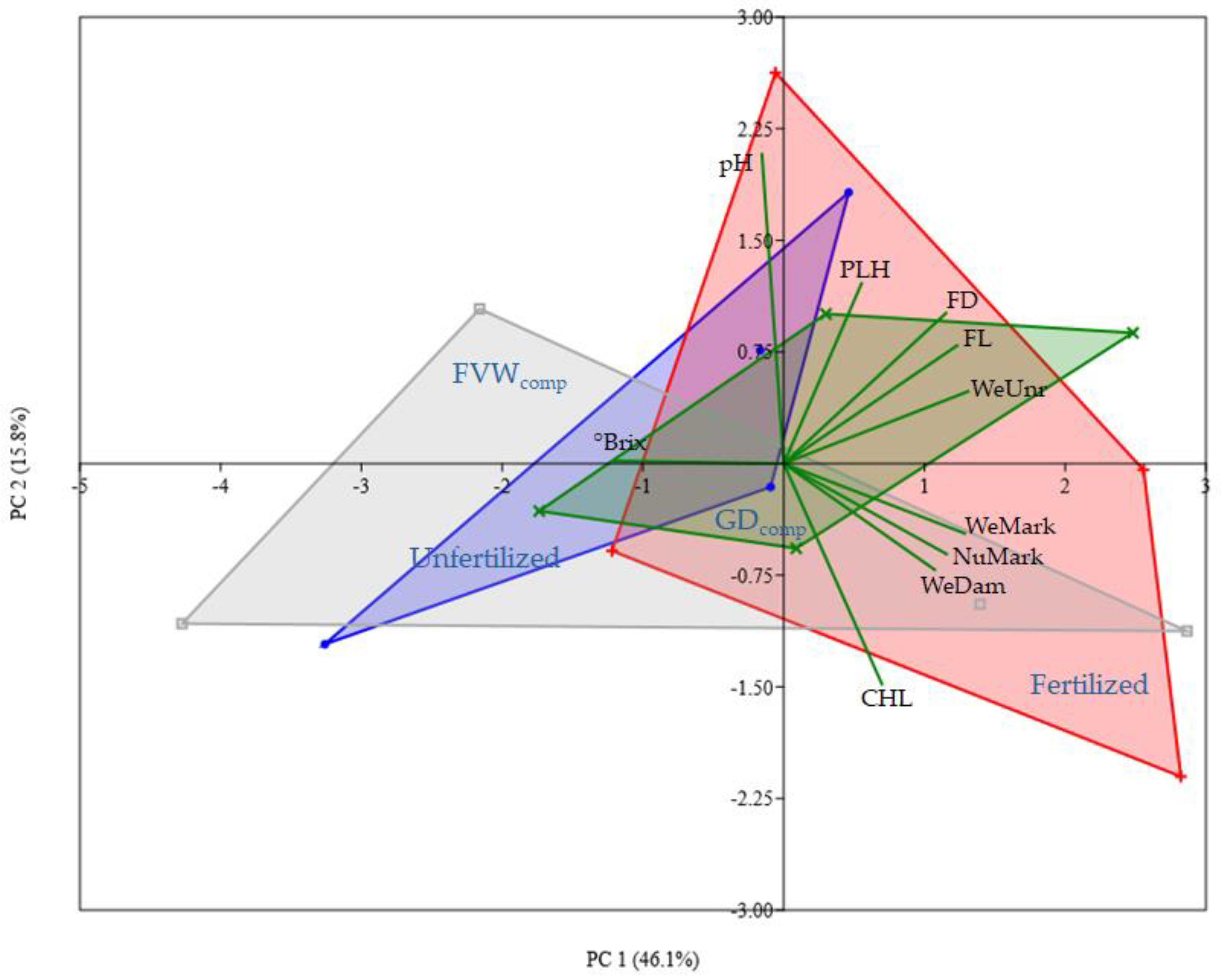
The most obvious finding from the results of the field trial is the lack of significance between different treatments. There is a trend that indicates a decreasing productivity according to the order Fertilised > FVW
comp > Unfertilized > GD
comp. Nevertheless, based on the data collected, it is not possible to state if this is due to an intrinsic difference among the fertilization strategies applied. A possible cause may be associated with the high experimental variability responsible for masking the real differences. However, the magnitude of the observed differences does not appear particularly consistent, and it can therefore be an indication of a real equivalence of effects, in particular, between chemical fertilization and compost administration. Although the tested compost was not able to completely equate the results obtained with the synthetic fertilizer, the yields were comparable. This observation is supported by the work of Anyega et al. [
26], who, by comparing composted BSF frass fertilizer (BSFFF), composted brewer’s spent grain, commercial organic fertilizer (Evergrow), mineral fertilizer, and some combinations, observed a substantial equivalence between BSFFF and chemical fertilization in terms of tomato yield in both greenhouse and in open field conditions. The two treatments were only overcome by the combination of BSFFF with synthetic fertilizer. The reader must be aware that only the repeated medium- and long-term administration of compost at agronomic or organic doses triggers improvement trajectories affecting both the physical and hydraulic properties of the soil [
68] and the bulk soil chemical and biological parameters, thus contributing to decreasing the amount of conventional fertilizer [
69].
The results obtained using FVWcomp were slightly higher than with GDcomp. This could be explained by a better composition in terms of micro- and macro-nutrients, which favored a higher production of marketable fruits. Based on the C/N ratio value, GDcomp appeared more mature and, therefore, more stable than FVWcomp. From a nutritional point of view, this means a slower nutrient release than FVWcomp. Conversely, FVWcomp, still not fully mature, could promote a faster release and, therefore, a prompt and greater availability of nutrients. The fact that the production of plants fertilized with GDcomp included the highest proportion of unripened fruits would confirm the effect.