Abstract
One of the most noticeable problems associated with the close location of piggeries is gaseous compounds emission. Ammonia and hydrogen sulfide emissions affect the quality of life of people living in the vicinity of such facilities. Among the diverse methods for managing and controlling malodorous substances, biological methods, which involve the utilization of microbiological agents, are widely employed. The use of bacterial strains is a relatively simple, low-cost, and ecological method. The study aimed to conduct a preliminary evaluation of the implementation of a novel consortium of deodorizing bacteria. The study involved the selection of bacteria, assessment of the antagonistic properties, implementation of the inoculum in a mesh-filled biofilter, and analysis of ammonia, hydrogen sulfide, and fine dust content in the air before and after passing through the mature biological bed. The results obtained demonstrate the effectiveness of the biofiltration bed in reducing ammonia levels, with a maximum decrease observed at 73.90%. For hydrogen sulfide, a removal efficiency of >72.08% was observed. Reduction in fine dust pollution also decreased from a level of 3.75 mg/m3 to 1.06 mg/m3. The study’s findings demonstrate the promising potential of utilizing a consortium of deodorizing bacteria as an effective approach to mitigating emissions from piggeries.
1. Introduction
The European Union is the world’s second-largest pork producer, trailing only behind China, and also holds the title of the largest pork exporter globally [1]. The leading pork producers among EU countries, listed in order, are Spain, Germany, France, Poland, the Netherlands, Denmark, and Italy [2]. In recent decades, a phenomenon of changing the structure of European farms, driven by the intensification of agriculture, has been observed. This shift encompasses not only crop production, but also extends to livestock farming. The industrialization of swine farming, which began in the late 1900s, also impacted these processes, resulting in the emergence of large-scale farms that consolidated acreage and resources [3]. It is noteworthy that until the 1960s, pig farming predominantly relied on extensive breeding conducted on small family-run farms [4]. The change in this trend has not been without environmental impact. Intensive livestock fattening units affect the quality of soil, water, and air in their surroundings, further contributing to global warming and increased resource consumption [5].
One of the most noticeable problems associated with the close location of piggeries is gaseous compounds emission. Malodorous substances affect the quality of life of people living in the vicinity of such facilities. The issue extends beyond psychological discomfort and subjective feelings and may pose health risks. It is especially significant in the context of prolonged exposure, which may contribute to the development of allergies, atopic dermatitis, asthma, or neurological damage [6,7,8]. The most common health complaints resulting from exposure to NH3 include irritation of the eyes, nose, and throat, headaches, nausea, diarrhea, hoarseness, sore throat, coughing, chest tightness, nasal congestion, palpitations, shortness of breath, feelings of stress, drowsiness, and mood changes [9]. H2S also poses a serious threat, capable of causing pneumonia, sudden unconsciousness (above 500 ppm), and even death from a single exposure when concentrations exceed 1000 ppm [10]. On the other hand, prolonged exposure to levels of H2S below 10 ppm can cause irritation of the eyes, nose, respiratory tract, and neurological symptoms [11].The primary source of atmospheric ammonia is animal production, through the volatilization and microbiological mineralization of organic nitrogen in animal manure [12]. Ammonia emissions are higher from industrial piggeries compared to the dairy cattle sector, although they still rank behind poultry farms [13]. Ammonia poses a dual challenge because it acts as both a greenhouse gas and displays high toxicity to humans and the environment [14]. Also noteworthy is the high toxicity of hydrogen sulfide, produced by microbial sulfate reduction processes in pig feces. The gas is often identified as the cause of many accidents and poisonings in animal production [15,16].
Currently, there are two main methods of controlling against increased gas emissions. The first, endogenous, includes preventive measures involving dietary adjustments and the usage of probiotics in animal feed [17]. The second method, exogenous, includes biological, chemical, and physical means of reducing emissions of the resulting gases outside production units [18]. Among the diverse methods for managing and controlling malodorous substances produced through intensive livestock production, biological methods, which involve the utilization of microbiological agents, are widely employed [19]. One of the oldest methods of biological air filtration is the use of classic biofilters. In these systems, pollutants are degraded by microbial consortia colonizing the filter bed. Another method involves the use of biological bioscrubbers, where the polluted air stream passes through a scrubbing solution. As a result, unwanted compounds and substances are captured in the water and degraded by microorganisms [20]. Another approach involves utilizing recombinant methods. Biotrickling is often regarded as an intermediate technology, combining aspects of both biofilters and bioscrubbers. Its mechanism involves the continuous circulation of a nutrient solution containing microorganisms within a reactor to degrade odor substances [21]. Technological solutions to reduce gas emissions from livestock housing are also an important aspect. These include the maintenance system, frequency of removal of manure from the ducts, reduction in water consumption, introduction of under-pipe scrapers, placement of ventilation inlets, and exhausts [22].
The use of bacterial strains is a relatively simple, low-cost, and environmentally friendly method of counteracting air pollution [23,24]. This approach aligns with the principles of sustainable agricultural production and agro-ecological practices aimed at mitigating the adverse effects of agriculture on ecosystems and enhancing the social well-being of communities residing near production sites [25].
The research hypothesis posits that the application of a selected bacterial consortium, which demonstrates no antagonistic interactions, can effectively decrease emissions of hydrogen sulfide and ammonia, leading to improved efficiency in animal production while concurrently mitigating the negative environmental impact. The aim of the study was to create a bacterial consortium and determine its effectiveness in reducing emissions of harmful gases (ammonia and hydrogen sulfide) and fine dust. The specific objective was to conduct a preliminary evaluation of the pilot implementation of the new bacteria consortium. The study involved several steps: selection of bacteria based on the literature data, assessment of the antagonistic properties of the proposed strains, implementation of the inoculum in a mesh-filled biofilter installed in operational piggeries, and analysis of ammonia, hydrogen sulfide, and fine dust (PM10) content in the air before and after passing through the mature biological bed. The identification and suggestion of new strains for commercial microbial consortia may play a significant role in enhancing air purification efficiency and promoting the advancement of environmentally friendly, non-intrusive agricultural practices.
2. Materials and Methods
2.1. The Selection of a Bacterial Consortium
The preparation of the bacterial strain list, which can be used to form deodorizing consortia, was guided by the following criteria:
- Literature data on the use of specific species of microorganisms in the removal of malodorous substances or published information on the metabolic characteristics of specific strains.
- Taxonomic affiliation of bacterial genera identified in samples collected from leachate water and biofilm from pig farm biofilters, as part of a previous in-house study [26].
- Environmental origin of the isolated bacteria: soil, activated sludge, wastewater treatment plant outflow water.
- Biosafety—strains classified as group BSL-1, which are non-pathogenic to humans or animals and are not phytopathogens.
- Culturing conditions and bacterial growth rate; the potential for relatively rapid proliferation of the strain to the desired quantity; composition of growth media. The list of proposed microorganisms comprises bacterial strains that can proliferate under aerobic conditions.
- Regulatory status: the use of strains in research and development is not restricted by the Nagoya Protocol.
An analysis of the available literature on the biological neutralization of malodorous compounds was conducted. Initially, scientific reports concerning the identification or implementation of bacterial strains were selected. Following this, a compilation of taxonomic affiliations and the origin of each microorganism was made. Potentially pathogenic organisms were eliminated from the compilation. Additionally, it was verified whether a given strain met the requirements of the Nagoya Protocol. Culturing conditions and the growth rate of bacteria were assessed during bacterial propagation for antagonism assessment tests, especially their ability to rapidly proliferate under aerobic conditions.
2.2. Evaluation of Bacterial Antagonism
To prepare test material from a 24 or 72 h culture of strains on medium Tryptic Soy Agar (TSA) (Merck, Darmstadt, Germany), bacterial suspensions were prepared at a concentration of 1.5 × 108 cfu/mL in phosphate-buffered saline (PBS) (Merck, Darmstadt, Germany). To a flask containing 150 mL of sterile and cooled TSA medium (2% agar), 1 mL of the prepared bacterial culture was added. The contents of the flask were thoroughly mixed, poured into Petri dishes, and incubated at 28 °C. After 24 or 72 h incubation in agar plates, 11 mm diameter columns were cut and transferred to plates with previously prepared (20 mL) TSA medium containing 0.2 mL of 24 or 72 h culture of another strain included in the consortium (agar slab method). The study involved the interaction of each strain within a consortium. After a 24- or 72 h incubation period, zones of inhibition in the growth of microorganism colonies were observed on the medium where slabs cut from agar containing other bacteria from the consortium were placed. As a positive control, slabs with deep cultures of bacteria were placed on a solidified TSA medium without added bacterial suspensions. The experiment was conducted in triplicate.
2.3. Pilot Implementation with Evaluation of Efficiency
2.3.1. Inoculation of Biofilter
The biological air purification unit is constructed as a modular system integrated into the ventilation ducts of the piggery. Within this module, a plastic mesh is installed to facilitate air circulation. The airflow through the mesh is regulated by fans positioned on the roof of the module. The entire system is equipped with sprinklers, pumps, and measuring devices to ensure optimal conditions for the growth of the desired microorganisms.
Biofilter parameters:
- Dimensions: 1800 mm (width) × 10,500 mm (length) × 900 mm (height).
- Bed area: 18.9 m2.
- Flow rate: Dependent on ventilation size: 0.2 to 1.0 m/s.
- Fan capacity: 10,000 to 70,000 m3/h.
- Sprinkling rate: 5 to 15 L/min/m2.
- Pig house air temperature: 20.5 °C to 24 °C.
- Bed load: Minimum 530 m3/h per 1 m2; maximum 3650 m3/h per 1 m2 of bed.
Bacterial suspensions at a concentration of 2.5 × 109 cfu/mL were introduced into the filter bed using a specially designed system:
- Tanks for the bacterial consortium suspension.
- Air supply tubes.
- Discharge tubes for the suspension of bacteria.
- Nozzles.
- Air compressor.
The compressed air, produced by a compressor, was conveyed to tanks containing bacterial inoculum. The resulting overpressure was utilized to transfer the inoculum through tubes (diameter: 6 mm) connected to low-pressure spray nozzles. These nozzles evenly disperse the inoculum across the surface of the biological filter bed.
2.3.2. Evaluation of the Efficiency of Ammonia, Hydrogen Sulfide, and Fine Dust Removal
The following measurement and test equipment was used during the measurements:
- Madur PGD-100 gas conditioner (Madur Electronics, Zgierz, Poland).
- Madur Photon gas analyzer (Madur Electronics, Zgierz, Poland).
- Bundling tube.
- TAD wing anemometer (Trotec, Marchtrenk, Austria).
- Silica gel scrubber set.
- P-10 ZA central dust meter unit (CJP-10) (ZAM Kęty, Kęty, Poland).
- Dehumidifier (Airpol, Poznań, Poland).
- Dust meter set with heated titanium probe (EMIO, Wrocław, Poland)
- WPT 3/6 electronic balance (Radwag, Radom, Poland).
- AWE-PW total organic carbon analyzer (LAT, Katowice, Poland).
- Vacuum pump (Busch Group, Schwarzwald, Germany).
Sampling for the determination of ammonia mass concentration was conducted using a side-track dust meter system equipped with a titanium aspiration track. An internal filtration system was employed, featuring a quartz flat filter in the main track and a scrubber system with absorbing solutions in the side track. Isokinetic aspiration was utilized for waste gases. The gas flowed through the filter material and was subsequently divided into two streams, with one passing through the scrubber system. The probe and titanium tee were heated, and the temperature stabilized. Measurements of the volume, pressures, and temperatures of the two gas streams were recorded. The aspiration time was set at 30 min. Before and following the measurements, blind samples were collected. Samples underwent the entire measurement procedure, excluding the aspiration stage. The blank samples obtained were treated identically to the measurement samples. The analysis was conducted using the spectrophotometric method outlined in the EN-ISO 7150-1 [27].
Sampling for the determination of hydrogen sulfide concentration was performed using a system equipped with a sampling probe with a particulate filter, a scrubber set with absorbent fluid, a gas dryer, and an aspirator. The aspirated compound was absorbed in the absorbent solution, which was transferred to the laboratory, where determinations were made. Determination of hydrogen sulfide content was conducted using the spectrophotometric method [28].
The dust concentration was measured using the gravimetric method following the requirements of PN-EN 13284-1:2018 [29], taking into account the requirements of PN-EN 15259:2007 [30], PN-EN 14790:2017 [31], and PN-EN ISO 16911-1:2013 [32]. Sampling for the determination of dust concentration was conducted using a dust meter system equipped with a titanium aspiration track. An internal filtration system employing a quartz flat filter was utilized. Following the measurement, the filter system underwent washing with distilled water and acetone to extract precipitates from the front of the filter. The mass of extracted precipitates was combined with the mass of dust captured on the flat filter. Isokinetic aspiration was employed for waste gases, and the dust meter system was temperature stabilized. Measurements of the gas stream volume, as well as its pressure and temperature, were conducted. Samples and sediments collected from the filter system were transferred to the laboratory for the determination of dust mass.
3. Results
3.1. Selection of a Bacterial Consortium
Various bacterial species were identified as a result of the first stage of bacterial strain screening. Among them, bacteria belonging to the Bacillus genus were distinguished, known for their ability to produce spore forms (endospores). These bacteria are recognized for their high resistance to adverse environmental conditions, making them potentially valuable candidates for application in biodegradation and environmental remediation processes.
Typical, genetically well-characterized strains were selected as the basis for the consortium (Table 1). These isolates were sourced from the German Collection of Microorganisms and Cell Cultures (DSMZ), a renowned repository known for its extensive collection of microbial strains.

Table 1.
Summary of selected strains.
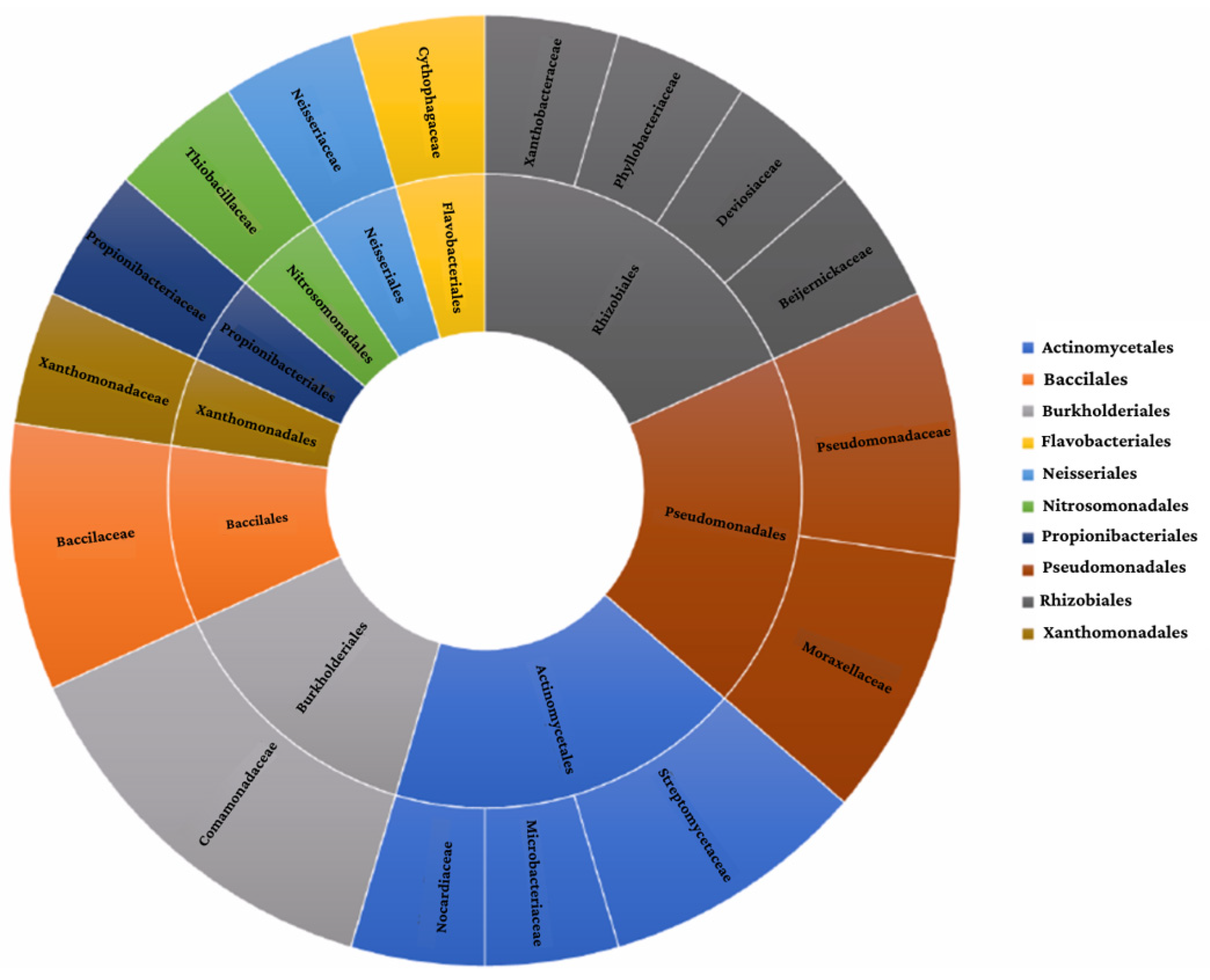
Based on a literature search, 21 strains belonging to 10 different orders were selected (Actinomycetales, Bacillales, Burkholderiales, Flavobacteriales, Neisseriales, Nitrosomonadales, Propionibacterials, Pseudomonadales, Rhizobiales, Xanthomonadales) (Figure 1).
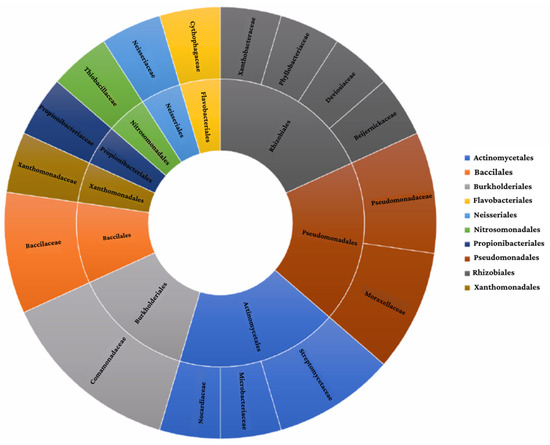
Figure 1.
Summary of the proposed consortium’s taxonomic classification.
3.2. Evaluation of Bacterial Antagonism
To uniquely identify each strain and enable tracking of test results, individual strains were assigned identification numbers (Table 2).

Table 2.
Summary of the ID numbers of the tested strains.
The test results did not generally confirm antagonistic interactions among the strains examined (Table 3). The presence of small areas of reduced bacterial colony growth near other strains suggests competitive interactions for nutrients in the medium rather than toxic effects. However, one interaction showing a distinct zone of growth inhibition was observed, specifically in the case of strain No. 4 when cultured on a medium containing strain No. 20.

Table 3.
Results of antagonistic interactions test among bacteria.
The following strains were excluded as a result of the research:
- Thiobacillus thioparus (too slow and weak growth on culture media).
- Thiopseudomonas denitrificans (demonstrating of antagonism).
- Xanthobacter viscosus (too slow and weak growth on culture media).
3.3. Pilot Inoculation of Biofilter
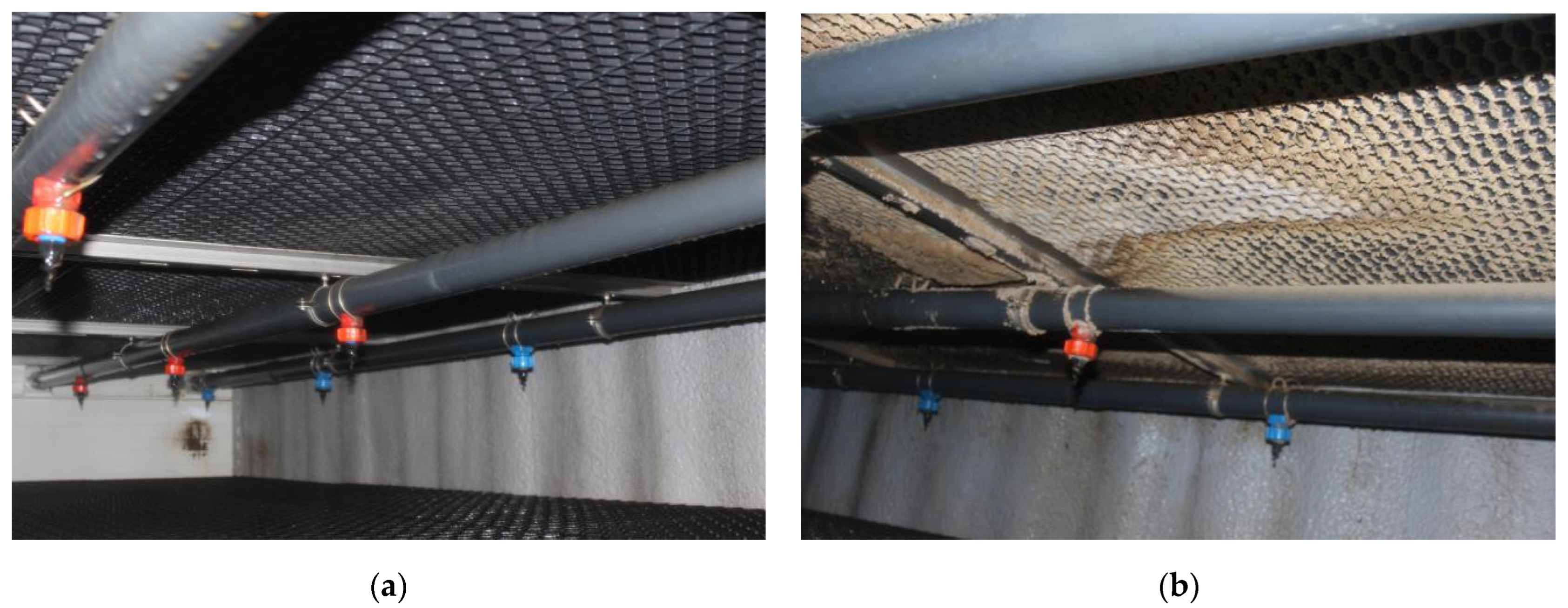
The initial application of bacteria to the filter bed took place approximately one week after the piglets were introduced into the facility. During this time, no sediment formation was detected on the bottom side of the bed across its entire surface. Additionally, there was a noticeable trend of increasing pH in the liquid flowing through the bed, with the pH value exceeding 8. After 11 days from the initial application of microorganisms, a slight deposition of dust was observed on the bottom side of the bed. Subsequently, the pH value of the liquid flowing through the bed stabilized within the range of 7.5 to 7.8. Approximately 8 weeks after the animals were introduced into the facility, visible contamination became evident. The outlet channel of the center stack also displayed signs of operation, while the other two outlet stacks showed significantly less contamination (Figure 2).
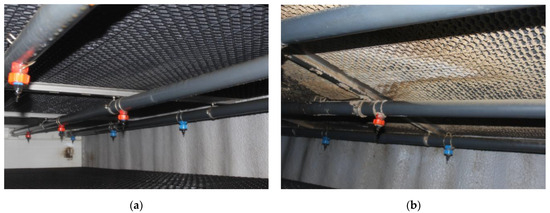
Figure 2.
Progress in the colonization of the biofilter bed by the bacterial consortium. (a) The upper part of the mesh filling 7 days after inoculation. (b) The upper part of the mesh filling 8 weeks after inoculation.
3.4. Evaluation of the Efficiency of Ammonia, Hydrogen Sulfide, and Fine Dust Removal
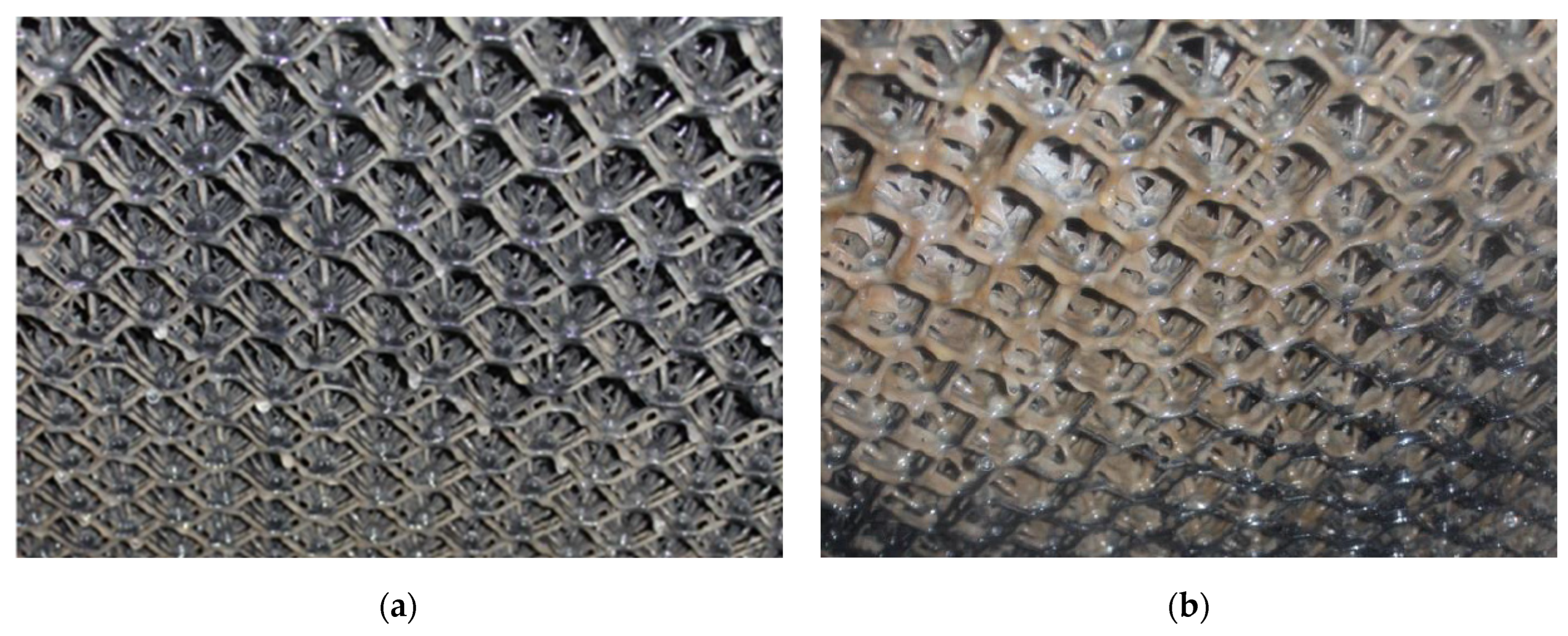
The research was conducted after the establishment of the bacterial consortium in the biofilter. A noticeable increase in bacterial sediment was observed in the central part of the bed after 8 weeks of its operation (Figure 3).
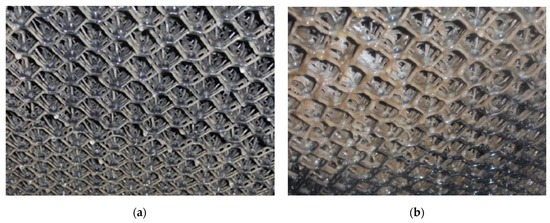
Figure 3.
Progress in the colonization of the filter bed, in the middle section. (a) The middle part of the mesh filling 19 days after inoculation. (b) The middle part of the mesh filling 8 weeks after inoculation.
The calculation of the efficiency of the modular biofilter’s performance, inoculated with the proposed bacterial consortium, was based on the percentage change analysis between the averaged air results prevailing in the piggery and the air emitted through the three stacks of the biofiltration bed (Table 4).

Table 4.
Results of ammonia, hydrogen sulfide, and fine dust reduction efficiency evaluation.
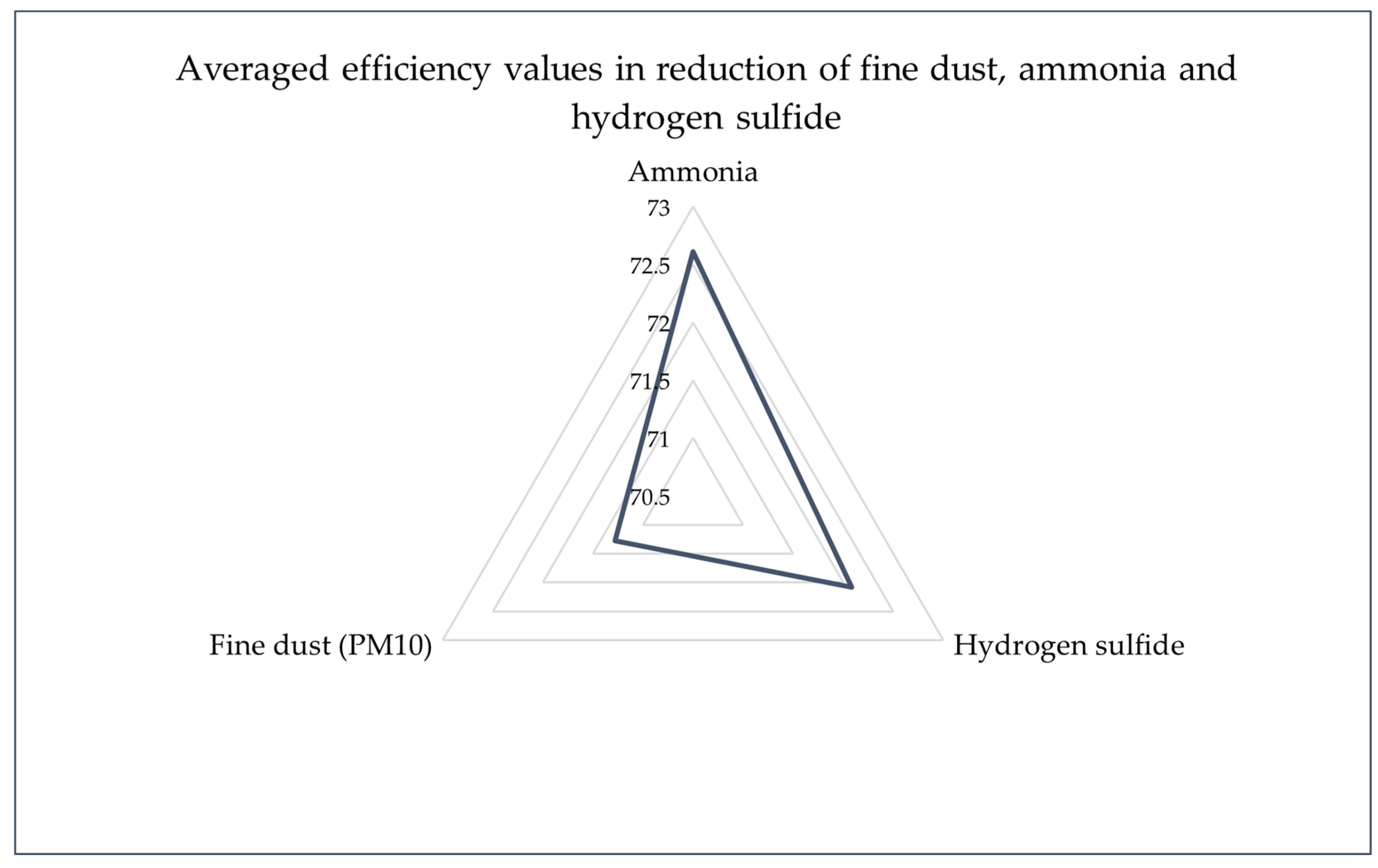
In the piggery air, the concentration of ammonia measured 13.45 mg/m3, which decreased to 3.60 mg/m3 in the emitted air, showcasing a notable reduction and an effectiveness of 73.23% in mitigating ammonia emissions (Emitter 1). Initial levels of hydrogen sulfide in the piggery air were recorded at 2.65 mg/m3. Remarkably, the emitted air showed levels below 0.74 mg/m3, indicating an effectiveness of over 72.08% in reducing hydrogen sulfide emissions (Emitter 1). Additionally, for Emitter 1, the concentration of fine dust in the piggery air stood at 3.75 mg/m3. Following the biofiltration process, the emitted air exhibited a reduced concentration of 1.09 mg/m3, demonstrating an effectiveness of 70.93% in fine dust pollution reduction. Emitter 2 demonstrated similar trends in pollutant reduction, with ammonia, hydrogen sulfide, and fine dust concentrations reduced by 70.71%, >72.08%, and 71.20%, respectively. Comparable reductions in pollutant concentrations were observed for Emitter 3, with ammonia, hydrogen sulfide, and fine dust levels showing reductions of 73.90%, >72.08%, and 71.73%, respectively (Figure 4).
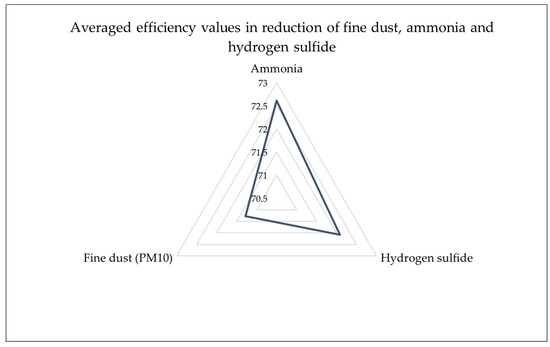
Figure 4.
Averaged efficiency values in reduction in fine dust, ammonia, and hydrogen sulfide (assuming the lowest values for the efficiency of hydrogen sulfide reduction).
4. Discussion
The research hypothesis posited that the application of a selected bacterial consortium, demonstrating no antagonistic interactions, would effectively reduce hydrogen sulfide and ammonia emissions, thereby improving the efficiency of animal production while mitigating the negative environmental impact. Considering achieving moderate effectiveness, while this outcome is significant, further research is needed to refine this approach and fully understand its potential.
Biofiltration of air in animal husbandry and agricultural production units has a well-documented history. The first biofilters were utilized in Europe in the late 1960s, and in the 1990s began to be implemented in North America [46]. In recent years, there have been increased efforts to mitigate gas and gaseous compound emissions from animal production units, alongside progressive improvements in air pollution control measures [47]. Nowadays, there is a scientific consensus indicating that biofilters play a crucial role in reducing greenhouse gas emissions. Further efforts to enhance their operation are desirable, not only to mitigate the effects of global warming but also to reduce the dissemination of malodorous substances [48,49].
Research and optimization of biofilms in closed intensive livestock farming systems have been the focus of scientists’ work for many years. However, to the best of the authors’ knowledge, this is one of the first studies to adopt a comprehensive approach, ranging from selecting a broad spectrum of strains to full-scale biofilm growth and evaluating the efficiency of removing ammonia, hydrogen sulfide, and fine dust. Screening for microorganisms typically involves isolating bacteria from feces, activated sludge, or active biofilters. In a prior in-house study, microbial communities residing in biofilters at an industrial Swiss fattening plant were identified using sequencing of 16S rRNA gene fragments (V3–V4) [26]. The current study relied on available literature data to select phylogenetically diverse species based on six initial assumptions. That approach and the exclusion of antagonistic and slow-growing strains allowed for the development of fast-growing deodorizing biofilm. Furthermore, this study investigates a biofilter with a non-traditional medium. In our study inoculation was conducted on a mesh filling. This approach is less common, as the most frequently utilized media include compost, peat, wood chips, activated charcoal, perlite, ceramic fill, and magma rocks [50]. This is particularly significant, considering the limitations associated with the utilization of natural organic packing materials. As noted by Tymczyna et al., the lifespan of a natural bed typically ranges from 3 to 5 years [51].
Our study demonstrated ammonia reductions ranging from 70.71% to 73.23%. These results are in line with reports associated with full-scale ammonia removal efficiency studies. Reduction levels in full-scale biofilters range between 40% and 100% and are typically lower than laboratory scale [52,53]. Research conducted by Baltrėnas et al. on an experimental biofilter utilizing various plate types (synthetic material and wood fiber) demonstrated the highest efficiency in ammonia reduction, reaching 87% at 28 °C [54]. Divergences may arise because our study was conducted in situ with the implementation of bacteria only. Reports indicating the high utility of fungal cultures align with the findings of Marycz et al., which demonstrated the effectiveness of employing fungal co-cultures in hydrophobic VOCs removal [55]. Nevertheless, it is worth noting that VOCs exhibit lower solubility in water compared to NH3 and H2S [23]. As noted by Ghaseni et al., despite advancements in both bacterial and fungal filtration, there still exists a research gap concerning the comparison of the efficiency of both types of biofilters [56]. It is important to recognize that over time, natural microbiological succession will occur within the biofilter we’re studying, resulting in changes to the species composition of the biofilms.
In our study, the reduction level for hydrogen sulfide remained consistently high, above 70%, similar to the findings for ammonia removal. Akdeniz et al.’s study demonstrated pilot hydrogen sulfide reduction ranging from 49.0% to 85.0%, depending on the biofilter type and location (dairy farm, pig fattening farm, and pig farm) [57]. Kumdhitiahutsawakul et al. emphasize that the flow rate is critical to the efficiency of hydrogen sulfide reduction, with efficiency decreasing as flow levels increase [58]. In contrast, a study conducted by Omri et al. achieved a reduction of 99% in H2S removal efficiency from effluent gases. The authors also highlight peat as an effective medium owing to its favorable environmental conditions for bacteria, characterized by high water retention and buffering properties [59]. A different approach was adopted in a study by Zheng et al., where a polyurethane foam cube fill with dimensions of 1.0 cm3, a density of 20 kg/m3, and a porosity of 95% was chosen as the filling material. This study investigated biofilters for co-treatment of H2S and NH3 in a domestic waste landfill site. The achieved reduction rates were 82.6–91.1% and 81.1–92.0%, respectively [60]. Considering similar investigations into the effectiveness of biofilters in reducing hydrogen sulfide emissions, it can be concluded that our results align with general trends related to the effective removal of this chemical compound.
The studies incorporated in our paper also assessed the degree of effectiveness in removing fine dust (PM10) by a pilot biofilter. This represents a somewhat novel approach, considering that biofilters are typically engineered to target the removal of ammonia, hydrogen sulfide, or VOCs, with less focus on PM10 concerns [61]. It is also worth noting that the problem of fine dust emissions may be associated with the propagation of dangerous pathogens that use matter particles as carriers. This problem can induce diseases in both humans and animals, affecting their welfare and disrupting agricultural production [62]. Our study observed PM10 emission reductions exceeding 70%. However, Melse et al. achieved even higher results, reporting over 90% reduction using a bed filled with wood chips [63].
Among the various studies examining the scientific efficacy of biofilters for air purification, there are different approaches to the microbiological aspect. These include spontaneous self-colonization of the bed, utilizing inoculum to support initial colonization from existing biological materials (e.g., activated sludge), or using pre-prepared inoculum [26,64,65]. In our study, the strains obtained from the German Collection of Microorganisms and Cell Cultures originated from various environments, including activated sludge and effluent water from wastewater treatment plants. The approach we proposed aimed at a broad application of bacteria from diverse habitats to optimize the consortium. It is noteworthy that laboratory-scale studies often employ inoculation with a single genus or even a single species of bacteria [66,67,68].
Currently, the utilization of molecular techniques, notably 16S rRNA sequencing, has become a prevalent analytical method in investigating environmental microbiota [69,70,71]. In our study, we did not perform validation of the growth of the inoculated consortium using microorganism sequencing growing on the biofilter’s medium. The reason for choosing such an approach includes the fact that the bacterial community is strongly influenced by diverse factors (temperature, humidity, antagonistic interactions, etc.). Considering the succession of microorganisms and changes in the proportion of individual bacterial groups as a natural and common phenomenon [72,73], we regarded the tested inoculum as a starter and a base for the rapid colonization of the substrate.
In summary, air biofiltration is a widely adopted method in agriculture and livestock production for air purification. However, there is a need for ongoing efforts and contributions to optimize this process. Several factors influence the efficacy of air purification, including the diversity and origin of microorganisms, the type of biofiltration system, the filling material used, the type of livestock, regulatory factors, seasonal variations, and geographic location. Continued research and development in this field are essential for maximizing the effectiveness of air biofiltration systems.
5. Conclusions
The study’s findings demonstrate the promising potential of utilizing a consortium of bacteria as an effective approach to mitigating harmful gases from piggeries. Reductions in ammonia, hydrogen sulfide, and particulate matter emissions are observed. The observed reduction in ammonia, hydrogen sulfide, and particulate matter suggests that this method could serve as an efficient tool in addressing gaseous compound challenges within the livestock industry.
The process of selecting suitable strains of bacteria based on the literature research and evaluating their antagonistic properties is crucial for the effectiveness of gaseous compound mitigation. These findings hold practical significance for both the livestock industry and local communities.
Implementing effective methods can enhance the quality of life for nearby residents and diminish the adverse environmental impacts associated with piggery operations.
Author Contributions
Conceptualization, Z.P. and G.G.; methodology, Z.P., A.-K.K. and B.B.-B.; validation, Z.P. and G.G.; formal analysis, Z.P., A.-K.K., B.B.-B., G.G. and P.K.; investigation, Z.P. and A.-K.K.; resources, P.K. and Z.P.; data curation, A.-K.K. and Z.P.; writing—original draft preparation, P.K.; writing—review and editing, P.K. and B.B.-B.; visualization, P.K.; supervision, Z.P. and B.B.-B.; project administration, Z.P.; funding acquisition, Z.P. and B.B.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Smart Growth Program: R&D and manufacturing of a prototype installation for purification of used air from pig breeding buildings, together with testing no. POIR.01.01.01–00-0330/17.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All relevant data supporting the findings of this article are included within the manuscript. Raw data, as well as any additional materials, are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bellini, S. 7. The Pig Sector in the European Union. In Understanding and Combatting African Swine Fever; Iacolina, L., Penrith, M.-L., Bellini, S., Chenais, E., Jori, F., Montoya, M., Ståhl, K., Gavier-Widén, D., Eds.; Brill|Wageningen Academic: Vienna, Austria, 2021; pp. 183–195. ISBN 978-90-8686-910-7. [Google Scholar]
- EUROSTAT. Agricultural Production—Livestock and Meat; European Statistical Office: Luxembourg, 2023. [Google Scholar]
- Park, H.-S.; Min, B.; Oh, S.-H. Research Trends in Outdoor Pig Production—A Review. Asian-Australas J. Anim. Sci. 2017, 30, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Tzanidakis, C.; Simitzis, P.; Arvanitis, K.; Panagakis, P. An Overview of the Current Trends in Precision Pig Farming Technologies. Livest. Sci. 2021, 249, 104530. [Google Scholar] [CrossRef]
- Pirlo, G.; Carè, S.; Casa, G.D.; Marchetti, R.; Ponzoni, G.; Faeti, V.; Fantin, V.; Masoni, P.; Buttol, P.; Zerbinatti, L.; et al. Environmental Impact of Heavy Pig Production in a Sample of Italian Farms. A Cradle to Farm-Gate Analysis. Sci. Total Environ. 2016, 565, 576–585. [Google Scholar] [CrossRef]
- Wroniszewska, A.; Zwoździak, J. Odor Annoyance Assessment by Using Logistic Regression on an Example of the Municipal Sector. Sustainability 2020, 12, 6102. [Google Scholar] [CrossRef]
- Chmielowiec-Korzeniowska, A.; Tymczyna, L.; Wlazło, Ł.; Trawińska, B.; Ossowski, M. Emissions of Gaseous Pollutants from Pig Farms and Methods for Their Reduction—A Review. Ann. Anim. Sci. 2022, 22, 89–107. [Google Scholar] [CrossRef]
- Piccardo, M.T.; Geretto, M.; Pulliero, A.; Izzotti, A. Odor Emissions: A Public Health Concern for Health Risk Perception. Environ. Res. 2022, 204, 112121. [Google Scholar] [CrossRef] [PubMed]
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia Emissions from Agriculture and Their Contribution to Fine Particulate Matter: A Review of Implications for Human Health. J. Environ. Manag. 2022, 323, 116285. [Google Scholar] [CrossRef] [PubMed]
- Austigard, Å.D.; Svendsen, K.; Heldal, K.K. Hydrogen Sulphide Exposure in Waste Water Treatment. J. Occup. Med. Toxicol. 2018, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Batterman, S.; Grant-Alfieri, A.; Seo, S.-H. Low Level Exposure to Hydrogen Sulfide: A Review of Emissions, Community Exposure, Health Effects, and Exposure Guidelines. Crit. Rev. Toxicol. 2023, 53, 244–295. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Winiwarter, W.; Bai, Z.; Wang, X.; Fan, X.; Zhu, Z.; Hu, C.; Ma, L. Strategies to Reduce Ammonia Emissions from Livestock and Their Cost-Benefit Analysis: A Case Study of Sheyang County. Environ. Pollut. 2021, 290, 118045. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Heber, A.J.; Lim, T.-T. Ammonia and Hydrogen Sulfide in Swine Production. In Air Quality and Livestock Farming; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-73833-8. [Google Scholar]
- Jo, G.; Ha, T.; Jang, Y.N.; Hwang, O.; Seo, S.; Woo, S.E.; Lee, S.; Kim, D.; Jung, M. Ammonia Emission Characteristics of a Mechanically Ventilated Swine Finishing Facility in Korea. Atmosphere 2020, 11, 1088. [Google Scholar] [CrossRef]
- Brglez, Š. Risk Assessment of Toxic Hydrogen Sulfide Concentrations on Swine Farms. J. Clean. Prod. 2021, 312, 127746. [Google Scholar] [CrossRef]
- Liu, S.; Ni, J.-Q.; Radcliffe, J.S.; Vonderohe, C. Hydrogen Sulfide Emissions from a Swine Building Affected by Dietary Crude Protein. J. Environ. Manag. 2017, 204, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cho, J.H.; Chen, Y.J.; Yoo, J.S.; Huang, Y.; Kim, H.J.; Kim, I.H. The Effect of Probiotic BioPlus 2B® on Growth Performance, Dry Matter and Nitrogen Digestibility and Slurry Noxious Gas Emission in Growing Pigs. Livest. Sci. 2009, 120, 35–42. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, X.; Yuan, Y.; Liao, Y.; Li, X. Deodorization Study of the Swine Manure with Two Yeast Strains. Biotechnol. Bioproc. E 2013, 18, 135–143. [Google Scholar] [CrossRef]
- Ma, H.; Li, F.; Niyitanga, E.; Chai, X.; Wang, S.; Liu, Y. The Odor Release Regularity of Livestock and Poultry Manure and the Screening of Deodorizing Strains. Microorganisms 2021, 9, 2488. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, B.; Jia, Y.; He, F.; Chen, W. Mitigation Strategies of Air Pollutants for Mechanical Ventilated Livestock and Poultry Housing—A Review. Atmosphere 2022, 13, 452. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Han, M.-F.; Jia, T.-P.; Hu, X.-R.; Zhu, H.-Q.; Tong, Z.; Lin, Y.-T.; Wang, C.; Liu, D.-Z.; Peng, Y.-Z.; et al. Emissions, Measurement, and Control of Odor in Livestock Farms: A Review. Sci. Total Environ. 2021, 776, 145735. [Google Scholar] [CrossRef]
- Bujny, J.; Maśliński, M. Zwalczanie uciążliwości zapachowych w świetle aktualnie obowiązujących przepisów prawnych. Finans. Komunal. 2018, 23–40. [Google Scholar]
- Barbusiński, K.; Parzentna-Gabor, A.; Kasperczyk, D. Removal of Odors (Mainly H2S and NH3) Using Biological Treatment Methods. Clean Technol. 2021, 3, 138–155. [Google Scholar] [CrossRef]
- Kwarciak-Kozłowska, A.; Gałwa-Widera, M. Chapter 11—Biofiltration as an Ecological Method of Removing Sewage Sludge Odors by Solar Drying. In Sustainable and Circular Management of Resources and Waste towards a Green Deal; Vara Prasad, M.N., Smol, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 151–161. ISBN 978-0-323-95278-1. [Google Scholar]
- Bezner Kerr, R.; Liebert, J.; Kansanga, M.; Kpienbaareh, D. Human and Social Values in Agroecology: A Review. Elem. Sci. Anthr. 2022, 10, 00090. [Google Scholar] [CrossRef]
- Paluszak, Z.; Kanarek, P.; Gryń, G.; Breza-Boruta, B. Deodorizing Bacterial Consortium: Community Analysis of Biofilms and Leachate Water Collected from an Air Biofiltration System in a Piggery. Environ. Sci. Pollut. Res. Int. 2024, 31, 18993–19001. [Google Scholar] [CrossRef] [PubMed]
- PN-ISO 7150-1:1984; Determination of Ammonium Part 1: Manual Spectrometric Method. International Organization for Standardization: Geneva, Switzerland, 1984.
- PN-Z-04015-13:1996; Protection of Air Purity—Tests of Sulfur and Its Compounds—Determination of Hydrogen Sulfide at Workplaces by Spectrophotometric Method. Polish Committee for Standardization: Warsaw, Poland, 1996.
- PN-EN 13284-1:2018-02; Stationary Source Emissions—Determination of Low Range Mass Concentration of Dust—Part 1: Manual Gravimetric Method. German Institute for Standardisation: Berlin, Germany, 2018.
- PN-EN 15259:2007; Jakość Powietrza—Pomiary Emisji Ze Źródeł Stacjonarnych—Wymagania Dotyczące Miejsc Pomiaru i Odcinków Pomiarowych, Celu i Planowania Pomiaru Oraz Sprawozdania. Comité Européen de Normalisation: Brussels, Belgium, 2007.
- PN-EN 14790:2017-04; Emisja Ze Źródeł Stacjonarnych—Oznaczanie Pary Wodnej w Przewodach—Standardowa Metoda Odniesienia. Polish Committee for Standardization: Warsaw, Poland, 2017.
- PN-EN ISO 16911-1:2013; Emisja Ze Źródeł Stacjonarnych—Manualne i Automatyczne Wyznaczanie Prędkości i Strumienia Objętości w Przewodach—Część 2: Automatyczne Systemy Pomiarowe. UNI: Rome, Italy, 2013.
- Heylen, K.; Lebbe, L.; De Vos, P. Acidovorax Caeni Sp. Nov., a Denitrifying Species with Genetically Diverse Isolates from Activated Sludge. Int. J. Syst. Evol. Microbiol. 2008, 58, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Bourque, D.; Bisaillon, J.-G.; Beaudet, R.; Sylvestre, M.; Ishaque, M.; Morin, A. Microbiological Degradation of Malodorous Substances of Swine Waste under Aerobic Conditions. Appl. Environ. Microbiol. 1987, 53, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Jolicoeur, P.; Morin, A. Isolation of Acinetobacter calcoaceticus Strains Degrading the Volatile Fatty Acids of Swine Wastes. Biol. Wastes 1987, 19, 133–140. [Google Scholar] [CrossRef]
- Walden, C.; Zhang, W. Bioaccumulation of Silver Nanoparticles in Model Wastewater Biofilms. Environ. Sci. Water Res. Technol. 2018, 4, 1163–1171. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Islam, M.; Mun, H.-S.; Sim, H.-J.; Kim, Y.-J.; Yang, C.-J. Effects of Bacillus Amyloliquefaciens as a Probiotic Strain on Growth Performance, Cecal Microflora, and Fecal Noxious Gas Emissions of Broiler Chickens. Poult. Sci. 2014, 93, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ahmed, S.T.; Islam, M.M.; Yang, C.J. Evaluation of Bacillus Amyloliquefaciens as Manure Additive for Control of Odorous Gas Emissions from Pig Slurry. Afr. J. Microbiol. Res. 2014, 8, 2540–2546. [Google Scholar] [CrossRef]
- Qi, W.; Mei, S.; Yuan, Y.; Li, X.; Tang, T.; Zhao, Q.; Wu, M.; Wei, W.; Sun, Y. Enhancing Fermentation Wastewater Treatment by Co-Culture of Microalgae with Volatile Fatty Acid- and Alcohol-Degrading Bacteria. Algal Res. 2018, 31, 31–39. [Google Scholar] [CrossRef]
- Patureau, D.; Helloin, E.; Rustrian, E.; Bouchez, T.; Delgenes, J.P.; Moletta, R. Combined Phosphate and Nitrogen Removal in a Sequencing Batch Reactor Using the Aerobic Denitrifier, Microvirgula Aerodenitrificans. Water Res. 2001, 35, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Kämpfer, P.; Müller, C.; Mau, M.; Neef, A.; Auling, G.; Busse, H.J.; Stolz, A. Description of Pseudaminobacter Gen. Nov. with Two New Species, Pseudaminobacter salicylatoxidans sp. Nov. and Pseudaminobacter defluvii sp. Nov. Int. J. Syst. Bacteriol. 1999, 49, 887–897. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, P.; Hao, B.; Yu, Z. Heterotrophic Nitrification and Aerobic Denitrification by the Bacterium Pseudomonas stutzeri YZN-001. Bioresour. Technol. 2011, 102, 9866–9869. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, M.; Jones, A.L.; Maldonado, L.A.; Salanitro, J. Rhodococcus aetherivorans sp. Nov., A New Species That Contains Methyl t-Butyl Ether-Degrading Actinomycetes. Syst. Appl. Microbiol. 2004, 27, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Ikeda, M. Deodorization of Pig Feces by Actinomycetes. Appl. Environ. Microbiol. 1978, 36, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. A Review of Microbiology in Swine Manure Odor Control. Agric. Ecosyst. Environ. 2000, 78, 93–106. [Google Scholar] [CrossRef]
- Nicolai, R.; Leffer, R. Biofilters Used to Reduce Emissions from Livestock Housing—A Literature Review. In Proceedings of the Workshop on Agricultural Air Quality: State of the Science, Potomac, MA, USA, 5–8 June 2006; pp. 5–8. [Google Scholar]
- Cao, T.; Zheng, Y.; Dong, H. Control of Odor Emissions from Livestock Farms: A Review. Environ. Res. 2023, 225, 115545. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Bodraya, T.; Lackner, M. Methane Biofiltration Processes: A Summary of Biotic and Abiotic Factors. Methane 2024, 3, 122–148. [Google Scholar] [CrossRef]
- Shang, B.; Zhou, T.; Tao, X.; Chen, Y. Greenhouse Gas Emissions from Biofilters for Composting Exhaust Ammonia Removal. Front. Bioeng. Biotechnol. 2022, 10, 918365. [Google Scholar] [CrossRef]
- Yang, N.; Wang, C.; Han, M.-F.; Li, Y.-F.; Hsi, H. Performance Improvement of a Biofilter by Using Gel-Encapsulated Microorganisms Assembled in a 3D Mesh Material. Chemosphere 2020, 251, 126618. [Google Scholar] [CrossRef]
- Tymczyna, L.; Chmielowiec-Korzeniowska, A.; Saba, L. Biological Treatment of Laying House Air with Open Biofilter Use. Pol. J. Environ. Stud. 2004, 13, 425–428. [Google Scholar]
- Rolewicz-Kalińska, A.; Lelicińska-Serafin, K.; Manczarski, P. Volatile Organic Compounds, Ammonia and Hydrogen Sulphide Removal Using a Two-Stage Membrane Biofiltration Process. Chem. Eng. Res. Des. 2021, 165, 69–80. [Google Scholar] [CrossRef]
- Melse, R.W.; Ploegaert, J.P.M.; Ogink, N.W.M. Biotrickling Filter for the Treatment of Exhaust Air from a Pig Rearing Building: Ammonia Removal Performance and Its Fluctuations. Biosyst. Eng. 2012, 113, 242–252. [Google Scholar] [CrossRef]
- Baltrėnas, P.; Januševičius, T.; Zagorskis, A.; Baltrėnaitė-Gedienė, E. Removal of Ammonia by Biofilters with Straight and Wavy Lamellar Plates. Int. J. Environ. Sci. Technol. 2021, 18, 1181–1190. [Google Scholar] [CrossRef]
- Marycz, M.; Brillowska-Dąbrowska, A.; Cantera, S.; Gębicki, J.; Muñoz, R. Fungal Co-Culture Improves the Biodegradation of Hydrophobic VOCs Gas Mixtures in Conventional Biofilters and Biotrickling Filters. Chemosphere 2023, 313, 137609. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, R.; Golbabaei, F.; Rezaei, S.; Pourmand, M.R.; Nabizadeh, R.; Jafari, M.J.; Masoorian, E. A Comparison of Biofiltration Performance Based on Bacteria and Fungi for Treating Toluene Vapors from Airflow. AMB Expr. 2020, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz, N.; Janni, K.A. Full-Scale Biofilter Reduction Efficiencies Assessed Using Portable 24-Hour Sampling Units. J. Air Waste Manag. Assoc. 2012, 62, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Kumdhitiahutsawakul, L.; Jirachaisakdeacha, D.; Kantha, U.; Pholchan, P.; Sattayawat, P.; Chitov, T.; Tragoolpua, Y.; Bovonsombut, S. Removal of Hydrogen Sulfide from Swine-Waste Biogas on a Pilot Scale Using Immobilized Paracoccus Versutus CM1. Microorganisms 2022, 10, 2148. [Google Scholar] [CrossRef] [PubMed]
- Omri, I.; Bouallagui, H.; Aouidi, F.; Godon, J.-J.; Hamdi, M. H2S Gas Biological Removal Efficiency and Bacterial Community Diversity in Biofilter Treating Wastewater Odor. Bioresour. Technol. 2011, 102, 10202–10209. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Li, L.; Chai, F.; Wang, Y. Factors Impacting the Performance and Microbial Populations of Three Biofilters for Co-Treatment of H2S and NH3 in a Domestic Waste Landfill Site. Process Saf. Environ. Prot. 2021, 149, 410–421. [Google Scholar] [CrossRef]
- Bist, R.B.; Chai, L. Advanced Strategies for Mitigating Particulate Matter Generations in Poultry Houses. Appl. Sci. 2022, 12, 11323. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, K.; Liu, J.; Jin, X.; Li, C. Distribution Characteristics of Bioaerosols inside Pig Houses and the Respiratory Tract of Pigs. Ecotoxicol. Environ. Saf. 2021, 212, 112006. [Google Scholar] [CrossRef]
- Melse, R.W.; Hol, J.M.G. Biofiltration of Exhaust Air from Animal Houses: Evaluation of Removal Efficiencies and Practical Experiences with Biobeds at Three Field Sites. Biosyst. Eng. 2017, 159, 59–69. [Google Scholar] [CrossRef]
- Lamprea Pineda, P.A.; Demeestere, K.; González-Cortés, J.J.; Alvarado-Alvarado, A.A.; Boon, N.; Devlieghere, F.; Van Langenhove, H.; Walgraeve, C. Effect of Inoculum Type, Packing Material and Operational Conditions on the Biofiltration of a Mixture of Hydrophobic Volatile Organic Compounds in Air. Sci. Total Environ. 2023, 904, 167326. [Google Scholar] [CrossRef] [PubMed]
- Roalkvam, I.; Drønen, K.; Dahle, H.; Wergeland, H.I. Comparison of Active Biofilm Carriers and Commercially Available Inoculum for Activation of Biofilters in Marine Recirculating Aquaculture Systems (RAS). Aquaculture 2020, 514, 734480. [Google Scholar] [CrossRef]
- Chaghouri, M.; Gennequin, C.; Tidahy, L.H.; Cazier, F.; Abi–Aad, E.; Veignie, E.; Rafin, C. Low Cost and Renewable H2S-Biofilter Inoculated with Trichoderma Harzianum. Environ. Technol. 2024, 45, 1508–1521. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-H.; Park, S.-W.; Lee, E.-J. Effect of Temperature on the Performance of a Biofilter Inoculated WithPseudomonas Putida to Treat Waste-Air Containing Ethanol. Korean J. Chem. Eng. 2005, 22, 922–926. [Google Scholar] [CrossRef]
- Jang, J.H.; Hirai, M.; Shoda, M. Performance of a Styrene-Degrading Biofilter Inoculated with Pseudomonas Sp. SR-5. J. Biosci. Bioeng. 2005, 100, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Allievi, M.J.; Silveira, D.D.; Cantão, M.E.; Filho, P.B. Bacterial Community Diversity in a Full Scale Biofilter Treating Wastewater Odor. Water Sci. Technol. 2018, 77, 2014–2022. [Google Scholar] [CrossRef]
- Ni, J.; Yang, H.; Chen, L.; Xu, J.; Zheng, L.; Xie, G.; Shen, C.; Li, W.; Liu, Q. Metagenomic Analysis of Microbial Community Structure and Function in a Improved Biofilter with Odorous Gases. Sci. Rep. 2022, 12, 1731. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lv, Y.-H.; Wang, C.; Jiang, G.-Y.; Han, M.-F.; Deng, J.-G.; Hsi, H.-C. Microbial Community Evolution and Functional Trade-Offs of Biofilm in Odor Treatment Biofilters. Water Res. 2023, 235, 119917. [Google Scholar] [CrossRef]
- Repečkienė, J.; Švedienė, J.; Paškevičius, A.; Tekorienė, R.; Raudonienė, V.; Gudeliūnaitė, E.; Baltrėnas, P.; Misevičius, A. Succession of Microorganisms in a Plate-Type Air Treatment Biofilter during Filtration of Various Volatile Compounds. Environ. Technol. 2015, 36, 881–889. [Google Scholar] [CrossRef]
- Jiang, W.; Tian, X.; Li, L.; Dong, S.; Zhao, K.; Li, H.; Cai, Y. Temporal Bacterial Community Succession during the Start-up Process of Biofilters in a Cold-Freshwater Recirculating Aquaculture System. Bioresour. Technol. 2019, 287, 121441. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).